Abstract
Background. Cocolonization by Streptococcus pneumoniae and Haemophilus influenzae among children has been noted in numerous studies, as has an inverse relationship involving colonization with these species and Staphylococcus aureus. Interactions among these pathogens could mediate unanticipated outcomes of clinical interventions, including changes in H. influenzae and S. aureus disease incidence following pneumococcal vaccine introduction. However, it remains unclear whether cocolonization patterns represent true interspecies interactions or whether they result from confounding factors.
Methods. We investigated polymicrobial carriage using longitudinal data from 369 Bedouin children and 400 Jewish children in Israel who were enrolled in a 7-valent pneumococcal conjugate vaccine (PCV7) trial. Children were swabbed 10 times between 2 and 30 months of age.
Results. The pathogens followed distinct age and seasonal distributions, but polymicrobial carriage associations persisted after controlling for these and other confounding factors. Receipt of PCV7 resulted in pneumococcal serotype replacement but did not influence total carriage of S. pneumoniae, H. influenzae, or S. aureus.
Conclusions. The fact that S. pneumoniae, H. influenzae, and S. aureus polymicrobial carriage patterns do not result from confounding by age and season supports the idea of active interspecies interactions. However, pneumococcal serotype replacement may prevent changes in H. influenzae and S. aureus carriage among PCV7 recipients.
Keywords: Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, pneumococcal conjugate vaccine, serotype replacement
Upper respiratory tract colonization is the source of transmission of Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus and precedes disease. S. pneumoniae and H. influenzae are more likely to cocolonize with one another than independently, suggesting these species have a cooperative relationship; in contrast, colonization with these species is associated with lower prevalence of S. aureus [1]. Negative associations between carriage of S. pneumoniae and S. aureus led to concerns that pneumococcal conjugate vaccine (PCV) could have unintended consequences, such as increasing carriage or disease caused by S. aureus [1–3]. However, it remains unclear whether observed patterns in cocolonization result from true interspecies interactions or confounding epidemiological risk factors.
Differences in the ages when children typically carry these pathogens may contribute to confounding [4]. Colonization by S. aureus is most frequent among neonates and declines in prevalence following the first weeks of life, increasing again as children approach school age [5–8]. In contrast, S. pneumoniae and H. influenzae colonization are most prevalent during later infancy [9, 10]. Another potential confounder, seasonality of carriage, has been minimally assessed in studies reporting cocolonization [1, 5, 11–14]. S. pneumoniae and H. influenzae are carried most often during the winter months, when the incidence of community-acquired S. aureus infections is typically lowest [9, 15]. Little is known regarding the seasonality of S. aureus colonization [16]. Last, most previous studies reporting associations involving S. aureus relied on nasopharyngeal isolates, whereas the anterior nares are the dominant anatomical niche for staphylococcal carriage [17].
Several epidemiological markers would indicate whether reported carriage associations result from interspecies interactions or confounding. First, polymicrobial carriage patterns should persist after adjustment for age, season, and other exposures expected to influence carriage. Earlier-life reductions in S. aureus carriage among populations acquiring S. pneumoniae and H. influenzae at younger ages would suggest that competitive interactions explain inverse associations between carriage of these pathogens and S. aureus [5, 18]. Additionally, differences in H. influenzae and S. aureus carriage among PCV recipients and nonrecipients would suggest that S. pneumoniae serotypes contained in PCV influence acquisition or clearance of the other species [1].
Comparative data from Jewish and Bedouin populations in southern Israel provide a unique opportunity to investigate these markers for interspecies interactions. Although they have access to the same healthcare services, Jewish and Bedouin children are socioeconomically distinct and have limited contact, living in predominantly urban areas and in scattered rural townships, respectively [19]. Geographic and climatic exposures are shared by the 2 groups and cannot account for differences in carriage rates or seasonality. While Bedouin children acquire S. pneumoniae earlier in life and carry pneumococci more frequently than nearby Jewish children [20], less is known about differences in H. influenzae and S. aureus carriage between the 2 populations. We used data from a randomized controlled trial of 7-valent PCV (PCV7) conducted among Jewish and Bedouin children to investigate S. pneumoniae, H. influenzae, and S. aureus cocolonization and risk factors, together with interspecies vaccine effects.
METHODS
Study Population and Design
The study design and pneumococcal and staphylococcal carriage data have been reported previously [21, 22]. The study received ethical approval from the Soroka University Medical Center, Maccabi Health Services, and the Israeli Ministry of Health. Participants were healthy Jewish and Bedouin children from southern Israel enrolled at public-sector mother-and-child primary healthcare centers. The Bedouin population is transitioning from a nomadic lifestyle to permanent settlements and tends to have lower socioeconomic status, larger family sizes, and higher rates of overcrowding relative to Jews in the region [19].
Jewish (n = 400) and Bedouin (n = 369) children were randomized to one of 4 study arms receiving PCV7 (containing serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F) with differing dosing schedules (Supplementary Table 1). Of the total study population, 382 Jewish and 351 Bedouin children were recruited at 2 months of age and received either a 3-dose primary series (at ages 2, 4, and 6 months), with or without a booster dose at 12 months; a 2-dose primary series (at ages 4 and 6 months), with a booster at 12 months; or a booster-only series with doses at ages 12 and 18 months. The remaining 18 Jewish and 18 Bedouin children were recruited at 18 months of age and given a single booster dose. Nasopharyngeal, oropharyngeal, and nasal swabs were collected to detect bacterial carriage at 10 scheduled visits over the first 30 months of life (ages 2, 4, 6, 7, 12, 13, 18, 19, 24, and 30 months). Of 7474 scheduled visits, 6909 (92.4%) were completed, and swabs were analyzed for 6764 (90.5%). Enrollment continued from August 2005 to April 2007, with the last follow-up occurring in March 2009, prior to the addition of PCV7 to the Israeli National Immunization Plan (July 2009). Children received all other routine immunizations according to standard schedules [21].
Laboratory Procedures
Procedures for swabbing, culturing, and pneumococcal serotyping have been reported previously [21, 22]. Carriage was defined by a positive oropharyngeal or nasopharyngeal swab for S. pneumoniae and H. influenzae, and by a positive nasal swab for S. aureus. Antimicrobial susceptibility for all 3 pathogens was assessed by broth dilution and disk diffusion according to US National Committee for Clinical Laboratory Standards protocols [23].
Statistical Analysis
We calculated the prevalence of S. pneumoniae (overall and for PCV7 and non-PCV7 serotypes separately), S. aureus, and H. influenzae carriage among children, stratifying by ethnicity (Jewish or Bedouin), study arm, vaccine doses received, age, and calendar week. We computed 95% confidence intervals (CIs) for prevalence estimates using a cluster bootstrap of individuals, thereby adjusting variance estimates to account for children's repeated sampling [24].
