Abstract
Background
Concentrated leukocytes in leukocyte- and platelet-rich plasma (L-PRP) may deliver increased levels of pro-inflammatory cytokines to activate the NF-κB signaling pathway, to counter the beneficial effects of growth factors on osteoarthritic cartilage. However, to date no relevant studies have substantiated that in vivo.
Material/Methods
Autologous L-PRP and pure platelet-rich plasma (P-PRP) were prepared, measured for componential composition, and injected intra-articularly after 4, 5, and 6 weeks post-anterior cruciate ligament transection. Caffeic acid phenethyl ester (CAPE) was injected intraperitoneally to inhibit NF-κB activation. All rabbits were sacrificed after 8 weeks postoperative. Enzyme-linked immunosorbent assays were performed to determine interleukin 1β (IL-1β) and prostaglandin E2 (PGE2) concentrations in the synovial fluid, Indian ink staining was performed for gross morphological assessment, and hematoxylin and eosin staining and toluidine blue staining were performed for histological assessment.
Results
Compared with L-PRP, P-PRP injections achieved better outcomes regarding the prevention of cartilage destruction, preservation of cartilaginous matrix, and reduction of IL-1β and PGE2 concentrations. CAPE injections reversed the increased IL-1β and PGE2 concentrations in the synovial fluid after L-PRP injections and improved the outcome of L-PRP injections to a level similar to P-PRP injections, while they had no influence on the therapeutic efficacy of P-PRP injections.
Conclusions
Concentrated leukocytes in L-PRP may release increased levels of pro-inflammatory cytokines to activate the NF-κB signaling pathway, to counter the beneficial effects of growth factors on osteoarthritic cartilage, and finally, result in a inferior efficacy of L-PRP to P-PRP for the treatment of osteoarthritis.
MeSH Keywords: Cytokines, Leukocytes, NF-kappa B, Osteoarthritis, Platelet-Rich Plasma, Rabbits
Background
Osteoarthritis is a degenerative joint disorder characterized by articular cartilage destruction that leads to pain and loss of function primarily in the knees and hips [1]. In clinical practice, challenges are still frequently encountered in the treatment of osteoarthritis. Although total hip arthroplasty and total knee arthroplasty have been well accepted as the gold standards, and have achieved favorable clinical outcomes in the aged population, the long-term outcomes of these surgical therapies in young adults are controversial due to the increased risk of revision that results from younger age [2]. Numerous clinical attempts have been made to alleviate major complaints such as pain, swelling, and muscle tightness. However, these therapies are barely effective on the prevention of osteoarthritis progression and promotion of articular cartilage regeneration, possibly because of the minimal blood supply, limited extracellular matrix formation and low cell density of this tissue [3]. To solve these problems, biological agents have been introduced as promising alternatives for the treatment of osteoarthritis; antagonists of interleukin 1β (IL-1β) and tumor necrosis factor α (TNF-α) have been used to inhibit the effects of these pro-inflammatory cytokines on cartilage destruction, and growth factors have been added to improve cartilage regeneration [4].
Platelet-rich plasma (PRP) is an autologous blood product that contains concentrated platelets. After activation, the α-granules of concentrated platelets in PRP release growth factors at concentrations significantly higher than the baseline blood levels, including platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), insulin-like growth factor (IGF), fibroblast growth factor (FGF), epidermal growth factor (EGF), and many others [5–7]. Many of these growth factors can stimulate chondrocyte and chondrogenic mesenchymal stem cell (MSC) proliferation, enhance chondrocyte and MSC survival, promote chondrocyte cartilaginous matrix secretion, induce MSC chondrogenic differentiation, and diminish the catabolic effects of pro-inflammatory cytokines [8–10]. Consequently, PRP has gained growing popularity in the treatment of osteoarthritis in the last decade [11–13].
Despite the increasing use of PRP, there is no standardized protocol for PRP preparation in clinical practice, and different protocols may result in PRP formulations that differ in componential composition, in particular, leukocyte concentration [14]. It has been shown that the significantly concentrated leukocytes in leukocyte- and platelet-rich plasma (L-PRP), compared with pure platelet-rich plasma (P-PRP), may release significantly higher levels of pro-inflammatory cytokines, such as IL-1β and TNF-α [15]. IL-1β and TNF-α have been described to have crucial roles in the physiopathology of osteoarthritis via inducing the nuclear translocation of NF-κB p65 to activate expression of a wide range of catabolic genes, including inducible nitric oxide synthase, cyclooxygenase-2, and matrix metalloproteinases, to disturb anabolism and enhance catabolism of chondrocytes [16–18]. Recently, the in vitro study by Cavallo et al. showed that L-PRP and P-PRP had significantly different leukocyte and pro-inflammatory cytokine concentrations, and induced distinct effects on human articular chondrocytes in terms of the production of destructive proteases and extracellular matrix synthesis [19]. Hence, high levels of IL-1β and TNF-α in L-PRP may activate the NF-κB signaling pathway to induce harmful effects on cartilage, to counter or overwhelm the beneficial effects of growth factors, and finally, make L-PRP unsuitable for the treatment of osteoarthritis. However, no relevant studies have substantiated that in vivo.
The objective of this study was to evaluate the efficacies of L-PRP and P-PRP for the treatment of osteoarthritis, and the in vivo effects of L-PRP and P-PRP on the NF-κB signaling pathway in a rabbit osteoarthritis model, in order to develop an alternative method for the treatment of osteoarthritis.
Material and Methods
Animal surgery
The study protocol was approved by the Animal Care and Use Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. Fifty mature New Zealand white rabbits (weighing 2.5–3.0 kg) were used in this study. The osteoarthritis model in rabbits was created by anterior cruciate ligament transection as described previously [20]. In brief, after achieving anesthetization with an intravenous injection of 60 mg/kg of ketamine hydrochloride and 6 mg/kg of xylazine, a 2 cm lateral para-patellar skin incision was made. Then, the patella was dislocated medially to expose the knee joint and the anterior cruciate ligament was transected visually with a #15 blade. The joint was then repositioned, irrigated with sterile saline and closed with 4-0 nylon. After surgery, all rabbits were housed in separated cages and had ad libitum access to food and water. All animals were sacrificed after 8 weeks postoperative.
