Abstract
Background:
Rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly prolonged event-free survival in first-line chemotherapy for patients with diffuse large B-cell lymphoma (DLBCL). But relapse and refractory DLBCL occur frequently. Although rituximab is effective, its role in salvage therapy after autologous transplant remains unclear. Maintenance therapy with rituximab in responding patients after first line chemotherapy may be a useful novel approach capable of eradicating minimal residual disease and to bring survival benefit. This systematic review and meta-analysis evaluated the effects of rituximab maintenance treatment and salvage therapy of patients with DLBCL.
Methods:
We performed a systematic review and meta-analysis of randomized controlled trials and compared rituximab maintenance or salvage therapy at relapse with observation. We searched the Cochrane Library, PubMed, EMBASE, conference proceedings, databases of ongoing trials, and references of published trials. Two reviewers independently assessed the quality of the trials and extracted data. Hazard ratios for time-to-event data were estimated and pooled.
Results:
Seven trials including 1470 DLBCL patients were included in this systematic review and meta-analysis. Patients treated with maintenance rituximab have better overall survival (OS) and event-free survival (EFS) than patients in the observation arm, but there was no statistical significance. Patients who received rituximab salvage therapy for relapse or refractory DLBCL have statistically significantly better OS [HR of death = 0.72, 95% CI (0.55-0.94), P = 0.02], progression-free survival (PFS) [HR = 0.61, 95% CI (0.52-0.72), P < 0.05], odds ratio (OR) [RR = 1.26, 95% CI (1.07-1.47), P = 0.004] than patients in the observation arm. The rate of infection-related adverse events was higher with rituximab treatment [RR = 1.37, 95% CI = (1.14 - 1.65) P =0.001].
Conclusions:
After first-line chemotherapy, the two rituximab-combined treatment strategies, including maintenance and salvage therapies can bring survival benefit. But due to the few studies, the low methodological quality assessment and the low outcome evidence quality, it's not confirmed that the two strategies are better than normal chemotherapy regimens. More high-quality randomized controlled trials are still needed to provide reliable evidence. The higher rate of infections after rituximab therapy should be taken into consideration when making treatment decisions.
Keywords: Diffuse Large B Cell Lymphoma, Meta-analysis, Review, Rituximab
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) is the most frequent histological type among malignant lymphomas, accounting for approximately 30% of cases.[1,2] DLBCL is highly chemosensitive and curable. The use of anti-CD20 antibody in addition to chemotherapy has significantly improved outcomes in patients with DLBCL. Rituximab in combination with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy has emerged as the standard of care for first-line DLBCL therapy, which can improve long-term survival.[3,4] However, relapse is detected in approximately 30% of patients despite R-CHOP therapy. Curative effect could be improved. After 6-8 cycles of first-line treatment, non-progressing patients enter in the so called “watch and wait” period in which periodical disease restaging is performed until the progression is reported then a salvage therapy is started, though it's got a poor long-term survival. Most patients relapsed within the first 2 or 3 years.[5,6] Now a new pattern called maintenance therapy refers to the use of systemic therapy, either by continuing the primary drug or switching to a new one, in patients who get objective response or stable disease from the first-line chemotherapy.[7,8] This was primarily tested in patients with refractory or relapsed (i. e., previously treated) follicular lymphoma, who had a survival benefit with rituximab maintenance therapy [HR for death = 0.58, 95% CI (0.42-0.79)].[9,10] To date, limited data from randomized clinical trials are available to guide the use of rituximab as maintenance therapy or salvage therapy for DLBCL patients who respond to induction therapy or relapse, and few long-term data have been published. The value of rituximab as maintenance or salvage therapy for DLBCL patients who respond to induction therapy or suffer relapse is yet to be determined.[11] We performed a systematic review of the literature and a meta-analysis of all randomized trials to evaluate the effects of rituximab maintenance treatment and salvage therapy for patients with DLBCL.
