Abstract
Background. For young South African women at risk for human immunodeficiency virus (HIV) infection, preexposure prophylaxis (PrEP) is one of the few effective prevention options available. Long-acting injectable PrEP, which is in development, may be associated with greater adherence, compared with that for existing standard oral PrEP formulations, but its likely clinical benefits and additional costs are unknown.
Methods. Using a computer simulation, we compared the following 3 PrEP strategies: no PrEP, standard PrEP (effectiveness, 62%; cost per patient, $150/year), and long-acting PrEP (effectiveness, 75%; cost per patient, $220/year) in South African women at high risk for HIV infection (incidence of HIV infection, 5%/year). We examined the sensitivity of the strategies to changes in key input parameters among several outcome measures, including deaths averted and program cost over a 5-year period; lifetime HIV infection risk, survival rate, and program cost and cost-effectiveness; and budget impact.
Results. Compared with no PrEP, standard PrEP and long-acting PrEP cost $580 and $870 more per woman, respectively, and averted 15 and 16 deaths per 1000 women at high risk for infection, respectively, over 5 years. Measured on a lifetime basis, both standard PrEP and long-acting PrEP were cost saving, compared with no PrEP. Compared with standard PrEP, long-acting PrEP was very cost-effective ($150/life-year saved) except under the most pessimistic assumptions. Over 5 years, long-acting PrEP cost $1.6 billion when provided to 50% of eligible women.
Conclusions. Currently available standard PrEP is a cost-saving intervention whose delivery should be expanded and optimized. Long-acting PrEP will likely be a very cost-effective improvement over standard PrEP but may require novel financing mechanisms that bring short-term fiscal planning efforts into closer alignment with longer-term societal objectives.
Keywords: HIV, preexposure prophylaxis, cost-effectiveness, South Africa, long-acting agents
(See the editorial commentary by Landovitz and Grinsztejn on pages 1519–20.)
Human immunodeficiency virus (HIV) infection continues to be a major cause of mortality in sub-Saharan Africa [1]. Despite growth in antiretroviral therapy (ART) coverage, the incidence of HIV infection among South African female teenagers continues to grow, with the prevalence of infection increasing from 2.4% to 17.4% between ages 14 and 24 years [2]. Preexposure prophylaxis (PrEP) has proven effective at preventing HIV infection [3, 4] and is being considered for low-income and middle-income countries where the incidence of infection is high [5, 6]. However, the success of current standard oral PrEP (Std-PrEP) hinges on daily adherence, with overall effectiveness of Std-PrEP in trials ranging from 0% to 94% [3, 4, 7–12]. Novel long-acting formulas of PrEP (LA-PrEP) provide sustained drug levels when administered bimonthly or quarterly and could help improve adherence [13, 14]. These formulations would, however, require a brief (approximately 1-month) period of short-term adherence, to rule out acute toxicity with a short-acting formulation, prior to long-acting dosing. Phase II clinical trials (the HIV Prevention Trials Network [HPTN] 077 study, the Centre for the AIDS Programme of Research in South Africa [CAPRISA] 014 study, the ÉCLAIR study, and the HPTN 076 study) are planned or ongoing for 2 LA-PrEP formulas, cabotegravir/GSK1265744 and rilpivirine/TMC278LA [15–17]. In animal studies, prophylaxis efficacies of rectal and vaginal formulations of these agents have reached 75%–100% [14, 18, 19].
Although modeling studies have already projected the cost-effectiveness and substantial clinical benefits of properly used Std-PrEP, the comparative cost and effectiveness of alternative PrEP formulations are unknown [20–23]. Our objective was to anticipate the development of newer PrEP formulations, to investigate effectiveness thresholds that would justify the additional cost over existing PrEP alternatives in a population of high-risk young women in South Africa, and to identify the key drivers and uncertainties behind that assessment.
METHODS
Analytic Overview
We used the Cost-Effectiveness of Preventing AIDS Complications–International (CEPAC-I) model to project clinical benefits, estimate upfront investments, and establish cost-effectiveness performance benchmarks for LA-PrEP for high-risk South African women aged 18–25 years. Leveraging our prior work on Std-PrEP [22, 23], we examine 3 strategies: (1) no PrEP, (2) Std-PrEP with 62% effectiveness [10], and (3) LA-PrEP with 75% effectiveness [14, 18, 24]. We examined the sensitivity of our findings to uncertainty in LA-PrEP effectiveness, HIV infection incidence, duration of PrEP use, and LA-PrEP programmatic cost. Model outcomes included lifetime risk of HIV infection (per 1000 women at high risk), 5-year mortality and cost, cost per infection averted, lifetime survival and cost, and incremental cost-effectiveness ratios (ICERs) in 2014 US dollars per life-year saved. All outcomes used for economic evaluation are reported using a 3% annual discount rate. We labeled programs as “very cost-effective” if their ICERs were less than the South African annual per capita gross domestic product (GDP; ie, $7000) and as “cost-effective” if their ICERs were <3 times the GDP [25, 26]. We also examined the 1-year and 5-year budget impacts of an LA-PrEP strategy in this population. We conducted our analysis from the HIV program perspective and excluded later medical care costs for HIV-uninfected women.
Model Overview
CEPAC-I is a state-transition simulation of HIV prevention, case detection, and disease progression that is used to project clinical, epidemiologic, and economic outcomes of HIV prophylaxis and treatment programs. Model users define cohort characteristics, HIV screening policies, treatment regimens, and disease-monitoring policies, which all influence the natural history of HIV disease progression. For this analysis, all model entrants were PrEP-eligible, HIV-uninfected, high-risk South African women.
PrEP Module
HIV-negative women (mean age, 18 years) entered the module and were subjected to an age-dependent incidence of HIV infection. To simulate HIV screening that might occur in communities, all women underwent HIV testing every 3 years, on average [6].
