Abstract
Background. MicroRNAs (miRs) are a class of short RNA molecules, which negatively regulate gene expression. The levels of circulating miR-15 family members are elevated in septic patients and may be associated with septic death. This study investigated whether inhibition of miR-195, a member of the miR-15 family, provided beneficial effects in sepsis.
Methods and Results. Sepsis was induced by injection of feces into the peritoneum in mice. miR-195 was upregulated in the lung and liver of septic mice. Silencing of miR-195 increased the protein levels of BCL-2, Sirt1, and Pim-1; prevented apoptosis; reduced liver and lung injury; and improved the survival in septic mice. Silencing of miR-195 provided similar protection in lipopolysaccharide-induced endotoxemic mice. In endothelial cells, upregulation of miR-195 induced apoptosis, and inhibition of miR-195 prevented lipopolysaccharide-induced apoptosis. miR-195 repressed expression of its protein targets, BCL-2, Sirt1, and Pim-1. Furthermore, overexpression of Pim-1 prevented apoptosis induced by lipopolysaccharide and miR-195 mimic. Inhibition of Pim-1 attenuated the protective effects of miR-195 silencing in septic mice.
Conclusions. Silencing of miR-195 reduced multiple-organ injury and improved the survival in sepsis, and the protective effects of miR-195 inhibition were associated with upregulation of Bcl-2, Sirt1, and Pim-1. Thus, inhibition of miR-195 may represent a new therapeutic approach for sepsis.
Keywords: sepsis, miR-195, apoptosis, multiple-organ injury
Sepsis and subsequent multiple organ failure remain the major cause of morbidity and mortality in intensive care units [1], despite remarkable advances in treatment of critical illness. We reported an incidence of severe sepsis in 19% of a cohort of Canadian community and teaching hospital intensive care units [2]. The mortality rate among these patients was 38%. The incidence of severe sepsis and septic shock has increased in the last 2 decades. However, there is no specific therapy available for sepsis.
Apoptosis has been demonstrated to play a critical role in sepsis. Apoptosis of immune cells (lymphocytes and dendritic cells) induces immune suppression [3], and apoptosis in other tissue cells directly contributes to microvascular dysfunction and organ failure in sepsis [4–7]. Thus, abrogation of apoptosis is a potential therapeutic strategy for sepsis [8, 9]. Antiapoptotic therapies that inhibit caspases [10–12] or target the death receptor pathway have achieved encouraging results in animal models [5, 6, 13]. However, the complexity of the signaling pathways in regulating apoptosis makes them challenging as possible therapeutic targets.
MicroRNAs (miRs) are a class of short RNA molecules, on average 22 nucleotides long, encoded within the genome and derived from endogenous small hairpin precursors. The miRs negatively regulate gene expression by targeting the 3′ untranslated region (UTR) of specific messenger RNA for transcript degradation or translational repression [14, 15]. Aberrant expression of miRs has been linked to a number of pathological conditions, including inflammatory diseases [16], cancer [17], and cardiovascular diseases [18]. Recent studies reported elevated levels of miR-15 family members in the circulation of septic patients [19, 20], which may be associated with septic death [21]. However, it remains unknown whether the miR-15 family plays a pathophysiological role in sepsis. The miR-15 family consists of 6 highly conserved miRs, including miR-15a, miR-15b, miR-16, miR-195, miR-322/424, and miR-497. They share a common seed region, with varying degrees of sequence homology in the nonseed region of the mature miR. The miR-15 family is increasingly found to play various roles in the regulation of cell cycle progression and cell apoptosis [22–26]. We recently reported that miR-195 promotes apoptosis in cardiomyocytes under diabetic conditions by downregulating its targets, Bcl-2 and Sirt1 [25, 27]. Because both Bcl-2 and Sirt1 have been shown to protect against organ injury in sepsis [28, 29], we hypothesized that inhibition of miR-195 may protect against organ injury in sepsis.
The serine/threonine kinase Pim-1 has been involved in a wide variety of cellular processes, such as cell cycle, apoptosis/cell survival, gene transcription, and general signal transduction pathways [30]. Pim-1 has been suggested as an important antiapoptotic factor that confers cardiac protection under stress [31]. Interestingly, computational algorithms reveal that there may be a putative binding site of miR-195 located in the 3′ UTR of Pim-1. However, it has never been reported whether miR-195 targets and represses Pim-1 expression, which may promote apoptosis in sepsis.
The purpose of this study was to investigate the role of miR-195 in apoptosis and multiple-organ injury during sepsis. Herein, we demonstrate that inhibition of miR-195 increased the protein levels of survival factors (Bcl-2, Sirt1, and Pim-1), prevented apoptosis, and reduced multiple-organ injury in septic mice. Thus, inhibition of the miR-15 family may be a new effective therapy for sepsis.
MATERIALS AND METHODS
Animals
This investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication, 8th Edition, 2011). All experimental procedures were approved by the Animal Use Subcommittee at the Western University, Canada. C57BL/6 mice were purchased from the Jackson Laboratory.
Endotoxemia was induced by injection of lipopolysaccharide (LPS; 4 mg/kg intraperitoneally; Sigma). Sepsis was induced by injection of feces in the peritoneum (FIP) in male C57BL/6 mice (age, 2 months; body weight, about 25 g) as described previously [32]. Briefly, fresh feces was collected from the cecum of donor mice and mixed in sterile saline (0.9% NaCl) at a concentration of 75 mg/mL and then stored in refrigerator at 4°C for 24 hours. The feces mixture was equilibrated to room temperature, and then an aliquot (50 mL/kg) was injected intraperitoneally into mouse. Mice were given sterile saline (1 mL) containing buprenorphine (4 µg/mL) subcutaneously immediately after feces injection and then at 6-hour intervals. Control mice received sterile saline (50 mL/kg) by intraperitoneal injection, followed by subcutaneous injection with sterile saline (1 mL) containing buprenorphine (4 µg/mL). At 4 hours (LPS) or 6 hours (FIP) after injection, plasma and organ tissues were collected for further analyses.
Pulmonary Microvascular Albumin Leak
Pulmonary microvascular leak in mice was assessed using the Evan's blue dye technique as previously described [33].
Modulation of miR-195
A chemically modified antisense oligonucleotide (antagomir; 5′-GCCAAUAUUUCUGUGCUGCUA-3′) and a synthetic miR-195 mimic (5′-UAGCAGCACAGAAAUAUUGGC-3′; GenePharm) were used to inhibit and upregulate miR-195, respectively. A scrambled oligonucleotide (GenePharm) was used as a control. Transfection was conducted by using the TransMessenger transfection reagent (Qiagen) according to the manufacturer's instructions. Twenty-four hours after transfection, cells were subjected to various treatments.
