Abstract
The dopamine (DA) neuron system most relevant for schizophrenia is the meso-limbic-cortical DA system inter alia densely innervating subcortical limbic regions. The field of dopamine D2 receptors and schizophrenia changed markedly with the discovery of many types of D2 heteroreceptor complexes in subcortical limbic areas as well as the dorsal striatum. The results indicate that the D2 is a hub receptor which interacts not only with many other G protein-coupled receptors (GPCRs) including DA isoreceptors but also with ion-channel receptors, receptor tyrosine kinases, scaffolding proteins and DA transporters. Disturbances in several of these D2 heteroreceptor complexes may contribute to the development of schizophrenia through changes in the balance of diverse D2 homo- and heteroreceptor complexes mediating the DA signal, especially to the ventral striato-pallidal γ-aminobutyric acid (GABA) pathway. This will have consequences for the control of this pathway of the glutamate drive to the prefrontal cortex via the mediodorsal thalamic nucleus which can contribute to psychotic processes. Agonist activation of the A2A protomer in the A2A–D2 heteroreceptor complex inhibits D2 Gi/o mediated signaling but increases the D2 β-arrestin2 mediated signaling. Through this allosteric receptor–receptor interaction, the A2A agonist becomes a biased inhibitory modulator of the Gi/o mediated D2 signaling, which may the main mechanism for its atypical antipsychotic properties especially linked to the limbic A2A–D2 heterocomplexes. The DA and glutamate hypotheses of schizophrenia come together in the signal integration in D2–N-methyl-d-aspartate (NMDA) and A2A–D2–metabotropic glutamate receptor 5 (mGlu5) heteroreceptor complexes, especially in the ventral striatum. 5-Hydroxytryptamine 2A (5-HT2A)–D2 heteroreceptor complexes are special targets for atypical antipsychotics with high potency to block their 5-HT2A protomer signaling in view of the potential development of pathological allosteric facilitatory 5-HT2A–D2 interaction increasing D2 protomer signaling. Neurotensin (NTS1)–D2 heterocomplexes also exist in the ventral and dorsal striatum, and likely also in midbrain DA nerve cells as NTS1-D2 autoreceptor complexes where neurotensin produces antipsychotic and propsychotic actions, respectively.
Keywords: allosteric receptor–receptor interactions, dopamine receptors, G protein-coupled receptors, heteroreceptor complexes, neurotensin receptors, psychotic disorders, schizophrenia, serotonin receptors
Introduction
The discovery of the central dopamine (DA), noradrenaline (NA) and serotonin [5-hydroxytryptamine (5-HT)] neurons was mainly based on Fuxe’s thesis work in 1964 and 1965 using the Falck–Hillarp technique for the cellular localization of catecholamines and 5-HT [Dahlstrom and Fuxe, 1964; Fuxe, 1965; Fuxe and Anden, 1965; Fuxe et al. 2007; Fuxe and Dahlstrom, 2009]. They were inter alia characterized by long monosynaptic pathways to the tel- and diencephalon from DA, NA and 5-HT nerve cell groups in the lower brain stem with a distinct and phylogentically conserved parcellation. The NA and 5-HT neurons formed global varicose terminal networks in the brain via massive formation of collaterals.
The DA neurons had a somewhat more restricted innervation pattern including a dense innervation of the dorsal and ventral striatum as well as of several other limbic regions [Dahlstrom and Fuxe, 1964; Fuxe, 1965; Fuxe and Anden, 1965, Fuxe et al. 2007; Anden et al. 1966]. The DA system most relevant for schizophrenia is the mesolimbic DA system inter alia densely innervating the nucleus accumbens and olfactory tubercle which has its origin from five different DA cell groups of the ventral tegmental area [Dahlstrom and Fuxe, 1964; Fuxe and Anden, 1965; Fuxe et al. 1970; Anden et al. 1966]. The cortical component of this DA system was discovered by Glowinski and his group preferentially innervating the limbic cortical areas including the prefrontal cortex [Thierry et al. 1973; Berger et al. 1974].
