Abstract
Neuropeptides (NPs), a unique and highly important class of signaling molecules across the animal kingdom, have been extensively characterized in the neuronal tissues of various crustaceans. Because many NPs are released into circulating fluid (hemolymph) and travel to distant sites in order to exhibit physiological effects, it is important to measure the secretion of these NPs from living animals. In this study, we report on extensive characterization of NPs released in the crab Cancer borealis by utilizing in vivo microdialysis to sample NPs from the hemolymph. We determined the necessary duration for collection of microdialysis samples, enabling more comprehensive identification of NP content while maintaining the temporal resolution of sampling. Analysis of in vivo microdialysates using a hybrid quadrupole-Orbitrap™ Q-Exactive mass spectrometer revealed that more than 50 neuropeptides from 9 peptide families—including the allatostatin, RFamide, orcokinin, tachykinin-related peptide and RYamide families–were released into the circulatory system. The presence of these peptides both in neuronal tissues as well as in hemolymph indicates their putative hormonal roles, a finding that merits further investigation. Preliminary quantitative measurement of these identified NPs suggested several potential candidates that may be associated with the circadian rhythm in Cancer borealis.
Keywords: neuropeptide, secretion, mass spectrometry, microdialysis, crustacean, hemolymph, in vivo sampling
Introduction
Neuropeptides (NPs) are one of the most diverse classes of signaling molecules, and they are present in a wide variety of organisms. They are known to have regulatory roles in many physiological processes, including food intake, reproduction, pain and stress1. To exert their hormonal effects on different organs, NPs are often secreted into circulating fluids to travel to different parts of the body2, 3. Characterization of these released NPs is essential towards understanding their actions.
Both tissue-based and fluid-based methods are commonly used in NP analysis. In tissue-based methods, the animal is sacrificed to permit dissection of the tissue of interest for analysis. However, there are several limitations to tissue-based techniques8, including an inability to obtain repeated samples from a single animal throughout the time course of a dynamic experiment. In addition, sampling NPs from tissue lacks the ability to distinguish inactive NPs from active forms of NPs, due to the nature of tissue homogenization and NP synthesis.
Due to the need to study secreted NPs, fluid-based methods have been developed as an alternative to tissue-based methods. These methods include sampling NPs from stimulated neuronal releasate10, 11, blood or hemolymph12. Direct analysis of signaling neuromodulators without sacrificing the animal via fluid-based methods also allows the study of biologically active molecules under different physiological conditions in a single animal. Because baseline values for NP content can vary greatly between animals, especially in wild-caught (as opposed to laboratory-raised) animals such as crabs, being able to compare NP concentrations in a single animal across an experimental manipulation will allow us to identify fold-changes in NP content that may otherwise be difficult to observe. Thus, hemolymph NP profiling from appropriate fluid-based samples would offer great insight into NP release in response to different stimuli or under different states in the same animal.
NPs can be sampled directly from hemolymph, obtained from the animal with the use of a needle and a syringe12. However, the presence of extracellular peptidases and a wide variety of molecules in addition to NPs, such as lipids, albumins, clotting factors and enzymes would make for an extremely complex sample. As a result, special treatment and several cleanup steps are often required for effective detection of NPs. Moreover, the stress caused by using a needle and a syringe may induce the release of stress-related NPs.
As an alternative, in vivo microdialysis allows for collection of extracellularly released molecules and enables real-time monitoring of substance release. Microdialysis shows great utility in the field of neuroscience, as it offers the ability to monitor dynamic changes of neurochemical content during different internal states of a single animal in a time-resolved fashion with minimal disturbance to the animal13. As a result, this technique could provide unique insight into our understanding of the effects of neuromodulator release on different behavior. The tip of the microdialysis probe consists of a semi-permeable dialysis membrane, which has a defined molecular weight cutoff (MWCO). The microdialysis probe is implanted into the tissue of interest and dialysate is collected at the outlet while perfusion fluid is pushed through the inlet, normally at a low flow rate14. Diffusion can occur between the perfusion fluid and the extracellular space as the perfusion fluid passes through the probe tip, and molecules such as NPs will diffuse into the perfusion fluid, driven down their concentration gradient. Microdialysis has been widely used to monitor a wide range of molecules including electrolytes15, amines and amino acids16, 17, NPs18, 19 and proteins17, 20.
As a complement to different methods of sampling secreted NPs, highly sensitive and effective detection methods to analyze peptide hormones present in circulating hemolymph are currently unavailable but highly important. Liquid chromatography (LC) –MS is well-suited to this purpose. Over the last two decades, biological MS has shown powerful capabilities in the discovery of NPs in crustacean neuronal tissues4, 6, 7, 10, 12. However, compared with tissue-based NP studies, fluid-based sampling methods coupled with MS for detection of NP release in crustaceans are still poorly developed. Chen et al.12 explored different NP extraction protocols from Cancer borealis hemolymph and subsequently detected 10 secreted NPs from five families, including RFamides, allatostatins, orcokinins, tachykinin-related peptides (TRPs), and crustacean cardioactive peptide (CCAP). Compared with the large number of NPs detected in tissues, the reduced number of NPs detected in the hemolymph suggested a need to develop methods with improved sample preparation and higher sensitivity.
By providing a cleaner sample due to collection through a dialysis membrane, microdialysis should provide a less complex sample and thus improve upon NP detection rates from hemolymph. So far, only a handful of reports have used in vivo microdialysis to investigate the NPs present in circulating fluid in the crustacean, although this sampling technique is more commonly used in NP analysis in the mammalian nervous system21, 22. Behrens et al.18 reported the identification of NPs from 10 families in microdialysates collected from the pericardial space (a hemolymph-filled cavity) of Cancer borealis using LC-ESI-QTOF and MALDI-TOF/TOF mass spectrometry. Moreover, a recent study by Schmerberg and Li19 reported improved relative recovery of NPs by utilizing affinity agents, antibody-coated magnetic nanoparticles, and suggested an increased potential for improved detection of NPs released into hemolymph with this method.
To provide a more complete picture of neurosecretion and information complementary to tissue-based NP analyses of the crustacean system, herein we employ an advanced high-resolution, accurate-mass (HRAM) MS platform to study NP secretion in hemolymph using fluid-based sampling methods. Comparison for NP identification is made between crude hemolymph NP extraction and an in vivo microdialysis sampling strategy in the Jonah crab, Cancer borealis. Appropriate sample preparation steps are performed for both types of samples, which are then analyzed by a hybrid quadrupole-Orbitrap™ Q-Exactive MS instrument. This new platform has greatly increased the confidence of NP identification by offering high resolution and high mass accuracy measurement and employing two complementary MS/MS spectral interpreting strategies (Mascot and PEAKS). Two in vivo sampling methods were also compared to aid in identification of secreted NPs. Microdialysate collection time was evaluated to achieve the best NPs coverage.
Materials and methods
Chemicals
Formic acid (FA) was purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were purchased from Fisher Scientific (Pittsburgh, PA, USA). ACS reagent-grade solvents and Mill-Q water were used for sample preparation. Optima grade solvents were used for sample analysis on the MS instrument.