Treatments of rabbit osteoarthritis
As anterior cruciate ligament transection has been reported to lead to cartilage degeneration in rabbit knees similar to human knee osteoarthritis after 4 weeks postoperative [20], rabbits were randomly divided into five groups of 5 male and 5 female rabbits each at 4 weeks postoperative. The control group received three weekly intra-articular injections of 300 μL saline, initiated 4-weeks postoperative for each knee joint. At the same time points, the L-PRP and P-PRP groups received three weekly intra-articular injections of 300 μL autologous L-PRP or P-PRP for each knee joint. A course of three weekly intra-articular injections of saline, L-PRP, or P-PRP was chosen to match the protocol that was used frequently in clinical practice [21–24]. Besides L-PRP or P-PRP intra-articular injections, the L-PRP+ caffeic acid phenethyl ester (CAPE) and P-PRP+CAPE groups received 21 daily intraperitoneal injections of 1 mL of 10 μmol/kg/day CAPE (Sigma-Aldrich, St. Louis, MO, USA), initiated 4-weeks postoperative, to inhibit the activation of the NF-κB signaling pathway [25]. All rabbits were sacrificed after 8 weeks postoperative. The study design is summarized in Figure 1.
Figure 1.
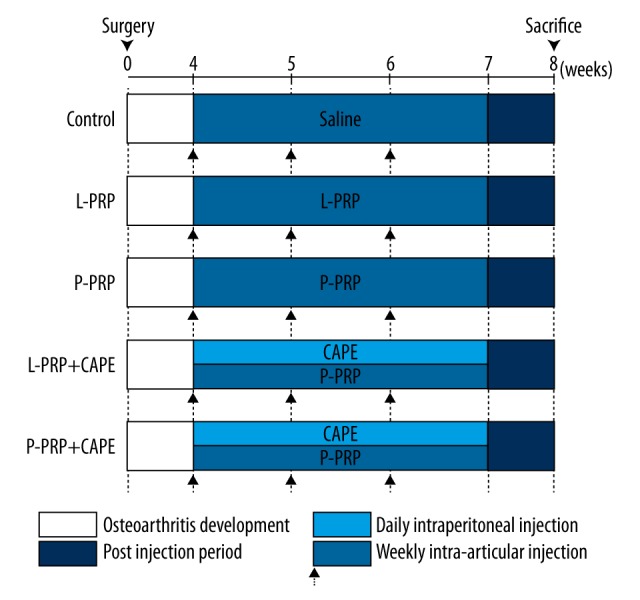
Study design. L-PRP – leukocyte- and platelet-rich plasma; P-PRP – pure platelet-rich plasma; CAPE – caffeic acid phenethyl ester.
Preparation of L-PRP and P-PRP
Whole blood used for L-PRP or P-PRP preparation was collected from rabbits of the L-PRP group and L-PRP+CAPE group, or the P-PRP group and P-PRP+CAPE group, through the central auricular artery into acid-citrate dextrose solution A (ACD-A) anticoagulant at a ratio of 9:1 (v/v). L-PRP was prepared with a buffy coat–based double-spin method, as described elsewhere [26]. In brief, 10 mL of whole blood was spun at 250× g for 10 minutes in a 15-mL centrifuge tube. After the first spin, the blood was separated into three components: erythrocytes at the bottom, buffy coat in the middle, and platelet-containing plasma at the top. Then, the top and middle layers were transferred to a new centrifuge tube and spun again at 1,000× g for 10 minutes. After the second spin, the supernatant platelet-poor plasma was discarded, and the precipitated platelets were resuspended in the remaining 1 mL of plasma to obtain L-PRP. P-PRP was prepared with a plasma-based double-spin method. In brief, a spin at 160× g for 10 minute was used to separate 15 mL of whole blood into three components, as above. Then, the platelet-containing plasma was transferred to a new tube and spun again at 1,000× g for 10 minutes. After discarding the supernatant platelet-poor plasma, the remaining plasma and precipitated platelets were blended evenly to obtained 1 mL of P-PRP: 0.6 mL of each PRP sample was used for intra-articular injections, 0.1 mL for whole blood analysis to determine leukocyte and platelet concentrations, and 0.3 mL for enzyme-linked immunosorbent assays (ELISA) to determine cytokine concentrations.
Quantification of components of L-PRP and P-PRP
Leukocyte and platelet concentrations in L-PRP and P-PRP were measured by whole blood analysis with an automatic hematology analyzer (XS-800i, Sysmex, Kobe, Japan) in the clinical laboratory of the hospital. Concentrations of PDGF-AB, TGF-β1, IL-1β, and TNF-α concentrations in L-PRP and P-PRP were determined by ELISA according to the protocols described previously [19]. In brief, L-PRP and P-PRP were incubated with 10% CaCl2 (final concentration 22.8 mM) at 37°C. Then, the supernatants were collected and assayed for growth factors and pro-inflammatory cytokine concentrations using commercial kits (Xitang, Shanghai, China) according to manufacturer’s instructions.
Quantification of IL-1β and prostaglandin E2 concentrations in the synovial fluid
After rabbits were euthanized at 8 weeks postoperative, the synovial fluid in knee joints was collected and measured for concentrations of IL-1β and prostaglandin E2 (PGE2) by ELISA with commercial kits (Xitang, Shanghai, China) according to manufacturer’s instructions.
Gross morphological assessment
After the rabbits were euthanized, femoral condyles were harvested and stained with Indian ink for 30 minutes. Then, gross morphological assessment was performed on both the medial and lateral sides, as described previously, according to the criteria shown in Table 1 [27].
Table 1.
Criteria of gross morphological assessment of cartilage degeneration.
| Grade | Indian ink staining |
|---|---|
| 1 | No staining by Indian ink |
| 2 | Surface retains ink as elongated specks or light gray patches |
| 3 | Surface retains ink as intense black patches |
| 4 | Loss of cartilage exposing the sub-cartilaginous bone |
| 4a | 0 mm < erosion ≤2 mm |
| 4b | 2 mm < erosion ≤5 mm |
| 4c | 5 mm < erosion |
Histological assessment
Femoral condyles were fixed with 4% paraformaldehyde for 72 hours, decalcified with 10% EDTA for 1 month, dehydrated with graded ethanol solutions, embedded in paraffin, and sectioned at 5 μm. Then, sections were stained with hematoxylin and eosin (HE) for general histological assessment, or with toluidine blue for assessment of cartilaginous matrix distribution.
Statistical analysis
Data were analyzed using the Statistical Package for Social Sciences version 22.0 (SPSS, Chicago, IL, USA) and presented as mean ± standard deviation (SD) or median and range as appropriate. One-way analysis of variance and Bonferroni post-hoc test or Wilcoxon rank sum test was performed to analyze the difference between groups as appropriate. Pearson correlation analysis was conducted to analyze the linear correlations between cytokine concentrations and platelet concentration, and leukocyte concentration of PRP formulations. A p value less than 0.05 was considered statistically significant.