To silence miR-195 expression in vivo, we used an anti-miR-195 miR construct (miRZip-195, System Biosciences). A construct containing a scramble hairpin (miRZip000) served as a control. Sixty micrograms of miRZip-195 or miRZip000 were mixed with 40 µL of nanoparticle-based transfection reagent (Altogen Biosystem) with a total volume of 500 µL of 5% glucose (weight/volume), as per the manufacturer's instruction. The mixture was injected into the tail vein of adult male mice (age, 2 months) as we recently described [27]. Two days later, mice were injected with LPS or feces.
Adenoviral Infection
Mouse lung microvascular endothelial cells were purchased from Cell Biologics (Cedarlane) and infected with adenoviral vectors containing the genes encoding human Pim-1(Ad-Pim1, SignaGen) or beta-gal (Ad-gal, Vector Biolabs) as a control (100 plaque-forming units/cell). All experiments were performed after 24 hours of adenoviral infection.
miR-195 Expression Assay
The levels of miR-195 relative to RNU6B (U6) in lung and liver tissues were measured using the miR plate assay kit (Signosis) as we described previously [25, 27].
Inhibition of Pim-1
Pim-1 inhibitor II or 4-[3-(4-chlorophenyl)-2,1-benzisoxa zol-5-yl]-2-pyrimidinamine (Santa Cruz Biotechnology) was used to block Pim-1 activity in vitro and in vivo. This drug binds and inhibits Pim-2 activity [34].
Assessment of Apoptosis
Caspase-3 activity in endothelial cells, and liver and lung tissues was measured by using a caspase-3 fluorescent assay kit (BIOMOL Research Laboratories) following the manufacturer's instructions. To localize cells undergoing nuclear DNA fragmentation in the liver and lung, in situ terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) was performed as described previously [35]. DNA fragmentation was determined in endothelial cells using a cellular DNA fragmentation enzyme-linked immunosorbent assay (ELISA) kit (Roche Applied Science, Germany) according to the manufacturer's instructions.
MPO Activity Measurement
Myeloperoxidase (MPO) activity in lung and liver tissues was determined as previously described [36].
Determination of Protein Expression
Tumor necrosis factor α (TNF-α) levels in plasma were measured using an ELISA kit as we described elsewhere [37]. The protein levels of Pim-1, Bcl-2, Sirt1, and GAPDH were determined by Western blot analysis.
Plasmids
The luciferase vector including the 3′ UTR of human Pim-1 containing the Pim-1-miR-195 response elements (wt-Luc-Pim-1) was purchased from Addgene. A mutant within the Pim-1–miR-195 response elements of the 3′ UTR of Pim-1 (mu-Luc-Pim-1) was generated by using site-directed gene mutagenesis, whose sequences contained 5′-CUCUUUUACCUcgacgUU-3′ (the 5 lowercase nucleotides are substitutes for GCUGC).
Luciferase Assays
Two hundred nanograms of plasmid DNA (wt-Luc-Pim-1 or mu-Luc-Pim-1) and miR-195 mimic, miR-195 antagomir, or a scrambled oligonucleotide were cotransfected on endothelial cells by using the Attractene Transfection Reagent (Qiagen) according to the manufacturer's instructions. The pRL-CMV Vector containing the CMV enhancer and early promoter elements to provide high-level expression of Renilla luciferase (Promega) served as an internal control. Luciferase assays were conducted by using a dual luciferase reporter assay system (Promega) 24 hours after transfection as we described previously [25].
Statistical Analysis
All data are specified as mean values ± SD. One-way or 2-way analysis of variance followed by Newman–Keuls test was performed for multi-group comparisons as appropriate. Student t test was used for 2-group comparison. Survival curves were created by the method of Kaplan and Meier, and compared by the log-rank test. A P value of <.05 was considered statistically significant.
RESULTS
miR-195 Is Upregulated in the Liver and Lung of Septic Mice
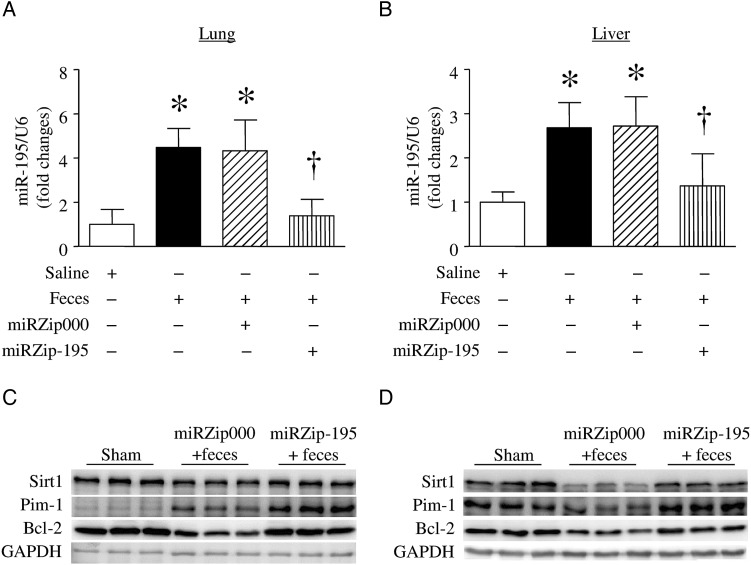
The levels of miR-16 and miR-195, 2 members of the miR-15 family, were increased in serum from a mouse model of cecal ligation and puncture (CLP)–induced sepsis [38]. In this study, we extended our analysis to determine the levels of miR-195 in liver and lung tissues in a mouse model of FIP-induced sepsis. Six hours after FIP injection, the levels of miR-195 were elevated in lung (Figure 1A) and liver (Figure 1B) tissues. Thus, miR-195 was upregulated in septic mouse organs.
Figure 1.
MicroRNA 195 (miR-195) expression and its target proteins in lung and liver. Mice received miRZip-195, miRZip000, or saline and then were challenged with feces injected into the peritoneum. A and B, miR-195 expression was measured in lung (A) and liver (B) tissues. Data are mean ± SD (n = 6). *P < .05 vs sham and †P < .05 vs miRZip000 + feces. C and D, Protein levels of BCL-2, Sirt1, and Pim-1 were determined by Western blot analysis. Data are representative Western blots for BCL-2, Sirt1, Pim-1, and GAPDH in lung (C) and liver (D) tissues from 3 of 6 in each group.
Knock Down of miR-195 Increases the Protein Levels of Survival Factors, Inhibits TNF-α Protein in Plasma, and Improves the Survival in Sepsis
We knocked down miR-195 in mice by delivering miRZip-195. Since miRZip-195 also expresses copGFP, we measured copGFP expression in lung and liver tissues to determine the efficiency of delivery. Two days after injection of miRZip-195, copGFP mRNA was detected in total RNAs isolated from lung and liver tissues by reverse-transcription polymerase chain reaction analysis (Supplementary Figure 1A); however, no copGFP DNA was detected in the same total RNAs (Supplementary Figure 1B). This result excluded the possibility of contamination with copGFP DNA in isolated RNAs and, thus, confirmed the presence of copGFP mRNA expression. We also examined green fluorescent protein (GFP) expression in frozen lung and liver tissue sections and observed significant GFP signals in mouse lung and liver 2 days after injection of miRZip-195. In contrast, no GFP signals were detectable in control mouse lung and liver tissues (Supplementary Figure 2). These results indicate a successful delivery of miRZip-195 into mouse lung and liver in vivo.