Animals
Jonah crabs, Cancer borealis, were purchased from Ocean Resources, Inc. (Sedgwick, ME, USA) and The Fresh Lobster Company (Gloucester, MA, USA). Crabs were maintained in an artificial seawater tank at 10–13°C with a 12 h/12 h light/dark cycle. The crabs were allowed to adjust to the tanks for at least one week after shipment before performing hemolymph extraction or microdialysis. Details of animal housing procedures were described elsewhere19. Animals were housed, treated and sacrificed following the animal care protocol in accordance with the University of Wisconsin-Madison’s animal care guidelines.
Hemolymph Extraction
Details of the procedure were previously described by Chen et al.12. Briefly, crabs were removed from the tank and cold-anesthetized on ice for 5 min. Hemolymph was withdrawn by inserting a 25 gauge needle attached to a 1 mL or 3 mL BD plastic syringe through the junction of the thorax and abdomen into the pericardial chamber. An aliquot of 750 microliters of freshly obtained hemolymph was spiked with an equal amount of acidified MeOH (90% MeOH, 9% glacial acetic acid, 1% water) immediately and mixed well to extract peptides and precipitate large proteins. Samples were subsequently purified by a 10 kDa molecular weight cutoff (MWCO) step and C18 spin column desalting step (Argos, Elgin, IL, USA). Eluates from the C18 spin columns were dried down and resuspended in 10 μL of 0.1% FA in water before MS injection.
Microdialysis Supplies
CMA/20 Elite probes with 4 mm membranes of polyarylether sulfone (PAES) were purchased from CMA Microdialysis (Harvard Apparatus, Holliston, MA, USA). A KD Harvard 22 (Harvard Apparatus, Holliston, MA, USA), and a Pump 11 Elite Nanomite Syringe Pump (Harvard Apparatus, Holliston, MA, USA) were used to drive perfusate through MD probes and tubing. Additional FEP (CMA) and PEEK (Upchurch-Scientific, Index Health and Science, Oak Harbor, WA, USA) tubing was used to lengthen the tubing of the microdialysis probe as needed. This was connected by flanged connectors from CMA and BASi (West Lafayette, IN, USA). Probes were rinsed with crab saline prior to implantation.
In Vivo Microdialysis Experiments
The procedure for in vivo microdialysis surgery on Jonah crabs was adapted from previous publications18, 19. After the probe was surgically implanted in the crab, the animal was allowed to recover for at least 24 hr before dialysate was collected for MS analysis. Physiological crab saline (440 mM NaCl; 11 mM KCl; 13 mM CaCl2; 26 mM MgCl2; 10 mM HEPES acid; pH 7.4, adjusted with NaOH) was used as perfusion solution. The flow rate was set at 0.5 μL / min by a programmable syringe pump. For circadian NP analysis, dialysate samples were collected every 2 hr with a refrigerated fraction collector (BASi Honeycomb, Bioanalytical Systems, Inc. Indianapolis, IN, USA). Upon collection, 3 μL of FA was added to each sample, which was then stored at −20°C immediately to improve NP stability23. The dialysates were concentrated ~6-fold in a SpeedVac (Thermo Fisher Scientific, Waltham, MA, USA). The concentrated dialysate was desalted using C18 ZipTips (EMD Millipore, Billerica, MA, USA), and eluted in 10 μL 0.1% FA in 50% acetonitrile (v/v). Similarly, for analysis of the optimal temporal resolution of in vivo microdialysis, samples were collected every 2, 4, 6, and 8 h with the same setup. They were immediately acidified to a final acid concentration of 5% and stored at −20°C. They were later concentrated 6-, 12-, 18, and 24-fold, respectively. The concentrated dialysate was desalted using C18 ZipTips and eluted in 0.1% FA in 50% acetonitrile (v/v). C18 Ziptip desalting was performed for an aliquot of concentrated dialysate that was correlated to every 2 h microdialysis fraction. The desalted dialysates were then all concentrated to a final volume of 10 μL prior to UPLC MS/MS analysis.
Instrumentation
The nanoLC-MS/MS experiment was performed using a Waters nanoAcquity UPLC system (Waters Corp, Milford, MA, USA) coupled to a quadrupole-Orbitrap™ Q-Exactive mass spectrometer (Thermo Scientific, Bremen, Germany). Chromatographic separations were performed on a home-packed C18 reversed phase capillary column (360 μm OD, 75 μm ID × 15 cm length, 1.7 μm particle size, 150 Å pore size, (BEH C18 material obtained from Waters UPLC column, part no. 186004661)). The mobile phases used were: 0.1% FA in water (A) and 0.1% FA in acetonitrile (B). An aliquot of 3.5 μL of desalted hemolymph/microdialysis sample dissolved in 0.1% FA in water was injected and loaded onto the column without trapping. A 108 min gradient was employed with 0–0.5 min, 0–10% B; 0.5–70 min, 10–35% B; 70–80 min, 35–75% B; 80–82 min, 75–95% B; 82–92 min, 95% B; 92–93 min, 95–0% B; 93–108 min, 100% A. Data was collected under positive electrospray ionization data dependent acquisition (DDA) mode with the top 10 most abundant precursor ions selected for HCD fragmentation. The MS scan range was from m/z 300 to 2000 at 70,000 resolution, and the MS/MS scan was at 17,500 resolution from m/z 120 to 6000 with an isolation width of 2 Da, collision energy 30.
Data Processing
MS raw data files were processed and analyzed by PEAKS Studio7 (Bioinformatics Solutions Inc., Waterloo, ON, CAN) and Mascot (Matrix Science Inc., Boston, MA, USA). C-terminal amidation, pyroglutamation, and methionine oxidation were specified as variable post-translational modifications (PTMs). Precursor ion mass tolerance was 20 ppm, and fragment ion mass tolerance was 0.01 Da. De novo sequencing and database search were conducted with no enzyme cleavage specified. The database used was constructed in-house with known crustacean neuropeptides and is available upon request. Peptide spectrum matches (PSMs) with a −10logP value cutoff of 15 in PEAKS and 10 in Mascot were considered for further manual validation.
Results and Discussion
Hemolymph Extraction
In total, 22 NPs were identified from a direct hemolymph preparation (Table 1) analyzed with ultrahigh performance liquid chromatography (UPLC)-tandem mass spectrometry (MS/MS). It is worth noticing that only 7 out of the 22 NPs also have decent matches with Mascot database searching, which may indicate that PEAKS is more suitable for identification of smaller NPs. As a result of improved instrumentation, more NPs were identified compared to a previous study using a similar sample preparation procedure with a modest resolution MALDI TOF/TOF instrument12. Out of these identified NPs, only CCAP and I/LNFTHKFa were detected in both studies. Besides the use of different instruments and different ionization methods in these two studies, the highly dynamic circulatory system in crustaceans could also be responsible for the poor reproducibility of NPs found in hemolymph samples.
Table 1.