We assumed that PrEP effectiveness, measured as a percentage reduction in HIV infection incidence, incorporated both intrinsic drug efficacy and anticipated client adherence. Women receiving one of the PrEP strategies underwent quarterly HIV screening to ensure that antiretroviral agents were not being prescribed suboptimally as prophylaxis to HIV-infected women [27]. In the base case, we assumed that women only received PrEP when their HIV infection risk was highest (ie, from the age at model entry through age 25 years) and relaxed this assumption in sensitivity analyses.
Disease Module
Women who became infected with HIV entered the disease module and traversed the natural history of HIV progression until they received a diagnosis of HIV infection via ongoing HIV screening efforts or via PrEP-associated HIV testing or until they developed a severe opportunistic infection [23]. Once HIV infection was detected, PrEP was discontinued, and the woman became eligible for guideline-concordant HIV care [28, 29], including ART initiation at a CD4+ T-cell count of < 500 cells/µL. In the model, ART suppressed viral load, increased the CD4+ T-cell count, and decreased the risk of opportunistic infections and death [29, 30]. Per South African standards, we assumed 2 sequential lines of ART: first-line efavirenz-based therapy and second-line protease inhibitor–based therapy. For women receiving PrEP who became infected with HIV, we assumed in the base case that there was no resistance-related reduction in the efficacy of first-line ART, because LA-PrEP uses different antiretroviral agents than those used for treatment in South Africa [28]. We then conducted sensitivity analyses around this assumption, implementing a 10% absolute decrease in the rate of virologic suppression during first-line ART for those who were infected.
Model Input Data
Demographic Parameters
We simulated a cohort of 1 million high-risk South African women with a mean age (±SD) of 18 ± 2 years and an annual incidence of HIV infection of 5.0% until age 26 years, as reported in the Preexposure Prophylaxis Trial for HIV Prevention Among African Women (FEM-PrEP; 5.0%) [11], the Vaginal and Oral Interventions to Control the Epidemic (VOICE) study (5.7%) [9], and the 2012 South African National Prevalence, Incidence, and Behaviour Survey (SANPIBS; 4.5%) [2]. At age ≥26 years, the annual incidence of HIV infection was age adjusted to that for the average female in South Africa (ie, 2.1% for women aged 26–44 years and 0.85% for women aged ≥45 years), as derived from the SANPIBS and the 2011 South African census (Table 1) [2, 31].
Table 1.
Selected Model Input Values
| Variable | Base Case Value | Range in Sensitivity Analysis | Reference |
|---|---|---|---|
| Baseline cohort characteristic | |||
| Age, y, mean ± SD | 18 ± 2 | … | Assumption |
| Female sex, % | 100 | … | Assumption |
| Annual HIV infection incidence, by age, % | |||
| ≤25 y | 5.0 | 2.5–9.0 | [2, 9, 11] |
| 26–44 y | 2.1 | 1.0–4.0 | [2, 31] |
| ≥45 y | 0.85 | … | [2, 31] |
| PrEP characteristic | |||
| Long-acting PrEP effectiveness, % | 75 | 0–100 | [14, 18, 24] |
| Standard PrEP effectiveness, % | 62 | 39–62 | [8, 10] |
| HIV test characteristic | |||
| Testing frequency during PrEP receipt | Every 3 mo | 1, 6, and 12 mo | [27] |
| Background testing frequency without PrEP receipt | Every 3 y | 1, 5, 7, and 10 y | Assumption |
| Clinical characteristic after HIV infection | |||
| Initial CD4+ T-cell count, cells/µL, mean ± SD | 559 ± 236 | … | [36–38] |
| ART efficacy of first- and second-line therapies | |||
| Patients with viral suppression at 48 wk, % | 92 | 50–100 | [29] |
| Rate of failure after 48 wk, per 100 person-years | 1.4 | … | [29] |
| Increase in CD4+ T-cell count at 48 wk, cells/µL, mean | 206 | … | [29] |
| Cost, 2014 $ | |||
| Discount rate, % | 3 | 0–5 | Assumption |
| PrEP program cost | |||
| Std-PrEP drug (TDF/FTC), monthly (annually) | 6.25 (75) | … | [33] |
| LA-PrEP drug, monthly (annually) | 12.50 (150) | … | Assumption |
| Chemistry panel, per test (annually) | 15.50 (31) | … | [35] |
| HIV test, per test (annually) | 1.20 (5) | 0.50–3.00 | [35] |
| Clinic visit, per visit (annually) | 10.40 (42) | … | [40] |
| Total Std-PrEP program cost, monthly | 12.30 | … | |
| Total LA-PrEP program cost, monthly | 18.60 | 1.90–37.10 | |
| Antiretroviral therapy (annually) | |||
| First line: TDF/FTC/EFV or TDF/3TC/EFV | 192 | 100–300 | [39] |
| Second line: AZT/3TC+LPV/r | 412 | 200–600 | [39] |
| HIV load testing, cost per test | 36 | 15–55 | [35] |
| CD4+ T-cell count testing, cost per test | 7 | 3–11 | [35] |
| Routine care cost, monthly (ranges by CD4+ T-cell count) | 20–157 | … | [35, 41] |
Abbreviations: 3TC, lamivudine; ART, antiretroviral therapy; AZT, zidovudine; EFV, efavirenz; FTC, emtricitabine; HIV, human immunodeficiency virus; LA-PrEP, long-acting preexposure prophylaxis; LPV/r, lopinavir/ritonavir; PrEP, preexposure prophylaxis; SD, standard deviation; Std-PrEP, standard preexposure prophylaxis; TDF, tenofovir.