We constructed 3 logistic regression models, with each of S. pneumoniae, H. influenzae, or S. aureus carriage as the outcome, to test whether interspecies associations in carriage of each pathogen persisted after accounting for potential confounders. The models included carriage of each of the 2 other pathogens, vaccine receipt (described below), age (log transformed), sex, ethnicity, antibiotic receipt in the prior month, number of children at the home or day care where participants spent most days (log transformed), and seasonal pattern (described below). We accounted for distinct seasonal and age patterns in Jewish and Bedouin children using interaction terms. We constructed 2 additional models examining these factors as predictors for PCV7-serotype and non–vaccine-serotype S. pneumoniae carriage. We controlled for age as a continuous variable. This was necessary because there would be complete separation between discrete age categories and number of vaccine doses under the trial design. As with the descriptive analyses, we fitted models on cluster-bootstrapped replicates to account for serial observations of individuals when estimating 95% CIs surrounding adjusted odds ratios (aORs).
Seasonality was accounted for using sine and cosine transformations of the sampling date with periods of 4, 6, and 12 months, allowing for an asymmetrically shaped seasonal curve. We also considered whether harmonics with a 3-month period should be included in the seasonal curve, and whether age should be modeled with a linear, quadratic, or cubic transformation. We compared these alternatives using the Bayesian information criterion and found that they did not improve model fit for S. pneumoniae, H. influenzae, or S. aureus. We accounted for differences in the age and seasonal patterns of carriage for Jewish and Bedouin children using interaction terms. We estimated vaccine effects as the aOR for carriage associated with having received any doses of PCV7. We also defined a second model for each pathogen, in which we estimated aORs associated with receiving 1, 2, or ≥3 doses (relative to 0 doses), controlling again for the previously listed confounding exposures. Analyses were implemented in R [25].
RESULTS
Population Characteristics
Study enrollment is summarized in Table 1 [21, 22]. Bedouin children inhabited households with an average (±SD) of 3.5 ± 1.3 persons per bedroom, compared with 2.7 ± 0.6 persons per bedroom in Jewish households (Table 1). While Bedouin children were less likely than Jewish children to attend day care (17% vs 79%), Bedouin children were on average (±SD) exposed to more children during the day (3.5 ± 2.3) than their Jewish counterparts (2.2 ± 1.1).
Table 1.
Description of the Study Population
| Characteristic | Bedouin (n = 369) | Jewish (n = 400) |
|---|---|---|
| Male sex | 195 (53) | 214 (54) |
| Day care attendance | 61 (17) | 316 (79) |
| Antibiotic use within 1 mo of any visit | 245 (66) | 211 (53) |
| Gestational age, wks | 39.4±1.6 | 39.3±1.5 |
| Persons per bedroom in household, no. | 3.5±1.3 | 2.7±0.6 |
| Children at place where child spends the day, no. | 3.5±2.3 | 2.2±1.1 |
| Visits completed, no. | 3308 | 3601 |
| Swabs analyzed/swabs collected (%)a | 3217/3308 (97) | 3547/3601 (99) |
| S. pneumoniae–positive swabs/ swabs analyzed (%)a,b,c | 2292/3217 (71) | 1409/3547 (40) |
| PCV7 serotype–positive swabs | 724 (32) | 438 (31) |
| Oropharyngeal carriage | 29 (1) | 26 (2) |
| Polymicrobial carriage | 1725 (75) | 950 (67) |
| H. influenzae cocolonization | 1628 (71) | 847 (60) |
| S. aureus cocolonization | 302 (13) | 201 (14) |
| Dual cocolonization | 205 (9) | 98 (7) |
| H. influenzae–positive swabs/swabs analyzed (%)d | 2081/3217 (65) | 1485/3547 (42) |
| Beta-lactamase positive | 386 (19) | 225 (15) |
| Unencapsulated types | 1933 (93) | 1430 (96) |
| Type B | 16 (1) | 14 (1) |
| Oropharyngeal carriage only | 277 (13) | 287 (19) |
| Nasopharyngeal carriage only | 67 (3) | 49 (3) |
| Polymicrobial carriage | 1687 (81) | 928 (62) |
| S. pneumoniae cocolonization | 1628 (78) | 847 (57) |
| S. aureus cocolonization | 264 (13) | 179 (12) |
| Dual cocolonization | 205 (10) | 98 (7) |
| S. aureus–positive swabs/swabs analyzed (%)e | 449/3308 (14) | 642/3547 (18) |
| Methicillin resistant | 87 (19) | 10 (2) |
| Polymicrobial carriage | 361 (80) | 282 (44) |
| S. pneumoniae cocolonization | 302 (67) | 201 (31) |
| H. influenzae cocolonization | 264 (59) | 179 (28) |
| Dual cocolonization | 205 (46) | 98 (15) |
Data are no. of participants (%) or mean±SD, unless otherwise indicated.
Abbreviations: H. influenzae, Haemophilus influenzae; PCV7, 7-valent pneumococcal conjugate vaccine; S. aureus, Staphylococcus aureus; S. pneumoniae, Streptococcus pneumoniae.
a Further details about antimicrobial susceptibility are presented in Supplementary Table 3.
b Serotype-specific frequencies are presented in Supplementary Table 2.
c The numbers of S. pneumoniae–positive swabs in each group are used as the denominators for the remaining characteristics subsumed under this row.
d The numbers of H. influenzae–positive swabs in each group are used as the denominators for the remaining characteristics subsumed under this row.
e The numbers of S. aureus–positive swabs in each group are used as the denominators for the remaining characteristics subsumed under this row.
Prevalence and Characteristics of S. pneumoniae, H. influenzae, and S. aureus Carriage
Jewish children carried S. pneumoniae at 40% of visits (95% CI, 37%–42%), in contrast to 71% of visits (95% CI, 69%–73%) by Bedouin children (Table 1). H. influenzae carriage occurred at 42% (95% CI, 39%–44%) and 65% (95% CI, 62%–67%) of visits by Jewish and Bedouin children, respectively. In contrast, S. aureus was significantly more prevalent among Jewish children (18% [95% CI, 16%–20%]) than Bedouin children (14% [95% CI, 12%–16%]). PCV7 serotypes represented an equivalent proportion (31%) of overall S. pneumoniae carriage in the 2 populations (Table 1; serotype frequencies are in Supplementary Table 2). Overall, 60% of pneumococcal isolates exhibited reduced susceptibility to ≥1 antibiotic [26, 27]. Resistance was more prevalent among Bedouin children than among Jewish children and among PCV7 serotypes than among non-PCV7 serotypes. Detailed antibiotic resistance data for the pneumococcal isolates are presented in Supplementary Table 3.
