Abstract
Background. The ubiquitous opportunistic pathogen Pneumocystis jirovecii causes pneumonia in immunocompromised individuals, including human immunodeficiency virus (HIV)–infected individuals, and pulmonary colonization with P. jirovecii is believed to be a cofactor in the development of chronic obstructive pulmonary disease. There is no vaccine for P. jirovecii; however, most adults are seropositive, indicating natural immune priming to this pathogen. We have shown that humoral response to a recombinant subunit of the P. jirovecii protease kexin (KEX1) correlates with protection from P. jirovecii colonization and pneumonia.
Methods. Here we evaluated the immunogenicity and protective capacity of the recombinant KEX1 peptide vaccine in a preclinical, nonhuman primate model of HIV-induced immunosuppression and Pneumocystis coinfection.
Results. Immunization with KEX1 induced a robust humoral response remained at protective levels despite chronic simian immunodeficiency virus/HIV–induced immunosuppression. KEX1-immunized macaques were protected from Pneumocystis pneumonia, compared with mock-immunized animals (P = .047), following immunosuppression and subsequent natural, airborne exposure to Pneumocystis.
Conclusions. These data support the concept that stimulation of preexisting immunological memory to Pneumocystis with a recombinant KEX1 vaccine prior to immunosuppression induces durable memory responses and protection in the context of chronic, complex immunosuppression.
Keywords: Pneumocystis, vaccine, HIV/SIV, macaque, nonhuman primate, humoral immunity
Pneumocystis jirovecii (formerly Pneumocystis carinii) is an opportunistic fungal pathogen and causative agent of life-threatening pneumonia in immunocompromised individuals, including human immunodeficiency virus (HIV)–infected persons. Pneumocystis pneumonia (PCP) and pulmonary colonization by Pneumocystis are associated with permanent, obstructive lung damage, and P. jirovecii colonization is believed to be a cofactor in the development of chronic obstructive pulmonary disease (COPD) [1–4]. Despite introduction of antiretroviral therapy (ART) and anti-Pneumocystis prophylaxis, PCP remains a significant cause of morbidity and mortality in HIV-infected individuals [5], particularly those with undiagnosed HIV infection. In developing countries where HIV infection rates are high, diagnosis is difficult, and access to ART is suboptimal [6]. Furthermore, there is an increased PCP incidence among persons receiving immunosuppressive therapies [7], including patients with cancer, transplant recipients [8], and individuals with inflammatory diseases [9], and among persons who are immunosuppressed because of aging, congenital immunosuppressive states, or acquired immunosuppressive states [10].
Exposure to P. jirovecii is common, as most individuals have P. jirovecii antigens detected by serological analysis by 4 years of age [11–13]. Pneumocystis–specific antibodies are important for protection, as demonstrated in animal models of Pneumocystis infection and clinical studies [14–21]. High titers of antibody to a recombinant subunit of the P. jirovecii protein kexin (KEX1) but not antibody to the P. jirovecii major surface glycoprotein correlated with a reduced incidence of PCP among HIV-infected subjects [15] and in a nonhuman primate (NHP) model of HIV and Pneumocystis coinfection [22]. Additionally, in HIV-negative smokers and patients with COPD, we found that low anti–P. jirovecii KEX1 antibody titers were independently associated with more-severe airway obstruction, suggesting that anti-KEX1 may contribute to protection from P. jirovecii colonization and progressive COPD [2]. Epidemiologic studies in macaques revealed a high prevalence of anti-KEX1 on serological analysis, similar to that among humans, suggesting that most macaques have been previously exposed to Pneumocystis and immunologically primed to KEX1 [23], thus making macaques an ideal model to test vaccine immunogenicity and efficacy. In the macaque model of simian immunodeficiency virus (SIV) and Pneumocystis coinfection, we established the following correlates of protection: plasma KEX1-specific immunoglobulin G (IgG) reciprocal end point titers of >10 000, early detectable KEX1-specific immunoglobulin A (IgA) antibodies in bronchoalveolar lavage (BAL) fluid, and peripheral blood KEX1-specific memory B cells [22].
The observation that most individuals have been primed to KEX1 suggests that protective immunologic responses could be achieved by boosting memory responses prior to immunosuppression. In this study, we tested the capacity of a recombinant KEX1 vaccine to boost the memory response in healthy macaques that had been naturally exposed to Pneumocystis, and we examined immune responses and protective efficacy following chronic immunosuppression by SIV/HIV (SHIV) infection and Pneumocystis exposure. KEX1 immunization induced significant and durable humoral responses that were well above those of previously established correlates of protection and were maintained despite SHIV-induced immunosuppression. KEX1 immunization prior to immunosuppression provided significantly longer protection against the development of PCP, compared with mock immunization. These studies present a strategy for immunization of healthy individuals at risk of subsequent immunosuppression and for protection against PCP in immunocompromised individuals.
METHODS
Vaccine Construction and Purification
A 270-nucleotide fragment of macaque-derived KEX1 was cloned into the pET28b(+) expression vector (Novagen) in Escherichia coli BL21(DE3) pLysS (ThermoFisher, Scientific) and used to produce an approximately 11-kDa recombinant protein, as confirmed by Western blot (Supplementary Figure 1). KEX1 was used for immunization, enzyme-linked immunosorbent assay (ELISA), and enzyme-linked immunospot (ELISpot) assay [23] (accession no. EU918304).
Animals, Study Design, and Sample Collection
Adult, Chinese-origin rhesus macaques (Macaca mulatta; 24 males) and cynomolgus macaques (Macaca fascicularis; 6 each of males and females) were purchased from vendors approved by the University of Pittsburgh. Studies were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Before study entry, all macaques were screened for anti–Pneumocystis KEX1 plasma antibody titers and pulmonary colonization with Pneumocystis (described below). Only Pneumocystis–negative macaques with an anti-KEX1 antibody titer of <6000 were used. Macaques were randomly assigned into one of 2 groups for immunization and challenge studies. Blood and bronchoalveolar lavage (BAL) fluid samples were collected at baseline and following immunization, as described elsewhere [1, 24, 25]. Plasma samples and BAL supernatants were collected and stored at −80°C until evaluation for the presence of anti-KEX1 antibodies, using ELISA (Supplementary Materials) [22, 23]. An ELISpot assay was used for detection of cells secreting anti-KEX1 antibody, to determine the presence of KEX1-specific memory B cells, according to previously described methods (Supplementary Materials) [22]. Flow cytometry of cells from peripheral blood and BAL fluid is described in the Supplementary Materials [22, 26, 27].
