Abstract
Nuclear factor erythroid-2 related factor 2 (Nrf2) appears to exert either a protective or detrimental effect on the heart; however, the underlying mechanism remains poorly understood. Herein, we uncovered a novel mechanism for turning off the Nrf2-mediated cardioprotection while switching on Nrf2-mediated cardiac dysfunction. In a murine model of pressure overload-induced cardiac remodeling and dysfunction via transverse aortic arch constriction (TAC), knockout of Nrf2 enhanced myocardial necrosis and death rate during an initial stage of cardiac adaptation when myocardial autophagy function is intact. However, knockout of Nrf2 turned out to be cardioprotective throughout the later stage of cardiac maladaptive remodeling when myocardial autophagy function became insufficient. TAC-induced activation of Nrf2 was dramatically enhanced in the heart with impaired autophagy which is induced by cardiomyocyte-specific knockout of autophagy related gene (Atg)5. Notably, Nrf2 activation coincided with upregulation of angiotensinogen (Agt) only in the autophagy impaired heart after TAC. Agt5 and Nrf2 gene loss of function approaches in combination with Jak2 and Fyn kinase inhibitors revealed that suppression of autophagy inactivated Jak2 and Fyn as well as nuclear translocation of Fyn while enhancing nuclear translocation of Nrf2 and Nrf2-driven Agt expression in cardiomyocytes. Taken together; these results indicate that the pathophysiological consequences of Nrf2 activation are closely linked with the functional integrity of myocardial autophagy during cardiac remodeling. When autophagy is intact, Nrf2 is required for cardiac adaptive responses; however, autophagy impairment most likely turns off Fyn-operated Nrf2 nuclear export thus activating Nrf2-driven Agt transcription, which exacerbates cardiac maladaptation leading to dysfunction.
Keywords: Nrf2, Autophagy, Cardiac Remodeling, Cardiac Dysfunction, Pressure Overload, Angiotensinogen
Nuclear factor erythroid-2 related factor 2 (Nrf2) is a member of the Cap ‘n’ Collar (CNC) family of basic leucine zipper (bZip) transcription factor. 1-3 Nrf2 binds to a cis-acting enhancer sequence known as the antioxidant response element (ARE) with a core nucleotide sequence of ‘5-RTGACNNNGC-3’ to control the basal and inducible expression of more than 200 genes that can be grouped into several categories including antioxidant genes, phase II detoxifying enzymes, transcriptional factors, transporters, scavenger receptors, and chaperone proteins. 1-3 Thus, the function of Nrf2 ranges broadly from the classical antioxidant defense to cell cycle regulation and protein quality control.
A cardioprotective role of Nrf2 has been demonstrated in several animal models including the TAC model of pressure overload-induced cardiac maladaptive remodeling and dysfunction. 4-8 At the molecular level, Nrf2 drives antioxidant and detoxifying defense to suppress oxidative stress-mediated cardiac injury and dysfunction. 4-7 Also, Nrf2 facilitates autophagic clearance of toxic ubiquitinated protein aggregates thereby protecting against proteocytotoxicity in the heart. 8 In contrast, a recent study has shown that knockout of Nrf2 prevents myocardial reductive stress, attenuates cardiac accumulation of ubiquitinated proteins and pathological hypertrophy, and ameliorates heart failure in the aged human missense (R120G) mutant of alpha B-crystallin (hCryABR120G) transgenic mouse, a mouse model of protein aggregate-induced cardiomyopathy. 9 Therefore, sustained activation of Nrf2 is proposed to cause reductive stress thereby contributing to hCryABR120G-induced cardiomyopathy. 9 However, Nrf2 cardiomyocyte-specific transgenic mice are healthy and resistant to cardiac maladaptive remodeling and dysfunction after 4 wk pressure overload, 8 arguing against the theory of Nrf2-mediated reductive stress. On the other hand, previous studies have demonstrated that Nrf2 activation leads to liver damage in a setting of autophagy impairment. 10, 11 In addition, autophagy insufficiency plays an essential role in mouse CryABR120G (mCryABR120G)-induced cardiomyopathy. 12 Given that the mCryABR120G and hCryABR120G have virtually identical amino sequences 13, 14 and aging is associated with autophagy insufficiency or impairment, 15 it is likely that the Nrf2-mediated adverse effects may be related to impaired autophagy in the aged hCryABR120G heart. Collectively, these findings indicate that Nrf2 may exert either a protective or detrimental effect on the heart depending on the nature of pathological settings. However, the precise mechanism for the dual effects of Nrf2 remains unknown.
Nrf2 is a protein whose half-life is estimated to be less than 20 min the cell. 2 Although precise molecular mechanisms of Nrf2 activation are not fully understood, it is general accepted that Keap1 (Kelch-like ECH associated protein 1) plays a central role in the regulation of Nrf2 protein stability and Nrf2-driven transcriptional activity. 2 Keap1 serves as an adaptor for the interaction of the Cul3-based E3-ubiquitin ligase complex with Nrf2 leading to Nrf2 ubiquitination and consequent proteosomal degradation. Several alternative mechanisms of Nrf2 regulation have been proposed, such as phosphorylation of Nrf2 by various protein kinases including mitogen-activated kinases (MAPK), protein kinase C (PKC), phosphatidylinositol 3-kinase (PI3K), and Fyn kinase. 2, 16 It has been documented that Fyn kinase is capable of phosphorylating Nrf2 in the nucleus, thereby leading to nuclear export of the phosphorylated Nrf2 for its degradation in vascular smooth muscle cells. 17 Yet, the pathophysiological significance of the Nrf2 regulation cascades in the heart remains to be explored.
In the present study, we found that the pathophysiological consequence of Nrf2 activation is linked to the functional integrity of autophagy in the heart. Nrf2 is required for cardiac adaptive responses when autophagy is intact; however, it becomes a mediator of cardiac maladaptive remodeling and dysfunction when autophagy is impaired. Autophagy impairment most likely turns off nuclear Fyn-operated Nrf2 export thus activating Nrf2-driven Agt transcription, which exacerbates cardiac maladaptation leading to dysfunction.
Methods
Littermates of wild type (WT) and Nrf2 knockout (Nrf2-/-), or floxedAtg5 (Atg5fl/fl), αMHC-MerCreMer (MerCreMer+), and MerCreMer+∷Atg5fl/fl mice were generated by cross-breeding of heterozygous Nrf2+/- or MerCreMer+∷Atg5fl/+ and Agt5fl/+ mice, respectively. Transverse aortic arch constriction (TAC) model, echocardiography, histopathology, immunohistochemistry, and biochemical assays were performed as previously described. 4, 7, 8
Detailed methods are provided in the Online-only Data Supplement.
