Abstract
Background
HDAC1 has been shown to be closely associated with the occurrence of tumors. We aimed to investigate the effects of siRNA-mediated HDAC1 knockdown on the biological behavior of esophageal carcinoma cell lines.
Material/Methods
HDAC1 expression in esophageal cancer cell lines TE-1, Eca109, and EC9706 was compared by Western blot analysis. These cells were transfected with siRNA-HDAC1 and cell proliferation was evaluated by MTT assay to select the optimum cell line for subsequent experiments. The effects of siRNA-HDAC1 on the migration and invasion of the selected cell line were assessed by transwell assay. The expression of cell cycle-related proteins cyclinD1, p21 and p27, and epithelial-mesenchymal transition (EMT)-related protein zonula occludens-1 (ZO-1), E-cadherin and vimentin was determined by Western blot analysis.
Results
HDAC1 expression in TE-1, Eca109 and EC9706 cells was significantly higher compared with normal esophageal cell line HEEC (P<0.01). MTT assay, Western blot and RT-PCR analyses demonstrated that the inhibitory effects of siRNA on HDAC1 expression and cell viability in TE-1 cells were the highest among all cell lines, which was therefore used in subsequent experiments. After TE-1 cells were transfected with siRNA-HDAC1, their migration and invasion were significantly lower compared with the controls (P<0.01). CyclinD1 and vimentin expression was significantly lower compared with the controls (P<0.01), whereas the expression of p21, p27, ZO-1 and E-cadherin was significantly higher (P<0.01).
Conclusions
The siRNA-mediated HDAC1 knockdown significantly inhibited the proliferation, migration and invasion of TE-1 cells probably by regulating the expression of cell cycle- and EMT-related proteins.
MeSH Keywords: Biohazard Release, Esophageal Achalasia, Histone Deacetylase 1
Background
The occurrence and development of esophageal cancer is a complex process involving a series of physiological changes including abnormal proliferation, apoptosis, invasion and metastasis of cells. Clinical manifestations of esophageal cancer include anorexia, weight loss, perforation, etc. Under normal physiological conditions, intracellular levels of acetylation and deacetylation remain in a dynamic equilibrium, whereas histone deacetylases (HDACs) are abnormally activated in a variety of tumors, and recruited in specific promoter regions, leading to the inhibition of transcription of a series of genes that are involved in the process of cell proliferation, differentiation, migration, invasion and metastasis [1]. HDACs are a class of enzymes that regulate gene transcription factors, and are divided into four classes including class I, II, III and IV. HDAC1, 2, 3 and 8 belong to class I. HDAC1 is the first mammalian HDAC that was discovered by Taunton et al. at the Harvard University in 1996. It is the most important member of the HDACs family with the closest association with tumor, and regulates gene transcription by acting on the corresponding histone.
Senese et al. have shown that HDAC1 deficiency in tumor cells can induce arrest of cell cycle in the G1 or G2/M phase, decreased or abnormal mitosis, and reduced cell proliferation [2]. The proportion of apoptotic cells is markedly increased. These finding suggest that the expression of HDAC1 is closely associated with cell cycle, proliferation and apoptosis of tumor cells. CyclinD1 is the first key protein synthesized in the G1 phase, and plays a crucial role in the transition between the G0/G1 and S phase. CyclinD1 binds to cyclin-dependent protein kinase (Cdk4) and forms cyclin-Cdk complexes, leading to the activation of protein kinase Cdk, which induces the entry of cells into the S phase and subsequent cell division through a series of regulatory actions. p21 and p27 are cyclin-dependent kinase inhibitors [3,4]. p21 gene is located on human chromosome 6, and inhibits the activity of cyclin-Cdk complexes and antigens involved in cell proliferation. Increased p21 expression greatly prevents the formation of cyclin-Cdk4 complexes and inhibits the proliferation of cells by cell cycle arrest in the G1 phase. p27 is located on chromosome 12, and blocks cell cycle in the G1 phase by inhibiting the binding of cyclinE to Cdk2. Previous studies have demonstrated that p21 and p27 are negative regulators of cell cycle, and are associated with the proliferation and metastasis of esophageal cancer cell. They also have good prognostic values in esophageal cancer [5,6].
