Abstract
Acute promyelocytic leukemia (APL) is a molecularly well-defined disease, characterized by a specific chromosomal translocation; the improvement in biologic and clinical knowledge and subsequent introduction of molecularly targeted therapies have transformed the management of APL, with survival rates now exceeding 80%. Minimal residual disease (MRD) assessment in APL is the most important tool for its treatment; the prognostic role of the molecular detection of promyelocytic leukemia retinoic acid receptor α (PML-RARα) transcript after consolidation therapy in the early identification of the following hematologic relapse is now well established and guides preemptive therapy. First experiences performed with a qualitative polymerase chain reaction (PCR) approach were replaced with more accurate real-time quantitative PCR (RQ-PCR), which guarantees a numeric quantification of MRD. The identification of arsenic trioxide (ATO) as a valid therapy not only in relapsed patients but also as an alternative to standard therapy alone or in association with all-trans-retinoic acid enlarges the setting of validation of MRD evaluation in APL patients, considering a possible different clearance of PML-RARα with innovative therapy different from the standard ones. MRD monitoring demonstrated its validity also in the setting of relapsed patients with interesting results in the autologous and allogeneic stem cell transplantation setting or with the use of other biological agents. The aim of this review is to report and discuss the actual state of the art of MRD in APL.
Keywords: Acute promyelocytic leukemia, All-trans-retinoic acid, Arsenic trioxide, Minimal residual disease, Molecular monitoring, Quantitative real-time polymerase chain reaction
Introduction
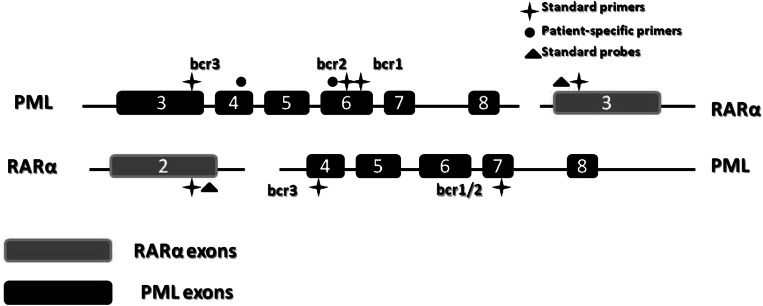
Acute promyelocytic leukemia (APL) is a molecularly well-defined disease, characterized by the presence of a specific chromosomal translocation—t(15;17) (q22;q21), which leads to the aberrant expression of the promyelocytic leukemia retinoic acid receptor α (PML-RARα) fusion gene (Fig. 1) [1]. In about 2% of cases, other aberrant translocations, such as t(11;17)(q23;q21), t(11;17)(q13;q21) and t(5;17)(q35;q21), and from an interstitial deletion event on chromosome 17 can involve fusions between retinoic acid receptor α (RARα) and other partner genes, such as the promyelocytic leukemia zinc finger (PLZF), nuclear mitotic apparatus protein (NuMA), nucleophosmin, and signal transducer and activator of transcription 5b (STAT5b) [2–6]. The aberrant expression of PML-RARα led to the exaltations of oncogenic signaling and consequently to the neoplastic transformation of myeloid cells [7].
Fig. 1.
Schematic representation of the PML/RARalpha hybrid with distinct isoforms. PML promyelocytic leukemia, RARα retinoic acid receptor α
An exquisite sensitivity to anthracyclines by APL blasts was shown for the first time by Jean Bernard in 1973, but those first encouraging results in APL treatment were impaired by the persistent and so far unresolved problem of early deaths and absence of valid alternative therapeutic strategies in case of relapse or refractory disease [8]. The evidence that all-trans-retinoic acid (ATRA) is highly effective in the induction of blast differentiation and its subsequent use in clinical practice induced a dramatic improvement in the outcome of APL, especially when associated with standard chemotherapy [9–11]. Further studies have widened the molecular knowledge around APL pathogenesis and have also demonstrated that arsenic trioxide (ATO) exhibits a significant antileukemic effect in relapsed and low-/intermediate-risk newly diagnosed patients [12, 13, 15]. It is of paramount importance that the baseline correctly identifies the fusion partner gene, as it is crucial to define the eventual sensitivity to molecularly targeted therapy: in fact, it has been demonstrated that morphological cases such as APL associated with PLZF and STAT5b are resistant to ATRA, while ATO activity is restricted only in cases of PML-RARα alteration [2, 14].
Recently, a cooperative German-Italian randomized trial demonstrated that the chemotherapy-free approach with ATRA plus ATO is superior to the association of ATRA plus standard chemotherapy in low-intermediate-risk APL [15]. New therapeutic strategies and refinement of molecular standardization have transformed APL from an aggressive fatal disease into one of the most curable neoplasms; indeed, a low percentage of patients (10–15%) failed to obtain a durable remission, and few patients were refractory to initial therapy [16]. To prevent morphologic relapse, potentially fatal because it is associated with concomitant coagulopathy, minimal residual disease (MRD) monitoring has been successfully standardized for the early identification of relapse [17].
Molecular methodologies have deeply changed the diagnostic and therapeutic approach to acute leukemias; during the last 3 decades diagnosis and follow-up criteria have evolved from simple morphologic evaluation to highly sensitive molecular methods. The standard reverse transcriptase polymerase chain reaction (RT-PCR) represents an important methodology for diagnosis assessment, but it was demonstrated to be less suitable for MRD monitoring during follow-up, because it was only informative about the disease status (positive or negative) and provided no real quantification of MRD.
The introduction of real-time quantitative PCR (RQ-PCR) overcame the limits of previous methodologies with sensitive and accurate quantification of gene expression. Parallel amplification of the target gene and one or more control genes represented a crucial innovation. The amplification of a control gene, mostly represented by the Abelson gene, avoided false-negative results related to suboptimal amplification of PCR results [18].
