Abstract
NRF2, a basic leucine zipper transcription factor encoded by the gene NFE2L2, is a master regulator of the transcriptional response to oxidative stress. NRF2 is structurally and functionally conserved from insects to humans, and it heterodimerizes with the small MAF transcription factors to bind a consensus DNA sequence (the antioxidant response element, or ARE) and regulate gene expression. We have used genome-wide chromatin immunoprecipitation (ChIP-seq) and gene expression data to identify direct NRF2 target genes in Drosophila and humans. These data have allowed us to construct the deeply conserved ancient NRF2 regulatory network – target genes that are conserved from Drosophila to human. The ancient network consists of canonical antioxidant genes, as well as genes related to proteasomal pathways, metabolism, and a number of less expected genes. We have also used enhancer reporter assays and electrophoretic mobility shift assays to confirm NRF2-mediated regulation of ARE (antioxidant response element) activity at a number of these novel target genes. Interestingly, the ancient network also highlights a prominent negative feedback loop; this, combined with the finding that and NRF2-mediated regulatory output is tightly linked to the quality of the ARE it is targeting, suggests that precise regulation of nuclear NRF2 concentration is necessary to achieve proper quantitative regulation of distinct gene sets. Together, these findings highlight the importance of balance in the NRF2-ARE pathway, and indicate that NRF2-mediated regulation of xenobiotic metabolism, glucose metabolism, and proteostasis have been central to this pathway since its inception.
INTRODUCTION
Oxidative stress from various reactive oxygen species (ROS) can damage all cellular macromolecules, including proteins, lipids, and DNA. The latter effect is particularly harmful because it can cause DNA mutations with potential long-term consequences. Accordingly, oxidative damage is a significant contributor to chronic diseases, from cancer to neurodegenerative disease [1, 2]. In response to oxidative stress, cells upregulate a battery of cytoprotective genes, including antioxidant and detoxifying enzymes, to protect against and recover from damage to the cell. NRF2, a basic leucine zipper (bZIP) transcription factor encoded by the gene NFE2L2 (nuclear factor (erythroid-derived 2)-like 2), is a master regulator of the transcriptional response to oxidative stress [3]. In the absence of oxidative stress, the nuclear concentration of NRF2 is kept low by direct interaction the inhibitory protein KEAP1 (kelch-like ECH-associated protein 1). KEAP1 inhibits NRF2 both by sequestering it in the cytoplasm and targeting it for polyubiquitination and proteasomal degradation [3]. Thus, under normal conditions, low levels of nuclear NRF2 maintain basal expression of antioxidant and detoxification genes, which is necessary for protecting cells against physiological levels of ROS. However, the KEAP1-NRF2 interaction is modulated by cellular redox state. Electrophilic stresses such as ROS modify redox-sensitive cysteine residues on KEAP1, and modification of these residues impairs KEAP1-mediated ubiquitination of NRF2. These reactive cysteine residues – KEAP1 has 25 such residues – allow KEAP1 to act as a stress sensor. In the presence of an oxidative insult, KEAP1’s ability to target NRF2 for degradation decreases and nuclear NRF2 concentration increases. In the nucleus, NRF2 heterodimerizes with another class of bZIP transcription factors, the small MAF proteins (MAFF, MAFG, MAFK), to bind a consensus DNA sequence (the antioxidant response element, or ARE) and upregulate antioxidant and detoxification gene batteries [3].
The need to cope with ROS from endogenous and exogenous sources is not limited to vertebrates, so it is not surprising that the KEAP1, NRF2, and MAF proteins are conserved from Drosophila melanogaster to humans [3]. In fact, the nematode Caenorhabditis elegans also has a functional ortholog of NRF2, Skn-1, although the mechanisms regulating its activity and DNA binding are not dependent on KEAP1 or the small MAF proteins [3]. Both the C. elegans and D. melanogaster NRF2 orthologs are activated by oxidants and both factors can upregulate antioxidant genes [3-5]; work in these model organisms also supports a role for Nrf2 in life-span extension [4-6]. The strong data connecting NRF2 to longevity are facilitated in part by the comparably short lifespan of D. melanogaster and C. elegans relative to vertebrate models, which is one of the multiple benefits of studying NRF2 function in invertebrate systems. The powerful genetic tools available in these model organisms have also provided insight into NRF2 function outside of the cytoprotective stress response, including roles in stem cell proliferation and neuroendocrine signaling [7, 8]. Considering the many similarities between the molecular and physiological responses to stress in invertebrate and mammalian model systems [9-11], it is clear that a comparative approach will continue to inform our models of NRF2 function in humans.
More comprehensive models of NRF2-mediated gene regulation are crucial because there is significant interest in using activators of the NRF2 pathway for chemoprevention and pharmacological intervention of many chronic diseases. However, more recent work indicates that NRF2 activation can be detrimental in certain contexts. Mutations that disrupt NRF2-KEAP1 interaction and lead to constitutive activation of this pathway are associated with multiple tumor types, particularly lung tumors [12-14]. This is partially because pathways activated by Nrf2 in tumors appear to promote metabolic growth and resistance to high levels of oxidative stress and chemotherapeutic treatments when mutationally hyperactivated [13, 15]. Continued exploration of the NRF2 regulatory network has demonstrated that NRF2-mediated regulation extends beyond just the genes responsible for scavenging ROS into genes associated with proteostasis, metabolism, and more [16]. Although it is possible that the antioxidant genes are the significant effectors in determining whether NRF2 activity crosses the threshold from beneficial to detrimental [17], non-canonical NRF2 targets are also likely to be important in both physiological and pathophysiological contexts. Thus, generation of broad and comprehensive models of the NRF2 regulatory network are essential.
We have used genome-wide chromatin immunoprecipitation (ChIP-seq) to begin identifying regions of the genome bound by NRF2 in both Drosophila and humans. The Drosophila cap-n-collar (cnc) gene encodes multiple isoforms of the Cnc protein, one of which (CncC) is functionally homologous to human NRF2. From the Drosophila and human ChIP-seq data, we were able to determine putative direct NRF2 target genes in both organisms and construct the deeply conserved, ancient NRF2 regulatory network, covering NRF2 target genes conserved from fly to man. These data provide a broad survey of putative direct NRF2 target genes, and serve as a valuable resource for evaluating the functional correspondence between Drosophila Cnc (hereafter referred to as Cnc/Nrf2) and human NRF2. Because NRF2 targets conserved over such a large evolutionary distance are likely to be functionally important, this approach provides a glimpse into the core gene sets and transcriptional regulatory mechanisms that are central to the NRF2 network. We use these data, in combination with expression profiling and functional validation data, to address three general questions: What are the target genes directly bound by Cnc/Nrf2 in Drosophila? Which Drosophila Cnc/Nrf2 target genes are conserved as NRF2 targets in humans? And, ultimately, what can we learn about the NRF2 regulatory network by focusing on the target genes conserved from invertebrates to humans?
MATERIALS and METHODS
Chemicals and materials
D,L-sulforaphane (SFN) was purchased from MP Biomedicals LLC (Santa Ana, CA) or Calbiochem/Millipore (Billerica, MA) and ethyl 7-chloro-4-hydroxy-8-methylquinoline-3-carboxylate (AI-1) was purchased from Calbiochem/Millipore. Luperox® (tert-butyl hydroperoxide solution; TBOOH) was purchased from Sigma-Aldrich Chemical Company (St. Louis, MO). Stock solutions of all test compounds were prepared in dimethyl sulfoxide (DMSO) and stored at −20°C. FuGENE® HD transfection reagent was purchased from Promega (Madison, WI). Total Exosome Isolation (from cell culture media) reagent was purchased from Invitrogen™-Life technologies™ (Carlsbad, CA). Purified human Nrf2 protein was purchased from ProteinOne (Surry Hills, NSW, Australia) and purified MAFG was purchased from ATGen, Ltd (Seoul, Korea). MISSION® LightSwitch Luciferase Assay Reagent™ was purchased from Sigma-Aldrich Chemical Company (St. Louis, MO). Expression vectors for NRF2 (pEF-NRF2), dominant-negative NRF2 (pEF-NRF2DN), and control vector (pEF) were kindly provided by Dr. Shinya Ito (Hospital for Sick Children, Toronto, Canada); an additional NRF2 expression vector (pCDNA3-Myc3-NRF2) [18] was also acquired from Addgene (Cambridge, MA).
Drosophila ChIP-seq and analysis
Drosophila Cnc/Nrf2 ChIP-seq experiments were performed as part of the modENCODE (model organism Encyclopedia of DNA Elements) project [19]. All ChIPs were performed with the iso-1 strain (y1; cn1, bw1, sp1) [20]. Chromatin was extracted from approximately 100 milligrams of animals at each developmental stage tested: 8-16 hour embryos, white prepupae, 2-3 day old males, and 2-3 day old females. Stage-specific chromatin collection and chromatin immunoprecipitation using a rabbit polyclonal anti-Cnc antibody (a gift from Kevin White) were performed as described previously [21]. Immunoprecipitated DNA was prepared for Illumina sequencing using the Epicentre Nextera DNA Sample Preparation Kit (Madison, WI). Briefly, Nextera library preparations were performed using the High Molecular Weight tagmentation buffer, and tagmented DNA was amplified using 12 cycles of PCR. Library DNA was sequenced on an Illumina HiSeq 2000 according to manufacturer’s standard protocols.
Biological ChIP-seq replicates were scored against an input DNA control (from non-immunoprecipitated chromatin). The MACS (v2) peak caller was used to identify and score (rank) potential binding sites/peaks [22]. For permissive peak calling (i.e., Class I peaks), biological replicates were pooled and peaks were called using MACSv2 with a p-value threshold of 1e-3. The Irreproducible Discovery Rate (IDR) framework was used for stringent peak calling (i.e., Class II peaks); IDR identifies high confidence binding events by leveraging the reproducibility and rank consistency of peak identifications across replicate experiments of a dataset as described previously [19, 23, 24]. Details of the IDR framework are available at https://sites.google.com/site/anshulkundaje/projects/idr. As indicated above, the Class I peaks consist of all binding sites (from any developmental stage) called using the permissive peak calling approach, and the Class II peaks consist of all binding sites (from any developmental stage) called using the stringent peak calling approach. Class III peaks consist of permissive peaks that are present (overlapping binding) across all four ChIP-seq datasets; in other words, a region that is called as a peak (MACSv2 p-value of 1e-3 or better) in embryos, white prepupae, adult males, and adult females would be a Class III peak. Correlations of average ChIP signals (aligned reads) across peaks were calculated using bamCorrelate [25]. Peaks were assigned to Drosophila target genes based on the the nearest transcription start site, and GO (Gene Ontology) and KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway enrichment was determined using DAVID [26]. Enriched DNA motifs within the Cnc/Nrf2 peaks were identified using i-cisTarget [27] to scan motifs from the JASPAR [28] and TRANSFAC [29] databases. Drosophila ChIP-seq data have been deposited in the NCBI GEO (Gene Expression Omnibus; http://www.ncbi.nlm.nih.gov/geo/) database under accession numbers GSE34354, GSE32661, GSE34182, and GSE34342, and peak calls are also available in Supplementary Table 2.