Results
Components of L-PRP and P-PRP
Components of L-PRP and P-PRP used in different groups at different time points are shown in Table 2. L-PRP used in the L-PRP group and L-PRP+CAPE group at 4, 5, and 6 weeks postoperative had similar concentrations of leukocytes, platelets, growth factors, and pro-inflammatory cytokines compared with each other (p>0.05). Also, P-PRP used in the P-PRP group, and P-PRP+CAPE group at 4, 5, and 6 weeks postoperative, were similar in leukocyte, platelet, growth factors, and pro-inflammatory cytokine concentrations compared with each other (p>0.05).
Table 2.
Components of platelet-rich plasma formulations used in different groups at different time points.
| Injection time | Group | Leukocyte concentration (106/ml) | Platelet concentration (1066/ml) | IL-1β concentration (pg/ml) | TNF-α concentration (pg/ml) | PDGF-AB concentration (ng/ml) | TGF-β1 concentration (ng/ml) |
|---|---|---|---|---|---|---|---|
| 4 weeks postoperatively | L-PRP | 51.24±19.53 | 2184.80±428.19 | 56.03±20.13 | 32.30±7.24 | 46.97±11.70 | 101.83±21.09 |
| L-PRP+CAPE | 58.73±23.48 | 2084.10±396.87 | 47.22±19.11 | 32.70±7.34 | 41.60±18.78 | 106.72±23.53 | |
| P-PRP | 0.10±0.11 | 2056.80±331.68 | 2.12±0.53 | 1.44±0.40 | 37.93±13.85 | 96.97±25.06 | |
| P-PRP+CAPE | 0.12±0.11 | 2036.10±314.06 | 2.54±0.92 | 1.43±0.54 | 41.52±17.35 | 101.55±28.12 | |
| 5 weeks postoperatively | L-PRP | 61.83±17.01 | 2030.00±265.67 | 52.18±22.61 | 30.78±6.04 | 50.01±13.57 | 113.74±16.63 |
| L-PRP+CAPE | 55.28±22.44 | 2032.60±270.18 | 56.09±18.99 | 32.28±7.24 | 43.00±15.85 | 99.27±32.77 | |
| P-PRP | 0.22±0.11 | 2007.30±263.81 | 2.55±1.11 | 1.64±0.56 | 38.59±11.81 | 96.00±29.79 | |
| P-PRP+CAPE | 0.15±0.14 | 1981.50±312.42 | 2.82±0.83 | 1.53±0.50 | 35.98±15.01 | 90.23±35.88 | |
| 6 weeks postoperatively | L-PRP | 56.92±26.83 | 1947.50±297.71 | 54.62±18.57 | 32.93±6.73 | 41.08±12.61 | 97.89±16.56 |
| L-PRP+CAPE | 64.86±24.39 | 1935.30±378.01 | 56.80±15.89 | 31.78±8.75 | 40.58±11.00 | 97.97±29.96 | |
| P-PRP | 0.14±0.10 | 1933.30±240.37 | 2.53±0.92 | 1.50±0.75 | 37.66±13.36 | 96.60±28.38 | |
| P-PRP+CAPE | 0.13±0.13 | 1880.10±284.31 | 2.76±0.83 | 1.35±0.52 | 37.14±14.80 | 88.02±21.17 |
L-PRP – leukocyte- and platelet-rich plasma; P-PRP – pure platelet-rich plasma; CAPE – caffeic acid phenethyl ester; IL-1β – interleukin-1β; TNF-α – tumor necrosis factor-a; PDGF-AB – platelet-derived growth factor AB; TGF-β1 – transforming growth factor-β1.
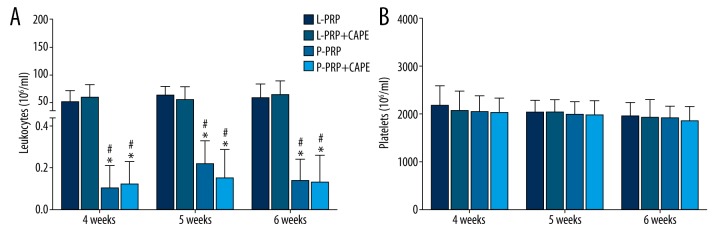
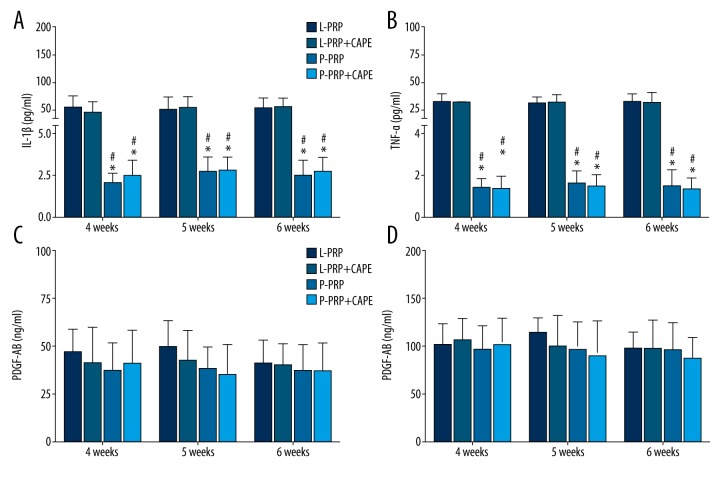
However, the leukocyte concentrations in L-PRP were significantly higher than P-PRP used at the same time point (p<0.001, Figure 2A). In accordance with the leukocyte concentrations, the IL-1β and TNF-α concentrations in L-PRP were significantly higher than P-PRP used at the same point (p<0.001, Figure 3A), whereas the platelet concentrations in L-PRP were similar to P-PRP used at the same time point (p>0.05, Figure 2B). A similar trend was observed in the results of growth factor concentrations, which demonstrated that L-PRP and P-PRP used at the same point had similar PDGF-AB and TGF-β1 concentrations (p>0.05, Figure 3B).
Figure 2.
Leukocyte and platelet concentrations in platelet-rich plasma formulations used in the study. (A) At each time point, the leukocyte concentrations in leukocyte- and platelet-rich plasma (L-PRP) used in the L-PRP group and L-PRP+ caffeic acid phenethyl ester (CAPE) group were higher than in pure platelet-rich plasma (P-PRP), which was used in the P-PRP group and P-PRP+CAPE group. (B) L-PRP and P-PRP were similar in platelet concentrations. Boxes and error bars represent mean ± standard deviation (n=10); * p<0.05 compared with L-PRP used in the L-PRP group at the same time point; # p<0.05 compared with L-PRP used in the L-PRP+CAPE group at the same time point.
Figure 3.
