Abstract
Background:
Subsequent neutrophil (polymorphonuclear neutrophil [PMN])-predominant inflammatory response is a predominant feature of ventilator-induced lung injury (VILI), and mesenchymal stem cell (MSC) can improve mice survival model of endotoxin-induced acute lung injury, reduce lung impairs, and enhance the repair of VILI. However, whether MSC could attenuate PMN-predominant inflammatory in the VILI is still unknown. This study aimed to test whether MSC intervention could attenuate the PMN-predominate inflammatory in the mechanical VILI.
Methods:
Sprague-Dawley rats were ventilated for 2 hours with large tidal volume (20 mL/kg). MSCs were given before or after ventilation. The inflammatory chemokines and gas exchange were observed and compared dynamically until 4 hours after ventilation, and pulmonary pathological change and activation of PMN were observed and compared 4 hours after ventilation.
Results:
Mechanical ventilation (MV) caused significant lung injury reflected by increasing in PMN pulmonary sequestration, inflammatory chemokines (tumor necrosis factor-alpha, interleukin-6 and macrophage inflammatory protein 2) in the bronchoalveolar lavage fluid, and injury score of the lung tissue. These changes were accompanied with excessive PMN activation which reflected by increases in PMN elastase activity, production of radical oxygen series. MSC intervention especially pretreatment attenuated subsequent lung injury, systemic inflammation response and PMN pulmonary sequestration and excessive PMN activation initiated by injurious ventilation.
Conclusions:
MV causes profound lung injury and PMN-predominate inflammatory responses. The protection effect of MSC in the VILI rat model is related to the suppression of the PMN activation.
Keywords: Inflammation, Mesenchymal Stem Cell, Neutrophil, Ventilator-induced Lung Injury
INTRODUCTION
Mechanical ventilation (MV), a crucial supported and therapeutic technique for surviving patients with acute or chronic respiratory failure, either with a very large or moderate or even small tidal volumes (VTs) could induce lung injury, which is known as ventilator-induced lung injury (VILI).[1,2]
Subsequent neutrophil (polymorphonuclear neutrophil [PMN])-predominant inflammatory response as well as diffuse alveolar damage and pulmonary and systemic vascular leak is the key features of VILI,[3,4,5] although the mechanisms of VILI with different impropriate ventilation parameters are not fully understood. Therapeutic methods referred to modulate the activation of PMNs, such as specific inhibitor of neutrophil elastase (NE)–N-[2-[[[4-(2,2-dimethyl-1-oxopropoxy) phenyl] sulfonyl] amino] benzoyl]-(S)-glycine monosodium salt (Sivelestat) administration,[6] macrophage inflammatory protein 2 (MIP-2) (one of PMN chemotactic factors) receptor knockout[7] are seemed to attenuate the severity of VILI. Hence, the management of PMN activation could be an important therapeutic approach to VILI.
Bone marrow-derived mesenchymal stem cells (MSCs) had been used to heal severe wounded or damage tissue as they preferentially localize to the injured sites as well as promoting the progress of repair and even adopting the phenotype of some kinds of cells in the local tissue after administration.[8,9] And the pulmonary studies showed that MSCs can immigrate into the lung, adopt the phenotype of lung cells and play positive roles in different models of lung injury.[10,11,12,13]
The aim of this study was to test the hypothesis that MSC infusion may be beneficial for VILI, which is linked these effects to the regulation of PMN; we used an in vivo rat model of high VT VILI to evaluate the effect of infusive MSC.
METHODS
Animals’ preparation
Ten specific pathogen-free (SPF) male Sprague-Dawley rats weighing 130–150 g (5–7 weeks old) and 30 SPF female Sprague-Dawley rats weighing 250–270 g were obtained from Animal Trial Center of Southern Medical University. All animals were maintained under SPF conditions in Animal Trial Center of Nanfang Hospital, Southern Medical University. Animals were handled according to the National Institutes of Health Guidelines on the use of laboratory animals. The experimental protocols were approved by the Animal Guidelines of Nanfang Hospital and Southern Medical University.