Over 90% of H. influenzae isolates were nontypeable in both populations, and <1% of isolates were the vaccine-targeted serotype b; 17% were β-lactamase positive. Nine percent of staphylococcal isolates were methicillin resistant (Table 1).
Variation in Carriage by Age and Season
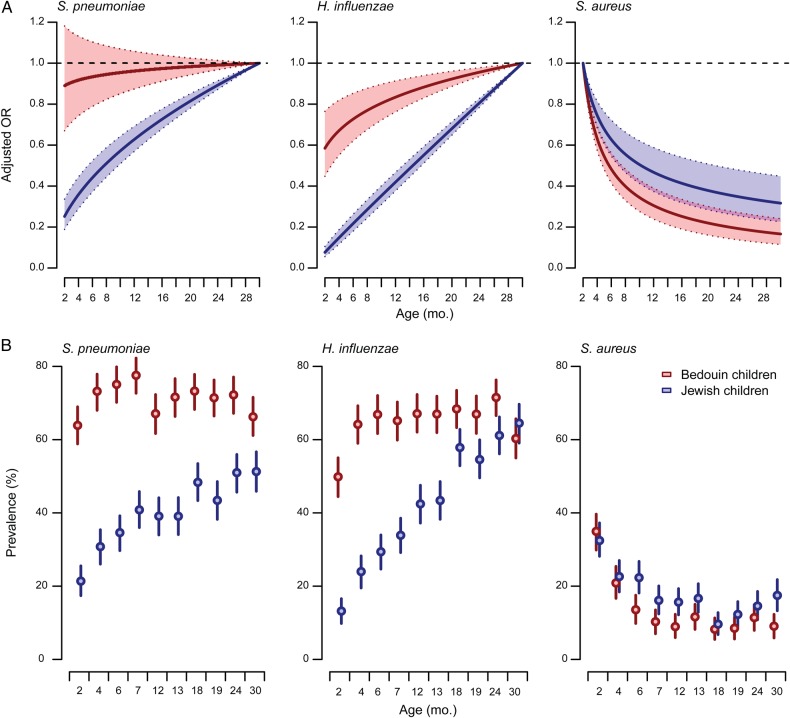
In addition to carrying these pathogens at higher overall rates, Bedouin children acquired S. pneumoniae and H. influenzae earlier than Jewish children. At 2 months of age, the prevalence of S. pneumoniae and H. influenzae was 64% (95% CI, 59%–69%) and 50% (95% CI, 44%–55%) among Bedouin children, respectively, in contrast to 21% (95% CI, 17%–26%) and 13% (95% CI, 10%–17%) among Jewish children (Figure 1). PCV7 receipt was not associated with a lower odds of carrying S. pneumoniae and did not alter the age distribution of carriage for this pathogen (data are presented below). Earlier S. pneumoniae and H. influenzae acquisition among Bedouin children corresponded to earlier decreases in S. aureus carriage. Whereas an equal proportion of Jewish children (32% [95% CI, 28%–37%]) and Bedouin children (35% [95% CI, 30%–40%]) carried S. aureus at age 2 months, for subsequent increases in age (log scale), Bedouin children experienced 25% greater reductions (95% CI, 10%–38%) in the adjusted odds of carrying S. aureus, compared with Jewish children (Table 2). By 30 months of age, the prevalence of S. aureus carriage was 53% lower (95% CI, 23%–72%) among Bedouin children, compared with Jewish children (aOR 0.55 [95% CI, 0.40, 0.77]).
Figure 1.
Variation in Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus colonization, by age, among Jewish and Bedouin children. Top, Plots illustrate variation in carriage odds by age within each ethnic group, with age at which carriage odds were predicted to be highest as a reference (30 months, S. pneumoniae and H. influenzae; 2 months, S. aureus). We identified that a logarithmic trend in carriage odds across ages provided the optimal fit to data (in comparison to linear and quadratic and cubic polynomial trends) via the Bayesian information criterion. Odds ratios (ORs) are estimated via cluster-bootstrapped logistic regression adjusting for seasonal trend, antibiotic receipt, child contacts, and ethnicity. Shaded regions delineate 95% confidence intervals (CIs). Bottom, Plots illustrate prevalence of carriage for each pathogen by study visit age. Error bars delineate 95% CIs.
Table 2.
Factors Associated With Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus Carriage
| Factor |
S. pneumoniae |
H. influenzae |
S. aureus |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | aOR (95% CI)a,b | OR (95% CI) | aOR (95% CI)a,b | OR (95% CI) | aOR (95% CI)a,b | |
| Ethnicity | ||||||
| Bedouin (reference) | … | … | … | … | … | … |
| Jewish | 0.27 (.23–.31) | 0.14 (.10–.21) | 0.39 (.34–.45) | 0.11 (.07–.17) | 1.36 (1.13–1.65) | 0.64 (.41–.98) |
| Age (per log mo)c | ||||||
| Bedouin | 1.02 (.92–1.12) | 0.98 (.88–1.09) | 1.22 (1.11–1.34) | 1.21 (1.07–1.37) | 0.52 (.45–.59) | 0.56 (.48–.66) |
| Jewish | 1.58 (1.43–1.76) | 1.36 (1.17–1.53) | 2.51 (2.24–2.81) | 2.45 (2.14–2.80) | 0.65 (.58–.74) | 0.75 (.65–.88) |
| Sex | ||||||
| Female (reference) | … | … | … | … | … | … |
| Male | 1.07 (.91–1.27) | 1.13 (.98–1.31) | 1.04 (.89–1.21) | 1.02 (.89–1.18) | 1.26 (1.04–1.53) | 1.26 (1.03–1.54) |
| Child contacts (per log no.) | 1.99 (1.74–2.27) | 1.49 (1.32. 1.70) | 1.93 (1.72–2.16) | 1.55 (1.38–1.74) | 0.83 (.72–.96) | 0.94 (.79–1.10) |
| Recent antibiotic use | 0.76 (.65–.88) | 0.44 (.37–.52) | 1.84 (1.56–2.15) | 1.57 (1.31–1.87) | 0.64 (.51–.80) | 0.80 (.63–1.01) |
| PCV7 receipt (I)b | ||||||
| No doses (reference) | … | … | … | … | … | … |
| Any doses | … | 1.05 (.85–1.30) | … | 0.94 (.77–1.14) | … | 0.83 (.67–1.04) |
| PCV7 receipt (II)b | ||||||
| 0 doses (reference) | … | … | … | … | … | … |
| 1 dose | … | 1.10 (.89–1.37) | … | 0.97 (.79–1.19) | … | 0.84 (.67–1.05) |
| 2 doses | … | 1.07 (.84–1.37) | … | 0.94 (.75–1.18) | … | 0.84 (.65–1.09) |
| ≥3 doses | … | 0.95 (.73–1.23) | … | 0.88 (.69–1.13) | … | 0.81 (.59–1.10) |
| Bacterial cocolonization | ||||||
| S. pneumoniae | … | … | 3.67 (3.26–4.13) | 2.82 (2.50–3.18) | 0.67 (.57–.77) | 0.84 (.71–.98) |
| H. influenzae | 3.67 (3.26–4.13) | 2.80 (2.48–3.15) | … | … | 0.56 (.48–.65) | 0.74 (.63–.87) |
| S. aureus | 0.67 (.57–.77) | 0.83 (.70–.97) | 0.56 (.48–.65) | 0.73 (.62–.86) | … | … |
Three models were fitted, with carriage of S. pneumoniae, H. influenzae, or S. aureus as the outcomes.