Cytokine concentrations in platelet-rich plasma formulations used in the study. (A, B) At each time point, the concentrations of interleukin-1β (IL-1β); (A) Tumor necrosis factor-α (TNF-α). (B) Leukocyte- and platelet-rich plasma (L-PRP) used in the L-PRP group and L-PRP+ caffeic acid phenethyl ester (CAPE) group were higher than in pure platelet-rich plasma (P-PRP), which was used in the P-PRP group and P-PRP+CAPE group; (C, D) L-PRP and P-PRP were similar in the concentrations of platelet-derived growth factor AB (PDGF-AB). (C) Transforming growth factor β1 (TGF-β1). (D) Boxes and error bars represent mean ± standard deviation (n=10); * p<0.05 compared with L-PRP used in the L-PRP group at the same time point; # p<0.05 compared with L-PRP used in the L-PRP+CAPE group at the same time point.
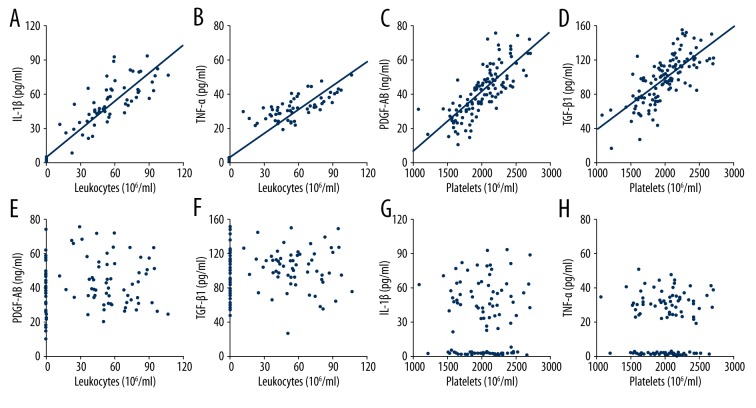
Additional analysis revealed that there were positive correlations between leukocyte concentration and IL-1β concentration in PRP formulations (r=0.936, p<0.001, Figure 4A), and TNF-α concentration in PRP formulations (r=0.942, p<0.001, Figure 4B). Also, positive correlations were observed between platelet concentration and PDGF-AB concentration in PRP formulations (r=0.769, p<0.001, Figure 4C), and TGF-β1 concentration in PRP formulations (r=0.729, p<0.001, Figure 4D). However, the correlations between leukocyte concentration and PDGF-AB concentration (r=0.117, p=0.204, Figure 4E), and TGF-β1 concentration (r=0.106, p=0.250, Figure 4F), and between platelet concentration and IL-1β concentration (r=0.058, p=0.531, Figure 4G), and TNF-α concentration (r=0.066, p=0.474, Figure 4H) were not significant.
Figure 4.
Correlations between components of platelet-rich plasma formulations. There was a significantly positive correlation between leukocyte concentration and IL-1β concentration (A) and TNF-α (B) concentration, and between platelet concentration and PDGF-AB concentration (C) and TGF-β1 concentration (D). The correlations between leukocyte concentration and PDGF-AB (E) and TGF-β1 (F) concentrations and the correlations between platelet concentration and IL-1β (G) and TNF-α (H) concentrations were not significant. IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α; PDGF-AB, platelet-derived growth factor AB; TGF-β1, transforming growth factor-β1.
These findings indicated that the L-PRP and P-PRP used in this study were constant in componential composition in terms of concentrations of leukocytes, platelets, pro-inflammatory cytokines, and growth factors. The significantly higher leukocyte concentration in L-PRP resulted in the significantly higher pro-inflammatory cytokine concentrations in L-PRP compared with P-PRP used at the same time point, and the similar platelet concentration between L-PRP and P-PRP used at the same time point resulted in the similar growth factor concentrations between them.
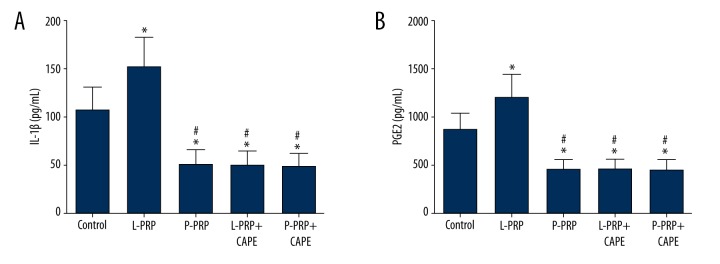
IL-1β and PGE2 concentrations in the synovial fluid
The mean IL-1β concentration in the synovial fluid collected from rabbits of the L-PRP group (151.22±31.27 pg/mL) was significantly higher than the control group (106.40±23.86 pg/mL, p<0.001), which, in turn, was significantly higher than the P-PRP group (50.40±14.29 pg/m, p<0.001), L-PRP+CAPE group (49.76±13.49 pg/mL, p<0.001), and P-PRP+CAPE group (47.91±13.00 pg/mL, p<0.001), which were similar compared with each other (p>0.05, Figure 5A). As shown in Figure 5B, a similar trend was observed in the results of PGE2 concentrations in the synovial fluid, which demonstrated that the mean PGE2 concentration was the highest in the L-PRP group (1198.91±231.41), significantly lower in the control group (857.05±174.07 pg/mL), and the lowest in the P-PRP group (446.59±97.38 pg/mL), L-PRP+CAPE group (451.73±101.24 pg/mL), and P-PRP+CAPE group (439.89±103.36 pg/mL).
Figure 5.
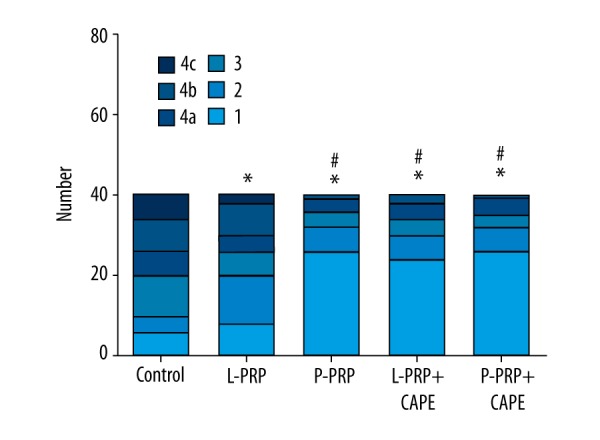
Gross morphological assessment of cartilage degeneration. Gross morphological assessment was performed on both the medial and lateral sides of femoral condyles according to the following criteria after Indian ink staining: grade 1, no staining by Indian ink; grade 2, surface retains ink as elongated specks or light gray patches; grade 3, surface retains ink as intense black patches; grade 4, loss of cartilage exposing the sub-cartilaginous bone; grade 4a, 0 mm < erosion ≤2 mm; grade 4b, 2 mm < erosion ≤5 mm; grade 4c, 5 mm < erosion. L-PRP, leukocyte- and platelet-rich plasma; P-PRP, pure platelet-rich plasma; CAPE, caffeic acid phenethyl ester. * p<0.05 compared with the control group; # p<0.05 compared with the L-PRP group (n=40).