METHODS
Search strategy
Two independent reviewers performed the literature search, study selection and extraction of data. Any disagreement between the two reviewers was resolved by consensus in meetings that involved all authors. The studies for our meta-analysis were retrieved from searches of the PubMed and Cochrane Library, EMBASE, conference proceedings, databases of ongoing trials, and references of published trials. Search terms included“randomized control trial”, “clinical trial”,“diffuse large B-cell lymphoma” iff “DLBCL”,“Rituximab” or “monoclonal antibodies”, “ituximab” or “monoc” and “salvage therapy”, and similar terms were cross-searched. We scanned references of all included trials and reviews identified for additional studies. We included all randomized controlled trials that compared rituximab maintenance therapy and salvage therapy with observation in patients with histologically confirmed DLBCL, regardless of publication status, date of publication, and language.
Inclusion and exclusion criteria
For maintenance therapy: the research type was randomized controlled trial; the meta-analysis included patients histologically diagnosed as stage I-IV DLBCL who have reached complete remission (CR)/unconfirmed complete remission (CRu)/partial remission (PR) after induced chemotherapy regardless of chemotherapy regimens, method of administration and dosage.
For salvage therapy: the research type was randomized controlled trials; the meta-analysis included patients histologically diagnosed as stage I-IV DLBCL who have suffered relapse of disease.
We excluded ongoing studies, interim analyses, nonrandomized studies, and studies with 10 or fewer patients per study arm.
Study selection and data extraction
Two investigators independently screen the titles and abstracts of all studies identified in the literature research to verify compliance with the inclusion and exclusion criteria. When this information was unsatisfactory, we performed a full-text analysis that considered the confined inclusion and exclusion criteria. The third investigator resolved disagreements between two investigators. The reviewers who screened the studies independently performed data extraction and quality assessment of all included articles.
Outcome measures
The primary outcome was overall survival (OS).
Secondary outcomes included progression-free survival (PFS), failure-free survival (FFS), odds ratio (OR) and adverse events.
Data synthesis and statistical analysis
Studies were grouped on the basis of strategy (maintenance or salvage) and analyzed separately. Further, we compared the pooled effect of the two strategies derived from the two analyses. For time-to-event data, the log hazard ratios (HRs) and their variances were estimated using the methods proposed by Parmar et al.,[12] when CIs of HRs were reported. Otherwise, median survival time, events in each arm, and P values of the log-rank or Cox proportional hazard regression model were used to estimate log HRs and their variances. The study heterogeneity was tested and P < 0.1 was defined as heterogenous. A fixed-effect model (Mantel-Haenszel) was applied in case of absence of heterogeneity between studies and otherwise a random-effect model was performed. The meta-analysis results were displayed as forest plots. All calculations were performed using Review Manager [RevMan], version 5.0 for Windows.
The results were described by forest plots, every square represented each study's OR or HR estimate. The pooled OR or HR was symbolized by a solid diamond at the bottom of the forest plot and the width of the square represented the 95% CI of OR or HR. The size of the square represents the weight that the corresponding study exerts in the meta-analysis. Potential sources of heterogeneity were explored through stratifying by type of induction therapy (chemotherapy, rituximab, chemotherapy + rituximab), rituximab schedule (one infusion every 2 months; four weekly infusions every 6 months), allocation concealment, blinding, and size of studies. All statistical tests were two-sided.
RESULTS
Description of trials
We identified 232 potentially relevant trials from our initial electronic search, and excluded 216 trials after a preliminary review. The remaining 16 studies were assessed in detail and 7 randomized controlled trials met the inclusion criteria, three of which involved maintenance therapy and the other four articles involved salvage therapy. All the included trials were published in full text. Table 1 summarized the baseline characteristics of the participants and the design of the studies included.
Table 1.