Results
Knockout of Nrf2 impairs acute cardiac adaptation; however, it ameliorates the progression of cardiac maladaptive remodeling leading to heart failure after pressure overload
In a murine TAC model, TAC-induced pressure overload initially results in an adaptive cardiac hypertrophy with preserved cardiac function (days 1-14) followed by maladaptive cardiac remodeling which eventually causes chronic heart failure (days 14-28). 4 Of note, up to 40% of wild type (WT) mice die from acute heart failure within the first 2 wks after TAC. 4 In this TAC model, we have demonstrated that knockout of Nrf2 exacerbates cardiac pathological hypertrophy, fibrosis, and apoptosis which results in cardiac dysfunction with increased mortality within the first 2 wks. 4 Thus, these findings indicate that Nrf2 is a critical mediator of cardiac adaptation. 4 Also, Nrf2 may act as a negative regulator of cardiac maladaptive remodeling and dysfunction in response to sustained pressure overload. Accordingly, we further determined the effect of Nrf2 deficiency on cardiac adaptation as well as cardiac maladaptive remodeling and dysfunction after TAC. TAC-induced death rate was increased by knockout of Nrf2 (Figure 1A), presumably due to the increased acute heart failure as previously reported. 4, 18 Since cardiomyocyte necrosis is one of the causes for heart failure, 19 we determined the impact of Nrf2 deficiency on TAC-induced myocardial necrosis. A time-course study revealed that TAC induced myocardial necrosis appeared on day 1, peaked on day 3, and declined to basal levels after one week (Figure S1). Thus, we compared myocardial necrosis of WT and Nrf2-/- mice 3 days after TAC. As shown in Figure 1B, loss of Nrf2 enhanced TAC-induced myocardial necrosis. These results indicate that Nrf2 is capable of protecting against TAC-induced acute heart failure via its ability to suppress myocardial necrosis during the initial stage of pressure overload-induced cardiac adaptation. These findings provide additional evidence to demonstrate a critical role of Nrf2 in mediating cardiac protection.
Figure 1.
The effect of TAC on death, myocardial necrosis and pathological hypetrophy in WT and Nrf2-/- mice. A, Survival rate. Nine wk old male littermates of WT and Nrf2-/- mice were subjected to sham or TAC operation. The number of dead mice over an 8 wk period was counted daily. *p<0.05 vs. Nrf2-/- group. B, The effect of Nrf2 knockout on myocardial necrosis after 3 days of TAC. Nine wk old male littermates of WT and Nrf2-/- mice were subjected to sham or TAC operation. Green indicates the cardiomyocyte membrane. Red indicates the necrotic cells. *p<0.05 vs. sham (-) in the same group. C, Representative images of hearts in WT and Nrf2-/- mice 4 wks after TAC surgery. D, Left panel: Representative echocardiographic images of WT and Nrf2-/- mice 4 wks after TAC surgery. Right panel: LVPW;d of WT and Nrf2-/- mice. *p<0.05 vs. sham (-) in the same group.
However, we found unexpectedly that knockout of Nrf2 attenuated cardiac hypertrophy and ameliorated progression of cardiac dysfunction by 8 wks after TAC (Figure 1C and D, Table 1). In addition, knockout of Nrf2 significantly inhibited cardiomyocyte hypertrophy and the fetal gene reprogramming and the downregulation of sarco-endoplasmic reticulum calcium ATPase2a (SERCA2a) for pathological cardiac hypertrophy, 20, 21 as well as myocardial fibrosis, apoptosis, and oxidative stress at 4 wks after TAC (Table 1 and Figures S2-4). These results clearly demonstrate a mediator role of Nrf2 in cardiac maladaptive remodeling and dysfunction in response to pressure overload.
Table 1. Echocardiography and pathology of WT and Nrf2-/- mice 4 and 8 wks after TAC.
| Echocardiography | WT | Nrf2-/- | ||
|---|---|---|---|---|
|
|
|
|||
| Sham | TAC | Sham | TAC | |
| 4 wks | ||||
| Echocardiography (n) | (10) | (24) | (13) | (17) |
| IVS;d (mm) | 0.8±0.04 | 1.3±0.22A | 0.8±0.16 | 1.0±0.17B,C |
| LVID;d (mm) | 4.1±0.35 | 4.0±0.38 | 4.1±0.38 | 4.1±0.47 |
| LVPW;d (mm) | 0.8±0.07 | 1.3±0.24A | 0.8±0.17 | 1.0±0.2B,C |
| FS% | 38.3±3.41 | 24.4±3.09A | 37.4±3.58 | 30.1±2.88B |
| EF% | 76.0±7.41 | 48.8±6.28A | 74.6±7.21 | 60.0±5.78B |
| Pathology (n) | (10) | (24) | (13) | (17) |
| BW (g) | 30.0±2.63 | 28.5±2.37 | 26.6±3.35 | 26.0±1.7 |
| HW (mg) | 150±12 | 225±40.68A | 133±18 | 186±39B,C |
| LW (mg) | 205±71 | 237±91 | 166±31 | 193±70 |
| TIBIA (mm) | 17.9±0.44 | 18.0±0.21 | 17.8±0.31 | 17.8±0.34 |
| HW/TIBIA (mg/mm) | 8.6±0.78 | 12.6±2.26A | 7.8±1.33 | 10.2±2B,C |
| HW/BW (mg/g) | 5.1±0.42 | 8.5±2.05A | 5.0±0.51 | 6.7±1.06B,C |
| 8 wks | ||||
| Echocardiography (n) | (8) | (11) | (8) | (12) |
| IVS;d (mm) | 0.9±0.1 | 1.4±0.17A | 0.9±0.14 | 1.2±0.21B,C |
| LVID;d (mm) | 4.4±0.29 | 5.2±0.34A | 4.4±0.46 | 4.6±0.61C |
| LVPW;d (mm) | 0.8±0.09 | 1.4±0.16A | 0.8±0.12 | 1.2±0.16B,C |
| FS% | 34.0±6.44 | 15.7±3.15A | 35.5±5.57 | 21.2±3.59B,C |
| EF% | 68.0±12.88 | 31.7±6.55A | 71.9±10.3 | 42.8±7.41B,C |
| Pathology (n) | (8) | (11) | (8) | (12) |
| BW (g) | 32.0±3.36 | 30.4±2.62 | 28.9±2.01 | 28.9±2.22 |
| HW (mg) | 155.3±12.8 | 338.4±43.9A | 161.4±20.1 | 258.8±38.3B,C |
| LW (mg) | 195.4±50.9 | 432.6±169.0A | 222.8±61.0 | 268.9±122C |
| TIBIA (mm) | 17.9±0.32 | 17.9±0.18 | 17.5±0.47 | 17.7±0.27 |
| HW/TIBIA (mg/mm) | 8.8±0.38 | 18.9±2.5A | 8.8±0.89 | 14.5±2.1B,C |
| HW/BW (mg/g) | 5.4±0.55 | 11.2±2.04A | 5.3±0.51 | 9.0±1.6B,C |
LVID;d, left ventricular internal diastolic dimension; LVID;s, left ventricular internal systolic dimension; LVPW;d, left ventricular diastolic posterior wall; FS, fractional shortening; EF, ejection fraction; BW, body weight; HW/Tibia, heart weight/tibia length ratio; LW, lung weight.