Gross morphological assessment
Gross morphological assessment was performed on both the medial and lateral sides, as described previously, according to the criteria shown in Table 1 (a total of 20 joints, 40 scores, in each group). As shown in Figure 5, the medians of gross morphological grading of the P-PRP group (median 1; range 1 to 4b), L-PRP+CAPE group (median 1; range 1 to 4b), and P-PRP+CAPE group (median 1; range 1 to 4b) were similar (p>0.05), but significantly better than the L-PRP group (median 2.5; range 1 to 4c, p<0.05), which, in turn, was significantly better than the control group (median 4a/4b; range 2 to 4c, p<0.05).
Histological assessment
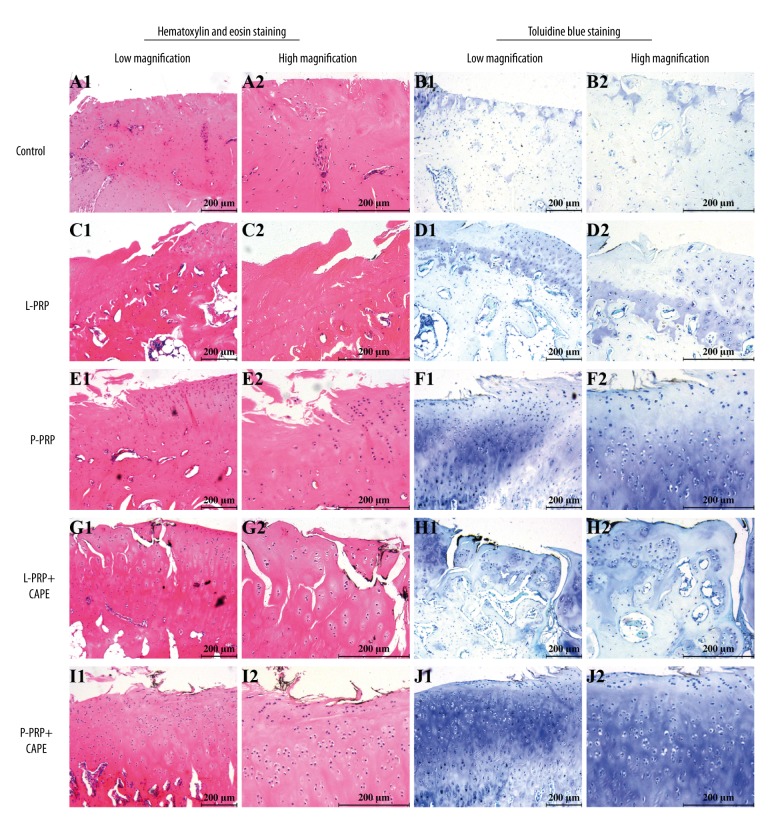
HE staining was performed for general histological assessment and toluidine blue staining was performed for assessment of cartilaginous matrix distribution. As shown in Figure 7, full-thickness cartilage defects and total loss of toluidine blue staining were observed in the control group (Figure 7A, 7B). In the L-PRP group, there was a marked reduction in the severity of cartilage loss compared with that of the control group, whereas the loss of toluidine blue staining was still extensively severe (Figure 7C, 7D). In the P-PRP group, less severe changes regarding loss of cartilage and toluidine blue staining were exhibited (Figure 7E, 7F). Also, samples from the L-PRP+CAPE and P-PRP+CAPE groups demonstrated a clear reduction in the severity of degenerative changes in cartilage compared with the control and L-PRP groups (Figure 7G–7J). Besides that, the height of cartilage in samples from the P-PRP, L-PRP+CAPE, and P-PRP+CAPE groups also seemed to be higher than the control and L-PRP groups. However, the therapeutic effects of P-PRP, L-PRP+CAPE, and P-PRP+CAPE on osteoarthritis regarding the prevention of cartilage destruction and preservation of toluidine blue staining seemed to be equal.
Figure 7.
Hematoxylin and eosin staining and toluidine blue staining for histological assessment. Representative HE stained sections (A, C, E, G, I) and toluidine blue stained sections (B, D, F, H, J) of femoral condyles after 8 weeks postoperative. L-PRP – leukocyte- and platelet-rich plasma; P-PRP – pure platelet-rich plasma; CAPE – caffeic acid phenethyl ester. Scales represent 200 μm.
Discussion
The comparison of platelet and growth factor levels between PRP formulations used in the study is important, because the growth factors released from platelet α-granules are believed to be the rationale behind PRP therapy, and small variations in their concentrations may result in distinct results [28–31]. Many growth factors have been detected in elevated concentrations in PRP, including PDGF-AB, TGF-β1, IGF, FGF, and EGF. PDGF-AB and TGF-β1 have been described previously to improve cell proliferation and cartilaginous matrix secretion in vitro [32,33], and increase cartilage regeneration in vivo [34]. Besides that, TGF-β1 may be capable of modulating the deleterious effects of IL-1β on cartilage by decreasing IL-1β receptor transcription and binding ability, while promoting IL-1 receptor antagonist synthesis [35]. Although IGF, FGF, and EGF also have beneficial effects on cartilage regeneration, they were shown to have much more variable concentrations in PRP. Moreover, exercise and nutritional status, which are hard to control, may affect IGF concentration in whole blood, and therefore, in PRP formulations [36]. Therefore, concentrations of IGF, FGF, and EGF in PRP formulations were not quantified in this study, and PDGF-AB and TGF-β1 concentrations, as well as platelet concentration, were measured to characterize the L-PRP and P-PRP used in this study. Our findings showed that the L-PRP and P-PRP used in this study were similar in platelet and growth factor concentrations. Additionally, we found that there were significantly positive correlations between platelet concentration and growth factor concentrations, and the similar platelet concentration in L-PRP and P-PRP might result in the similar growth factor concentrations between them, which were in accordance with a previous study [15]. These findings imply that the L-PRP and P-PRP used in this study are similar in the levels of anabolic molecules, and therefore, should have similar effects on the promotion of cartilage anabolism in osteoarthritis. However, our in vivo results indicated that L-PRP was not as effective as P-PRP in the treatment of osteoarthritis in rabbits, possibly because L-PRP not only concentrated platelets and growth factors that have beneficial effects on cartilage regeneration, but also concentrated leukocytes and pro-inflammatory cytokines compared with P-PRP.