The aim of this review is to highlight the principal indications and new insights in MRD monitoring in APL and to overview the rationale of MRD-based preemptive therapy. This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Minimal Residual Disease Monitoring in Newly Diagnosed Patients Treated with Standard Regimens
A specific molecular hallmark in APL and the possibility to monitor the MRD with accurate and standardized RT-PCR allow strict molecular monitoring during the frontline treatment and follow-up phase.
Cooperative groups in Italy [Gruppo Italiano Malattie EMatologiche dell’Adulto (GIMEMA)] and Spain [Programa para el Tratamiento de Hemopatias Malignas (PETHEMA)] have largely contributed to the correct definition of the standard of care in APL. In 1997, the GIMEMA group introduced a combination of ATRA and anthracycline as first-line induction therapy (AIDA trial), followed by three cycles of standard chemotherapy consolidation; after the third consolidation, a qualitative evaluation of MRD showed the absence of the PML/RARα transcript in 98% of patients. This protocol guaranteed event-free survival (EFS) rates of 83% at 1 year and 79% at 2 years [19]. The PETHEMA group introduced a similar trial slightly different from AIDA by the absence of cytarabine and etoposide in consolidation. RT-PCR MRD evaluation was performed at the end of induction treatment and consolidation, with a complete molecular remission rate of 51% and 93%, respectively. In this trial similar overall survival (OS) (82%) and EFS (79%) compared to the AIDA protocol were reported: the authors concluded that cytarabine and etoposide have a minor role in the treatment of newly diagnosed APL [20].
Fenaux et al. investigated the role of maintenance therapy in APL patients [9]. Patients in complete remission (CR) were randomized to receive 2-year maintenance therapy with continuous low-dose chemotherapy (6-mercaptopurine, methotrexate), intermittent ATRA (15 days every 3 months), a combination of both agents or no maintenance therapy. The authors concluded that a maintenance therapy combining chemotherapy and intermittent ATRA reduced the incidence of relapse in APL patients [9].
Although the association of ATRA and anthracyclines guaranteed high response rates, a minority of patients did not reach CR or early relapse. A predictive model was introduced to identify risk of relapse earlier. Three risk categories were proposed considering the clinical characteristics at diagnosis [high risk: white blood cell count (WBC) ≥ 10 × 109/l; intermediate risk: WBC ≤ 10 × 109/l and platelet count ≤ 40 × 109/l; low risk: WBC ≤ 10 × 109/l and platelet count ≥ 40 × 109/l] [21], and consolidation therapy was adapted to reduce the toxicity and rate of mortality in CR. It should be mentioned that, at the present time, the prognostic relevance based on platelet levels and the WBC assumes a minor role and is not yet considered strictly prognostic with the possible exception of the WBC. At the same time, a specific correlation between MRD positivity and the subsequent risk of clinical relapse has been reported [17, 22–27]; in particular, it has become clear that the assessment at the end of consolidation is the more appropriate point for performing MRD evaluation compared to the end of induction. Otherwise, molecular characterization of morphologic-resembling APL with an atypical transcript clarifies the correlation between the molecular pattern and clinical resistance to treatment [2–6].
Considering the prognostic value of molecular monitoring for the early identification of relapse, in 1999 the GIMEMA group showed for the first time that molecularly guided preemptive therapy confers an advantage compared to patients treated at the time of hematological overt relapse; they reported cumulative 2-year progression-free survival (PFS) of 85% in patients preemptively treated based on molecular relapse, which was statistically significant when compared with the results from previous series in which treatment was initiated in the presence of hematological relapse (2-year PFS: 44%). Molecular relapse was considered as the reappearance of a positive RT-PCR (sensitivity 10−4) in two consecutive tests performed on bone marrow (BM). The small number of patients and absence of quantitative evaluation of PML-RARα did not allow stratification of the risk of relapse [28]. In 2007, the PETHEMA group also confirmed that salvage therapy in the presence of molecular relapse guaranteed a better outcome compared to treatment at the time of hematological relapse [29] (Table 1). Also in this study MRD was assessed with qualitative RT-PCR; it is remarkable that the RT-PCR methods used in those studies showed an inferior sensitivity (10−4) of almost two logs compared to other techniques used for other types of leukemia in the same period. This difference is largely attributable to the intrinsically lower sensitivity of RT-PCR for the PML/RARα transcript in respect to other AML genes, such as BRC-ABL [30] or AML/ETO [31]. This inferior sensitivity can explain the high probability of subsequent hematological relapse demonstrated in different experiences [27, 32]. Conversely, when more sensitive MRD detection for PML-RARa methods is used (10−6), a clear correlation between molecular and hematological relapse became less clear. Tobal et al. showed that some APL patients in long-term remission may show RT-PCR MRD positivity without ever experiencing a further hematological relapse [33].
Table 1.
