Abstract
Carbapenem-resistant Klebsiella pneumoniae strains classified as multilocus sequence type 258 (ST258) are among the most widespread multidrug-resistant hospital-acquired pathogens. Treatment of infections caused by these organisms is difficult, and mortality is high. The basis for the success of ST258, outside of antibiotic resistance, remains incompletely determined. Here we tested the hypothesis that ST258 K. pneumoniae has enhanced capacity to circumvent killing by human neutrophils, the primary cellular defense against bacterial infections. There was limited binding and uptake of ST258 by human neutrophils, and correspondingly, there was limited killing of bacteria. On the other hand, transmission electron microscopy revealed that any ingested organisms were degraded readily within neutrophil phagosomes, thus indicating that survival in the neutrophil assays is due to limited phagocytosis, rather than to microbicide resistance after uptake. Our findings suggest that enhancing neutrophil phagocytosis is a potential therapeutic approach for treatment of infection caused by carbapenem-resistant ST258 K. pneumoniae.
Keywords: Klebsiella pneumoniae, neutrophil, phagocytosis
Antibiotic resistance is a major problem for treatment of healthcare infections worldwide. Staphylococcus aureus and Klebsiella pneumoniae are among the most prevalent bacterial pathogens isolated from healthcare-associated infections in the United States [1], and many of these organisms are resistant to multiple antibiotics. Notably, carbapenem-resistant K. pneumoniae isolates classified as multilocus sequence type 258 (ST258) are resistant to all β-lactam antibiotics and most other antibiotics used historically for treatment of K. pneumoniae infections (eg, aminoglycoside and fluoroquinolone antibiotics) [2–5]. Thus, mortality for invasive infections caused by carbapenem-resistant K. pneumoniae is relatively high (approximately 30%–70%), although these infections occur primarily in individuals with significant comorbidities [5–8]. ST258 is the most widespread lineage of carbapenem-resistant K. pneumoniae [3, 4, 9–11], but the molecular basis for success of this lineage, outside of antibiotic resistance, remains incompletely determined.
Neutrophils (or polymorphonuclear leukocytes [PMNs]) are the most prominent cellular component of the innate immune response and are essential for defense against bacterial infections [12]. Indeed, neutrophils have long been known to be important for defense against K. pneumoniae in animal infection models in vivo [13–15], and humans with neutropenia are susceptible to severe or fatal infections caused by this pathogen [16, 17]. Myeloperoxidase and neutrophil elastase have been shown to be critical for killing of K. pneumoniae by neutrophils [18, 19]. On the other hand, previous studies have shown that K. pneumoniae capsule polysaccharide (CPS) and lipopolysaccharide promote resistance to neutrophil phagocytosis in vitro and/or killing by serum complement [20].
Although progress has been made, our understanding of the interaction of neutrophils with ST258 K. pneumoniae is incomplete. More specifically, it is not known whether these organisms are ingested and killed readily by neutrophils. To address this deficiency in knowledge, we investigated phagocytosis and killing of the epidemic carbapenem-resistant ST258 K. pneumoniae strain by human neutrophils.
MATERIALS AND METHODS
Ethics Statement
All studies with human blood and PMNs were performed in accordance with a protocol approved by the Institutional Review Board for Human Subjects, National Institute of Allergy and Infectious Diseases. All subjects gave written informed consent to participate in the study.
Bacterial Strains and Culture
We selected 6 K. pneumoniae clinical isolates for these studies based upon known multilocus sequence type, date of isolation, and antibiotic resistance profile (Table 1). K. pneumoniae were grown in Luria-Bertani (LB) broth. Working bacterial cultures were inoculated from overnight cultures (1:200 dilution), incubated at 37°C with shaking (220 rpm), and bacteria were harvested at mid exponential growth phase (OD600 = 0.75) for use in PMN assays. Staphylococcus aureus strain LAC (pulsed-field type USA300) was grown in tryptic soy broth containing 0.5% glucose as described elsewhere [21].
Table 1.
Characteristics of Klebsiella pneumoniae Clinical Isolates
| Strain ID, Name | MLST | Location | Year | Capsule Type | KPC Type |
|---|---|---|---|---|---|
| 34826 | 258 | PA | 2011 | cps-1 | None |
| 13043 | 258 | NY | 2004 | cps-2 | None |
| 34446 | 258 | PA | 2010 | cps-1 | KPC-2 |
| 30660, NJST258_1a | 258 | NJ | 2010 | cps-2 | KPC-3 |
| 28577 | 379 | NJ | 2009 | cps-2 | KPC-3 |
| 30684, NJST258_2b | 258 | NJ | 2010 | cps-2 | KPC-3 |
Isolation of Human PMNs
PMNs were isolated from heparinized venous blood of healthy individuals, using dextran sedimentation and Hypaque-Ficoll (GE Healthcare Life Sciences) gradient centrifugation as described previously [22]. PMNs were suspended at 107 PMNs/mL in Roswell Park Memorial Institute (RPMI) 1640 medium (Life Technologies) buffered with 10 mM HEPES (RPMI/H, pH 7.2) and kept at ambient temperature until use.