Immunization of Macaques
Eighteen macaques were intramuscularly immunized with 100 μg of recombinant KEX1 and aluminum hydroxide (Imject Alum, Thermo Scientific) mixed in a 1:1 ratio. Eighteen mock-immunized animals received sham inoculation with protein derived from pET28b(+) expression vector and alum. Animals were rested for 12 weeks, after which KEX1-immunized animals were boosted with 50 μg of KEX1 and alum, and mock-immunized animals were sham inoculated as described above. Following a 10-week resting period, macaques were intravenously inoculated with 1 × 105 SHIV89.6P (kindly provided by Keith Reimann, Beth Israel Deaconess Medical Center), which induces CD4+ T-cell lymphopenia and AIDS-like disease [28, 29]. Viral infection was monitored at weekly time points for 4 weeks and monthly thereafter up to 36 weeks after infection. Immunologic parameters (described below and in the Supplementary Materials) were monitored at monthly intervals after infection.
Pneumocystis Challenge and Determination of Pneumocystis Infection
Pneumocystis cannot be reliably cultured in vitro. Thus, Pneumocystis challenge of KEX1- and mock-immunized rhesus macaques was performed via natural airborne transmission by cohousing these animals with animals coinfected with SIV and Pneumocystis, as described previously [22, 23, 30]. Following SHIV infection, Pneumocystis colonization status was evaluated at monthly intervals by nested polymerase chain reaction (PCR) analysis of BAL fluid samples, as described previously [22, 23, 30]. To control for the DNA quality in BAL fluid samples, PCR for detection of β-globin was also performed [23, 24]. A diagnosis of PCP was made on the basis of detection of Pneumocystis in BAL fluid by first-round PCR and/or microscopy-based detection of Pneumocystis clusters, using Pneumocystis–specific (3FC) immunohistochemical (IHC) staining, in lung tissue at study termination (Supplementary Materials) [31, 32]. Pneumocystis colonization was defined as detection of Pneumocystis DNA in the nested round of PCR only, as described previously [23, 25, 33, 34]. During coinfection with SHIV and Pneumocystis, animals exhibiting evidence of end-stage AIDS (ie, persistent anorexia, weight loss of >20%, or symptoms of opportunistic infections) were euthanized. All other animals were euthanized at study termination (ie, 40 weeks after SHIV infection).
Statistical Analysis
Statistical analyses were performed using Prism software (GraphPad, La Jolla, California). An unpaired Student t test was used to compare baseline plasma anti-KEX1 IgG reciprocal end point titers between KEX1-immunized and mock-immunized animals. Paired Student t tests or Wilcoxon signed rank tests were performed as indicated to evaluate data between 2 time points. One-way repeated measures analyses of variance (ANOVAs) were performed to assess changes in plasma anti-KEX1 IgG at all subsequent time points following immunizations. Two-way repeated measures ANOVAs were performed to assess differences in cell activation between control and immunized monkeys for an entire time series.
RESULTS
Humoral Immune Responses of Macaques to KEX1 Vaccination
Prior to immunizations, plasma samples from 36 macaques were evaluated for preexisting anti-KEX1 antibodies. Baseline mean titers (±SD) were not different between 18 KEX1-immunized macaques (1808 ± 1515) and 18 mock-immunized macaques (1388 ± 1540; P = .415).
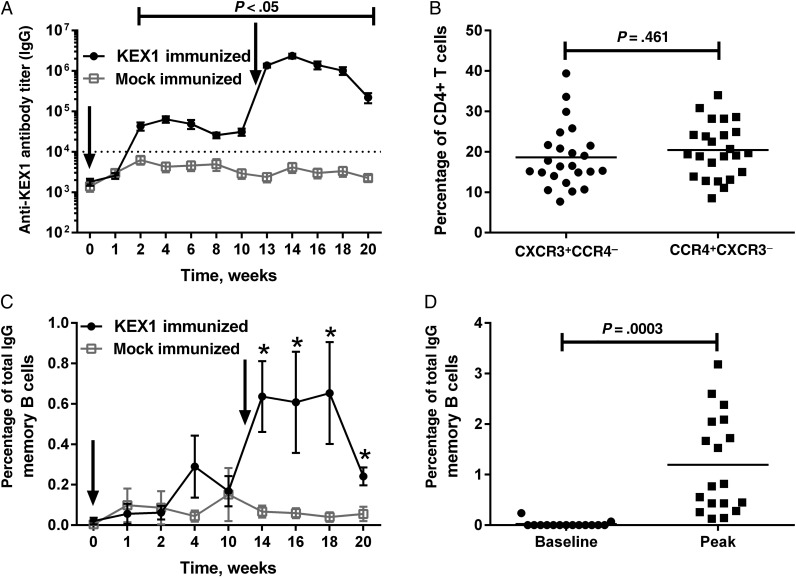
In KEX1-immunized animals, mean plasma anti-KEX1 IgG titers (±SD) peaked 4 weeks following the initial immunization (64 022 ± 48 679) and 2 weeks following the boost (2.35 × 106 ± 1.35 × 106; Figure 1A). The first peak was 35.4-fold greater than that before immunization (P < .0001); the second peak was 36.7-fold greater than the initial peak (P < .0001) and approximately 1300-fold greater than the titer before immunization (P < .0001). At the time of SHIV infection, mean plasma anti-KEX1 IgG titers (±SD) had declined somewhat but were significantly higher in KEX1-immunized animals (338 444 ± 345 501), compared with mock-immunized macaques (1707 ± 1385; P = .0002). Peripheral blood KEX1-specific memory B-cell responses peaked in KEX1-immunized animals 4 weeks after boost (Figure 1C); however, individual animals peaked at different time points, from 2 to 10 weeks after boost. When considering all individual peak antigen-specific memory B-cell responses, the mean peak frequency of circulating anti-KEX1 IgG memory B cells in KEX1-immunized macaques (1.19% of total IgG memory B cells) was significantly increased over baseline values (P = .003; Figure 1D) and was 5.7-fold higher than the mean peak KEX1 memory B-cell frequency for mock-immunized animals (0.209% of total IgG memory B cells; P = .01).