Two days after injection of miRZip-195, mice received FIP. Six hours after FIP injection, delivery of miRZip-195 but not miRZip000 decreased the levels of miR-195 in lung and liver tissues, confirming knock down of miR-195 (Figure 1A and 1B). FIP injection decreased Bcl-2 and Sirt1 expression, known as targets of miR-195, in lung (Figure 1C) and liver (Figure 1D), which were restored by silencing miR-195. Silencing of miR-195 also increased the levels of Pim-1, an important survival factor, in liver and lung tissues of FIP-induced mice (Figure 1C and 1D).
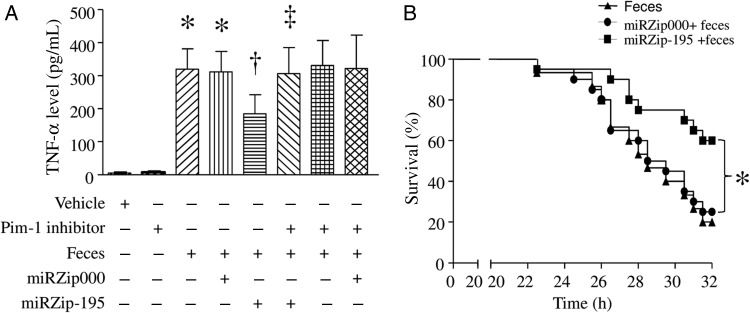
The levels of TNF-α were elevated in plasma from mice with FIP-induced sepsis (Figure 2A), suggesting a systemic inflammatory response. However, delivery of miRZip-195 but not miRZip000 significantly reduced the levels of TNF-α in plasma of septic mice.
Figure 2.
Tumor necrosis factor α (TNF-α) levels in plasma and survival in septic mice. After receiving miRZip-195 or miRZip000, mice were challenged with feces injected into the peritoneum or with saline in the presence of Pim-1 inhibitor II or vehicle. A, Six hours after feces injection, the levels of TNF-α n plasma were measured. Data are mean ± SD (n = 6). *P < .05 vs sham, †P < .05 vs miRZip000 + feces, and ‡P < .05 vs miRZip-195 + feces. B, Survival was monitored for 32 hours. *P < .05 (n = 15 in each group).
To determine whether silencing of miR-195 improved survival, we monitored mortality for 32 hours after FIP injection. As shown in Figure 2B, FIP injection decreased survival in mice, and silencing of miR-195 significantly increased survival in FIP-injected mice. As a control, miRZp000 did not change survival.
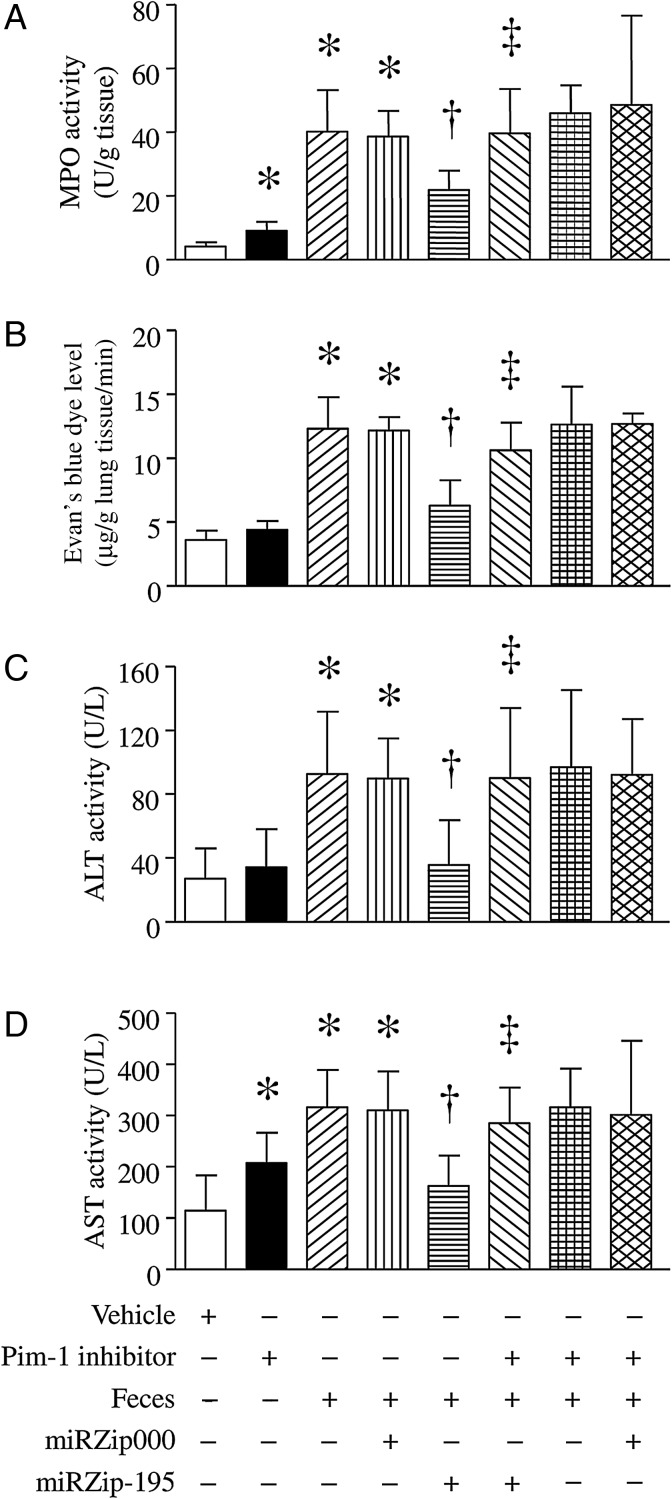
Inhibition of miR-195 Prevents Multiple-Organ Injury in Sepsis
We determined whether inhibition of miR-195 reduced septic lung injury. As shown in Figure 3A and 3B, the MPO activity and pulmonary microvascular albumin leak were significantly increased in the lung of FIP-injected mice. However, delivery of miRZip-195 but not miRZip000 reduced MPO activity in the lung and prevented pulmonary microvascular albumin leak in FIP-induced septic mice. miR-195 silencing also provided protective effects in the liver of septic mice. FIP injection dramatically elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities in plasma; however, silencing of miR-195 prevented the elevation of ALT and AST activities in plasma of septic mice (Figure 3C and 3D). Similar protective effects of miRZip-195 were observed in LPS-injected mice (Supplementary Figure 3A–D). These results demonstrate that silencing of miR-195 protects liver and lung against sepsis-induced injury.