, p<0.05 vs. WT sham;
, p<0.05 vs. Nrf2-/- sham;
, p<0.05 vs. WT TAC.
Taken together, our findings reveal that Nrf2 is required for cardiac adaptation and paradoxically it becomes a mediator of cardiac maladaptive remodeling and dysfunction in response to pressure overload.
Pathophysiological consequences of Nrf2 activation are linked to the functional integrity of autophagy in the heart after pressure overload
Since autophagy impairment may be linked to the Nrf2-mediated adverse effects aforementioned, we postulated that there is an intimate relationship between autophagy function and Nrf2-mediated actions in the heart. To test this hypothesis, we examined the temporal autophagy functional states in the heart after TAC. We found that myocardial autophagy flux, a more accurate parameter reflecting autophagy function, 22 was intact at 2 wks, suppressed at 4 wks, and blocked at 8 wks after TAC (Figure 2). These results indicate that TAC eventually induces autophagy impairment in the heart. Given that the loss of Nrf2 exaggerated cardiac maladaptive remodeling and led to an onset of cardiac dysfunction at 2 wks after TAC 4 when the autophagic flux remains normal (Figure 2), these results indicate that Nrf2 activation is cardioprotective in the pressure overloaded heart when myocardial autophagy function is intact. Also, since the observed Nrf2-mediated cardiac adverse remodeling at 4 wks after TAC (Supplementary Figures S2-S4.)
Figure 2.
The effect of TAC on myocardial autophagy in mice. Male WT C57BL/6J mice at 9-10 wks of age were subjected to sham or TAC operation as indicated. A, Western blot analysis of myocardial LC3-I, LC3-II, and p62 expression. Left panel: representative immunoblots from 4 separate experiments. Right panel: densitometric analysis of LC3-II and p62 protein levels. n=4, * or # p<0.05 vs. control (0). There are no differences of LC3-II or p62 expression between control (0) and sham groups at each time point as indicated. B, Myocardial autophagic flux after TAC. Upper panel: representative immunoblots of LC3-I and LC3-II from 4 separate experiments. middle panel: densitometric analysis of LC3-II protein levels; bottom panel: quantified autophagic flux. n=4. ns, non-significant; CQ, chloroquine. There are no differences of LC3-II expression between control (0) and sham groups at each time point as indicated.
4) was associated with insufficient autophagy in the heart (Figure 2), and the Nrf2-mediated cardiac pathological hypertrophy and dysfunction at 8 wks after TAC (Table 1) was associated with impaired autophagy in the heart (Figure 2); it is conceivable that Nrf2 mediates TAC-induced cardiac pathological remodeling and dysfunction in a setting of autophagy impairment. Collectively, these findings in the heart highlight a crucial link between autophagy functional states and the Nrf2-mediated dual effects.
Nrf2 expression and activity are enhanced in the failing heart with autophagy impairment after pressure overload
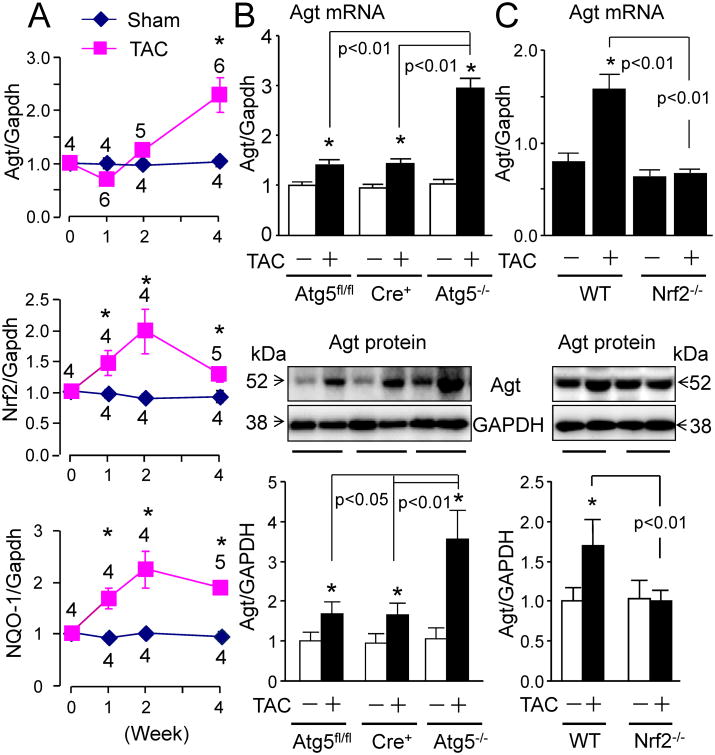
To further investigate the interplay between autophagy and Nrf2 activation in cardiac dysfunction, we determined the impact of myocardial specific autophagy impairment on Nrf2 expression and activity in the heart at 4 wks after TAC utilizing tamoxifen-inducible cardiomyocyte-restricted Atg5 knockout (Atg5-/-) mice. Tamoxifen (20 mg/kg/d, i.p.) for 21 days ablated Atg5 expression in the heart of MerCreMer+∷Atg5fl/fl mice (Figure 3A) without any adverse health problems as previously reported. 23 The ablation of Atg5 induced accumulation of p62 and suppression of LC3-II expression in the heart (Figure 3A), indicating myocardial autophagy impairment due to the loss of Atg5. Echocardiography showed that TAC-induced cardiac dysfunction was worsened in the cardiac specific Atg5 depleted and autophagy impaired mice, compared with the control groups of Atg5fl/fl and MerCreMer+ mice (data not shown) as previously described. 24 Tamoxifen treatment per se did not affect TAC-induced cardiac hypertrophy and dysfunction and death in WT mice (Supplementary Figure S5 and Supplementary Table S2). Of interest, TAC-induced expression of Nrf2 and its downstream target gene NAD(P)H dehydrogenase, quinone-1 (NQO1) were enhanced by the ablation of Atg5 (Figure 3B and C), indicating that myocardial autophagy impairment results in a further production of Nrf2 in the pressure overloaded heart. Considering the Nrf2-mediated cardiac adverse remodeling and dysfunction in the autophagy impaired heart (Tables 1 and Supplementary Figures S2-S4), it is likely that autophagy impairment switches on this remodeling and dysfunction via a yet unknown mechanism in the pressure overloaded heart.