Figure 1.
KEX1-specific humoral immune responses following immunization and boost with KEX1/alum in normal macaques. A, Mean plasma KEX1-specific immunoglobulin G (IgG) titer, as determined by enzyme-linked immunosorbent assay. Dashed line indicates KEX1-specific IgG titer correlate of protection. Arrows indicate time of immunizations. B, To evaluate skewing of T-helper cell phenotype, peripheral blood lymphocytes were analyzed by flow cytometry for surface markers associated with either T-helper type 1 (Th1; CXCR3+CCR4−) or T-helper type 2 (Th2; CCR4+CXCR3−) CD4+ T cells and expressed as a percentage of total peripheral blood CD4+ T cells. The frequency of peripheral blood CD4+ Th1 and Th2 cells at the time of SHIV-infection was similar (P = .461). C, Kinetics of KEX1-specific memory B-cell responses, determined by B-cell enzyme-linked immunospot analysis and expressed as the mean percentage of total IgG B cells. D, Comparison of peak KEX1-specific memory B cells to baseline data (KEX1-immunized animals only). Paired Student t tests or Wilcoxon signed rank tests were performed to compare data baseline to data at indicated time points. *P < .05, compared with baseline.
Effects KEX1 Immunizations on Total Circulating CD4+ T-Helper Type 1 (Th1) and Th2 Cell Populations
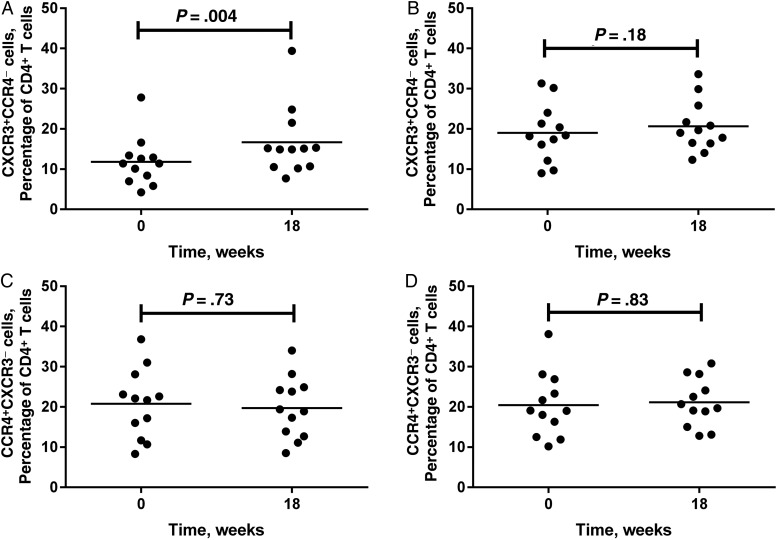
A subset of 24 animals was evaluated for T-helper cell response skewing following immunization. A transient decline in the frequency of CD4+ Th1 cells (CXCR3+CCR4−; P < .0001) and a concomitant increase in the frequency of CD4+ Th2 cells (CCR4+CXCR3−; P < .0001) occurred 1 week following initial immunization (data not shown). This was seen in KEX1-immunized animals and mock-immunized animals, suggesting that the effects were associated with adjuvant administration. By 6 weeks after boost, the frequency of CD4+ Th2 cells was similar to the frequency at baseline in KEX1-immunized animals (P = .73; Figure 2C) and mock-immunized animals (P = .83; Figure 2D). No significant change in the frequency of Th1 cells was seen in mock-immunized animals at this time (P = .18; Figure 2B); however, a modest but significant increase in frequency of peripheral blood Th1 cells was sustained at this time in KEX1-immunized macaques (P = .004; Figure 2A). There was no significant difference between the frequencies of Th1 and Th2 cells at time of SHIV infection (P = .46; Figure 1B), suggesting a balanced CD4+ T-helper cell response.
Figure 2.
Frequencies of T-helper type 1 (Th1) and Th2 cells in peripheral blood prior to and following KEX1 and mock immunizations. To evaluate skewing of the T-helper cell phenotype, peripheral blood lymphocytes were analyzed by flow cytometry for surface markers associated with either Th1 (CXCR3+CCR4−) or Th2 (CCR4+CXCR3−) CD4+ cells and expressed as a percentage of total peripheral blood CD4+ T cells. Comparisons were made between data before immunization and data 18 weeks following initial immunization (6 weeks after boost). Frequencies of T-helper populations for KEX1-immunized animals are given in panels A and C; frequencies of T-helper populations for mock-immunized animals are given in panels B and D.
Antigen-Specific IgG and IgA Responses Detected in BAL Fluid Following Systemic KEX1 Immunization
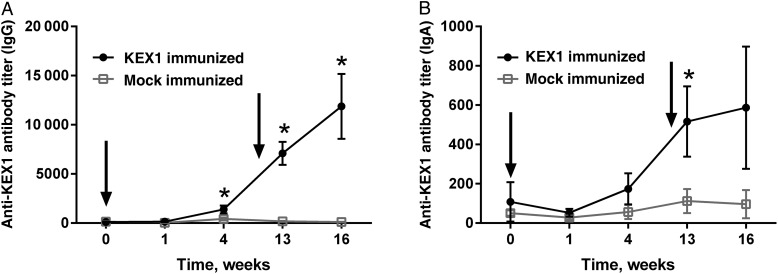
In KEX1-immunized macaques, increases in anti-KEX1 IgG titers were seen in BAL fluid 4 weeks following initial immunization (P = .006; Figure 3A). One week after boost, significant boosting of anti-KEX1 IgG titers occurred (mean [±SD], 7089 ± 4973; P < .0001) and remained significantly increased at 4 weeks following boost (mean [±SD], 11 881 ± 13 998; P = .002). Additionally, following booster immunizations, KEX1-immunized animals had a significant increase in IgA titers present in BAL fluid by 1 week following immunization (P = .001; Figure 3B). No significant increase from baseline in mean anti-KEX1 IgG or IgA titers occurred in mock-immunized animals (P > .05; data not shown).