The epithelial-mesenchymal transition (EMT) is a multistage complex process involving multiple genes during which epithelial cells gain migratory and invasive properties to become mesenchymal stem cells or fibroblasts. Numerous studies have shown that EMT is important for the occurrence, development and metastasis of tumors, and might be crucial for the start of cancer invasion and metastasis [7]. Vimentin is an intermediate filament (IF) protein that is frequently expressed in mesenchymal tumors. It has been known that increased expression of vimentin stimulates the invasion and metastasis of tumors, indicating that the reverse of the expression of EMT-related proteins may significantly inhibit the development of tumors. ZO-1 is a tight junction protein that controls the formation of tight junctions. Functional abnormity of ZO-1 plays an important role in the occurrence and development of a wide range of diseases. E-cadherin is an epithelial marker whose expression deficiency is associated with the invasion, migration and poor prognosis of tumors [8].
Material and methods
Reagents and instruments
Esophageal cancer cell lines TE-1, Eca109 and EC9706 and normal esophageal cell line HEEC were purchased from the cell bank of the Chinese Academy of Sciences (catalog number: GNHu40; Shanghai, China). Tetrazolium reagent (MTT) and crystal violet were purchased from Sigma (St. Louis, MO, USA). Rabbit anti-cyclin D1, p21, p27 and ZO-1 antibodies were purchased from Epitomics (Burlingame, CA, USA). Rabbit anti-E-cadherin and vimentin antibodies were purchased from Abcam (Cambridge, MA, USA). GADPH and cell cycle assay kit were purchased from Beyotime Institute of Biotechnology (Shanghai, China). Transwell chambers were purchased from Corning (Corning, NY, USA). Fetal bovine serum, DMEM culture medium and trypsin were purchased from Gibco. The siRNA targeting HDAC1 (siRNA-HDAC1) and negative control (siRNA-NC) were synthesized by GenePharma Biotech. (Shanghai, China). The sequences were as follows: 5′-GCCGGUCAUGUCCAAAGUATT-3′, 5′-UACUUUGGACAUGACCGGCTT-3′; 5′-GCCCTGAGGGC CCGAACTGTTACT-3′, 5′-CAGACGCACGGCTTTGACCTTCTT -3′. CO2 incubator and biological safety cabinet were purchased from Thermo Scientific Company. Flow cytometer was purchased from BD Company. Mini double vertical electrophoresis and mini trans-blot electrophoretic transfer cell were purchased from Beijing Liuyi Instrument Factory (Beijing, China). Molecular Imager® ChemiDocTM XRS System was manufactured by Bio-Rad Laboratories.
Western blot analysis
The expression of HDAC1 in esophageal cancer cell lines TE-1, Eca109 and EC9706 and normal esophageal cell HEEC was compared by Western blot analysis. Briefly, aliquots of 100 μl of cells were inoculated into each well of 96-well plates and cultured at 37°C in a 5% CO2 incubator. Four plates were prepared for each cell line. Cells at 70% confluence were collected. Total protein was extracted using protein extraction kits (Beyotime Institute of Biotechnology), and quantified using a BCA kit (Beyotime Institute of Biotechnology). Equal amounts of total protein (20 μg) were separated by SDS-PAGE electrophoresis and transferred to polyvinylidene difluoride membranes. The membrane was blocked in TBS buffer containing 5% skim milk and 0.1% Tween20 at room temperature for 1 h and incubated with the appropriate primary antibody (1:200) overnight at pH 7.6 at 4°C with gentle shaking. The peroxidase-labeled secondary antibodies (1:200) were added and the membranes were incubated at 37°C for 1h. The membranes were washed 3 times with TBST for 5 min each and subjected to ECL detection. The intensity of bands was detected by a Molecular Imager® ChemiDocTM XRS System (Bio-Rad Laboratories). The gray value of bands was analyzed by Image Lab 2.0 software (Bio-Rad Laboratories Inc).