Figure 3.
Lung and liver injuries in septic mice. After receiving miRZip-195 or miRZip000, mice were challenged with feces injected into the peritoneum or with saline in the presence of Pim-1 inhibitor II or vehicle. Six hours later, myeloperoxidase (MPO) activity was measured in lung tissues (A), and pulmonary microvascular albumin leak was determined (B). Alanine aminotransferase (ALT) activity (C) and aspartate aminotransferase (AST) activity (D) were measured in plasma. Data are mean ± SD (n = 6). *P < .05 vs sham or vehicle, †P < .05 vs miRZip000 + feces, and ‡P < .05 vs miRZip-195 + feces.
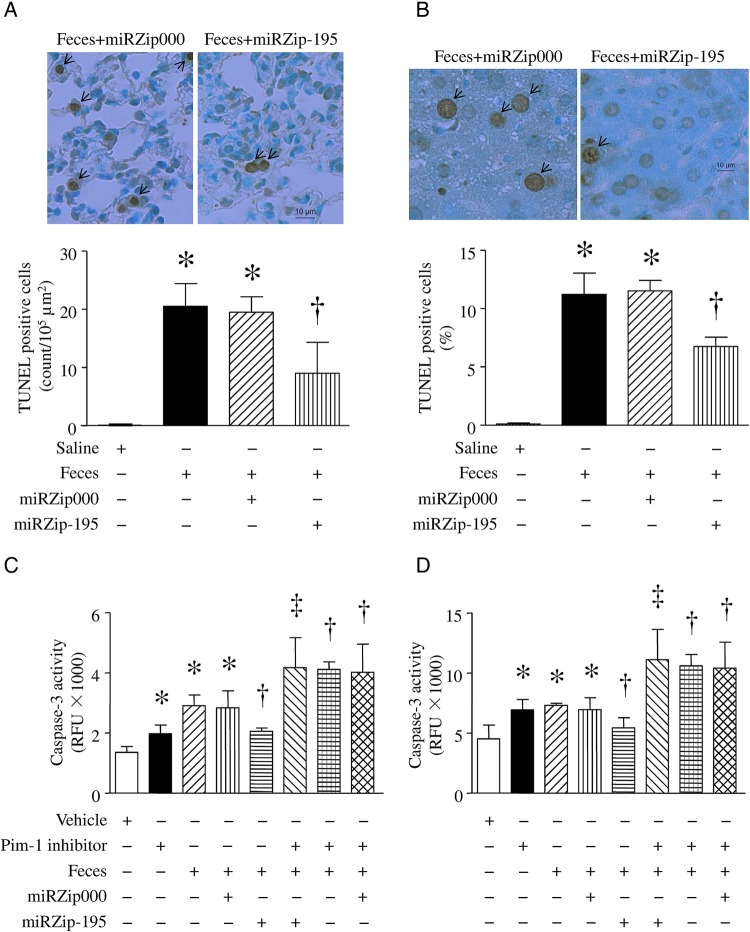
Silencing of miR-195 Inhibits Apoptosis in Sepsis
Apoptosis contributes to organ dysfunction during sepsis. We therefore analyzed apoptosis in lung and liver tissue 6 hours after FIP injection. Sepsis significantly increased caspase-3 activity and TUNEL staining–positive cells in lung (Figure 4A and 4C) and liver (Figure 4B and 4D) tissues. However, the increases in caspase-3 activity and TUNEL-positive cells were significantly attenuated by silencing miR-195 (Figure 4). In contrast, miRZip000 did not change caspase-3 activity in FIP-induced mice (Figure 4C and 4D). A similar inhibitory effect of miR-195 silencing on apoptosis was observed in lung and liver tissues from mice injected with LPS (Supplementary Figure 3E and 3F).
Figure 4.
Apoptosis in lung and liver of septic mice. After receiving miRZip-195 or miRZip000, mice were challenged with feces injected into the peritoneum or with saline in the presence of Pim-1 inhibitor II or vehicle. Six hours later, apoptosis was assessed by using a TUNEL staining assay and measuring caspase-3 activity in lung (A and C) and liver (B and D). A and B, Upper panels are representative microphotographs for TUNEL-positive signals, and lower panels are the quantifications of TUNEL-positive cells (arrows indicate positive staining). C and D, Caspase-3 activity. Data are mean ± SD (n = 6). *P < .05 vs sham or vehicle, †P < .05 vs miRZip000 + feces, and ‡P < .05 vs miRZip-195 + feces. Abbreviation: RFU, relative fluorescence units.
Upregulation of miR-195 Induces Apoptosis In Vitro
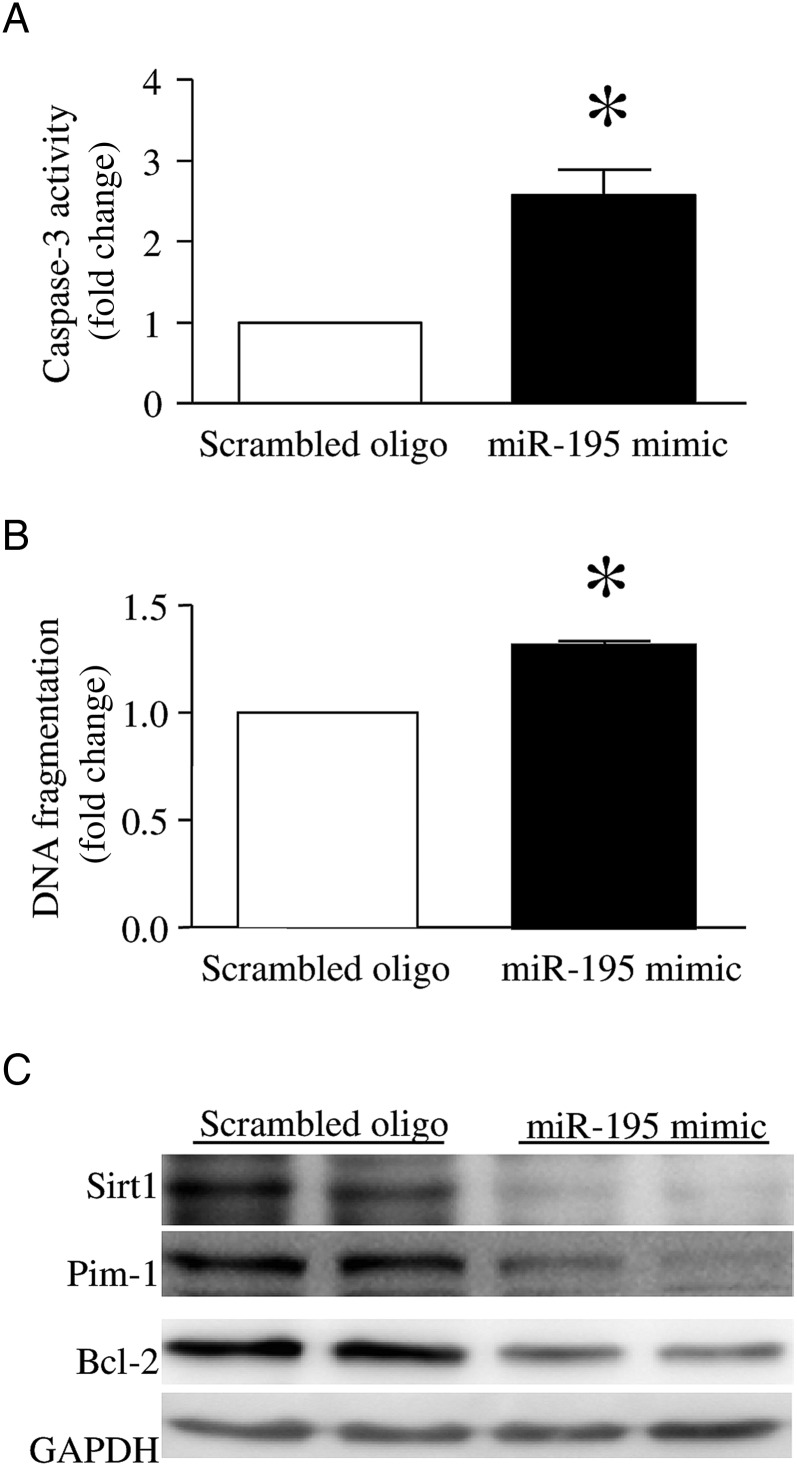
To provide direct evidence to support the role of miR-195 in apoptosis in vitro, we introduced miR-195 mimic or a scrambled oligonucleotide into endothelial cells. Twenty-four hours after transfection with miR-195 mimic, there was induced caspase-3 activity and increased DNA fragmentation, compared with a scrambled oligonucleotide-transfected endothelial cells (Figure 5A and 5B). This result suggests that upregulation of miR-195 sufficiently induces apoptosis in endothelial cells.