Neuropeptides identified in Cancer borealis hemolymph extract
| Neuropeptide Family | Neuropeptide Seqeunce | M+H | Mascot Score | PEAKS Score |
|---|---|---|---|---|
| AST (Allatostatin) | SYWKQCAFNAVSCFa (C-type AST) | 1650.7192 | 30.08 | |
| DPYAFGLGKRPDMYAFGLa (A-type AST) | 2017.0000 | 26.30 | 31.93 | |
| CCAP (Crustacean cardioactive Peptide) CPRP (CHH precursor-related peptide) |
PFCNAFTGCa RASQGLGKMEa TPLGDLSGSVGHPV |
956.3753 1075.5677 1335.6903 |
22.13 22.00 |
45.12 65.68 21.30 |
| FLP (FMRFamide-like peptide) | I/LNFTHKFa | 905.4992 | 30.64 | |
| NRNFLRFa | 965.5428 | 31.77 | 59.99 | |
| DRNFLRFa | 966.5268 | 78.28 | ||
| SPRNFLRFa | 1035.5847 | 65.59 | ||
| SDRNFLRFa | 1053.5588 | 26.88 | 91.08 | |
| TNRNFLRFa | 1066.5905 | 27.85 | 92.93 | |
| ENRNFLRFa | 1094.5854 | 17.10 | ||
| LNPSNFLRFa | 1106.6105 | 69.08 | ||
| EMPSLRLRFa | 1147.6405 | 23.79 | ||
| Orcokinin | VYGPRDIANLY | 1280.6634 | 24.69 | |
| PDH (Pigment dispersing hormone) | NSILGIPR | 869.5203 | 54.52 | |
| NSI/LLGAPRVa | 925.5578 | 77.68 | ||
| NSILGAPRV | 926.5418 | 33.41 | ||
| NSILGIPKVM(O)N | 1201.6609 | 26.68 | ||
| RPCH (Red pigment concentrating hormone) Others |
pQLNFSPGWa AVLLPKKTEKK EEPEAPa |
930.4468 1254.8144 670.3042 |
33.65 | 46.14 60.92 55.35 |
Legend: “a” at the end of a peptide indicates C-terminal amidation. “(O)” indicates an oxidized methionine. “pQ” or “pE” indicates pyroglutamic acid.
The presence of a wide variety of molecules in addition to NPs, such as lipids, peptidases, and clotting factors, makes the analysis of NPs from hemolymph very difficult. Although more NPs were identified in this study than were previously found in hemolymph, compared with tissue-based studies, the number is still relatively small. There are also many peaks in the MS spectra of hemolymph samples that could not be assigned to any known NPs or high probability de novo matches, which may lead to discovery of novel peptides or other hemolymph components in the future. The complex composition of crude hemolymph extract may suppress the signal of NPs on the MS instrument.
In Vivo Microdialysis (MD)
Performing MD surgery on crabs has proven to be challenging mainly due to the crab shell. Since it was first introduced in 200818, a rather sophisticated procedure has been developed in our group. Beyond technical challenges related to probe implantation, however, the low concentration of circulating hormones has continued to make the detection of NP content in dialysate difficult. Compared with the number of NPs identified from neuronal tissues, much less is known about the circulating peptides. In previous work employing MD and earlier iterations of MS-based techniques, over 30 NPs were determined to be present in the circulating hemolymph of Cancer borealis with samples collected over more than 10 hours. However, most of these NPs were identified based on accurate mass matching, with only 3 that have been confirmed by tandem MS due to their low abundances.
One of the most powerful advantages of MD sampling is that the collection is concurrent with different internal states or activity in the animal. As a result, it allows correlation of neurochemical content with physiology or behavior to provide important function-related information. Temporal resolution, which is defined by the shortest time period over which a fluctuation can be observed, is an important parameter associated with microdialysis. In order to optimize temporal resolution and increase NP identification numbers, we first evaluated the collection time required to provide a more comprehensive identification of secreted NPs in Cancer borealis.
Microdialysates collected for 2, 4, 6, and 8 h at a flow rate of 0.5 μL/min would produce samples with volumes of 60, 120, 180, and 240 μL respectively, followed by desalting using C18 Ziptips and resuspension in 10 μL of 0.1% FA in water. An aliquot (3.5 μL) of each resulting sample was injected onto UPLC-MS/MS. Data was then processed as described above. The number of NPs identified from these dialysates increased as the collection duration increased, as expected (Figure 1), since increasing collection time increased the concentration of the final sample submitted for analysis. Twenty-two previously known crustacean NPs were identified from a 2 h collection; similarly 36 were observed in a 4 h sample, 39 in a 6 h collection, and only 3 RFamide peptides in an 8 h collection. The NPs identified from these samples overlapped quite well, and yielded overall 52 peptides from 9 NP families as shown in Table 2. This represents the most comprehensive characterization of the secreted crustacean neuropeptidome.
Figure 1.
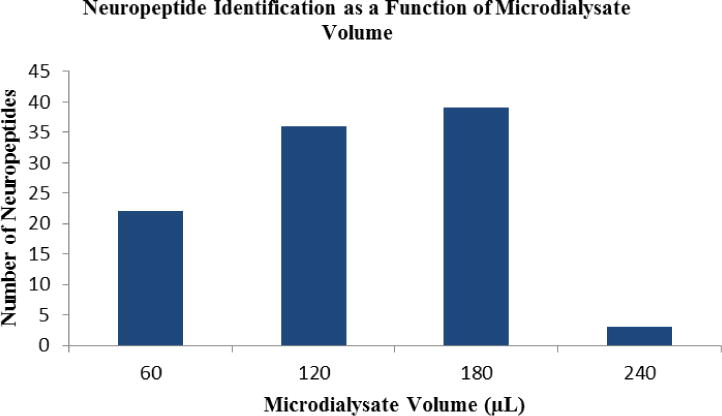
Number of neuropeptides identified from 60, 120, 180, and 240 μL dialysates from Cancer borealis hemolymph.
Table 2.