PrEP Effectiveness
Animal studies of LA-PrEP demonstrated prevention efficacies ranging from 75% to 100% (Table 1) [14, 18, 24]. We used an LA-PrEP effectiveness of 75% in the base case, to approximate 1 missed LA-PrEP dose per year. Trial-based effectiveness estimates for Std-PrEP have varied from 0% (in the FEM-PrEP and VOICE study) in nonadherent populations to 94% (in the Partners PrEP study) in fully adherent populations [4, 9, 11, 12]. Acknowledging this wide range, we set a relatively high effectiveness estimate of 62% (from the TDF2 study [10]) for Std-PrEP in the base case (the effectiveness among heterosexual women ranges from 49% to 75%). We intentionally used 62% instead of a lower reported effectiveness because this sets a reasonable yet high standard against which we could compare the value of LA-PrEP (data in Supplementary Figure 4 compare LA-PrEP to Std-PrEP with 49% effectiveness). We chose the TDF2 study specifically because it was conducted with a target study population similar to that used in our analysis. PrEP toxicity was excluded from the base case because the majority of PrEP trials have reported no difference in the rate of serious adverse events across study groups [3, 4, 7, 9, 10] and because a study on the safety and tolerability of LA-PrEP reported no severe adverse events [32].
PrEP Costs
We estimated the monthly drug cost of Std-PrEP (tenofovir-emtricitabine) as $6.25 [33]. Although the cost of LA-PrEP is unknown, pricing of long-acting versus short-acting contraceptives showed that long-acting injectables cost nearly the same as or less than daily pills [34]. However, we conservatively approximated the LA-PrEP drug cost to be double that of Std-PrEP (ie, $12.50 monthly). The programmatic cost of both LA-PrEP and Std-PrEP incorporated the cost of drug, biannual chemistry panels, and clinic visits. Each biannual chemistry panel costs $15.50 [35], and a quarterly routine clinic visit costs $10.40. Thus, in our model the base case total monthly programmatic cost for patients receiving Std-PrEP was $12.30 and that for LA-PrEP was $18.60. We conducted sensitivity analyses examining variations in overall programmatic cost, noting that, for LA-PrEP, only 70% of the overall cost was related to the cost of the drug itself (Table 1).
Other Inputs
Other key inputs are provided in Table 1 [2, 8–11, 14, 18, 24, 27, 29, 31, 33, 35–41].
Sensitivity Analyses
In sensitivity analyses, we varied LA-PrEP effectiveness, HIV infection incidence, age at PrEP discontinuation, and LA-PrEP programmatic cost. We then chose extreme values within realistic ranges of key PrEP-related parameters and combined them to create plausibly optimistic and pessimistic scenarios for LA-PrEP, compared with no PrEP and Std-PrEP.
Budget Impact
To inform financial planning, we conducted 1-year and 5-year budget impact assessments of alternative PrEP strategies. We adopted the perspective of the South African HIV prevention program, restricting our attention to undiscounted PrEP program cost (including drug, clinic visit, and chemistry panel cost) and assumed a 50% uptake. We estimated the number of PrEP-eligible South African women aged 18–25 years [31] by deducting the percentage of women with prevalent HIV infection (range, 2.4%–28.4%) from age-stratified population estimates [2]. Beyond the first year, we introduced women aged 18 years who were newly eligible for PrEP and removed the expected number of women with incident cases, given Std-PrEP or LA-PrEP effectiveness. We then multiplied this figure by the annual Std-PrEP or LA-PrEP program cost and subtracted the annual ART-associated cost savings resulting from PrEP-averted infections.
RESULTS
Base Case
Clinical Outcomes
The lifetime risk of HIV infection in high-risk South African women with no PrEP was 630 cases/1000. With an average of 8 years of PrEP use, the risk of infection declined, ranging from 540 cases/1000 with Std-PrEP (effectiveness, 62%) to 510 cases/1000 with LA-PrEP (effectiveness, 75%). The undiscounted per-person life expectancy starting from model entry (mean age, 18 years)—including women with and those without HIV infection—was shortest with the no PrEP strategy (47.7 years). Life expectancy for the PrEP strategies extended from 50.1 years (for Std-PrEP) to 50.4 years (for LA-PrEP). As a point of validation, this range of life expectancy was consistent with reports for South African HIV-infected adults receiving ART [42, 43]. PrEP also averted HIV-related deaths: over a 5-year period, LA-PrEP averted 16 HIV-related deaths per 1000 high-risk women, compared with no PrEP (Table 2).
Table 2.
Base Case Results
| Variable | No PrEP | Std-PrEP (62%, $12.30/mo) | LA-PrEP (75%, $18.60/mo) |
|---|---|---|---|
| Lifetime HIV infection risk,a cases/1000 high-risk women | 630 | 540 | 510 |
| Undiscounted per-person life expectancy,b y | 47.7 | 50.1 | 50.4 |
| 5-y HIV infections averted, compared with no PrEP, no./1000 high-risk women | … | 127 | 156 |
| 5-y HIV-related deaths averted, compared with no PrEP, no./1000 high-risk women | … | 15 | 16 |
| Discounted 5-y cost per high-risk woman,c $ | 260 | 840 | 1130 |
| Discounted PrEP cost per lifetime HIV infection averted, $ | … | 10 100 | 12 400 |
| Discounted life expectancy, y | 23.9 | 24.8 | 25.0 |
| Discounted per person lifetime cost,c $ | 5730 | 5270 | 5300 |
| ICER vs no PrEP, $/life-year saved | … | Cost savingd | Cost savingd |
| ICER vs Std-PrEP, $/life-year saved | NA | … | 150 |
Abbreviations: HIV, human immunodeficiency virus; ICER, incremental cost-effectiveness ratio; LA-PrEP, long-acting preexposure prophylaxis; NA, not applicable; PrEP, preexposure prophylaxis; Std-PrEP, standard preexposure prophylaxis.
a Lifetime HIV infection risk was projected from the cohort starting age of 18 years.
b Per-person life expectancy was projected from the cohort starting age of 18 years. A per-person undiscounted life expectancy of 50 years corresponds here to an overall life expectancy of 68 years.
c Costs were measured in 2014 $ and discounted at a rate of 3%.
d PrEP was more effective and less expensive than the comparator strategy.