Isolation, expansion, and differentiation of mesenchymal stem cell
MSC was isolated from the male rats’ bone marrows after decapitated sacrifice and expanded as described in previous literature.[14] The expression of surface markers was determined by flow cytometry (FACS) to ascertain the purity of the population. Thereafter, we found that MSC cultures contained a small number of cells expressing cluster of differentiation 45 (CD45) (<0.9%) and CD34 (<0.7%), and mostly cells expressing CD29 (>99.9%) and CD44 (>97.8%). And the chondrocytes-target differentiation trial was carried as Xu et al[15] concerned to determine the ability of differentiation of the cultured MSC. The cells were cultured for 14 days and then stained with toluidine blue. These data illustrated that the purity and the differentiation property of cultured MSC.
In vivo animal model and protocol
Thirty female rats were randomized into the following experimental groups: control group (unventilated and saline treated, n = 6); MSC group (unventilated and MSC treated, n = 6); VT20 group (ventilated with high VT and saline treated, VT = 20 mL/kg, positive end-expiratory pressure = 2 mmHg, respiratory rate = 30 beats/min, insipratory-to-expiratory ratio = 1/2 and fraction of inspire O2 = 0.21, n = 6), MSC + VT20 group (MSC pretreated and ventilated with high VT, n = 6), and VT20 + MSC group (ventilated with high VT and MSC treated, n = 6).
All animals were anesthetized with pentobarbital sodium (40 mg/kg body weight) intra-peritoneal and then placed in the supine position on a platform device. Thereafter, a tracheotomy was performed, and a 14-gauge catheter (B. Braun, Germany) was inserted into the trachea and then a sterile catheter was inserted into the right carotid. And the blood was sampled for gas exchange and inflammatory and anti-inflammatory mediators just after catheterization as the baseline time point. And then the animals of ventilator groups were connected to a small animal mechanical ventilator (HX100E, Chengdu Time Technology, China) for 2 hours ventilation.
Animals from MSC + VT20 group or VT20 + MSC group were given MSCs (3 × 106/animal suspended with 0.5 mL saline[16]) 2 hours before ventilation or immediately after the ventilation via tail vein, respectively. And rats from MSC group received the MSC 2 hours after being catheterized. And the same tidal saline (0.5 mL) was injected to animal in control and VT20 groups at the same time. After receiving ventilation for 2 hours with high VT, had their tracheotomy closed and sutured and were sent back to their cages breathing room air. Four hours after MV, blood was sampled, and the carotid catheter was removed. And the animals from the control group and MSC group breathed room air for 2 hours and then have their tracheotomy closed and sent back to their cages for 4 hours. The rats were sacrificed 6 hours after catheterization (i.e. 4 hours after ventilation initiation in ventilator groups) by exsanguinations. During the 6 hours, the rats were monitored every 30 minutes to ensure adequate sedation and pentobarbital sodium was administered as required. And the blood was sampled for gas exchange (Mallin AkroBt Sensor Systems Ltd., USA) and inflammatory and anti-inflammatory mediators every 2 hours after catheterized during the experiment.
Bronchoalveolar lavage fluid
Four hours after ventilation, a bronchoalveolar lavage fluid (BALF) was performed using 2 mL 1 × PBS for 3 times (total 6 mL). The recovered rate of volume was 80% and 40 μl aliquots of each sample were used for cells counting, and the remaining fluid was centrifuged and the supernatant was analyzed for protein concentration by Biorad Assay (Biorad, Hercules, CA, USA).
Enzyme-linked immunosorbent assay for cytokine analysis
The levels of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and MIP-2 were determined in serum and BALF supernatants using ELISA kits. The kits were from R and D Systems (Minneapolis, MN, USA). The absorbance of each sample was measured at 450 nm using a microplate reader (BioRad 680, USA).
Neutrophil elastase activity in bronchoalveolar lavage fluid and serum
NE activity in BALF and serum was determined spectrophotometrically using N-methoxysuccinyl-Ala-Ala-Pro-Val p-nitroanilide as a specific synthetic substrate for NE as described.[6] Briefly, 20 μl of BALF or serum was incubated with 200 μl of 0.1 mmol/L tris-HCl buffer (pH 8.0) containing 0.5 mmol/L NaCl and 1 mmol/L substrate at 37°C for 24 hours and the amount of liberated p-nitroanilide was determined spectrophotometrically at 405 nm. NE activity was calculated from the standard curve of p-nitroanilide absorbance.
