Introduction
Diabetes mellitus is associated with decrements in cognitive function and changes in brain structure. People with both type 1 and type 2 diabetes have been shown to have mild to moderate reductions in cognitive function as measured by neuropsychological testing compared to non-diabetic controls. Type 2 diabetes (T2DM) has also been associated with 50% increased risk of dementia.1 Whether such an association is true for people with type 1 diabetes(T1DM) is not yet known.
Interestingly, diabetes has been known to have an effect on the brain for more than one hundred years. In the early twentieth century, researchers and clinicians recognized that people with diabetes frequently complained of poor memory and attention. In 1922 Mile et al,2 showed that people with diabetes performed poorly on cognitive tasks examining memory and attention. The term ‘diabetic encephalopathy’ was introduced in 1950 to describe central nervous system related complications of diabetes.3 Other terms like functional cerebral impairment and central neuropathy have also been used in literature to describe diabetes related cognitive dysfunction.4 Mijnhout et al,4 have proposed the term —‘diabetes-associated cognitive decline’ (DACD) to describe diabetes related mild to moderate reductions in cognitive function.
With the growing epidemic of diabetes and the ever increasing number of people who live to old age, diabetes related cognitive dysfunction could have challenging future public health implications. In this review, we will examine the research that has been done over the last two decades to increase our understanding of how diabetes affects brain function and structure. At the conclusion, we will make suggestions of future research that could help us address the challenges we may face as more people live longer with diabetes than ever before.
Cognitive dysfunction in type 1 diabetes
Longitudinal studies
In the Diabetes Control and Complications Trial (DCCT) and its follow-up Epidemiology of Diabetes Interventions Complications (EDIC) study, patients with T1DM underwent comprehensive battery of cognitive tests at study enrollment (at mean age of 27 years) and 18 years later. The results demonstrated that patients with worse metabolic control (glycated hemoglobin values >8.8%) showed moderate declines in motor speed and psychomotor efficiency as compared to those with better control (glycated hemoglobin <7.4%).5 Frequency of severe hypoglycemia was not associated with decline in any cognitive domain in this population. Similar results were seen in the Stockholm Diabetes Intervention Study (SDIS), where at 10-year follow up cognitive function was similar in both treatment groups, and was not related to the number of severe hypoglycemic episodes.6
T1DM is commonly diagnosed during childhood and adolescence. This is a period of rapid developmental changes in the central nervous system and there has been concern that the younger brain maybe more susceptible to extremes of glycemia.7 In a sub analysis of the DCCT cohort where only participants who were 13–19 years at time of entry in the DCC were included, Musen and colleagues8 reported that severe hypoglycemia was not associated with cognitive decline and higher A1C values were associated with declines in the psychomotor and mental efficiency domain, as was found in the population as a whole. In another prospective study Ryan and colleagues9 found that at 7 year follow up, adults with T1DM (age 34 at entry) showed significant declines on measures of psychomotor efficiency compared to non-diabetic controls. No differences were seen in domains of learning, memory, or problem-solving tasks. Proliferative retinopathy, autonomic neuropathy and duration of diabetes were associated with cognitive decline.
Cross-sectional studies
Cross-sectional studies have shown that relative to non-diabetic controls, subject with type 1 diabetes have performance deficits in multiple cognitive domains including information processing speed, psychomotor efficiency, memory, attention, visuospatial abilities and executive function.10–14
Perantie et al11 reported that children with T1DM who experienced severe hypoglycemic episodes before the age of five had deficits in spatial intelligence and delayed recall, suggesting developing brain at very young ages maybe susceptible to effect of hypoglycemia. Other factors including poor glycemic control and presence of microvascular complication like neuropathy and retinopathy have also been associated with cognitive dysfunction in subjects with T1DM.10; 11
Systematic reviews and Meta-analysis
Brands et al15 performed a meta-analysis to examine the nature and magnitude of cognitive impairment in T1DM. This analysis included 33 studies with participants who were mostly less 50 years of age. The authors reported that compared to non-diabetic controls, people with T1DM had mild to moderate declines (effect size ranging from d −0.3 to −0.7) in multiple domains including intelligence, speed of information processing, psychomotor efficiency, attention, cognitive flexibility, and visual perception. Lowered cognitive performance in diabetic patients appeared to be associated with the presence of microvascular complications but not with the occurrence of severe hypoglycemic episodes or with poor metabolic control. Gaudieri et al16 preformed a meta-analysis which included data from 19 studies in children with T1DM. They found that children with T1DM had a decrement in a broad range of domains, however the magnitude of decrement was greater in children who were diagnosed with diabetes at < than 7 years of age compared with those with later onset. This observation again suggests that early age of onset may be an important variable of cognitive dysfunction in children with T1DM.
In summary, results from both longitudinal and cross-sectional studies show that T1DM is associated with mild to modest decrements in cognitive function. Domains of psychomotor speed, mental flexibility, attention and general intelligence are most commonly affected. Data from prospective studies suggest that hypoglycemia is not a risk factor for cognitive decline; however this may not be true for children with young age at onset of diabetes. Early age of onset and presence of microvascular complications are important risk factor for cognitive decline. Longitudinal studies looking at cognitive function in elderly subject with T1DM are lacking and necessary since age and duration of diabetes are important contributors to the changes in cognitive function found in T2DM. More research is needed to understand the clinical implications of these mild-moderate decrements in cognitive functioning in the daily lives of people with T1DM.
Cognitive dysfunction in type 2 diabetes
Longitudinal studies
Several longitudinal studies have evaluated the impact of T2DM on cognitive function. These studies have been done mostly in middle age to older adults and have examined the magnitude and rate of change in cognitive function in non-demented subjects with T2DM compared to non-diabetic controls. All of these studies have included a relatively short follow up period of less than six years.17–21 The neuropsychological examination done as part of the research range from limited testing to extensive batteries that examined all major cognitive domains. Most studies attempted to control for confounding factors like age, education, stroke, hypertension, visual impairment, dyslipidemia, heart disease, exercise and depression but were unable to address the underlying mechanisms responsible for any differences they found between the subjects with diabetes and the controls without the disease.