Figure 3.
The effect of cardiomyocyte-restricted knockout of Atg5 on TAC-induced activation of Nrf2 in the murine heart. Male 6 wk-old mice with different genotypes as indicated were i.p. injected with tamoxifen (20 mg/kg/d) for 3 wks. After 2 wks for washing out tamoxifen from the body, these mice were subjected to sham or TAC operation for 4 wks. A, The efficacy of tamoxifen-induced knockout of Atg5 in the heart. Representative immunoblots of myocardial Atg5, p62, LC3-I and LC3-II from 8 separate experiments. B, qPCR analysis of myocardial Nrf2 and NQO1 mRNAs in the heart 4 wks after sham (-) and TAC. *p<0.05 vs. sham (-) in the same group. C, Western blot analysis of myocardial Nrf2 and NQO1 proteins in the heart 4 wks after sham and TAC. n=3 for each sham group and n=4∼5 for each TAC group. *p<0.05 vs. sham (-) in the same group.
Nrf2 drives Agt expression in the pressure overloaded heart with functional insufficiency of autophagy
A recent study has documented that Nrf2-operated upregulation of Agt is causative for renal damage associated with type 1 diabetes, 25 a state which induces autophagy impairment. 26, 27 Because it is well established that the upregulation of Agt is a primary cause of cardiac pathological remodeling and dysfunction, 28, 29 we postulated that Nrf2 drives the upregulation of Agt in the autophagy insufficient heart thereby leading to cardiac pathological remodeling and dysfunction in response to pressure overload. Thus, we first performed a temporal study of Nrf2 activation and Agt expression in the heart of wild type mice after TAC. We found that Agt expression was upregulated by 2 wks and remained so at 4 wks after TAC; however, Nrf2 expression and activation was apparent at 1 wk, peaked at 2 wks, and then Nrf2 mRNA expression contrastingly declined closer to the basal level while NQO1 mRNA expression (reflecting Nrf2 transcriptional activity) was largely retained by 4 wks after TAC (Figure 4A). These expression patterns indicate that Nrf2 activation per se may not be able to upregulate Agt expression in the heart, and Nrf2-mediated upregulation of Agt is dependent on a yet unknown mechanism in the pressure overloaded heart. In addition, it is likely that posttranscriptional as opposed to transcriptional activation of Nrf2 plays a critical role in the upregulation of Agt in the pressure overloaded heart. Given that the myocardial autophagy function is intact within 2 wks, and then declines and becomes insufficient at 4 wks after TAC (Figure 2), we questioned whether autophagy function is critical for Nrf2-operated upregulation of Agt in the heart. We found that ablation of Atg5 in cardiomyocytes dramatically enhanced 4 wk TAC-induced Agt expression (Figure 4B), whereas knockout of Nrf2 blocked 4 wk TAC-induced Agt expression in the heart (Figure 4C). Considering that TAC-induced upregulation of LC3-II in the heart is mostly blocked in cardiomyocyte-restricted Agt5-/- mice, 24 loss of Atg5 in cardiomyocytes impairs autophagy in the pressure overloaded heart. In addition, we have demonstrated that Nrf2 does not directly regulate autophagy per se and may facilitate autophagy-mediated clearance in the heart. 8 Thus, knockout of Nrf2 may have a minimal or even negative impact on autophagy activation in the heart. Hence, 4 wk TAC-induced autophagy insufficiency in the heart is either worsened by the knockout of Atg5 or slightly exaggerated by knockout of Nrf2. Collectively, results suggest that autophagy deficiency drives Nrf2-mediated upregulation of Agt in the heart.
Figure 4.
Nrf2-mediated upregulation of Agt in the autophagy impaired heart after TAC. A, TAC-induced expression of Agt, Nrf2, and NQO-1 in the heart. Male WT mice at 9 wks of age were subjected to sham or TAC operation. Left ventricles of mice before and after 1, 2, and 4 wks post-surgery were subjected to qPCR analysis of Agt, Nrf2, and NQO-1 mRNA expression. *p<0.05 vs. Sham. Sample numbers for each group are indicated in the figure. B, The effect of cardiomyocyte-restricted knockout of Atg5 on Agt expression in the heart 4 wks after sham (-) or TAC (+). Male mice with different genotypes were treated as described in Figure 3. Upper panel: qPCR analysis of Agt mRNA expression in the heart. Middle panel: representative immunoblots, and Bottom panel: densitometric analysis of Agt protein in the heart. n=4, *p<0.05 vs. sham (-) in the same group. C, Effect of Nrf2 knockout on Agt expression in the heart 4 wks after sham (-) or TAC (+). Male 6 wk-old littermates of WT and Nrf2 received the same treatment as described in Figure 3. Upper panel: qPCR analysis of Agt mRNA expression in the heart. Middle panel: representative immunoblots, and Bottom panel: densitometric analysis of Agt protein in the heart. n=4, *p<0.05 vs. WT sham (-).
Autophagy impairment suppresses TAC-induced activation of Jak2/Fyn pathway which operates Nrf2 nuclear export for degradation in the pressure overloaded heart
To explore the signaling mechanism by which Nrf2 upregulates Agt in the autophagy deficient heart after TAC, we determined the effect of Nrf2 knockout on TAC-induced myocardial activation of Jak2/Stat3 pathway, which has been shown to control Agt expression in cardiomyocytes. 30 As shown in Figure 5A, phosphorylation of Jak2 and Stat3 was enhanced in the heart of control Atg5fl/fl and MerCreMer+ mice at 4 wks after TAC when myocardial autophagy is insufficient (Figure 2). Compared with the control, 4 wk TAC-induced enhancement of Jak2 and Stat3 activities was blocked in cardiomyocyte-specific Atg5 knockout mice (Figure 5A). These results demonstrate that autophagy is required for TAC-induced activation of Jak2/Stat3 pathway in the heart as observed in other cell types. 31 On the other hand, knockout of Nrf2 minimally regulated the 4 wk TAC-induced activation of Jak2/Stat3 pathway (Figure 5B). These results indicate that Nrf2 mediates TAC-induced upregulation of Agt in the heart with autophagy insufficiency via a mechanism independent of Jak2/Stat3 pathway or dependent on a yet unappreciated signaling mechanism due to Jak2 inhibition.