Abbreviations: CI, confidence interval; H. influenzae, Haemophilus influenzae; PCV7, 7-valent pneumococcal conjugate vaccine; S. aureus, Staphylococcus aureus; S. pneumoniae, Streptococcus pneumoniae.
a Multivariate analyses additionally control for sine and cosine transformations of calendar week with 4-, 6-, and 12-month periods, stratified by ethnicity (Figure 2).
b Adjusted odds ratios (aORs), calculated by logistic regression, are presented with adjustment for having received any dose of PCV7 (I) and differ by <5% after adjustment for receipt of 1, 2, or ≥3 doses (II).
c Unadjusted odds ratios (ORs), calculated by logistic regression, are estimated from substrata of the data from Bedouin and Jewish children only for this variable.
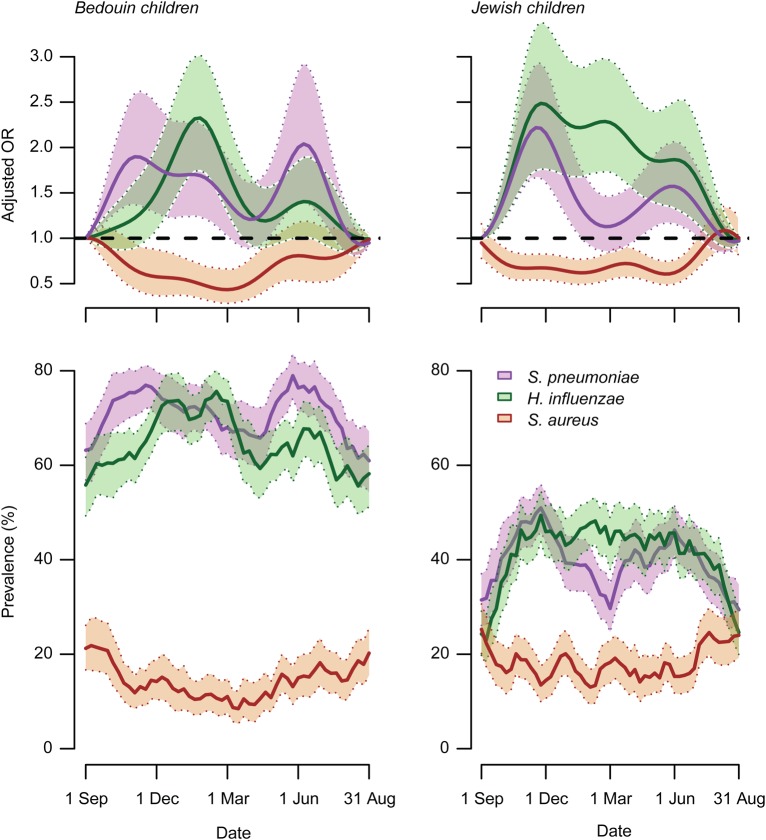
Pathogen carriage rates varied throughout the year (Figure 2). S. pneumoniae carriage peaked biannually in November and June. Biannual patterns persisted after controlling for seasonally varying exposures, such as recent antibiotic use and number of daily child contacts at the time of swabbing, and were observed in both antibiotic-resistant and antibiotic-susceptible pneumococci (Supplementary Figure 1). H. influenzae carriage increased concurrently with S. pneumoniae carriage from September to November in the 2 populations, but H. influenzae showed less of a biannual pattern (Figure 2). The late-summer nadir in S. pneumoniae and H. influenzae carriage coincided with a seasonal peak in S. aureus carriage within both populations (Figure 2). Lower S. aureus carriage was sustained during the autumn-winter peaks in S. pneumoniae and H. influenzae among Bedouin and Jewish children. In contrast to S. pneumoniae, no biannual pattern was noted in S. aureus carriage.
Figure 2.
Seasonal variation in Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus colonization among Bedouin and Jewish children. Top, Plots illustrate variation in carriage odds for each pathogen throughout the calendar year, with carriage during the first week of September as a reference. Seasonal trends are constructed from sine and cosine transformations of calendar week at which sampling occurred, considering 4-, 6-, and 12-month periods of oscillation. Odds ratios (ORs) are estimated via cluster-bootstrapped logistic regression adjusting for age, antibiotic receipt, child contacts, and ethnicity. Shaded regions delineate 95% confidence intervals (CIs). Bottom, Plots illustrate prevalence of carriage for each pathogen, aggregated with a 3-week moving average across calendar weeks. Shaded regions delineate 95% CIs.
Polymicrobial Carriage
If host factors and epidemiological exposures accounted for differences in age and seasonal patterns of S. pneumoniae, H. influenzae, and S. aureus carriage, cocolonization associations would not be expected to persist after adjustment for age, season, and other confounding risk factors. We found instead that interspecies bacterial carriage remained a significant predictor for carriage of each pathogen in multivariate models controlling for age, season, and other exposures (Table 2), suggesting that the patterns of carriage across ages and seasons do not explain characteristic interspecies carriage associations. S. pneumoniae and H. influenzae were each associated with 2.8-fold higher (95% CI, 2.5-fold–3.2-fold) adjusted odds of carrying the other pathogen and with an 0.8-fold lower (95% CI, 0.7-fold–1.0-fold) and 0.7-fold lower (95% CI, 0.6-fold–0.9-fold) adjusted odds of carrying S. aureus, respectively. Associations persisted in analyses stratified by vaccine- or non–vaccine-serotype pneumococcal carriage (Table 3). However, it is likely that our study was underpowered to provide statistically significant estimates for the inverse association with S. aureus in these strata.
Table 3.