FACS analysis
Characteristics of MSC, intracellular reactive oxygen species (ROS) production, and the CD11b expression of PMN were determined by FACS. Briefly, the prepared cell suspensions of MSC were incubated with specific antibodies using phycoerythrin (PE)-anti-CD34, PE-anti-CD45, fluorescein isothiocyanate (FITC)-anti-CD29, and FITC-anti-CD44 (e-BIOSCIENCE, USA) before measurement of PMN characteristics. And the mixture of blood or BALF with LDS-751 was incubated with saturating concentrations of FITC-labeled mouse CD11b antibody (Sigma, USA), and 80 mmol/L 2’,7’-dichlorofluorescin diacetate (Molecular Probes, USA) before measurement of CD11b and intracellular ROS production of PMN. And the analysis was performed on an FACS using CellQuest software (Becton Dickinson, USA).
Intercellular reactive oxygen species production measurement
The inferior lobe of the right lung was isolated after the animals were killed and used for ROS (OH) measurement. Corresponding to the lung tissue weight, nine volumes of PBS containing antiproteases (5 μg/mL each of aprotinin, leupeptin, and pepstatin) were added. After homogenization on ice, the tissue samples were centrifuged (10 minutes, 4000 r/min at 4°C). The supernatant was used for ROS production measurement with Fenton reaction kit (Nanjin Jiancheng Bioengineering Institute, China). And the serum ROS production was also measured 6 hours after ventilation initiation.
Histological analysis
Lung tissues were removed immediately after animals were sacrificed, and the lung section with hematoxylin and eosin (HE) staining was made to assess the severity of lung injury under light microscopy using a modified lung injury score in three random sections of at least five lungs from each group of rats.[6] Briefly, the following pathological processes were scored on a scale of 0–4: (1) alveolar congestion, (2) hemorrhage, (3) leukocyte infiltration or neutrophil aggregation in the airspace or vessel walls, and (4) alveolar wall thickness. A score of 0 represented normal findings and scores of 1, 2, 3, and 4 represented mild (<25%), moderate (25%–50%), severe (50%–75%) and very severe (>75%) lung involvement, respectively. The overall score was based on the sum of all scores. And the number of PMN in lung tissue were determined per high-power fields under light microscopy (Olympus, Japan) at high-power magnification (HE, original magnification × 1000), and 10 random fields for each section and the means of 10 fields were adopted for each animals.
Statistical analysis
Normality test was conducted to determine the distribution of measurement data, and the data of the normal distribution were expressed as mean ± standard deviation (SD). Differences of measurement data were determined by one-way or repeated-measure analysis of variance with Bonferroni's post-test using SPSS version 13.0 (SPSS Inc., USA). Differences were accepted as statistically significant when P < 0.05.
RESULTS
Mesenchymal stem cell treatment attenuates ventilator-induced lung injury and pulmonary polymorphonuclear neutrophil sequestration
The arterial blood oxygen pressure decreased after receiving VT20 MV (all P < 0.05, vs. control group) and increased while administrated with MSC, but no significant difference was found among VT20 group, MSC + VT group and VT20 + MSC group in the same time point except that between MSC + VT20 group and VT20 group at the end of ventilation (T2) [Table 1]. Administration of MSC alone to normal rats caused no detectable alterations in lung structure. Lungs from VT20 group without MSC showed vascular hyperpermeability and PMNs infiltration increased, alveolar septae thickened and alveolar bloody effusion at the end of the trial. MSC preadministration attenuated these pathological changes significantly while MSC postadministration partially attenuated the PMN infiltration in the lung tissue. Though a part of the alveolar septae thickened severe in VT20 + MSC group, PMN pulmonary sequestration, and alveolar bloody effusion were less as compared to the VT20 group. And there was a significantly difference in injury score among VT20 group, MSC + VT20 group and VT20 + MSC groups [Figure 1].
Table 1.