Figure 3.
Mean KEX1-specific antibody titers in bronchoalveolar lavage fluid following immunization and boost. KEX1-specific immunoglobulin G (IgG; A) and immunoglobulin A (IgA; B) titers are shown. Error bars show standard errors of the means. There was no significant difference between peak and baseline anti-KEX1 titers in the mock-immunized group. Paired Student t tests or Wilcoxon signed rank tests were performed to compare data baseline to data at indicated time points. *P < .05, compared with baseline.
KEX1-Specific Humoral Response Following SHIV-Induced Immunosuppression
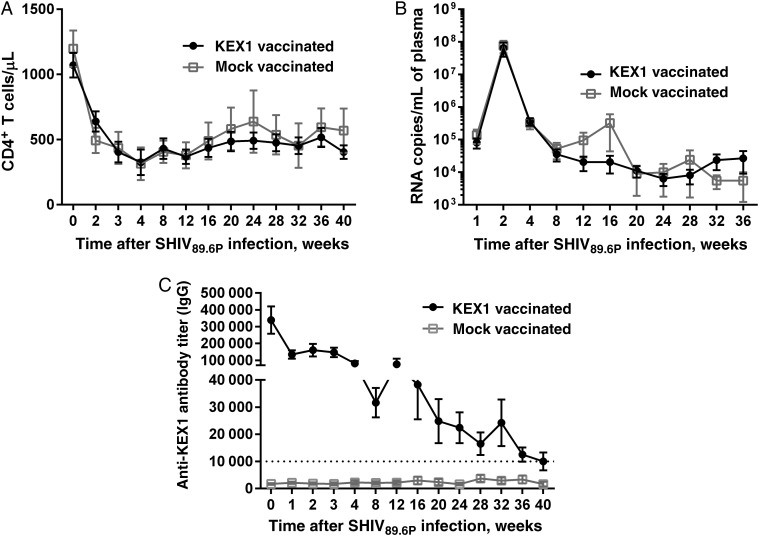
Following SHIV infection, numbers of circulating CD4+ T cells declined precipitously in both groups by 2 weeks after infection (P < .0001; Figure 4A). Mean CD4+ T-cell numbers (±SD) remained depressed and, 36 weeks after SHIV infection, were 517 ± 301 cells/µL in KEX1-immunized animals (P = .0004, compared with baseline) and 594 ± 563 cells/µL in mock-immunized animals (P < .0001, compared with baseline). Plasma virus loads were also not different between KEX1-immunized animals and mock-immunized animals (peak viral load, P = .80; viral set point, P = .79; Figure 4B). We previously reported that mean plasma KEX1 titers of 10 000 correlated with protection from natural Pneumocystis infection in SHIV-infected macaques [22]. In the current study, the mean plasma anti-KEX1 IgG titer in KEX1-immunized macaques remained >30 000 up to 16 weeks after SHIV infection (Figure 4C), well above the correlate of protection [22], and remained at ≥10 000 through 40 weeks after SHIV infection (Figure 4B). In mock-immunized animals, the mean plasma anti-KEX1 IgG titer remained below 4000 through 40 weeks after SHIV infection.
Figure 4.
Vaccine-induced humoral immune responses following simian/human immunodeficiency virus (SHIV) infection. At 10 weeks after boost, macaques were infected with SHIV89.6P. A, Mean peripheral blood CD4+ T-cell levels for KEX1-immunized animals and mock-immunized macaques, as determined by flow cytometric analysis. B, Mean plasma virus loads for KEX1-immunized animals and mock-immunized animals. C, Mean plasma KEX1-specific immunoglobulin G (IgG) titer for KEX1-immunized macaques and mock-immunized macaques following SHIV infection, as determined by enzyme-linked immunosorbent assay. Dashed line indicates KEX1-specific titer correlate of protection. Error bars show standard errors of the means.
KEX1 Immunization Protects Against PCP in SHIV-Infected Macaques
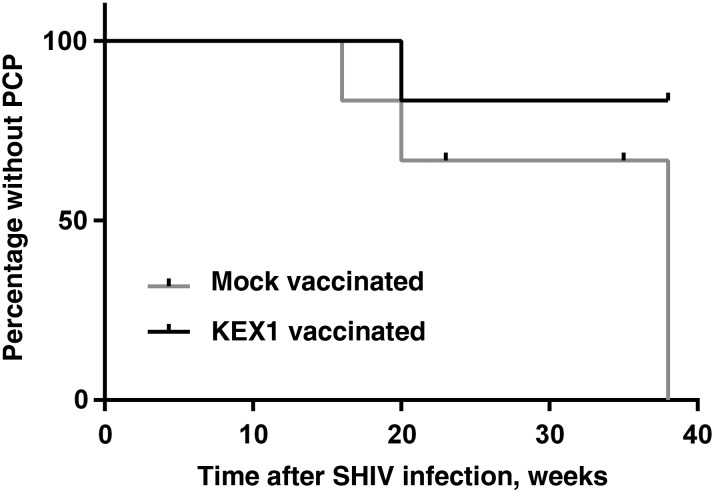
In a pilot study, 6 KEX1-immunized macaques and 6 mock-immunized macaques were infected with SHIV and challenged with Pneumocystis by natural, airborne transmission [22, 23, 30]. The mean KEX1 titer (±SD) for the KEX1-immunized group at the time of SHIV infection was 157 500 ± 177 022 and that of the mock-immunized group was 2067 ± 1475 (P = .002, by the Mann–Whitney test). None of the monkeys had evidence of Pneumocystis colonization at the time of SHIV infection. There was also no difference in the mean age of KEX1-immunized and mock-immunized animals (8.0 years and 7.7 years, respectively; P = .73; Supplementary Tables 1 and 2). Both groups were simultaneously exposed to Pneumocystis, and colonization was detectable in some animals by 4 weeks after SHIV infection (Supplementary Figures 2 and 3), when peripheral blood CD4+ T cells had declined, as previously reported [23]. Two mock-immunized animals required euthanasia prior to study termination, for clinical reasons other than PCP, and were censored in the Kaplan–Meier analysis. At study termination (40 weeks after challenge), 4 of 6 (67%) of the remaining mock-immunized macaques had developed PCP, compared with 1 of 6 KEX1-immunized macaques (17%), indicating that KEX1-immunized animals were protected from developing PCP (P = .047, by Kaplan–Meier analysis; Figure 5), despite comparable immunosuppression (ie, CD4+ T-cell count decline) and viral loads. The presence of Pneumocystis in the lungs was confirmed by IHC analysis at study termination (Supplementary Figure 4). Among the KEX1-immunized animals, the 5 protected animals did not develop signs or symptoms of PCP (up to 40 weeks after SHIV infection), and all were negative by IHC analysis for Pneumocystis organisms in the lung (Supplementary Figure 4). Of the 4 mock-immunized animals with evidence of PCP, 2 became first-round PCR positive for Pneumocystis by 16–20 weeks following SHIV infection. The remaining 2 were also colonized with Pneumocystis and had IHC-based evidence of Pneumocystis present in the lung (Supplementary Figure 4).