Figure 5.
Jak2/Fyn pathway in the autophagy impaired heart after TAC. Male mice with different genotypes were treated as described in Fig. 3. Western blot analysis of p-Jak2, Jak2, p-Stat3, Stat3, p-Fyn, and Fyn in A) Agt5fl/fl, MerCreMer+, and cardiomyocyte-restricted Atg5-/- as well as B) WT and Nrf2-/- mice 4 wks after sham (-) and TAC. Results are representative immunoblots from 4 separate experiments.
Considering the observed link between posttranscriptional activation of Nrf2 and Agt upregulation (Figure 4), we postulated that Jak2 dependent posttranscriptional regulation of Nrf2 is critical for the upregulation of Agt in the autophagy impaired heart. It has been reported that nuclear Fyn kinase, a downstream substrate of Jak2, 32 once activated, is capable of causing Nrf2 nuclear export for its degradation. 17 Therefore, we hypothesized that autophagy insufficiency leads to suppression of Jak2 dependent Fyn phosphorylation as well as the subsequent Nrf2 nuclear export and degradation, thereby resulting in increased nuclear accumulation of Nrf2 to activate Agt expression in the heart. To test this hypothesis, we determined the impact of Nrf2 and/or Atg5 knockout on Ang II-induced activation of Jak2/Stat3 and Fyn kinases as well as the protein expression of Agt in cultured rat neonatal cardiomyocytes. As shown in Figure 6A and B, Ang II upregulated LC3-II expression (without affecting bafilomycin A1-induced accumulation of LC3-II; data not shown), induced phosphorylation of Jak2, Stat3, and Fyn, and upregulated Agt protein expression in the control group. These results indicate that the Ang II-induced upregulation of Agt associates with the activation of autophagy as well as Jak2/Stat3 and Fyn kinases in cardiomyocytes. Knockdown of Atg5 alone resulted in downregulation of LC3-II expression and upregulation of p62 (Figure 6), indicating that the knockdown of Atg5 impaires autophagy in cardiomyocytes as observed in vivo (Figure 3A). The Atg5 knockdown-induced autophagy impairment blocked the Ang II-induced activation of Jak2, Stat3, and Fyn kinases while dramatically enhancing Ang II-induced Agt expression (Figure 6), demonstrating that autophagy impairment suppresses the activation of Jak2/Stat3 pathway and Fyn kinase but facilitates upregulation of Agt in cardiomyocytes as we observed in the heart (Figure 4B and Figure 5A). Knockdown of Nrf2 alone had minimal impact on the Ang II-induced activation of autophagy, Jak2/Stat3, and Fyn kinases but suppressed the Ang II-induced upregulation of Agt (Figure 6). While knockdown of Nrf2 did not affect the Atg5 deficiency-induced inactivation of Jak2/Stat3 and Fyn kinases, it blocked the Atg5 deficiency-induced enhancement of Agt expression (Figure 6). These results demonstrate that Nrf2 is a critical mediator of Agt expression in cardiomyocytes regardless of autophagy functional status. Importantly; these findings also strongly support the aforementioned notion that Jak2/Fyn signaling plays a key role in the control of Nrf2-mediated upregulation of Agt in autophagy-impaired cardiomyocytes. To further establish a causative link between Jak2, Fyn kinases, and Nrf2-mediated Agt expression, we used Jak2 and Fyn kinase inhibitors, AG490 and PP2, in rat neonatal cardiomyocytes. A time-course study showed that Ang II activated Nrf2-driven NQO1 expression and Agt transcription, reaching a peak at 6∼12 h and declining to the basal levels by 48 h (Figure 7A). Both AG490 and PP2 enhanced Ang II-induced upregulation of NQO1 and Agt (Figure 7B). These results reveal an intimate interaction between Jak2-Fyn kinases, Nrf2 activation, and Agt expression in cardiomyocytes, in which Jak2 and Fyn kinases serve as negative feedback regulators of Nrf2 activation and Agt expression when autophagy is intact. Notably, knockdown of Atg5 dramatically enhanced Ang II-induced mRNA expression of NQO1 and Agt and this enhancement could not be further increased by inhibiting Fyn kinase; whereas, both the Fyn kinase inhibitor PP2-potentiated and Atg5 deficiency-heightened Agt expression were blocked by knockdown of Nrf2 (Figure 7C). These results indicate that Fyn kinase suppresses Agt expression by inactivating Nrf2, serving as a negative feedback mechanism for the control of Agt expression in autophagy intact cardiomyocytes; however, autophagy impairment could turn off the negative feedback regulation thus exaggerating Agt expression in cardiomyocytes. To explore the pathophysiological relevance of Fyn-Nrf2 axis, we studied the nuclear location of Fyn kinase and Nrf2 as well as the role of Nrf2 in driving Agt expression in the heart after TAC. Immunochemical staining showed that there is no detectable level of nuclear phosphorylated (p)-Fyn or Nrf2 in 4 wk sham hearts of Atg5fl/fl, MerCreMer+, and Atg5-/- mice (data not shown). However, 4 wk TAC led to increased levels of nuclear p-Fyn in the heart of control Agt5fl/fl and MerCreMer+ mice but not in the heart of Atg5-/- mice (Figure 8A). Also, 4 wk TAC led to increased levels of nuclear Nrf2 in the heart of control Agt5fl/fl and MerCreMer+ mice and it was more dramatic in the heart of Atg5-/- mice (Figure 8A). This reciprocal relationship between nuclear p-Fyn and Nrf2 as well as the enhanced expression and transcriptional activity of Nrf2 in Atg5-/- hearts suggest that the inactivation of Fyn is responsible for the nuclear accumulation of Nrf2 leading to upregulation of Agt expression in myocardium. Indeed, knockout of Nrf2 blocked the TAC-induced upregulation of Agt expression in autophagy impaired hearts in which Atg5 is specifically knocked out in cardiomyocytes (Figure 8B).
Figure 6.