Factors Associated With Vaccine-Serotype and Non–Vaccine-Serotype Streptococcus pneumoniae Carriage
| Factor | PCV7 Serotypes |
Non-PCV7 Serotypes |
||
|---|---|---|---|---|
| OR (95% CI) | aOR (95% CI)a,b | OR (95% CI) | aOR (95% CI)a,b | |
| Ethnicity | ||||
| Bedouin (reference) | … | … | … | … |
| Jewish | 0.49 (.41–.59) | 0.27 (.16–.44) | 0.40 (.34–.46) | 0.27 (.17–.42) |
| Age (per log-month)c | ||||
| Bedouin | 0.82 (.73–.92) | 1.01 (.85–1.19) | 1.14 (1.03–1.25) | 1.00 (.88–1.15) |
| Jewish | 1.24 (1.07–1.43) | 1.36 (1.13–1.64) | 1.54 (1.37–1.74) | 1.25 (1.07–1.46) |
| Sex | ||||
| Female (reference) | … | … | … | … |
| Male | 1.22 (1.02–1.47) | 1.20 (1.01–1.44) | 0.96 (.82–1.12) | 1.01 (.88–1.16) |
| Child contacts (per log no.) | 1.31 (1.14–1.52) | 1.05 (.91–1.21) | 1.72 (1.53–1.94) | 1.40 (1.24–1.57) |
| Recent antibiotic used | 1.27 (1.04–1.54) | 1.04 (.86–1.26) | 0.63 (.53–.74) | 0.44 (.37–.53) |
| PCV7 receipt (I)b | ||||
| No doses (reference) | … | … | … | … |
| Any doses | … | 0.78 (.61–1.00) | … | 1.23 (.99–1.53) |
| PCV7 receipt (II)b | ||||
| 0 doses (reference) | … | … | … | … |
| 1 dose | … | 1.01 (.79–1.28) | … | 1.07 (.86–1.35) |
| 2 doses | … | 0.73 (.55–.98) | … | 1.32 (1.03–1.70) |
| ≥3 doses | … | 0.54 (.40–.74) | … | 1.41 (1.09–1.83) |
| Bacterial cocolonization | ||||
| H. influenzae | 2.34 (2.01–2.73) | 1.99 (1.69–2.35) | 2.37 (2.11–2.66) | 0.88 (.75–1.03) |
| S. aureus | 0.80 (.65–.99) | 0.90 (.73–1.12) | 0.73 (.63–.84) | 1.83 (1.63–2.06) |
Three models were fitted, with carriage of S. pneumoniae, H. influenzae, or S. aureus as the outcomes.
Abbreviations: CI, confidence interval; H. influenzae, Haemophilus influenzae; PCV7, 7-valent pneumococcal conjugate vaccine; S. aureus, Staphylococcus aureus; S. pneumoniae, Streptococcus pneumoniae.
a Multivariate analyses additionally control for sine and cosine transformations of calendar week, with 4-, 6-, and 12-month periods, stratified by ethnicity (Figure 2).
b Adjusted odds ratios (aORs), calculated by logistic regression, control for 1, 2, and ≥3 doses of PCV7 received (I) and differ by <5% after controlling for receipt of any PCV7 dose (II).
c Unadjusted odds ratios (ORs), calculated by logistic regression, are estimated from substrata of the data from Bedouin and Jewish children only for this variable.
d Differences in antimicrobial susceptibility profiles of PCV7 serotypes and non-PCV7 serotypes are reported in Supplementary Table 3.
Pneumococcal Vaccine Effects on S. pneumoniae, H. influenzae, and S. aureus Carriage
Children who had received PCV7 had lower odds of carrying vaccine-serotype pneumococci, compared with children who had not received PCV7 (aOR, 0.8 [95% CI, 0.6–1.0]; Table 3 and Figure 3). In a separate model accounting for PCV7 doses received, incrementally lower odds of vaccine-serotype carriage were noted among children who received 2 doses (aOR, 0.7 [95% CI, 0.5–1.0]) and ≥3 doses (aOR 0.5 [95% CI, 0.4–0.7]), compared with unvaccinated children. Children who received PCV7 also tended to have higher odds of carrying nonvaccine pneumococcal serotypes, compared with children who did not receive PCV7 (aOR, 1.2 [95% CI, 1.0–1.5]). We noted incrementally higher odds of non–vaccine-serotype carriage after receiving 2 doses (aOR, 1.3 [95% CI, 1.0–1.7]) and ≥3 doses (aOR, 1.4 [95% CI, 1.1–1.8]). As a result of this replacement in carried pneumococcal serotypes, PCV7 receipt was not associated with any net change in children's odds for carrying S. pneumoniae, nor did we identify associations between vaccination and H. influenzae or S. aureus carriage (Table 3). Similarly, differences were seen primarily in vaccine-serotype and non–vaccine-serotype pneumococcal carriage when comparing the prevalence of each pathogen across the study arms within each age and ethnic group (Supplementary Table 4).
Figure 3.
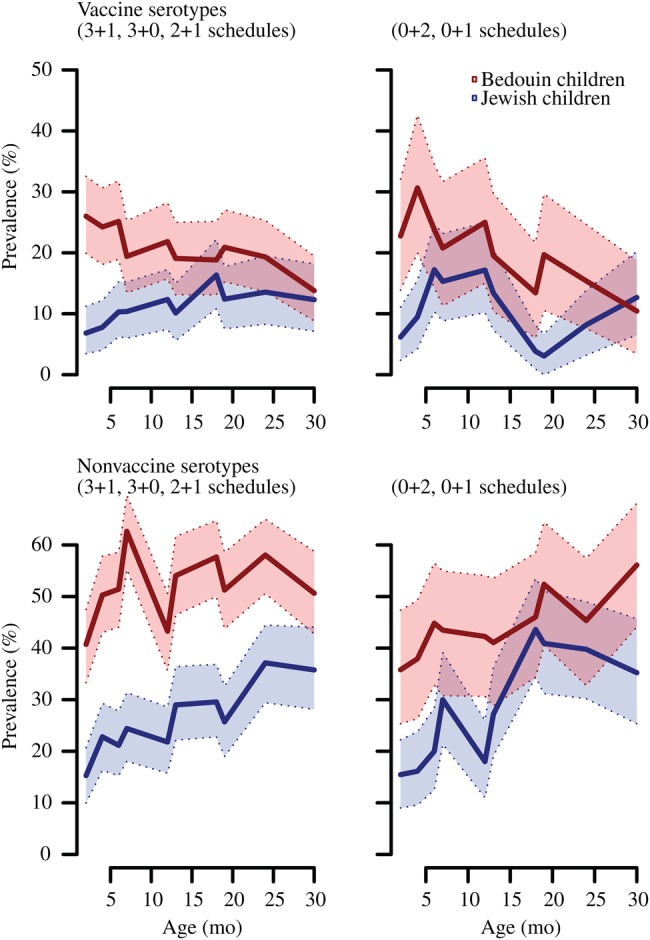
Vaccine and non–vaccine-serotype Streptococcus pneumoniae carriage among Bedouin and Jewish children. Plots illustrate prevalence of vaccine-serotype (top) and non–vaccine-serotype (bottom) carriage across study visit ages. Children are aggregated across study arms with primary-series schedules (left, “3 + 1” and “3 + 0” for 3-dose primary series at 2, 4, and 6 months with or without booster at 12 months; and “2 + 1” for 2-dose primary series at 4 and 6 months with booster at 12 months) and delayed-dosing schedules (right, “0 + 2” for doses at 12 and 18 months and “0 + 1” for 1 dose at 18 months). Shaded areas delineate 95% confidence intervals estimated by a cluster bootstrap of individuals.