Figure 5.
Time to development of Pneumocystis pneumonia (PCP) in KEX1-immunized animals, compared with mock-immunized animals. Despite comparable immunosuppression, KEX1-immunized animals were significantly protected from developing PCP following simian/human immunodeficiency virus (SHIV) infection, compared with mock-immunized controls (P = .047, by Kaplan–Meier analysis). A diagnosis of PCP was made by detection of Pneumocystis DNA by polymerase chain reaction (first-round) analysis of bronchoalveolar lavage fluid and/or by detection of Pneumocystis in lung tissue by immunohistochemical analysis.
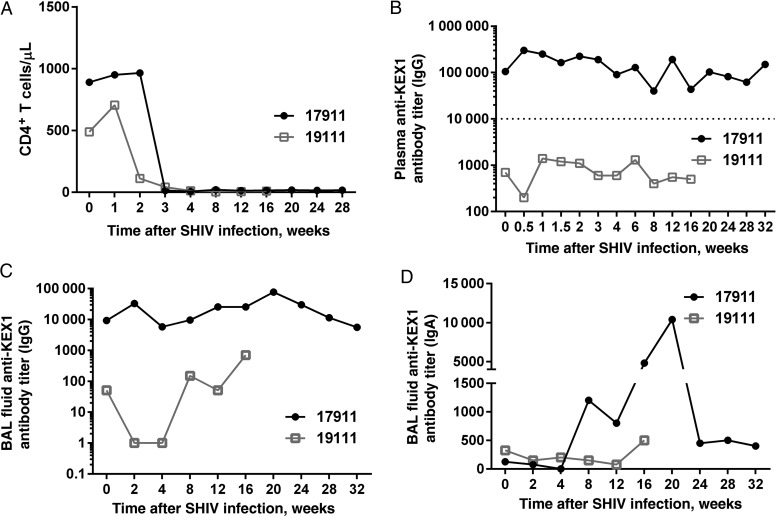
As expected with genetically distinct NHPs, responses to SHIV infection were variable within the cohort, with some animals experiencing a rapid decrease in CD4+ T-cell count and persistently high viral loads and others appearing capable of effectively controlling virus replication for a longer duration. One animal in the KEX1-immunized group (monkey 17911) experienced rapid progression of SHIV infection (CD4+ T cell count, <20 cells/µL by 2 weeks after SHIV infection) and developed evidence of PCP (ie, positive results of first-round PCR) by 20 weeks after SHIV infection; however, euthanasia was not required until 31 weeks after infection. When compared to a monkey with rapid disease progression in the mock-immunized group (monkey 19111), there were notable differences in immune responses to infection and in survival time between the 2 animals (Figure 6). Despite a profound loss of CD4+ T cells by 3 weeks after SHIV infection and evidence of Pneumocystis colonization by 4 weeks, the humoral immune response of monkey 17911 to Pneumocystis infection was robust, in comparison with that for monkey 19111, and may have contributed to prolonged survival time, despite evidence of PCP. Monkey 19111 (from the mock-immunized group) succumbed more quickly to PCP, required euthanasia (19 weeks after infection) owing to rapid clinical decline, and was unable to mount an effective immune response to Pneumocystis infection. IHC analysis confirmed ubiquitous presence of Pneumocystis organisms in lung tissue. Summary data for individual animals are presented in Supplementary Figures 2 and 3.
Figure 6.
CD4+ T-cell depletion and KEX1-antibody responses in KEX1-immunized animals and mock-immunized animals with rapid progression of disease. A, Comparable rapid decreases in CD4+ T-cell counts occurred in animals 17911 (KEX1 immunized) and 19111 (mock immunized). B–D, However, plasma anti-KEX1 immunoglobulin G (IgG; B) and bronchoalveolar lavage (BAL) fluid IgG (C) and immunoglobulin A (IgA; D) responses were notably greater in animal 17911, compared with animal 19111. These data suggest that immune responses to KEX1 immunization prior to simian/human immunodeficiency virus (SHIV) infection can be maintained at comparably high levels despite severe CD4+ T-cell depression. Dashed line indicates KEX1-specific IgG titer correlate of protection [22].
DISCUSSION
We examined the immunogenicity of the Pneumocystis vaccine candidate KEX1 and the durability of humoral immunity, induced by vaccination in immunosuppressed macaques, as a model of chronic HIV infection and other complex immunocompromised hosts. In addition, we tested the efficacy of this vaccine in SHIV-infected NHPs as a model of HIV infection. We found that immunization of healthy macaques with KEX1 induced robust humoral memory responses, which exceeded previously determined correlates of protection [22], and that this response was maintained during chronic SHIV-induced immunosuppression. The Pneumocystis vaccine candidate, KEX1, is a protein to which most adults have some level of preexisting immunity and are likely restimulated regularly as a result of environmental reexposure to this ubiquitous opportunistic organism. We surmise that induction of extraordinarily high anti-KEX1 antibody titers (>1 × 106) following a modest immunization regimen and that long-lasting B-cell memory, despite subsequent immunosuppression, may be due to high prevalence of KEX1-positive serological findings in NHPs, thus bypassing the need for immunologic priming by immunization. The high frequency of KEX1-positive serological results in adults (both HIV positive and HIV negative) [2, 15, 35] is comparable to that found in extensive studies of normal rhesus and cynomolgus macaques [23, 36], thus we predict similarly strong responses to this vaccine regimen in human subjects, even those who subsequently become immunocompromised.