Summary of molecular monitoring reported in clinical trials
| Reference | Number of evaluated patients | MRD status at the end of the third consolidation (no. of patients) | Relapse rate on the basis of MRD status | Efficacy of preemptive therapy for obtaining mCR |
|---|---|---|---|---|
| Lo-Coco et al. [17] | 35/35 (100%) |
Positive: 13 Negative: 22 |
11/13 (84.6%) 0/22 (0%) |
Not assessed |
| Miller et al. [22] | 32/32 (100%) |
Positive:13 Negative: 19 |
13/13 (100%) 3/19 (15%) |
Not assessed |
| Huang et al. [23] | 62/97 (64%) |
Positive: 11 Negative: 51 |
5/11 (45%) 0/51 (0%) |
Not assessed |
| Fukutani et al. [24] | 27/27 (100%) |
Positive: 13 Negative: 14 |
10/13 (77%) 0/14 (0%) |
Not assessed |
| Burnett et al. [26] | 76/239 (32%) |
Positive: 7 Negative: 69 |
4/7 (57%) 39/69 (27%) |
Not assessed |
| Diverio et al. [27] | 163/163 (100%) |
Positive: 21 Negative: 142 |
20/21 (95%) 8/142 (6%) |
Not assessed |
| Lo-Coco et al. [28] | 14/253 (5%) | 14 patients with positive MRD selected from AIDA trials | – | CR: 12/14 (85%) |
| Esteve et al. [29] | 16/549 (3%) | 16 patients with positive MRD selected from LPA96 and LPA99 trials | – | CR: 14/16 (87%) |
APL acute promyelocytic leukemia, CR complete remission, mCR molecular complete remission, MRD minimal residual disease
In order to overcome limitation bias related to qualitative PCR, standardized quantification of the PML/RARα copy number based on RQ-PCR has become the new alternative for MRD monitoring. Also with RQ-PCR, Santamaria et al. confirmed the correlation between high levels of normalized copy numbers of the PML-RARα transcript and risk of relapse when it was evaluated at the end of consolidation (high risk of relapse when the normalized copy number is >10, persistence in hematological remission when the value is <1) [34]. These data confirmed that the prognostic relevance of MRD became effective when it was identified after consolidation therapy, and it is concordant with what was demonstrated in previous experiences [17, 32, 35]. RQ-PCR offers several advantages compared to the qualitative method: Flora et al. [36] demonstrated that RQ-PCR enhanced sensitivity and reduced the risk of sample contamination. Moreover, it allowed performing quality control of the process by the quantification of an independent control gene amplification (ABL gene), avoiding obtaining a false negativity related to a suboptimal amplification of the PCR result. Finally, the quantification of the gene transcript allowed evaluating the increase of the MRD over time, which is essential for making the correct treatment decision [37, 38].
The validation of the novel RQ-PCR approach came from a large UK study that analyzed samples from 406 patients receiving ATRA and anthracycline-based therapy, most of them enrolled in the AML 15 trial [39]. A total of 6727 BM and peripheral blood (PB) samples were analyzed. With the higher sensitivity of RQ-PCR, 95% of patients achieved complete molecular remission at the end of consolidation; patients with a positive RQ-PCR were preemptively treated with ATO. Quantitative MRD assessment in BM was shown to be the most powerful predictor of relapse-free survival (RFS) in multivariable analysis [hazard ratio 17.87; 95% confidence interval (CI) 6.88–46.41], superior to the presenting WBC (hazard ratio 1.02; 95% CI 1.00–1.03). Also in this article the evaluation of MRD in PB was not significant because of the lack of sensitivity, which limits the opportunity to use PB for monitoring [40].
Regarding the management of high-risk patients, a risk-tailored treatment has been established; high-risk patients benefit from a more intensive consolidation therapy. Data from two PETHEMA trials (LPA96 and LPA99) showed that an increased dose of anthracyclines enhanced the antileukemic efficacy [41]. Although risk stratification was performed using clinical parameters, it is of interest that six out of seven MRD-positive patients were high risk, and a linear correlation between clinical and molecular parameters has been shown [43]. The GIMEMA trial AIDA-2000 confirmed the validity of a risk-tailored therapy and demonstrated that the introduction of cytarabine in consolidation had a favorable role [44].
Minimal Residual Disease Monitoring In Newly Diagnosed Patients Treated with ATO in Frontline Therapy
The efficacy of ATO in relapsed/refractory APL is well defined; during the last years, the possibility of its use in the first-line therapy was assessed, and this new approach opens a new scenario in the molecular monitoring of APL. It has been demonstrated that molecular clearance of APL blast cells using ATO as induction therapy is different compared to ATRA alone or ATRA plus chemotherapy regimens [43].
The variability in PML-RARα clearance between ATRA and ATO is probably influenced by the different mechanism of action of the two drugs on APL blast: while ATRA barely promotes blast differentiation, ATO at high concentration (1–2 × 10−6 M) induces apoptosis, mainly by activating the mitochondria-mediated intrinsic apoptotic pathway. Indeed, ATO at low concentrations (0.25–0.5 × 10−6 M) and with a longer treatment course promotes the differentiation of APL cells [44].
For this reason, data regarding MRD assessment derived from conventional treatment protocols may not be applicable in this particular setting.
First evidence of ATO efficacy in newly diagnosed patients came from developing countries where standard regimens are associated with significant economic costs that make them unaffordable. In 2006, an Iranian group published their experience with 111 patients with both newly diagnosed and relapsed APL patients who received ATO in monotherapy. Induction was performed with ATO at 0.15 mg/kg/day until hematological remission, followed by consolidation with the same schedule for 28 total infusions. The authors reported a 1- and 2-year disease-free survival (DFS) of 88.3% and 63.7%, respectively; in patients with relapsed disease, 19/24 (79%) obtained a second remission. MRD monitoring was performed with a semi-sensitive reverse transcription method on PB after the consolidation phase and 12 months after CR [45]. Long-term results after 5 years of this trial reported a morphologic CR rate of 85.8%, while DFS was 64.4 ± 4%. MRD was performed with the same method utilized in the first trial [44]. The Indian group produced a similar trial in which induction, consolidation and maintenance were performed with ATO as a single agent: in 2006 they reported an interim analysis in which hematologic CR was achieved in 86.1% of patients. At a median follow-up of 25 months, the 3-year EFS, DFS and OS were 74.87%, 87.21% and 86.11%, respectively. Side effects were mild and reversible [47]. The long-term follow-up showed that the 5-year EFS, DFS and OS were 69%, 80% and 74.2%, respectively [48], and confirmed the safety profile of ATO. Chendamarai et al. published on the specific role of MRD monitoring in ATO-treated patients as frontline therapy: the evaluation was performed using a quantitative RT-PCR on the PB sample [49]. They showed in multivariate analysis that a positive RQ-PCR at the end of the induction was associated with an increased risk of relapse. RQ-PCR negativity in low-risk patients (WBC ≤ 5 × 109/l; platelet count ≥ 20 × 109/l) was predictive of subsequent relapse. After the achievement of molecular remission, the MRD monitoring strategy predicted relapse in 60% of cases, with an overall sensitivity and specificity of 60% and 93.2%, respectively. Therefore, high-risk patients and those with RQ-PCR positivity after induction benefit from serial RQ-PCR monitoring for 3 years after completion of therapy [49]. Considering these results, it can be assumed that although ATO is efficacious in inducing morphological RC, the probability of relapse seems to be higher in this group in comparison with other standard approaches. Regarding ATO's role in consolidation for APL-naïve patients, Powell et al. investigated its role in a randomized trial: after a standard induction with ATRA, daunorubicin and cytarabine, patients were randomly assigned to receive consolidation therapy with two courses of ATRA and daunorubicin or two courses of ATO. They reported a better 3-year EFS and DFS in the ATO group in comparison with standard consolidation (80% vs. 63% and 90% vs. 70%, respectively) [50].