DISCUSSION
Cross-sectional studies in diverse settings have identified positive correlations in carriage of S. pneumoniae and H. influenzae among children, in contrast to inverse correlations between these pathogens and S. aureus [1]. These findings raised concern that using PCVs to prevent certain S. pneumoniae serotypes from colonizing the nasopharynx could have unforeseen consequences. Comparative longitudinal data on bacterial carriage among Bedouin and Jewish children in southern Israel allowed us to investigate whether (1) polymicrobial carriage patterns are attributable to the confounding influences of age and season and (2) PCV7 causes interspecies replacement in bacterial carriage. We noted similarities in age and seasonal patterns of S. pneumoniae and H. influenzae carriage, contrasting starkly with those for S. aureus carriage. However, interspecies correlations persisted in our analysis after accounting for these covarying factors. Because nonvaccine serotypes offset reductions in PCV7 serotype carriage among vaccine recipients, immunization did not influence individuals' odds for carrying S. pneumoniae. Because H. influenzae and S. aureus carriage did not differ according to vaccine receipt, factors mediating S. pneumoniae associations with these pathogens may not relate specifically to vaccine-targeted pneumococcal serotypes [3, 11].
Differences in age distributions of S. pneumoniae, H. influenzae, and S. aureus between Bedouin and Jewish populations indicate that epidemiological exposures, rather than host factors alone, contribute to variation in carriage risk by age. The higher prevalence of S. pneumoniae and H. influenzae in Bedouin communities is of particular importance to transmission dynamics and age of acquisition [28]. In this regard, several observations from our study and others comport with the hypothesis that competition involving S. pneumoniae and H. influenzae influences the population distribution of S. aureus carriage. We found higher S. aureus carriage among Jewish children, in contrast to higher S. pneumoniae and H. influenzae carriage among Bedouin children. Similarly, in the United States, S. aureus colonization is more prevalent among white, non-Hispanic children and adults in comparison to their black or Hispanic counterparts, among whom S. pneumoniae and H. influenzae carriage is more prevalent [10, 29, 30]. We also observed reductions in S. aureus carriage at younger ages among Bedouin children, who carried S. pneumoniae and H. influenzae earlier and more frequently than Jewish children. In Western Australia, earlier acquisition of S. pneumoniae and H. influenzae among Aboriginal infants (compared with non-Aboriginal infants) similarly corresponded to steeper declines in S. aureus carriage during the first 2 years of life, despite equal S. aureus carriage in the 2 populations at 1 month of age [18]. Previous S. pneumoniae and H. influenzae carriage were associated with longer times to S. aureus acquisition among US infants [30]. Beyond carriage patterns over the first 30 months of life described in the present study, decreases in S. pneumoniae and H. influenzae carriage coincide with increases in S. aureus carriage during later childhood and adolescence [5, 17, 31, 32].
S. aureus carriage exhibited a distinct seasonal pattern within both the Bedouin and Jewish populations, peaking in the summer, when S. pneumoniae and H. influenzae carriage were at their lowest. Seasonal patterns in S. aureus carriage outside clinical settings have received little attention [16]. Higher summer incidence of community-acquired S. aureus infections, particularly skin and soft-tissue infections, including impetigo contagiosa, has been attributed to sweating during hot or humid weather, which increases the risk for disease in colonized individuals [15, 16]. We find that this seasonal pattern is also associated with a trend toward higher carriage in the summer, suggesting that transmission may be facilitated by the same hygiene factors predisposing S. aureus carriers to infection during warm weather. In our study, S. pneumoniae carriage peaked biannually, with a secondary low phase in March. This pattern affected antibiotic-susceptible and antibiotic-resistant pneumococcal strains alike, suggesting that midseason declines were not exclusively attributable to antibiotic prescribing.
Previous findings that the inverse association between S. aureus and S. pneumoniae occurs only in human immunodeficiency virus–uninfected children suggest that interspecies interference may be immune mediated [13, 14]. Indeed, nonspecific effectors elicited in response to one bacterium could play a role in clearing other microbiota. We noted significantly lower carriage of S. aureus among Bedouin children first at 6 months of age, although their carriage of S. pneumoniae and H. influenzae was markedly higher even at 2 months of age. This delayed effect of S. pneumoniae or H. influenzae exposure on S. aureus carriage suggests that acquired immune responses resulting from earlier carriage may mediate inverse interspecies associations with S. aureus carriage. For instance, interleukin 17–expressing CD4+ T cells triggered in response to S. pneumoniae and H. influenzae facilitate phagocytic clearance of S. aureus [33–36]. A previous finding that S. aureus acquisition is delayed among infants who had previously carried other bacterial species further suggests acquired immunity as a determinant for polymicrobial carriage patterns [30]. In turn, positive associations between S. pneumoniae and H. influenzae may relate to mutualistic interactions between the species reported in animal models [37, 38].
Several previous studies assessed the effects of PCV on S. aureus and H. influenzae out of concern that immunological pressure from the vaccine would open a niche for these species. Most studies of healthy asymptomatic children have shown no interspecies effect on colonization [1]. However, intermittently higher nasopharyngeal S. aureus carriage was noted in the Netherlands during a prelicensure PCV7 trial [39], and increases in nasopharyngeal S. aureus and H. influenzae carriage coincided with the rollout of routine PCV7 administration in that country [40]. There have also been conflicting reports of an increased risk for S. aureus carriage in the ear canal or nasopharynx during acute otitis media among children who received PCV [41, 42]. In contrast to these studies, we assessed asymptomatic S. aureus colonization in the anterior nares, the dominant anatomical niche for healthy staphylococcal carriage. We found that nonvaccine pneumococcal serotypes replaced those targeted by PCV7, so that we observed no change in total carriage of S. pneumoniae or, in turn, H. influenzae and S. aureus. Serotype replacement in carriage has been observed in numerous settings where PCV has been implemented [1, 43–45] and may suggest that interactions underlying interspecies correlates of S. pneumoniae carriage are not specific to vaccine-targeted serotypes. Other pneumococcal factors, such as the pilus, have been suggested to mediate interspecies associations with S. aureus [46].