Phagocytosis of K. pneumoniae by Human PMNs
Synchronized phagocytosis assays were performed with adherent PMNs as described previously [22] but with modifications. K. pneumoniae were opsonized in fresh 5% (vol/vol) normal human serum (NHS) for 30 minutes at 37°C, washed in phosphate-buffered saline (PBS), and resuspended in RPMI/H at 5 × 107 colony-forming units (CFU)/mL. PMNs (5 × 105) were added to serum-coated glass coverslips in 24-well tissue culture plates and allowed to adhere at room temperature for 15 minutes. PMNs were chilled on ice and combined with 5 × 106 K. pneumoniae, and assay plates were centrifuged at 524 g for 8 minutes at 4°C to synchronize phagocytosis. Samples were incubated at 37°C for the indicated times and then washed with cold PBS. Cells were fixed with 4% paraformaldehyde for 10 minutes on ice, followed by 10 minutes at room temperature. Fixative was removed by aspiration, and samples were washed 3 times in PBS and incubated in blocking buffer (5% goat serum in PBS) for 30 minutes at room temperature. To detect uningested bacteria, cells were incubated with a 1:100 dilution of antibody specific for K. pneumoniae (αKp; see below) for 1 hour at room temperature, washed three times with washing buffer (1.25% goat serum in PBS) and stained with AlexaFluor 594 conjugated to goat anti-rabbit antibody (Molecular Probes) for 1 hour at room temperature. To detect ingested bacteria, PMNs were permeabilized with 0.3% Triton X-100 for 10 minutes at room temperature prior to incubation with αKp, and stained with AlexaFluor 488 conjugated to goat anti-rabbit antibody (Molecular Probes) for 1 hour at room temperature. Samples were visualized with a Zeiss Axioskop 2 Plus fluorescence microscope, and a minimum of 50 PMNs were scored from at least 5 separate fields of view. Data are expressed as association index (total number of bacteria bound plus those ingested per PMN) or phagocytic index (total number of intracellular bacteria per PMN). Brightness and contrast of fluorescence images were adjusted equally in Adobe Photoshop CC (Adobe Systems).
Production of PMN Reactive Oxygen Species (ROS)
PMNs were incubated with 25 μM 2′,7-dihydrodichlorofluorescein diacetate (DCF; Molecular Probes) for 30 minutes at room temperature in RPMI/H. K. pneumoniae, zymosan (Sigma) (prepared as described elsewhere [23]), and S. aureus USA300 were opsonized in fresh 5% (vol/vol) normal human serum (NHS) for 30 minutes at 37°C, washed in PBS, and resuspended in RPMI/H. DCF-containing PMNs (1 × 106) and K. pneumoniae (1 × 107), S. aureus (1 × 107), or zymosan (3 × 106) were combined in wells of a 96-well microtiter plate, centrifuged to synchronize phagocytosis as described above, and transferred to a microplate fluorometer (Spectramax Gemini, Molecular Devices). ROS production was measured as described previously [22]. Data are expressed as a change in millifluorescence units (ΔFL) per minute, and each data point represents the rate of ROS production within the indicated 10-minute period. Alternatively, PMN ROS production was measured using luminol-enhanced chemiluminescence as reported previously [24] but with modifications. PMNs (106) in RPMI/H, luminol (50 mM), catalase (2000 U/mL), and SOD (50 U/mL) were combined with 107 K. pneumoniae in serum-coated wells of a white 96-well microtiter plate and centrifuged to synchronize phagocytosis as described above. Assay plates were transferred to a microplate luminometer (Synergy Mx, BioTek Instruments), and luminescence was measured every 2 minutes for up to 3 hours.
Bactericidal Activity Assays
Killing of bacteria by PMNs was determined as described previously [25] but with modifications. PMNs (5 × 105) were combined with 5 × 106 K. pneumoniae in serum-coated wells of a 24-well tissue culture plate, and plates were centrifuged at 524 g for 8 minutes at 4°C to synchronize phagocytosis. At indicated times, PMNs were lysed with 0.1% saponin (15 minutes on ice), and K. pneumoniae were plated on LB agar. Colonies were enumerated the following day, and percentage survival was calculated with the equation [CFU +PMN at t/CFU-PMN at t] × 100.
PMN Viability and Apoptosis Assays
PMNs (106) were combined with K. pneumoniae (106 or 107, as indicated) in serum-coated wells of a 96-well tissue culture plate, and phagocytosis was synchronized as described above. A total of 500 ng/mL of anti-Fas antibody (EMD Millipore) was added 20 minutes following phagocytosis as indicated. Surface expression of phosphatidylserine was measured by flow cytometry analysis of annexin V–fluorescein isothiocyanate (Annexin V–FITC Apoptosis Detection Kit II, BD Biosciences), and cell membrane permeability (a measure of viability) was determined with propidium iodide staining [22]. PMN lysis (10 bacteria/neutrophil) was assessed by release of cytoplasmic lactate dehydrogenase according to the kit manufacturer's instructions (Cytotoxicity Detection kit; Roche Diagnostics) [26].
Transmission Electron Microscopy (TEM)
PMNs (5 × 105) were aliquoted into wells of a 24-well tissue culture plate containing serum-coated Thermonox coverslips (Thermo Fisher Scientific) and allowed to adhere at room temperature for 20 minutes. Cells were chilled on ice for 10 minutes, and 5 × 106 NHS-opsonized K. pneumoniae were added to the wells. Plates were centrifuged as described above to synchronize phagocytosis. Assays were incubated at 37°C for up to 2 hours. TEM images were processed as described previously [26]. Images were adjusted for brightness and contrast in Adobe Photoshop CC.
Antibody Production
We produced αKp in rabbits in accordance with a protocol approved by the Institutional Animal Care and Use Committee, Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases. Fifteen K. pneumoniae clinical isolates of varied multilocus sequence types (ST258, ST11, ST14, ST15, ST16, ST23, ST37, ST65, ST101, and ST234) were grown to late exponential phase (OD600 = 1.0) in LB and RPMI 1640 medium. Bacteria were washed 4 times in PBS and inactivated by UV treatment for 120 seconds. Bacteria in 2.5 mL of PBS were emulsified in complete Freund's adjuvant (2.5 mL). Female New Zealand White rabbits (n = 2; Western Oregon Rabbit) were inoculated subcutaneously with 2 × 108 killed bacteria in adjuvant emulsion. Rabbits received booster inoculations (2 × 108 killed bacteria in incomplete Freund's adjuvant) every 4 weeks for 20 weeks. Sera were harvested 22 weeks following the first immunization, and immunogenicity was confirmed by immunoblot analysis.