Neuropeptides identified in Cancer borealis hemolymph via in vivo microdialysis
| Family | Sequence | M+H | Mascot Score | PEAKS Score |
|---|---|---|---|---|
| AST (Allatostatin) | MFAPLAWPKGGARWa | 1586.8413 | 28.63 | |
| M(O)FAPLAWPKGGARWa | 1602.8362 | 42.7 | ||
| M(O)FAPLAWPKGGARW | 1603.8202 | 23.34 | 34.25 | |
| LMFAPLAWPKGGARWa | 1699.9253 | 19.27 | ||
| LM(O)FAPLAWPKGGARW | 1716.9043 | 25.93 | ||
| CPRP (CHH precursor-related peptide) | LSGSLGHPVE | 995.5156 | 39.56 | 52.95 |
| DLSGSLGHPVE | 1110.5426 | 21.55 | ||
| TPLGDLSGSLGHPVE | 1478.7485 | 42.90 | ||
| RGALEPNTPLGDLSGSLGHPVE | 2216.1306 | 14.97 | 48.27 | |
| FLPs (FMRFamide-like peptide) | pQRNFLRFa | 962.5319 | 21.47 | |
| NRNFLRFa | 965.5428 | 14.99 | ||
| DRNFLRFa | 966.5268 | 23.69 | 49.13 | |
| NPSDFLRFa | 994.5105 | 24.00 | 42.21 | |
| GNRNFLRFa | 1022.5643 | 17.07 | ||
| LETNFLRFa | 1038.5731 | 33.81 | ||
| SDRNFLRFa | 1053.5588 | 29.60 | 68.30 | |
| LDRNFLRFa | 1079.6109 | 25.92 | 46.37 | |
| DGGRNFLRFa | 1080.5697 | 41.13 | ||
| QNRNFLRFa | 1093.6014 | 24.87 | ||
| ENRNFLRFa | 1094.5854 | 23.05 | 28.94 | |
| GSDRNFLRFa | 1110.5803 | 28.79 | 46.41 | |
| TGNRNFLRFa | 1123.6119 | 50.59 | ||
| LGDRNFLRFa | 1136.6323 | 35.31 | ||
| DGNRNFLRFa | 1137.5912 | 32.06 | ||
| GYSKNYLRFa | 1146.6054 | 23.45 | 25.44 | |
| ALDRNFLRFa | 1150.6480 | 19.26 | ||
| SENRNFLRFa | 1181.6174 | 19.49 | 33.25 | |
| DENRNFLRFa | 1209.6123 | 48.08 | ||
| LTGNRNFLRFa | 1236.6960 | 24.05 | ||
| LDGPLAPFLRFa | 1244.7150 | 9.80 | ||
| YGSDRNFLRFa | 1273.6436 | 26.14 | ||
| Orcokinin | NFDEIDRSGFG | 1256.5542 | 35.07 | 18.22 |
| DFDEIDRSGFG | 1257.5382 | 25.86 | 27.4 | |
| NFDEIDRSGFGF | 1403.6226 | 32.22 | ||
| SSEDMPSSLGFGFN | 1474.6155 | 10.17 | ||
| NFDEIDRSGFGFA | 1474.6597 | 20.16 | 46.77 | |
| DFDEIDRSGFGFA | 1475.6437 | 18.00 | 21.50 | |
| DFDEIDRSGFGFV | 1503.6750 | 22.31 | ||
| Orcomyotropin | FPAFTTGFGHS | 1168.5422 | 26.69 | 34.47 |
| FDAFTTGFGHS | 1186.5164 | 51.3 | 50.03 | |
| PDH (Pigment dispersing hormone) | NSELINSILGLPKVM(O)NEAa | 1957.0423 | 15.25 | 37.74 |
| RPCH (Red pigment concentrating hormone) | pQLNFSPGWa | 930.4468 | 21.68 | |
| RYamide | SGFYANRYa | 976.4635 | 33.77 | 52.83 |
| SGFYADRYa | 977.4476 | 37.64 | ||
| pQGFYSQRYa | 1030.4741 | 22.37 | 36.60 | |
| LSGFYANRYa | 1089.5476 | 14.24 | ||
| LEWYSQRYa | 1143.5582 | 23.53 | ||
| TRP (Tachykinin-related peptide) | TPSGFLGMRa | 964.5033 | 35.2 | 36.74 |
| APSGFLGMRa | 934.4927 | 38.64 | 42.92 | |
| APSGFLGM(O)RGa | 1007.5091 | 34.29 |
Legend: “a” at the end of a peptide indicates C-terminal amidation. “(O)” indicates an oxidized methionine. “pQ” or “pE” indicates pyroglutamic acid.
Two MS/MS interpretation platforms, Mascot and PEAKS, were used. It was proven to be difficult to choose a score cutoff for NPs, as the algorithms for these programs are designed for larger molecules, such as proteins. Therefore, most scores do not accurately represent the quality of a peptide-spectrum match (PSM). The results were further manually examined for accurate matches of b- and y-ions. The overlap of NP identifications between Mascot and PEAKS was moderate, with 22 out of the 52 listed peptides being identified using both algorithms. The peptides identified from this study represent the largest number of circulating peptides characterized via mass spectrometry using in vivo microdialysis sampling from any crustacean.
However, when the collection time was increased to 8 h or longer, the number of identifiable NPs decreased dramatically, with fewer than 10 NPs identified. With the same sample handling process, one possible explanation would be the tolerance of MS instruments to compounds like salt and other interfering compounds, including small organics, present in the hemolymph (Figure S1). As the collection time increases, the total amount of dialysate collected also increases. Desalting was performed for an aliquot of concentrated dialysate which corresponded to 2 h microdialysis fraction; thus, dialysate with longer collection duration yielded larger volume of desalting elution solution. All desalted dialysate samples for 2 h, 4 h, 6 h and 8 h were then further concentrated and resuspended into the same volume prior to UPLC MS/MS analysis. Increasing the volume of the sample prior to concentration and analysis has the clear advantage of increasing NP concentration, and thus improving detection sensitivity, but it also leads to increased concentrations of other components, including salts. Concentrating the sample from an 8 h or longer collection time may have led to accumulation of various compounds including salts that could interfere with NP detection on the MS instrument. Similarly, the concentration of NPs and other components may reach a good balance by MS detection in the 6 h sample. With the addition of more salts and other components of hemolymph in more concentrated samples, the NP signal is likely to be suppressed or masked by other interfering signals. Based on our study, 180 μL of microdialysate obtained at 0.5 μL/min seems to be a good volume to obtain for the purpose of identification of secreted NPs in the crustacean.
The low endogenous concentration of NPs, usually present in vivo at the nM-pM range23, accounts for some of the challenges associated with detection of these compounds. In microdialysis, the concentration collected is even lower as it is governed by passive diffusion. NPs have a lower relative recovery rate (20%–40%)19 in comparison to small molecules due to their larger sizes, which increase the hindrance of passing through the dialysis membrane. Flow rate is closely related to recovery rate, and the temporal resolution of microdialysis collection is directly affected by the sensitivity of detection technique.
Allatostatin
As demonstrated by a number of studies4, 7, 24, 25, ASTs are widely distributed across many neuronal tissues in various crustacean species. In Cancer borealis, ASTs were identified from the pericardial organ (PO)26, brain24, and the stomatogastric ganglion (STG)27. A-type ASTs share C-terminal motif –YXFGLa, and B-type ASTs have a WX6Wa motif on the C-terminus. Three ASTs sequenced from microdialysate in our study had sequence similarity to LMFAPLAWPKGGARWa (m/z 1699.93) isolated from crab PO, with all of three sequenced from microdialysates possessing an oxidized methionine. This particular modification may be critical for functional reasons, or may be an artifact of sample processing. Interestingly, only B-type ASTs were observed in microdialysate, whereas no B-type ASTs were found in hemolymph extracts. This may result from quick degradation of B-type ASTs due to the presence of related peptidases in crude hemolymph. ASTs are known to inhibit the pyloric motor pattern and stomatogastric neurotransmission28, 29, and the presence of these potentially novel ASTs in the hemolymph further supports the functional roles of these circulating peptides.