Figure 5.
Effects of microRNA 195 (miR-195) mimic on apoptosis and BCL-2, Sirt1, and Pim-1 expression in lung microvascular endothelial cells. Endothelial cells were transfected with miR-195 mimic or a scrambled oligonucleotide (oligo) as a control. Twenty-four hours later, apoptosis was determined by measuring caspase-3 activity (A) and DNA fragmentation (B). C, Representative Western blots for BCL, Sirt1, Pim-1, and GAPDH from 3 different experiments in duplicate. Data are mean ± SD (n = 3). *P < .05.
miR-195 Targets and Inhibits Pim-1 Expression In Vitro
Transfection with miR-195 mimic decreased the protein levels of its targets Bcl-2 and Sirt1 in endothelial cells. The miR-195 mimic also significantly reduced Pim-1 levels in endothelial cells (Figure 5C). These results suggest that miR-195 negatively regulates Bcl-2, Sirt1, and Pim-1 expression in endothelial cells.
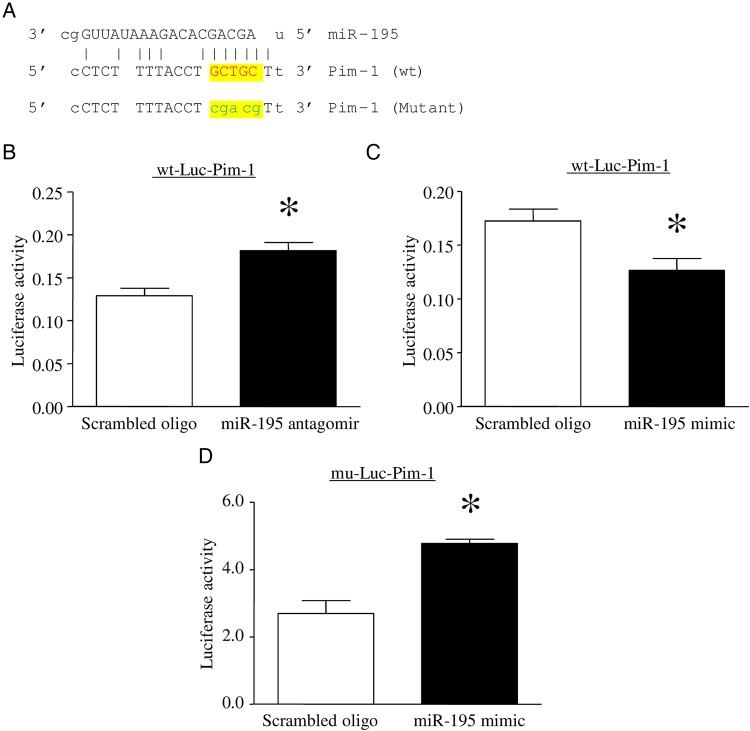
While Bcl-2 and Sirt1 have been identified as direct targets of miR-195, multiple computational algorithms found one putative binding site located in the 3′ UTR of Pim-1. As shown in Figure 6A, the alignments between miR-195 and the region within the 3′ UTR of human Pim-1 represent the putative target sequences that can confer inhibition of translation by miR-195. To clarify whether Pim-1 is a direct target of miR-195, we used a reporter vector containing a luciferase gene followed by the 3′ UTR of human Pim-1 mRNA (wt-Luc-Pim1). Inhibition of miR-195 increased (Figure 6B), whereas upregulation of miR-195 inhibited the luciferase activity in wt-Luc-Pim1 transfected endothelial cells (Figure 6C). To verify this, we mutated the miR-195 putative binding site on the 3′ UTR of Pim-1 in wt-Luc-Pim1 by substitution (Figure 6A). The mutation abrogated the inhibitory effect of miR-195 mimic on the luciferase activity in endothelial cells (Figure 6D). These results provide direct evidence that miR-195 targets and inhibits Pim-1 expression.
Figure 6.
Determination of Pim-1 as a direct target of microRNA 195 (miR-195). A, A segment of the Pim-1 3′ untranslated region (UTR) was inserted downstream of the luciferase-coding sequence. Sequence alignment of miR-195 and the 3′ UTR of Pim-1 shows the complementarity at the 5′ end of miR-195, where the crucial seed region is located, and sequence alignment of miR-195 and the mutated 3′ UTR of Pim-1 shows no complementarity at the 5′ end of miR-195. The 5 lowercase nucleotides in mutant Pim-1 are substitutes for GCTGC in wild-type (wt) Pim-1. B–D, Lung microvascular endothelial cells were cotransfected with the plasmid containing the segment of wt 3′ UTR of Pim-1 (wt-Luc-Pim-1) or the segment of mutated 3′ UTR of Pim-1 (mu-Luc-Pim-1) and an miR-195 mimic, miR-195 antagomir, or a scrambled oligonucleotide (oligo) as a control. Dual luciferase activity assay was performed. Data are mean ± SD from 4 different experiments. *P < .05.