Drosophila-human ortholog identification
Orotholog identification was performed using DIOPT (DRSC Integrative Ortholog Prediction Tool), a program that integrates ortholog predictions from 10 commonly used orthology tools [30]. Human orthologs of Drosophila genes were using two strategies based on the DIOPT orthology score, which indicates the number of tools that support an orthologous gene-pair relationship (max = 10). For the first strategy, orthologous genes were called using all human matches per Drosophlia gene, filtering out matches with a DIOPT score <3 (unless a Drosophila gene had no matches at this stringency, in which case matches with a score of 1 or 2 were included). For the second strategy, only the best match (highest score) was used when there was more than one human match per Drosophlia gene; when multiple genes tied for best match all were included.
Human ChIP-seq, exon arrays, and analysis
Human NRF2 ChIP-seq data were from Chorley et al. [31] and can be accessed at the NCBI GEO database (http://www.ncbi.nlm.nih.gov/geo/) under accession GSE37589. Kernel density values for comparing ChIP signals from ‘vehicle’ and ‘sulforaphane’ ChIPs were calculated using QuEST and genome-wide correlations were performed using ACT [32, 33]. For gene expression analysis, human lymphoblastoid cells (Coriell; Camden, NJ) were cultured as recommended by Coriell, were treated with 10 uM D,L-sulforaphane from Calbiochem and RNA was extracted from untreated cells and treated cells after 8 hours and 24 hours. Total RNA was applied to Affymetrix Exon 1.0 array and hybridization and scanner where perfomed as per manufacturer’s instructions. Intensity values were normalized across all arrays using the RMA (robust, multi-array average) algorithm [32] in Affymetrix Expression Console. Gene expression data have been submitted to the NCBI GEO database under accession GSE66164 (reviewer link: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=yperykwinhsrdmb&acc=GSE66164).
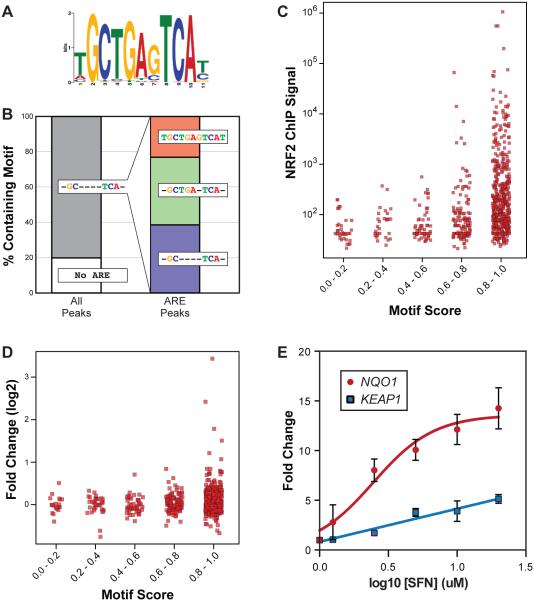
Enriched DNA motifs within the NRF2 peaks were identified using MEME-ChIP (parameters: 11mers, 0 or 1 per sequence) [34], and GO/KEGG enrichment of human NRF2 target genes (or deeply conserved target genes) was determined using Enrichr [35]. For Figures 6C and 6D, ChIP peaks were sorted into bins based on the similarity of their strongest the ARE to the NFE2L2 position weight matrix from JASPAR (ID: MA0150.1) using a first-order transcription factor flexible model [36].
Figure 6. Relationship between ARE sequence, NRF2 DNA binding, and gene expression in human cells.
(A) The top enriched motif in the human NRF2 ChIP-seq data (see Methods). This sequence matches the NRF2/ARE consensus. (B) Breakdown of the percent of NRF2 bound regions containing various iterations of the ARE consensus sequence. (C) Dot plot representing relationship between ChIP-seq signal and the ARE motif score in each NRF2 bound region. ARE motif score is determined by similarity to the sequence in (A). ChIP peaks were sorted into bins based on the similarity of their strongest ARE to the ARE position weight matrix from JASPAR (ID: MA0150.1) using a first-order transcription factor flexible model [36]. (D) Same as C, only representing the relationship between motif score and SFN-induced expression changes for the target genes associated with NRF2 binding regions. (E) Dose-response experiment using reporter constructs driven by NRF2-targeted enhancers from the NQO1 and KEAP1 loci. Human IMR32 cells were treated with a range of SFN concentrations (0 to 20 uM), and fold change was calculated relative to DMSO control (0 uM SFN). Lines best fitting the data (nonlinear for NQO1, linear for KEAP1) are represented.
Luciferase reporter assays
Human regulatory elements (enhancer regions) were cloned into the LightSwitch optimized luciferase reporter vector system (more details at http://switchgeargenomics.com/products/utr-reporter-collection/) (Supplemental Table 1). Enhancer/promoter elements that contained a putative transcriptional start site were cloned into the pLightSwitch promoter vector. Putative long-range enhancer elements were cloned into the pLightSwitch long range vector which contains a minimal TK promoter. Constructs containing mutations to the ARE binding motif were generated using a modification of the QuikChange (Stratagene) protocol and sequence verified.
The human hepatocellular carcinoma HepG2 and neuroblastoma IMR32 cell lines were purchased from the American Type Culture Collection (ATCC). For general maintenance, cells were grown and maintained in EMEM culture media (ATCC), supplemented with 10% fetal bovine serum (Biowest, Logan, UT) and 1% (v/v) penicillin/streptomycin (Invitrogen-Life Technologies, Carlsbad, CA), and gown at 37°C in humidified 5% CO2 air.
For transfection experiments, cells were maintained in Opti-MEM (Invitrogen) supplemented with 10% FBS. White 96 well plates were seeded with 15,000 cells/well and simultaneously transfected using the SwitchGear Genomic High-throughput transfection protocol. Each transfection included 0.15μL of FuGENE® HD transfection reagent and 47.5-50 ng/well of reporter plasmid. For experiments with overexpression of NRF2 or NRF2DN, expression plasmids (pCDNA3, pCDNA3-Myc3-NRF2, pEF, pEF-NRF2, or pEF-NRF2DN) were co-transfected at 2.5 ng/well with 47.5 ng/well of reporter plasmid; reporter plasmids were transfected at 50 ng/well for all other experiments. The experimental set up employed when DMSO, SFN, or AI-1 were utilized is as follows: each construct was transfected in triplicate (at minimum) and plates were incubated at 37°C. The transfected cells were treated with DMSO, SFN or AI-1 24 hours post transfection. Then the cells were incubated for an additional 24 hours at 37°C before being frozen at −80°C. The experimental set up employed when no chemical induction of NRF2 was utilized is as follows: each construct was transfected in triplicate and plates were incubated at 37°C for 24 hours, after which cells were frozen at −80°C. Plates were removed from the freezer and allowed to reach room temperature. 100 μL of MISSION® LightSwitch Luciferase Assay Reagent was added to each well, and plates were incubated at room temperature for 30 min and read on a SpectraMax M3 (Molecular Devices, Sunnyvale, CA) according to the manufacturers instructions.
Electrophoretic mobility shift assays (EMSA)
Purified myc-tagged NRF2 (75nM), MAFG (37.5nM), IRDye-700 labeled double stranded DNA, and competitor oligonucleotides were incubated for 30 min at room temperature with poly(dI-dC) in the binding buffer (20mM HEPES, 4mM MgCl2, 100μg/ml BSA, 4% glycerol, 20mM KCl, 5mM DTT, and 1mM EDTA). Orange loading dye (LI-COR, Lincoln, NE) was added and samples were electrophoresed through a native acrylamide gel in 1X TBE. A labeled (IRDye-700) double-stranded oligonucleotide containing the human NQO1 ARE was used for the electrophoretic mobility shift assay (EMSA): 5’-AATCCGCAGTCACAGTGACTCAGCAGAATCTGAGCCTAG-3’. The sequences of cold competitor oligonucleotides can be found in Supplemental Table 1. Gels were imaged using the Odyssey infrared imaging system (LI-COR, Lincoln, NE).
Isolation and quantification of exosomes
Cells used for exosome isolation were grown in exosome-depleted FBS (System Biosciences; Mountain View, CA). Exosomes were isolated from cell culture media according to the manufacturers instructions using the Total Exosome Isolation (from cell culture media) reagent (Invitrogen-Life Technologies). Briefly, IMR32 cells were seeded at 1.25×105 cell /well in a 24 well plate, 24 hours after seeding cells were treated with either SFN, TBOOH, or DMSO for 24 ours. 48 hours after seeding (24 hours after adding treatment) the cell culture media was collected and centrifuged at 2000xG for 20 minutes and a final cell count was obtained (cell/ml) for each individual well. The cell-free supernatant containing the exosomes was transferred to a clean tube and 0.5 volumes of the Total Exosome Isolation reagent was added. Samples were thoroughly mixed and stored at 4°C overnight, and then finally samples were centrifuged at 10,000xG for 1 hour. Supernatant was discarded and the exosomes were resuspended in 50μL phosphate buffered saline. Samples were analyzed for exosomes (30-100nm) using a NanoSight and the Nanoparticle Tracking Analysis (Malvern Instruments, Worcestershire, UK). Exosome counts (particles/mL) were normalized to cell count (cells/ml) to obtain exosomes/cell.