RESULTS
Phagocytosis of ST258 by Human Neutrophils
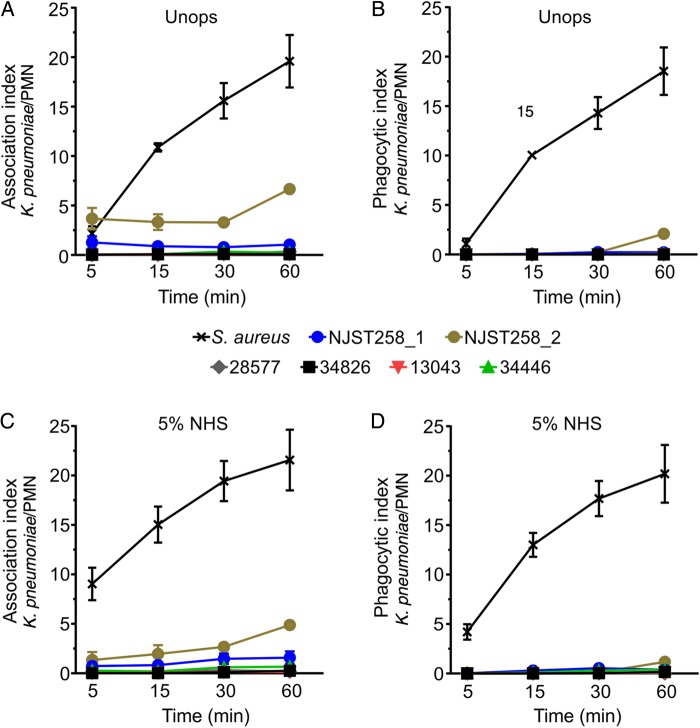
As a first step toward determining whether the success of ST258 is due at least in part to evasion of innate host defense, we tested the ability of neutrophils to phagocytose selected ST258 clinical isolates (Figures 1 and 2). Compared with the epidemic community-associated methicillin S. aureus (CA-MRSA) clone USA300 [21], which is known to be ingested rapidly by human neutrophils, binding and phagocytosis of ST258 was limited (Figures 1 and 2A–D). For example, the mean phagocytic index (±SD), defined as the number of ingested bacteria per PMN, for serum opsonized USA300 was 20.2 ± 2.9, whereas that for K. pneumoniae strain NJST258_2 was 1.2 ± 0.3 (Figure 2D). Phagocytosis of ST258 by adherent PMNs was largely unaffected by opsonization with NHS, since results with opsonized and unopsonized bacteria were similar (compare data in Figure 2B and 2D). These findings are consistent with previous phagocytosis studies, using adherent neutrophils, in which we reported comparable phagocytosis of NHS-opsonized and unopsonized S. aureus [27]. Collectively, these results indicate that binding and uptake of ST258 by human neutrophils is limited. Moreover, neutrophils failed to phagocytose heat-killed ST258 or organisms killed by exposure to UV light (Supplementary Figure 1). These later observations provide strong support to the idea that the phagocytosis resistance-phenotype of ST258 and related organisms is conferred passively by a structure or structures on the bacterial surface—such as the capsule polysaccharide—as has been reported previously for Klebsiella species [28–32].
Figure 1.
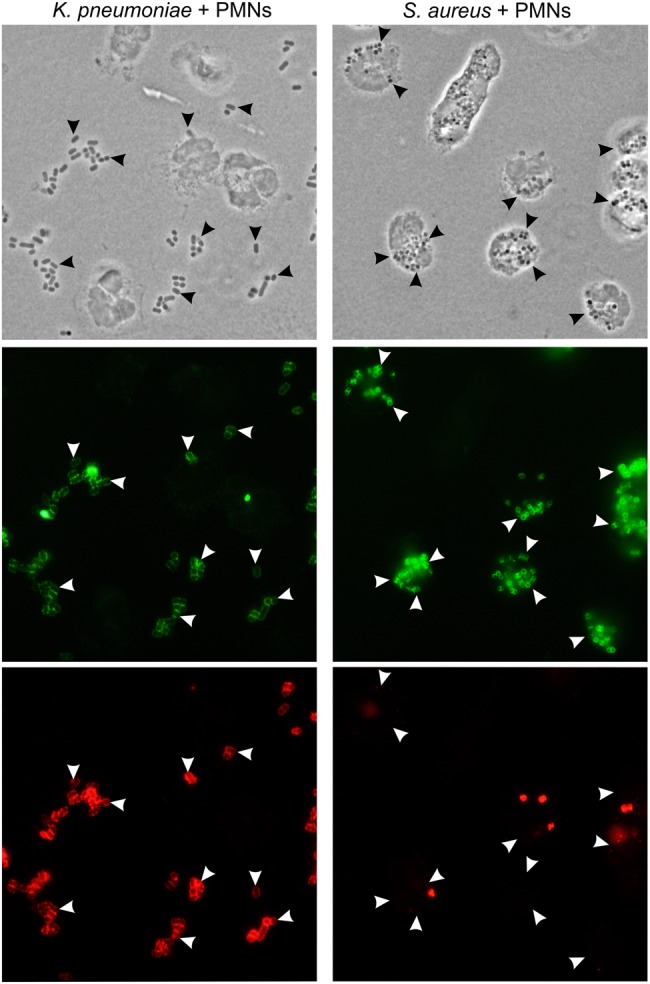
Phagocytosis of multilocus sequence type 258 (ST258) and Staphylococcus aureus. Human neutrophils were combined with Klebsiella pneumoniae strain NJST258_2 (left panels) or S. aureus strain USA300 (right panels), and binding and phagocytosis were evaluated using immunofluorescence microscopy. Top panels are phase-contrast images (arrowheads indicate selected bacteria), middle panels (green staining) show all bacteria, and bottom panels show extracellular bacteria (red staining). Images are representative of the phagocytosis data quantitated in Figure 2. Abbreviation: PMN, polymorphonuclear leukocyte.