Overexpression of Pim-1 Prevents Apoptosis Induced by miR-195 or LPS
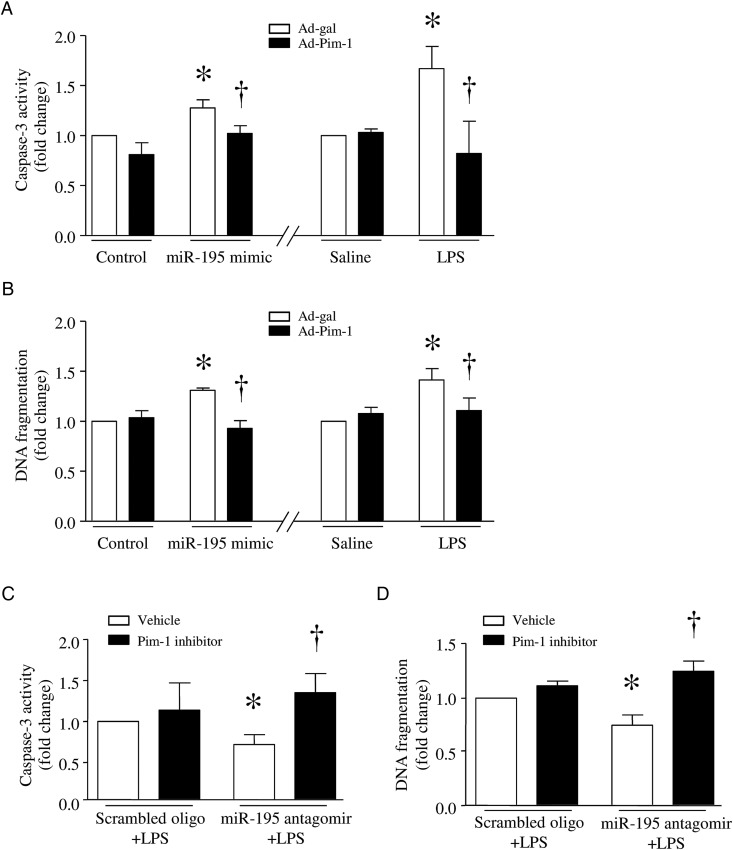
To investigate the role of Pim-1 in apoptosis, we first infected endothelial cells with Ad-Pim1 or Ad-gal and then transfected them with miR-195 mimic or a scrambled oligonucleotide as a control. Twenty-four hours after infection, the Pim-1 level was increased in Ad-Pim1-infected endothelial cells. Upregulation of Pim-1 inhibited caspase-3 activity and DNA fragmentation induced by the miR-195 mimic (Figure 7A and 7B).
Figure 7.
Role of Pim-1 in apoptosis. A and B, Lung microvascular endothelial cells were infected with Ad-Pim-1 or Ad-gal as a control. Twenty-four hours after infection, cells were transfected with microRNA 195 (miR-195) mimic or a scrambled oligonucleotide (oligo). In a separate experiment, after infection with Ad-Pim-1, cells were incubated with lipopolysaccharide (LPS) or saline for 24 hours. Caspase-3 (A) and DNA fragmentation (B) were measured. C and D, Endothelial cells were transfected with miR-195 antagomir or a scrambled oligo, followed by incubation with LPS or saline in combination with Pim-1 inhibitor II or vehicle. Caspase-3 (C) and DNA fragmentation (D) were measured. Data are mean ± SD (n = 4). *P < .05 vs Ad-gal + scrambled oligo, Ad-gal + saline, or vehicle + scrambled oligo; †P < .05 vs Ad-gal + miR-195 mimic, Ad-gal + LPS, or vehicle + miR195 antagomir.
Similarly, infection with Ad-Pim1 prevented LPS-induced apoptosis, whereas Pim-1 inhibitor II (10 µmol/L) further increased apoptosis in LPS-stimulated endothelial cells (Figure 7A and 7B). To demonstrate that downregulation of Pim-1 is one of the mechanisms by which miR-195 induces apoptosis, we transfected endothelial cells with miR-195 antagomir or a scrambled oligonucleotide as a control and then incubated them with LPS in the presence of Pim-1 inhibitor II or vehicle for 24 hours. miR-195 antagomir inhibited apoptosis in LPS-stimulated endothelial cells. However, this effect of miR-195 antagomir was significantly attenuated by Pim-1 inhibitor II (Figure 7C and 7D). Thus, miR-195 inhibition prevents LPS-induced apoptosis, at least partly, through Pim-1 upregulation.
Inhibition of Pim-1 Attenuates the Protective Effects of miR-195 Silencing in Sepsis
To determine whether the effects of miR-195 silencing were mediated through upregulation of Pim-1, we delivered miRZip-195 into mice and then gave them a FIP injection in the presence of Pim-1 inhibitor II (10 mg/kg intraperitoneally) or vehicle. Six hours later, administration of Pim-1 inhibitor II increased MPO activity (Figure 3A), AST activity (Figure 3D), and caspase-3 activity (Figure 4C and 4D) in sham-treated animals and abrogated the protective effects of miR-195 silencing on MPO activity (Figure 3A), pulmonary microvascular albumin leak in lung (Figure 3B), and caspase-3 activity (Figure 4C and 4D), as well as TNF-α (Figure 2A), ALT, and AST activities in plasma of FIP-injected mice (Figure 3C and 3D). These results further suggest that silencing of miR-195 exerts protection at least in part by increasing Pim-1 expression in sepsis.
DISCUSSION
We found that sepsis increased miR-195 expression and decreased the levels of its targets, Bcl-2, Sirt1, and Pim-1, in liver and lung tissues. Upregulation of miR-195 sufficiently induced apoptosis and repressed Bcl-2, Sirt1, and Pim-1 expression in vitro. Thus, inhibition of miR-195 prevented apoptosis and reduced lung and liver injuries in vivo during sepsis, leading to an improvement in survival. To our knowledge, this is the first study that demonstrates that inhibition of miR-195 protects against multiple-organ injury in sepsis.
Previous studies have revealed that levels of circulating miR-15 family members are elevated in septic patients [19, 20] and mice [38]. The present study demonstrates upregulation of miR-195, one member of the miR-15 family, in liver and lung tissues of septic mice. miR-195 has been implicated in promoting apoptosis in a variety of cell types. For instances, miR-195 promoted apoptosis in mouse podocytes during diabetes [24], and it also induced apoptosis in human embryonic stem cell–derived neural progenitor cells [39]. Our recent study demonstrated that miR-195 contributed to palmitate-induced apoptosis in cardiomyocytes [25] and silencing of miR-195 reduced diabetic cardiomyopathy [27]. The present study provides evidence that upregulation of miR-195 was sufficient to induce apoptosis in endothelial cells. The role of miR-195 may be relevant to the apoptosis in other cells, including immune cells, epithelial cells, and other tissue cells, that has been found in both animal [4–7] and human [40, 41] studies. Indeed, we show that inhibition of miR-195 prevented apoptosis in liver and lung tissues of septic mice induced by LPS or FIP. Thus, miR-195 plays an important role in promoting apoptosis during sepsis.