Despite these limitations, data from these prospective studies have shown that people with T2DM perform less well than controls in the cognitive domains of information-processing speed, memory, attention and executive function.18–21 Mental flexibility and global cognitive function17 have also shown to be effected in some, but not in all studies.18
In the Utrecht Diabetic Encephalopathy Study cohort, Van denBerg21 and colleagues reported that subjects with T2DM performed poorly in the cognitive domains of information-processing speed, attention and executive functions both at baseline and at the 4 year of follow up compared to non-diabetic controls, however there was no evidence of accelerated cognitive decline in subjects with T2DM. In contrast, other studies have found evidence of accelerated decline in cognitive function over a follow up of 3–6 years in subjects with T2DM.19; 20 However, only one of these studies found reduced performance in the people with T2DM at baseline compared to controls,19 raising questions about when in the course of diabetes and aging these reductions in cognitive function develop.
Decrements in cognitive function in subjects with T2DM have been associated with increased duration of diabetes19 and poor glycemic control.18 The ACCORD Memory in Diabetes (MIND) Study22 sought to directly determine if the level of glycemic control impacts cognitive performance over time in nearly 3000 subjects with T2DM. In this study, subjects were either randomized to intensive glycemic control where the target was a HbA1c <6% or to a standard strategy targeting HbA1c to 7%–7.9%. At baseline, Cukierman-Yafee et al23 showed that there was an inverse relationship between cognitive function and glycemic control as measures by HbA1c. However, after 40 months of follow up there was no significant difference in the cognitive function between the intensive and standard treatment arms. Interestingly, Hugenschmidt et al24 found that there was an association between the presence of diabetic retinopathy at baseline and changes in cognitive function over time in T2DM subjects participating in both the ACCORD – MIND and the ACCORD – Eye substudies. In this analysis, baseline diabetic retinopathy and severity of retinopathy was associated with decline in global cognitive function and processing speed over 40 months. Similar association was not seen for domains of executive function and memory.
Retinal vessels and cerebral small vessels have similar embryology and anatomy,25 raising the possibility that changes in the microvascular may be responsible for both the retinopathy and the cognitive changes.
In another longitudinal study,26 a cohort of healthy community-dwelling elderly subjects underwent extensive battery of cognitive test at baseline and after 4 years. Despite similar initial cognitive function, diabetic subjects tended to have an unfavorable evolution of cognitive performance over 4 years compared with subjects who had normal glucose or impaired fasting glucose. After 4 years people with diabetes showed decrements in the cognitive domains of memory, attention and psychomotor speed.
Dementia due to both Alzheimer’s disease and vascular disease have also been linked to type 2 diabetes in longitudinal studies. Rawkings and colleagues27 recently reported that diabetes in midlife was associated with a 19% greater cognitive decline over 20 years compared with no diabetes in the ARIC (Atherosclerosis Risk in Communities) study cohort. In this study cognitive decline was noted primarily in the domains of processing speed and executive function and was associated with duration of diabetes. In a large prospective population-based cohort study of more than 6000 elderly subjects, the presence of T2DM almost doubled the risk of dementia.28
Cross-sectional studies
Cross-sectional studies have also shown that subjects with T2DM performed poorly in several cognitive domains including attention, executive function, information-processing, memory, psychomotor efficiency, verbal fluency and learning.29–33 These reductions have been associated with poor glycemic control,29 longer duration of diabetes,29; 31 and the presence of microvascular complications like diabetic retinopathy34 and peripheral neuropathy.35 Epidemiological studies have also shown that comorbidities like hypertension, dyslipidemia and depression36–38 are associated with poor cognitive function in subject with T2DM.
While long duration of diabetes appears to be an important risk factor for cognitive dysfunction, even patients in early phases of the disease including prediabetes appear to be affected.33; 39 Yau et al40 assessed cognitive function in adolescents (average age 16 years) with type 2 diabetes and found that adolescents with diabetes had lower performance in intellectual function, verbal memory and psychomotor efficiency compared to non-diabetic control. However, the type of cognitive deficits found in subject with T2DM also appear to be more pronounced in people who are 60 year and older.41
Systematic reviews and Meta-analysis
Longitudinal and cross sectional studies have clearly demonstrated an association between diabetes and mild to moderate cognitive dysfunction in T2DM, but less is known about the strength of association between diabetes and dementia. To address this question, investigators have performed systematic reviews and meta-analyses of small studies to increase the likelihood of finding an association. In one such systematic review, Biessels et al1 reported that risk of dementia was increased by 50–100% in people with T2DM relative to people without diabetes. This approach has also been used to identify the cognitive domains particularly impacted by diabetes. One systematic review which included data from 27 studies, found that processing speed, attention, memory and cognitive flexibility were the most commonly effected domains in subjects with T2DM with effect sizes ranging from 0—1.9.42 Palta et al43 performed a meta-analyses of data from 24 studies in which cognitive function was compared between subjects with T2DM and controls. They found reductions of small to moderate effect size in people with T2DM, which ranged from −0.26 to −0.36 in the domains of motor function executive function, processing speed verbal memory and visual memory.
In summary, T2DM is associated with mild to moderate cognitive deficits mostly in the domains of memory, psychomotor speed, and executive function. Changes in cognitive function compared to non-diabetic control can be seen early in the course of T2DM; however duration diabetes, glycemic control and presence of microvascular complication are important risk factors. There is also increasing body of evidence showing that in the elderly population, T2DM increases the risk of dementia.
Imaging studies on diabetes and brain structure
Various neuroimaging techniques have been employed to study impact of diabetes on brain structure and function. This approach has also been used to define the structural correlates of cognitive dysfunction in diabetes and to provide insights into the mechanisms underlying the CNS complications of the disease. Here we will review studies which have used magnetic resonance based techniques including structural MRI, diffusion tensor imaging (DTI), magnetic resonance spectroscopy (MRS) and functional MRI (fMRI) to assess brain structure and function in diabetes (Table 1).
Table 1.