Figure 2.
Phagocytosis of multilocus sequence type 258 (ST258) clinical isolates by human neutrophils. A–D, Human neutrophils were combined with Klebsiella pneumoniae clinical isolates or Staphylococcus aureus strain USA300 (positive control), and synchronized phagocytosis assays were performed as described in “Methods” section. Bacteria were either left unopsonized (Unops; A and B) or were opsonized with 5% normal human serum (NHS; C and D). The association index is the number of bacteria surface bound or ingested per neutrophil. Phagocytic index is the number of ingested bacteria per neutrophil. Data are the mean ± standard error of the mean of 3–5 separate experiments. Abbreviation: PMN, polymorphonuclear leukocyte.
Production of ROS
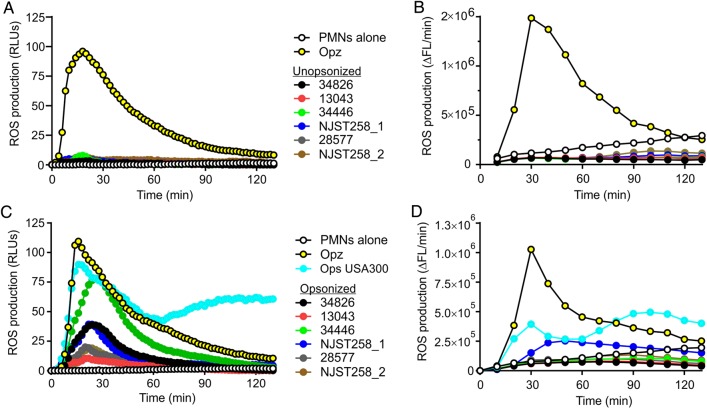
We next evaluated the ability of the ST258 clinical isolates to elicit production of neutrophil ROS during phagocytic interaction (Figure 3). There was little or no neutrophil ROS production elicited by unopsonized bacteria (Figure 3A and 3B), a finding compatible with the phagocytosis data (Figure 2). On the other hand, at least 3 of 6 serum-opsonized K. pneumoniae isolates tested—34826, 34446, and NJST258_1—elicited notable production of ROS by neutrophils in at least 1 of 2 assays used to measure ROS production (Figure 3C and 3D). Although these results seem at variance with the phagocytosis data (few ingested bacteria), the level of ROS production by these clinical isolates was generally less than that by opsonized zymosan or opsonized USA300, which are phagocytosed readily by human neutrophils (Figure 3) [23]. It is also possible that molecules shed from uningested K. pneumoniae elicit production of neutrophil ROS, and K. pneumoniae is known to contain cell envelope components that activate neutrophils [33].
Figure 3.
Production of neutrophil reactive oxygen species (ROS). Human neutrophils were combined with unopsonized (A and B) or normal human serum–opsonized (C and D) Klebsiella pneumoniae clinical isolates, opsonized zymosan (Opz; a positive control), or Staphylococcus aureus strain USA300 (Ops USA300) as indicated, and production of ROS was measured as described in “Methods” section. A and C, Luminol-enhanced chemiluminescence. B and D, Oxidation of 2′-7′dichlorofluorecin diacetate to 2′-7′dichlorofluorescein. Results are the mean of 3–6 separate experiments. Abbreviations: ΔFL/min, change in millifluorescence units per minute; PMN, polymorphonuclear leukocyte; RLU, relative light unit.
Killing of ST258 by Human Neutrophils
We next evaluated the ability of human neutrophils to kill ST258 and related K. pneumoniae clinical isolates. Net survival for the majority of clinical isolates tested (5 of 6) was ≥100% during phagocytic interaction with neutrophils (Figure 4A and 4B). Although these findings are consistent with the observed limited phagocytosis of K. pneumoniae by human neutrophils (Figures 1 and 2), they are somewhat unexpected. This is because neutrophils are known to be the primary cellular defense against bacteria, including K. pneumoniae [13], and this microbe is not widely considered a common cause of infections in healthy individuals [34].
Figure 4.