The effect of Nrf2 and Atg5 knockdown on Ang II-induced activation of the Jak2/Fyn/Agt signaling axis in cardiomyocytes. Rat neonatal cardiomyocytes infected with adenovirus of control scramble shRNA (Ad-shCtr) and Ad-shNrf2 were further transfected with control siRNA (siCtr) and siAgt5 prior to Ang II treatment as described in the Online Supplement. A, Representative immunoblots from 4 separate experiments. B, Densitometric analysis. n=4, *p<0.05 vs. the control (vehicle treated Ad-shCtr+siCtr cells). C, The efficacy of Nrf2 and Atg knockdown. Upper panel: qPCR analysis of Nrf2 mRNA expression and Western blot analysis of Nrf2 target gene NQO1 in rat neonatal cardiomyocytes infected with Ad-shCtr and Ad-shNrf2. n=4, *p<0.05 vs. Ad-shCtr infected cells. Lower panel: representative immunoblot of LC3 and Agt5 in the rat neonatal cardiomyocytes transfected with siCtr and siAtg5 from 4 separate experiments.
Figure 7.
The effect of Fyn kinase inhibition on Ang II-induced Nrf2 activation and Agt expression in cardiomyocytes with a setting of autophagy impairment. A, A time-course study of Ang II-induced mRNA expression of NQO1 and Agt. Serum starved rat neonatal cardiomyocytes were treated with Ang II (1 M) in serum free DMEM as indicated. n=4, *p<0.05 vs. Ang II (0 h) in the same group. B, The effect of Jak2 inhibitor AG490 and Fyn kinase inhibitor PP2 on Ang II-induced mRNA expression of NQO1 and Agt. Serum starved rat neonatal cardiomyocytes were treated with Ang II (1 μM), AG490 (1 μM), and PP2 (1 μM) in serum free DMEM as indicated for 48 h. n=4, *p<0.05 vs. vehicle, PP2, AG490, or Ang II alone treated groups. ns; non-significant. C, The effect of PP2 on Ang II-induced mRNA expression of NQO1 and Agt in the settings of Nrf2 and/or Atg5 knockdown. Rat neonatal cardiomyocytes were treated as in Figure 6 and stimulated with Ang II and PP2 as in Figure 7B. n=4, *p<0.05 vs. Ang II-treated Ad-shCtr+siCtr group. ns; non-significant.
Figure 8.
The role of Jak2/Fyn signaling in regulating Nrf2 nuclear accumulation in the pressure overloaded heart. A, Immunochemical staining of p-Fyn and Nrf2 in the heart at 4 wks after TAC. Left panel: representative images from 4 separate experiments. Right panel: % positive nuclei values for p-Fyn and Nrf2. B, The effect of Nrf2 deficiency on Agt expression in the heart with cardiomyocyte-specific knockout of Atg5 at 4 wks after TAC. Left panel, Western blot analysis of Agt protein expression. n=4, *p<0.05 vs. Sham (-) in the same group. Right panel, qPCR analyses of Agt mRNA expression. n=8, *p<0.05. vs. Sham(-) in the same group. Nrf2+/+∷Atg5-/-; cardiomyocyte-restricted Atg5 knockout mice. Nrf2-/-∷Atg5-/-; double Nrf2 and cardiomyocyte-restricted Atg5 knockout mice. C, Schematic depiction of the results and proposed hypothesis.
Discussion
In the present study, there are several novel findings which address the contradictory roles of Nrf2 in cardioprotection and cardiac dysfunction as follows: (1) Nrf2 activation is cardioprotective when myocardial autophagy function is sufficient; however, it exacerbates cardiac maladaptive remodeling and dysfunction when myocardial autophagy function is insufficient; (2) Nrf2 is capable of inhibiting myocardial necrosis in the heart with a sufficient function of autophagy, whereas it could enhance angiotensin II signaling in the heart with an insufficient function of autophagy; and (3) autophagy impairment results in Nrf2 nuclear accumulation and the subsequent activation of Nrf2-driven transcription of Agt in cardiomyocytes, most likely due to the inactivation of Jak2/Fyn signaling for Nrf2 export and degradation. Our findings uncover for the first time that autophagy plays a critical role in controlling the pathophysiological consequences of Nrf2 activation, unveiling the nature of the Nrf2-mediated controversial actions in the heart.
The heart is capable of initiating a rapid adaptation to compensate for a large variety of mechanical, hemodynamic, hormonal, and pathological stimuli, which is characterized by cardiac hypertrophy with preserved left ventricle (LV) function. However, the cardiac compensation is limited and, as a result, the adaptive responses become maladaptive and LV dysfunction and heart failure death ensue if the stress persists. 33 The molecular mechanism of cardiac compensation and decompensation has only been partly understood. Our previous studies indicate that, in a setting of pressure overload, Nrf2 is critical for the initial cardiac adaptation most likely via its ability to suppress oxidative stress as well as its antioxidant independent capability to facilitate clearance of toxic ubiquitinated proteins in cardiomyocytes. 4, 8 Considering a critical role of Nrf2 in inhibiting cardiomyocyte necrosis, 7, 8 it wasn't surprising to find that Nrf2 knockout enhances myocardial necrosis during the cardiac adaptation process. These results collectively indicate that Nrf2 is a crucial mediator of cardiac compensation and serves as an inhibitor of the transition from cardiac compensated to maladaptive remodeling and dysfunction. Also, Nrf2 may play a subsequent critical role in the decompensation which leads to heart failure. However, the suppression of cardiac maladaptive remodeling and dysfunction in Nrf2 knockout mice is intriguing. Given that Nrf2 activation was cardioprotective during the cardiac adaptation phase while being detrimental to the heart during the decompensation phase in the same TAC model, it is conceivable that there must be a yet unappreciated mechanism that could turn off Nrf2-mediated cardioprotection and switch on Nrf2-operated cardiac maladaptation in the pathogenesis of pressure overload-induced heart failure. In this regard, our findings support a notion that myocardial autophagy impairment is responsible for switching on Nrf2-mediated cardiac maladaptive remodeling and dysfunction in response to pressure overload. At the molecular level, autophagy impairment switches off Jak2/Fyn signaling for Nrf2 export and degradation thus leading to nuclear accumulation of Nrf2 to drive Agt expression in the heart. Since the upregulation of myocardial Agt inevitably elevates the local levels of angiotensin (Ang) II, in turn, activation of Ang II-Ang II receptor type 1 (AT1R) axis which causes cardiac adverse remodeling and dysfunction, 34, 35 it is predictable that the activation of Nrf2-Agt axis eventually leads to cardiac adverse remodeling and dysfunction. Taken together, these results suggest that autophagy impairment is a contributing mechanism for initiating cardiac decompensation in which Nrf2 activates Agt-Ang II signaling.