The antipsychotic DA receptor was identified as the DA D2 receptor [Seeman, 2010], which is the major target for typical and atypical antipsychotic drugs [Ginovart and Kapur, 2012]. The D2 receptor operates both as a postjunctional, mainly extrasynaptic DA receptor involved in mediating DA transmission, and as extrasynaptic autoreceptor complexes at the soma-dendritic and nerve terminal level. The D2 receptors are linked to the mesolimbic DA neurons by being autoreceptors in the DA nerve cells of the ventral tegmental area and by participating in mediating extrasynaptic DA volume transmission in the mesolimbic terminal fields inter alia in the nucleus accumbens [Fuxe et al. 2010a].
The field of D2 receptors and schizophrenia has changed markedly with the discovery of many types of D2 heteroreceptor complexes in subcortical limbic areas like the nucleus accumbens (Figure 1). They offer novel targets for antipsychotic drugs [Fuxe et al. 2010, 2014a,b] and are discussed in this article in terms of mediating the therapeutic effects versus the side effects of antipsychotic drugs. The molecular mechanisms involved in the allosteric receptor–receptor interactions in these D2 containing heteroreceptor complexes that produce their dynamic signaling panorama are also covered.
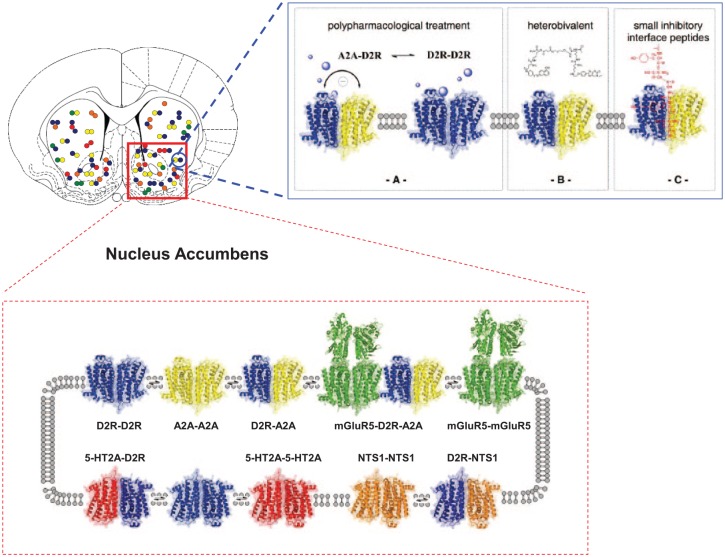
Figure 1.
Schematic representation of different types of dopamine D2 heteroreceptor complexes in subcortical limbic areas like the nucleus accumbens and their potential role as a drug target for schizophrenia treatment. Top and bottom left panel. In the dorsal and ventral striatum (here indicated in the rat brain as bregma level 1.0 mm), dopamine D2R homo and heteroreceptor complexes allow direct physical interactions between the receptors, making possible the allosteric receptor–receptor interactions between them. The stoichiometry balance between these homo/heteromers determines the final functional output of dopamine D2R signalling and thus the cellular response. Top right panel. Illustration of treatment of schizophrenia based on the A2AR–D2R heteroreceptor complexes. In (A), the A2A receptor agonist is illustrated to activate both the A2A protomer in the A2A-D2R heteroreceptor complexes and the A2A protomer of the A2AR homoreceptor complexes. A2A receptor agonists may be used as antipsychotic drugs through their allosteric antagonism of Gi/o mediated D2R signalling in the A2A–D2R heteroreceptor complex in the soma-dendritic terminal regions of the ventral-striato-pallidal GABA pathway. The activation of the A2A homoreceptor complexes may also contribute to the antipsychotic actions of A2A agonists through their Golf–AC–PKA pathways increasing the excitability of the ventral-pallidal GABA pathway. In (B), the use of a heterobivalent compound is illustrated built up of a D2R antagonist and an A2A agonist. This compound should specifically target the A2A–D2R heteroreceptor complexes and exert antipsychotic actions. In (C), the use of a small inhibitory interface peptide which specifically targets the A2A–D2R receptor interface is illustrated. This action is postulated to increase development of positive schizophrenic symptoms by setting free the D2R protomer from the brake exerted by the A2A protomer on the limbic A2A–D2R heteroreceptor complexes.