RESULTS and DISCUSSION
Overview of Cnc/Nrf2 genome-wide binding in Drosophila
To get a genome-wide view of the Cnc/Nrf2 regulatory network in Drosophila, we performed ChIP-seq using an antibody against the C-terminal region of Cnc/Nrf2 that recognizes all Cnc isoforms (Figure 1B). Chromatin was isolated from embryos (16-24 hours after egg laying), white prepupae (at the transition between larval and pupal stages), or 2-3 day old adults (male or female). Although it is a stress-responsive transcription factor, CncC – the Nrf2 ortholog – also regulates cytoprotective genes under basal (non-stress) conditions [5]. We chose to monitor genome-wide binding in the absence of stress in an attempt to capture both canonical cytoprotective Cnc/Nrf2 target genes as well as non-canonical developmentally regulated target genes.
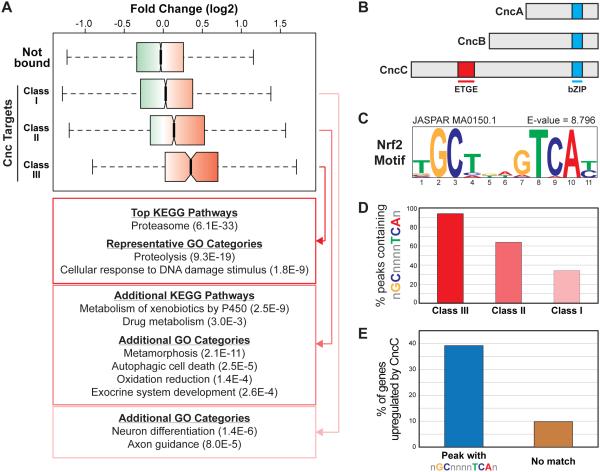
Figure 1. Genes targeted by Cnc/Nrf2 in Drosophila.
(A) Boxplot representing the range of gene expression changes in response to CncC overexpression for genes not bound by Cnc and the Class I, II, and III Cnc/Nrf2 targets. Gene expression profiling data are from [45] and and can be found under GEO Accession Number GSE30087; fold change represents expression in adult flies transiently overexpressing CncC relative to wild-type adult flies. (B) Schematic representation of the three Cnc isoforms. The Keap1 interaction region (ETGE) and DNA binding region (bZIP) are highlighted. (C) The NRF2 consensus motif (ARE), which is the top enriched motif in the Class III peaks as determined by i-cisTarget. (D) Graph representing the fraction of Class I, II, or III peaks containing a match to the general ARE consensus GCnnnnTCA (E) Graph representing the percent of CncC-induced genes as defined in [45] that are targeted by Cnc/Nrf2 via a peak that contains an ARE versus those targeted by Cnc/Nrf2 via a peak that does not contain an ARE.
Cnc/Nrf2 bound regions were first identified using MACS (Model-based Analysis of ChIP-Seq) a standard peak calling algorithm [22] (Supplemental Table 2). In general, Cnc/Nrf2 binding patterns are highly consistent across developmental stages, with highly correlated ChIP signals across the 12,645 identified Cnc/Nrf2 bound regions (Supplemental Figure S1A-B). To identify developmental stage-specific Cnc/Nrf2 binding, we next focused on the top 1000 ChIP peaks from each dataset (or all 666 peaks for white prepuae, because there were fewer than 1000 peaks in this dataset). We defined stage-specific peaks as those that fall within the top 1000 peaks of one stage and are not called as a peak in any other stage (Supplemental Table 2). Consistent with the correlations described above, Cnc/Nrf2 displays very little stage-specific binding using this thresholding approach (Supplemental Figure S1C). Thus, much of the Cnc/Nrf2 binding signal from these whole animal ChIPs is relatively consistent across developmental stages.
Nevertheless, developmental stage-specific Cnc/Nrf2 binding is present in these data. Interestingly, the most enriched gene ontology (GO) categories in all cases of stage-specific binding are often related to developmental patterning, developmental specification, or morphogenesis (Supplemental Table 2). For example, the most significant category for genes associated with embryo-specific Cnc/Nrf2 binding is ‘pattern specification process’ (Bonferroni corrected p-value <0.05), and includes important developmental genes such as hairy, schnurri, Stat92E, and βFTZ-F1. CncB, one of the smaller isoforms of Cnc (Figure 1B), plays an important role in patterning of the embryonic head [37, 38], so it is possible that these developmentally labile peaks represent binding by the Cnc isoforms that are not functionally homologous to Nrf2. We also found evidence for sex-specific Cnc/Nrf2 binding that could influence sexually dimorphic growth patterns: there is a female-specific binding event at the Hr4 (DHR4) locus, and a male-specific binding event at the Hnf4 (dHNF4) locus. Hr4 and Hnf4 both encode nuclear receptor transcription factors that influence metabolism, growth, and lipid storage [39-41], and these data suggest Cnc/Nrf2 could influence sex-specific differences in these pathways.
Overall, however, stage-specific binding makes up a small fraction of Cnc/Nrf2 genome-wide binding. Importantly, genes involved cytoprotective response to stress are not enriched in any of the stage-specific Cnc/Nrf2 target gene sets. Thus, we next shifted our focus to the full catalog of Cnc/Nrf2 binding.
Cnc/Nrf2 targets cytoprotective gene sets in Drosophila
To fully catalog Cnc/Nrf2 bound regions in Drosophila, peaks were identified using both standard and stringent peak calling algorithms to identify three classes of binding sites (see Methods) (Supplemental Table 2). Class I is the most inclusive, consisting of regions bound by Cnc/Nrf2 at any developmental stage using standard peak calling (12,645 peaks; these are the peaks discussed in the previous section). Class II peaks consist of regions bound by Cnc/Nrf2 at any developmental stage using stringent peak calling (3,028 peaks). And Class III peaks consist of regions consistently bound by Cnc/Nrf2 across all four datasets (233 peaks). Class III peaks are the most stringent as this class consists of regions of the genome bound by Cnc/Nrf2 in a manner that is unaffected by these vastly different developmental contexts. Although the number of binding events in Class I and Class II categories is large, this is consistent with the widespread binding seen in many ChIP-seq studies, and these categories likely consists of both functional binding and binding that has little regulatory impact (i.e., nonfunctional binding) [42-44].
Although transcription factor genome-wide binding is often widespread, it is still possible to identify biologically relevant sets of direct target genes within these data. To explore the Cnc/Nrf2 target genes, each Cnc/Nrf2 peak was assigned to an associated target gene based on the nearest transcription start site. Because more than one binding event can be associated with a given gene, there are fewer genes than peaks in each category: 4,271 Class I target genes, 2,018 Class II target genes, and 227 Class III target genes. We then used previously published gene expression profiling data to explore how the expression of the genes in each class is impacted by overexpression of CncC, the Cnc isoform that is functionally homologous to NRF2, in adult male flies [45]. Each target gene class is significantly more responsive to CncC overexpression than genes not bound by Cnc/Nrf2, and this is especially evident in the Class III target genes (Figure 1A). Thus, a significant fraction of the genes near Cnc/Nrf2 bound regions represent bona fide target genes. Further support for this comes from analysis of the most overrepresented GO categories and KEGG pathways within these gene sets (Figure 1A). The Class III gene set is highly enriched for genes that encode proteins associated with proteasome-mediated proteolysis and a number of stress-responsive genes, particularly those associated with the response to DNA damage. In addition, when we expand our search to cover Class II, genes associated with xenobiotic metabolism, redox homeostasis, and autophagy are enriched. Interestingly, a number of less expected categories are also evident in the Class I and II categories, including genes associated with metamorphosis/neuroendocrine signaling, which is consistent with a recent study highlighting a novel role for Cnc/Nrf2 in Drosophila metamophosis [8]. Thus the expected NRF2 target gene categories are enriched in the putative Cnc/Nrf2 target genes as identified by ChIP-seq. Overall these results indicate that a significant fraction of the Cnc/Nrf2-bound regions are true, functional binding events.
To further characterize the DNA regions targeted by Cnc/Nrf2, we then looked at the DNA motifs enriched in these binding regions. Cnc/Nrf2 bound regions were queried for matches to position weight matrices (PWMs) from publicly accessible DNA motif databases including JASPAR [28] and TRANSFAC [29] (see Methods), and the most significantly enriched PWM in the Class III regions is the ARE motif (GCnnnnTCA) (Figure 1C); this same PWM was also significantly enriched in the Class II regions, and a similar motif based on the NRF2 binding site was enriched in the Class I regions (not shown). In total, over 90% of the Class III regions contain at least one sequence matching the GCnnnnTCA ARE consensus, and >60% of the Class II regions contain this consensus (Figure 1D). These results are consistent with previous results demonstrating that Cnc/Nrf2 interacts with ARE sequences in vivo [37]. We then used this information to see if Cnc/Nrf2 bound regions containing an ARE are more likely to be responsive to CncC overexpression. Indeed, the ARE-containing peaks are far more likely to be associated with CncC-mediated expression than Cnc/Nrf2 peaks that lack an ARE, and can account for ~40% of all genes significantly upregulated by CncC overexpression (Figure 1E). These results further validate the Drosophila ChIP-seq data, and suggest that a large part of Cnc/Nrf2-mediated gene regulation in flies is mediated via its interaction with the sequences matching the ARE consensus.
Overall these data highlight the homology between the Drosophila and human NRF2 regulatory networks. The Drosophila ortholog of NRF2 interacts with ARE sequences to regulate antioxidant and proteostasis genes, which is very consistent with NRF2’s role in humans. Both the clear homology and the identification of conserved noncanonical NRF2 targets suggest the possibility that these target gene may have important biological roles.
Summary of ancient NRF2 target genes
To move beyond the high level similarities between the Drosophila and human NRF2 regulatory networks, we next cataloged all orthologous Drosophila and human genes that are identified by ChIP-seq as direct NRF2 target genes in both species. For these comparisons we used data from human lymphoblastoid cell line (LCL) cultures treated sulforaphane (SFN), a naturally occurring isothiocyanate that activates the NRF2 pathway [46], because these cells displayed more robust NRF2 binding than vehicle treated cells [31]. However, the NRF2 binding in SFN and vehicle treated cells is highly correlated (Supplemental Figure 2), so NRF2 targets similar regions under basal conditions.