Recently ATO was successfully tested in first-line therapy as an alternative to the standard chemotherapy approach; first experiences using ATRA in association with ATO in untreated patients were reported by Estey et al. [51] and recently updated by Ravandi et al. [52], suggesting the potential efficacy and good safety profile of this association.
All this evidence demonstrated that ATO is effective also in first-line therapy, either as induction or consolidation therapy, and not only in the setting of relapsed patients. Lo-Coco et al. demonstrated that frontline therapy with ATRA and ATO for low-/intermediate-risk patients might be superior to the standard association of ATRA plus chemotherapy, with a better safety profile. In this experience the authors also investigated whether ATO can induce a different pattern of clearance of the molecular burden; MRD evaluation with RT-PCR was also performed after the induction phase in addition to conventional measurement at the end of the third consolidation, but no statistical differences were noted between the ATO and standard group [15]. The identification of this novel targeted therapy raises new questions about the correct management of APL: the ATRA-ATO association in frontline therapy seems to be effective, but for high-risk patients, according to Sanz [21], the use of a chemotherapeutic approach probably cannot be abandoned tout court. Future experiences may better clarify the role of ATO in first-line therapy for high-risk patients.
MRD Monitoring in Relapsed Patients
The rationale of molecular monitoring in APL patients is to detect disease relapse early and consequently to provide a preemptive intervention; the preemptive approach has been validated and shown to be a significant advantage in the long-term outcome, even though ATO therapy has not yet been identified as the optimal therapy for relapsed patients [28, 29]. Two studies showed the predictive value of the molecular detection of relapse: Diverio et al. prospectively detected 21 positive PCRs in the entire cohort of 163 untreated patients receiving the AIDA protocol. Of them, 20/21 experienced a further hematological relapse with a short time interval (3 months, range 1–14) [27]. Similarly, Jurcic et al. demonstrated that in 7/10 cases of molecular relapse (either after the first or subsequent remission), a further hematological relapse occurred [32].
These data, associated with the evidence that most relapses occur during the first 3 years after consolidation, led to the indication in current guidelines that molecular monitoring should be performed every 3 months for the first 3 years after the end of consolidation [9, 11, 13–52]. With the increased sensitivity of last generation RQ-PCR tools, concerns about the possibility of monitoring MRD by PB were posed: although some studies proposed interesting experiences with MRD monitoring with PB, lack of validation impaired the real clinical utility of this approach. In particular, a comparative analysis between PB and BM evaluation showed the superiority of BM with an average 1.5 log sensitivity [34, 40].
The optimal treatment of relapsed APL was progressively refined together with the improvement of first-line therapy: in the AIDA and PETHEMA studies [19, 20], treatment strategies at molecular relapse were different (autologous stem cell transplantation, further chemotherapy schedules) and case tailored, and different authors have approached this problem. In the pre-ATO era, salvage therapy with ATRA conferred only a partial advantage in the outcome of patients: Lo-Coco et al. reported that early intervention when molecular relapse occurred with ATRA was efficacious (2-year OS of 92%), but suboptimal results were obtained when the same regimens were administered in overt hematological relapse (44%) [28].
The introduction of ATO as a specific therapy for relapsed APL improved the clinical outcome of relapsed patients: preliminary evidence of ATO efficacy was provided by Chinese groups in the 1990s [53]. In 1999 Niu et al. demonstrated the efficacy of ATO in APL patients (11 newly diagnosed and 47 relapsed patients). Surprisingly, ATO guaranteed 85.1% ORR, but with a 2-year DFS of 41.6%. Furthermore, a molecular assessment was performed with RT-PCR, but the high percentage of molecular positivity (14/15) probably was largely sustained by the premature evaluation performed immediately after the achievement of the hematological response [54].
These results were subsequently confirmed by other experiences [55–57], and, consequently, ATO was approved as the standard therapy for relapsed APL [13], with reported CR rates ranging between 80–90%.
Regarding the molecular clearance of PML-RARα with ATO, recently Shen et al. [58] evaluated the clearance of PML-RARα in patients with APL with RQ-PCR: 61 patients were randomized to receive ATRA, ATO or their combination. Although CR was similar in the three groups (≥90%), the median time to achieve morphologic and molecular CR was significantly shorter in the combination arm, and this difference also lasted after consolidation. In particular, clearance of PML-RARα was higher with ATO monotherapy compared to ATRA, but only few data were available in the literature.