Immunization of immunocompetent macaques with recombinant KEX1 resulted in generation of high levels of plasma anti-KEX1 IgG antibodies and sustained anti-KEX1 IgG–releasing memory B cells. Following boost, mean peak titers exceeded 2 × 106, which greatly exceeded plasma anti-KEX1 IgG titers previously shown to be associated with protection from natural Pneumocystis infection [22]. We also report a significant increase in the KEX1-specific memory B-cell population, which was greater than the KEX1-specific memory B-cell frequency previously reported to correlate with protection from natural Pneumocystis colonization [22], suggesting stimulation of immunological memory. Importantly, we report stimulation of anti-KEX1 IgG and IgA antibodies in the lung [22]. We previously demonstrated that early detection of anti-KEX1 IgA in the lung correlated with protection from Pneumocystis infection and subsequent evidence of COPD [22]. This is supported by evidence that local Pneumocystis–specific antibodies likely participate in host defense against Pneumocystis [14, 37, 38].
Achieving a balanced helper T-cell response following immunization has important implications for immunizing individuals at risk for acquiring HIV, as type 1 T-helper cell responses are critical for viral control [39]; thus, we serially analyzed Th1 and Th2 responses following immunization and boost. Early after immunization with KEX1/alum, we found moderate, transient skewing of CD4+ T-cell responses toward type 2, though this skewing was not augmented by subsequent immunizations and resolved to the preimmunization balance by 10 weeks after vaccination. Thus, we did not observe persistent Th2 skewing as a result of alum-based vaccination.
As anticipated, SHIV infection of macaques resulted in a precipitous decline in numbers of peripheral blood CD4+ T cells. Despite the decline in CD4+ helper T-cell counts, the mean circulating anti-KEX1 IgG titer remained ≥10 000 (a previously established correlate of protection) until approximately 10 months after SHIV infection, a point when some animals were approaching end-stage AIDS. During SHIV infection, it is possible that restimulation of humoral responses from Pneumocystis colonization/exposure accounts for a portion of the anti-KEX1 antibody response; however, high anti-KEX1 antibody titers persisted in immunized animals, even when Pneumocystis colonization was not evident by PCR (Supplementary Figure 2). Thus, we conclude that immunization prior to SHIV infection accounts for at least a portion of persistently high antibody titers observed in these animals. Additionally, circulating KEX1-specific memory B cells were maintained above preimmunization levels, despite immunosuppression indicating induction of strong and durable memory responses to KEX1 vaccination.
In addition to CD4+ T-cell loss, HIV-infected individuals have a number of B-cell compartment perturbations, which likely influence responses to immunizations. Hyperactivation, exhaustion, and reduced memory populations and reduced capacity to respond to stimulation in vitro have all been shown in B-cell populations in HIV infection and in NHP models of HIV infection [40–45] and may contribute to impaired immunization responsiveness in vivo. Several studies indicate that HIV-infected individuals have reduced antibody responses to common recall antigens, including influenza [46], hepatitis A [47], tetanus [48], and pneumococcal [49] vaccines, compared with non–HIV-infected individuals. Conflicting reports on maintenance of recall responses may be related to the type of antigen assessed (protein vs carbohydrate), the level of circulating CD4+ T cells, and/or the age of study subjects [48, 50]. We reported that, despite B-cell perturbations mimicking HIV-induced B-cell dysregulation, some SHIV-infected macaques were capable of maintaining significant levels of peripheral blood KEX1-specific memory B cells and were protected from Pneumocystis infection, compared with those susceptible to Pneumocystis infection [22, 26]. These studies suggested maintenance of durable memory B-cell responses to KEX1 antigen and supported the feasibility of KEX1 as a Pneumocystis vaccine candidate. The current study supports the concept that peptide vaccine–induced B-cell memory responses can be maintained following immunosuppression. similar to that induced by HIV infection.
In challenge studies, KEX1-immunized animals were significantly less likely to develop PCP following SHIV-induced immunosuppression and Pneumocystis exposure, compared with mock-immunized animals. Of the mock-immunized animals that were either euthanized due to severe PCP or remained in the study for the full duration, all had histological evidence of PCP and thereby met the criteria for PCP diagnosis. KEX1-associated immune responses to Pneumocystis exposure were low in mock-immunized animals, as would be expected for animals with low plasma titers (<10 000 for IgG) prior to SHIV infection [22] and negligible B-cell memory responses, and thus ineffective at controlling Pneumocystis infection.
The model of P. jirovecii infection (natural transmission by airborne exposure) used for this study presents a number of challenges. Although it more accurately emulates the likely route by which susceptible humans acquire P. jirovecii infection, it lacks dose-associated precision. Furthermore, because macaques are outbred, genetically distinct animals, they demonstrate variable responses to SHIV infection, and as in human HIV infection, we observed a spectrum of disease progression within a single cohort. However, variation in responses also reveals pertinent information for characterizing different responses to Pneumocystis infection. We have noted 2 animals with rapidly progressing disease that had comparable CD4+ T-cell depletion and peak viral loads, 1 KEX1-immunized animal and 1 mock-immunized animal. The KEX1-immunized macaque maintained high KEX1 titers in plasma and BAL fluid throughout SHIV infection until euthanasia was required (31 weeks after SHIV infection), despite severely depressed CD4+ T-cell numbers. The comparable mock-immunized animal did not produce effective humoral immune responses to Pneumocystis infection, quickly developed PCP, and required euthanasia at 19 weeks after SIV infection.
Importantly, protection from PCP development in KEX1-immunzed animals was not due to lack of Pneumocystis exposure. Transient Pneumocystis colonization was evident in KEX1-immunized animals, but the majority (83%) did not develop PCP. Immune responses to Pneumocystis exposure were evident by transient increases in anti-KEX1 antibodies, which may have contributed to control of colonization. Anti-KEX1 antibody responses were evident in some mock-immunized controls, but responses were low (titers, <6000), compared with those for KEX1-immunized animals, and these responses were insufficient at preventing PCP.