There are several issues requiring future studies. Firstly, the precise mechanism by which Fyn suppresses Nrf2-mediated Agt expression in the heart has not been completely delineated in the present study. A plausible explanation is likely that the phosphorylated Nrf2 via Fyn 36 could not bind to the promoter of Agt and activate Agt expression when autophagy is intact; however, autophagy impairment blocks the Fyn-mediated phosphorylation of Nrf2, which in turn enable Nrf2-driven expression of Agt. Secondly, the Nrf2-Agt axis may not be the sole mechanism for driving cardiac maladaptive remodeling and dysfunction in a setting of autophagy impairment. We have observed that autophagy impairment compromises ubiquitin-proteasome system (UPS) performance in a p62/SQSTM1 dependent manner in cardiomyocytes. 37 Since p62 is a target gene of Nrf2 and a substrate of autophagic-lysosomal pathway (ALP), 10, 38 the activation Nrf2-p62-UPS dysfunction pathway may be an alternative mechanism. Finally, whether the Nrf2-mediated dichotomy is restricted to this TAC model is unclear. Thus, the long-term impact of Nrf2 knockout on cardiac remodeling and dysfunction in diverse pathological settings, such as myocardial infarction, hypertensive cardiac hypertrophy, and diabetic cardiomyopathy, is warranted. Further investigation of these subjects will lead to a better understanding of Nrf2-mediated cardiac maladaptive remodeling and dysfunction in the setting of autophagy impairment.
Supplementary Material
Perspectives.
As summarized in Figure 8C, Nrf2 activation exacerbates maladaptive remodeling and dysfunction in a setting of autophagy impairment. At the molecular level, autophagy impairment suppresses the activation of Jak2/Fyn pathway which facilitates nuclear export of Nrf2 for degradation, leading to nuclear accumulation of Nrf2 and activation of Nrf2-driven expression of Agt to enhance angiotensin II formation in the heart. Subsequently, the activation of angiotensin II signaling contributes to the progression of cardiac maladaptive remodeling and dysfunction. Our findings highlight a unique coupling between Nrf2 signaling and autophagy function in the pathogenesis of cardiac dysfunction, and also suggest that the simultaneous targeting of Nrf2 and autophagy may be a novel therapeutic approach for the prevention of adverse cardiac remodeling and dysfunction associated with hypertensive heart disease.
Novelty and Significance.
What Is New?
This is the first report to document that autophagy impairment switches on Nrf2-mediated cardiac maladaptive remodeling and dysfunction secondary to pressure overload in mice.
What Is Relevant?
The findings of this study suggest that simultaneously targeting of Nrf2 and autophagy may be a novel approach for the prevention of cardiac maladaptive remodeling and dysfunction.
Summary
Nrf2 activation is cardioprotective when myocardial autophagy is intact, whereas it becomes detrimental to the heart when myocardial autophagy is impaired. Autophagy impairment most likely turns off nuclear Fyn-operated Nrf2 export and degradation thus activating Nrf2-driven Agt transcription, which exacerbates cardiac maladaptation leading to heart failure.
Acknowledgments
Source of Funding: This research was supported by grants by NIH NCCAM PO20 GM103641 and 2PO1AT003961-06A1, the National Natural Science Foundation of China (No. 81370267), and the Shandong University National Qianren Scholar Fund and Taishan Scholar Fund.
Footnotes
Disclosures: None.
References
- 1.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the keap1-nrf2-are pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Ichikawa T, Janicki JS, Cui T. Targeting the nrf2 pathway against cardiovascular disease. Expert Opin Ther Targets. 2009;13:785–794. doi: 10.1517/14728220903025762. [DOI] [PubMed] [Google Scholar]
- 3.Itoh K, Mimura J, Yamamoto M. Discovery of the negative regulator of nrf2, keap1: A historical overview. Antioxidants & redox signaling. 2010;13:1665–1678. doi: 10.1089/ars.2010.3222. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Ichikawa T, Villacorta L, Janicki JS, Brower GL, Yamamoto M, Cui T. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arterioscler Thromb Vasc Biol. 2009;29:1843–1850. doi: 10.1161/ATVBAHA.109.189480. [DOI] [PubMed] [Google Scholar]
- 5.Sussan TE, Rangasamy T, Blake DJ, Malhotra D, El-Haddad H, Bedja D, Yates MS, Kombairaju P, Yamamoto M, Liby KT, Sporn MB, Gabrielson KL, Champion HC, Tuder RM, Kensler TW, Biswal S. Targeting nrf2 with the triterpenoid cddo-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc Natl Acad Sci USA. 2009;106:250–255. doi: 10.1073/pnas.0804333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Zhang C, Xing Y, Janicki JS, Yamamoto M, Wang XL, Tang DQ, Cui T. Up-regulation of p27(kip1) contributes to nrf2-mediated protection against angiotensin ii-induced cardiac hypertrophy. Cardiovasc Res. 2011;90:315–324. doi: 10.1093/cvr/cvr010. [DOI] [PubMed] [Google Scholar]
- 7.Li S, Wang W, Niu T, Wang H, Li B, Shao L, Lai Y, Li H, Janicki JS, Wang XL, Tang D, Cui T. Nrf2 deficiency exaggerates doxorubicin-induced cardiotoxicity and cardiac dysfunction. Oxid Med Cell Longev. 2014;2014:748524. doi: 10.1155/2014/748524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W, Li S, Wang H, Li B, Shao L, Lai Y, Horvath G, Wang Q, Yamamoto M, Janicki JS, Wang XL, Tang D, Cui T. Nrf2 enhances myocardial clearance of toxic ubiquitinated proteins. J Mol Cell Cardiol. 2014;72:305–315. doi: 10.1016/j.yjmcc.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kannan S, Muthusamy VR, Whitehead KJ, Wang L, Gomes AV, Litwin SE, Kensler TW, Abel ED, Hoidal JR, Rajasekaran NS. Nrf2 deficiency prevents reductive stress-induced hypertrophic cardiomyopathy. Cardiovasc Res. 2013;100:63–73. doi: 10.1093/cvr/cvt150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor nrf2 through inactivation of keap1. Nat Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 11.Taguchi K, Fujikawa N, Komatsu M, Ishii T, Unno M, Akaike T, Motohashi H, Yamamoto M. Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proc Natl Acad Sci USA. 2012;109:13561–13566. doi: 10.1073/pnas.1121572109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhuiyan MS, Pattison JS, Osinska H, James J, Gulick J, McLendon PM, Hill JA, Sadoshima J, Robbins J. Enhanced autophagy ameliorates cardiac proteinopathy. J Clin Invest. 2013;123:5284–5297. doi: 10.1172/JCI70877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Osinska H, Klevitsky R, Gerdes AM, Nieman M, Lorenz J, Hewett T, Robbins J. Expression of r120g-alphab-crystallin causes aberrant desmin and alphab-crystallin aggregation and cardiomyopathy in mice. Circ Res. 2001;89:84–91. doi: 10.1161/hh1301.092688. [DOI] [PubMed] [Google Scholar]