The inclusion of leukocytes in PRP formulations is debatable because of concerns of the potential effects of leukocytes on tissue healing. Several leukocyte subsets, such as M2 macrophages, may have an anti-inflammatory function, aid in removal of debris from damaged tissue to initiate tissue repair, and suppress fibrosis [37]. Therefore, some authors advocated that increased macrophage infiltration and the ratio of M1/M2 macrophages might lead to increased collagen deposition and reduced fibrosis in skeletal muscle repair [38]. However, M2 macrophages are not present in the whole blood or PRP, due to the fact that they are differentiate from monocytes that have migrated into injury tissues [39,40]. Besides that, other leukocyte subsets, such as neutrophils, monocytes, and lymphocytes, are essential elements of the immune system, and may release excessive amounts of catabolic molecules to induce harmful effects on tissue healing [41]. In a study by McCarrel et al. [42], tendon explants treated with PRP formulations with higher leukocyte concentration demonstrated increased pro-inflammatory cytokine synthesis and decreased extracellular matrix synthesis, and increasing platelet concentration was not able to counter the catabolic effects of leukocytes. These findings suggest that a reduction in leukocyte concentration may be more important than the platelet to leukocyte ratio in enhancing tissue repair.
Our findings support a previous study finding demonstrating the significantly lower levels of pro-inflammatory cytokines in P-PRP compared with L-PRP, and the significantly positive correlations between leukocyte concentration and pro-inflammatory cytokine concentration in PRP formulations [15]. The deleterious effects of pro-inflammatory cytokines on cartilage in the physiopathology of osteoarthritis have become elucidated in the past few decades. IL-1β, which can be locally produced by both the synovial cells and articular chondrocytes, was detected in high levels in the synovial fluids of osteoarthritis patients [43] and shown to stimulate the expression of catabolic molecules with subsequent degradation of cartilaginous matrix [19]. In addition to its catabolism-promoting effect, IL-1β markedly inhibits the synthesis of extracellular matrix components [44]. Furthermore, an excess of IL-1β may downregulate the expression of type II receptor and phosphorylation of Smad3 and MAPKs to overwhelm the favorable effects of growth factors on the promotion of cartilage anabolism and the modulation of the effects of catabolic molecules [45]. Besides IL-1β, other pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-17, also attribute to the physiopathology of osteoarthritis [46], and may act in synergy to induce more severe articular cartilage destruction in vivo compared with acting independently [47]. Also, studies have demonstrated that antagonists of these pro-inflammatory cytokines are effective with respect to relieving osteoarthritis symptoms, providing substantial proof for the harmful effects of IL-1β and TNF-α on cartilage [48,49]. These findings imply that the concentrated leukocytes in L-PRP may release increased levels of pro-inflammatory cytokines to counter the beneficial effects of growth factors and result in the inferior effects of L-PRP compared with P-PRP, as observed in our study.
Considerable evidence has suggested that the NF-κB signaling pathway is intimately involved in the disturbed metabolism and enhanced catabolism of osteoarthritic cartilage that is induced by IL-1β and TNF-α, and inhibition of NF-κB or cyclooxygenase-2, a downstream inflammation-related gene of the NF-κB signaling pathway, may be targets for novel drugs for the treatment of osteoarthritis [18,50]. However, the effects of PRP formulations that increase or decrease levels of IL-1β and TNF-α on the NF-κB signaling pathway have never been evaluated. Some studies on other platelet products, such as platelet lysate and PRP clot releasate, indicated that molecules released from platelets might inhibit the activation of the NF-κB signaling pathway [51,52]. However, the absence of viable leukocytes and platelets, as well as pro-inflammatory cytokines, in these products makes them have distinct characteristics compared with the PRP formulations used in clinical practice, especially those with increased levels of pro-inflammatory cytokines. IL-1β and TNF-α activate the NF-κB pathway via the canonical pathway, which involves the nuclear translocation of NF-κB p65 and the subsequent upregulated production of downstream catabolic molecules, including IL-1β and PGE2 [53,54]. Therefore, the concentrations of IL-1β and PGE2 in the synovial fluid were determined in our study. Our results demonstrated that intra-articular injections of L-PRP increased IL-1β and PGE2 concentrations in the synovial fluid, which were reversed by intraperitoneal injections of CAPE, an inhibitor of NF-κB activation. Interestingly, IL-1β and PGE2 concentrations were decreased after P-PRP injections, but not decreased further after P-PRP+CAPE injections. These findings suggest that P-PRP may be similar in its capacity of inhibiting NF-κB activation in osteoarthritis to the previously mentioned platelet products, while L-PRP may be similar in the capacity of activating NF-κB to IL-1β and TNF-α.
The contrary effects of L-PRP and P-PRP on the NF-κB signaling pathway may play a mechanistic role in their distinct efficacies for the treatment of osteoarthritis. Our results demonstrated that the combined use of L-PRP and CAPE yielded better outcomes in the treatment of osteoarthritis in rabbits than using L-PRP alone, while neither the combined use of L-PRP and CAPE nor the combined use of P-PRP and CAPE achieved any better results than using P-PRP alone. These findings reveal that inhibiting NF-κB activation by using inhibitors of NF-κB activation enhances the efficacy of L-PRP for the treatment of osteoarthritis to a level similar to P-PRP. Hence, the capacity for inhibiting NF-κB activation may play an equally important role as the capacity for promoting cartilage regeneration in the beneficial outcomes of PRP formulations for the treatment of osteoarthritis. Also, concentrated leukocytes in L-PRP may release increased levels of pro-inflammatory cytokines to activate the NF-κB signaling pathway, to counter or overwhelm the beneficial effects of growth factors on cartilage metabolism, and finally, result in an inferior capacity of L-PRP compared to P-PRP for the treatment of osteoarthritis.
In summary, L-PRP and P-PRP, with similar platelet and growth factors concentrations but different leukocyte and pro-inflammatory cytokine concentrations, induced distinct in vivo effects on the NF-κB signaling pathway and osteoarthritis, with P-PRP showing better efficacy for the treatment of osteoarthritis in rabbits. Further studies are needed to substantiate these findings in larger animals or human volunteers, to inform the development of an alternative method for the treatment of osteoarthritis in clinical practice.
Conclusions
Increased levels of pro-inflammatory cytokines released from concentrated leukocytes in L-PRP may activate the NF-κB signaling pathway to counter the beneficial effects of growth factors on osteoarthritic cartilage, and finally, result in a lower efficacy of L-PRP compared with P-PRP for the treatment of rabbit knee osteoarthritis. Therefore, P-PRP may be more suitable for the treatment of osteoarthritis.
Figure 6.