The FMRFamide –like peptides
Since the discovery of FMRFamide30 from the clam, Macorocallista nimbosa30, a large group of FMRFamide-like peptides (FLPs) have been found in both vertebrates and invertebrates. A C-terminal RFamide motif is shared by crustacean FLPs, which can be further categorized into several subfamilies25. These subfamilies include myosuppressins, characterized by a C-terminal HVFLRFamide; the neuropeptide Fs, which share sequence homology with the vertebrate neuropeptide Y and have the C-terminal motif RXRFamide; and sulfakinins, known as invertebrate homologs of vertebrate gastrin and cholecystokinin (CCK). In addition to these subfamilies, a large number of FLPs identified from decapod crustaceans possess the C-terminal motif –FLRFamide31.
In our study, 23 FLPs all sharing a C-terminal -FLRFamide were identified in hemolymph microdialysate. Similar with hemolymph extraction, more members of the FLP family were identified than members of any other family. Moreover, FLPs identified from hemolymph extraction and microdialysis overlap with each other quite well. The occurrence of a wide array of FLPs in circulating hemolymph may indicate that these NPs play diverse functional roles and might be involved in many different neuroendocrine processes.
Mass spectral investigation of neuronal tissues, especially mass spectrometric imaging techniques, has provided important evidence about the wide distribution of –FLRFamides in the nervous systems of crustaceans24, 32, 33. Physiological assessment of the identified –FLRFamides has revealed a broad array of possible neuromodulatory roles including cardioexcitation, modulation of muscle contraction and regulation of feeding behavior34, 35. Ten of the secreted FLRFamides identified in this work have been previously found in the POs24. The fact that these NPs are present in both hemolymph and POs may suggest that they were released from the POs into the circulatory system to have hormonal effects on the crustacean heart or more distant organs. Studies have shown that SDRNFLRFamide (m/z 1053.5588) and TNRNFLRFamide (m/z 1066.5905) (Figure 2) exhibited excitatory effects on different muscles in the stomach and heart34, 35. The co-release of these neuropeptides may suggest that they belong to related pathways to coordinate various muscle contractions involved in behavior or a physiological process. The identification of FLPs in hemolymph microdialysate that were previously identified in the crab’s main neurosecretory organ, the PO, provides more specific information on which NPs may be important neuromodulators.
Figure 2.
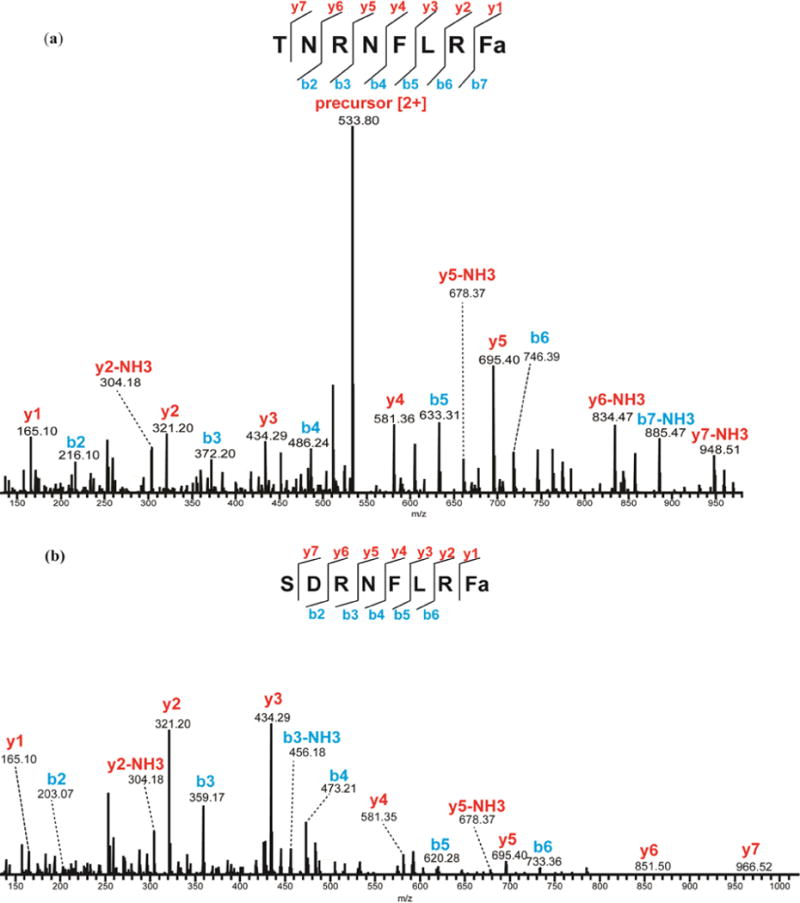
MS/MS spectra of (a) TNRNFLRFamide in hemolymph extract and (b) SDRNFLRFamide in microdialysate by HCD fragmentation. The presence of b- and y-ions is indicated by lines above (y-ions) or below (b-ions) the corresponding amino acid residues in the peptide sequence.
Orcokinins and orcomyotropin
NFDEIDRSGFGFN was the first identified orcokinin and was first found in the crayfish, Orconectes limosus36. The orcokinins occur widely across different crustacean species. Seven neuropeptides from the orcokinin family and two from the orcomyotropin family were found to be secreted into hemolymph in our study. These findings are in good correlation with previous MS and immunohistochemical studies conducted on the neuronal tissues of Cancer borealis24. Hoa-Orcokinin, SSEDMPSSLGFGFN (m/z 1474.51) and VYGPRDIANLY (m/z 1280.44) were previously sequenced in the brain, the stomatogastric nervous system (STNS), and the sinus gland (SG) of the lobster Homarus americanus37 by investigating orcokinin precursors. However, these two neuropeptides were confirmed to be present in Cancer borealis for the first time in this study.
Crustacean hyperglycemic hormone precursor-related peptides
The crustacean hyperglycemic hormone precursor-related peptides (CPRPs) found in hemolymph in this study are apparently truncated, which seems to be common in CPRP sequences7. The detection of 5 truncated CPRPs derived from the same full-length CPRP in the circulating fluid suggest that they may be co-released with crustacean hyperglycemic hormone (CHH), likely secreted from another important neurosecretory organ, the SG, in Cancer borealis24. The CHH is a well-known regulator of hemolymph glucose levels in crustaceans; however, the functions of CPRPs are unknown. The detection of these truncated forms of CPRP, which is encoded by the CHH prohormone, in hemolymph microdialysate provides potential functional clues about these novel forms of CPRPs in energy homeostasis and feeding regulation. Further investigation will be needed to determine the precise roles of these secreted NPs.
The CHH superfamily, well known for its multifunctional roles in the X-organ and SG system38, however, was not detected in our study. CHHs are relatively large compared to other NPs, ranging from 70 to 80 amino acids in length. When sampling with microdialysis, as molecular weight increases, so does the hindrance for analyte diffusion into the microdialysis probe39, 40. As a result, it is very likely that the level of CHHs collected via microdialysis with the particular probe employed is too low to be efficiently detected with MS.
RYamide
Almost all the known RYamides have been characterized by MS-based strategies. The first RYamide was observed in the releasate of the POs of Cancer borealis in 200310, and since then there have been numerous reports documenting the identification of an array of RYamides from various decapod crustacean species7, 32, 41, 42. However, much less is known about the bioactivity of RYamides in crustaceans. The presence of RYamides in neuroendocrine organs in conjunction with this evidence of their existence in the circulatory system (representative ones shown in Figure 3) provides additional insight for investigation of their potential bioactivities.