Cost and Cost-effectiveness Outcomes
Over a 5-year horizon, the per-person costs of PrEP were substantially higher ($840 for Std-PrEP and $1130 for LA-PrEP) than for no PrEP ($260). The cost of averting 1 HIV infection over a lifetime was estimated as $10 100 for Std-PrEP and $12 400 for LA-PrEP. When all 3 strategies were compared over a lifetime, including the cost of ART, the Std-PrEP strategy was the least expensive ($5270). Compared with no PrEP, both Std-PrEP and LA-PrEP were less expensive and more effective, and therefore cost saving. Compared with Std-PrEP, the ICER of LA-PrEP was $150/life-year saved (Table 2).
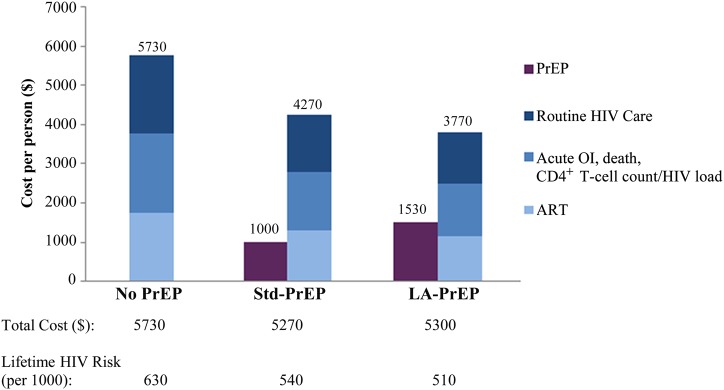
The 3 strategies differed in terms of the components of their costs. Increasingly aggressive PrEP approaches required a greater investment in PrEP-related program cost (Figure 1). However, these investments were offset by savings in HIV-related cost (Figure 1). Compared with no PrEP, spending an average of $1530 per person on LA-PrEP resulted in $1960 less spent per person on total HIV care.
Figure 1.
Average discounted per-person lifetime cost distribution of the no preexposure prophylaxis (PrEP), standard PrEP (Std-PrEP), and long-acting PrEP (LA-PrEP) strategies. Discounted per-person lifetime costs (in 2014 $) are provided on the vertical axis. Costs associated with PrEP administration (drug costs, safety labs, and clinic visits) are shown in dark purple. Costs associated with human immunodeficiency virus (HIV) care are blue. Antiretroviral therapy (ART) costs are lightest blue. HIV routine care costs, such as clinic visits, are dark blue. Costs associated with laboratory monitoring and AIDS-defining events for HIV-infected people are medium blue. Total costs (sum of overall PrEP costs and overall HIV costs), as well as lifetime risk of HIV infection associated with each strategy, are in the rows below the figure. These values demonstrate the interaction between the PrEP investment and its prevention impact. Investments in PrEP programs resulted in lower HIV-related costs and substantially fewer HIV infections. Abbreviation: OI, opportunistic infection.
Strategies also differed in terms of their cost and benefit trajectories over time. When we focused on the first 5 years after program implementation, increasingly aggressive PrEP strategies were costlier (Table 2). Viewed over a longer horizon, however, PrEP interventions were less costly. The LA-PrEP strategy required a higher initial investment (Figure 2A); its discounted cost greatly exceeded that of the no PrEP strategy and slightly exceeded that of the Std-PrEP strategy over the first 5–15 years. However, the higher eventual cost related to increased numbers of HIV infections and treatment with the no PrEP strategy caused the cumulative discounted cost curves to converge (the curves crossed at approximately year 29; data not shown). Beyond that point, the LA-PrEP strategy had lower cumulative cost. LA-PrEP also reduced the number and delayed the occurrence of HIV-related deaths (Figure 2A) and the number of women requiring ART (Figure 2B).
Figure 2.
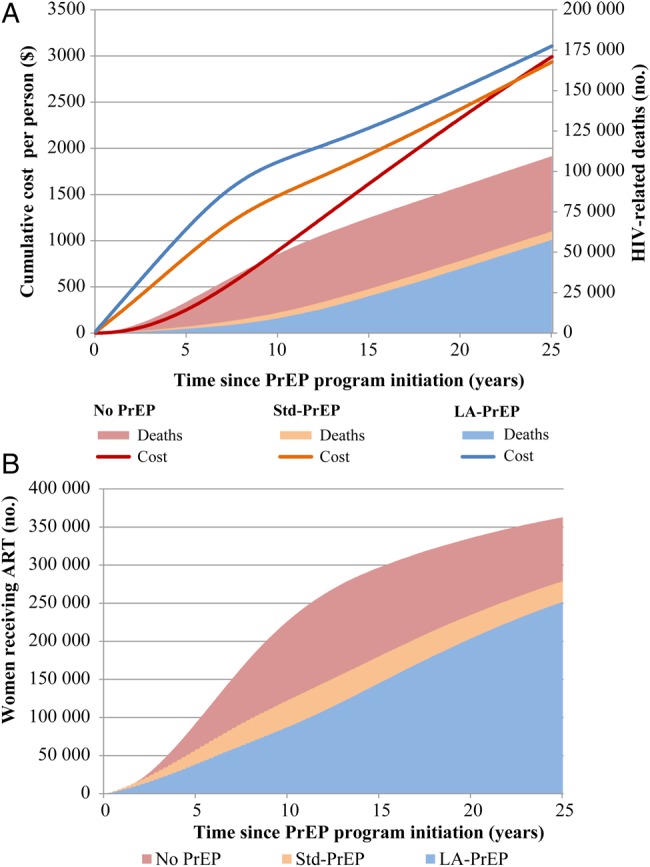
Cumulative discounted costs, human immunodeficiency virus (HIV)–related deaths, and women receiving antiretroviral therapy (ART) for the no preexposure prophylaxis (PrEP), standard PrEP (Std-PrEP), and long-acting PrEP (LA-PrEP) strategies. A, The cumulative discounted cost per high-risk woman is shown on the left vertical axis, and the cumulative number of HIV-related deaths is on the right vertical axis. Lines correspond to the per-person cumulative cost on the left axis: the red line is the no PrEP strategy, the orange line is the Std-PrEP strategy, and the blue line is the LA-PrEP strategy. The changes in the slopes of the blue and orange lines occurring around year 8 correspond to the time at which PrEP was stopped and required investments therefore decreased. Costs of the LA-PrEP strategy (blue line) and the no PrEP strategy (red line) converge over time and ultimately cross at year 29 (data not shown). The pink shaded area corresponds to the number of HIV-related deaths that occurred with no PrEP (109 000 deaths by year 25). The orange shaded area corresponds to the number of HIV-related deaths that occurred with Std-PrEP (63 000 deaths by year 25). The blue shaded area corresponds to the number of HIV-infected deaths that occurred with an LA-PrEP program (58 000 deaths by year 25). B, The cumulative number of women who received ART is shown on the left vertical axis. The red area corresponds to the number of women in the no PrEP group who received ART (363 000 women were receiving ART by year 25). The orange area corresponds to the number in the Std-PrEP group who received ART (279 000 women were receiving ART by year 25). The blue area corresponds to the number of women in the LA-PrEP group who were receiving ART (252 000 women were receiving ART by year 25).