Interleukin 1β (IL-1β) and prostaglandin E2 (PGE2) concentrations in the synovial fluid. IL-1β (A) and PGE2 (B) concentrations in the synovial fluid were quantified by enzyme-linked immunosorbent assay. The mean IL-1β and PGE2 concentrations in the synovial fluid collected from rabbits of the L-PRP group were significantly higher than the control group, which, in turn, was significantly higher than the P-PRP group, L-PRP+CAPE group, and P-PRP+CAPE group, which were similar compared with each other. L-PRP, leukocyte- and platelet-rich plasma; P-PRP, pure platelet-rich plasma; CAPE, caffeic acid phenethyl ester. Boxes and error bars represent mean ± standard deviation (n=20); * p<0.05 compared with the control group; # p<0.05 compared with the L-PRP group.
Footnotes
Conflicts of interest
There are no conflicts of interest for any of the authors.
Source of support: This work was supported by grants from the National Natural Science Foundation of China (No. 81401799 and No. 81301589) and Shanghai Youth Start-up Grant (No. 14YF1412100)
References
- 1.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646–56. [PMC free article] [PubMed] [Google Scholar]
- 2.Santaguida PL, Hawker GA, Hudak PL, et al. Patient characteristics affecting the prognosis of total hip and knee joint arthroplasty: A systematic review. Can J Surg. 2008;51:428–36. [PMC free article] [PubMed] [Google Scholar]
- 3.Muir H. The chondrocyte, architect of cartilage. Biomechanics, structure, function and molecular biology of cartilage matrix macromolecules. Bioessays. 1995;17:1039–48. doi: 10.1002/bies.950171208. [DOI] [PubMed] [Google Scholar]
- 4.Chevalier X, Eymard F, Richette P. Biologic agents in osteoarthritis: Hopes and disappointments. Nat Rev Rheumatol. 2013;9:400–10. doi: 10.1038/nrrheum.2013.44. [DOI] [PubMed] [Google Scholar]
- 5.Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39:266–71. doi: 10.1177/0363546510387517. [DOI] [PubMed] [Google Scholar]
- 6.Magalon J, Bausset O, Serratrice N, et al. Characterization and comparison of 5 platelet-rich plasma preparations in a single-donor model. Arthroscopy. 2014;30:629–38. doi: 10.1016/j.arthro.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 7.Appel TR, Potzsch B, Muller J, et al. Comparison of three different preparations of platelet concentrates for growth factor enrichment. Clin Oral Implants Res. 2002;13:522–28. doi: 10.1034/j.1600-0501.2002.130512.x. [DOI] [PubMed] [Google Scholar]
- 8.Ellman MB, An HS, Muddasani P, Im HJ. Biological impact of the fibroblast growth factor family on articular cartilage and intervertebral disc homeostasis. Gene. 2008;420:82–89. doi: 10.1016/j.gene.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandl A, Angele P, Roll C, et al. Influence of the growth factors PDGF-BB, TGF-beta1 and bFGF on the replicative aging of human articular chondrocytes during in vitro expansion. J Orthop Res. 2010;28:354–60. doi: 10.1002/jor.21007. [DOI] [PubMed] [Google Scholar]
- 10.Fortier LA, Barker JU, Strauss EJ, et al. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469:2706–15. doi: 10.1007/s11999-011-1857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kon E, Mandelbaum B, Buda R, et al. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: From early degeneration to osteoarthritis. Arthroscopy. 2011;27:1490–501. doi: 10.1016/j.arthro.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Filardo G, Kon E, Buda R, et al. Platelet-rich plasma intra-articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2011;19:528–35. doi: 10.1007/s00167-010-1238-6. [DOI] [PubMed] [Google Scholar]
- 13.Kon E, Buda R, Filardo G, et al. Platelet-rich plasma: intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc. 2010;18:472–79. doi: 10.1007/s00167-009-0940-8. [DOI] [PubMed] [Google Scholar]
- 14.Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27:158–67. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39:2135–40. doi: 10.1177/0363546511417792. [DOI] [PubMed] [Google Scholar]
- 16.Burguera EF, Vela-Anero A, Magalhaes J, et al. Effect of hydrogen sulfide sources on inflammation and catabolic markers on interleukin 1beta-stimulated human articular chondrocytes. Osteoarthritis Cartilage. 2014;22:1026–35. doi: 10.1016/j.joca.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 17.Campbell KA, Minashima T, Zhang Y, et al. Annexin A6 interacts with p65 and stimulates NF-kappaB activity and catabolic events in articular chondrocytes. Arthritis Rheum. 2013;65:3120–29. doi: 10.1002/art.38182. [DOI] [PubMed] [Google Scholar]
- 18.Davidson RK, Jupp O, de Ferrars R, et al. Sulforaphane represses matrix-degrading proteases and protects cartilage from destruction in vitro and in vivo. Arthritis Rheum. 2013;65:3130–40. doi: 10.1002/art.38133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavallo C, Filardo G, Mariani E, et al. Comparison of platelet-rich plasma formulations for cartilage healing: An in vitro study. J Bone Joint Surg Am. 2014;96:423–29. doi: 10.2106/JBJS.M.00726. [DOI] [PubMed] [Google Scholar]
- 20.Yoshioka M, Coutts RD, Amiel D, Hacker SA. Characterization of a model of osteoarthritis in the rabbit knee. Osteoarthritis Cartilage. 1996;4:87–98. doi: 10.1016/s1063-4584(05)80318-8. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez M, Anitua E, Azofra J, et al. Intra-articular injection of an autologous preparation rich in growth factors for the treatment of knee OA: A retrospective cohort study. Clin Exp Rheumatol. 2008;26:910–13. [PubMed] [Google Scholar]
- 22.Napolitano M, Matera S, Bossio M, et al. Autologous platelet gel for tissue regeneration in degenerative disorders of the knee. Blood Transfus. 2012;10:72–77. doi: 10.2450/2011.0026-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spakova T, Rosocha J, Lacko M, et al. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91:411–17. doi: 10.1097/PHM.0b013e3182aab72. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28:1070–78. doi: 10.1016/j.arthro.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Altug ME, Serarslan Y, Bal R, et al. Caffeic acid phenethyl ester protects rabbit brains against permanent focal ischemia by antioxidant action: A biochemical and planimetric study. Brain Res. 2008;1201:135–42. doi: 10.1016/j.brainres.2008.01.053. [DOI] [PubMed] [Google Scholar]
- 26.Jia WT, Zhang CQ, Wang JQ, et al. The prophylactic effects of platelet-leucocyte gel in osteomyelitis: An experimental study in a rabbit model. J Bone Joint Surg Br. 2010;92:304–10. doi: 10.1302/0301-620X.92B2.22042. [DOI] [PubMed] [Google Scholar]