Figure 3.
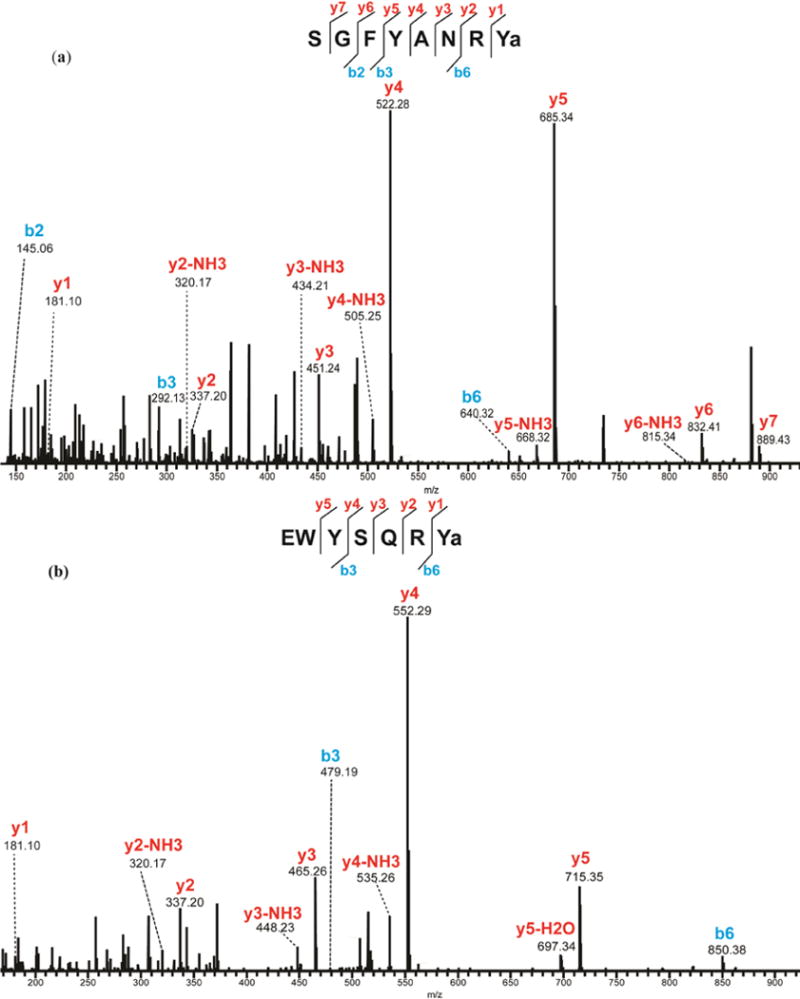
MS/MS spectra of identified RYamide from microdialysate under HCD fragmentation. (a) SGFYANRYamide and (b) EWYSQRYamide. The presence of b- and y-ions is indicated by lines above (y-ions) or below (b-ions) the corresponding amino acid residues in the peptide sequence.
Tachykinin-related peptides (TRP)
TRPs in crustaceans have sequence similarity to vertebrate tachykinins, and possess the C-terminal motif –FXGXRamide. The secreted neuropeptides identified here appear to be related to the mature APSGFLGMRamide (CabTRP-I) and TPSGFLGMRamide (CabTRP-II shown in Figure 4). These two peptides have been well described previously including the oxidized version24. As the invertebrate homologs of mammalian substance P, TRPs have been reported to be involved in various physiological processes43. Studies in Jonah crabs44 have shown that TRP containing neurons in the STG form networks that could generate rhythms to modulate chewing and filtering behaviors. Co-transmission of TRP with proctolin and GABA could stimulate a distinct pyloric motor pattern in the STG44.
Figure 4.
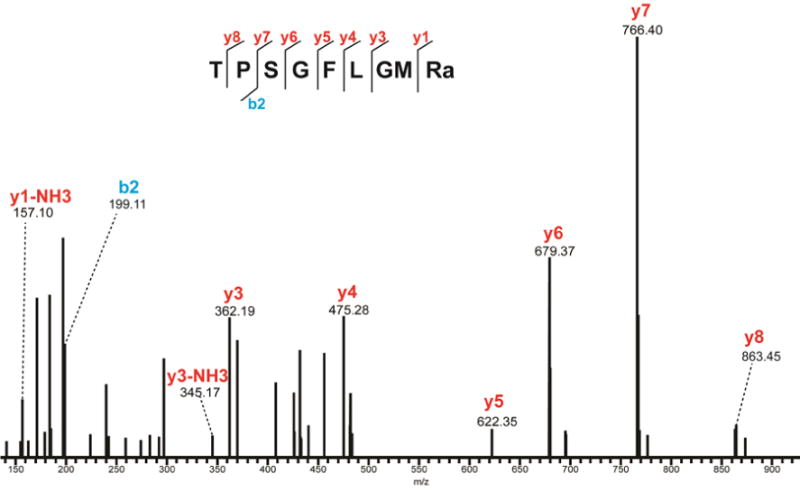
MS/MS spectrum of CabTRP-II from microdialysate under HCD fragmentation. Peaks are annotated with their corresponding b- and y-ions. The presence of b- and y-ions is indicated by lines above (y-ions) or below (b-ions) the corresponding amino acid residues in the peptide sequence.
New insights into well-characterized neuropeptides
In addition to the NPs described above, crustacean cardioactive peptide (CCAP) and peptides from the pigment dispersing hormone (PDH) and red pigment concentrating hormone (RPCH) families were also detected in circulating hemolymph.
The peptide PFCNAFTGCamide is commonly referred as CCAP. Its presence in the decapod crustacean nervous system has been revealed by a number of MS studies7, 24, 41. Its presence in circulating hemolymph has also been confirmed12. Its canonical function is to stimulate cardiac activity45, although it is also active in the STNS46. Peptide related to the full length NSELINSILGLPKVMNEAamide was also identified. PDH regulates the light sensitivity of the retina by migrating eye pigments43, 47. Red pigment concentrating hormone (RPCH), with a function antagonistic to that of PDH, was also identified here. Like PDH, RPCH is well known to be produced inside the eyestalk, in the SG, of decapod crustaceans24, 48. The co-existence of PDH and RPCH in the hemolymph suggests that they may have coordinated functions in other parts of the body in addition to light-related regulatory actions in the eyestalk. Indeed, the role of these NPs in coordination of circadian responses throughout the body has been postulated. This work provides an alternative approach for neuropeptide studies by sampling secreted neuropeptides in vivo. Investigation of secreted neuropeptides, in conjunction with neuronal tissue studies, offers a list of targets for further function-related studies.
Investigation of Circadian NP Changes from In Vivo Microdialysis in the Crab
As a proof-of-principle experiment, we then examined these possible neuromodulatory candidates for circadian rhythm-associated changes that may occur with daily light period changes. Using a method described elsewhere49, we monitored dynamic changes of several identified NPs in microdialysate, throughout 12 h: 12 h light/dark cycle. Due to the preliminary nature of these studies, statistical analysis was not possible with the current number of animals available.