It is well recognized that apoptotic immune cell death is the principal cause of immune suppression, leading to secondary infection during sepsis [3]. Apoptosis in other tissue cells directly contributes to organ dysfunction [4–7]. Thus, the present study shows that inhibition of miR-195 attenuated inflammation, prevented pulmonary microvascular albumin leak, reduced organ injury (eg, liver and lung), and improved the survival during sepsis. These findings highlight a critical role of miR-195 in multiple-organ dysfunction during sepsis. This was indeed supported by clinical observations, which showed that increased miR-15 family members in circulation were associated with septic death [21]. Thus, inhibition of miR-195 may represent a novel therapeutic intervention for septic organ failure.
Although the underlying mechanisms by which silencing of miR-195 prevents apoptosis and reduces organ injury in sepsis remain to be determined, multiple factors might be involved. First, miR-195 targeted and repressed Bcl-2 and Sirt1 expression in vitro, and importantly, silencing of miR-195 increased the levels of Bcl-2 and Sirt1 in mouse models of LPS- and FIP-induced sepsis. Bcl-2 is a well-known antiapoptotic protein [42]. It was reported that transgenic overexpression of Bcl-2 resulted in decreased apoptosis among T cells, B cells, intestinal epithelial cells, and myeloid cells and improved survival among septic mice [28]. Intraperitoneal injection of recombinant Bcl-2 prevented apoptosis in the intestine and heart and reduced mortality among mice with CLP-induced sepsis [43]. Thus, the protective effects of miR-195 silencing may be related to upregulation of Bcl-2 in sepsis. Second, Sirt1 belongs to class III histone/protein deacetylases [44]. A previous study reported that Sirt1 promoted termination of nuclear factor κB–dependent transcription in monocytes and leukocytes during LPS stimulation [45], suggesting that it may protect organs by suppressing proinflammatory responses in sepsis. Indeed, administration of resveratrol, an activator of Sirt1, reduced acute lung injury in a mouse model of LPS-induced sepsis [46] and protected against liver injury in a rat model of CLP-induced sepsis [47]. Knockout of Sirt1 increased inflammasome activation and lung inflammatory responses in mice with CLP-induced sepsis [29]. The present study shows that silencing of miR-195 upregulated Sirt1 in septic mice. Thus, an increased Sirt1 level may attenuate liver and lung injury in sepsis. Third, we found that Pim-1 is a direct target of miR-195. Pim-1 has been suggested as a survival factor in the cardiovascular system [31]. Similarly, we show that downregulation of Pim-1 correlated with apoptosis in miR-195 mimic–induced endothelial cells. In support of the antiapoptotic role of Pim-1, we demonstrate that overexpression of Pim-1 reduced apoptosis, whereas inhibition of Pim-1 enhanced apoptosis in response to LPS or miR-195 mimic. Importantly, inhibition of Pim-1 attenuated the protective effects of miR-195 silencing in mouse models of LPS- and FIP-induced sepsis. Thus, inhibition of Pim-1 may represent another mechanism underlying miR-195–mediated apoptosis and organ injury during sepsis.
It is currently unknown whether other members of the miR-15 family play a similar role in sepsis, although they may share similar targets. However, this will require further studies for clarification. Whereas we are focusing on Bcl-2, Sirt1, and Pim-1 as targets of miR-195 in sepsis, other targets cannot be excluded.
In summary, the present study has demonstrated an important role of miR-195 in promoting apoptosis and multiple-organ injury during sepsis. Thus, inhibition of miR-195 may represent a potentially therapeutic intervention for sepsis.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgment. Some data from this article have been filed in an application for a US patent.
Financial support. This work was supported by the Canadian Institutes of Health Research (grant MOP-133657), the National Natural Scientific Foundation of China (grant 81470499), the University of Western Ontario Department of Medicine (Program of Experimental Medicine Research Award), the Canadian Institutes of Health Research (new investigator award to T. P.), and the National Institutes of Health (grant R01 HL-087861 to G.-C. F.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med 2001; 29:S109–16. [DOI] [PubMed] [Google Scholar]
- 2.Martin CM, Priestap F, Fisher H et al. . A prospective, observational registry of patients with severe sepsis: the Canadian Sepsis Treatment and Response Registry. Crit Care Med 2009; 37:81–8. [DOI] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med 2009; 15:496–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou M, Simms HH, Wang P. Adrenomedullin and adrenomedullin binding protein-1 attenuate vascular endothelial cell apoptosis in sepsis. Ann Surg 2004; 240:321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuda N, Takano Y, Kageyama S et al. . Silencing of caspase-8 and caspase-3 by RNA interference prevents vascular endothelial cell injury in mice with endotoxic shock. Cardiovasc Res 2007; 76:132–40. [DOI] [PubMed] [Google Scholar]
- 6.Matsuda N, Teramae H, Yamamoto S, Takano K, Takano Y, Hattori Y. Increased death receptor pathway of apoptotic signaling in septic mouse aorta: effect of systemic delivery of FADD siRNA. Am J Physiol Heart Circ Physiol 2010; 298:H92–101. [DOI] [PubMed] [Google Scholar]
- 7.Londono D, Carvajal J, Strle K, Kim KS, Cadavid D. IL-10 Prevents apoptosis of brain endothelium during bacteremia. J Immunol 2011; 186:7176–86. [DOI] [PubMed] [Google Scholar]
- 8.Wesche-Soldato DE, Swan RZ, Chung CS, Ayala A. The apoptotic pathway as a therapeutic target in sepsis. Curr Drug Targets 2007; 8:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hattori Y, Takano K, Teramae H, Yamamoto S, Yokoo H, Matsuda N. Insights into sepsis therapeutic design based on the apoptotic death pathway. J Pharmacol Sci 2010; 114:354–65. [DOI] [PubMed] [Google Scholar]
- 10.Hotchkiss RS, Tinsley KW, Swanson PE et al. . Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci U S A 1999; 96:14541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hotchkiss RS, Chang KC, Swanson PE et al. . Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol 2000; 1:496–501. [DOI] [PubMed] [Google Scholar]
- 12.Neviere R, Fauvel H, Chopin C, Formstecher P, Marchetti P. Caspase inhibition prevents cardiac dysfunction and heart apoptosis in a rat model of sepsis. Am J Respir Crit Care Med 2001; 163:218–25. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda N, Yamamoto S, Takano K et al. . Silencing of fas-associated death domain protects mice from septic lung inflammation and apoptosis. Am J Respir Crit Care Med 2009; 179:806–15. [DOI] [PubMed] [Google Scholar]