- 27.Amiel D, Toyoguchi T, Kobayashi K, et al. Long-term effect of sodium hyaluronate (Hyalgan) on osteoarthritis progression in a rabbit model. Osteoarthritis Cartilage. 2003;11:636–43. doi: 10.1016/s1063-4584(03)00119-5. [DOI] [PubMed] [Google Scholar]
- 28.Batten ML, Hansen JC, Dahners LE. Influence of dosage and timing of application of platelet-derived growth factor on early healing of the rat medial collateral ligament. J Orthop Res. 1996;14:736–41. doi: 10.1002/jor.1100140509. [DOI] [PubMed] [Google Scholar]
- 29.Torricelli P, Fini M, Filardo G, et al. Regenerative medicine for the treatment of musculoskeletal overuse injuries in competition horses. Int Orthop. 2011;35:1569–76. doi: 10.1007/s00264-011-1237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weibrich G, Hansen T, Kleis W, et al. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone. 2004;34:665–71. doi: 10.1016/j.bone.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Anitua E, Sanchez M, Zalduendo MM, et al. Fibroblastic response to treatment with different preparations rich in growth factors. Cell Prolif. 2009;42:162–70. doi: 10.1111/j.1365-2184.2009.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heldin CH, Eriksson U, Ostman A. New members of the platelet-derived growth factor family of mitogens. Arch Biochem Biophys. 2002;398:284–90. doi: 10.1006/abbi.2001.2707. [DOI] [PubMed] [Google Scholar]
- 33.Yaeger PC, Masi TL, de Ortiz JL, et al. Synergistic action of transforming growth factor-beta and insulin-like growth factor-I induces expression of type II collagen and aggrecan genes in adult human articular chondrocytes. Exp Cell Res. 1997;237:318–25. doi: 10.1006/excr.1997.3781. [DOI] [PubMed] [Google Scholar]
- 34.Stewart K, Pabbruwe M, Dickinson S, et al. The effect of growth factor treatment on meniscal chondrocyte proliferation and differentiation on polyglycolic acid scaffolds. Tissue Eng. 2007;13:271–80. doi: 10.1089/ten.2006.0242. [DOI] [PubMed] [Google Scholar]
- 35.Redini F, Mauviel A, Pronost S, et al. Transforming growth factor beta exerts opposite effects from interleukin-1 beta on cultured rabbit articular chondrocytes through reduction of interleukin-1 receptor expression. Arthritis Rheum. 1993;36:44–50. doi: 10.1002/art.1780360108. [DOI] [PubMed] [Google Scholar]
- 36.Berg U, Gustafsson T, Sundberg CJ, et al. Local changes in the insulin-like growth factor system in human skeletal muscle assessed by microdialysis and arterio-venous differences technique. Growth Horm IGF Res. 2006;16:217–23. doi: 10.1016/j.ghir.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013;93:875–81. doi: 10.1189/jlb.1012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H, Hicks JJ, Wang L, et al. Customized platelet-rich plasma with transforming growth factor beta1 neutralization antibody to reduce fibrosis in skeletal muscle. Biomaterials. 2016;87:147–56. doi: 10.1016/j.biomaterials.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 39.Bielecki T, Dohan Ehrenfest DM, et al. The role of leukocytes from L-PRP/L-PRF in wound healing and immune defense: New perspectives. Curr Pharm Biotechnol. 2012;13:1153–62. doi: 10.2174/138920112800624373. [DOI] [PubMed] [Google Scholar]
- 40.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–37. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russell RP, Apostolakos J, Hirose T, et al. Variability of platelet-rich plasma preparations. Sports Med Arthrosc. 2013;21:186–90. doi: 10.1097/JSA.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 42.McCarrel TM, Minas T, Fortier LA. Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J Bone Joint Surg Am. 2012;94:e143(1–8). doi: 10.2106/JBJS.L.00019. [DOI] [PubMed] [Google Scholar]
- 43.Wood DD, Ihrie EJ, Dinarello CA, Cohen PL. Isolation of an interleukin-1-like factor from human joint effusions. Arthritis Rheum. 1983;26:975–83. doi: 10.1002/art.1780260806. [DOI] [PubMed] [Google Scholar]
- 44.Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986;322:547–49. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pujol JP, Chadjichristos C, Legendre F, et al. Interleukin-1 and transforming growth factor-beta 1 as crucial factors in osteoarthritic cartilage metabolism. Connect Tissue Res. 2008;49:293–97. doi: 10.1080/03008200802148355. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Peng H, Meng Z, Wei M. Correlation of IL-17 level in synovia and severity of knee osteoarthritis. Med Sci Monit. 2015;21:1732–36. doi: 10.12659/MSM.893771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henderson B, Pettipher ER. Arthritogenic actions of recombinant IL-1 and tumour necrosis factor alpha in the rabbit: Evidence for synergistic interactions between cytokines in vivo. Clin Exp Immunol. 1989;75:306–10. [PMC free article] [PubMed] [Google Scholar]
- 48.Bacconnier L, Jorgensen C, Fabre S. Erosive osteoarthritis of the hand: Clinical experience with anakinra. Ann Rheum Dis. 2009;68:1078–79. doi: 10.1136/ard.2008.094284. [DOI] [PubMed] [Google Scholar]
- 49.Grunke M, Schulze-Koops H. Successful treatment of inflammatory knee osteoarthritis with tumour necrosis factor blockade. Ann Rheum Dis. 2006;65:555–56. doi: 10.1136/ard.2006.053272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ou Y, Tan C, An H, et al. Selective COX-2 inhibitor ameliorates osteoarthritis by repressing apoptosis of chondrocyte. Med Sci Monit. 2012;18(6):BR247–52. doi: 10.12659/MSM.882901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pereira RC, Scaranari M, Benelli R, et al. Dual effect of platelet lysate on human articular cartilage: A maintenance of chondrogenic potential and a transient proinflammatory activity followed by an inflammation resolution. Tissue Eng Part A. 2013;19:1476–88. doi: 10.1089/ten.TEA.2012.0225. [DOI] [PubMed] [Google Scholar]
- 52.van Buul GM, Koevoet WL, Kops N, et al. Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am J Sports Med. 2011;39:2362–70. doi: 10.1177/0363546511419278. [DOI] [PubMed] [Google Scholar]
- 53.Ledoux AC, Perkins ND. NF-kappaB and the cell cycle. Biochem Soc Trans. 2014;42:76–81. doi: 10.1042/BST20130156. [DOI] [PubMed] [Google Scholar]
- 54.Marcu KB, Otero M, Olivotto E, et al. NF-κB signaling: multiple angles to target OA. Curr Drug Targets. 2010;11:599–613. doi: 10.2174/138945010791011938. [DOI] [PMC free article] [PubMed] [Google Scholar]