Figure 5 shows a few representative NPs with dynamic changes in response to light/dark cycle alternation. As the light went on, dramatic changes were observed for secreted PDH, CCAP and RPCH (Figure 5). CCAP and PDH both exhibited decreases in their relative hemolymph levels. While CCAP exhibited a slower decrease (Figure 5b), occurring over a period of ~6 hrs, PDH decreased more rapidly (Figure 5c). RPCH, however, was observed to exhibit a unique oscillating pattern (Figure 5d) during the light period. Throughout the daily light/dark transition, not only do the eyes need to be adapted to light changes–which could correlate with the changes of PDH and RPCH, but the whole body also needs to have a coordinated response. In other words, the circadian rhythm must integrate information about light levels, obtained primarily from the eyes, with whole body changes. For instance, the crab tends to be less active when the light is on, and thus may need a lower level of cardiac activity. The observed decrease in hemolymph CCAP thus may prepare the body to adapt to such environmental changes by decreasing cardiac excitability. The observed changes in these secreted peptides provide strong evidence that locally released peptides travel to different organs via the hemolymph to have hormonal effects. These findings lay the groundwork for further investigation of the potential circadian effects of these NPs at the organism level.
Figure 5.
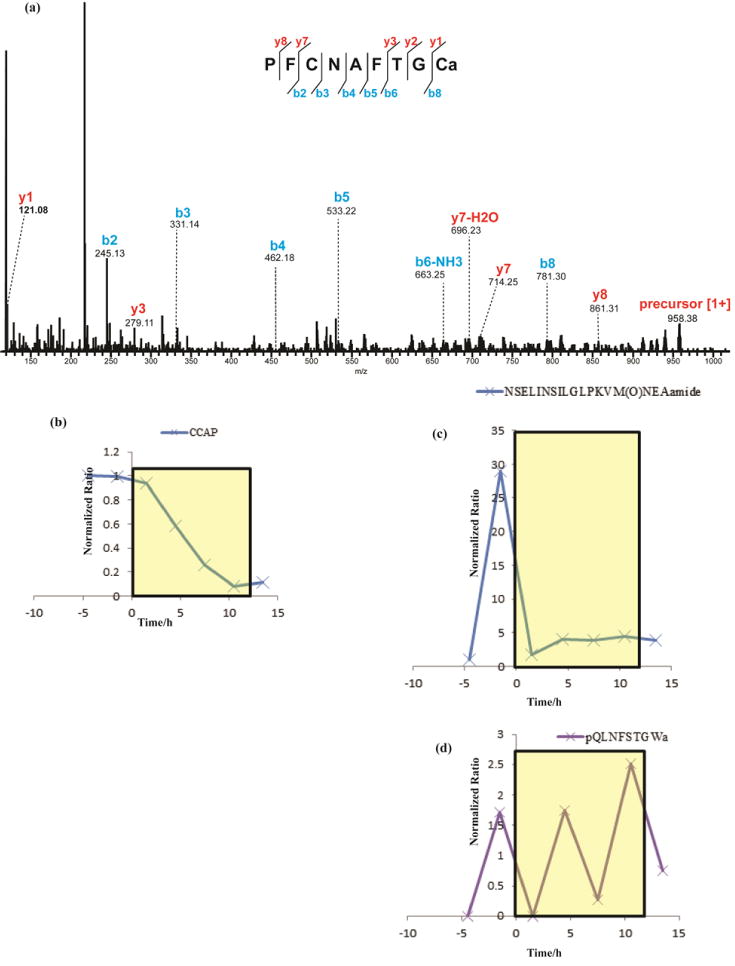
Preliminary comparison of CCAP, PDH, and RPCH in 12 h: 12 h light/dark cycle. Yellow box indicates lights on, starting at time zero and continuing for 12 h. Relative quantity changes were normalized against that of a peptide standard. (a) MS/MS spectrum of CCAP under HCD fragmentation. The presence of b- and y-ions is indicated by lines above (y-ions) or below (b-ions) the corresponding amino acid residues in the peptide sequence; (b) CCAP decreased slowly as the light went on; (c) changes for two PDH isoforms, both of which decreased during the light period. The one with a methionine showed a very sharp decrease right after light went on; (d) RPCH exhibited an oscillating change pattern.
Conclusions
It is important to study NP secretion to increase our understanding of neuromodulation in a well-defined nervous system. In this work, we were able to identify over 50 circulating NPs from several main neuropeptide families, including ASTs, FLPs, orcokinins, TRPs, PDH, CPRPs, RYamides, and CCAP. These results agree with studies determining the neuropeptidomes of various neuronal tissues. The fact that these NPs are present in both neuronal tissues and the circulatory system suggests that they have roles as neuromodulators and hormones. In vivo microdialysis provides the advantage of sampling while the animal is alert and allows for the correlation of neurochemical content dynamics with different behaviors or other changes. From the identified secreted NPs, we found several that could be potentially responsible for the adaptation to light and dark changes.
Supplementary Material
Acknowledgments
This work was supported by a National Institutes of Health (NIH) grant R01DK071801 and a National Science Foundation (NSF) grant (CHE-1413596) (to LL). The Q-Exactive Orbitrap mass spectrometer was purchased through the support of an NIH shared instrument grant (NIH-NCRR S10RR029531). CMS was supported in part by the NIH training grant 5T32GM08349. LL acknowledges an H.I. Romnes Faculty Research Fellowship.