Identification of fly-human orthologs can be complicated by fact that duplication events sometimes lead to multiple human orthologs for a single Drosophila gene. For example, the gene encoding heme oxygenase in Drosophila (Ho) is orthologous to both heme oxygenase 1 (HMOX1) and 2 (HMOX2) in humans. Thus, it is often appropriate to allow for a single fly gene to map to multiple human genes, which is what we have done for the analysis presented below. Nevertheless, we performed parallel analysis using only the most significant human ortholog for a given fly gene – human HMOX1 would not be considered orthologous to fly Ho, in this case, as HMOX2 is the more significant hit – and all patterns were consistent across both ortholog calling strategies. Regardless of the ortholog calling method used, we find highly significant overlap between NRF2 target genes identified in LCL ChIP-seq experiments [31] and in fly ChIP-seq experiments (Figure 2A). For example, using all significant orthologs (i.e., including both HMOX1 and HMOX2 from the example above), of the 840 human NRF2 targets, 255 fall into the Class I Drosophila Cnc/Nrf2 target gene category, 144 are Class II targets, and 23 are Class III (Figure 2A and Supplemental Table 3). Enrichment numbers are still significant, though slightly lower, when only the ‘best hit’ orthologs are included (Figure 2A and Supplemental Table 3). Overall, the significant overlap between Drosophila and human NRF2 target genes indicates that NRF2 function in these disparate organisms is highly conserved at the level of regulatory network structure.
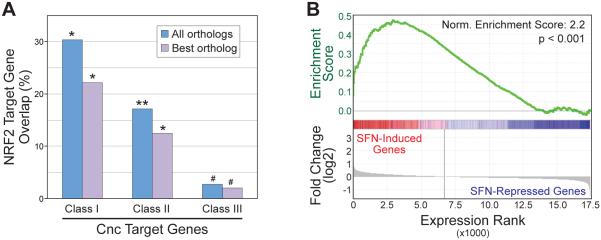
Figure 2. Deeply conserved human NRF2 targets are upregulated by sulforaphane in human cells.
(A) Percent of NRF2 target genes overlapping human orthologs of Drosophila Class I, II, or III genes. Orthologs were identified using either the top scoring ortholog only (best ortholog), or all orthologs scoring >2 as described in the text. *P<0.0005, **P<0.001, #P<0.05, based on hypergeometric test. (B) Gene Set Enrichment Analysis (GSEA) comparing conserved human NRF2 target genes (human orthologs of Drosophila Class I genes) and gene expression changes after treatment of LCL cells with sulforaphane (SFN).
We then used gene expression profiling to explore how conserved NRF2 target genes correlate with the gene expression changes that occur after treatment of LCL cells with sulforaphane (SFN). Affymetrix Exon Arrays were used to get a comprehensive view of SFN-induced changes to the transcriptome 8 and 24 hours after SFN treatment (Supplemental Table 4). Gene Set Enrichment Analysis (GSEA) [47] demonstrates that the conserved NRF2 target gene set is enriched in the group of genes that are upregulated 24 hours post-SFN addition (Figure 2B). A similar enrichment pattern was seen with the 8 hour SFN data (not shown). These results indicate that the conserved NRF2 target genes are generally upregulated after NRF2 activation. In addition, these target genes strongly overlap targets of the transcription factor MAFK (p=7.5×10−17) as determined by the ENCODE project [35, 48]. MAFK, one of the small Maf proteins, is a known NRF2 cofactor, so this overlap provides further validation of these genes as NRF2 targets.
A closer look at the deeply conserved NRF2 target genes provides insight into some essential components of the NRF2 regulatory network. The most stringent set, the 23 genes that are targeted by NRF2 in humans and at all stages of development in Drosophila is dominated by proteasome-associated genes, genes associated with lipid or glucose metabolism, and genes encoding proteins involved in oxidation-reduction reactions (Table 1). GCLC, which encodes the catalytic subunit of glutamate-cysteine ligase and regulates production of the antioxidant glutathione (GSH), has long been recognized as an important NRF2 target gene [49] so its conservation is not unexpected. Along these lines, it is also not surprising to find additional genes involved in redox reactions or homeostasis on this list. Finding genes involved in proteasome-mediated degradation and proteostasis is also consistent with known NRF2 functions [20, 50]; however their prominence on this list, and that NRF2 has the potential to regulate proteasomal activity at multiple levels – from expression of both 19S (PSMA3, PSMA4) and 20S (PSMD4, PSMD6) proteasomal subunits, to genes associated with proteasome assembly (POMP) and ubiquitin recruitment/recognition (ADRM1, VCP) – is unanticipated. Involvement of NRF2 in lipid metabolism has been suggested by several studies [31, 51, 52] and the presence of conserved lipid metabolism genes supports this notion. The NRF2 inhibitor KEAP1 is also on this list, highlighting the importance of the NRF2-KEAP1 negative feedback loop [53]. A subset of interesting genes from this list is explored in more detail below.
Table 1.
Drosophila Class III genes and human orthologs
| Drosophila Gene |
Human Ortholog |
Full Gene Name (Human) | Function |
|---|---|---|---|
| Keap1 | KEAP1 | Kelch-like ECH-associated protein 1 |
Inhibitor of Nrf2 |
| Gclc | GCLC | Glutamate-cysteine ligase, catalytic subunit |
Oxidation/Reduction |
| Cyp6v1 | TBXAS1 | Thromboxane A synthase 1 | Lipid metabolism Oxidation/Reduction |
| Cpr | POR | P450 oxidoreductase | Lipid metabolism Oxidation/Reduction |
| CG9436 | AKR1D1 | Aldo-keto reductase family 1, member D1 |
Lipid metabolism Oxidation/Reduction |
| CG31523 | ELOVL1 | ELOVL fatty acid elongase 1 | Lipid metabolism |
| Sap-r | PSAP | Prosaposin | Lipid metabolism |
| Lpin | LPIN1 | Lipin 1 | Lipid metabolism Transcription/Nuclear receptor signaling |
| PCB | PC | Pyruvate carboxylase | Lipid metabolism Glycolysis/Gluconeogenesis |
| Aldh-III | ALDH3A1 | Aldehyde dehydrogenase 3 family, member A1 |
Glycolysis/Gluconeogenesis |
| Rpn13 | ADRM1 | Adhesion regulating molecule 1 | Proteasome-mediated processes |
| Pomp | POMP | Proteasome maturation protein | Proteasome-mediated processes |
| Prosalpha7 | PSMA3 | Proteasome subunit, alpha type, 3 | Proteasome-mediated processes |
| Prosalpha3 | PSMA4 | Proteasome subunit, alpha type, 4 | Proteasome-mediated processes |
| Rpn10 | PSMD4 | Proteasome 26S subunit, non- ATPase, 4 |
Proteasome-mediated processes |
| Rpn7 | PSMD6 | Proteasome26S subunit, non- ATPase, 6 |
Proteasome-mediated processes |
| Npl4 | NPLOC4 | Nuclear protein localization 4 homolog |
Proteasome-mediated processes |
| TER94 | VCP | Valosin containing protein | Proteasome-mediated processes Autophagy |
| Smr | NCOR2 | Nuclear receptor corepressor 2 | Transcription/Nuclear receptor signaling |
| Kr-h1 | ZNF143 | Zinc finger protein 143 | Transcription |
| bru-2 | CELF2 | CUGBP, Elav-like family member 2 | mRNA processing |
| CG10353 | ANO4 | Anoctamin 4 | Ion transport |
| Rab8 | RAB10 | RAB10, member RAS oncogene family |
Intracellular protein transport |
Similar functional categories are enriched when we look at the most inclusive set of conserved NRF2 target genes (Class I Drosophila genes also bound by NRF2 in humans). Gene ontology (GO) analysis of this larger gene set revealed continued enrichment for genes associated with redox homeostasis, carbohydrate metabolism, and proteostasis (Table 2), although many genes not fitting in these categories are also present (Supplemental Table 3). The redox homeostasis genes are represented across multiple GO categories (cellular iron ion homeostasis; response to arsenic-containing substance; regulation of transcription from RNA polymerase II promoter in response to stress; response to oxidative stress) and include genes encoding superoxide dismutase (SOD1), heme oxygenase (HMOX1), both the catalytic and modifier subunits of glutamate-cysteine ligase (GCLC and GCLM, respectively), and ferrochelatase (FECH), among others. Enrichment for proteostasis genes (encompassing the ‘regulation of ligase activity’ and ‘protein catabolic process’ categories) is largely driven by the proteasomal genes described above, but also includes genes such as UBC, which encodes ubiquitin C, and RFFL, which encodes an E3 ubiquitin ligase.
Table 2.
Enriched GO categories for human orthologs of Drosophila Class I genes
| Gene Ontology Description | P-value |
|---|---|
| Cellular iron ion homeostasis (GO:0006879) | 2.1×10−5 |
| Response to arsenic-containing substance (GO:0046685) |
3.6×10−5 |
| Carbohydrate biosynthetic process (GO:0016051) | 6.8×10−5 |
| Regulation of ligase activity (GO:0051340) | 8.9×10−5 |
| Protein catabolic process (GO:0030163) | 0.00015 |
| Regulation of transcription from RNA polymerase II promoter in response to stress (GO:0043618) |
0.00072 |
| Response to oxidative stress (GO:0006979) | 0.00096 |
The carbohydrate metabolism group includes genes encoding key enzymes such as pyruvate carboxylase (encoded by PC; also in Table 1), fructose-bisphosphate aldolase A (encoded by ALDOA), and 6-phosphogluconate dehydrogenase (encode by PGD, often referred to as 6PGD). Both PC and 6PGD are important for regeneration of NADPH, which is essential for reducing GSH and maintaining redox homeostasis, a function that likely explains why these genes are evolutionarily conserved NRF2 targets. However, induction of either PC or 6PGD can also have negative consequences as increased activity of either enzyme gives cancer cells a metabolic (and proliferative) advantage relative to normal cells [54, 55]. Interestingly, KEAP1 mutations that lead to constitutive activation of NRF2 drive metabolic reprogramming events, particularly in the direction of the pentose phosphate pathway (including 6PGD), that give cancer cells a growth advantage over normal cells [15]. Thus, this deeply conserved carbohydrate metabolism gene set, which includes genes important for NRF2’s cytoprotective functions, provides a possible avenue for aberrant NRF2 activity to drive rapid proliferation of cancer cells.