PaO2 changes from different groups (mean±SD, mmHg)
| Groups (n=6) | T0 baseline | T2 at the end of MV | T4 2 hours after MV | T6 4 hours after MV |
|---|---|---|---|---|
| Control | 113.50 ± 7.15 | 115.50 ± 8.87 | 114.00 ± 7.62 | 112.50 ± 8.69 |
| MSC | 113.00 ± 11.08 | 113.33 ± 11.09 | 111.67 ± 12.48 | 112.00 ± 10.79 |
| VT20 | 119.50 ± 12.08 | 57.00 ± 4.43* | 58.33 ± 4.84* | 57.00 ± 5.18* |
| MSC+ VT20 | 119.00 ± 16.05 | 108.00 ± 24.75† | 104.67 ± 24.52 | 104.83 ± 25.69 |
| VT20+ MSC | 120.17 ± 10.85 | 83.17 ± 23.17 | 83.67 ± 19.05 | 80.33 ± 16.12* |
SD: Standard deviation; MV: Mechanical ventilation; MSC: Mesenchymal stem cell; VT: Tidal volume. *P < 0.05 versus control group; †P < 0.05 versus VT20 group.
Figure 1.
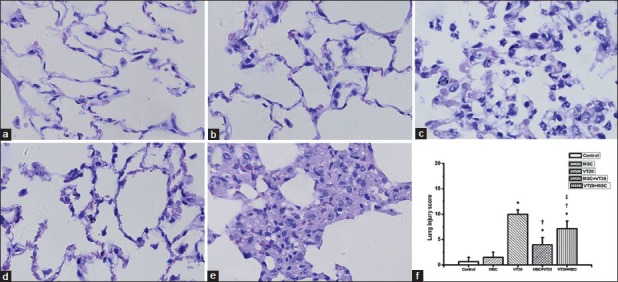
Pathological changes in different groups ([a-e] HE, original magnification ×1000) and lung injury score of pathological change ([f] Smith score). Administrated alone does not cause detectable morphologic change in lung tissue in the mesenchymal stem cell (MSC) group (b) as compared to the control group (a). VT20 caused vascular hyperpermeability and severe neutrophils infiltration, alveolar septae thickened and alveolar bloody effusion in the lung tissue (c). MSCs administration attenuated the lung injury caused by VT20 ([d] MSC+VT20 group; [e] VT20 + MSC group). *P < 0.05 vs. control group; †P < 0.05 vs. VT20 group; ‡P < 0.05, vs. MSC + VT20 group.
Mesenchymal stem cell intervention attenuates lung and systemic inflammation
The inflammatory marker levels of TNF-α, IL-6, MIP-2, and anti-inflammatory marker level of IL-10 in serum and BALF were shown in Figures 2 and 3. The levels of IL-6, TNF-α, and MIP-2 were increased in BALF and serum in VT20 group. The increasing levels of the three inflammatory markers in the BALF and serum in the VT20 group were significantly reduced by MSC intervention. Thus, MSC intervention significantly decreased levels of IL-6, TNF-α, and MIP-2 either in BALF or serum increased by VT20 ventilation. Similarly, VT20 ventilation significantly increased the IL-10 concentration; but MSC intervention did not affect the IL-10 concentration increased by VT20 MV.
Figure 2.
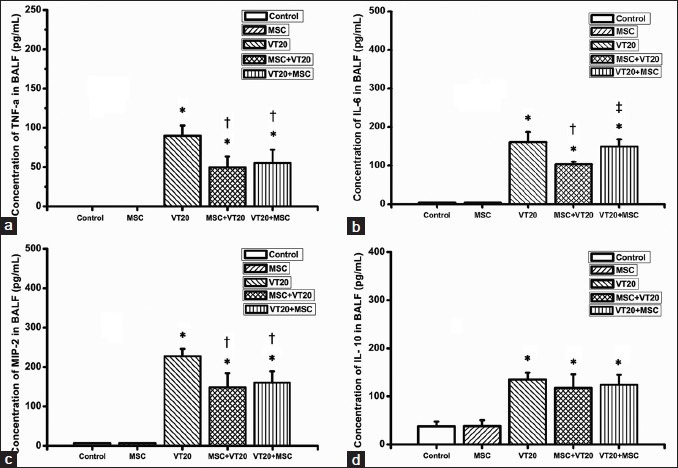
Comparison of pulmonary proinflammatory mediators and anti-inflammatory mediator concentrates 4 hours after ventilation. ([a] tumor necrosis factor-alpha in bronchoalveolar lavage fluid (BALF); [b] interleukin-6 (IL-6) in BALF; [c] macrophage inflammatory protein 2 in BALF; [d] IL-10 in BALF). *P < 0.05 vs. control group; †P < 0.05 vs. VT20 group; ‡P < 0.05 vs. MSC + VT20 group.
Figure 3.