MTT assay
Aliquots of 100 μl of cells were inoculated into each well of 96-well plates and cultured at 37°C in a 5% CO2 incubator. Cells at 70% confluence were transfected with 100 nmol siRNA-HDAC1 or 50 nmol siRNA-NC using Lipofectamine 2000. A total of 20 μl of 5 mg/ml MTT was added to the appropriate well after 24, 48, 72 and 96 h, respectively. Medium was discarded after 4 h, and 150 μl of DMSO was added to each well. The optical density of dissolved MTT crystals was measured by a plate reader (BioRad) at 560 nm.
Cell migration assay
Cells were transfected with 100 nmol siRNA-HDAC1 or 50 nmol siRNA-NC as described in 2.3. After 72 h, cells were digested and added into the upper chamber of transwell. DMEM medium containing 5% FBS was added into the lower chamber. After 24 h of culture, migrated cells were washed, fixed and stained with crystal violet. The transmembrane cells were counted under an inverted microscopy. Mean values were obtained from five randomly selected fields for each well.
Cell invasion assay
Matrigel (BD Biosciences) was spread evenly on the micro-film of a transwell chamber. Other steps were performed as described in 2.4. Mean values of transmembrane cells were obtained from five randomly selected fields for each well.
Detection of the expression of cell cycle-and EMT-related proteins
Cells were transfected with 100 nmol siRNA-HDAC1 or 50 nmol siRNA-NC as described in 2.4. After 72 h, cells were collected and treated with an appropriate volume of RIPA lysis buffer. The mixture was vortexed for 30 sec every 10 min. After 40 min, the mixture was centrifuged at 10000 rpm for 10 min at 4°C. The supernatant was carefully transferred to new EP tubes and the concentration of total protein was measured using a BCA kit (Beyotime Institute of Biotechnology).
Equal amounts of total protein (20 μg) were subjected to Western blot analyses as described in 2.2 in order to determine the expression of cell cycle- and EMT-related proteins. The first and second antibodies were both diluted in 1: 100 before use.
Statistical analysis
Data was analyzed by SPSS11.0 (SPSS inc., Chicago, IL, USA). Difference between groups was analyzed by t tests. P values smaller than 0.05 were considered statistically significant.
Results
Comparison of HDAC1 expression in difference esophageal cancer cell lines and the selection of optimal cell line
As shown in Figure 1A, HDAC1 expression in cell line TE-1, Eca109 and EC9706 was significantly higher than that in normal esophageal HEEC cells (P <0.01 or 0.05). Moreover, the highest HDAC1 expression was observed in TE-1 and Eca109 (P<0.01). After transfection of siRNA-HDAC1, HDAC1 expression in TE-1 and Eca109 were significantly decreased as shown by results of Western blot analyses (Figure 1B). As shown in Figure 1C, results of MTT assay demonstrated that the relative cell viability of TE-1 was significantly lower compared with Eca109 cells at 72 and 96 h after transfection, indicating that the inference of siRNA-HDAC1 on HDAC1 expression in TE-1 cells was the highest among the three cancer cells lines. The inhibition of siRNA-HDAC1 on cell viability was also the highest in TE-1 cells, which were therefore chosen for subsequent experiments.
Figure 1.