GO analysis also allows for exploration beyond biological process-related categories. One interesting finding from analysis of GO ‘cellular component’ categories is that the deeply conserved NRF2 targets are enriched for genes encoding proteins found in extracellular exosomes (p = 0.003) (Supplementary Figure 3). Exosomes are secreted nanovesicles that mediate cell signaling via intercellular transfer of proteins, mRNAs, and microRNAs [56]. Consistent with NRF2 playing a role exosome loading and secretion, we find that cells exposed to either oxidative stress or SFN release significantly more exosomes than vehicle-treated cells (Supplemental Figure 3). Additionally, a previous study demonstrated that exosomes released by cells exposed to oxidative stress communicate a protective signal to unstressed cells [57], and there is significant evidence indicating that NRF2 activity in astrocytes leads to release or secretion of factors that protect neurons (where NRF2 activity is intrinsically low) [58, 59]. Thus, these findings suggest that NRF2 could drive cell non-autonomous cytoprotective signaling via loading and release of exosomes.
Taken together, these data suggest that, beyond antioxidant genes, the ancient NRF2 network is built upon a core set of genes encompassing many components of the ubiquitin-proteasome system, as well as a number of genes associated with glucose metabolism, lipid metabolism, and more. Accordingly, it is clear that in order to understand what happens when NRF2 is activated pharmacologically or aberrantly activated in various disease states, we must understand how NRF2 regulates more than just the canonical antioxidant target genes.
NRF2 cis-regulatory activity at ancient target genes
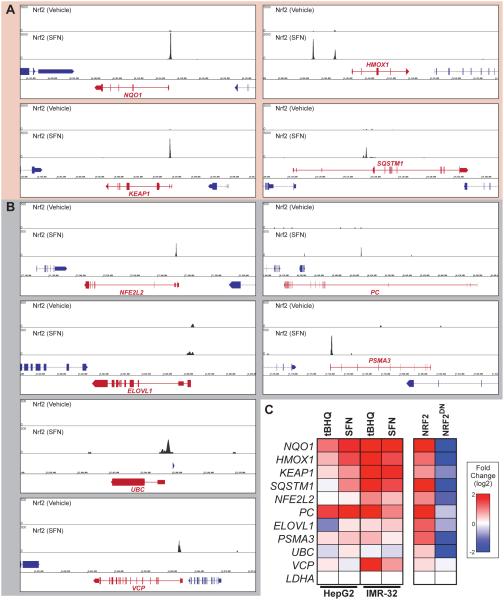
To determine if NRF2 binding near these conserved target genes has a regulatory impact, we used ChIP-seq in human cells (Figure 3A-B) to identify NRF2 occupied AREs near 9 conserved antioxidant and non-antioxidant target genes, and then created luciferase reporter constructs to test their functionality in NRF2 responsive cell lines. Putative enhacers were tested for genes associated with redox homeostasis (HMOX1, KEAP1, NFE2L2), lipid or carbohydrate metabolism (PC, ELOVL1), proteostasis (PSMA3, UBC), and genes linking proteostasis and autophagy (SQSTM1, VCP). All tested regions were predicted to act as functional enhancers based their overlap with strong histone H3 lysine 27 acetylation signals in multiple cell lines (not shown) [60]. In almost all cases, with the exception of the UBC enhancer and one outlier sample from the ELOVL1 enhancer, we find that the NRF2 activators SFN and tert-butylhydroquinone (tBHQ) upregulate the NRF2 targeted enhancers (Figure 3C). Because assays performed in IMR-32 cells were more robust and consistent, we then performed a series of more focused assays in this cell type. Importantly, all of the enhancers are also induced to varying degrees by overexpression of NRF2 and repressed by overexpression of a dominant negative version of NRF2 (NRF2DN) (Figure 3C, right panel), suggesting that NRF2 directly regulates these enhancers. Although all of the tested enhancers are responsive to NRF2 activity in one or more contexts the responses are not uniform, with some enhancers responding much more robustly than others. Thus, conclusions based on NRF2’s regulation of AREs at its canonical target genes (NQO1, HMOX1, etc.) may not be directly applicable to all NRF2 target genes.
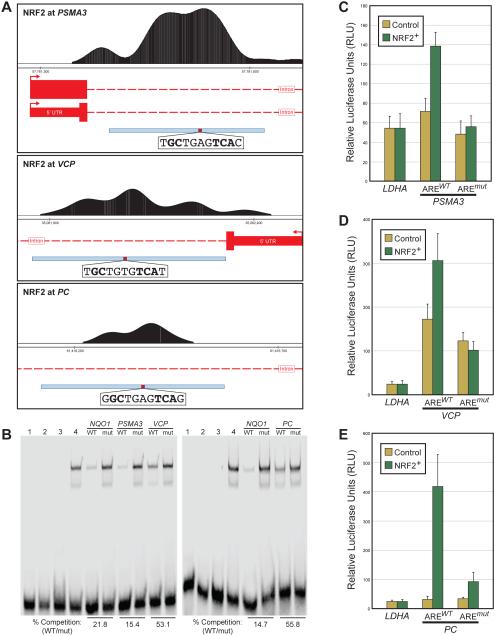
Figure 3. Enhancers at deeply conserved human target genes are regulated by NRF2 in human cells.
(A) Human NRF2 ChIP-seq signal from LCL cells treated with DMSO or sulforaphane (SFN) as indicated. Select ancient NRF2 target genes with highly significant binding are represented (ChIP y-axis scale = 0-5000). (B) ChIP-seq signal as in (A) at select ancient NRF2 target genes with moderate binding (ChIP y-axis scale = 0-500). (C) Heatmap representing the response to sulforaphane (SFN), tert-butylhydroquinone (tBHQ), overexpression of NRF2, or overexpression of a dominant negative version of NRF2 (NRF2DN) for reporter constructs driven by the enhancer regions highlighted in panels (A) and (B). NQO1 is a positive control for human NRF2, but is not a conserved target because insects do not have an orthologous gene; the remaining nine are enhancers at deeply conserved NRF2 target genes.
More detailed testing of a number of these NRF2 targeted enhancers provides further support for direct NRF2/ARE-mediated regulation of these deeply conserved target genes. For enhancers at the PSMA3, VCP, and PC loci determined by ChIP-seq (Figure 4A), we identified an ARE that is specifically bound by NRF2-MAFG, as determined by competition for binding with a labeled NQO1 ARE probe in electrophoretic mobility shift assays (EMSAs) (Figure 4B). To test if these AREs mediated NRF2 driven enhancer activity, we mutated the identified ARE sequences in their respective reporter constructs. To maximize the potential for NRF2-mediated gene induction, wild-type and ARE-mutant enhancer activity was monitored under basal conditions and with NRF2 overexpression in the presence of SFN (Figure 4C-D). All three enhancers contained two possible AREs; in each case one of the two was a stronger match to the ARE consensus (Supplmental Table 5), so the stronger match was tested for binding and functional activity. Importantly, in all cases enhancer induction is lost upon mutation of the strongest ARE, indicating that NRF2 directly regulates each enhancer in a manner that is primarily dependent on a single ARE motif (Figure 4B-E).
Figure 4. Direct NRF2 regulation of enhancers at PSMA3, VCP, and PC in human cells.
(A) NRF2 ChIP-seq data from human LCL cells treated with suforaphane (SFN). Top: NRF2 ChIP-seq peak and ARE sequence at the PSMA3 locus. Middle: NRF2 ChIP-seq peak and ARE sequence at the VCP locus. Bottom: NRF2 ChIP-seq peak and ARE sequence at the PC locus. Gene models are in red, with tall boxes representing coding regions, short boxes representing untranslated regions, and dashed lines representing introns. Enhancer regions tested in reporter assays are represented by the blue box at the bottom of each panel, with location of the tested ARE highlighted in red. ARE sequences are provided, with orientation of the ARE sequence matching orientation of the target gene. (B) Electrophoretic mobility shift assays in which, for both gels, lane 1 contains a labeled NQO1 ARE probe with no protein, lane 2 contains the NQO1 probe with purified MAFG only, lane 3 contains the NQO1 probe with purified NRF2 only, and lane for contains the NQO1 probe with NRF2 and MAFG. Binding is only seen when both proteins are present in the reaction. Wild-type (WT) and mutant (mut) versions of the AREs from PSMA3, VCP, and PC were used as cold competitors as indicated. Although competition strength varies depending on the ARE, all WT probes compete with the labeled NQO1 probe, and this competition is lost when the AREs are mutated, indicating that the AREs from PSMA3, VCP, and PC are bound by NRF2-MAFG. (C) Reporter assays in which the region highlighed in the (A) was cloned upstream of the luciferase reporter gene. Control represents human IMR32 cells that were transfected with a control vector (pEF) and treated with DMSO, and NRF2+ represents cells that were transfected with an NRF2 expression plasmid (pEF-NRF2) and treated with SFN. The PSMA3 enhancer is upreguated in NRF2+, and this induction is ARE-dependent (lost in a construct with a mutated ARE). (D) Same as (C) for the VCP locus. This enhancer is also upreguated in NRF2+ in an ARE-dependent manner. (E) Same as (C) for the PC locus. This enhancer is also upreguated in NRF2+ in an ARE-dependent manner.
The above results confirm that NRF2 directly interacts with and regulates enhancers at: 1) the PSMA3 locus, which encodes a component of the 20S core proteasome [61], 2) the VCP locus, which encodes an AAA+ ATPase involved in the regulation of proteostasis and autophagy [62-64], and 3) the PC locus, which encodes pyruvate carboxylase, an enzyme that converts pyruvate to oxaloacetate a key reaction in intermediary metabolism [65]. Direct regulation of PSMA3 by NRF2 provides further evidence that NRF2 is the key direct transcriptional regulator of genes encoding proteasomal subunits [50, 66], and this is a strongly conserved NRF2 function. NRF2-mediated regulation of an enhancer at the VCP locus extends the proteostasis branch of the network beyond proteasome subunits into additional regulators of protein degradation. VCP (also known as p97) enhances proteasomal protein degradation by using energy from ATP hydrolysis to remodel or unfold proteins targeted for the proteasome [62]. Thus, there is a role for NRF2 in regulating components that clear macromolecules damaged by oxidative stress as well as in regulating gene pathways that protect from oxidative stress. NRF2-mediated regulation of an enhancer at PC highlights the importance of an emerging role for NRF2 in controlling intermediary metabolism and regeneration of NADPH, which is a necessary cofactor for many antioxidant reactions [16].