5-HT, 5-hydroxytryptamine; AC, adenylyl cyclase; GABA, γ-aminobutyric acid; mGlu5, metabotropic glutamate receptor 5; NTS1, neurotensin 1; PKA, protein kinase A.
G protein-coupled receptor, especially D2, heteroreceptor complexes
The accepted biological principle appears to be that G protein-coupled receptor (GPCR) can form homo and heteroreceptor complexes with allosteric receptor–receptor interactions [Fuxe et al. 1983, 2010, 2014a; Portoghese, 2001; Agnati et al. 2009; Borroto-Escuela et al. 2014a; Perreault et al. 2014]. This takes place through direct physical interactions between co-expressed GPCRs either by physical association between the same receptor (homo-oligomer) or different GPCR types (hetero-oligomer). It can take place with or without the participation of adapter proteins. There are several examples demonstrating that, for instance, D1 and D2 heteromerization takes place, which through allosteric receptor–receptor interactions, alters their G protein activation, receptor signaling, receptor trafficking and receptor recognition leading inter alia to changes in DA receptor pharmacology and function.
One emerging new concept in neuropsychopharmacology is that a dysfunction of allosteric receptor–receptor interactions contributes to disease progression, for instance, in schizophrenia. We aim to improve our understanding of receptor–receptor interactions in D2 heteroreceptor complexes in the brain and their functional relevance in schizophrenia. So far their stoichiometry and topology is unknown within the heteromer formed as well as the number of adapter proteins participating, including their architecture. They should therefore be described as heteroreceptor complexes [Fuxe and Borroto-Escuela, 2016]. The overall architecture of the global GPCR heterodimer network shows that D2R are hub components forming more than 10 heterodimer pairs [Borroto-Escuela et al. 2014a].
It should be underlined that D2 receptors can also form complexes with ion channel receptors like striatal D2–N-methyl-d-aspartate (NMDA) heteroreceptor complexes in which allosteric inhibitory D2–NMDA receptor subtype 2B (NR2B) interactions exist [Liu et al. 2006]. They are located to glutamate synapses. Putative D2 receptor tyrosine kinase heteroreceptor complexes may also exist in which D2 induced activation of mitogen-activated protein kinases (MAPK) takes place via transactivation of receptor tyrosine kinase (RTK) [Wang et al. 2005]. D2–epidermal growth factor receptor (EGFR) heterocomplexes may be formed. Fibroblast growth factor receptor (FGFR)–A2A–D2 complexes were postulated to exist [Flajolet et al. 2008; Borroto-Escuela et al. 2013a,c; Fuxe et al. 2014a]. Thus, D2 receptors may increase trophism and structural plasticity via allosteric transactivation of RTK in D2–RTK complexes. In addition, the DA D2 receptor can also establish direct interactions with the DA transporter, which facilitates the cell surface expression of the DA transporter and leads to increased DA uptake [Lee et al. 2007].
These results indicate that the D2 is a hub receptor which interacts not only with a large number of other GPCRs including DA isoreceptors [Borroto-Escuela et al. 2014a], but also with ion channel receptors, RTKs and DA transporters. Early on it was also postulated that GPCR can interact with nonreceptor proteins to enhance the integrative process at the molecular level in the plasma membrane [Fuxe and Agnati, 1987]. Of particular interest is the recently discovered D2R–Disc1 protein complex by Fang Liu and colleagues which contributes to antipsychotic-like effects [Su et al. 2014].