Given the robust responses induced and the protective efficacy of the vaccine, an important question to be addressed in subsequent studies is whether vaccination is effective after SHIV infection, during a phase of infection when CD4+ T cells are stabilized above the level at which patients are at risk of acquiring PCP. Our preliminary studies suggest a robust KEX1-specific immune responses can be stimulated in SHIV-infected, immunosuppressed animals, and studies to address the protective capacity of this immunization strategy are currently underway.
In summary, we report a method for immunization against PCP in a preclinical model of HIV infection. The durability of memory responses in immunosuppressed animals suggests that this vaccination approach may be useful for the development of vaccination strategies for other pathogens for which there is immunologic memory.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Irina Lebedeva, Sangita Patil, Margaret Beucher, Andrew Burton, Nicholas Giaccobi, Siobhan Guyach, Alexandria McManus, Rebecca Tarantelli, and ToniAnn Zullo, for technical support; Dr Chris Janssen, for technical and veterinary support; and Drs Judd Shellito, Alistair Ramsay, and David Welsh, for helpful discussions of this work.
Financial support. This work was supported by the National Institutes of Health (grants 5R01HL064563, R56AI091576, and 2P01HL076100).
Potential conflict of interest. H. M. K. and K. A. N. are coinventors of kexin-associated technologies related to methods of vaccination for fungal infections and may be entitled to royalties. Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kling HM, Shipley TW, Guyach S, Tarantelli R, Morris A, Norris KA. Trimethoprim-sulfamethoxazole treatment does not reverse obstructive pulmonary changes in pneumocystis-colonized nonhuman primates with SHIV infection. J Acquir Immune Defic Syndr 2014; 65:381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris A, Netravali M, Kling HM et al. Relationship of pneumocystis antibody response to severity of chronic obstructive pulmonary disease. Clin Infect Dis 2008; 47:e64–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris A, Sciurba FC, Lebedeva IP et al. Association of chronic obstructive pulmonary disease severity and Pneumocystis colonization. Am J Respir Crit Care Med 2004; 170:408–13. [DOI] [PubMed] [Google Scholar]
- 4.Morris A, George MP, Crothers K et al. HIV and chronic obstructive pulmonary disease: is it worse and why? Proc Am Thorac Soc 2011; 8:320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller RF, Huang L, Walzer PD. Pneumocystis pneumonia associated with human immunodeficiency virus. Clin Chest Med 2013; 34:229–41. [DOI] [PubMed] [Google Scholar]
- 6.Lowe DM, Rangaka MX, Gordon F, James CD, Miller RF. Pneumocystis jirovecii pneumonia in tropical and low and middle income countries: a systematic review and meta-regression. PLoS One 2013; 8:e69969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Worth LJ, Dooley MJ, Seymour JF, Mileshkin L, Slavin MA, Thursky KA. An analysis of the utilisation of chemoprophylaxis against Pneumocystis jirovecii pneumonia in patients with malignancy receiving corticosteroid therapy at a cancer hospital. Br J Cancer 2005; 92:867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eitner F, Hauser IA, Rettkowski O et al. Risk factors for Pneumocystis jiroveci pneumonia (PcP) in renal transplant recipients. Nephrol Dial Transplant 2011; 26:2013–7. [DOI] [PubMed] [Google Scholar]
- 9.Mori S, Cho I, Sugimoto M. A cluster of Pneumocystis jirovecii infection among outpatients with rheumatoid arthritis. J Rheumatol 2010; 37:1547–8. [DOI] [PubMed] [Google Scholar]
- 10.Roux A, Gonzalez F, Roux M et al. Update on pulmonary Pneumocystis jirovecii infection in non-HIV patients. Med Mal Infect 2014; 44:185–98. [DOI] [PubMed] [Google Scholar]
- 11.Peglow SL, Smulian AG, Linke MJ et al. Serologic responses to Pneumocystis carinii antigens in health and disease. J Infect Dis 1990; 161:296–306. [DOI] [PubMed] [Google Scholar]
- 12.Respaldiza N, Medrano FJ, Medrano AC et al. High seroprevalence of Pneumocystis infection in Spanish children. Clin Microbiol Infect 2004; 10:1029–31. [DOI] [PubMed] [Google Scholar]
- 13.Vargas SL, Hughes WT, Santolaya ME et al. Search for primary infection by Pneumocystis carinii in a cohort of normal, healthy infants. Clin Infect Dis 2001; 32:855–61. [DOI] [PubMed] [Google Scholar]
- 14.Pascale JM, Shaw MM, Durant PJ et al. Intranasal immunization confers protection against murine Pneumocystis carinii lung infection. Infect Immun 1999; 67:805–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gingo MR, Lucht L, Daly KR et al. Serologic responses to pneumocystis proteins in HIV patients with and without Pneumocystis jirovecii pneumonia. J Acquir Immune Defic Syndr 2011; 57:190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Empey KM, Hollifield M, Schuer K, Gigliotti F, Garvy BA. Passive immunization of neonatal mice against Pneumocystis carinii f. sp. muris enhances control of infection without stimulating inflammation. Infect Immun 2004; 72:6211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garvy BA, Wiley JA, Gigliotti F, Harmsen AG. Protection against Pneumocystis carinii pneumonia by antibodies generated from either T helper 1 or T helper 2 responses. Infect Immun 1997; 65:5052–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gigliotti F, Garvy BA, Haidaris CG, Harmsen AG. Recognition of Pneumocystis carinii antigens by local antibody-secreting cells following resolution of P. carinii pneumonia in mice. J Infect Dis 1998; 178:235–42. [DOI] [PubMed] [Google Scholar]