Studies examining relationship between diabetes and brain volume
| Study | Groups: Number | Mean age patient (years) | Study design | Outcome and results (diabetes compared to control) | Association with diabetes related risk factors |
|---|---|---|---|---|---|
| Musen et al., 200644 | T1DM: 82 Controls:36 |
33 | Cross sectional | lower GMD primarily in the posterior, temporal, and cerebellar regions | Lower GMD associated with poor glycemic control and higher frequency of severe hypoglycemia |
| Hughes et al., 201345 | T1DM: 104 Controls: 151 |
49 | Cross sectional | Smaller GMV in frontal lobe | No significant association between diabetes-associated variables and reduced GMV |
| Wessels et al., 200646 | T1DM: 13 DR, 18 NDR Controls: 21 |
42 | Cross sectional | Reduced GMD in the right inferior frontal gyrus and right occipital lobe | Reduced GMD seen in patients with diabetic retinopathy |
| Hershey et al., 201053 | T1DM: 95 Controls: 49 |
12 | Cross sectional | No difference in hippocampal volumes between the groups | Greater exposure to severe hypoglycemia was associated with larger hippocampal volumes |
| Moran et al., 201366 | T2DM: 350 Controls: 363 |
67 | Cross sectional | Reduced GMV seen in hippocampal, frontal, cingulate, and temporal regions. Reduced WMV seen in the frontal and temporal regions |
|
| den Heijer et al., 200367 | T2DM: 41 Controls: 465 |
77 | Cross sectional | Smaller hippocampal and amygdalar volumes | Insulin resistance associated with amygdalar atrophy in non-diabetic controls |
| Brundel et al., 201068 | T2DM: 56 Controls:30 |
70 | Cross sectional | Reduced cortical grey matter, most pronounced in the temporal lobe | Atrophy in the hippocampal region associated with the presence of small vessel disease |
| Manschot et al., 200769 | T2DM: 122 Controls: 56 |
66 | Cross sectional | Increased cortical and subcortical atrophy | Retinopathy associated with more pronounced cortical atrophy |
| van Elderen et al., 201071 | T2DM: 89 Controls: 438 |
75 | longitudinal | Reduced brain volume at baseline with increase rate of volume loss during follow up | Fasting glucose level and insulin treatment associated with rate of brain volume loss |
| de Bresser et al., 201074 | T2DM: 55 Controls: 28 |
66 | longitudinal | Reduced brain volume at baseline with greater increase over time in lateral ventricular volume | Increasing age and hypertension associated with greater progression of cerebral atrophy |
Abbreviations: T1DM, type 1 diabetes; T2DM, type 2 diabetes; GMD, gray matter density; GMV, Gray matter volume; DR, diabetic retinopathy; NDR, No diabetic retinopathy; WMV, White matter volume
Type 1 diabetes
Structural MRI techniques are most commonly used to examine the impact of diabetes on total and regional brain volumes. Structural MRI studies have shown lower gray and white volumes in subject with T1DM compared to non-diabetic controls. Musen 2006 et al44 used voxel-based morphometry to examine brain changes in 82 patients with T1DM. Compared to non-diabetic control, subjects with diabetes had lower gray matter density primarily in the posterior, temporal, and cerebellar regions of the brain. Lower gray matter density was associated with poor glycemic control, higher frequency of severe hypoglycemic events, age of onset and duration of diabetes. In another study, the frontal lobe appeared to be the location of reduced volumes in patients with type 1 diabetes relative to controls.45 Reduced white matter volumes has been identified in subjects with T1DM by Wessels et al and this volume loss was associated with lower performances on tests for attention, speed of information processing, and executive function.14 Age of onset, duration of diabetes and presence retinopathy have also been associated with structural changes in imaging studies in T1DM.46; 47 Patients with T1DM have also been shown to have increased white matter lesions (WML) which may represent vascular abnormalities in intraparenchymal cerebral arterioles(Figure 1).48; 49 In subjects with T1DM increased severity of WML compared to controls has been reported in some50 but not all studies.51
Figure 1.
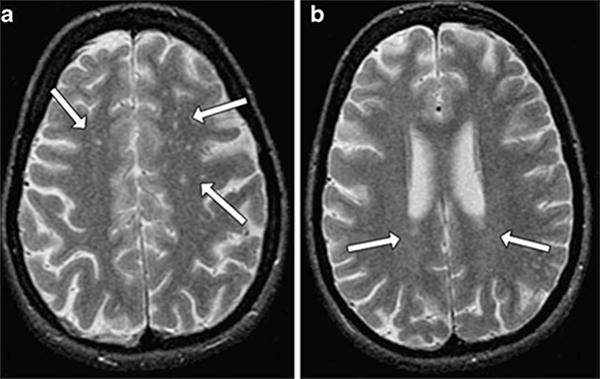
Example of white matter hyperintensities on MRI images. A Arrows indicate deep white matter hyperintensities. B Arrows indicate periventricular hyperintensities.
Source: Weinger et al., 200751 Reproduced with permission from the publisher, Springer-Verlag.
Compared to studies in people with T2DM there are limited data available on hippocampal volume in adults with T1DM. A small study in adults with T1DM did not find any difference in hippocampal volumes between adults with T1DM and controls.52 Hershey et al examined a large sample of youth with type 1 diabetes and compared them with their siblings without diabetes.53 Over all there were no difference in hippocampal volumes between the groups but hippocampal gray matter volume was larger in those children with type 1 diabetes with history of three or more severe hypoglycemic episodes in the past. Overall studies show that T1DM is associated with reduction in brain volume compared to non-diabetic controls; the distribution of brain areas involved appear to be variable and the changes in brain structure has been associated with decline in cognitive performance.
Diffusion tensor imaging (DTI) can identify white matter microstructural deficits by measuring the directionally restrained diffusion of water (anisotropy) within fiber tracts. Specifically, a reduction in fractional anisotropy (FA) due to the loss of restriction of water movement is expected when fiber bundles are damaged by the pathology. In a DTI study in subjects who had diabetes for at least 15 years, Kodl et al54 reported white matter microstructural deficits in the posterior corona radiata and the optic radiation which correlated with lower performance in cognitive tests thought to be associated with white matter function. In vivo brain magnetic resonance spectroscopy (1H-MRS) can noninvasively quantify concentration of various metabolites. Mangia et al,55 using MRS report lower NAA and glutamate concentration in gray matter rich occipital lobe of patients with T1DM. Lower NAA is thought to be marker of neuronal low or dysfunction.
Other than providing invaluable information about tissue structure and microstructure, MRI is a method of choice also to evaluate brain function, and is being increasingly utilized in diabetes research. Current MRI approaches employed for functional brain mapping detect task-evoked energy requirements and accompanying hemodynamic responses. The most common fMRI technique is the Blood Oxygenation Level Dependent (BOLD) contrast,56 which detect signal changes induced by the alterations in the local content of deoxyhemoglobin (dHb) which intrinsically acts as an endogenous contrast agent. Since the dHb content critically depends on a complex interplay of hemodynamic and metabolic parameters, caution is warranted when interpreting fMRI results in diabetes, as an altered neurovascular coupling cannot be always ruled out due to the possible vascular complications of the disease.