Baseline characteristics for included trials
| Investigators | No. of patients in meta-analysis | Age (year) | Stage | Quality of allocation concealment | Quality of sequence generation | Treatment status | Rituximab administration protocol | Median follow-up (month) |
|---|---|---|---|---|---|---|---|---|
| Habermann et al, 2006[13] | 352 | 60-92 | I-IV | Unclear | Adequate | Maintenance therapy | 375 mg/m2/w X 4w every 6 mo for 2 y | 42 |
| Haioun et al, 2009[14] | 269 | 18-60 | I-IV | Unclear | Adequate | Maintenance therapy | 375 mg/m2/w X4w | 60 |
| Jäger et al, 2013[15] | 440 | >18 | I-IV | Unclear | Unclear | Maintenance therapy | 375 mg/m2/2w X2 y | 60 |
| Aviles et al, 2010[16] | 100 | 32-63 | III-IV | Unclear | Adequate | Salvage therapy | R-ESHAP/ESHAP | 64.5 |
| Olivieri et al, 2006[17] | 46 | 18-65 | I-IV | Unclear | Unclear | Salvage therapy | R-DHAP+HDT+ASCT/DHAP+HDT+ASCT | NA |
| Sieniawski et al, 2007[18] | 38 | 23-62 | I-IV | Unclear | Unclear | Salvage therapy | R-DHAP+ASCT/DHAP+ASCT | 60 |
| Vellenga et al, 2008[19] | 225 | 25-65 | I-IV | Unclear | Adequate | Salvage therapy | R-DHAP-RVIM-RDHAP+ASCT/DHAP-VIM-DHAP+ASCT | 31 |
ID: identity; ASCT: autologous stem cell transplantation; HDT: high-dose chemotherapy; VIM: etoposide-ifosfamide-methotrexate. ; ESHAP:etoposide-methylprednisolone-cytosine-arabinoside-platinum; DHAP: dexamethasone-Cisplatin-Cytarabine
Patient characteristics
Three trials included patients with DLBCL who receive rituximab maintenance therapy immediately after induce chemotherapy. One trial[13] included untreated DLBCL patients 60 years or older who got CR/CRu/PR after R-CHOP or CHOP. One trial[14] included untreated 18–60 years old patients with CD20 (+) who got CR/CRu/PR after first-line therapy. Two trials[14,15] made a subgroup analysis according age-adjusted international prognostic index (aaIPI).
Four trials[16,17,18,19] reported salvage therapy with rituximab on relapse or refractory disease, while one trial[16] reported patients who received rituximab and normal-dose chemotherapy. Three trials[17,18,19] reported patients who received rituximab and high-dose chemotherapy followed by autologous stem cell transplantation (ASCT). Other common exclusion criteria of the original trials were poor performance status, active infection, symptomatic central nervous system disease, and a history of serious medical conditions. The follow-up periods ranged from 31 to 64.5 months.
Trial design
In three trials,[13,14,15] patients were randomly assigned to a type of induction therapy and subsequently underwent a second random assignment to maintenance therapy or observation.
In the salvage trials,[16,17,18,19] all patients were treated with a type of induction therapy and were randomly assigned to salvage therapy or observation after relapse or refractory.
Quality assessment
The quality of included reports was scored using the Jadad composite scale,[20] which assessed the trials according to the following three questions: (1) whether they reported an appropriate randomization method; (2) whether double blindness was mentioned in the trial and whether the trial was appropriately performed; (3) whether they reported withdrawals and dropouts. The quality scale ranged from 0 to 5 points, with a low-quality report receiving a score of 2 or less and a high-quality report receiving a score of at least 3.
Overall survival
Habermann (2006)[13] reported patients who were treated with rituximab maintenance therapy had better OS than patients in the observation group [HR = 0.96, 95% CI (0.63-1.47), P = 0.85], regardless of R-CHOP or CHOP as induction therapy, but no significant differences were observed. Jäger et al.,[15] reported at a median follow-up of 45 months, in the rituximab maintenance vs. observation groups: three-year OS as 92.0% vs. 90.3% [HR = 0.78 (95% CI: 0.49-1.34)].
Three trials (409 patients) about salvage therapy were eligible for the meta-analysis of OS. No statistical heterogeneity between studies was examined; a random-effect model was used. Patients who were treated with rituximab salvage therapy had statistically significantly better OS than patients in the observation group [HR of death = 0.72, 95% CI = 0.55 - 0.94, P = 0.02] [Figure 1].