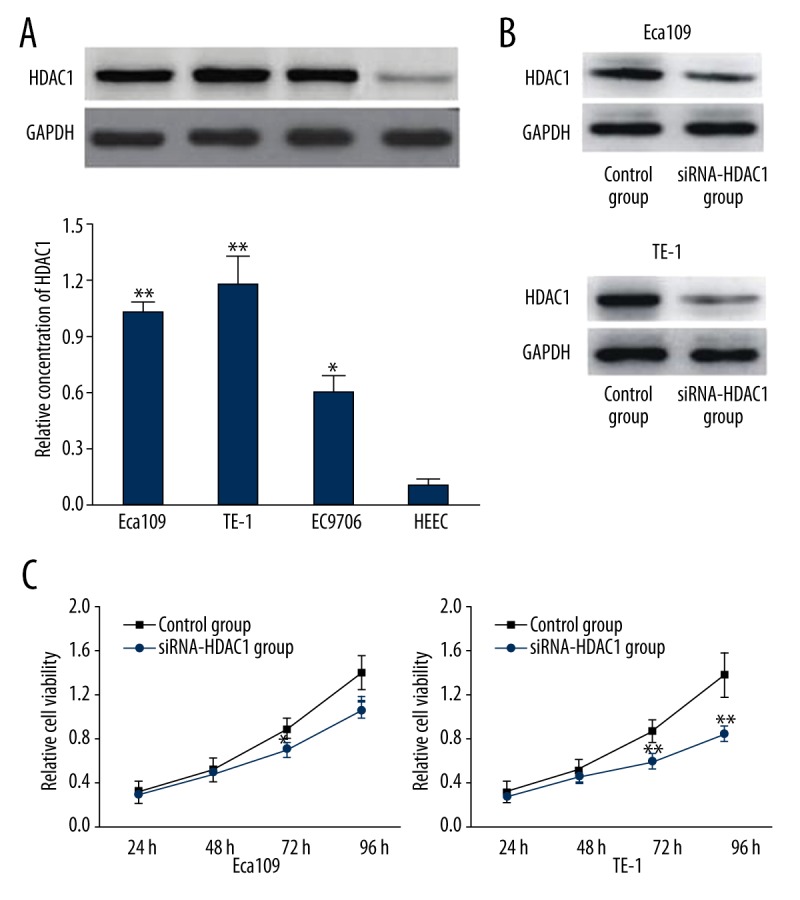
(A) Western-blot analysis showing the expression of HDAC1 in esophageal cancer cell lines Eca109, TE-1, and EC9706, and normal esophageal HEEC cells (control). ** Indicates P<0.01, * indicates P<0.05 when compared with the control group. (B) Western blot analysis of HDAC1 expression in Eca109 and TE-1 transfected with siRNA-HDAC1. (C) MTT assay showing inhibitory effects of siRNA-HDAC1 on cell viability of Eca109 and TE-1. ** Indicates P<0.01, * indicates P<0.05 when compared with the control group.
Effects of siRNA-HDAC1 on the migration and invasion of esophageal cancer cells
As shown in Figure 2A, cell migration assay demonstrated that the number of migrated TE-1 cells in siRNA-HDAC1 group was significantly decreased (208.48±27.84 vs. 69.63±8.49, P <0.01) compared with the control group. Results of cell invasion assay showed that the transmembrane TE-1 cells in siRNA-HDAC1 group was also significantly lower than that in the control group (132.48±12.43 vs. 38.62±3.29, P<0.01). These results suggested that the migration and invasion abilities of TE-1 cells were significantly reduced after the downregulation of HDAC1 expression by siRNA-HDAC1.
Figure 2.
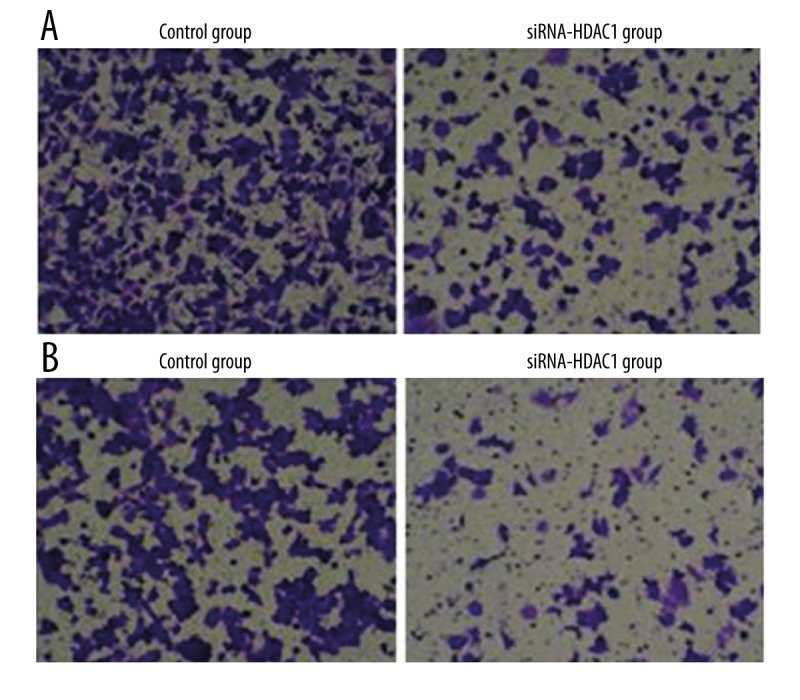
(A) Cell migration assay showing the number of migrated TE-1 cells in the siRNA-HDAC1 group was significantly lower compared with the control group (P<0.01). (B) Cell invasion assay showing the number of migrated TE-1 cells in the siRNA-HDAC1 group was significantly lower compared with the control group (P<0.01).