Even during a so-called “resting-state” condition, i.e. in absence of external stimuli or tasks, there are physiological variations in brain activity and accompanying hemodynamic events that manifest as fluctuations in the BOLD signal. In fact, it has been long recognized that the engagement of brain areas to a task occur on top of a complex baseline state. Synchronized neural activity exists between distinct brain locations in any given period of time, an observation which leads to the concepts of brain functional connectivity and resting-state (RS) networks. Such brain networks are remarkably consistent across healthy subjects.57 Some of these networks are clearly linked to neurobiological relevant functions, as the visual, auditory, motor, sensory networks, while the interpretation of other networks still remains less clearly defined. Another network referred to as “default mode” network (DMN),58–60 has attracted considerable interest in the clinical neuroscience community for its possible interpretation as the baseline cognitive state of a subject and its link to memory and executive function in normal and pathological conditions. The default mode network involves the anterior cingulate cortex and the posterior cingulate cortex, which are known to be involved in attention-related processes,61 and a number of other regions that are transiently or consistently deactivated during different types of cognitive tasks.62
The impact of T1DM on brain functional connectivity is still poorly characterized as compared to T2DM. Few recent functional connectivity studies have been conducted on T1DM patients with neuropathic pain63 and with or without microangiopathy.64 Such studies revealed abnormalities in networks involving attention,63 working memory, auditory and language processing, and motor and visual areas.64 In particular, reduced functional connectivity in the attention network was found in diabetics with microangiopathy compared to controls, but not in patients who did not have microangiopathy.64 In a subsequent study by the same group,65 subclinical macroangiopathy was also found to be a factor that likely contribute to development of diabetes-related cognitive changes in T1DM. More extensive studies aimed at establishing the impact of other clinical features of the disease including hyperglycemia or hypoglycemia episodes have yet to be performed.
Type 2 diabetes
People with T2DM have also been shown to have brain atrophy including lower total and regional white and gray matter volumes compared to non-diabetic controls.66 In a large cross sectional study Moran et al66 reported that subjects with T2DM had lower total gray, white, and hippocampal volumes. Regions with loss of gray matter include the medial temporal, anterior cingulate, and medial frontal lobes. White matter loss was found in the frontal and temporal regions. These investigators determined that brain volume loss was associated with poor performance in cognitive testing in these patients with type 2 diabetes. Other studies have suggested that atrophy may be greater in the hippocampal region in patient with T2DM.67; 68 Patients withT2DM have also been shown to have increased white WML.66; 69 Brain atrophy and WML has been associated with cognitive dysfunction in some69 but not all studies.70
In prospective studies, subjects with T2DM showed an accelerated progression of brain atrophy and WML over 3–4 years71–74 relative to controls. Diabetes related risk factors including hypertension, duration of diabetes, glycemic control and retinopathy have been associated with brain structural changes in this patient population.24; 69; 74 However, in a more recent study using ultra-highfield MRI at 7 tesla, Brundel and colleagues75 did not find any differences in the presence and number microvascular lesions (microinfarcts and microbleeds) in patients with T2DM compared to controls, nor did they find that microvascular lesions were associated to performance on cognitive testing. As in T1DM, studies in T2DM also show the distribution of volume loss across brain areas is variable but medial temporal lobe appears to be more susceptible. Future work will need to be done to determine if particular groups of patients with type 2 diabetes are at greater risk for changes in brain structure and function.
Using diffusion magnetic resonance imaging Reijmer et al reported microstructural abnormalities and disruptions in the white matter network in people with T2DM compared with controls. These abnormalities were related to slowing of information-processing speed.76; 77 Reduced white and grey matter microstructural integrity has also been shown in obese adolescents with type 2 diabetes,40 suggesting that these structural changes are related to diabetes specific factors other than the atherosclerotic vascular disease related changes seen in older people with diabetes.
Decreased connectivity of the posterior cingulate cortex (PCC) within the default mode network is not only commonly observed in patients with Alzheimer’s disease78 and mild cognitive impairment,79 but is observed also in subjects with T2DM.80 Abnormal functional connectivity of the PCC to selected brain regions in patients with T2DM also appear to correlate with lower fractional anisotropy (FA) in the cingulum bundle and uncinate fasciculus,81 and with insulin resistance.82
Patients with T2DM not only demonstrate reduced functional connectivity within the resting state default mode network, but also show abnormal involvement of the default mode network during task performance,83 including a reduced activation of the dorsolateral prefrontal cortex during encoding and reduced deactivation of the default mode network during recognition, with these effects being possibly exacerbated by acute hyperglycemia.
Other alterations of brain functional connectivity have been reported in T2DM84 which resemble those observed in individuals at risk for Alzheimer’s disease,79(including a reduced resting-state connectivity between the hippocampus and other brain regions.80; 84 In a study by Zhou et al, the decline in cognitive performance in T2DM was associated with a reduction in functional connectivity of the hippocampus.84 These are interesting observations, because patients with T2DM have an increased incidence of both Alzheimer’s disease85–89 and vascular type dementia86; 89; 90 therefore abnormal functional connectivity might constitute an early marker of subsequent cognitive decline for patients withT2DM. Future longitudinal studies are however necessary to determine whether these changes are predictive of cognitive dysfunction.
