Abstract
Insulin is essential for glucose homeostasis, but reducing its activity delays the aging process in model organisms. In this issue of Cell Metabolism, Lee, et al. show how these effects of insulin signaling intersect when glucose is fed to C. elegans.
Insulin has essential functions as an anabolic hormone, and in maintenance of glucose homeostasis. After consumption of food, particularly sugary foods, bloodstream glucose levels rise. This triggers release of insulin, which stimulates glucose uptake (Fig. 1) (Shepherd and Kahn, 1999). Under conditions of insulin insufficiency or resistance, circulating glucose levels are elevated, eventually leading to damage in vascular and renal tissues and other diabetic complications. A major cause of this damage is an increase in intracellular reactive oxygen species (ROS) resulting from metabolic perturbations associated with hyperglycemia (Brownlee, 2005).
Figure 1.
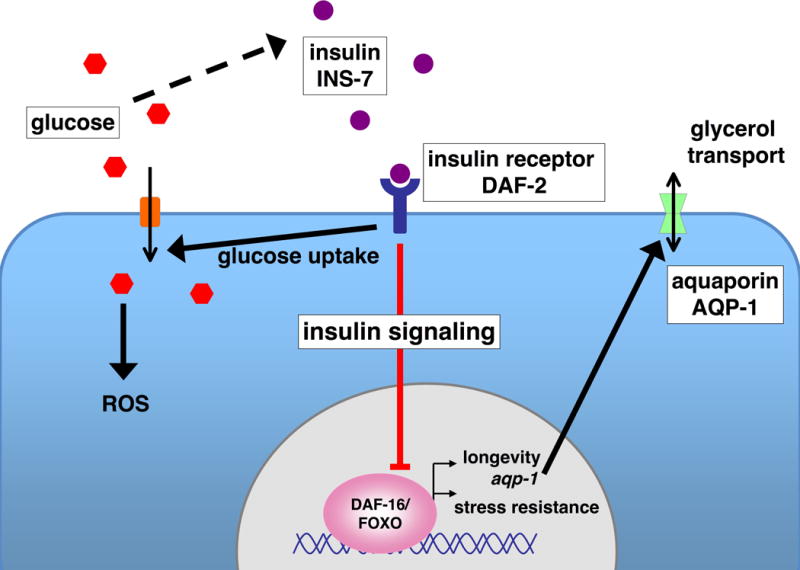
Glucose stimulates release of insulin, which induces glucose uptake. Insulin-like signaling also inhibits FOXO/DAF-16, which positively regulates aqp-1 and other stress resistance and longevity genes. High glucose levels may increase cellular ROS. In C. elegans these events all appear to occur in the intestine, although insulin-like signaling responses vary among tissues.
While insulin is essential for survival, it also seems to play a role in aging. Studies in model organisms have shown that aging can be delayed by reductions in signaling from insulin or related factors (insulin-like signaling) (Russell and Kahn, 2007). Specifically, insulin-like signaling inhibits the transcription factor FOXO (DAF-16 in C. elegans), which acts to promote stress resistance and healthy longevity (Figure). Analyses of knockout mice and long-lived human cohorts support a link between reduced insulin signaling, FOXO, and longevity (Russell and Kahn; Lee, 2009), suggesting that this longer life might be available not only to simple organisms like nematodes, but also to us. The question remains, however: how can the essential functions of insulin be reconciled with the expected benefits of lowering its activity? In this issue of Cell Metabolism, Lee, et al. (2009) begin to unravel how these insulin functions influence each other. They show that high glucose levels shorten C. elegans lifespan by increasing insulin-like signaling, and that glycerol may be an important mediator of glucose metabolism.
As numerous studies have investigated how insulin-like signaling affects longevity, it is reassuring to see compelling evidence that glucose decreases lifespan by acting on this pathway, in the organism where this story originated (Lee, 2009). Glucose feeding reduced lifespan in wild type animals, and essentially negated the longevity benefits associated with either mutations in the insulin receptor DAF-2, or RNA interference (RNAi) against the insulin-like peptide INS-7. While this reduction of daf-2 mutant lifespan might seem to suggest a daf-2-independent effect of glucose, it must be remembered that the insulin receptor is only partially impaired in these mutants, so that increased insulin activity deriving from elevated glucose would likely be devastating. Glucose also inhibited the propensity of daf-2 mutants to develop into dauer larvae, a diapause state that allows the animal to withstand adverse conditions. Importantly, glucose did not further reduce the truncated lifespans associated with the absence of either DAF-16 or heat-shock factor (HSF-1), which cooperates with DAF-16. Finally, glucose increased expression of several insulin-like genes, and led to other gene expression changes that overlapped with effects of inhibiting DAF-16.