The optimal consolidation therapy after ATO-induced second remission is still a controversial point, and this is largely due to the restricted number of patients with resistant disease. Interesting data were reported by Lo-Coco et al. with gemtuzumab ozogamycin in the second or more advanced molecular relapse or in patients in first molecular relapse not eligible for conventional therapies. MRD evaluation was assessed after two doses at 6 mg/m2; all patients achieved a molecular CR after the third dose with a median duration of molecular response of 15 months [59].
The best transplant procedure in relapsed/refractory APL was not identified: autologous HSCT guarantees a better safety profile but is free from any graft versus leukemia effect; furthermore, the hypothetical risk of stem cell harvest contamination may impair the use of this procedure [13]. For this reason, MRD evaluation before the stem cell harvest is a critical point and should guide the following therapeutic strategies. Meloni et al. demonstrated the need for an MRD evaluation on stem cell harvesting before autologous HSCT, because all patients who underwent this procedure with positive MRD relapsed, while none of the negative patients showed the same behavior [60].
According to the current guidelines, it seems reasonable that the choice of the allogeneic HSCT should be reserved for patients who failed to achieve a second CR or for patients with a short first molecular remission [52, 61]. The MRD monitoring also has a place during follow-up in patients who have undergone allogeneic transplantation, and it is interesting that in a paper by Lo-Coco et al. [62] the subsequent negativity of MRD was obtained without the use of ATRA or ATO, but only with the enhancement of the graft versus leukemia effect obtained by reducing immunosuppressive therapy.
Conclusion
Unlike other acute myeloid leukemias, biological and clinical improvements in the APL understanding have revolutionized the outcome of a traditionally fatal disease. The homogeneity of the molecular hallmark in APL patients allowed confirming the diagnosis of APL and further introducing the possibility of preemptive therapy that has demonstrated its validity in reducing mortality. The assessment of molecular remission in BM is now the standard of care in APL treatment, considering the evidence cited above, although the effect on the mortality and morbidity of sequential monitoring has not been evaluated in a randomized trial. Some authors have raised the question whether standard MRD monitoring for low-risk patients has significance after the post-consolidation time point, considering the low risk of relapse and costs related to molecular monitoring after this time point [63]. Some considerations should be made regarding this critical point: it has to be considered that also some low-risk patients can have a slow clearance of the molecular burden and as in high-risk patients an early discontinuation of molecular monitoring can induce a higher rate of relapses. Moreover, all these considerations born from the results obtained with standard therapy, including antracyclines and ATRA, also have to be considered; the introduction of a novel treatment strategy with the association of ATRA and ATO could induce a different clearance of the molecular burden; subsequently, the time for MRD assessment could be completely different from what has been demonstrated. Burnett et al. recently published an MRD evaluation in ATRA plus ATO as frontline therapy: the results demonstrated that all patients (including high-risk patients) treated with ATRA plus ATO who achieved a molecular remission did not experience a further relapse of disease. If these data are confirmed by other experiences, it may redefine the role of MRD monitoring in this setting of patients [64].
Although the role of MRD monitoring is well established, it is limited in particular settings: about 10% of morphologic-resembling APL presented an aberrant rearrangement pattern involving other responsible genes, in some cases (PLZF-RARα and STAT5b- RARα) with a well-known insensitivity to retinoids [65]. Currently, there are virtually no data on molecular monitoring in PLZF-RARα and STAT5b-RARα-positive diseases, which have been associated with a poor prognosis, although Jovanovic et al. reported the possibility of MRD monitoring in this subset of patients and designed a specific RQ-PCR for MRD evaluation, with interesting results [66]. In conclusion, MRD monitoring remains an important tool in APL management; cooperative randomization trials could provide more information regarding the optimal MRD management, considering the advent of novel and less toxic therapeutic strategies.
Acknowledgments
No funding or sponsorship was received for this study or publication of this article. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole and have given final approval for the version to be published.
Disclosures.
F. De Angelis and M. Breccia have nothing to disclose.
Compliance with ethics guidelines.
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
References
- 1.Alcalay M, Zangrilli D, Fagioli M, et al. Expression pattern of the RARa-PML fusion gene in acute promyelocytic leukemia. Proc Natl Acad Sci USA. 1992;89(11):4840–4844. doi: 10.1073/pnas.89.11.4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grimwade D, Biondi A, Mozziconacci MJ, et al. Characterization of acute promyelocytic leukemia cases lacking the classic t (15;17): results of the European Working Party. Groupe Francais de Cytogenetique Hematologique, Groupe de Francais d’Hematologie Cellulaire, UK Cancer Cytogenetics Group and BIOMED 1 European Community-Concerted Action "Molecular Cytogenetic Diagnosis in Haematological Malignancies". Blood. 2000;96(4):1297–1308. [PubMed] [Google Scholar]