- 14.Rajasekaran NS, Connell P, Christians ES, Yan LJ, Taylor RP, Orosz A, Zhang XQ, Stevenson TJ, Peshock RM, Leopold JA, Barry WH, Loscalzo J, Odelberg SJ, Benjamin IJ. Human alpha b-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell. 2007;130:427–439. doi: 10.1016/j.cell.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linton PJ, Gurney M, Sengstock D, Mentzer RM, Jr, Gottlieb RA. This old heart: Cardiac aging and autophagy. J Mol Cell Cardiol. 2015;83:44–54. doi: 10.1016/j.yjmcc.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bryan HK, Olayanju A, Goldring CE, Park BK. The nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem pharmacol. 2013;85:705–717. doi: 10.1016/j.bcp.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Li W, Febbraio M, Reddy SP, Yu DY, Yamamoto M, Silverstein RL. Cd36 participates in a signaling pathway that regulates ros formation in murine vsmcs. J Clin invest. 2010;120:3996–4006. doi: 10.1172/JCI42823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Connell TD, Swigart PM, Rodrigo MC, Ishizaka S, Joho S, Turnbull L, Tecott LH, Baker AJ, Foster E, Grossman W, Simpson PC. Alpha1-adrenergic receptors prevent a maladaptive cardiac response to pressure overload. J Clinl Invest. 2006;116:1005–1015. doi: 10.1172/JCI22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest. 2007;117:2431–2444. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKinsey TA, Olson EN. Toward transcriptional therapies for the failing heart: Chemical screens to modulate genes. J Clin Invest. 2005;115:538–546. doi: 10.1172/JCI24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Periasamy M, Bhupathy P, Babu GJ. Regulation of sarcoplasmic reticulum ca2+ atpase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc Res. 2008;77:265–273. doi: 10.1093/cvr/cvm056. [DOI] [PubMed] [Google Scholar]
- 22.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koitabashi N, Bedja D, Zaiman AL, Pinto YM, Zhang M, Gabrielson KL, Takimoto E, Kass DA. Avoidance of transient cardiomyopathy in cardiomyocyte-targeted tamoxifen-induced mercremer gene deletion models. Circ Res. 2009;105:12–15. doi: 10.1161/CIRCRESAHA.109.198416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 25.Abdo S, Shi Y, Otoukesh A, Ghosh A, Lo CS, Chenier I, Filep JG, Ingelfinger JR, Zhang SL, Chan JS. Catalase overexpression prevents nuclear factor erythroid 2-related factor 2 stimulation of renal angiotensinogen gene expression, hypertension, and kidney injury in diabetic mice. Diabetes. 2014;63:3483–3496. doi: 10.2337/db13-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouyang C, You J, Xie Z. The interplay between autophagy and apoptosis in the diabetic heart. J Mol Cell Cardiol. 2014;71:71–80. doi: 10.1016/j.yjmcc.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Watada H, Fujitani Y. Minireview: Autophagy in pancreatic beta-cells and its implication in diabetes. Mol Endocrinol. 2015;29:338–348. doi: 10.1210/me.2014-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wollert KC, Drexler H. The renin-angiotensin system and experimental heart failure. Cardiovasc Res. 1999;43:838–849. doi: 10.1016/s0008-6363(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 29.Mori J, Zhang L, Oudit GY, Lopaschuk GD. Impact of the renin-angiotensin system on cardiac energy metabolism in heart failure. J Mol Cell Cardiol. 2013;63:98–106. doi: 10.1016/j.yjmcc.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Booz GW, Day JN, Baker KM. Interplay between the cardiac renin angiotensin system and jak-stat signaling: Role in cardiac hypertrophy, ischemia/reperfusion dysfunction, and heart failure. J Mol Cell Cardiol. 2002;34:1443–1453. doi: 10.1006/jmcc.2002.2076. [DOI] [PubMed] [Google Scholar]
- 31.Wang S, Li B, Qiao H, Lv X, Liang Q, Shi Z, Xia W, Ji F, Jiao J. Autophagy-related gene atg5 is essential for astrocyte differentiation in the developing mouse cortex. EMBO reports. 2014;15:1053–1061. doi: 10.15252/embr.201338343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sayeski PP, Ali MS, Safavi A, Lyles M, Kim SO, Frank SJ, Bernstein KE. A catalytically active jak2 is required for the angiotensin ii-dependent activation of fyn. J Biol Chem. 1999;274:33131–33142. doi: 10.1074/jbc.274.46.33131. [DOI] [PubMed] [Google Scholar]
- 33.Selvetella G, Hirsch E, Notte A, Tarone G, Lembo G. Adaptive and maladaptive hypertrophic pathways: Points of convergence and divergence. Cardiovasc Res. 2004;63:373–380. doi: 10.1016/j.cardiores.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 34.Mazzolai L, Nussberger J, Aubert JF, Brunner DB, Gabbiani G, Brunner HR, Pedrazzini T. Blood pressure-independent cardiac hypertrophy induced by locally activated renin-angiotensin system. Hypertension. 1998;31:1324–1330. doi: 10.1161/01.hyp.31.6.1324. [DOI] [PubMed] [Google Scholar]
- 35.Mazzolai L, Pedrazzini T, Nicoud F, Gabbiani G, Brunner HR, Nussberger J. Increased cardiac angiotensin ii levels induce right and left ventricular hypertrophy in normotensive mice. Hypertension. 2000;35:985–991. doi: 10.1161/01.hyp.35.4.985. [DOI] [PubMed] [Google Scholar]
- 36.Jain AK, Jaiswal AK. Gsk-3beta acts upstream of fyn kinase in regulation of nuclear export and degradation of nf-e2 related factor 2. J Biol Chem. 2007;282:16502–16510. doi: 10.1074/jbc.M611336200. [DOI] [PubMed] [Google Scholar]
- 37.Tian Z, Wang C, Hu C, Tian Y, Liu J, Wang X. Autophagic-lysosomal inhibition compromises ubiquitin-proteasome system performance in a p62 dependent manner in cardiomyocytes. PLoS One. 2014;9:e100715. doi: 10.1371/journal.pone.0100715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jain A, Lamark T, Sjottem E, Larsen KB, Awuh JA, Overvatn A, McMahon M, Hayes JD, Johansen T. P62/sqstm1 is a target gene for transcription factor nrf2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.