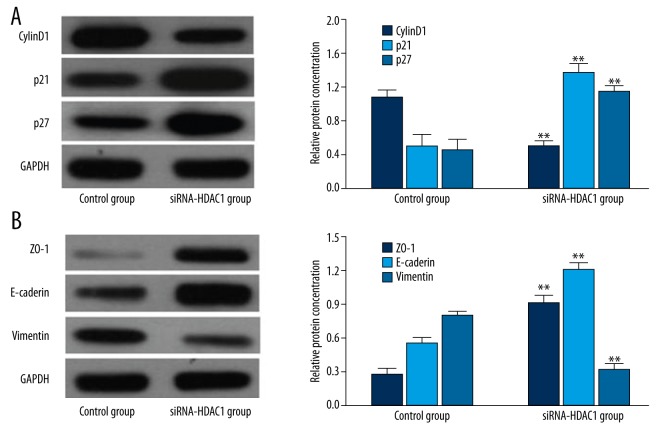
Effects of siRNA-HDAC1 on the expression of cell cycle-and EMT-related proteins
As shown in Figure 3, the expression of cyclinD1 in TE-1 cells transfected with siRNA-HDAC1 was significantly lower than that in the control group (P<0.01), whereas p21 and p27 expression was significantly higher (P<0.01). The expression of ZO-1 and E-cadherin in TE-1 cells transfected with siRNA-HDAC1 was significantly upregulated compared with the control group (P<0.01), whereas vimentin expression was significantly downregulated (P<0.01).
Figure 3.
(A) Western blot analysis comparing the expression of cell cycle-related proteins in TE-1 cells transfected with siRNA-HDAC1 and the control group (P<0.01). (B) Western blot analysis comparing the expression of EMT-related proteins in TE-1 cells transfected with siRNA-HDAC1 and the control group (P<0.01).
Discussion
Numerous studies have confirmed the close association between HDAC1 dysfunction and the development of a variety of tumors [9]. Quint et al. have compared the expression of HDAC1 in 170 cases of primary hepatocellular carcinoma and adjacent normal tissues by immunohistochemical staining, and found that HDAC1 in hepatic cancer tissue is significantly higher than that in normal tissues and was closely related to the grade of tumors [10]. Cheng et al. have demonstrated that HDAC1 expression in hepatocellular carcinoma and tumor-associated fibroblasts is significantly higher compared with liver tissue and fibroblasts [11]. Studies have shown that HDACs inhibitors suppress the proliferation of a variety of cancer cells including liver cancer, lung cancer, cervical cancer, prostate cancer, breast cancer, colon cancer, etc. and induce their differentiation or apoptosis [9,12].
These findings have clearly shown that HDAC1 anomalies are closely associated with the occurrence and development of malignant tumors. However, its role in esophageal cancer has not yet been studied. In this study, it was found that HDAC1 expression in TE-1, Eca109 and EC9706 cells was significantly increased compared with normal esophageal cells. Further, the interferences of siRNA-HDAC1 on HDAC1 expression and cell viability in TE-1, Eca109 and EC9706 cells transfected with siRNA-HDAC1were compared by Western blot analysis and MTT assay. TE-1 cells with the highest interferences of siRNA-HDAC1 were chosen for subsequent experiments.