- 3.Chen Z, Brand NJ, Chen A, et al. Fusion between a novel Kruppellike zinc finger gene and the retinoic acid receptor-a locus due to avariant t (11;17) translocation associated with acute promyelocytic leukaemia. EMBO J. 1993;12:1161–1167. doi: 10.1002/j.1460-2075.1993.tb05757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wells RA, Catzavelos C, Kamel-Reid S. Fusion of retinoic acid receptor a to NuMA, the nuclear mitotic apparatus protein, by avariant translocation in acute promyelocytic leukaemia. Nat Genet. 1997;17:109–113. doi: 10.1038/ng0997-109. [DOI] [PubMed] [Google Scholar]
- 5.Redner RL, Rush EA, Faas S, Rudert WA, Corey SJ. The t (5;17) variant of acute promyelocytic leukemia expresses a nucleophosmin retinoic acid receptor fusion. Blood. 1996;87(3):882–886. [PubMed] [Google Scholar]
- 6.Arnould C, Philippe C, Bourdon V, Grgoire MJ, Berger R, Jonveaux P. The signal transducer and activator of transcription STAT5b gene is a new partner of retinoic acid receptor a in acute promyelocytic-like leukaemia. Hum Mol Genet. 1999;8(9):1741–1749. doi: 10.1093/hmg/8.9.1741. [DOI] [PubMed] [Google Scholar]
- 7.Lo-Coco F, Hasan SK. Understanding the molecular pathogenesis of acute promyelocytic leukaemia. Best Pract Res Clin Haematol. 2014;27:3–9. doi: 10.1016/j.beha.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Bernard J, Weil M, Boiron M, et al. Acute promyelocytic leukemia: results of treatment by daunorubicin. Blood. 1973;41(4):489–496. [PubMed] [Google Scholar]
- 9.Fenaux P, Chastang C, Chevret S, et al. A randomized comparison of all transretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia. The European APL Group. Blood. 1999;94(4):1192–1200. [PubMed] [Google Scholar]
- 10.Ades L, Guerci A, Raffoux E, et al. Very long-term outcome of acute promyelocytic leukemia after treatment with all-trans retinoic acid and chemotherapy: the European APL Group experience. Blood. 2010;115(9):1690–1696. doi: 10.1182/blood-2009-07-233387. [DOI] [PubMed] [Google Scholar]
- 11.Lo-Coco F, Avvisati G, Vignetti M, et al. Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation for adults younger than 61 years: results of the AIDA-2000 trial of the GIMEMA Group. Blood. 2010;116(17):3171–3179. doi: 10.1182/blood-2010-03-276196. [DOI] [PubMed] [Google Scholar]
- 12.Lengfelder E, Lo-Coco F, Ades L, et al. Arsenic trioxide-based therapy of relapsed acute promyelocytic leukemia: registry results from the European LeukemiaNet. Leukemia. 2015;29(5):1084–1091. doi: 10.1038/leu.2015.12. [DOI] [PubMed] [Google Scholar]
- 13.Sanz MA, Grimwade D, Tallman MS, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113(9):1875–1891. doi: 10.1182/blood-2008-04-150250. [DOI] [PubMed] [Google Scholar]
- 14.Guidez F, Parks S, Wong H, et al. RARalpha-PLZF overcomes PLZF-mediated repression of CRABPI, contributing to retinoid resistance in t (11;17) acute promyelocytic leukemia. Proc Natl Acad Sci USA. 2007;104:18694–18699. doi: 10.1073/pnas.0704433104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111–121. doi: 10.1056/NEJMoa1300874. [DOI] [PubMed] [Google Scholar]
- 16.Fenaux P, Chomienne C, Degos L. Treatment of acute promyelocytic leukaemia. Best Pract Res Clin Haematol. 2001;14(1):153–174. doi: 10.1053/beha.2000.0121. [DOI] [PubMed] [Google Scholar]
- 17.Lo-Coco F, Diverio D, Pandolfi PP, et al. Molecular evaluation of residual disease as a predictor of relapse in acute promyelocytic leukemia. Lancet. 1992;340(8833):1437–1438. doi: 10.1016/0140-6736(92)92625-P. [DOI] [PubMed] [Google Scholar]
- 18.Beillard E, Pallisgaard N, van der Velden VH, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using ‘real-time’ quantitative reverse transcriptase polymerase chain reaction (RQ-PCR)—a Europe against cancer program. Leukemia. 2003;17(12):2474–2486. doi: 10.1038/sj.leu.2403136. [DOI] [PubMed] [Google Scholar]
- 19.Mandelli F, Diverio D, Avvisati G, et al. Molecular remission in PML/RAR alpha-positive acute promyelocytic leukemia by combined all-trans retinoic acid and idarubicin (AIDA) therapy. Gruppo Italiano-Malattie Ematologiche Maligne dell’Adulto and Associazione Italiana di Ematologia ed Oncologia Pediatrica Cooperative Groups. Blood. 1997;90:1014–1021. [PubMed] [Google Scholar]
- 20.Sanz MA, Martin G, Rayon C, et al. A modified AIDA protocol with anthracycline-based consolidation results in high antileukemic efficacy and reduced toxicity in newly diagnosed PML/RARalpha-positive acute promyelocytic leukemia PETHEMA group. Blood. 1999;94:3015–3021. [PubMed] [Google Scholar]
- 21.Sanz MA, Lo-Coco F, Martín G, et al. Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups. Blood. 2000;96(4):1247–1253. [PubMed] [Google Scholar]
- 22.Miller WH, Jr, Levine K, DeBlasio A, Frankel SR, Dmitrovsky E, Warrell RP., Jr Detection of minimal residual disease in acute promyelocytic leukemia by a reverse transcription polymerase chain reaction assay for the PML/RARa fusion mRNA. Blood. 1993;82(6):1689–1694. [PubMed] [Google Scholar]
- 23.Huang W, Sun G-L, Li X-S, et al. Acute promyelocytic leukemia: clinical relevance of two major PML-RARα isoforms and detection of minimal residual disease by retrotranscriptasepolymerase chain reaction. Blood. 1993;82(4):1264–1269. [PubMed] [Google Scholar]