Overall, these results indicate NRF2’s regulatory reach undoubtedly extends beyond antioxidant genes [16], and this is a deeply conserved feature of the NRF2 network. Importantly, a subset of these genes may be involved in the phenotypic selection for tumor mutations that lead to constitutive NRF2 activity. For example, increased PC activity allows tumor cells to use glucose-derived pyruvate as a precursor for anaplerotic reactions; this can promote glutamine independence and give cancer cells a proliferative advantage over normal cells [54, 67-69]. Therefore it is important that NRF2 output is regulated in a way that ensures its target genes are expressed at the proper level at an appropriate time.
Autoregulatory potential within ancient NRF2 regulatory network
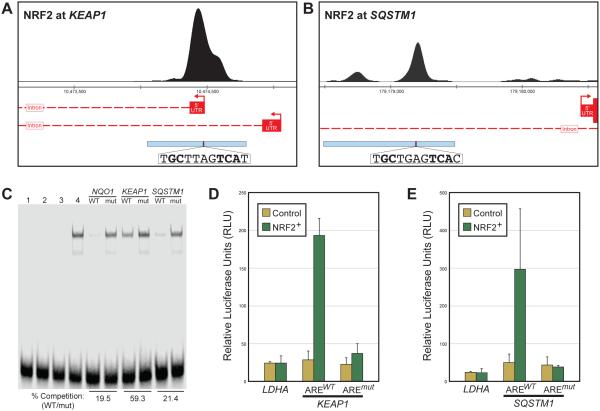
As discussed above, NRF2 activation can be detrimental in certain contexts, so regulation of NRF2’s nuclear concentration is paramount. Consistent with this, one of the most significant peaks in the human ChIP-seq data and in all stages of the Drosophila Cnc/Nrf2 ChIP-seq data is at the KEAP1 locus (see Figure 3A and Supplemental Figure 4). The prominence of this NRF2 binding event across organisms underscores the importance of the finding that NRF2 activates KEAP1 as part of a negative feedback loop in both Drosophila and humans [5, 53]. The NRF2-KEAP1 negative feedback loop is mediated in part via an ARE in a 5’ untranslated region (5’UTR) of KEAP1 [53]. The NRF2 ChIP-seq peak encompasses this KEAP1 ARE as well as the entire 5’UTR (Figure 5A). Because the original reporter assays describing NRF2 regulation of KEAP1 did not cover this entire peak region, we generated a reporter construct with the 867 bp of DNA under the ChIP peak driving luciferase expression (Figure 5A). Using EMSAs and reporter assays similar to those described above, we found that this enhancer region drives NRF2-responsive expression, and this expression is entirely dependent on the previously identified ARE motif within the 5’UTR (Figure 5C-D). Therefore, NRF2 upregulates KEAP1 to form a highly conserved negative feedback loop that is mediated by a single ARE in humans. Interestingly, although nematodes lack a KEAP1 ortholog, a similar negative feedback loop is place in C. elegans, where SKN-1 upregulates its own repressor, encoded by the gene wdr-23 [70]. The repeated occurrence of this autoregulatory network motif as the NRF2 network evolved suggests that one of the more important functions of NRF2 is to limit its own activity.
Figure 5. Direct NRF2 regulation of enhancers at KEAP1 and SQSTM1 in human cells.
(A) Human NRF2 ChIP-seq peak and ARE sequence at the KEAP1 locus; gene models and tested enhancer regions are indicated as described in Figure 4A. (B) Human NRF2 ChIP-seq peak and ARE sequence at the SQSTM1 locus; gene models and tested enhancer regions are indicated as described in Figure 4A. (C) Electrophoretic mobility shift assays as described in Figure 4B, with AREs from KEAP1 and SQSTM1 enhancers used as cold competitors; both are able to compete with labeled NQO1 probe in an ARE-dependent manner (lost with mutation of ARE), though the KEAP1 ARE is a weaker competitor than the SQSTM1 ARE. (D) Luciferease reporter assay as described in Figure 4C, only with enhancer from the KEAP1 locus. (E) Same as (D) with enhancer from the SQSTM1 locus. Both the KEAP1 (D) and SQSTM1 (E) enhancers are upreguated in NRF2+ in an ARE-dependent manner.
Although negative feedback is a key component of the ancient NRF2 network, a separate conserved target gene provides a route to positive feedback in the network. NRF2 binds a region between 1100-1800 bp upstream of the most proximal transcription start site of the gene SQSTM1, which encodes a protein often referred to as p62/SQSTM1 (the Drosophila ortholog is Ref(2)P) (Figure 5B). SQSTM1 is an adaptor protein that selectively targets proteins for autophagic degradation; one of the proteins it inactivates is KEAP1, thus SQSTM1 indirectly increases NRF2 activity [40, 41, 71]. One of the peaks in the NRF2 binding cluster at the SQSTM1 locus is consistent with the location of an NRF2-targeted ARE identified in previous enhancer bashing experiments [71]. There is one prominent peak centered on this conserved, perfect ARE (TGCTGAGTCAC), as well as a less substantial secondary peak centered on a conserved, near-perfect ARE (CGCTGACTCAC); the near-perfect ARE overlaps a previously mapped AP-1 motif [72]. We tested the regulatory activity of this region using a reporter construct that encompasses both NRF2 binding peaks. NRF2 activates this enhancer, and NRF2-mediated activation is entirely dependent on the perfect ARE (Figure 5D), further highlighting the importance of this ARE sequence upstream of SQSTM1 [71]. How the SQSTM1-mediated positive feedback and KEAP1-mediated negative feedback loops cooperate to finely tune NRF2 regulatory activity remains to be determined.
One interesting feature of regulatory networks with prominent negative feedback loops is that they have the potential to regulate gene expression in a graded, tunable manner [73]. Gene expression is often thought of as being ‘on’ or ‘off’ and, indeed, bistable, on/off gene regulation is often essential in contexts related to cell specification and differentiation. However, switch-like responses are not necessarily ideal in stress-responsive regulatory networks, where responses should often be proportional to the level of stress. Consistent with this, we find evidence for a graded output mechanism for NRF2 when looking at the relationship between ARE motif quality, in vivo DNA binding, and regulatory output. The most significantly enriched DNA motif within the human ChIP-seq data – tGCTGAgTCA[t/c] – fits the general ARE consensus of GCnnnnTCA (Figure 6A). Overall, approximately 80% of the NRF2 bound regions contain at least one sequence matching the general ARE consensus (Figure 6B). A closer examination of these ARE-containing regions reveals that >20% perfectly match the more stringent motif (TGCTGAGTCA) identified in Figure 6A, and ~40% are a close match to the stringent consensus, with mismatches only in one of the three more variable ARE positions (positions 1, 7, or 11 from Figure 6A), and ~40% match the GCnnnnTCA consensus but have mismatches in other key positions (Figure 6B). Thus, NRF2 bound regions contain a broad spectrum of ARE sequences, from those that match a strict consensus (higher affinity binding) to weaker AREs and, in a minority of cases, no AREs. The bound regions lacking AREs likely represent indirect or nonspecific binding by NRF2 [44]. To examine the relationship between ARE sequence and NRF2 activity, all NRF2 bound regions were first separated into bins based on how closely given region’s strongest ARE matches the canonical ARE motif (see Figure 1B for canonical motif). We then explored whether motif quality influences NRF2 binding in vivo (measured by ChIP-seq signal) and, indeed, NRF2 binding signals increase progressively as motif quality increases (Figure 6C and Supplemental Figure 5). A similar pattern is seen when we look at how NRF2 target genes respond to SFN treatment, where we see an overall increase in gene induction with increasing motif quality (Figure 6D). However the trend is more complex with gene expression, likely because a gene’s expression level is the result of cooperation or collaboration between multiple transcription factors and regulatory enhancers, and chromatin state regulators [44]. Accordingly, many genes targeted by NRF2 are not significantly induced, and there is a clear subset of genes that is far more responsive to SFN than the others in the strongest ARE bin.
One gene that stands out as strikingly more responsive to SFN at both 8 hours and 24 hours is the canonical NRF2 target NQO1; this category also includes expected targets such as HMOX1 and ME1. Interestingly, we found that the properties of the NRF2-targeted enhancer at NQO1 are quite unique in comparison to the NRF2-targeted enhancer at KEAP1, a moderately SFN-responsive target (KEAP1 expression increases 25% after 24 hours, versus >300% for NQO1). Both enhancers were tested for their response to a range of SFN concentrations; the NQO1 enhancer’s dose-response relationship is sigmoidal and saturating, whereas the KEAP1 enhancer’s output is graded and linear across the range of SFN concentrations (Figure 6E). Mechanistically, this difference may be the result of cooperativity at the NQO1 enhancer that is lacking at the KEAP1 enhancer: in contrast to the KEAP1 enhancer (see Figure 5), the NRF2 bound NQO1 enhancer is not entirely dependent on a single ARE for NRF2-mediated induction (Supplemental Figure 6). The NQO1 enhancer only contains a single sequence matching the general ARE consensus (TGCTGAGTCAC), so residual activity when this is mutated is dependent on a non-ARE sequence. Indeed, at least part of this residual activity is likely driven by an AP-1-like sequence (TGACTG) two bases away from the ARE [74, 75], although it is not clear whether NRF2 (via binding a suboptimal motif) or another transcription factor is interacting with this motif [75]. Regardless of the transcription factor(s) involved, the non-ARE regulatory sequences likely cooperate with the ARE to drive nonlinear response of the NQO1 enhancer.
Taken together these results demonstrate that not all NRF2 targeted enhancers are equivalent. Some, like the enhancer targeting NQO1, have evolved to respond strongly to small increases in NRF2 activity, possibly because the energetic cost of producing too much NQO1 and other genes in this group (like HMOX1) outweighs the potential damage a cell can incur if too little is produced. On the other hand, there are also enhancers like those targeting KEAP1 and PC (not shown) that respond to NRF2 activity in a much more linear, graded manner. For these targets, the consequences of overproduction – full shutdown of the NRF2 pathway for KEAP1, metabolic reprogramming for PC – necessitate a more constrained mode of transcriptional activation. In fact, considering the prominent NRF2-KEAP1 negative feedback loop, the graded correlation between ARE quality and NRF2 binding, and the fact that most NRF2 target genes are not induced to the same level as genes like NQO1 and HMOX1, it is possible that most enhancers in the NRF2 network are fine tuned to respond linearly to NRF2 activation.