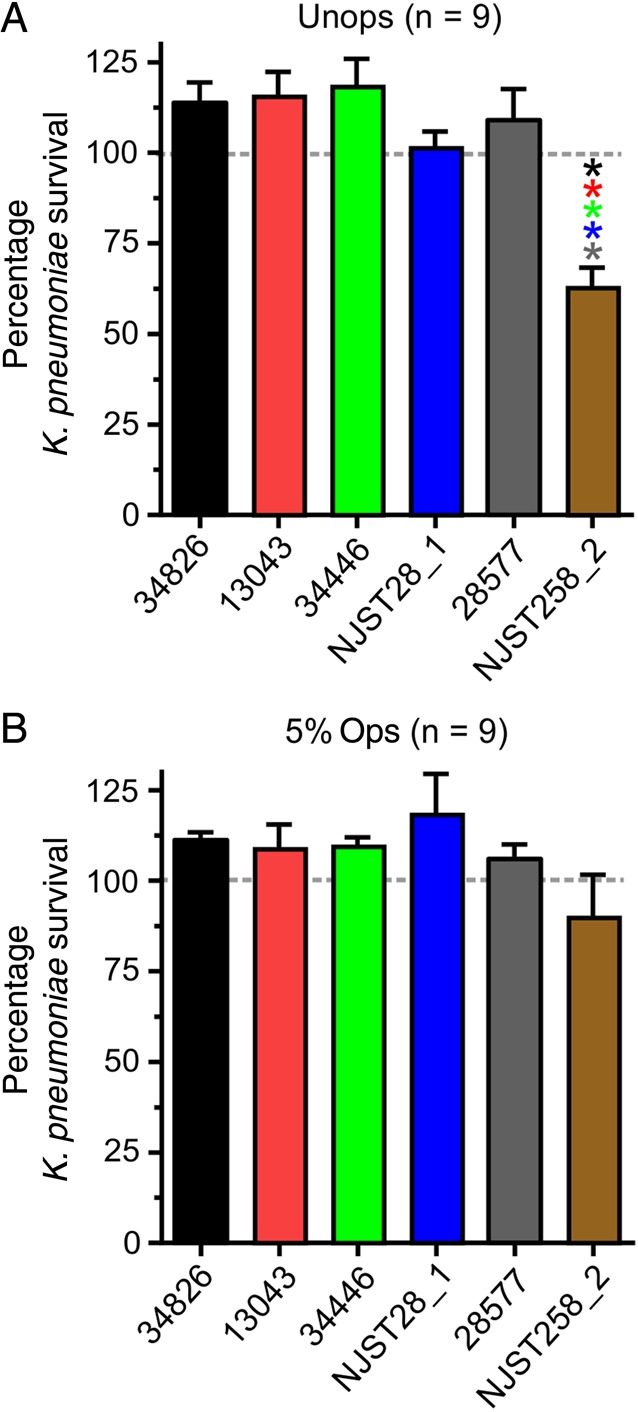
Survival of multilocus sequence type 258 (ST258) during phagocytic interaction with human neutrophils. Neutrophils were combined with unopsonized (Unops; A) or normal human serum–opsonized (Ops; B) Klebsiella pneumoniae clinical isolates, phagocytosis was synchronized, and percentage survival was determined. Results are the mean ± standard error of 9 separate experiments. P < .05 vs the bar with the corresponding asterisk color. Statistics were performed using repeated-measures analysis of variance and the Tukey post hoc test (GraphPad Prism, version 6.05).
As noted above, not all clinical isolates fully circumvented killing by human neutrophils. In neutrophil bactericidal activity assays with unopsonized bacteria, survival of strain NJST258_2 was decreased, compared with the other strains/clinical isolates tested (mean survival [±SD] was 62.8% ± 5.4% at 1 hour; Figure 4A). Correspondingly, this isolate had the highest neutrophil association and phagocytic indices among the isolates tested (Figure 2). There was clear fusion of cytoplasmic granules with K. pneumoniae–containing phagosomes, and this process would enrich phagosomes with molecules that kill and degrade microbes (Figure 5A and 5B). Indeed, ingested NJST258_2 was readily degraded within neutrophil phagosomes (Figure 5B and 5C). Taken together, these data indicate that the high level of K. pneumoniae survival in these assays is a reflection of limited neutrophil phagocytosis, rather than survival after phagocytosis.
Figure 5.
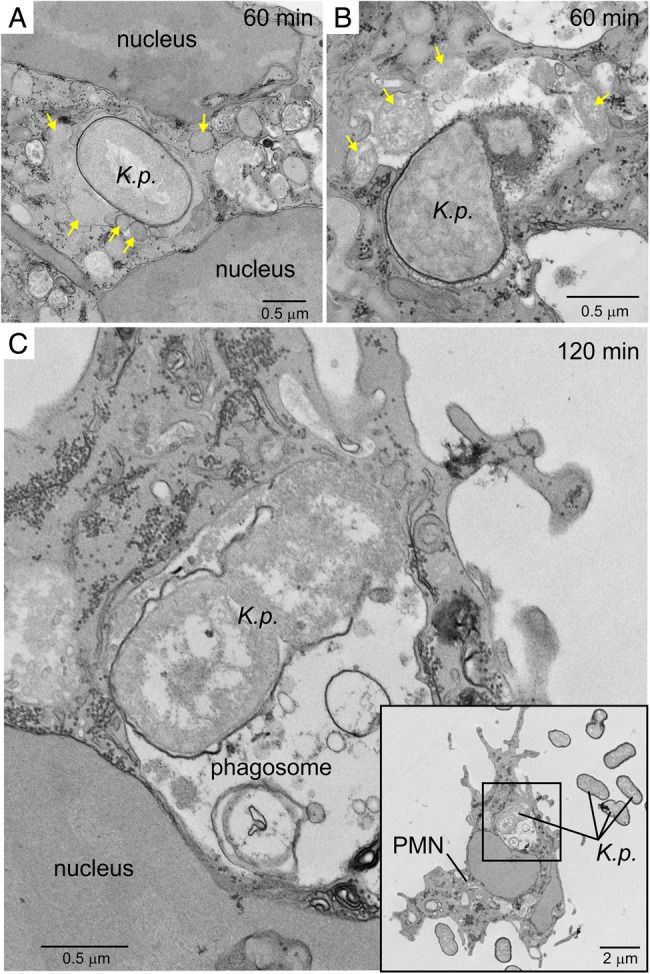
Ultrastructural analysis of ingested Klebsiella pneumoniae. Human neutrophils were cultured with unopsonized K. pneumoniae strain NJST258_2, phagocytosis was synchronized, and samples were analyzed by TEM. A and B, ingested K. pneumoniae 60 minutes after start of the assay. Yellow arrows indicate neutrophil granules, including those that have fused with the K. pneumoniae–containing phagosome. C, Ingested K. pneumoniae 120 minutes after the start of phagocytosis. Destruction of bacteria is evident in panels B and C. Inset, Black square represents the area of the micrograph from which panel C was derived. Abbreviations: K.p., K. pneumoniae; PMN, polymorphonuclear leukocyte.