One of the genes that was downregulated by both glucose and DAF-16 is aqp-1, which encodes an aquaporin glycerol channel (Lee, 2009). Lack of aqp-1 mimicked many effects of glucose, including reduction of wild type but not daf-16(−) lifespan, downregulation of DAF-16 and HSF-1 targets, upregulation of ins-7, and modulation of DAF-16 activity. Glycerol was elevated by glucose feeding and also reduced lifespan, suggesting that AQP-1 and glycerol may function downstream of glucose in a pathway that affects lifespan through insulin-like signaling. These provocative results suggest that glycerol might be involved in glucose metabolism in mammals. In mice, aquaporin channels allow movement of glycerol from adipocytes to the liver (both corresponding to the C. elegans intestine), and knockout of an adipocyte aquaporin channel is associated with abnormal glycerol metabolism, insulin resistance, and obesity (Maeda et al., 2008).
Other recent studies have also shown that glucose feeding shortens C. elegans lifespan, but suggested involvement of additional mechanisms (Schlotterer et al., 2009; Schulz et al., 2007). Schulz, et al. (2007) reported that a glucose mimetic that cannot be metabolized increases lifespan, an effect attributed to stress pathway stimulation by ROS arising from increased respiration. ROS are also increased by glucose, however (Schlotterer et al., 2009), indicating that further analysis will be required to understand the possible effects of glucose metabolism on longevity. In the latter study, overexpression of glyoxalase-1 protected against the lifespan-shortening effects of glucose. This enzyme detoxifies methylglyoxal, a glucose metabolite involved in diabetic complications. This observation should also be explored further, because only one transgenic strain was analyzed, but it suggests that C. elegans might be amenable to analysis of glucose toxicity mechanisms that lead to diabetic complications.
While caution should be exercised in extrapolating from simple model organisms to people, the results of (Lee, 2009) could have profound implications for understanding how insulin affects us. While the “good” effects of insulin are undoubtedly essential, including prevention of hyperglycemia and resultant tissue damage, it seems that one might want to get by with needing as little insulin as possible, in order to minimize its inhibition of the lifespan-extending effects identified in animal models. The present study may also have implications for understanding effects of calorie restriction (CR), a condition that prolongs life in essentially every organism examined (Bishop and Guarente, 2007). By identifying a specific lifespan-inhibitory effect of glucose, its results raise the question of whether the effects of limiting calories and glucose might be distinguishable. Evidently we can’t have our cake and eat it too just yet, but hope remains that a better understanding of how low insulin activity increases lifespan could allow these pro-longevity mechanisms to be harnessed without impairing the essential activities of insulin. In the meantime, this work provides additional motivation to skip dessert.
References
- Bishop NA, Guarente L. Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nat Rev Genet. 2007;8:835–844. doi: 10.1038/nrg2188. [DOI] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Murphy CT, Kenyon C. Glucose Shortened the Lifespan of Caenorhabditis elegans by Down-Regulating Aquaporin Gene Expression. Cell Metabolism. 2009 doi: 10.1016/j.cmet.2009.10.003. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N, Funahashi T, Shimomura I. Metabolic impact of adipose and hepatic glycerol channels aquaporin 7 and aquaporin 9. Nature Clinical Practice. 2008;4:627–634. doi: 10.1038/ncpendmet0980. [DOI] [PubMed] [Google Scholar]
- Russell SJ, Kahn CR. Endocrine regulation of ageing. Nature Reviews. 2007;8:681–691. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- Schlotterer A, Kukudov G, Bozorgmehr F, Hutter H, Du X, Oikonomou D, Ibrahim Y, Pfisterer F, Rabbani N, Thornalley P, Sayed A, Fleming T, Humpert P, Schwenger V, Zeier M, Hamann A, Stern D, Brownlee M, Bierhaus A, Nawroth P, Morcos M. C. elegans as model for the study of high glucose mediated lifespan reduction. Diabetes. 2009 doi: 10.2337/db09-0567. published on line. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metabolism. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Shepherd PR, Kahn BB. Glucose transporters and insulin action–implications for insulin resistance and diabetes mellitus. The New England Journal of Medicine. 1999;341:248–257. doi: 10.1056/NEJM199907223410406. [DOI] [PubMed] [Google Scholar]