References
- 1.Schmerberg CM, Li L. Protein Pept Lett. 2013;20:681–694. doi: 10.2174/0929866511320060007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mykles DL, Adams ME, Gade G, Lange AB, Marco HG, Orchard I. Physiol Biochem Zool. 2010;83:836–846. doi: 10.1086/648470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marder E, Bucher D. Annu Rev Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- 4.Ye H, Hui L, Kellersberger K, Li L. J Am Soc Mass Spectrom. 2013;24:134–147. doi: 10.1007/s13361-012-0502-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye H, Greer T, Li L. J Proteomics. 2012;75:5014–5026. doi: 10.1016/j.jprot.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia C, Lietz CB, Ye H, Hui L, Yu Q, Yoo S, Li L. J Proteomics. 2013;91:1–12. doi: 10.1016/j.jprot.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hui L, D’Andrea BT, Jia C, Liang Z, Christie AE, Li L. Gen Comp Endocrinol. 2013;184:22–34. doi: 10.1016/j.ygcen.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Sweedler JV. Annu Rev Anal Chem (Palo Alto Calif) 2008;1:451–483. doi: 10.1146/annurev.anchem.1.031207.113053. [DOI] [PubMed] [Google Scholar]
- 9.Fricker LD, Lim J, Pan H, Che FY. Mass Spectrom Rev. 2006;25:327–344. doi: 10.1002/mas.20079. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Kelley WP, Billimoria CP, Christie AE, Pulver SR, Sweedler JV, Marder E. J Neurochem. 2003;87:642–656. doi: 10.1046/j.1471-4159.2003.02031.x. [DOI] [PubMed] [Google Scholar]
- 11.Billimoria CP, Li L, Marder E. J Neurochem. 2005;95:191–199. doi: 10.1111/j.1471-4159.2005.03355.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen R, Ma M, Hui L, Zhang J, Li L. J Am Soc Mass Spectrom. 2009;20:708–718. doi: 10.1016/j.jasms.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy RT. Curr Opin Chem Biol. 2013;17:860–867. doi: 10.1016/j.cbpa.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson CJ, Venton BJ, Kennedy RT. Anal Chem. 2006;78:1391–1399. doi: 10.1021/ac0693722. [DOI] [PubMed] [Google Scholar]
- 15.Chung YT, Ling YC, Yang CS, Sun YC, Lee PL, Lin CY, Hong CC, Yang MH. Anal Chem. 2007;79:8900–8910. doi: 10.1021/ac070981z. [DOI] [PubMed] [Google Scholar]
- 16.Timmerman W, Westerink BH. Synapse. 1997;27:242–261. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 17.Vasicek TW, Jackson MR, Poseno TM, Stenken JA. ACS Chem Neurosci. 2013;4:737–746. doi: 10.1021/cn400025m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behrens HL, Chen R, Li L. Anal Chem. 2008;80:6949–6958. doi: 10.1021/ac800798h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmerberg CM, Li L. Anal Chem. 2013;85:915–922. doi: 10.1021/ac302403e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bajpai G, Simmen RC, Stenken JA. Mol Biosyst. 2014;10:806–812. doi: 10.1039/c3mb70308h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sundstrom I, Arts J, Westerlund D, Andren PE. Analyst. 2010;135:405–413. doi: 10.1039/b917940b. [DOI] [PubMed] [Google Scholar]
- 22.Sun L, Stenken JA, Brunner JE, Michel KB, Adelsberger JK, Yang AY, Zhao JJ, Musson DG. Anal Biochem. 2008;381:214–223. doi: 10.1016/j.ab.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 23.Li Q, Zubieta JK, Kennedy RT. Anal Chem. 2009;81:2242–2250. doi: 10.1021/ac802391b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma M, Wang J, Chen R, Li L. J Proteome Res. 2009;8:2426–2437. doi: 10.1021/pr801047v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cape SS, Rehm KJ, Ma M, Marder E, Li L. J Neurochem. 2008;105:690–702. doi: 10.1111/j.1471-4159.2007.05154.x. [DOI] [PubMed] [Google Scholar]
- 26.Ma M, Szabo TM, Jia C, Marder E, Li L. Peptides. 2009;30:1660–1668. doi: 10.1016/j.peptides.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szabo TM, Chen R, Goeritz ML, Maloney RT, Tang LS, Li L, Marder E. J Comp Neurol. 2011;519:2658–2676. doi: 10.1002/cne.22654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skiebe P, Schneider H. J Exp Biol. 1994;194:195–208. doi: 10.1242/jeb.194.1.195. [DOI] [PubMed] [Google Scholar]
- 29.Jorge-Rivera J, Marder YE. J Exp Biol. 1997;200:2937–2946. doi: 10.1242/jeb.200.23.2937. [DOI] [PubMed] [Google Scholar]
- 30.Price DA, Greenberg MJ. Science. 1977;197:670–671. doi: 10.1126/science.877582. [DOI] [PubMed] [Google Scholar]
- 31.Christie AE, Stemmler EA, Dickinson PS. Cell Mol Life Sci. 2010;67:4135–4169. doi: 10.1007/s00018-010-0482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma M, Gard AL, Xiang F, Wang J, Davoodian N, Lenz PH, Malecha SR, Christie AE, Li L. Peptides. 2010;31:27–43. doi: 10.1016/j.peptides.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma M, Sturm RM, Kutz-Naber KK, Fu Q, Li L. Biochem Biophys Res Commun. 2009;390:325–330. doi: 10.1016/j.bbrc.2009.09.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilkens JL, Shinozaki T, Yazawa T, ter Keurs HE. J Exp Biol. 2005;208:737–747. doi: 10.1242/jeb.01430. [DOI] [PubMed] [Google Scholar]
- 35.Jorge-Rivera JC, Marder E. J Comp Physiol A. 1996;179:741–751. doi: 10.1007/BF00207353. [DOI] [PubMed] [Google Scholar]
- 36.Stangier J, Hilbich C, Burdzik S, Keller R. Peptides. 1992;13:859–864. doi: 10.1016/0196-9781(92)90041-z. [DOI] [PubMed] [Google Scholar]
- 37.Dickinson PS, Stemmler EA, Barton EE, Cashman CR, Gardner NP, Rus S, Brennan HR, McClintock TS, Christie AE. Peptides. 2009;30:297–317. doi: 10.1016/j.peptides.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webster SG, Keller R, Dircksen H. Gen Comp Endocrinol. 2012;175:217–233. doi: 10.1016/j.ygcen.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 39.Kendrick KM. J Neurosci Methods. 1990;34:35–46. doi: 10.1016/0165-0270(90)90040-m. [DOI] [PubMed] [Google Scholar]
- 40.Kendrick KM. Methods Enzymol. 1989;168:182–205. doi: 10.1016/0076-6879(89)68013-5. [DOI] [PubMed] [Google Scholar]
- 41.Ma M, Bors EK, Dickinson ES, Kwiatkowski MA, Sousa GL, Henry RP, Smith CM, Towle DW, Christie AE, Li L. Gen Comp Endocrinol. 2009;161:320–334. doi: 10.1016/j.ygcen.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu Q, Kutz KK, Schmidt JJ, Hsu YW, Messinger DI, Cain SD, de la Iglesia HO, Christie AE, Li L. J Comp Neurol. 2005;493:607–626. doi: 10.1002/cne.20773. [DOI] [PubMed] [Google Scholar]
- 43.Liang Z, Yu Q, Ouyang C, Li L. Colloquium Series on Neuropeptides Submitted Morgan&Claypool Life Sciences. 2014:88–92. [Google Scholar]
- 44.Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E. Trends Neurosci. 2001;24:146–154. doi: 10.1016/s0166-2236(00)01723-9. [DOI] [PubMed] [Google Scholar]
- 45.Stangier J, Hilbich C, Beyreuther K, Keller R. Proc Natl Acad Sci U S A. 1987;84:575–579. doi: 10.1073/pnas.84.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cruz-Bermudez ND, Marder E. J Exp Biol. 2007;210:2873–2884. doi: 10.1242/jeb.002949. [DOI] [PubMed] [Google Scholar]
- 47.Meelkop E, Temmerman L, Schoofs L, Janssen T. Prog Neurobiol. 2011;93:125–147. doi: 10.1016/j.pneurobio.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 48.Stemmler EA, Gardner NP, Guiney ME, Bruns EA, Dickinson PS. J Mass Spectrom. 2006;41:295–311. doi: 10.1002/jms.989. [DOI] [PubMed] [Google Scholar]
- 49.Liang Z, Schmerberg CM, Li L. ACS Chemical Neuroscience Submitted. 2014 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