Sensitivity Analyses
We summarize our extensive sensitivity analyses in the section below, reporting on key instances variation in the input data assumptions had a material impact on our policy findings or were clinically important. A fuller report is provided in the Supplementary Materials.
LA-PrEP Effectiveness
Clinical benefits increased with increases in LA-PrEP effectiveness (Table 3). Lifetime risk of HIV infection decreased from 510 cases/1000 high-risk women (base case effectiveness, 75%) to 490 and 470 cases/1000 high-risk women (effectiveness, 85% and 95%, respectively); similarly, more HIV-related deaths were averted as effectiveness increased. Average lifetime cost also decreased, dropping as low as $4530 per woman at 95% effectiveness; as total lifetime cost decreased, PrEP accounted for a greater proportion of total cost.
Table 3.
Sensitivity Analysis Results
| LA-PrEP Strategy | Lifetime Risk of HIV Infection,a Infections/1000 High-Risk Women | 5-y Averted HIV-Related Deaths, No./1000 High-risk Womenb |
Discounted Per-Person Lifetime Cost,c $ | PrEP Cost, % of Total Cost | Discounted PrEP Cost per Lifetime HIV Infection Averted,c $ | ICER, $/Life-Year Saved |
||
|---|---|---|---|---|---|---|---|---|
| Versus No PrEP | Versus Std-PrEP | Versus No PrEP | Versus Std-PrEP | |||||
| Base cased | 510 | 16 | 1 | 5300 | 28 | 12 400 | Cost savinge | 150 |
| Effectiveness, % | ||||||||
| 65 | 530 | 15 | 0 | 5660 | 26 | 14 200 | Cost saving | 18 560 |
| 85 | 490 | 17 | 2 | 4920 | 31 | 10 800 | Cost saving | Cost saving |
| 95 | 470 | 18 | 3 | 4530 | 34 | 9600 | Cost saving | Cost saving |
| HIV infection annual incidence until age 25 y, % | ||||||||
| 2.5 | 490 | 8 | 0 | 4830 | 32 | 21 800 | 680 | 4790 |
| 9.0 | 550 | 27 | 2 | 6000 | 24 | 8200 | Cost saving | Cost saving |
| PrEP through age | ||||||||
| 19 y | 600 | 8 | 0 | 5640 | 8 | 15 800 | Cost saving | 590 |
| 35 y | 430 | 16 | 1 | 5840 | 48 | 14 100 | 60 | 1770 |
| 45 y | 340 | 16 | 1 | 6240 | 60 | 13 000 | 280 | 2 490 |
| Program cost, % of basecase | ||||||||
| 50 ($9/mo) | 510 | 16 | 1 | 4540 | 16 | 6200 | Cost saving | Cost saving |
| 150 ($28/mo) | 510 | 16 | 1 | 6040 | 37 | 18 400 | 280 | 7870 |
| Scenario | ||||||||
| Optimistic (85% efficacy, 50% cost, 9.0% incidence) | 520 | 29 | 4 | 4620 | 16 | 3600 | Cost saving | Cost saving |
| Pessimistic (65% efficacy, 150% cost, 2.5% incidence) | 500 | 8 | 0 | 5780 | 39 | 37 500 | 2420 | 165 360 |
Abbreviations: HIV, human immunodeficiency virus; ICER, incremental cost-effectiveness ratio; LA-PrEP, long-acting preexposure prophylaxis; PrEP, preexposure prophylaxis; Std-PrEP, standard preexposure prophylaxis.
a Lifetime HIV infection risk was projected from the cohort starting mean age of 18 years.
b Averted deaths were rounded down to the nearest integer.
c Costs were measured in 2014 $.
d The base case used LA-PrEP with an effectiveness of 75% in a population of women with 5.0% annual HIV infection incidence at a monthly cost of $18.60. PrEP was provided through age 25 y.
e PrEP was more effective and less expensive than the comparator strategy.
HIV Infection Incidence
When annual incidence was reduced from 5.0% to 2.5% (the population average risk in South Africa), LA-PrEP no longer achieved cost savings, and the PrEP cost per averted infection increased to $21 800; however, LA-PrEP was very cost-effective as compared to no PrEP, with an ICER of $680/life-year saved (<10% annual per capita GDP). Among very high-risk women—similar to those observed in the CAPRISA 004 study (HIV infection incidence, 9.0%/year) [8]—LA-PrEP averted 27 deaths/1000 women over 5 years and cost $8200 per averted infection. Supplementary Figures 3 and 4 provide 2-way sensitivity analyses of LA-PrEP as compared to Std-PrEP at alternative Std-PrEP efficacies (62% and 49%) and in average-risk, high-risk, and very high-risk populations.