Our study has several limitations. By following children only to age 30 months, we did not see how declining S. pneumoniae and H. influenzae prevalence during later childhood related to S. aureus acquisition [5, 17, 31, 32]. Swabbing was too infrequent to identify all carriage episodes, which would have permitted us to investigate the impacts of previous or concurrent colonization on the rates that children acquired or cleared other species [30]. Whereas immunomodulation by viral infections contributes to variation in bacterial density and host susceptibility, we did not investigate how respiratory viruses influenced age and seasonal bacterial carriage patterns [47, 48]. Similarly, we assessed only 3 bacterial pathogens, although the upper respiratory tract microbiome is diverse and interactions with other species may be important determinants of carriage. Last, our analysis did not address exposures reported to influence bacterial carriage, such as breastfeeding and tobacco smoke, which have been found inconsistently to be associated with bacterial carriage [49, 50].
We found that, although S. pneumoniae, H. influenzae, and S. aureus follow distinct age and seasonal carriage patterns among children, characteristic polymicrobial carriage patterns among these pathogens do not owe to confounding by age and season. Differences among Jewish and Bedouin children in the prevalence and age distribution of S. aureus carriage suggest that S. pneumoniae and H. influenzae exposure may impact staphylococcal colonization. PCV7 did not influence H. influenzae and S. aureus carriage, suggesting that bacterial interactions are not specific to pneumococcal serotypes contained in the vaccine. Multiple-species carriage should be monitored in trials of next-generation cellular and protein-based pneumococcal vaccines conferring pan-serotype protection, as interspecies effects may arise when vaccine-driven immunological pressure does not differentially affect pneumococcal serotypes. Clinical implications of polymicrobial carriage for otitis media, pneumonia, and invasive disease risk remain an important research area.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Financial support. This work was supported by Wyeth/Pfizer (grant 0887X-101801 to R. D.); the National Institute of Allergy and Infectious Diseases (R56AI110449 to D. M. W.) and the National Institute on Aging (P30AG021342 to D. M. W., a Scholar at the Claude D. Pepper Older Americans Independence Center at Yale School of Medicine), National Institutes of Health; the Yale Center for Clinical Investigation (ULI TR000142 to D. M. W.); the Bill and Melinda Gates Foundation (OPP1114733 to D. M. W.); and Yale School of Medicine (Wilber Downs Fellowship to J. A. L.).
Potential conflicts of interest. D. M. W. reports receipt of investigator-initiated research funds from Pfizer by Yale University for other studies and has received consulting fees from Merck and Pfizer. R. D. has received grants and research support from Berna/Crucell, Wyeth/Pfizer, Merck, and Protea; has been a scientific consultant for Berna/Crucell, GlaxoSmithKline, Novartis, Wyeth/Pfizer, Merck, and Protea; has been a speaker for Berna/Crucell, GlaxoSmithKline, and Wyeth/Pfizer; and is a shareholder of Protea/NASVAX. G. R.-Y. has received consulting fees from Neopharm and Pfizer. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Shak JR, Vidal JE, Klugman KP. Influence of bacterial interactions on pneumococcal colonization of the nasopharynx. Trends Microbiol 2013; 21:129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol 2009; 7:887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kopterides P, Falagas ME. Potential consequences of the pneumococcal conjugate vaccine. N Engl J Med 2006; 355:95–6. [DOI] [PubMed] [Google Scholar]
- 4.Regev-Yochay G, Bogaert D, Malley RI et al. Does pneumococcal conjugate vaccine influence Staphylococcus aureus carriage in children? Clin Infect Dis 2008; 47:289–90. [DOI] [PubMed] [Google Scholar]
- 5.Bogaert D, Van Belkum A, Sluijter M et al. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet 2004; 363:1871–2. [DOI] [PubMed] [Google Scholar]
- 6.Leshem E, Maayan-Metzger A, Rahav G et al. Transmission of Staphylococcus aureus from mothers to newborns. Pediatr Infect Dis J 2012; 31:360–3. [DOI] [PubMed] [Google Scholar]
- 7.Peacock SJ, Justice A, Griffiths D et al. Determinants of acquisition and carriage of Staphylococcus aureus in infancy. J Clin Microbiol 2003; 41:5718–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebon A, Labout JAM, Verbrugh HA et al. Dynamics and determinants of Staphylococcus aureus carriage in infancy: The generation R study. J Clin Microbiol 2008; 46:3517–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray BM, Turner ME, Dillon HC. Epidemiologic studies of Streptococcus pneumoniae in infants. The effects of season and age on pneumococcal acquisition and carriage in the first 24 months of life. Am J Epidemiol 1982; 116:692–703. [DOI] [PubMed] [Google Scholar]
- 10.Stephenson WP, Doern G, Gantz N, Lipworth L, Chapin K. Pharyngeal carriage rates of Haemophilus influenzae, type b and non-b, and prevalence of ampicillin-resistant Haemophilus influenzae among healthy day-care children in central Massachusetts. Am J Epidemiol 1985; 122:868–75. [DOI] [PubMed] [Google Scholar]
- 11.Regev-Yochay G, Dagan R, Raz M et al. Association between carriage of Streptococcus pneumoniae and Staphylococcus aureus in Children. JAMA 2004; 292:716–20. [DOI] [PubMed] [Google Scholar]
- 12.Chien Y-W, Vidal JE, Grijalva CG et al. Density interactions among Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus in the nasopharynx of young Peruvian children. Pediatr Infect Dis J 2013; 32:72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madhi SA, Adrian P, Kuwanda L, Cutland C, Albrich WC, Klugman KP. Long-term effect of pneumococcal conjugate vaccine on nasopharyngeal colonization by Streptococcus pneumoniae—and associated interactions with Staphylococcus aureus and Haemophilus influenzae colonization—in HIV-Infected and HIV-uninfected children. J Infect Dis 2007; 196:1662–6. [DOI] [PubMed] [Google Scholar]
- 14.McNally LM, Jeena PM, Gajee K et al. Lack of association between the nasopharyngeal carriage of Streptococcus pneumoniae and Staphylococcus aureus in HIV-1-infected South African children. J Infect Dis 2006; 194:385–90. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan SL, Hulten KG, Gonzalez BE et al. Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin Infect Dis 2005; 40:1785–91. [DOI] [PubMed] [Google Scholar]
- 16.Leekha S, Diekema DJ, Perencevich EN. Seasonality of staphylococcal infections. Clin Microbiol Infect 2012; 18:927–33. [DOI] [PubMed] [Google Scholar]
- 17.Wertheim HFL, Melles DC, Vos MC et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 2005; 5:751–62. [DOI] [PubMed] [Google Scholar]
- 18.Watson K, Carville K, Bowman J et al. Upper respiratory tract bacterial carriage in Aboriginal and non-Aboriginal children in a semi-arid area of Western Australia. Pediatr Infect Dis J 2006; 25:782–90. [DOI] [PubMed] [Google Scholar]
- 19.Israeli Central Bureau of Statistics. Society and population. http://www1.cbs.gov.il Accessed 2 November 2015.