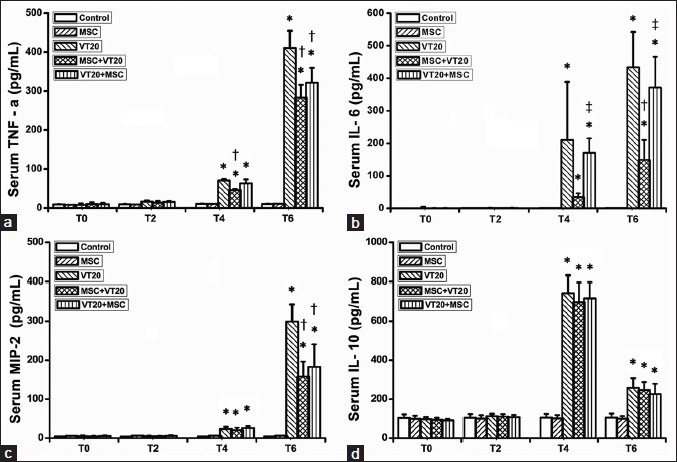
Comparison of systemic proinflammatory mediators and anti-inflammatory mediator concentrates ([a] serum tumor necrosis factor-alpha; [b] serum interleukin-6 [IL-6]; C: Serum macrophage inflammatory protein 2; [d] serum IL-10) (*P < 0.05, vs. control group in the same time point; †P < 0.05 vs. VT20 group in the same time point; ‡P < 0.05 vs. MSC + VT20 in the same time point. T0: Baseline; T2: At the end of 2 hours ventilation; T4: 2 hours after ventilation; T6: 4 hours after ventilation).
Mesenchymal stem cell attenuates polymorphonuclear neutrophil-dominated reactive oxygen species production and neutrophil elastase activity
MSC administration attenuated the VT20 induced increased intracellular NE activity of PMN, intracellular ROS production of PMN and serum ROS either in blood or BALF. And MSC preadministration especially lowered the serum ROS production as compared to the MSC postadministration in VT20 + MSC group. However, there was no significant difference among different groups about the surface molecular CD11b expression of PMN either in blood or BALF [Figure 4].
Figure 4.
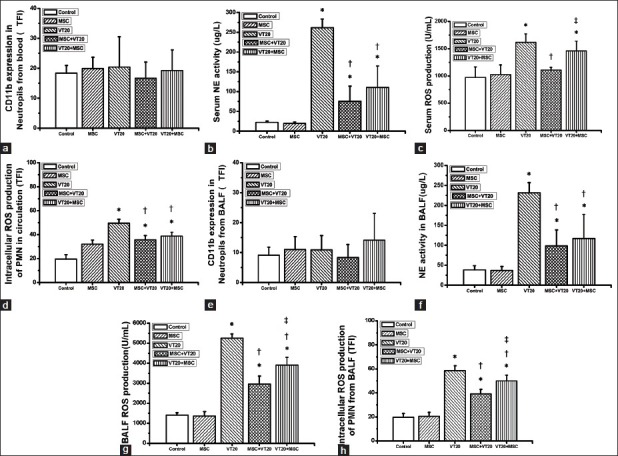
Effect of mesenchymal stem cell on polymorphonuclear neutrophil (PMN) activation 4 hours after ventilation. CD11b expression in surface of PMN from blood (a) or bronchoalveolar lavage fluid (BALF) (e); serum neutrophil elastase (NE) activity (b) or BALF NE activity (f); reactive oxygen species (ROS) production in serum (c) or BALF (g); intracellular ROS production of PMN from blood (d) or BALF (h). TFI: Total fluorescence intensity. *P < 0.05 vs. control group; †P < 0.05 vs. VT20 group; ‡P < 0.05 vs. MSC + VT20 group.
DISCUSSION
The main finding of this study was as follows: (1) MV with high VT could induce VILI, which was associated with increased systemic and lung inflammatory mediators (TNF-α, IL-6, and MIP-2), and a subsequent activation of PMN, which includes increasing the number of PMN immigrating to the alveolar space and the release of NE and oxidants; (2) MSC administration, especially pre-treatment attenuated the VILI and its subsequent PMN-predominant inflammatory response.