Duration of LA-PrEP Use
In the base case analysis, we assumed that LA-PrEP was administered from model entry (ie, at a mean age of 18 years) through age 25 years. If LA-PrEP was administered for longer durations—through age 35 or 45 years—a greater cumulative per-person investment (from $5300 to $5840 or $6240) would be required, LA-PrEP program cost would account for a larger fraction of total outlay (48%–60%), and lifetime risk of HIV would decrease from 510 to 430 or 340 cases per 1000 high-risk women (Supplementary Figure 1). Alternatively, if PrEP “fatigue” resulted in its use only through age 19 years, lifetime cost for LA-PrEP would be higher than in the base case, despite reduced PrEP expenditure, because 2 years of PrEP use would not avert enough infections to decrease lifetime cost substantially as compared to the no PrEP strategy.
LA-PrEP Cost
When the overall LA-PrEP program cost decreased by 50% of the base case value to $9/month, cost savings were achieved as early as 13 years after program implementation, per-person lifetime cost was $4540, and the PrEP cost per infection averted was $6200. If LA-PrEP overall program cost was 150% of the base case ($28/month)—as might occur if the dosing frequency were every 2 months—the ICER for LA-PrEP as compared to no PrEP was very cost-effective, at $280/life-year saved (4% of GDP), and was cost-effective as compared to Std-PrEP, at $7870/life-year saved (112% of GDP; Supplementary Figures 2 and 3); at 150% of cost, LA-PrEP cost $18 400 per infection averted. At a monthly cost exceeding $45 and $310, LA-PrEP ceased to be cost-effective as compared to Std-PrEP and no PrEP, respectively.
Resistance-Related Reduction in ART Efficacy
A sensitivity analysis adding a 10% absolute reduction in virologic suppression due to resistance among persons who experience breakthrough HIV infections during LA-PrEP produced no material impact on any major outcome or on the resultant policy recommendation.
Optimistic and Pessimistic LA-PrEP Scenarios
In a best-case scenario for LA-PrEP use, highly effective LA-PrEP (effectiveness, 85%) was administered at low cost ($9 monthly) and successfully targeted to very high-risk (annual incidence, 9%) women. Use of LA-PrEP in this optimistic scenario (Table 3), averted 29 deaths/1000 women in the first 5 years, cost $4620 per woman over her lifetime, and cost $3600 per infection averted as compared to no PrEP. In a pessimistic scenario, less effective LA-PrEP (effectiveness, 65%) was administered at higher cost ($28 monthly) to the general population of women with average risk of HIV infection (annual incidence, 2.5%). Even under this pessimistic scenario, LA-PrEP had an ICER of $2420/life-year saved as compared to no PrEP (very cost-effective, 35% of GDP) but it was not cost-effective as compared to Std-PrEP ($165 360/life-year saved). LA-PrEP reduced the lifetime risk of HIV infection to 500 cases/1000 high-risk women and cost $37 500 per infection averted, compared with no PrEP.
Budget Impact Analysis
An estimated 3 million South African women ages 18–25 years are eligible for PrEP each year [31]. Providing LA-PrEP to half this population, at an average per-person programmatic cost of $220 annually, would cost $327 million in the first year (Std-PrEP cost, $217 million) and $1.6 billion over 5 years (Std-PrEP cost, $1.1 billion).
DISCUSSION
New long-acting antiretroviral formulations may soon provide a PrEP option that does not require daily adherence to pills. Anticipating the development of long-acting options, we compared the potential clinical benefits, additional cost, cost-effectiveness, and budget impact of existing and novel PrEP strategies. We found that, in high-risk populations (annual HIV infection incidence, 5.0% [9, 11]), currently available oral PrEP formulations, with an effectiveness of 62%, would avert deaths, extend life expectancy, reduce the lifetime risk of HIV infection, and save money in the long term, compared with no PrEP. LA-PrEP, with an effectiveness of 75%, could avert even more deaths, provide longer life expectancy, and result in an even lower lifetime risk of HIV infection. LA-PrEP would also be cost saving as compared to no PrEP in the long term. Compared with standard PrEP, it would have an attractive ICER ($150/life-year saved)—just 2% of South Africa's GDP per capita. This is substantially less than reported cost-effectiveness ratios for ART in South Africa, ranging from $1240 to $2400/life-year saved (updated to 2014 US dollars) [29, 41, 44, 45].
Viewed on an individual basis, LA-PrEP would cost about $12 400 (discounted) per infection averted, more than twice the per-person lifetime discounted cost of care in the absence of PrEP ($5730). However, the upfront investment in LA-PrEP would be offset in the long term by reduced HIV transmission and a lower overall cost of HIV treatment.
Despite long-term cost savings, the scale-up of a PrEP program, even if successfully targeted, would be substantial. According to the South African National Strategic Plan, $2.8 billion is needed in the 2015–2016 fiscal year to meet the goals for prevention and treatment of sexually transmitted diseases, tuberculosis, and HIV infection, a value that already results in a $460 million gap in available funding [46]. Approximately 20% ($560 million) is earmarked for HIV prevention and testing initiatives, likely primarily targeted to the 28 million individuals aged 10–40 years. Yet, our estimated annual budget for LA-PrEP, assuming 50% uptake in high-risk women aged 18–25 years, is $327 million for just 1.5 million women. Thus, for LA-PrEP to truly be sustainable and scalable, creative fiscal strategies will be required. Concessionary loans, which would spread the cost of a PrEP program over a long period, might be one avenue of financing [47].