Lei et al. have confirmed the overexpression of HDAC1 in invasive hepatic cancer tissues and significant inhibition of siRNA-mediated HDAC1 knockdown on cell migration. They have also found that HDAC1 inhibitor maspin can reverse EMT in prostate cancer cells [13], suggesting that HDAC1 is not only closely related to the occurrence and development of liver cancer, but also affects its metastasis. Increasingly more evidences have shown that EMT plays an important role in the beginning of tumor invasion and metastasis [14]. As a key protein in the G1 phase, cyclinD1 is the first protein synthesized in the G1 phase, and is crucial for the transition between the G0/G1 to S phase. CyclinD1 binds to cyclin-dependent protein kinase (Cdk4) and forms cyclin-Cdk complexes, leading to the activation of protein kinase Cdk, which induces the entry of cells into the S phase and subsequent cell division through a series of regulatory actions [15]. In this study, the expression of cyclinD1 in TE-1 cells transfected with siRNA-HDAC1 was significantly downregulated, suggested that HDAC1 might affect the proliferation of cells through regulating the process of cell cycle.
ZO-1 is a tight junction protein that controls the formation of tight junctions. Functional abnormity of ZO-1 plays an important role in the occurrence and development of a wide range of diseases. E-cadherin is an epithelial marker whose expression deficiency is associated with the invasion, migration and poor prognosis of tumors including esophageal carcinoma [16]. Vimentin is an IF protein that is frequently expressed in mesenchymal tumors. Increased expression of vimentin has been shown to stimulate the invasion and metastasis of tumors [17]. These findings suggest that the reverse of the expression of EMT-related proteins may significantly inhibit the invasion and metastasis of tumors. Previous studies have revealed the close association between HDAC1 expression and EMT. Peulen et al. have found that HDAC1 and Snail are important for EMT induced by E-cadherin deficiency during the metastasis of pancreatic cancer cells [18]. In this study, it was shown that the expression of ZO-1 and E-cadherin was upregulated in TE-1 cells transfected with siRNA-HDAC1, whereas vimentin expression was downregulated [9,19].
Conclusions
Our study revealed the overexpression of HDAC1 in esophageal cancer cell lines. The treatment of siRNA targeting HDAC1 significantly reduced HDAC1 expression in TE-1 cells, and markedly inhibited their migration and invasion abilities. Moreover, siRNA downregulated the expression of cyclinD1 and upregulated p21 and p27 expression in TE-1 cells transfected with siRNA-HDAC1. siRNA also increased the expression of EMT-related protein ZO-1 and E-cadherin and reduced vimentin expression in TE-1 cells, indicating that siRNA might inhibit the growth of esophageal cancer cells by regulating the expression of cell cycle- and EMT-related proteins. This study may provide preliminary theoretic basis for the development of molecular targeted therapy for esophageal cancer.
Footnotes
Conflict of interests
The authors declare no conflicts of interest.