It was also proposed that scaffolding proteins and neuronal adhesion molecules encoded by susceptibility genes for schizophrenia [Bradshaw and Porteous, 2012; De Bartolomeis et al. 2013, 2014; Bourgeron, 2015] importantly participate in the modulation of integration of signals in D2 heteroreceptor complexes [Fuxe et al. 2014b]. Disturbances in the scaffolding protein structure can lead to changes in the balance of protein complexes in which they participate. This process in turn may contribute to the development of schizophrenia through changes in the balance of different D2 heteroreceptor complexes mediating the DA signal in the ventral striato-pallidal γ-aminobutyric acid (GABA) pathway. This will have consequences for the control of this pathway of the glutamate drive to the prefrontal cortex via the mediodorsal thalamic nucleus [Fuxe et al. 2008].
Role of A2A–D2 heteroreceptor complexes in modulating D2 recognition and signaling
It was found early on that A2A agonists can reduce the affinity of the D2 receptor agonist binding sites and counteract the D2 Gi/o mediated signaling over the adenylyl cyclase (AC)–protein kinase A (PKA) pathways [Yang et al. 1995; Fuxe et al. 1998; Hillion et al. 2002; Borroto-Escuela et al. 2010a, 2010b]. D2 activation is also known to inhibit the calcium influx via the L-type voltage dependent calcium channels (Cav 3.1) through the phospholipase C (PLC) and protein phosphatase 2 (PP2) B pathway, which leads to dephosphorylation and closure of the Cav3.1 channels [Hernandez-Lopez et al. 2000]. In addition, this action is counteracted by A2A agonists through an allosteric antagonistic receptor–receptor interaction which inhibits the Gi/o activation with failure to release Gβγ), which therefore no longer can activate the above intracellular pathway [Azdad et al. 2009]. The D2 receptor mediated suppression of NMDA-induced depolarized plateau potential is mediated by inhibition of the Cav3.1 channels, an action which is reversed by A2A receptor activation. As a consequence, an upstate is obtained in the striato-pallidal GABA neurons [Surmeier et al. 2007], the ventral component representing an antireward system [Fuxe et al. 2014a]. Early work also showed that activation of A2A receptors also inhibits the D2 receptor induced increases in intracellular calcium levels [Yang et al. 1995; Salim et al. 2000]. In addition, A2A antagonists enhanced and A2A agonists diminished the D2 agonist induced decrease in firing rates in nerve cells of the striatum from hemiparkinson rats [Stromberg et al. 2000].
It is less clear how the allosteric A2A–D2 interaction modulates the D2 signaling over β-arrestin 2-AKT-protein phosphatase 2A pathway [Beaulieu et al. 2015]. It was proposed that the A2A protomer activation can favor a conformational change in the D2 protomer that favors the binding of β-arrestin 2 to the D2 protomer, while its Gi/o binding is reduced [Borroto-Escuela et al. 2011; Fuxe et al. 2014a] which is in line with the discussion above. Such a change towards G protein independent D2 signaling with β-arrestin 2 can help explain the demonstration of co-aggregation, co-internalization and co-desensitization of the A2A–D2 heteroreceptor complexes upon agonist co-activation of the two protomers [Hillion et al. 2002].
D2 agonists induce a negative modulation of AKT via the β-arrestin 2 signaling pathway through reduction of its phosphorylation which results in an increased activation of GSK3. Instead, D2 antagonists enhance phosphorylation of AKT and this brings down glycogen synthase kinase 3 (GSK3) activity [Beaulieu et al. 2004]. GSK3 phosphorylates a number of protein targets involved in glutamate transmission and gene expression and translation.
Taken together, the findings indicate that agonist activation of the A2A protomer in the A2A–D2 heteroreceptor complex inhibits D2 Gi/o mediated signaling but increases the D2 β-arrestin 2 mediated signaling. This is in contrast to D2 receptor antagonists which block all the D2 signaling pathways. Thus, through the allosteric receptor-receptor interaction, the A2A agonist becomes a biased inhibitory modulator of the Gi/o mediated D2 signaling.