- 24.Fukutani H, Naoe T, Ohno R, et al. Prognostic significance of the RT-PCR assay of PML/RARA transcripts in acute promyelocytic leukemia. The Leukemia Study Group of the Ministry of Health and Welfare (Kouseisho) Leukemia. 1995;9(4):588–593. [PubMed] [Google Scholar]
- 25.Roman J, Martin C, Torres A, et al. Absence of detectable PML/RARα fusion transcripts in long-term remission patients after bone marrow transplantation for acute promyelocytic leukemia. Bone Marrow Transpl. 1997;19(7):679–683. doi: 10.1038/sj.bmt.1700712. [DOI] [PubMed] [Google Scholar]
- 26.Burnett AK, Grimwade D, Solomon E, Wheatley K, Goldstone AH. Presenting white blood cell count and kinetics of molecular remission predict prognosis in acute promyelocytic leukemia treated with all-trans retinoic acid: result of the randomized MRC trial. Blood. 1999;93:4131–4143. [PubMed] [Google Scholar]
- 27.Diverio D, Rossi V, Avvisati G, et al. Early detection of relapse by prospective RT-PCR analysis of the PML/RARa fusion gene in patients with acute promyelocytic leukemia enrolled in the GIMEMA-AIEOP multicenter ‘‘AIDA’’ trial. Blood. 1998;92(3):784–789. [PubMed] [Google Scholar]
- 28.Lo-Coco F, Diverio D, Avvisati G, et al. Therapy of molecular relapse in acute promyelocytic leukemia. Blood. 1999;94(7):2225–2229. [PubMed] [Google Scholar]
- 29.Esteve J, Escoda L, Martín G, et al. Outcome of patients with acute promyelocytic leukemia failing to front-line treatment with all-trans retinoic acid and anthracycline-based chemotherapy (PETHEMA protocols LPA96 and LPA99): benefit of an early intervention. Leukemia. 2007;21(3):446–452. doi: 10.1038/sj.leu.2404501. [DOI] [PubMed] [Google Scholar]
- 30.Cross NC, Hughes TP, Feng L, et al. Minimal residual disease after allogenic bone marrow transplantation for chronic myeloid leukaemia in first chronic phase: correlations with acute graft-versus-host disease and relapse. Br J Haematol. 1993;84:67–74. doi: 10.1111/j.1365-2141.1993.tb03026.x. [DOI] [PubMed] [Google Scholar]
- 31.Jurlander J, Caligiuri MA, Ruutu T, et al. Persistence of the AML1/ETO fusion transcript in patients treated with allogeneic bone marrow transplantation for t (8;21) leukemia. Blood. 1996;88:2183–2191. [PubMed] [Google Scholar]
- 32.Jurcic JG, Nimer SD, Scheinberg DA, et al. Prognostic significance of minimal residual disease detection and PML/RARa isoform type: long-term followup in acute promyelocytic leukemia. Blood. 2001;98:2651–2656. doi: 10.1182/blood.V98.9.2651. [DOI] [PubMed] [Google Scholar]
- 33.Tobal K, Liu YJ. RT-PCR method with increased sensitivity shows persistence of PML-RARA fusion transcripts in patients in long-term remission of APL. Leukemia. 1998;12(9):1349–1354. doi: 10.1038/sj.leu.2401133. [DOI] [PubMed] [Google Scholar]
- 34.Santamaria C, Chillón MC, Fernández C, et al. Using quantification of the PML-RARalpha transcript to stratify the risk of relapse in patients with acute promyelocytic leukemia. Haematologica. 2007;92(3):315–322. doi: 10.3324/haematol.10734. [DOI] [PubMed] [Google Scholar]
- 35.Burnett AK, Grimwade D, Solomon E, et al. Presenting white blood cell count and kinetics of molecular remission predict prognosis in acute promyelocytic leukemia treated with all-trans retinoic acid: result of the Randomized MRC Trial. Blood. 1999;93(12):4131–4143. [PubMed] [Google Scholar]
- 36.Flora R, Grimwade D. Real-time quantitative RT-PCR to detect fusion gene transcripts associated with AML. Methods Mol Med. 2004;91:151–173. doi: 10.1385/1-59259-433-6:151. [DOI] [PubMed] [Google Scholar]
- 37.Lo-Coco F, Diverio D, Falini B, et al. Genetic diagnosis and molecular monitoring in the management of acute promyelocytic leukemia. Blood. 1999;94:12–22. [PubMed] [Google Scholar]
- 38.Grimwade D, Lo-Coco F. Acute promyelocytic leukemia: a model for the role of molecular diagnosis and residual disease monitoring in directing treatment approach in acute myeloid leukemia. Leukemia. 2002;16:1959–1973. doi: 10.1038/sj.leu.2402721. [DOI] [PubMed] [Google Scholar]
- 39.Burnett AK, Hills RK, Grimwade D, et al. United Kingdom National Cancer Research Institute Acute Myeloid Leukaemia Subgroup. Inclusion of chemotherapy in addition to anthracycline in the treatment of acute promyelocytic leukaemia does not improve outcomes: results of the MRC AML15 trial. Leukemia. 2013;27(4):843–851. doi: 10.1038/leu.2012.360. [DOI] [PubMed] [Google Scholar]
- 40.Grimwade D, Jovanovic JV, Hills RK, et al. Prospective minimal residual disease monitoring to predict relapse of acute promyelocytic leukemia and to direct pre-emptive arsenic trioxide therapy. J Clin Oncol. 2009;27:3650–3658. doi: 10.1200/JCO.2008.20.1533. [DOI] [PubMed] [Google Scholar]
- 41.Sanz MA, Montesinos P, Vellenga E, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans retinoic acid and anthracycline monochemotherapy: long-term outcome of the LPA 99 multicenter study by the PETHEMA Group. Blood. 2008;112(8):3130–3134. doi: 10.1182/blood-2008-05-159632. [DOI] [PubMed] [Google Scholar]
- 42.Sanz MA, Martín G, González M, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans-retinoic acid and anthracycline monochemotherapy: a multicenter study by the PETHEMA group. Blood. 2004;103(4):1237–1243. doi: 10.1182/blood-2003-07-2462. [DOI] [PubMed] [Google Scholar]