Injurious MV induced impairment in gas exchange, interstitial pulmonary edema, and even membrane hyaline formation. These findings were in keeping with previous studies showing worsening gas and pathological change after high VT ventilation.[17,18] This indicated that MSC administration can attenuate the ongoing alveolar epithelial insults. However, as we did not track the infused MSC in situ in this study dynamically, we could not acknowledge the defined relationship of the degree of lung morphological change and the MSC infusion. Hence, further research is needed to investigate the exact relationship of the lung injury and the MSC through tracking the MSC in situ.
PMN is traditionally regarded as one of key innate immunologic and inflammatory cells in acute severe diseases with acute systemic or local inflammation. The PMN recruitment and NE release play a central role in the pathogenesis of VILI including cell damage, extracellular matrix degradation, and alveolar-capillary hyperpermeability.[7] And we again ascertained the predominant feature of dramatically increase of PMN pulmonary sequestration and NE release in the VILI model. Our further research illustrated that MSC infusion significantly attenuated the degree of PMN pulmonary sequestration and NE release induced by impropriate ventilation as well as inflammatory chemokines and cytokines (including TNF-α, IL-6 and MIP-2), and do not affect the IL-10s (one type of anti-inflammatory cytokine), which consisted with previous VILI research.[11] It was reported that modulating the activation of PMN like specific NE inhibitor-sivelestat administration could reduce PMN sequestration by suppressing the release of PMN chemotactic factors (MIP-2/IL-8) and inflammatory cytokines (TNF-α and IL-6).[6,8] Here, this finding supported the notion that MSC might attenuate the PMN sequestration and NE release by suppressing the release of early-stage proinflammatory cytokines (such as TNF-α and IL-6) and PMN chemotactic factor (MIP-2).
In addition, we also found that the ROS production either in serum or from the PMN was significantly increased in the VILI rat model, which again illustrated that the excess activation of PMN exist in the VILI animal models.[19,20] And our data referred to MSC intervention also showed the lowering effect of MSC on the VILI-induced ROS production of PMN. However, our data referred to the expression of CD11b (a classical biological receptor linked to phagocytosis of PMN) did not influence by ventilation and MSC; here, further research is called to explain the phenomenon.
Some study limitation should be noted here: first, our target inflammatory cell was PMN as it became the main ingredients of pulmonary sequestrated inflammatory cells, whose infiltration into the lung might play a pivotal role in the VILI pathogenesis. But the other cells like macrophages might play an important role in the VILI as the protective effects of MSC in the model may also partially relate with the inhibition of macrophages activation.[21] Second, though our results showed that the PMN activity was associated with the protective effect of MSC in the model, we still cannot determine the exact mechanism of protective effects of MSC to the VILI, as the involving pathways and related molecules are so many and the interaction with pathways is still complex and puzzle.[22] Third, no lung infection preventive action was adopted in the current study; hence, we do not exclude the possibility of lung infection during the study.
In conclusion, we have shown that MSC systemic administered attenuated the excess activation of PMN while reduced the severity of VILI. That may provide a basis for the development of an innovative approach for the prevention and treatment of VILI, which continues to be an important complication of patients with acute and chronic respiratory failure undergoing ventilation. And our finding also indicated that MSC administration could be a hopeful therapy in subsequent inflammatory states induced by first “nonmicro” attack which attenuates organ injury via modulating the dominant inflammatory cell, like PMN.
Footnotes
Edited by: Xin Chen
Source of Support: This study was supported by a grant from Natural Science Foundation of Guangdong Province (No. S2012040006274).
Conflict of Interest: None declared.