A2A–D2 heteroreceptor complexes and their relevance for schizophrenia and its treatment
DA receptor antagonists are well known to be the major antipsychotic drugs [Seeman, 2010; Ginovart and Kapur, 2012]. The A2A agonist is known to be an atypical antipsychotic-like drug based on work in rat models of schizophrenia [Rimondini et al. 1997; Andersen et al. 2002; Fuxe et al. 2008]. In view of the above discussion, the antipsychotic-like effects of A2A agonists are mainly due to their ability to counteract Gi/o mediated D2 signaling over the Gαi–AC–PKA and the Gβγ–PLC pathways. The potential enhancement of D2 signaling over the β-arrestin 2 mediated pathway probably has no major role in the antipsychotic-like effects of A2A agonists as lithium, which interferes with the formation/maintenance of the β-arrestin 2–Akt–PP2A pathway, is mainly effective in treatment of bipolar disorders [Beaulieu et al. 2004, 2008, 2015]. Based on these results it also becomes unclear if the common feature of antipsychotic drugs of the D2 antagonist type to counteract AKT-GSK3 signaling [Masri et al. 2008] has therapeutic relevance. The failure of A2A agonists to counteract this pathway may instead lead to reduced side effects.
The A2AR agonists may be used as antipsychotic drugs through their allosteric antagonism of Gi/o mediated D2 signaling in the A2AR–D2R heteroreceptor complex in the soma-dendritic-terminal regions of the ventral striato-pallidal GABA pathway. This can help restore the glutamate drive from the mediodorsal thalamic nucleus to the prefrontal cortex [Fuxe et al. 2008]. A2AR agonists robustly lower the D2R agonist binding affinity in the nucleus accumbens shell and core [Diaz-Cabiale et al. 2001], and bring down D2 Gi/o protein coupling.
The A2AR–D2R heteroreceptor complex is also present on the glutamate terminals of the local circuits in the striato-pallidal GABA neurons and reduces the D2R-induced inhibition of glutamate release upon agonist induced activation of the A2A receptors. This increase in glutamate release may also contribute to enhancement of the excitability of the ventral striato-pallidal GABA (antireward) pathway which also contributes to the antipsychotic activity of A2AR agonists.
According to the DA hypothesis of schizophrenia, an overactivity of the mesolimbic DA neurons exists which leads to an enhanced D2 mediated inhibition of the ventral striato-pallidal GABA neurons, which represents an antireward system. Therefore, in schizophrenia, salience develops also to irrelevant stimuli which may result in dysfunctional inner speech and to delusions in order to make sense out of the pathological salience given to stimuli of no relevance [Kapur, 2003; Ginovart and Kapur, 2012]. The major target for the current antipsychotic drugs is therefore the D2 receptor located on and inhibiting the activity in the ventral striato-pallidal antireward neurons. Through its blockade by the D2 receptor blocking activity of typical and atypical antipsychotic drugs, activity will be restored in this antireward system and, via the mediodorsal thalamic nucleus, the glutamate drive to the prefrontal cortex will be restored. This event will transfer the antireward information to the prefrontal cortex and the malfunction of salience will diminish as will the psychotic symptoms. The drawback of D2 receptor antagonists is that they can also reduce incentive salience below normal levels leading to depressive symptoms [Ginovart and Kapur, 2012]. A2A agonists may have an advantage versus D2 receptor antagonists by being a negative allosteric modulator of D2 recognition and Gi/o mediated D2 signaling. Thus, the inhibitory allosteric mechanism activated by A2A agonists allows the development of reduced D2 signaling over Gi/o, but a potentially normal baseline level of D2 signaling may be maintained in the therapeutic dose range. This may be more difficult to obtain with D2 receptor antagonists acting at the orthosteric DA binding site, unless they have a rapid dissociation rate from the postjunctional DA receptors However, reversibility of D2R antagonism does not differ between atypical and typical antipsychotics [Sahlholm et al. 2014].