- 43.Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111(5):2505–2515. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- 44.Chen GQ, Shi XG, Tang W, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL), I: As2O3 exerts dose dependent dual effects on APL cells. Blood. 1997;89:3345–3353. [PubMed] [Google Scholar]
- 45.Ghavamzadeh A, Alimoghaddam K, Ghaffari SH, et al. Treatment of acute promyelocytic leukemia with arsenic trioxide without ATRA and/or chemotherapy. Ann Oncol. 2006;17(1):131–134. doi: 10.1093/annonc/mdj019. [DOI] [PubMed] [Google Scholar]
- 46.Ghavamzadeh A, Alimoghaddam K, Rostami S, et al. Phase II study of single-agent arsenic trioxide for the front-line therapy of acute promyelocytic leukemia. J Clin Oncol. 2011;29(20):2753–2757. doi: 10.1200/JCO.2010.32.2107. [DOI] [PubMed] [Google Scholar]
- 47.Mathews V, George B, Lakshmi KM, et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: durable remissions with minimal toxicity. Blood. 2006;107(7):2627–2632. doi: 10.1182/blood-2005-08-3532. [DOI] [PubMed] [Google Scholar]
- 48.Mathews V, George B, Chendamarai E, et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: long-term follow-up data. J Clin Oncol. 2010;28(24):3866–3871. doi: 10.1200/JCO.2010.28.5031. [DOI] [PubMed] [Google Scholar]
- 49.Chendamarai E, Balasubramanian P, George B, et al. Role of minimal residual disease monitoring in acute promyelocytic leukemia treated with arsenic trioxide in frontline therapy. Blood. 2012;119(15):3413–3419. doi: 10.1182/blood-2011-11-393264. [DOI] [PubMed] [Google Scholar]
- 50.Powell BL, Moser B, Stock W, et al. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: north American Leukemia Intergroup Study C9710. Blood. 2010;116(19):3751–3757. doi: 10.1182/blood-2010-02-269621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Estey E, Garcia-Manero G, Ferrajoli A, et al. Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood. 2006;107:3469–3473. doi: 10.1182/blood-2005-10-4006. [DOI] [PubMed] [Google Scholar]
- 52.Ravandi F, Estey E, Jones D, et al. Effective treatment of acute promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J Clin Oncol. 2009;27:504–510. doi: 10.1200/JCO.2008.18.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen ZX, Chen GQ, Ni JH, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89(9):3354–3360. [PubMed] [Google Scholar]
- 54.Niu C, Yan H, Yu T, et al. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood. 1999;94(10):3315–3324. [PubMed] [Google Scholar]
- 55.Au WY, Lie AK, Chim CS, et al. Arsenic trioxide in comparison with chemotherapy and bone marrow transplantation for the treatment of relapsed acute promyelocytic leukaemia. Ann Oncol. 2003;14:752–757. doi: 10.1093/annonc/mdg208. [DOI] [PubMed] [Google Scholar]
- 56.Lazo G, Kantarjian H, Estey E, et al. Use of arsenic trioxide (As2O3) in the treatment of patients with acute promyelocytic leukemia: the M.D. Anderson experience. Cancer. 2003;97:2218–2222. doi: 10.1002/cncr.11314. [DOI] [PubMed] [Google Scholar]
- 57.Raffoux E, Rousselot P, Poupon J, et al. Combined treatment with arsenic trioxide and all-trans retinoic acid in patients with relapsed acute promyelocytic leukemia. J Clin Oncol. 2003;21:2326–2334. doi: 10.1200/JCO.2003.01.149. [DOI] [PubMed] [Google Scholar]
- 58.Shen ZX, Shi ZZ, Fang J, et al. All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci USA. 2004;101:5328–5335. doi: 10.1073/pnas.0400053101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lo-Coco F, Cimino G, Breccia M, et al. Gemtuzumab ozogamicin (Mylotarg) as a single agent for molecularly relapsed acute promyelocytic leukemia. Blood. 2004;104(7):1995–1999. doi: 10.1182/blood-2004-04-1550. [DOI] [PubMed] [Google Scholar]
- 60.Meloni G, Diverio D, Vignetti M, et al. Autologous bone marrow transplantation for acute promyelocytic leukemia in second remission: prognostic relevance of pretransplant minimal residual disease assessment by reverse-transcription polymerase chain reaction of the PML/RAR alpha fusion gene. Blood. 1997;90:1321–1325. [PubMed] [Google Scholar]
- 61.Tallman MS. Treatment of relapsed or refractory acute promyelocytic leukemia. Best Pract Res Clin Haematol. 2007;20:57–65. doi: 10.1016/j.beha.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 62.Lo-Coco F, Romano A, Mengarelli A, et al. Allogeneic stem cell transplantation for advanced acute promyelocytic leukemia: results in patients treated in second molecular remission or with molecularly persistent disease. Leukemia. 2003;17(10):1930–1933. doi: 10.1038/sj.leu.2403078. [DOI] [PubMed] [Google Scholar]
- 63.Grimwade D, Jovanovic JV. Hills RK Can we say farewell to monitoring minimal residual disease in acute promyelocytic leukaemia? Best Pract Res Clin Haematol. 2014;27(1):53–61. doi: 10.1016/j.beha.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 64.Burnett AK, Russell NH, Hills RK, et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16(13):1295–1305. doi: 10.1016/S1470-2045(15)00193-X. [DOI] [PubMed] [Google Scholar]
- 65.Licht JD, Chomienne C, Goy A, et al. Clinical and molecular characterization of a rare syndrome of acute promyelocytic leukemia associated with translocation (11;17) Blood. 1995;85(4):1083–1094. [PubMed] [Google Scholar]
- 66.Jovanovic JV, Rennie K, Culligan D, et al. Development of real-time quantitative polymerase chain reaction assays to track treatment response in retinoid resistant acute promyelocytic leukemia. Front Oncol. 2011;1:35. doi: 10.3389/fonc.2011.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]