REFERENCES
- 1.Gajic O, Lee J, Doerr CH, Berrios JC, Myers JL, Hubmayr RD. Ventilator-induced cell wounding and repair in the intact lung. Am J Respir Crit Care Med. 2003;167:1057–63. doi: 10.1164/rccm.200208-889OC. [DOI] [PubMed] [Google Scholar]
- 2.Frank JA, Gutierrez JA, Jones KD, Allen L, Dobbs L, Matthay MA. Low tidal volume reduces epithelial and endothelial injury in acid-injured rat lungs. Am J Respir Crit Care Med. 2002;165:242–9. doi: 10.1164/ajrccm.165.2.2108087. [DOI] [PubMed] [Google Scholar]
- 3.Uhlig S. Ventilation-induced lung injury and mechanotransduction: Stretching it too far? Am J Physiol Lung Cell Mol Physiol. 2002;282:L892–6. doi: 10.1152/ajplung.00124.2001. [DOI] [PubMed] [Google Scholar]
- 4.Wolters PJ, Wray C, Sutherland RE, Kim SS, Koff J, Mao Y, et al. Neutrophil-derived IL-6 limits alveolar barrier disruption in experimental ventilator-induced lung injury. J Immunol. 2009;182:8056–62. doi: 10.4049/jimmunol.0801323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai TS, Cai SX, Guo ZH. Serum and lung endothelin-1 increased in a canine model of ventilator-induced lung injury. Chin Med J. 2010;123:1021–7. [PubMed] [Google Scholar]
- 6.Sakashita A, Nishimura Y, Nishiuma T, Takenaka K, Kobayashi K, Kotani Y, et al. Neutrophil elastase inhibitor (sivelestat) attenuates subsequent ventilator-induced lung injury in mice. Eur J Pharmacol. 2007;571:62–71. doi: 10.1016/j.ejphar.2007.05.053. [DOI] [PubMed] [Google Scholar]
- 7.Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med. 1998;157:294–323. doi: 10.1164/ajrccm.157.1.9604014. [DOI] [PubMed] [Google Scholar]
- 8.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 9.Nagai A, Kim WK, Lee HJ, Jeong HS, Kim KS, Hong SH, et al. Multilineage potential of stable human mesenchymal stem cell line derived from fetal marrow. PLoS One. 2007;2:e1272. doi: 10.1371/journal.pone.0001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M, Ikehara S. Bone-marrow-derived mesenchymal stem cells for organ repair. Stem Cells Int. 2013;2013:132642. doi: 10.1155/2013/132642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curley GF, Hayes M, Ansari B, Shaw G, Ryan A, Barry F, et al. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax. 2012;67:496–501. doi: 10.1136/thoraxjnl-2011-201059. [DOI] [PubMed] [Google Scholar]
- 12.Chimenti L, Luque T, Bonsignore MR, Ramírez J, Navajas D, Farré R. Pre-treatment with mesenchymal stem cells reduces ventilator-induced lung injury. Eur Respir J. 2012;40:939–48. doi: 10.1183/09031936.00153211. [DOI] [PubMed] [Google Scholar]
- 13.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–63. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 14.Wang ZH, Guo KY, Yang YL, Liu K, Li J, Zhou XY, et al. Effect of bone-marrow mesenchymal stem cells on the immune function of aging rats (in Chinese) J South Med Univer. 2011;31:146–50. [PubMed] [Google Scholar]
- 15.Xu J, Woods CR, Mora AL, Joodi R, Brigham KL, Iyer S, et al. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L131–41. doi: 10.1152/ajplung.00431.2006. [DOI] [PubMed] [Google Scholar]
- 16.Ohnishi S, Yanagawa B, Tanaka K, Miyahara Y, Obata H, Kataoka M, et al. Transplantation of mesenchymal stem cells attenuates myocardial injury and dysfunction in a rat model of acute myocarditis. J Mol Cell Cardiol. 2007;42:88–97. doi: 10.1016/j.yjmcc.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–36. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 18.Nin N, Lorente JA, de Paula M, El Assar M, Vallejo S, Peñuelas O, et al. Rats surviving injurious mechanical ventilation show reversible pulmonary, vascular and inflammatory changes. Intensive Care Med. 2008;34:948–56. doi: 10.1007/s00134-007-0959-6. [DOI] [PubMed] [Google Scholar]
- 19.Kaynar AM, Houghton AM, Lum EH, Pitt BR, Shapiro SD. Neutrophil elastase is needed for neutrophil emigration into lungs in ventilator-induced lung injury. Am J Respir Cell Mol Biol. 2008;39:53–60. doi: 10.1165/rcmb.2007-0315OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Syrkina O, Jafari B, Hales CA, Quinn DA. Oxidant stress mediates inflammation and apoptosis in ventilator-induced lung injury. Respirology. 2008;13:333–40. doi: 10.1111/j.1440-1843.2008.01279.x. [DOI] [PubMed] [Google Scholar]
- 21.Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E (2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–9. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, Chai J, Yu Y, Hou Y. Advances in the research of the role of mesenchymal stem cell in wound healing (in Chinese) Chin J Burns. 2014;30:134–7. [PubMed] [Google Scholar]