When discussing the atypical antipsychotic properties of A2A agonists, it should also be considered that they target A2A homoreceptor complexes and other A2A heteroreceptor complexes like the A2A isoreceptor complexes which may exist in the same nerve cells in balance with the A2A–D2 heteroreceptor complexes and D2 homoreceptor complexes (Figure 2) [Fuxe et al. 2010b, 2014a, 2014b, 2016; Borroto-Escuela et al. 2015a]. Thus, the activation of the A2A homoreceptor complexes may also contribute to the antipsychotic-like actions of A2A agonists through their Golf–AC–PKA pathways increasing the excitability of the striato-pallidal GABA pathway via the phosphorylation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) (GluA1) and NMDA (GluNB) receptors [Filip et al. 2012].
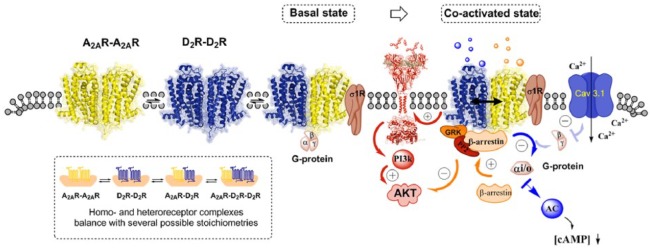
Figure 2.
Illustration of the complexity of the balance between A2A–D2R heteroreceptor and homoreceptor complexes in key brain regions for schizophrenia such as the nucleus accumbens. In the lower left panel, several possible receptor stoichiometries from heterodimers to higher order heteromers of various types are shown. In the co-activated state, the antipsychotic-like effects of A2A agonists are mainly due to their ability to counteract Gi/o mediated D2 signalling over the Gα−AC–PKA and the Gβγ–PLC pathways. Also, A2A activation blocks the formation of βγ dimer from Gi/o and thus counteracts the D2 mediated inhibition of the Cav3.1 channels over the PLC and PP2B pathway. It is less clear how the allosteric A2A–D2 interactions modulate the D2 Akt signalling. D2 agonist induces a negative modulation of Akt via the β-arrestin 2 signalling pathway but induces positive modulations of Akt via receptor tyrosine kinase (RTK)–phosphoinositide 3 kinase (PI3k) pathways. Furthermore, agonist activation of the A2A protomer in the A2A–D2 heteroreceptor complexes enhanced D2 β-arrestin 2 mediated signalling. The adapter proteins (sigma-1 receptor) also participate in modulating the organization and function of the A2A and D2 homoreceptors and their higher order heteroreceptor complexes.
AC, adenylyl cyclase; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A; PLC, phospholipase C; PP2, protein phosphatase 2.
Understanding the balance of A2A and D2 homo-and heteroreceptor complexes
There is a need to understand the complexity of the balance between A2AR–D2R and its homomers, iso A2AR- and iso D2R-heteromers and other A2A- and D2 heteromers in key brain regions for schizophrenia like the nucleus accumbens ( Figure 2). Which complexes are in the ventral striato-pallidal GABA pathway modulating the glutamate drive to the prefrontal cortex? If so, which receptor complexes are in the same striato-pallidal GABA neuron of this pathway? If so, which receptor complexes are located in the same synapses formed on this neuron?
Altered plasticity of A2A–D2 receptor–receptor interactions after amphetamine challenge, and in the amphetamine sensitized state, a rat model of schizophrenia
An increase in D2 receptor dimerization was observed after acute amphetamine exposure and in the amphetamine-induced sensitized state as also found in schizophrenia [Wang et al. 2010]. Changes in the A2A–D2 receptor–receptor interactions were therefore postulated in the ventral and dorsal striatum and recently demonstrated [Pintsuk et al. 2016]. A major finding was the development of a facilitatory A2A–D2 receptor–receptor interaction in the dorsal striatum in the amphetamine sensitized state versus the saline sensitized state as demonstrated by an A2A agonist induced increase in affinity in the high affinity D2 receptors ex vivo. This is in contrast to the usual antagonistic interactions found in untreated rats. Such a change in the A2A–D2 interaction towards a pathological enhancement of inhibitory D2 signaling in the dorsal striato-pallidal GABA neurons reducing motor inhibition mediated via this pathway. It can contribute to the development of the sensitized state (increased locomotion) produced by long-term amphetamine treatment [Seeman et al. 2002]. It may involve habit formation and compulsive behaviors. Changes in the stoichiometry of the A2A–D2 heteroreceptor complexes in the dorsal striatum inter alia due to the increased D2 homodimerization together with the subchronic amphetamine induced DA release may be part of the mechanisms involved in this change in the allosteric receptor-receptor interactions [Pintsuk et al. 2016]. Such a change may also exist in schizophrenia.