Our study has several limitations. First, while the efficacy of oral PrEP has been well established in clinical trials, its effectiveness in real-world medical practice has not. Further, the effectiveness and cost of LA-PrEP are unknown. We conducted broad sensitivity analyses around potential target populations and LA-PrEP prophylactic efficacy and cost values, but the point estimates will remain uncertain until more data are available. We did not include the impact of potential fatigue associated with injectables or loss to follow up in the LA-PrEP (or Std-PrEP) program, as has been associated with long-acting contraceptives [48]. However, we examined shorter LA-PrEP horizons (through age 19 years); we believe that LA-PrEP, compared with Std-PrEP, would be less likely associated with disengagement, because LA-PrEP would require much less intensive maintenance and follow-up care. Nevertheless, the LA-PrEP safety profile has yet to be established. The prolonged pharmacokinetic tail of long-acting nanoformulations bears careful monitoring for toxicities (eg, fulminant drug-related liver or cutaneous drug reactions) and their potential contribution to the need for discontinuation and for medical care.
We also accounted only for first-generation, age-dependent transmission to HIV-negative women; inclusion of later-generation infections, especially in young women, would serve to make LA-PrEP even more cost-effective, with negligible impact on the early required budget. Further, we restricted our attention to women >18 years in this analysis. Expanding our assessment to include sexually active adolescent girls—a group with a similarly high HIV infection incidence—would likely both strengthen our cost-effectiveness findings and heighten the budget impact concerns regarding LA-PrEP.
Taken as a whole, our findings suggest a 2-part policy response. First, the delivery of existing oral PrEP formulations, which are very cost-effective, should be expanded and optimized for young, high-risk South African women. Scale-up of PrEP should be accompanied by improved community education and adherence interventions to ensure its effectiveness in the population. Data from large cohorts of women still report annual HIV infection incidences as strikingly high as 9.0%. An immediate need for effective prevention will persist until the full scale-up of treatment to all ART-eligible persons can be achieved [49, 50]. For young women at high risk for HIV infection, oral PrEP is one of the very few effective and cost-saving HIV prevention alternatives available.
Second, the research and development effort should be expanded to bring a viable LA-PrEP formulation to market. The evidence currently at our disposal suggests that long-acting PrEP could be a very cost-effective alternative to standard formulations. Depending on cost, discontinuation rates, and toxicities that are as-yet unanticipated, it might offer a small but still acceptable improvement (as injectable contraception is to oral contraceptive pills). However, even under the most optimistic scenario, a long-acting PrEP formulation is still several years away from implementation; moreover, a long-acting PrEP formulation will place an even greater strain on existing HIV prevention budgets. For this reason, the research effort to confirm the clinical effectiveness of newer LA-PrEP formulations must go hand in hand with a development effort to identify novel financing mechanisms that bring short-term fiscal planning efforts into closer alignment with longer-term societal objectives.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Ethan Borre for his technical assistance with this manuscript.
R. P. W. had access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Disclaimer. The funding sources had no role in the design, analysis, or interpretation of the study or in the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grants R01 AI058736 and AI093269), the National Institute of Mental Health (grant R01 MH105203), and Massachusetts General Hospital (Research Scholars Award to R. P. W.).
Potential conflicts of interest. R. P. W. received grants from the NIH during the conduct of the study. R. A. P. received grants from the NIH during the conduct of the study. A. D. P. received grants from the National Institute of Mental Health during the conduct of the study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.UNAIDS. The gap report. http://www.unaids.org Accessed 27 May 2015.
- 2.Shishana O, Rehle T, Simbayi LC et al. South African national HIV prevalence, incidence and behaviour survey, 2012. Cape Town, South Africa: HSRC Press, 2014. [Google Scholar]
- 3.Baeten JM, Donnell D, Ndase P et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant RM, Lama JR, Anderson PL et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNAIDS. Success with PrEP: next steps to support policy decisions in southern and eastern Africa. http://www.unaids.org/ Accessed 28 July 2015.
- 6.World Health Organization. Consolidated Guidelines on HIV Prevention, Diagnosis, Treatment, and Care for Key Populations. Geneva, Switzerland: WHO Press, 2014. [PubMed] [Google Scholar]
- 7.Choopanya K, Martin M, Suntharasamai P et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013; 381:2083–90. [DOI] [PubMed] [Google Scholar]
- 8.Karim QA, Karim SS, Frohlich JA et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010; 329:1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrazzo JM, Ramjee G, Richardson BA et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015; 372:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thigpen MC, Kebaabetswe PM, Paxton LA et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–34. [DOI] [PubMed] [Google Scholar]
- 11.Van Damme L, Corneli A, Ahmed K et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnell D, Baeten JM, Bumpus NN et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr 2014; 66:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolgin E. Long-acting HIV drugs advanced to overcome adherence challenge. Nat Med 2014; 20:323–4. [DOI] [PubMed] [Google Scholar]
- 14.Radzio J, Spreen W, Yueh YL et al. The long-acting integrase inhibitor GSK744 protects macaques from repeated intravaginal SHIV challenge. Sci Transl Med 2015; 7:270ra5. [DOI] [PubMed] [Google Scholar]
- 15.HPTN 076 - Phase II safety and acceptability of an investigational injectable product, TMC278 LA, for pre-exposure prophylaxis (PrEP) [NCT02165202]. http://www.ClinicalTrials.gov. Accessed 16 June 2015.
- 16.A phase IIa study to evaluate the safety, tolerability and pharmacokinetics of the investigational injectable HIV integrase inhibitor, GSK1265744, in HIV-uninfected men and women [NCT02178800]. http://www.ClinicalTrials.gov Accessed 16 June 2015.
- 17.A phase IIa study to evaluate the safety, tolerability and acceptability of long acting injections of the HIV integrase inhibitor, GSK1265744, in HIV uninfected men (ECLAIR) [NCT02076178]. http://www/ClinicalTrials.gov Accessed 16 June 2015.