CONCLUSION
Together, these data demonstrate that the Keap1-Nrf2-Maf regulatory axis is strongly conserved from Drosophila melanogaster to humans, even at the level of network structure. The regulatory reach of NRF2 clearly extends well beyond antioxidant genes, covering metabolic genes, proteostasis genes, autoregulatory pathways, and more; this property of the NRF2 network is also an ancient one. Importantly, because NRF2 targets a variety of distinct gene batteries, it is also likely that NRF2 employs more than one regulatory strategy for transcriptional activation in response to stress. Although most models of NRF2-mediated gene regulation are based on canonical NRF2 target genes like NQO1, which regulated by a switch-like ARE enhancer module, it is likely that many NRF2 targeted enhancer exhibit a graded response to NRF2 activity. Thus, models NRF2-mediated regulation must be developed in an enhancer- or gene-specific manner, and cannot rely entirely on expectations based on NRF2-mediated regulation of the canonical antioxidant target genes.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Evan Odean (University of Minnesota), Lijia Ma (University of Chicago), Anshul Kundaje (Stanford), and Katherine Harris (SwitchGear Genomics) for technical assistance. This work was supported by funding from the University of Minnesota Foundation (M.S.) and the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health [Z01-ES-100475 and ES065079 (D.A.B)].
REFERENCES
- [1].Saeidnia S, Abdollahi M. Toxicological and pharmacological concerns on oxidative stress and related diseases. Toxicology and applied pharmacology. 2013;273:442–455. doi: 10.1016/j.taap.2013.09.031. [DOI] [PubMed] [Google Scholar]
- [2].Jimenez-Del-Rio M, Velez-Pardo C. The bad, the good, and the ugly about oxidative stress. Oxidative medicine and cellular longevity. 2012;2012:163913. doi: 10.1155/2012/163913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sykiotis GP, Bohmann D. Stress-activated cap'n'collar transcription factors in aging and human disease. Science signaling. 2010;3:re3. doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].An JH, Blackwell TK. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes & development. 2003;17:1882–1893. doi: 10.1101/gad.1107803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Developmental cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tullet JM, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hochmuth CE, Biteau B, Bohmann D, Jasper H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell stem cell. 2011;8:188–199. doi: 10.1016/j.stem.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Deng H, Kerppola TK. Regulation of Drosophila metamorphosis by xenobiotic response regulators. PLoS genetics. 2013;9:e1003263. doi: 10.1371/journal.pgen.1003263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pickering AM, Staab TA, Tower J, Sieburth D, Davies KJ. A conserved role for the 20S proteasome and Nrf2 transcription factor in oxidative stress adaptation in mammals, Caenorhabditis elegans and Drosophila melanogaster. The Journal of experimental biology. 2013;216:543–553. doi: 10.1242/jeb.074757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pickering AM, Vojtovich L, Tower J. Oxidative stress adaptation with acute, chronic, and repeated stress. Free radical biology & medicine. 2013;55:109–118. doi: 10.1016/j.freeradbiomed.2012.11.001. KJ, A. D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Landis G, Shen J, Tower J. Gene expression changes in response to aging compared to heat stress, oxidative stress and ionizing radiation in Drosophila melanogaster. Aging. 2012;4:768–789. doi: 10.18632/aging.100499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cancer Genome Atlas Research, N. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jaramillo MC, Zhang DD. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes & development. 2013;27:2179–2191. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pi J, Freeman ML, Yamamoto M. Nrf2 in toxicology and pharmacology: the good, the bad and the ugly? Toxicology and applied pharmacology. 2010;244:1–3. doi: 10.1016/j.taap.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, Yamamoto M, Motohashi H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer cell. 2012;22:66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- [16].Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends in biochemical sciences. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- [17].Harris IS, Treloar AE, Inoue S, Sasaki M, Gorrini C, Lee KC, Yung KY, Brenner D, Knobbe-Thomsen CB, Cox MA, Elia A, Berger T, Cescon DW, Adeoye A, Brustle A, Molyneux SD, Mason JM, Li WY, Yamamoto K, Wakeham A, Berman HK, Khokha R, Done SJ, Kavanagh TJ, Lam CW, Mak TW. Glutathione and Thioredoxin Antioxidant Pathways Synergize to Drive Cancer Initiation and Progression. Cancer cell. 2015 doi: 10.1016/j.ccell.2014.11.019. [DOI] [PubMed] [Google Scholar]
- [18].Furukawa M, Xiong Y. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase. Molecular and cellular biology. 2005;25:162–171. doi: 10.1128/MCB.25.1.162-171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Slattery M, Ma L, Spokony RF, Arthur RK, Kheradpour P, Kundaje A, Negre N, Crofts A, Ptashkin R, Zieba J, Ostapenko A, Suchy S, Victorsen A, Jameel N, Grundstad AJ, Gao W, Moran JR, Rehm EJ, Grossman RL, Kellis M, White KP. Diverse patterns of genomic targeting by transcriptional regulators in Drosophila melanogaster. Genome research. 2014;24:1224–1235. doi: 10.1101/gr.168807.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brizuela BJ, Elfring L, Ballard J, Tamkun JW, Kennison JA. Genetic analysis of the brahma gene of Drosophila melanogaster and polytene chromosome subdivisions 72AB. Genetics. 1994;137:803–813. doi: 10.1093/genetics/137.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Negre N, Brown CD, Ma L, Bristow CA, Miller SW, Wagner U, Kheradpour P, Eaton ML, Loriaux P, Sealfon R, Li Z, Ishii H, Spokony RF, Chen J, Hwang L, Cheng C, Auburn RP, Davis MB, Domanus M, Shah PK, Morrison CA, Zieba J, Suchy S, Senderowicz L, Victorsen A, Bild NA, Grundstad AJ, Hanley D, MacAlpine DM, Mannervik M, Venken K, Bellen H, White R, Gerstein M, Russell S, Grossman RL, Ren B, Posakony JW, Kellis M, White KP. A cis-regulatory map of the Drosophila genome. Nature. 2011;471:527–531. doi: 10.1038/nature09990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, Liu XS. Model-based analysis of ChIP-Seq (MACS) Genome biology. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Boyle AP, Araya CL, Brdlik C, Cayting P, Cheng C, Cheng Y, Gardner K, Hillier LW, Janette J, Jiang L, Kasper D, Kawli T, Kheradpour P, Kundaje A, Li JJ, Ma L, Niu W, Rehm EJ, Rozowsky J, Slattery M, Spokony R, Terrell R, Vafeados D, Wang D, Weisdepp P, Wu YC, Xie D, Yan KK, Feingold EA, Good PJ, Pazin MJ, Huang H, Bickel PJ, Brenner SE, Reinke V, Waterston RH, Gerstein M, White KP, Kellis M, Snyder M. Comparative analysis of regulatory information and circuits across distant species. Nature. 2014;512:453–456. doi: 10.1038/nature13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li Q, Brown JB, Huang H, Bickel PJ. Measuring reproducibility of high-throughput experiments. Annals of Applied Statistics. 2011;5:1752–1779. [Google Scholar]
- [25].Ramirez F, Dundar F, Diehl S, Gruning BA, Manke T. deepTools: a flexible platform for exploring deep-sequencing data. Nucleic acids research. 2014;42:W187–191. doi: 10.1093/nar/gku365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- [27].Herrmann C, Van de Sande B, Potier D, Aerts S. i-cisTarget: an integrative genomics method for the prediction of regulatory features and cis-regulatory modules. Nucleic acids research. 2012;40:e114. doi: 10.1093/nar/gks543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mathelier A, Zhao X, Zhang AW, Parcy F, Worsley-Hunt R, Arenillas DJ, Buchman S, Chen CY, Chou A, Ienasescu H, Lim J, Shyr C, Tan G, Zhou M, Lenhard B, Sandelin A, Wasserman WW. JASPAR 2014: an extensively expanded and updated open-access database of transcription factor binding profiles. Nucleic acids research. 2014;42:D142–147. doi: 10.1093/nar/gkt997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Matys V, Fricke E, Geffers R, Gossling E, Haubrock M, Hehl R, Hornischer K, Karas D, Kel AE, Kel-Margoulis OV, Kloos DU, Land S, Lewicki-Potapov B, Michael H, Munch R, Reuter I, Rotert S, Saxel H, Scheer M, Thiele S, Wingender E. TRANSFAC: transcriptional regulation, from patterns to profiles. Nucleic acids research. 2003;31:374–378. doi: 10.1093/nar/gkg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hu Y, Flockhart I, Vinayagam A, Bergwitz C, Berger B, Perrimon N, Mohr SE. An integrative approach to ortholog prediction for disease-focused and other functional studies. BMC bioinformatics. 2011;12:357. doi: 10.1186/1471-2105-12-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chorley BN, Campbell MR, Wang X, Karaca M, Sambandan D, Bangura F, Xue P, Pi J, Kleeberger SR, Bell DA. Identification of novel NRF2-regulated genes by ChIP-Seq: influence on retinoid X receptor alpha. Nucleic acids research. 2012;40:7416–7429. doi: 10.1093/nar/gks409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Valouev A, Johnson DS, Sundquist A, Medina C, Anton E, Batzoglou S, Myers RM, Sidow A. Genome-wide analysis of transcription factor binding sites based on ChIP-Seq data. Nature methods. 2008;5:829–834. doi: 10.1038/nmeth.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jee J, Rozowsky J, Yip KY, Lochovsky L, Bjornson R, Zhong G, Zhang Z, Fu Y, Wang J, Weng Z, Gerstein M. ACT: aggregation and correlation toolbox for analyses of genome tracks. Bioinformatics. 2011;27:1152–1154. doi: 10.1093/bioinformatics/btr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Machanick P, Bailey TL. MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics. 2011;27:1696–1697. doi: 10.1093/bioinformatics/btr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, Ma'ayan A. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC bioinformatics. 2013;14:128. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mathelier A, Wasserman WW. The next generation of transcription factor binding site prediction. PLoS computational biology. 2013;9:e1003214. doi: 10.1371/journal.pcbi.1003214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Veraksa A, McGinnis N, Li X, Mohler J, McGinnis W. Cap 'n' collar B cooperates with a small Maf subunit to specify pharyngeal development and suppress deformed homeotic function in the Drosophila head. Development. 2000;127:4023–4037. doi: 10.1242/dev.127.18.4023. [DOI] [PubMed] [Google Scholar]