- 14.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116:281–97. [DOI] [PubMed] [Google Scholar]
- 15.Zamore PD, Haley B. Ribo-gnome: the big world of small RNAs. Science 2005; 309:1519–24. [DOI] [PubMed] [Google Scholar]
- 16.O'Connell RM, Rao DS, Baltimore D. microRNA regulation of inflammatory responses. Annu Rev Immunol 2012; 30:295–312. [DOI] [PubMed] [Google Scholar]
- 17.Schoof CR, Botelho EL, Izzotti A, Vasques Ldos R. MicroRNAs in cancer treatment and prognosis. Am J Cancer Res 2012; 2:414–33. [PMC free article] [PubMed] [Google Scholar]
- 18.Quiat D, Olson EN. MicroRNAs in cardiovascular disease: from pathogenesis to prevention and treatment. J Clin Invest 2013; 123:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang JF, Yu ML, Yu G et al. . Serum miR-146a and miR-223 as potential new biomarkers for sepsis. Biochem Biophys Res Commun 2010; 394:184–8. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Zhang P, Chen W, Feng D, Jia Y, Xie LX. Evidence for serum miR-15a and miR-16 levels as biomarkers that distinguish sepsis from systemic inflammatory response syndrome in human subjects. Clin Chem Lab Med 2012; 50:1423–8. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Zhang P, Chen W, Feng D, Jia Y, Xie L. Serum microRNA signatures identified by Solexa sequencing predict sepsis patients’ mortality: a prospective observational study. PLoS One 2012; 7:e38885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cimmino A, Calin GA, Fabbri M et al. . miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A 2005; 102:13944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo CJ, Pan Q, Li DG, Sun H, Liu BW. miR-15b and miR-16 are implicated in activation of the rat hepatic stellate cell: an essential role for apoptosis. J Hepatol 2009; 50:766–78. [DOI] [PubMed] [Google Scholar]
- 24.Chen YQ, Wang XX, Yao XM et al. . MicroRNA-195 promotes apoptosis in mouse podocytes via enhanced caspase activity driven by BCL2 insufficiency. Am J Nephrol 2011; 34:549–59. [DOI] [PubMed] [Google Scholar]
- 25.Zhu H, Yang Y, Wang Y, Li J, Schiller PW, Peng T. MicroRNA-195 promotes palmitate-induced apoptosis in cardiomyocytes by down-regulating Sirt1. Cardiovasc Res 2011; 92:75–84. [DOI] [PubMed] [Google Scholar]
- 26.Guo ST, Jiang CC, Wang GP et al. . MicroRNA-497 targets insulin-like growth factor 1 receptor and has a tumour suppressive role in human colorectal cancer. Oncogene 2013; 32:1910–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng D, Ma J, Yu Y et al. . Silencing of miR-195 reduces diabetic cardiomyopathy in C57BL/6 mice. Diabetologia 2015; 58:1949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hotchkiss RS, Swanson PE, Knudson CM et al. . Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol 1999; 162:4148–56. [PubMed] [Google Scholar]
- 29.Gao R, Ma Z, Hu Y, Chen J, Shetty S, Fu J. Sirt1 restrains lung inflammasome activation in a murine model of sepsis. Am J Physiol Lung Cell Mol Physiol 2015; 308:L847–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bachmann M, Moroy T. The serine/threonine kinase Pim-1. Int J Biochem Cell Biol 2005; 37:726–30. [DOI] [PubMed] [Google Scholar]
- 31.Siddiqi S, Sussman MA. Cell and gene therapy for severe heart failure patients: the time and place for Pim-1 kinase. Expert Rev Cardiovasc Ther 2013; 11:949–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tyml K, Li F, Wilson JX. Septic impairment of capillary blood flow requires nicotinamide adenine dinucleotide phosphate oxidase but not nitric oxide synthase and is rapidly reversed by ascorbate through an endothelial nitric oxide synthase-dependent mechanism. Crit Care Med 2008; 36:2355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green TP, Johnson DE, Marchessault RP, Gatto CW. Transvascular flux and tissue accrual of Evans blue: effects of endotoxin and histamine. J Lab Clin Med 1988; 111:173–83. [PubMed] [Google Scholar]
- 34.Pierce AC, Jacobs M, Stuver-Moody C. Docking study yields four novel inhibitors of the protooncogene Pim-1 kinase. J Med Chem 2008; 51:1972–5. [DOI] [PubMed] [Google Scholar]
- 35.Shen E, Li Y, Shan L et al. . Rac1 is required for cardiomyocyte apoptosis during hyperglycemia. Diabetes 2009; 58:2386–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rui T, Feng Q, Lei M et al. . Erythropoietin prevents the acute myocardial inflammatory response induced by ischemia/reperfusion via induction of AP-1. Cardiovasc Res 2005; 65:719–27. [DOI] [PubMed] [Google Scholar]
- 37.Peng T, Lu X, Lei M, Moe GW, Feng Q. Inhibition of p38 MAPK decreases myocardial TNF-alpha expression and improves myocardial function and survival in endotoxemia. Cardiovasc Res 2003; 59:893–900. [DOI] [PubMed] [Google Scholar]
- 38.Wu SC, Yang JC, Rau CS et al. . Profiling circulating microRNA expression in experimental sepsis using cecal ligation and puncture. PLoS One 2013; 8:e77936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y, Jiang H, Gu J, Tang Y, Shen N, Jin Y. MicroRNA-195 targets ADP-ribosylation factor-like protein 2 to induce apoptosis in human embryonic stem cell-derived neural progenitor cells. Cell Death Dis 2013; 4:e695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hotchkiss RS, Swanson PE, Freeman BD et al. . Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med 1999; 27:1230–51. [DOI] [PubMed] [Google Scholar]
- 41.Hotchkiss RS, Tinsley KW, Swanson PE et al. . Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol 2001; 166:6952–63. [DOI] [PubMed] [Google Scholar]
- 42.Zhou F, Yang Y, Xing D. Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis. FEBS J 2011; 278:403–13. [DOI] [PubMed] [Google Scholar]
- 43.Iwata A, de Claro RA, Morgan-Stevenson VL et al. . Extracellular administration of BCL2 protein reduces apoptosis and improves survival in a murine model of sepsis. PLoS One 2011; 6:e14729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev 2006; 20:2913–21. [DOI] [PubMed] [Google Scholar]
- 45.Liu TF, Yoza BK, El Gazzar M, Vachharajani VT, McCall CE. NAD+-dependent SIRT1 deacetylase participates in epigenetic reprogramming during endotoxin tolerance. J Biol Chem 2011; 286:9856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li T, Zhang J, Feng J et al. . Resveratrol reduces acute lung injury in a LPS induced sepsis mouse model via activation of Sirt1. Mol Med Rep 2013; 7:1889–95. [DOI] [PubMed] [Google Scholar]
- 47.Xu W, Lu Y, Yao J et al. . Novel role of resveratrol: suppression of high-mobility group protein box 1 nucleocytoplasmic translocation by the upregulation of sirtuin 1 in sepsis-induced liver injury. Shock 2014; 42:440–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.