Figure 1.
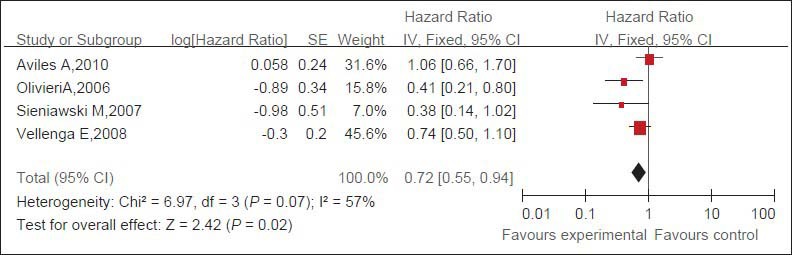
Pooled HRs of OS of patients with refractory or relapsed DLBCL and of control patients. Black squares represent the point estimate, their sizes represent their weight in the pooled analysis, and the horizontal bars represent the 95% CI. The black diamond at the bottom represents the pooled point estimate. HR: Hazard ratio for death; CI: confidence interval; experimental: salvage therapy with rituximab
Secondary outcomes EFS PFS OR
Habermann (2006)[13] reported patients who were treated with rituximab maintenance therapy had statistically significantly better 3-year FFS than patients in the observation group [HR = 0.63, 95% CI (0.44-0.90), P = 0.009]. Haioun (2009)[14] observed an increased 4-year EFS in the rituximab arm compared with the observation arm. In one subgroup analysis, there was a significant improvement in EFS for patients who experienced a CR following ASCT and received maintenance rituximab compared with those who received observation only [HR = 0.38, 95% CI (0.19-0.90), P = 0.02]. In another subgroup analysis according to aaIPI, the two groups (aaIPI = 2/aaIPI = 3) all have improvement in EFS, but no statistical significance was observed [HR = 0.66, 95% CI (0.27-1.29), P = 0.20; HR = 0.69, 95% CI (0.31-2.01), P = 0.70]. Jäger et al.[15] reported in the rituximab maintenance group vs. observation group, three-year EFS was 80.1% vs. 76.5% [HR = 0.78 (95% CI: 0.57-1.08); P = 0.067]; three-year PFS was 86.3% vs. 79.0% [HR = 0.62 (95% CI: 0.43-0.90)]. In the patient subgroups stratified by treatment arm and IPI risk group (≤1 vs. ≥2), EFS was significantly longer in those patients with an IPI risk score ≤ 1 and was longer for rituximab maintenance than observation [HR = 1.67 (95% CI: 1.18–2.35); P = 0.012].
Three trials[16,17,19] (371 patients) about salvage therapy were eligible for the meta-analysis of PFS. Due to no statistical heterogeneity between the studies, a random-effect model was used. The rituximab group has statistically significantly better PFS than the observation group [HR = 0.61, 95% CI (0.52-0.72), P < 0.05] [Figure 2].
Figure 2.
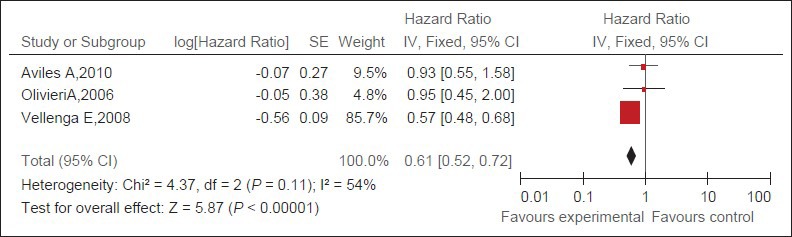
Pooled HRs of PFS of patients with refractory or relapsed DLBCL and of control patients. Black squares represent the point estimate, their sizes represent their weight in the pooled analysis, and the horizontal bars represent the 95% CI. The black diamond at the bottom represents the pooled point estimate. HR: Hazard ratio for death; CI: confidence interval; experimental: salvage therapy with rituximab.