- [38].McGinnis N, Ragnhildstveit E, Veraksa A, McGinnis W. A cap 'n' collar protein isoform contains a selective Hox repressor function. Development. 1998;125:4553–4564. doi: 10.1242/dev.125.22.4553. [DOI] [PubMed] [Google Scholar]
- [39].Parisi M, Li R, Oliver B. Lipid profiles of female and male Drosophila. BMC research notes. 2011;4:198. doi: 10.1186/1756-0500-4-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Palanker L, Tennessen JM, Lam G, Thummel CS. Drosophila HNF4 regulates lipid mobilization and beta-oxidation. Cell metabolism. 2009;9:228–239. doi: 10.1016/j.cmet.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].King-Jones K, Charles JP, Lam G, Thummel CS. The ecdysone-induced DHR4 orphan nuclear receptor coordinates growth and maturation in Drosophila. Cell. 2005;121:773–784. doi: 10.1016/j.cell.2005.03.030. [DOI] [PubMed] [Google Scholar]
- [42].Li XY, MacArthur S, Bourgon R, Nix D, Pollard DA, Iyer VN, Hechmer A, Simirenko L, Stapleton M, Luengo Hendriks CL, Chu HC, Ogawa N, Inwood W, Sementchenko V, Beaton A, Weiszmann R, Celniker SE, Knowles DW, Gingeras T, Speed TP, Eisen MB, Biggin MD. Transcription factors bind thousands of active and inactive regions in the Drosophila blastoderm. PLoS biology. 2008;6:e27. doi: 10.1371/journal.pbio.0060027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].MacQuarrie KL, Fong AP, Morse RH, Tapscott SJ. Genome-wide transcription factor binding: beyond direct target regulation. Trends in genetics : TIG. 2011;27:141–148. doi: 10.1016/j.tig.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Slattery M, Zhou T, Yang L, Dantas Machado AC, Gordan R, Rohs R. Absence of a simple code: how transcription factors read the genome. Trends in biochemical sciences. 2014;39:381–399. doi: 10.1016/j.tibs.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Misra JR, Horner MA, Lam G, Thummel CS. Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes & development. 2011;25:1796–1806. doi: 10.1101/gad.17280911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hur W, Gray NS. Small molecule modulators of antioxidant response pathway. Current opinion in chemical biology. 2011;15:162–173. doi: 10.1016/j.cbpa.2010.12.009. [DOI] [PubMed] [Google Scholar]
- [47].Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gerstein MB, Kundaje A, Hariharan M, Landt SG, Yan KK, Cheng C, Mu XJ, Khurana E, Rozowsky J, Alexander R, Min R, Alves P, Abyzov A, Addleman N, Bhardwaj N, Boyle AP, Cayting P, Charos A, Chen DZ, Cheng Y, Clarke D, Eastman C, Euskirchen G, Frietze S, Fu Y, Gertz J, Grubert F, Harmanci A, Jain P, Kasowski M, Lacroute P, Leng J, Lian J, Monahan H, O'Geen H, Ouyang Z, Partridge EC, Patacsil D, Pauli F, Raha D, Ramirez L, Reddy TE, Reed B, Shi M, Slifer T, Wang J, Wu L, Yang X, Yip KY, Zilberman-Schapira G, Batzoglou S, Sidow A, Farnham PJ, Myers RM, Weissman SM, Snyder M. Architecture of the human regulatory network derived from ENCODE data. Nature. 2012;489:91–100. doi: 10.1038/nature11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wild AC, Moinova HR, Mulcahy RT. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. The Journal of biological chemistry. 1999;274:33627–33636. doi: 10.1074/jbc.274.47.33627. [DOI] [PubMed] [Google Scholar]
- [50].Kwak MK, Wakabayashi N, Greenlaw JL, Yamamoto M, Kensler TW. Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Molecular and cellular biology. 2003;23:8786–8794. doi: 10.1128/MCB.23.23.8786-8794.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Shin S, Wakabayashi J, Yates MS, Wakabayashi N, Dolan PM, Aja S, Liby KT, Sporn MB, Yamamoto M, Kensler TW. Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide. European journal of pharmacology. 2009;620:138–144. doi: 10.1016/j.ejphar.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Pi J, Leung L, Xue P, Wang W, Hou Y, Liu D, Yehuda-Shnaidman E, Lee C, Lau J, Kurtz TW, Chan JY. Deficiency in the nuclear factor E2-related factor-2 transcription factor results in impaired adipogenesis and protects against diet-induced obesity. The Journal of biological chemistry. 2010;285:9292–9300. doi: 10.1074/jbc.M109.093955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lee OH, Jain AK, Papusha V, Jaiswal AK. An auto-regulatory loop between stress sensors INrf2 and Nrf2 controls their cellular abundance. The Journal of biological chemistry. 2007;282:36412–36420. doi: 10.1074/jbc.M706517200. [DOI] [PubMed] [Google Scholar]
- [54].Cheng T, Sudderth J, Yang C, Mullen AR, Jin ES, Mates JM, DeBerardinis RJ. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:8674–8679. doi: 10.1073/pnas.1016627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Shan C, Elf S, Ji Q, Kang HB, Zhou L, Hitosugi T, Jin L, Lin R, Zhang L, Seo JH, Xie J, Tucker M, Gu TL, Sudderth J, Jiang L, DeBerardinis RJ, Wu S, Li Y, Mao H, Chen PR, Wang D, Chen GZ, Lonial S, Arellano ML, Khoury HJ, Khuri FR, Lee BH, Brat DJ, Ye K, Boggon TJ, He C, Kang S, Fan J, Chen J. Lysine acetylation activates 6-phosphogluconate dehydrogenase to promote tumor growth. Molecular cell. 2014;55:552–565. doi: 10.1016/j.molcel.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lee Y, El Andaloussi S, Wood MJ. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Human molecular genetics. 2012;21:R125–134. doi: 10.1093/hmg/dds317. [DOI] [PubMed] [Google Scholar]
- [57].Eldh M, Ekstrom K, Valadi H, Sjostrand M, Olsson B, Jernas M, Lotvall J. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PloS one. 2010;5:e15353. doi: 10.1371/journal.pone.0015353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].ENCODE An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Tomko RJ, Jr., Hochstrasser M. Molecular architecture and assembly of the eukaryotic proteasome. Annual review of biochemistry. 2013;82:415–445. doi: 10.1146/annurev-biochem-060410-150257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Meyer H, Bug M, Bremer S. Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nature cell biology. 2012;14:117–123. doi: 10.1038/ncb2407. [DOI] [PubMed] [Google Scholar]
- [63].Franz A, Ackermann L, Hoppe T. Create and preserve: proteostasis in development and aging is governed by Cdc48/p97/VCP. Biochimica et biophysica acta. 2014;1843:205–215. doi: 10.1016/j.bbamcr.2013.03.031. [DOI] [PubMed] [Google Scholar]
- [64].Ju JS, Fuentealba RA, Miller SE, Jackson E, Piwnica-Worms D, Baloh RH, Weihl CC. Valosin-containing protein (VCP) is required for autophagy and is disrupted in VCP disease. The Journal of cell biology. 2009;187:875–888. doi: 10.1083/jcb.200908115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Jitrapakdee S, Maurice M, Rayment I, Cleland WW, Wallace JC, Attwood PV. Structure, mechanism and regulation of pyruvate carboxylase. The Biochemical journal. 2008;413:369–387. doi: 10.1042/BJ20080709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Aiken CT, Kaake RM, Wang X, Huang L. Oxidative stress-mediated regulation of proteasome complexes. Molecular & cellular proteomics : MCP. 2011;10 doi: 10.1074/mcp.M110.006924. R110 006924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Galluzzi L, Kepp O, Vander Heiden MG, Kroemer G. Metabolic targets for cancer therapy. Nature reviews. Drug discovery. 2013;12:829–846. doi: 10.1038/nrd4145. [DOI] [PubMed] [Google Scholar]
- [68].Fan TW, Lane AN, Higashi RM, Farag MA, Gao H, Bousamra M, Miller DM. Altered regulation of metabolic pathways in human lung cancer discerned by (13)C stable isotope-resolved metabolomics (SIRM) Molecular cancer. 2009;8:41. doi: 10.1186/1476-4598-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sellers K, Fox MP, Bousamra M, 2nd, Slone SP, Higashi RM, Miller DM, Wang Y, Yan J, Yuneva MO, Deshpande R, Lane AN, Fan TW. Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. The Journal of clinical investigation. 2015 doi: 10.1172/JCI72873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Leung CK, Wang Y, Deonarine A, Tang L, Prasse S, Choe KP. A negative-feedback loop between the detoxification/antioxidant response factor SKN-1 and its repressor WDR-23 matches organism needs with environmental conditions. Molecular and cellular biology. 2013;33:3524–3537. doi: 10.1128/MCB.00245-13. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [71].Jain A, Lamark T, Sjottem E, Larsen KB, Awuh JA, Overvatn A, McMahon M, Hayes JD, Johansen T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. The Journal of biological chemistry. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Duran A, Linares JF, Galvez AS, Wikenheiser K, Flores JM, Diaz-Meco MT, Moscat J. The signaling adaptor p62 is an important NF-kappaB mediator in tumorigenesis. Cancer cell. 2008;13:343–354. doi: 10.1016/j.ccr.2008.02.001. [DOI] [PubMed] [Google Scholar]
- [73].Stewart-Ornstein J, Nelson C, DeRisi J, Weissman JS, El-Samad H. Msn2 coordinates a stoichiometric gene expression program. Current biology : CB. 2013;23:2336–2345. doi: 10.1016/j.cub.2013.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Moehlenkamp JD, Johnson JA. Activation of antioxidant/electrophile-responsive elements in IMR-32 human neuroblastoma cells. Archives of biochemistry and biophysics. 1999;363:98–106. doi: 10.1006/abbi.1998.1046. [DOI] [PubMed] [Google Scholar]
- [75].Lee JM, Moehlenkamp JD, Hanson JM, Johnson JA. Nrf2-dependent activation of the antioxidant responsive element by tert-butylhydroquinone is independent of oxidative stress in IMR-32 human neuroblastoma cells. Biochemical and biophysical research communications. 2001;280:286–292. doi: 10.1006/bbrc.2000.4106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.