- 18.Andrews CD, Spreen WR, Mohri H et al. Long-acting integrase inhibitor protects macaques from intrarectal simian/human immunodeficiency virus. Science 2014; 343:1151–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snyder O, Vincent H, Lachau-Durant S, Kraus G, Williams P, Garcia J. Preclinical evaluation of TMC-278 LA, a long-acting formulation of rilpivirine, demonstrates significant protection from vaginal HIV infection. AIDS Res Hum Retrov 2014; 30.S1:A11–2. [Google Scholar]
- 20.Alistar SS, Owens DK, Brandeau ML. Effectiveness and cost effectiveness of oral pre-exposure prophylaxis in a portfolio of prevention programs for injection drug users in mixed HIV epidemics. PLoS One 2014; 9:e86584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez GB, Borquez A, Case KK, Wheelock A, Vassall A, Hankins C. The cost and impact of scaling up pre-exposure prophylaxis for HIV prevention: a systematic review of cost-effectiveness modelling studies. PLoS Med 2013; 10:e1001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paltiel AD, Freedberg KA, Scott CA et al. HIV preexposure prophylaxis in the United States: impact on lifetime infection risk, clinical outcomes, and cost-effectiveness. Clin Infect Dis 2009; 48:806–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walensky RP, Park JE, Wood R et al. The cost-effectiveness of pre-exposure prophylaxis for HIV infection in South African women. Clin Infect Dis 2012; 54:1504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrews CD, Yueh YL, Spreen WR et al. A long-acting integrase inhibitor protects female macaques from repeated high-dose intravaginal SHIV challenge. Sci Transl Med 2015; 7:270ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Commission on Macroeconomics and Health. Macroeconomics and health: investing in health for economic development. World Health Organization, 2001. [Google Scholar]
- 26.World Bank. World Development Indicators. http://data.worldbank.org/. Accessed 2 June 2015. [Google Scholar]
- 27.US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2014 clinical practice guidelines. http://www.cdc.gov/hiv/pdf/PrEPguidelines2014.pdf Accessed 28 July 2015.
- 28.South African National AIDS Council. The South African Antiretroviral Treatment Guidelines. Pretoria, South Africa: Department of Health for the Republic of South Africa, 2010. [Google Scholar]
- 29.Walensky RP, Ross EL, Kumarasamy N et al. Cost-effectiveness of HIV treatment as prevention in serodiscordant couples. N Engl J Med 2013; 369:1715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.April MD, Wood R, Berkowitz BK et al. The survival benefits of antiretroviral therapy in South Africa. J Infect Dis 2014; 209:491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Statistics South Africa. Census 2011: Census in brief. Pretoria: Statistics South Africa, 2012. [Google Scholar]
- 32.Spreen W, Williams P, Margolis D et al. Pharmacokinetics, safety, and tolerability with repeat doses of GSK1265744 and rilpivirine (TMC278) long-acting nanosuspensions in healthy adults. J Acquir Immune Defic Syndr 2014; 67:487–92. [DOI] [PubMed] [Google Scholar]
- 33.Clinton Health Access Initiative. Antiretroviral (ARV) ceiling price list 2014. http://www.clintonhealthaccess.org/ Accessed 28 July 2015.
- 34.RED BOOK 2015: Truven Health Analytics Inc. MicroMedex Solutions. Accessed 23 March 2015.
- 35.aids2031 Costs and Financing Working Group. The long-term costs of HIV/AIDS in South Africa. Washington, DC: Results for Development Institute, 2010. [Google Scholar]
- 36.Chirwa LI, Johnson JA, Niska RW et al. CD4(+) cell count, viral load, and drug resistance patterns among heterosexual breakthrough HIV infections in a study of oral preexposure prophylaxis. AIDS 2014; 28:223–6. [DOI] [PubMed] [Google Scholar]
- 37.Grant RM, Liegler T, Defechereux P et al. Drug resistance and plasma viral RNA level after ineffective use of oral pre-exposure prophylaxis in women. AIDS 2015; 29:331–7. [DOI] [PubMed] [Google Scholar]
- 38.Laeyendecker O, Redd AD, Nason M et al. Antibody maturation in women who acquire HIV infection while using antiretroviral preexposure prophylaxis. J Infect Dis 2015; 212:754–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization. WHO global pricing mechanism. http://www.who.int Accessed 18 June 2015.
- 40.World Health Organization. WHO-CHOICE unit cost estimates for service delivery. 2008.
- 41.Cleary S, Boulle A, McIntyre D, Coetzee D. Cost-effectiveness of antiretroviral treatment for HIV-positive adults in a South African township. Cape Town, South Africa: University of Cape Town and Health Systems Trust, 2004. [Google Scholar]
- 42.Bor J, Herbst AJ, Newell ML, Barnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science 2013; 339:961–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson LF, Mossong J, Dorrington RE et al. Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLoS Med 2013; 10:e1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braithwaite RS, Tsevat J. Is antiretroviral therapy cost-effective in South Africa? PLoS Med 2006; 3:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walensky RP, Wolf LL, Wood R et al. When to start antiretroviral therapy in resource-limited settings. Ann Intern Med 2009; 151:157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen S, Guthrie T. Financing the South African national strategic plan for HIV, STIs, and TB 2012-2016: An analysis of funding, funding gaps, and financial considerations. Pretoria, South Africa: SANAC Costing Technical Team & Strategic Development Consultants, 2014. [Google Scholar]
- 47.Katz I, Routh S, Bitran R, Hulme A, Avila C. Where will the money come from? Alternative mechanisms to HIV donor funding. BMC Public Health 2014; 14:956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smit JA, Beksinska ME. Hormonal contraceptive continuation and switching in South Africa: implications for evaluating the association of injectable hormonal contraceptive use and HIV. J Acquir Immune Defic Syndr 2013; 62:363–5. [DOI] [PubMed] [Google Scholar]
- 49.Insight Start Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Temprano ANRS Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373:808–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.