- 19.Gigliotti F, Haidaris CG, Wright TW, Harmsen AG. Passive intranasal monoclonal antibody prophylaxis against murine Pneumocystis carinii pneumonia. Infect Immun 2002; 70:1069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gigliotti F, Hughes WT. Passive immunoprophylaxis with specific monoclonal antibody confers partial protection against Pneumocystis carinii pneumonitis in animal models. J Clin Invest 1988; 81:1666–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells J, Haidaris CG, Wright TW, Gigliotti F. Active immunization against Pneumocystis carinii with a recombinant. P. carinii antigen. Infect Immun 2006; 74:2446–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kling HM, Shipley TW, Patil SP et al. Relationship of Pneumocystis jiroveci humoral immunity to prevention of colonization and chronic obstructive pulmonary disease in a primate model of HIV infection. Infect Immun 2010; 78:4320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kling HM, Shipley TW, Patil S, Morris A, Norris KA. Pneumocystis colonization in immunocompetent and simian immunodeficiency virus-infected cynomolgus macaques. J Infect Dis 2009; 199:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Croix DA, Board K, Capuano S III et al. Alterations in T lymphocyte profiles of bronchoalveolar lavage fluid from SIV- and Pneumocystis carinii-coinfected rhesus macaques. AIDS Res Hum Retroviruses 2002; 18:391–401. [DOI] [PubMed] [Google Scholar]
- 25.Board KF, Patil S, Lebedeva I et al. Experimental Pneumocystis carinii pneumonia in simian immunodeficiency virus-infected rhesus macaques. J Infect Dis 2003; 187:576–88. [DOI] [PubMed] [Google Scholar]
- 26.Kling HM, Shipley TW, Norris KA. Alterations in peripheral blood B-cell populations in SHIV89.6P-infected macaques (Macacca fascicularis). Comp Med 2011; 61:269–77. [PMC free article] [PubMed] [Google Scholar]
- 27.Roederer M. Compensation in flow cytometry. Curr Protoc Cytom 2002; 22:1.14.1–1.14.20. [DOI] [PubMed] [Google Scholar]
- 28.Reimann KA, Li JT, Veazey R et al. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol 1996; 70:6922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reimann KA, Li JT, Voss G et al. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J Virol 1996; 70:3198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shipley TW, Kling HM, Morris A et al. Persistent Pneumocystis colonization leads to the development of chronic obstructive pulmonary disease (COPD) in a non-human primate model of AIDS. J Infect Dis 2010; 202:302–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radio SJ, Hansen S, Goldsmith J, Linder J. Immunohistochemistry of Pneumocystis carinii infection. Mod Pathol 1990; 3:462–9. [PubMed] [Google Scholar]
- 32.Fischer S, Gill VJ, Kovacs J et al. The use of oral washes to diagnose Pneumocystis carinii pneumonia: a blinded prospective study using a polymerase chain reaction-based detection system. J Infect Dis 2001; 184:1485–8. [DOI] [PubMed] [Google Scholar]
- 33.Patil SP, Board KF, Lebedeva IP, Norris KA. Immune responses to Pneumocystis colonization and infection in a simian model of AIDS. J Eukaryot Microbiol 2003; 50(Suppl):661– 2. [DOI] [PubMed] [Google Scholar]
- 34.Savoia D, Millesimo M, Cassetta I, Forno B, Caramello P. Detection of Pneumocystis carinii by DNA amplification in human immunodeficiency virus-positive patients. Diagn Microbiol Infect Dis 1997; 29:61–5. [DOI] [PubMed] [Google Scholar]
- 35.Bloomfield GS, Khazanie P, Morris A et al. HIV and noncommunicable cardiovascular and pulmonary diseases in low- and middle-income countries in the ART era: what we know and best directions for future research. J Acquir Immune Defic Syndr 2014; 67(suppl 1):S40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demanche C, Wanert F, Barthelemy M et al. Molecular and serological evidence of Pneumocystis circulation in a social organization of healthy macaques (Macaca fascicularis). Microbiology 2005; 151:3117–25. [DOI] [PubMed] [Google Scholar]
- 37.Laursen AL, Jensen BN, Andersen PL. Local antibodies against Pneumocystis carinii in bronchoalveolar lavage fluid. Eur Respir J 1994; 7:679–85. [DOI] [PubMed] [Google Scholar]
- 38.Jalil A, Moja P, Lambert C et al. Decreased production of local immunoglobulin A to Pneumocystis carinii in bronchoalveolar lavage fluid from human immunodeficiency virus-positive patients. Infect Immun 2000; 68:1054–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soghoian DZ, Streeck H. Cytolytic CD4(+) T cells in viral immunity. Expert Rev Vaccines 2010; 9:1453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med 1983; 309:453–8. [DOI] [PubMed] [Google Scholar]
- 41.Malaspina A, Moir S, Kottilil S et al. Deleterious effect of HIV-1 plasma viremia on B cell costimulatory function. J Immunol 2003; 170:5965–72. [DOI] [PubMed] [Google Scholar]
- 42.Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol 2009; 9:235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moir S, Ho J, Malaspina A et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med 2008; 205:1797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Titanji K, De Milito A, Cagigi A et al. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood 2006; 108:1580–7. [DOI] [PubMed] [Google Scholar]
- 45.Jiang W, Lederman MM, Mohner RJ et al. Impaired naive and memory B-cell responsiveness to TLR9 stimulation in human immunodeficiency virus infection. J Virol 2008; 82:7837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malaspina A, Moir S, Orsega SM et al. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J Infect Dis 2005; 191:1442–50. [DOI] [PubMed] [Google Scholar]
- 47.Weissman S, Feucht C, Moore BA. Response to hepatitis A vaccine in HIV-positive patients. J Viral Hepat 2006; 13:81–6. [DOI] [PubMed] [Google Scholar]
- 48.Choudhury SA, Matin F. Subnormal and waning immunity to tetanus toxoid in previously vaccinated HIV-infected children and response to booster doses of the vaccine. Int J Infect Dis 2013; 17:e1249–51. [DOI] [PubMed] [Google Scholar]
- 49.Hart M, Steel A, Clark SA et al. Loss of discrete memory B cell subsets is associated with impaired immunization responses in HIV-1 infection and may be a risk factor for invasive pneumococcal disease. J Immunol 2007; 178:8212–20. [DOI] [PubMed] [Google Scholar]
- 50.Rinaldi S, Zangari P, Cotugno N et al. Antibody but not memory B-cell responses are tuned-down in vertically HIV-1 infected children and young individuals being vaccinated yearly against influenza. Vaccine 2014; 32:657–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.