K. pneumoniae Delays Neutrophil Apoptosis
Spent and or aged neutrophils are normally removed from the host by programmed cell death, including phagocytosis-induced cell death, and these processes facilitate the resolution of the inflammatory response [12, 22, 35, 36]. Bacterial pathogens such as S. aureus can alter this normal process and cause neutrophil lysis, which in turn causes release of cytotoxic molecules (and pathogen escape) that can damage host tissues [25, 26, 37, 38]. Inasmuch as neutrophil apoptosis and turnover are known to be altered by bacteria and molecules produced by bacteria, and the majority of K. pneumoniae strains tested here are neither ingested nor killed efficiently, we assessed neutrophil viability following 6 hours exposure to these organisms (Figure 6).
Figure 6.
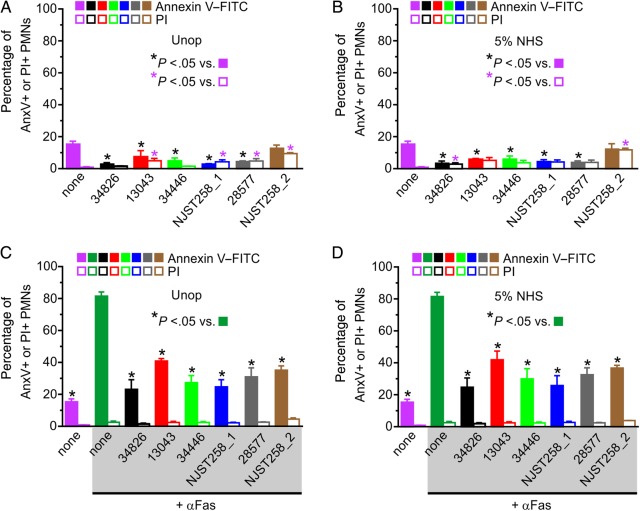
Klebsiella pneumoniae alters neutrophil viability and turnover. A and C, Unopsonized (Unop; A and C) or normal human serum (NHS)–opsonized (B and D) K. pneumoniae clinical isolates were cultured for 6 hours with human neutrophils at a 1:1 ratio of K. pneumoniae colony-forming units to neutrophils. C and D, Antibody specific for Fas (αFas; 500 ng/mL) was added 20 minutes following phagocytosis to accelerate polymorphonuclear leukocyte (PMN) apoptosis. Surface association of annexin V–fluorescein isothiocyanate (FITC) only (AnxV+; not PI+) and staining of nuclear DNA with propidium iodide (PI+) were measured by flow cytometry. Data are the mean ± standard error of the mean of at least 3 separate experiments. Statistical analyses were performed using 1-way analysis of variance and the Dunnett post hoc test.
First, there was essentially no lysis of human neutrophils after 6 hours of interaction between K. pneumoniae and neutrophils, as assessed by release of lactate dehydrogenase (0.0%–0.14% lysis for all isolates/strains tested; n = 3). In accordance with these results, there was limited uptake of propidium iodide—an indicator of membrane permeabilization—by neutrophils exposed to ST258 and related K. pneumoniae isolates for up to 6 hours (Figure 6A and 6B). Moreover, surface expression of phosphatidylserine, a marker of early apoptosis, remained either unchanged or was slightly decreased by neutrophils under these conditions (Figure 6A and 6B). Unexpectedly, interaction of neutrophils with these K. pneumoniae isolates inhibited surface expression of phosphatidylserine induced by anti-Fas antibody—an agonist known to induce neutrophil apoptosis (Figure 6C and 6D). These results provide an additional dimension to the ability of K. pneumoniae to alter innate host responses, although the significance of these in vitro findings to human infections in vivo requires further investigation.
DISCUSSION
Infections caused by carbapenem-resistant K. pneumoniae are often fatal, largely because patients are frequently immunocompromised and because the organism is multidrug resistant. Thus, few therapeutic options are available. Alternative approaches to prevention and treatment of infections, such as an active and/or passive vaccine, are needed. Vaccine approaches for protection against Klebsiella infections were reported extensively in the 1980s [39–44], but the organisms predated the contemporary carbapenem-resistant ST258 strains. More-recent studies tested immunogenicity and safety of a capsular polysaccharide vaccine against Klebsiella [45]. Despite these early successful efforts, there is no Klebsiella vaccine approved for use in the United States. As a step toward developing alternative therapeutics (such as vaccines) for carbapenem-resistant K. pneumoniae, we investigated the ability of neutrophils from healthy individuals to ingest and kill ST258 K. pneumoniae.