- 20.Dagan R, Givon-Lavi N, Greenberg D, Fritzell B, Siegrist CA. Nasopharyngeal carriage of Streptococcus pneumoniae shortly before vaccination with a pneumococcal conjugate vaccine causes serotype-specific hyporesponsiveness in early infancy. J Infect Dis 2010; 201:1570–9. [DOI] [PubMed] [Google Scholar]
- 21.Dagan R, Givon-Lavi N, Porat N, Greenberg D. The effect of an alternative reduced-dose infant schedule and a second year catch-up schedule with 7-valent pneumococcal conjugate vaccine on pneumococcal carriage: a randomized controlled trial. Vaccine 2012; 30:5132–40. [DOI] [PubMed] [Google Scholar]
- 22.Adler A, Givon-Lavi N, Moses AE, Block C, Dagan R. Carriage of community-associated methicillin-resistant Staphylococcus aureus in a cohort of infants in southern Israel: Risk factors and molecular features. J Clin Microbiol 2010; 48:531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 22nd informational supplement. Wayne, PA: National Committee for Clinical Laboratory Standards, 2013.
- 24.Sherman M, le Cessie S. A comparison between bootstrap methods and generalized estimating equations for correlated outcomes in generalized linear models. Commun Stat Simul Comput 1997; 26:901–25. [Google Scholar]
- 25.R Core Team. R: A language and environment for statistical computing. https://www.r-project.org/. Accessed 1 November 2015.
- 26.Song JH, Dagan R, Klugman KP, Fritzell B. The relationship between pneumococcal serotypes and antibiotic resistance. Vaccine 2012; 30:2728–37. [DOI] [PubMed] [Google Scholar]
- 27.Dagan R, Barkai G, Givon-Lavi N et al. Seasonality of antibiotic-resistant Streptococcus pneumoniae that causes acute otitis media: a clue for an antibiotic-restriction policy? J Infect Dis 2008; 197:1094–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson RM, May RM. Infectious diseases of humans: dynamics and control. Oxford, United Kingdom: Oxford Univ Press, 1991. [Google Scholar]
- 29.Kuehnert MJ, Kruszon-Moran D, Hill HA et al. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001–2002. J Infect Dis 2006; 193:172–9. [DOI] [PubMed] [Google Scholar]
- 30.Patel JA, Alvarez-Fernandez P, Jennings K, Loeffelholz M, McCormick D, Chonmaitree T. Factors affecting Staphylococcus aureus colonization of the nasopharynx in the first 6 months of life. Pediatr Infect Dis J 2015; 34:826–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackenzie GA, Leach AJ, Carapetis JR, Fisher J, Morris PS. Epidemiology of nasopharyngeal carriage of respiratory bacterial pathogens in children and adults: cross-sectional surveys in a population with high rates of pneumococcal disease. BMC Infect Dis 2010; 10:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Datta F, Erb T, Heininger U et al. A multicenter, cross-sectional study on the prevalence and risk factors for nasal colonization with Staphylococcus aureus in patients admitted to children's hospitals in Switzerland. Clin Infect Dis 2008; 47:923–6. [DOI] [PubMed] [Google Scholar]
- 33.Reiss-Mandel A, Regev-Yochay G. Staphylococcus aureus and Streptococcus pneumoniae interaction and response to pneumococcal vaccination. Hum Vaccin Immunother 2015; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci U S A 2005; 102:4848–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trzciński K, Thompson CM, Srivastava A, Basset A, Malley R, Lipsitch M. Protection against nasopharyngeal colonization by Streptococcus pneumoniae is mediated by antigen-specific CD4+ T cells. Infect Immun 2008; 76:2678–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin L, Ibrahim AS, Xu X et al. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog 2009; 5:e1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Margolis E, Yates A, Levin BR. The ecology of nasal colonization of Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus: the role of competition and interactions with host's immune response. BMC Microbiol 2010; 10:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weimer KED, Armbruster CE, Juneau RA, Hong W, Pang B, Swords WE. Coinfection with Haemophilus influenzae promotes pneumococcal biofilm formation during experimental otitis media and impedes the progression of pneumococcal disease. J Infect Dis 2010; 202:1068–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Gils EJM, Hak E, Veenhoven RH et al. Effect of seven-valent pneumococcal conjugate vaccine on Staphylococcus aureus colonisation in a randomised controlled trial. PLoS One 2011; 6:e20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spijkerman J, Prevaes SMPJ, van Gils EJM et al. Long-term effects of pneumococcal conjugate vaccine on nasopharyngeal carriage of S. pneumoniae, S. aureus, H. influenzae and M. catarrhalis. PLoS One 2012; 7:e39730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veenhoven R, Bogaert D, Uiterwaal C et al. Effect of conjugate pneumococcal vaccine followed by polysaccharide pneumococcal vaccine on recurrent acute otitis media: A randomised study. Lancet 2003; 361:2189–95. [DOI] [PubMed] [Google Scholar]
- 42.Cohen R, Levy C, Thollot F et al. Pneumococcal conjugate vaccine does not influence Staphylococcus aureus carriage in young children with acute otitis media. Clin Infect Dis 2007; 45:1583–7. [DOI] [PubMed] [Google Scholar]
- 43.Flasche S, van Hoek AJ, Sheasby E et al. Effect of pneumococcal conjugate vaccination on serotype-specific carriage and invasive disease in England: A cross-sectional study. PLoS Med 2011; 8:e1001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang SS, Hinrichsen VL, Stevenson AE et al. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics 2009; 124:e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dagan R. Serotype replacement in perspective. Vaccine 2009; 27:c22–4. [DOI] [PubMed] [Google Scholar]
- 46.Regev-Yochay G, Lipsitch M, Basset A et al. The pneumococcal pilus predicts the absence of Staphylococcus aureus co-colonization in pneumococcal carriers. Clin Infect Dis 2009; 48:760–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weinberger DM, Grant LR, Steiner CA et al. Seasonal drivers of pneumococcal disease incidence: Impact of bacterial carriage and viral activity. Clin Infect Dis 2014; 58:188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walter ND, Taylor TH, Shay DK et al. Influenza circulation and the burden of invasive pneumococcal pneumonia during a non-pandemic period in the United States. Clin Infect Dis 2010; 50:175–83. [DOI] [PubMed] [Google Scholar]
- 49.Greenberg D, Givon-Lavi N, Broides A, Blancovich I, Peled N, Dagan R. The contribution of smoking and exposure to tobacco smoke to Streptococcus pneumoniae and Haemophilus influenzae carriage in children and their mothers. Clin Infect Dis 2006; 42:897–903. [DOI] [PubMed] [Google Scholar]
- 50.Duffy LC, Faden H, Wasielewski R, Wolf J, Krystofik D. Exclusive breastfeeding protects against bacterial colonization and day care exposure to otitis media. Pediatrics 1997; 100:e7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.