Functional connectivity of other brain regions outside the default mode network and hippocampus have been also associated with cognitive dysfunction in T2DM. For example, in a recent study by Cui et al,91 a decreased amplitude of low frequency fluctuations (possibly indicative of reduced functional connectivity) was observed in the postcentral gyrus and occipital lobe of patients with T2DM compared to controls. Interestingly, this finding was present in the absence of structural brain changes and was associated with worse memory performance and executive functioning. Disturbances of low frequency fluctuations have been observed in several additional brain areas.92; 93 For instance, smaller fluctuations in the bilateral middle temporal gyrus have been associated with higher A1C values, impaired β-cell function and poor neurocognitive performances.92
It is likely that the microvascular complications of diabetes largely contribute to the development of brain functional abnormalities, which possibly even precede the cognitive decline observed in T2DM. Indeed, when diabetics with or without microangiopathy were compared to non-diabetic controls, reductions of functional connectivity were observed only in patients with microangiopathy.64 In addition, diabetic retinopathy is considered to be an independent risk factor for cognitive decline in diabetes.94
The pathophysiology underlying the cognitive decline and brain structural changes in subjects with diabetes is not well understood. Poor glycemic control, vascular disease, oxidative stress, genetic predisposition, insulin resistance and amyloid disposition have been proposed as possible contributors, these proposed mechanisms are discussed in detail in other published reviews.1; 95; 96
Conclusion
Both type 1 and type 2 diabetes are associated with mild to moderate decrements in cognitive function. They are significant differences in the underlying pathophysiology of cognitive impairment between type 1 and type 2 diabetes. T1DM is usually diagnosed at an early age and may have effects on brain development. Chronic hyperglycemia and microvascular complications are important risk factors common to both type 1 and type 2 diabetes. T2DM is usually diagnosed at an older age and is commonly associated with obesity, insulin resistance, hypertension and dyslipidemia, all of which can have negative impact on brain. The underlying mechanism and the risk factors that may lead to the development of more severe cognitive dysfunction like dementia in some but not all people with diabetes are not well understood. Large longitudinal studies, in especially in older people with diabetes, are needed to better understand the impact, progression and risk factors that drive the development of diabetes related cognitive dysfunction. Both type 1 and type 2 diabetes have also been associated with structural and functional changes in the brain. However the direct relationship between structural or functional changes seen in specific brain areas to specific cognitive task has not been well identified.
More studies are needed to understand the impact of mild to moderate decrements in cognitive function in the daily lives people with diabetes. This mild to moderate degree of cognitive impairment likely does cause not clinically significant problems in the day to day activities of most people with diabetes. However it may present problems during more stressful and challenging situations. People at the extremes of age are more likely to be at increased risk of developing clinically significant decline in cognitive function. Cognitive impairment in children with early onset T1DM appears to negatively affect their academic performance.97 In elderly people with T2DM, cognitive dysfunction is associated with poor diabetes self-management, requiring more assistance with personal care and increased risk of hospitalization.98 More research is needed to develop specific diagnostic criteria or severity scores to identify people who are at increased risk of developing accelerated or clinically significant cognitive decline. Specific therapeutic interventions or preventive measures to prevent cognitive decline have not been developed. The DCCT/EDIC study provides some evidence that good glycemic control has beneficial effects on cognitive decline in people with T1DM. However the ACCORD Memory in Diabetes (MIND) Study, with relatively shorter duration of follow up did not show benefit of intensive control on cognitive function in T2DM. It is also unclear if reduction of vascular risk factors in T2DM will have beneficial effects on cognitive function in T2DM. Overall results of available studies do not support universal screening for cognitive impairment in all subjects with diabetes. Increased awareness about the risk of cognitive impairment in diabetes among medical providers in warranted and screening may be considered if a treatment regimen is to be intensive to ensure the patient can adhere to the regimen. Patients and their families should be counseled about risk factors associated with cognitive decline. Screening for cognition dysfunction should be considered in subjects with cognitive complaints or in older subjects with T2DM, especially if there is evidence of deterioration in everyday functional ability. Large prospective intervention studies with long-term follow up with neuroimaging and neuropsychological assessments are needed to develop strategies to prevent and treat this brain related complication of diabetes.
Acknowledgments
Grant support: Amir Moheet is supported by CTSA 5KL2TR000113.
LITERATURE CITED
- 1.Biessels GJ. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurology The. 5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 2.Miles WR, Root HF. Psychologic tests applied to diabetic patients. Arch Intern Med. 1922;30:767–777. [Google Scholar]
- 3.Dejong R. The Nervous System Complications of Diabetes Mellitus, with Special Reference to Cerebrovascular Changes. J Nerv Ment Dis. 1950;111:181–206. [Google Scholar]
- 4.Mijnhout GS, Scheltens P, Diamant M, et al. Diabetic encephalopathy: A concept in need of a definition. Diabetologia. 2006;49:1447–8. doi: 10.1007/s00125-006-0221-8. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson A, Musen G, Ryan C, et al. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med. 2007;356:1842–52. doi: 10.1056/NEJMoa066397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reichard P, Pihl M, Rosenqvist U, et al. Complications in IDDM are caused by elevated blood glucose level: the Stockholm Diabetes Intervention Study (SDIS) at 10-year follow up. Diabetologia. 1996;39:1483–1488. doi: 10.1007/s001250050602. [DOI] [PubMed] [Google Scholar]