Although K. pneumoniae capsular polysaccharide has been reported to inhibit phagocytosis by neutrophils [28, 31], these host phagocytes are known to be important for protection against infections caused by K. pneumoniae [13, 14, 16, 17]. Thus, the finding that there was limited phagocytosis and killing of ST258 strains, using adherent neutrophils in vitro, was surprising to us. In addition, neutrophil phagocytosis is optimal under the synchronized phagocytosis assay conditions used here (ie, microbes not normally ingested in suspension are often phagocytosed readily by neutrophils primed by adherence) [27]. Resistance of ST258 strains to phagocytosis and killing by neutrophils was independent of carbapenem resistance, since 2 of the ST258 clinical isolates tested here—34826 and 13043—lacked genes encoding K. pneumoniae carbapenemase, and survival of these isolates was comparable to that of the resistant strains in assays with neutrophils (Table 1 and Figure 4). The few organisms that were ingested by neutrophils triggered fusion of granules with phagosomes, and the ingested bacteria were readily destroyed (Figure 5).
A number of bacterial pathogens produce molecules that cause neutrophil lysis, or the pathogens cause leukocyte lysis after phagocytosis, with both processes linked to virulence. Such pathogenic processes likely contribute to failure of immunotherapeutics, as with an opsonophagocytosis vaccine approach for S. aureus [46]. However, the vast majority of human neutrophils remained viable for at least 6 hours in the presence of ST258 K. pneumoniae organisms (Figure 6). These findings are compatible with the phenotype of K. pneumoniae as an asymptomatic colonizer of humans.
The implication of our observations collectively is that an antibody-based approach directed to enhance opsonophagocytosis of carbapenem-resistant K. pneumoniae by neutrophils merits consideration as a vaccine or therapeutic. Inasmuch as the most widespread organisms are ST258, a limited number of capsule polysaccharide variants exist, and an active or passive vaccine based on capsular polysaccharide seems reasonable. A potential shortcoming of this approach is with patients who are neutropenic or have defects in phagocytes, but this is likely to impact only a subset of individuals who have infections caused by ST258 or related organisms. More work is needed, especially with animal infection and vaccine protection models, to determine the usefulness of a capsule polysaccharide (or other surface component) vaccine for protection against carbapenem-resistant K. pneumoniae.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Financial support. This work was supported by the National Institutes of Health (NIH; grant R01AI090155 to B. N. K.) and the Intramural Research Program, National Institute of Allergy and Infectious Diseases, NIH.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Magill SS, Edwards JR, Bamberg W et al. Multistate point-prevalence survey of health care-associated infections. New Engl J Med 2014; 370:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.da Silva RM, Traebert J, Galato D. Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae: a review of epidemiological and clinical aspects. Expert Opin Biol Ther 2012; 12:663–71. [DOI] [PubMed] [Google Scholar]
- 3.Kitchel B, Rasheed JK, Patel JB et al. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrob Agents Chemother 2009; 53:3365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordmann P, Naas T, Poirel L. Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 2011; 17:1791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daikos GL, Tsaousi S, Tzouvelekis LS et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 2014; 58:2322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Endimiani A, Depasquale JM, Forero S et al. Emergence of blaKPC-containing Klebsiella pneumoniae in a long-term acute care hospital: a new challenge to our healthcare system. J Antimicrob Chemother 2009; 64:1102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falagas ME, Tansarli GS, Karageorgopoulos DE, Vardakas KZ. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg Infect Dis 2014; 20:1170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez-Simmonds A, Greenman M, Sullivan SB et al. Population structure of Klebsiella pneumoniae causing bloodstream infections at a New York City tertiary care hospital: diversification of multidrug-resistant isolates. J Clin Microbiol 2015; 53:2060–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuzon G, Naas T, Truong H et al. Worldwide diversity of Klebsiella pneumoniae that produce beta-lactamase blaKPC-2 gene. Emerg Infect Dis 2010; 16:1349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adler A, Carmeli Y. Dissemination of the Klebsiella pneumoniae carbapenemase in the health care settings: tracking the trails of an elusive offender. mBio 2011; 2:e00280–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Mathema B, Chavda KD, DeLeo FR, Bonomo RA, Kreiswirth BN. Carbapenemase-producing Klebsiella pneumoniae: molecular and genetic decoding. Trends Microbiol 2014; 22:686–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLeo FR, Nauseef WM. Granulocytic Phagocytes. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 8th ed Vol. 1. Philedelphia, PA: Elsevier; 2014:78–92. [Google Scholar]
- 13.Rehm SR, Gross GN, Pierce AK. Early bacterial clearance from murine lungs. Species-dependent phagocyte response. J Clin Invest 1980; 66:194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bullen JJ, Leach PA, Lange L. Abolition of resistance to Klebsiella pneumoniae by anti-polymorphonuclear leucocyte IgG. Immunology 1980; 39:47–52. [PMC free article] [PubMed] [Google Scholar]
- 15.Fukutome T, Mitsuyama M, Takeya K, Nomoto K. Importance of antiserum and phagocytic cells in the protection of mice against infection by Klebsiella pneumoniae. J Gen Microbiol 1980; 119:225–9. [DOI] [PubMed] [Google Scholar]
- 16.Valdez JM, Scheinberg P, Young NS, Walsh TJ. Infections in patients with aplastic anemia. Semin Hematol 2009; 46:269–76. [DOI] [PubMed] [Google Scholar]