Four trials[16,17,18,19] (309 patients) about salvage therapy were eligible for the meta-analysis of OR. There is no statistical heterogeneity between studies; a random-effect model was used. The rituximab group has statistically significantly better OR than the observation group [RR = 1.26, 95% CI (1.07-1.47), P = 0.004] [Figure 3].
Figure 3.
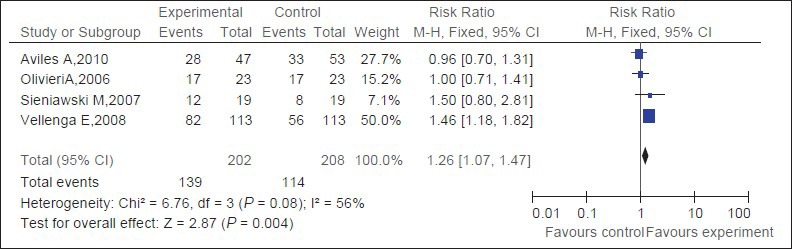
Pooled OR of patients with refractory or relapsed DLBCL and of control patients. Black squares represent the point estimate, their sizes represent their weight in the pooled analysis, and the horizontal bars represent the 95% CI. The black diamond at the bottom represents the pooled point estimate. OR : overall remission rate; CI: confidence interval; experimental: salvage therapy with rituximab
Adverse events
The main adverse events were Grade 3 or 4 leukocytopenia and infection, which were reported in three trials.[15,16,18] Specifically, patients who underwent rituximab as second-line therapy had more infection-related adverse events than patients in the observation arm [RR = 1.37, 95% CI = (1.14 - 1.65) P < 0.05] [Figure 4].
Figure 4.
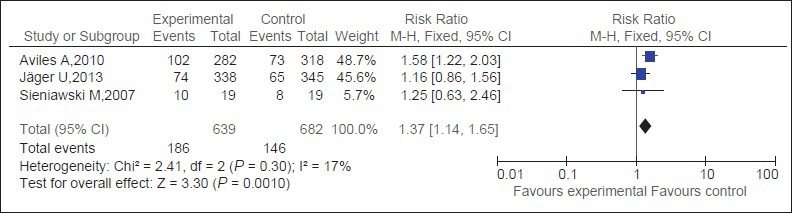
Infection-related adverse events in patients with DLBCL treated with rituximab compared with observation. Black squares represent the point estimate, their sizes represent their weight in the pooled analysis, and the horizontal bars represent the 95% CI. The black diamond at the bottom represents the pooled point estimate. CI: confidence interval; experimental: salvagetherapy with rituximab
In first-line treatment of DLBCL, the addition of the monoclonal antibody rituximab to standard chemotherapy has consistently been shown to improve results with regard to overall remission rates, PFS and OS.[21]
In contrast, to date a few prospective randomized trials exist to determine the value of adding rituximab to maintenance therapy or salvage chemotherapy after first-line treatment for relapsed or refractory DLBCL. To our knowledge, there is no published meta-analysis about this question.
In this meta-analysis seven studies were included, three of which were about rituximab maintenance therapy. Due to the heterogeneity between studies, we made a systematic review which demonstrated that rituximab maintenance treatment improved OS and FFS after induction chemotherapy regardless of whether conventional chemotherapy or immuno-chemotherapy was chosen in induction therapy, but there was no statistical significance observed about OS, despite this effect on FFS was statistically significant, especially for the subgroup patients whose induction chemotherapy did not contain rituximab. Maintenance treatment after high-dose chemotherapy followed by ASCT in first-line treatment with rituximab prolonged EFS in either aaIPI 2 or 3 and CRu/PR subgroup, but there was no statistical significance, except for CR subgroup. So whether rituximab maintenance is a good option after first-line chemotherapy needs more prospective, randomized controlled study to provide evidence.