- 7.Arbelaez AM, Semenkovich K, Hershey T. Glycemic extremes in youth with T1DM: the structural and functional integrity of the developing brain. Pediatric Diabetes. 2013;14:541–553. doi: 10.1111/pedi.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musen G, Jacobson AM, Ryan CM, Cleary PA, Waberski BH, Weinger K, Dahms W, Bayless M, Silvers N, Harth J, White N, Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Impact of diabetes and its treatment on cognitive function among adolescents who participated in the Diabetes Control and Complications Trial. Diabetes Care. 2008;31:1933–1938. doi: 10.2337/dc08-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan CM, Geckle MO, Orchard TJ. Cognitive efficiency declines over time in adults with Type 1 diabetes: effects of micro- and macrovascular complications. Diabetologia. 2003;46:940–8. doi: 10.1007/s00125-003-1128-2. [DOI] [PubMed] [Google Scholar]
- 10.Ryan CM, Williams TM, Finegold DN, et al. Cognitive dysfunction in adults with type 1 (insulin-dependent) diabetes mellitus of long duration: effects of recurrent hypoglycaemia and other chronic complications. Diabetologia. 1993;36:329–34. doi: 10.1007/BF00400236. [DOI] [PubMed] [Google Scholar]
- 11.Perantie DC, Lim A, Wu J, et al. Effects of prior hypoglycemia and hyperglycemia on cognition in children with type 1 diabetes mellitus. Pediatric Diabetes. 2008;9:87–95. doi: 10.1111/j.1399-5448.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- 12.Northam E, Rankins D, Lin A, et al. Central nervous system function in youth with type 1 diabetes 12 years after disease onset. Diabetes Care. 2009;32:445–50. doi: 10.2337/dc08-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohmann S, Popow C, Rami B, et al. Cognitive functions and glycemic control in children and adolescents with type 1 diabetes. Psychol Med. 2010;40:95–103. doi: 10.1017/S0033291709005777. [DOI] [PubMed] [Google Scholar]
- 14.Wessels AM, Rombouts SA, Remijnse PL, et al. Cognitive performance in type 1 diabetes patients is associated with cerebral white matter volume. Diabetologia. 2007;50:1763–1769. doi: 10.1007/s00125-007-0714-0. [DOI] [PubMed] [Google Scholar]
- 15.Brands AM, Biessels GJ, de Haan EH, et al. The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care. 2005;28:726–735. doi: 10.2337/diacare.28.3.726. [DOI] [PubMed] [Google Scholar]
- 16.Gaudieri PA. Cognitive function in children with type 1 diabetes: a meta-analysis. Diabetes Care. 2008;31:1892–1897. doi: 10.2337/dc07-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nooyens AC, Baan CA, Spijkerman AM, et al. Type 2 diabetes and cognitive decline in middle-aged men and women: the Doetinchem Cohort Study. Diabetes Care. 2010;33:1964–1969. doi: 10.2337/dc09-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanaya AM. Change in cognitive function by glucose tolerance status in older adults: a 4-year prospective study of the Rancho Bernardo study cohort. Arch Intern Med. 2004;164:1327–33. doi: 10.1001/archinte.164.12.1327. [DOI] [PubMed] [Google Scholar]
- 19.Gregg EW, Yaffe K, Cauley JA, et al. Is diabetes associated with cognitive impairment and cognitive decline among older women? Study of Osteoporotic Fractures Research Group. Arch Intern Med. 2000;160:174–80. doi: 10.1001/archinte.160.2.174. [DOI] [PubMed] [Google Scholar]
- 20.Hassing L, Grant M, Hofer S, et al. Type 2 diabetes mellitus contributes to cognitive decline in old age: a longitudinal population-based study. Journal of the International Neuropsychological Society. 2004;10:599–607. doi: 10.1017/S1355617704104165. [DOI] [PubMed] [Google Scholar]
- 21.van den Berg E, Reijmer YD, de Bresser J, et al. A 4 year follow-up study of cognitive functioning in patients with type 2 diabetes mellitus. Diabetologia. 2010;53:58–65. doi: 10.1007/s00125-009-1571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Launer L, Miller M, Williamson J, et al. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet neurology. 2011;10:969–77. doi: 10.1016/S1474-4422(11)70188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cukierman-Yaffe T, Gerstein H, Williamson J, et al. Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes Care. 2009;32:221–6. doi: 10.2337/dc08-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hugenschmidt CE, Lovato JF, Ambrosius WT, et al. The Cross-sectional and Longitudinal Associations of Diabetic Retinopathy With Cognitive Function and Brain MRI Findings: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial. Diabetes Care. 2014;37:3244–3252. doi: 10.2337/dc14-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patton N, Aslam T, MacGillivray T, et al. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat. 2005;206:319–348. doi: 10.1111/j.1469-7580.2005.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fontbonne A, Berr C, Ducimetire P, et al. Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: results of the Epidemiology of Vascular Aging Study. Diabetes Care. 2001;24:366–70. doi: 10.2337/diacare.24.2.366. [DOI] [PubMed] [Google Scholar]
- 27.Rawlings AM, Sharrett AR, Schneider ALC, et al. Diabetes in Midlife and Cognitive Change Over 20 Years A Cohort Study. Ann. Intern Med. 2014;161:785–U68. doi: 10.7326/M14-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ott A, Stolk RP, van Harskamp F, et al. Diabetes mellitus and the risk of dementia - The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 29.Manschot SM, Brands AMA, van der Grond J, et al. Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes. 2006;55:1106–1113. doi: 10.2337/diabetes.55.04.06.db05-1323. [DOI] [PubMed] [Google Scholar]
- 30.Reaven GM, Thompson LW, Nahum D, et al. Relationship between hyperglycemia and cognitive function in older NIDDM patients. Diabetes Care. 1990;13:16–21. doi: 10.2337/diacare.13.1.16. [DOI] [PubMed] [Google Scholar]
- 31.Ebady S. Investigation on the relationship between diabetes mellitus type 2 and cognitive impairment. Diabetes Res Clin Pract. 2009;82:305. doi: 10.1016/j.diabres.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 32.Grodstein F, Wilson RS, Chen J, et al. Type 2 diabetes and cognitive function in community-dwelling elderly women. Diabetes Care. 2001;24:1060–1065. doi: 10.2337/diacare.24.6.1060. [DOI] [PubMed] [Google Scholar]
- 33.Ruis C, Biessels GJ, Gorter KJ, et al. Cognition in the Early Stage of Type 2 Diabetes. Diabetes Care. 2009;32:1261–1265. doi: 10.2337/dc08-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding J, Strachan MWJ, Reynolds R, et al. Diabetic retinopathy and cognitive decline in older people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes. 2010;59:2883. doi: 10.2337/db10-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perlmuter LC, Hakami MK, Hodgson-Harrington C, et al. Decreased cognitive function in aging non-insulin-dependent diabetic patients. Am J Med. 1984;77:1043–8. doi: 10.1016/0002-9343(84)90186-4. [DOI] [PubMed] [Google Scholar]