- 17.Satlin MJ, Calfee DP, Chen L et al. Emergence of carbapenem-resistant Enterobacteriaceae as causes of bloodstream infections in patients with hematologic malignancies. Leuk Lymphoma 2013; 54:799–806. [DOI] [PubMed] [Google Scholar]
- 18.Hirche TO, Gaut JP, Heinecke JW, Belaaouaj A. Myeloperoxidase plays critical roles in killing Klebsiella pneumoniae and inactivating neutrophil elastase: effects on host defense. J Immunol 2005; 174:1557–65. [DOI] [PubMed] [Google Scholar]
- 19.Belaaouaj A, McCarthy R, Baumann M et al. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat Med 1998; 4:615–8. [DOI] [PubMed] [Google Scholar]
- 20.Tomas JM, Benedi VJ, Ciurana B, Jofre J. Role of capsule and O antigen in resistance of Klebsiella pneumoniae to serum bactericidal activity. Infect Immun 1986; 54:85–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voyich JM, Braughton KR, Sturdevant DE et al. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunol 2005; 175:3907–19. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi SD, Voyich JM, Buhl CL, Stahl RM, DeLeo FR. Global changes in gene expression by human polymorphonuclear leukocytes during receptor-mediated phagocytosis: cell fate is regulated at the level of gene expression. Proc Natl Acad Sci U S A 2002; 99:6901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeLeo FR, Allen LA, Apicella M, Nauseef WM. NADPH oxidase activation and assembly during phagocytosis. J Immunol 1999; 163:6732–40. [PubMed] [Google Scholar]
- 24.Bylund J, Bjornsdottir H, Sundqvist M, Karlsson A, Dahlgren C. Measurement of respiratory burst products, released or retained, during activation of professional phagocytes. Methods Mol Biol 2014; 1124:321–38. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi SD, Braughton KR, Whitney AR et al. Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils. Proc Natl Acad Sci U S A 2003; 100:10948–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi SD, Braughton KR, Palazzolo-Ballance AM et al. Rapid neutrophil destruction following phagocytosis of Staphylococcus aureus. J Innate Immun 2010; 2:560–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu T, Porter AR, Kennedy AD, Kobayashi SD, DeLeo FR. Phagocytosis and killing of Staphylococcus aureus by human neutrophils. J Innate Immun 2014; 6:639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Domenico P, Salo RJ, Cross AS, Cunha BA. Polysaccharide capsule-mediated resistance to opsonophagocytosis in Klebsiella pneumoniae. Infect Immun 1994; 62:4495–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams P, Lambert PA, Brown MR, Jones RJ. The role of the O and K antigens in determining the resistance of Klebsiella aerogenes to serum killing and phagocytosis. J Gen Microbiol 1983; 129:2181–91. [DOI] [PubMed] [Google Scholar]
- 30.Baer H, Ehrenworth L. The pathogenicity of Klebsiella pneumoniae for mice: the relationship to the quantity and rate of production of type-specific capsular polysaccharide. J Bacteriol 1956; 72:713–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall HE, Humphries JC. The relationship between insusceptibility to phagocytosis and virulence of certain Klebsiella pneumoniae strains. J Infect Dis 1958; 103:157–62. [DOI] [PubMed] [Google Scholar]
- 32.Kabha K, Nissimov L, Athamna A et al. Relationships among capsular structure, phagocytosis, and mouse virulence in Klebsiella pneumoniae. Infect Immunity 1995; 63:847–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Capsoni F, Minonzio F, Venegoni E et al. In vitro and ex vivo effect of RU41740 on human polymorphonuclear leukocyte function. Int J Immunopharmacol 1988; 10:121–33. [DOI] [PubMed] [Google Scholar]
- 34.Ko WC, Paterson DL, Sagnimeni AJ et al. Community-acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. Emerg Infect Dis 2002; 8:160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watson RW, Redmond HP, Wang JH, Condron C, Bouchier-Hayes D. Neutrophils undergo apoptosis following ingestion of Escherichia coli. J Immunol 1996; 156:3986–92. [PubMed] [Google Scholar]
- 36.Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest 1989; 83:865–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenlee-Wacker MC, Rigby KM, Kobayashi SD, Porter AR, DeLeo FR, Nauseef WM. Phagocytosis of Staphylococcus aureus by human neutrophils prevents macrophage efferocytosis and induces programmed necrosis. J Immunol 2014; 192:4709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeLeo FR. Modulation of phagocyte apoptosis by bacterial pathogens. Apoptosis 2004; 9:399–413. [DOI] [PubMed] [Google Scholar]
- 39.Roe EA, Jones RJ. Vaccination against Klebsiella aerogenes. J Hyg (Lond) 1984; 93:355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones RJ, Roe EA. Vaccination against 77 capsular types of Klebsiella aerogenes with polyvalent Klebsiella vaccines. J Med Microbiol 1984; 18:413–21. [DOI] [PubMed] [Google Scholar]
- 41.Roe EA, Jones RJ, Dyster RE. Passive immunization of mice against Klebsiella aerogenes. Br J Exp Pathol 1986; 67:25–32. [PMC free article] [PubMed] [Google Scholar]
- 42.Cryz SJ Jr, Furer E, Germanier R. Safety and immunogenicity of Klebsiella pneumoniae K1 capsular polysaccharide vaccine in humans. J Infect Dis 1985; 151:665–71. [DOI] [PubMed] [Google Scholar]
- 43.Cryz SJ Jr, Furer E, Germanier R. Protection against fatal Klebsiella pneumoniae burn wound sepsis by passive transfer of anticapsular polysaccharide. Infect Immun 1984; 45:139–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cryz SJ Jr, Furer E, Germanier R. Purification and vaccine potential of Klebsiella capsular polysaccharides. Infect Immun 1985; 50:225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campbell WN, Hendrix E, Cryz S Jr., Cross AS. Immunogenicity of a 24-valent Klebsiella capsular polysaccharide vaccine and an eight-valent Pseudomonas O-polysaccharide conjugate vaccine administered to victims of acute trauma. Clin Infect Dis 1996; 23:179–81. [DOI] [PubMed] [Google Scholar]
- 46.DeLeo FR, Otto M. An antidote for Staphylococcus aureus pneumonia? J Exp Med 2008; 205:271–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.