We didn’t observed significant heterogeneity among studies in the analysis of rituximab salvage therapy, so we made a meta-analysis, which demonstrated that rituximab combining high-dose chemotherapy and ASCT salvage therapy statistically significant improved OS, PFS, OR and disease control compared with observation in patients with refractory or relapsed DLBCL who responded to induction therapy. Despite different kinds of induction therapy and salvage chemotherapy regimens, this meta-analysis showed that rituximab-combined salvage therapy improved OS, PFS, OR than conventional high-dose chemotherapy with ASCT; the differences were statistically significant.
Three studies reported the adverse events. The most common adverse events were infections. We all know that rituximab may cause immunosuppression through several mechanisms, such as neutropenia and hypogammaglobulinemia.[22,23] These effects might be of even greater clinical significance when rituximab is administered in maintenance or salvage therapy.[24] Meantime, the financial costs of this rituximab should be taken into consideration.
This study has several other limitations. Heterogeneity is a potential problem that affects the results. Many factors might cause significant heterogeneity, such as different induction therapy, second-line treatment regimens, rituximab administration protocols, aaIPI and ASCT. Furthermore, due to very few studies, low methodological quality assessment and low outcome evidence quality, it's not confirmed that rituximab-combined salvage therapy is better than normal chemotherapy regimens. More high-quality randomized controlled trials are needed to provide reliable evidence.
Rituximab maintenance as a new strategy is rising in the treatment of DLBCL after first-line therapy. However, to date there is still lack of trials comparing the strategy of rituximab maintenance therapy to rituximab second-line treatment. So for DLBCL, the doubt persists whether to give rituximab maintenance directly when first-line therapy ends or to follow “watch and wait” procedure until disease progression. It needs more prospective, randomized controlled study to provide the evidence.
Footnotes
Edited by: De Wang
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–42. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 2.van Oers MH, Klasa R, Marcus RE, Wolf M, Kimby E, Gascoyne RD, et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood. 2006;108:3295–301. doi: 10.1182/blood-2006-05-021113. [DOI] [PubMed] [Google Scholar]
- 3.Cabanillas F. Rituximab in DLBCL: 6 years on. Lancet Oncol. 2011;12:984–5. doi: 10.1016/S1470-2045(11)70251-0. [DOI] [PubMed] [Google Scholar]
- 4.Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, et al. MabThera International Trial Group. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–91. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi H, Hirakawa T, Inokuchi K. Importance of relative dose intensity in chemotherapy for diffuse large B-Cell lymphoma. J Clin Exp Hematop. 2011;51:1–5. doi: 10.3960/jslrt.51.1. [DOI] [PubMed] [Google Scholar]
- 6.Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–90. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Zang J, Xu J, Bai C, Qin Y, Liu K, et al. Maintenance therapy with continuous or switch strategy in advanced non-small cell lung cancer: a systematic review and meta-analysis. Chest. 2011;140:117–26. doi: 10.1378/chest.10-2745. [DOI] [PubMed] [Google Scholar]
- 8.Michallet AS, Lebras L, Coiffier B. Maintenance therapy in diffuse large B-cell lymphoma. Curr Opin Oncol. 2012;24:461–5. doi: 10.1097/CCO.0b013e3283562036. [DOI] [PubMed] [Google Scholar]
- 9.Vidal L, Gafter-Gvili A, Leibovici L, Dreyling M, Ghielmini M, Hsu Schmitz SF, et al. Rituximab maintenance for the treatment of patients with follicular lymphoma: systematic review and meta-analysis of randomized trials. J Natl Cancer Inst. 2009;101:248–55. doi: 10.1093/jnci/djn478. [DOI] [PubMed] [Google Scholar]