- 36.Kivipelto M, Helkala EL, Hnninen T, et al. Midlife vascular risk factors and late-life mild cognitive impairment: A population-based study. Neurology. 2001;56:1683–9. doi: 10.1212/wnl.56.12.1683. [DOI] [PubMed] [Google Scholar]
- 37.DeCarli C, Miller BL, Swan GE, et al. Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute Twin Study. Arch Neurol. 2001;58:643–7. doi: 10.1001/archneur.58.4.643. [DOI] [PubMed] [Google Scholar]
- 38.Hill CD, Stoudemire A, Morris R, et al. Similarities and differences in memory deficits in patients with primary dementia and depression-related cognitive dysfunction. J Neuropsychiatry Clin Neurosci. 1993;5:277–82. doi: 10.1176/jnp.5.3.277. [DOI] [PubMed] [Google Scholar]
- 39.Yaffe K, Blackwell T, Kanaya AM, et al. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63:658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 40.Yau PL, Javier DC, Ryan CM, et al. Preliminary evidence for brain complications in obese adolescents with type 2 diabetes mellitus. Diabetologia. 2010;53:2298–2306. doi: 10.1007/s00125-010-1857-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryan CM, Geckle M. Why is learning and memory dysfunction in Type 2 diabetes limited to older adults? Diabetes-Metabolism Research and Reviews. 2000;16:308–315. doi: 10.1002/1520-7560(2000)9999:9999<::aid-dmrr141>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 42.van den Berg E, Kloppenborg RP, Kessels RPC, et al. Type 2 diabetes mellitus, hypertension, dyslipidemia and obesity: A systematic comparison of their impact on cognition. Biochimica Et Biophysica Acta-Molecular Basis of Disease. 2009;1792:470–481. doi: 10.1016/j.bbadis.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 43.Palta P, Schneider ALC, Biessels GJ, et al. Magnitude of Cognitive Dysfunction in Adults with Type 2 Diabetes: A Meta-analysis of Six Cognitive Domains and the Most Frequently Reported Neuropsychological Tests Within Domains. Journal of the International Neuropsychological Society. 2014;20:278–291. doi: 10.1017/S1355617713001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Musen G, Lyoo I, Sparks C, et al. Effects of type 1 diabetes on gray matter density as measured by voxel-based morphometry. Diabetes. 2006;55:326–33. doi: 10.2337/diabetes.55.02.06.db05-0520. [DOI] [PubMed] [Google Scholar]
- 45.Hughes TM, Ryan CM, Aizenstein HJ, et al. Frontal gray matter atrophy in middle aged adults with type 1 diabetes is independent of cardiovascular risk factors and diabetes complications. J Diabetes Complications. 2013;27:558–564. doi: 10.1016/j.jdiacomp.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wessels AM, Simsek S, Remijnse PL, et al. Voxel-based morphometry demonstrates reduced grey matter density on brain MRI in patients with diabetic retinopathy. Diabetologia. 2006;49:2474–80. doi: 10.1007/s00125-006-0283-7. [DOI] [PubMed] [Google Scholar]
- 47.Marzelli MJ, Mazaika PK, Barnea-Goraly N, et al. Neuroanatomical Correlates of Dysglycemia in Young Children With Type 1 Diabetes. Diabetes. 2014;63:343–353. doi: 10.2337/db13-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28:652–9. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- 49.Jeerakathil T, Wolf P, Beiser A, et al. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke. 2004;35:1857–61. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 50.Dejgaard A, Gade A, Larsson H, et al. Evidence for diabetic encephalopathy. Diabetic Med. 1991;8:162–7. doi: 10.1111/j.1464-5491.1991.tb01564.x. [DOI] [PubMed] [Google Scholar]
- 51.Weinger K, Jacobson AM, Musen G, et al. The effects of type 1 diabetes on cerebral white matter. Diabetologia. 2008;51:417–25. doi: 10.1007/s00125-007-0904-9. [DOI] [PubMed] [Google Scholar]
- 52.Lobnig BM, Krmeke O, Optenhostert-Porst C, et al. Hippocampal volume and cognitive performance in long-standing Type 1 diabetic patients without macrovascular complications. Diabetic Med. 2006;23:32–9. doi: 10.1111/j.1464-5491.2005.01716.x. [DOI] [PubMed] [Google Scholar]
- 53.Hershey T, Perantie D, Wu J, et al. Hippocampal volumes in youth with type 1 diabetes. Diabetes. 2010;59:236–41. doi: 10.2337/db09-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kodl C, Franc D, Rao J, et al. Diffusion tensor imaging identifies deficits in white matter microstructure in subjects with type 1 diabetes that correlate with reduced neurocognitive function. Diabetes. 2008;57:3083–9. doi: 10.2337/db08-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mangia S, Kumar AF, Moheet AA, et al. Neurochemical profile of patients with type 1 diabetes measured by H-1-MRS at 4 T. Journal of Cerebral Blood Flow and Metabolism. 2013;33:754–759. doi: 10.1038/jcbfm.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ogawa S, Lee TM, Kay AR, et al. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 60.Greicius MD, Krasnow B, Reiss AL, et al. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Badgaiyan RD, Posner MI. Mapping the cingulate cortex in response selection and monitoring. Neuroimage. 1998;7:255–260. doi: 10.1006/nimg.1998.0326. [DOI] [PubMed] [Google Scholar]
- 62.McKiernan KA, Kaufman JN, Kucera-Thompson J, et al. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- 63.Cauda F, D’Agata F, Sacco K, et al. Altered resting state attentional networks in diabetic neuropathic pain. Journal of Neurology Neurosurgery & Psychiatry. 2010;81:806–811. doi: 10.1136/jnnp.2009.188631. [DOI] [PubMed] [Google Scholar]
- 64.van Duinkerken E, Schoonheim MM, Sanz-Arigita EJ, et al. Resting-state brain networks in type 1 diabetic patients with and without microangiopathy and their relation to cognitive functions and disease variables. Diabetes. 2012;61:1814–1821. doi: 10.2337/db11-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Duinkerken E, Ijzerman RG, van der Zijl NJ, et al. Differential impact of subclinical carotid artery disease on cerebral structure and functioning in type 1 diabetes patients with versus those without proliferative retinopathy. Cardiovascular Diabetology. 2014;13:58. doi: 10.1186/1475-2840-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moran C, Phan TG, Chen J, et al. Brain Atrophy in Type 2 Diabetes Regional distribution and influence on cognition. Diabetes Care. 2013;36:4036–4042. doi: 10.2337/dc13-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.den Heijer T, Vermeer SE, van Dijk EJ, et al. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia. 2003;46:1604–10. doi: 10.1007/s00125-003-1235-0. [DOI] [PubMed] [Google Scholar]
- 68.Brundel M, van den Heuvel M, de Bresser J, et al. Cerebral cortical thickness in patients with type 2 diabetes. J Neurol Sci. 2010;299:126–130. doi: 10.1016/j.jns.2010.08.048. [DOI] [PubMed] [Google Scholar]