Source of support: Departmental sources
References
- 1.Isik A, Firat D, Peker K, et al. A case report of esophageal perforation: Complication of nasogastric tube placement. Am J Case Rep. 2014;15:168–71. doi: 10.12659/AJCR.890260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senese S, Zaragoza K, Minardi S, et al. Role for histone deacetylase 1 in human tumor cell proliferation. Mol Cell Biol. 2007;27(13):4784–95. doi: 10.1128/MCB.00494-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen KW, Ou TM, Hsu CW, et al. Current systemic treatment of hepatocellular carcinoma: A review of the literature. World J Hepatol. 2015;7(10):1412–20. doi: 10.4254/wjh.v7.i10.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klasser GD, Echandi L, Shannon M. Hepatocellular carcinoma metastasis to the condyle: a case report and review of the literature. J Am Dent Assoc. 2014;145(10):1063–67. doi: 10.14219/jada.2014.70. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda Y, Wakai T, Hirose Y, et al. p27 Is a critical prognostic biomarker in non-alcoholic steatohepatitis-related hepatocellular carcinoma. Int J Mol Sci. 2013;14(12):23499–515. doi: 10.3390/ijms141223499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peker K, Sayar I, Gelincik I, et al. The diagnostic importance of matrix metalloproteinase-7 and nestin in gastrointestinal stromal tumors. Med Sci Monit. 2014;20:674–80. doi: 10.12659/MSM.890303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landskron G, De la Fuente M, Thuwajit P, et al. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilbig A, Oettle H. Transforming growth factor beta in pancreatic cancer. Curr Pharm Biotechnol. 2011;12(12):2158–64. doi: 10.2174/138920111798808356. [DOI] [PubMed] [Google Scholar]
- 9.Isik A, Peker K, Firat D, et al. Importance of metastatic lymph node ratio in non-metastatic, lymph node-invaded colon cancer: a clinical trial. Med Sci Monit. 2014;20:1369–75. doi: 10.12659/MSM.890804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quint K, Agaimy A, Di Fazio P, et al. Clinical significance of histone deacetylases 1, 2, 3, and 7: HDAC2 is an independent predictor of survival in HCC. Virchows Arch. 2011;459(2):129–39. doi: 10.1007/s00428-011-1103-0. [DOI] [PubMed] [Google Scholar]
- 11.Cheng CC, Liu YH, Lai YC, et al. Hypoacetylation in association with histone 3 modulation in human hepatocellular carcinoma. In Vivo. 2015;29(2):237–42. [PubMed] [Google Scholar]
- 12.Zhai X, Zhu H, Wang W, et al. Abnormal expression of EMT-related proteins, S100A4, vimentin and E-cadherin, is correlated with clinicopathological features and prognosis in HCC. Med Oncol. 2014;31(6):970. doi: 10.1007/s12032-014-0970-z. [DOI] [PubMed] [Google Scholar]
- 13.Choi HK, Pokharel YR, Lim SC, et al. Inhibition of liver fibrosis by solubilized coenzyme Q10: Role of Nrf2 activation in inhibiting transforming growth factor-beta1 expression. Toxicol Appl Pharmacol. 2009;240(3):377–84. doi: 10.1016/j.taap.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 14.Zheng NG, Mo SJ, Li JP, et al. Anti-CSC effects in human esophageal squamous cell carcinomas and Eca109/9706 cells induced by nanoliposomal quercetin alone or combined with CD 133 antiserum. Asian Pac J Cancer Prev. 2014;15(20):8679–84. doi: 10.7314/apjcp.2014.15.20.8679. [DOI] [PubMed] [Google Scholar]
- 15.Kim WD, Kim YW, Cho IJ, et al. E-cadherin inhibits nuclear accumulation of Nrf2: implications for chemoresistance of cancer cells. J Cell Sci. 2012;125(Pt 5):1284–95. doi: 10.1242/jcs.095422. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Zhou Q. [E-cadherin/beta-catenin and the invasion and metastasis of lung cancer]. Zhongguo Fei Ai Za Zhi. 2010;13(3):254–59. doi: 10.3779/j.issn.1009-3419.2010.03.11. [in Chinese] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sadamori H, Yagi T, Shigeyasu K, et al. Advanced hepatocellular carcinoma with lymph node metastases showing epithelial to mesenchymal transition effectively treated with systemic chemotherapy: Report of a case. Hepatol Res. 2013;43(12):1368–73. doi: 10.1111/hepr.12080. [DOI] [PubMed] [Google Scholar]
- 18.Peulen O, Gonzalez A, Peixoto P, et al. The anti-tumor effect of HDAC inhibition in a human pancreas cancer model is significantly improved by the simultaneous inhibition of cyclooxygenase 2. PLoS One. 2013;8(9):e75102. doi: 10.1371/journal.pone.0075102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernardo MM, Meng Y, Lockett J, et al. Maspin reprograms the gene expression profile of prostate carcinoma cells for differentiation. Genes Cancer. 2011;2(11):1009–22. doi: 10.1177/1947601912440170. [DOI] [PMC free article] [PubMed] [Google Scholar]