- 10.Vidal L, Gafter-Gvili A, Salles G, Dreyling MH, Ghielmini M, Hsu Schmitz SF, et al. Rituximab maintenance for the treatment of patients with follicular lymphoma: an updated systematic review and meta-analysis of randomized trials. J Natl Cancer Inst. 2011;103:1799–806. doi: 10.1093/jnci/djr418. [DOI] [PubMed] [Google Scholar]
- 11.Lindenmeyer LP, Hegele V, Caregnato JP, Wust D, Grazziotin L, Stoll P. Follow-up of patients receiving rituximab for diffuse large B cell lymphoma: an overview of systematic reviews. Ann Hematol. 2013;92:1451–9. doi: 10.1007/s00277-013-1811-4. [DOI] [PubMed] [Google Scholar]
- 12.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–7. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 14.Haioun C, Mounier N, Emile JF, Ranta D, Coiffier B, Tilly H, et al. Rituximab versus observation after high-dose consolidative first-line chemotherapy with autologous stem-cell transplantation in patients with poor-risk diffuse large B-cell lymphoma. Ann Oncol. 2009;20:1985–92. doi: 10.1093/annonc/mdp237. [DOI] [PubMed] [Google Scholar]
- 15.Jäger U, Trneny M, Melzer H. A multicentre, randomized, phase III study of rituximab as maintenance treatment versus observation alone in patients with aggressive B-cell lymphoma: The AGMT NHL13 trial. EHA Congress Abstracts. 2013:P309. [Google Scholar]
- 16.Aviles A, Neri N, Huerta-Guzman J, de Jesus Nambo M. ESHAP versus rituximab-ESHAP in frail patients with refractory diffuse large B-cell lymphoma. Clin Lymphoma Myeloma Leuk. 2010;10:125–8. doi: 10.3816/CLML.2010.n.017. [DOI] [PubMed] [Google Scholar]
- 17.Mey UJ, Olivieri A, Orlopp KS, Rabe C, Strehl JW, Gorschlueter M, et al. DHAP in combination with rituximab vs DHAP alone as salvage treatment for patients with relapsed or refractory diffuse large B-cell lymphoma: a matched-pair analysis. Leuk Lymphoma. 2006;47:2558–66. doi: 10.1080/10428190600926572. [DOI] [PubMed] [Google Scholar]
- 18.Sieniawski M, Staak O, Glossmann JP, Reineke T, Scheuss H, Diehl V, et al. Rituximab added to an intensified salvage chemotherapy program followed by autologous stem cell transplantation improved the outcome in relapsed and refractory aggressive non-Hodgkin lymphoma. Ann Hematol. 2007;86:107–15. doi: 10.1007/s00277-006-0210-5. [DOI] [PubMed] [Google Scholar]
- 19.Vellenga E, van Putten WL, van ‘t Veer MB, Zijlstra JM, Fibbe WE, van Oers MH, et al. Rituximab improves the treatment results of DHAP-VIM-DHAP and ASCT in relapsed/progressive aggressive CD20+NHL: a prospective randomized HOVON trial. Blood. 2008;111:537–43. doi: 10.1182/blood-2007-08-108415. [DOI] [PubMed] [Google Scholar]
- 20.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary. Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 21.Coiffier B. Rituximab in combination with CHOP improves survival in elderly patients with aggressive non-Hodgkin's lymphoma. Semin Oncol. 2002;29:18–22. doi: 10.1053/sonc.2002.32749. [DOI] [PubMed] [Google Scholar]
- 22.Cattaneo C, Spedini P, Casari S, Re A, Tucci A, Borlenghi E, et al. Delayed-onset peripheral blood cytopenia after rituximab: frequency and risk factor assessment in a consecutive series of 77 treatments. Leuk Lymphoma. 2006;47:1013–7. doi: 10.1080/10428190500473113. [DOI] [PubMed] [Google Scholar]
- 23.Lim SH, Esler WV, Zhang Y, Zhang J, Periman PO, Burris C, et al. B-cell depletion for 2 years after autologous stem cell transplant for NHL induces prolonged hypogammaglobulinemia beyond the rituximab maintenance period. Leuk Lymphoma. 2008;49:152–3. doi: 10.1080/10428190701742506. [DOI] [PubMed] [Google Scholar]
- 24.Filanovsky K, Shvidel L, Shtalrid M, Haran M, Duek A, Berrebi A. Predictive factors to hypogammaglobulinemia and non-neutropenic infection complications after rituximab/chemotherapy treatment. Blood. 2007;110:1288. [Google Scholar]