- 69.Manschot SM, Biessels GJ, de Valk H, et al. Metabolic and vascular determinants of impaired cognitive performance and abnormalities on brain magnetic resonance imaging in patients with type 2 diabetes. Diabetologia. 2007;50:2388–97. doi: 10.1007/s00125-007-0792-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmidt R, Launer L, Nilsson L, et al. Magnetic resonance imaging of the brain in diabetes: the Cardiovascular Determinants of Dementia (CASCADE) Study. Diabetes. 2004;53:687–92. doi: 10.2337/diabetes.53.3.687. [DOI] [PubMed] [Google Scholar]
- 71.van Elderen SGC, de Roos A, de Craen AJM, et al. Progression of brain atrophy and cognitive decline in diabetes mellitus: a 3-year follow-up. Neurology. 2010;75:997. doi: 10.1212/WNL.0b013e3181f25f06. [DOI] [PubMed] [Google Scholar]
- 72.Reijmer YD, van den Berg E, de Bresser J, et al. Accelerated cognitive decline in patients with type 2 diabetes: MRI correlates and risk factors. Diabetes-Metabolism Research and Reviews. 2011;27:195–202. doi: 10.1002/dmrr.1163. [DOI] [PubMed] [Google Scholar]
- 73.Kooistra M, Geerlings MI, Mali WPTM, et al. Diabetes mellitus and progression of vascular brain lesions and brain atrophy in patients with symptomatic atherosclerotic disease. The SMART-MR study. J Neurol Sci. 2013;332:69–74. doi: 10.1016/j.jns.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 74.de Bresser J, Tiehuis AM, van den Berg E, et al. Progression of Cerebral Atrophy and White Matter Hyperintensities in Patients With Type 2 Diabetes. Diabetes Care. 2010;33:1309–1314. doi: 10.2337/dc09-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brundel M, Reijmer YD, van Veluw SJ, et al. Cerebral Microvascular Lesions on High-Resolution 7-Tesla MRI in Patients With Type 2 Diabetes. Diabetes. 2014;63:3523–3529. doi: 10.2337/db14-0122. [DOI] [PubMed] [Google Scholar]
- 76.Reijmer YD, Brundel M, de Bresser J, et al. Microstructural White Matter Abnormalities and Cognitive Functioning in Type 2 Diabetes A diffusion tensor imaging study. Diabetes Care. 2013;36:137–144. doi: 10.2337/dc12-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reijmer YD, Leemans A, Brundel M, et al. Disruption of the Cerebral White Matter Network Is Related to Slowing of Information Processing Speed in Patients With Type 2 Diabetes. Diabetes. 2013;62:2112–2115. doi: 10.2337/db12-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Greicius MD, Krasnow B, Reiss AL, et al. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sorg C, Riedl V, Muhlau M, et al. Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Musen G, Jacobson AM, Bolo NR, et al. Resting-state brain functional connectivity is altered in type 2 diabetes. Diabetes. 2012;61:2375–2379. doi: 10.2337/db11-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoogenboom WS, Marder TJ, Flores VL, et al. Cerebral white matter integrity and resting-state functional connectivity in middle-aged patients with type 2 diabetes. Diabetes. 2014;63:728–738. doi: 10.2337/db13-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen YC, Jiao Y, Cui Y, et al. Aberrant brain functional connectivity related to insulin resistance in type 2 diabetes: a resting-state fMRI study. Diabetes Care. 2014;37:1689–1696. doi: 10.2337/dc13-2127. [DOI] [PubMed] [Google Scholar]
- 83.Marder TJ, Flores VL, Bolo NR, et al. Task-induced brain activity patterns in type 2 diabetes: a potential biomarker for cognitive decline. Diabetes. 2014;63:3112–3119. doi: 10.2337/db13-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou H, Lu W, Shi Y, et al. Impairments in cognition and resting-state connectivity of the hippocampus in elderly subjects with type 2 diabetes. Neurosci Lett. 2010;473:5–10. doi: 10.1016/j.neulet.2009.12.057. [DOI] [PubMed] [Google Scholar]
- 85.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes–systematic overview of prospective observational studies. Diabetologia. 2005;48:2460–2469. doi: 10.1007/s00125-005-0023-4. [DOI] [PubMed] [Google Scholar]
- 86.Curb JD, Rodriguez BL, Abbott RD, et al. Longitudinal association of vascular and Alzheimer’s dementias, diabetes, and glucose tolerance. Neurology. 1999;52:971–975. doi: 10.1212/wnl.52.5.971. [DOI] [PubMed] [Google Scholar]
- 87.Luchsinger JA, Tang MX, Stern Y, et al. Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol. 2001;154:635–641. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 88.Kuusisto J, Koivisto K, Mykkanen L, et al. Association between features of the insulin resistance syndrome and Alzheimer’s disease independently of apolipoprotein E4 phenotype: cross sectional population based study. Br Med J. 1997;315:1045–1049. doi: 10.1136/bmj.315.7115.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ott A, Stolk RP, Hofman A, et al. Association of diabetes mellitus and dementia: the Rotterdam Study. Diabetologia. 1996;39:1392–1397. doi: 10.1007/s001250050588. [DOI] [PubMed] [Google Scholar]
- 90.Yoshitake T, Kiyohara Y, Kato I, et al. Incidence and risk factors of vascular dementia and Alzheimer’s disease in a defined elderly Japanese population: the Hisayama Study. Neurology. 1995;45:1161–1168. doi: 10.1212/wnl.45.6.1161. [DOI] [PubMed] [Google Scholar]
- 91.Cui Y, Jiao Y, Chen YC, et al. Altered spontaneous brain activity in type 2 diabetes: a resting-state functional MRI study. Diabetes. 2014;63:749–760. doi: 10.2337/db13-0519. [DOI] [PubMed] [Google Scholar]
- 92.Xia W, Wang S, Sun Z, et al. Altered baseline brain activity in type 2 diabetes: a resting-state fMRI study. Psychoneuroendocrinology. 2013;38:2493–2501. doi: 10.1016/j.psyneuen.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 93.Wang C, Fu K, Liu H, et al. Spontaneous Brain Activity in Type 2 Diabetics Revealed by Amplitude of Low-Frequency Fluctuations and Its Association with Diabetic Vascular Disease: A Resting-State fMRI Study. Plos One. 2014;9:e108883. doi: 10.1371/journal.pone.0108883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baker LD, Cross DJ, Minoshima S, et al. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol. 2011;68:51–57. doi: 10.1001/archneurol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev. 2008;29:494–511. doi: 10.1210/er.2007-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379:2291–2299. doi: 10.1016/S0140-6736(12)60360-2. [DOI] [PubMed] [Google Scholar]
- 97.Ryan C, Vega A, Drash A. Cognitive deficits in adolescents who developed diabetes early in life. Pediatrics. 1985;75:921–27. [PubMed] [Google Scholar]
- 98.Sinclair AJ, Girling AJ, Bayer A. Cognitive dysfunction in older subjects with diabetes mellitus: impact on diabetes self-management and use of care services. Diabetes Res Clin Pract. 2000;50:203–212. doi: 10.1016/s0168-8227(00)00195-9. [DOI] [PubMed] [Google Scholar]