In the ventral striatum, the receptor plasticity in the A2A–D2 receptor–receptor interactions was altered in an opposite way compared with the dorsal striatum in the amphetamine sensitized rats versus the saline sensitized rats. Thus, in the amphetamine sensitized rats, the antagonistic A2A–D2 receptor interactions were significantly restored versus the saline sensitized rats after an acute amphetamine challenge [Pintsuk et al. 2016]. These differential changes observed can be explained by postulating that the A2A–D2 heteroreceptor complexes are different in terms of stoichiometry and geometry in these two areas, leading to differential changes to amphetamine treatment in the allosteric receptor–receptor interactions. It may also involve an increased formation of A2A receptors in the ventral but not the dorsal striatum through the subchronic amphetamine treatment. This can result in an increased formation of A2A homoreceptor complexes and A2A–D2 heteroreceptor complexes in the ventral striatum and thus in the return of the antagonistic A2A–D2 receptor-receptor interactions in this region [Pintsuk et al. 2016].
These results strongly support the view that the atypical antipsychotic-like actions of A2A agonists are related at least in part to the activation of antagonistic A2A–D2 receptor–receptor interactions in the ventral striatum, including the nucleus accumbens. The extrapyramidal side effects such as parkinsonian-like effects should be reduced since the antagonistic A2A–D2 receptor–receptor interactions in the dorsal striatum is replaced by facilitatory A2A–D2 interactions in this model of schizophrenia, contributing to the increased locomotion observed.
Are the A2A-D2 heteroreceptor complexes altered in schizophrenia and how should they be targeted?
The increased activity in the mesolimbic DA neurons [Dahlstrom and Fuxe, 1964; Fuxe, 1965; Anden et al. 1966; Fuxe et al. 1970] is a major component of the DA hypothesis of schizophrenia [Seeman, 2010]. It should be noted that the A2A receptors are not located in the ascending midbrain DA neurons, including the mesolimbic DA neurons. Therefore, any change in the A2A receptors and in the A2A–D2 heteroreceptor complexes in schizophrenia is likely located postjunctionally in the limbic regions innervated by the mesolimbic DA neurons and their cortical component.
A deficit in the formation of A2A–D2 heteroreceptor complexes or in their antagonistic receptor–receptor interactions may contribute to the development of positive schizophrenic symptoms in view of the reduced brake on limbic D2 signaling, which is already enhanced by the increased DA release from the activated mesolimbic DA neurons.
In the amphetamine model of schizophrenia discussed above, however, even stronger antagonistic A2A–D2 interactions developed upon A2A agonist treatment ex vivo. This does not support any deficit of this interaction in the brains of schizophrenic patients. Instead it emphasizes that A2A agonist treatment in schizophrenia can produce significant therapeutic effects by targeting especially the A2A–D2 heteroreceptor complexes but also A2A homoreceptor complexes and potentially other A2A heteroreceptor complexes. Thus, A2A agonists may target many A2A heteroreceptor complexes.
Pharmacological approaches to target ventral striatal A2A–D2 heteroreceptor complexes for functional studies and treatment development for schizophrenia
Current A2A agonists target both A2A homoreceptor complexes and A2A protomers of A2A–D2 heteroreceptor complexes. Preferential A2A agonists for A2A protomers in heteromers versus A2A protomers in homomers have not yet been developed. Combined treatment with A2A agonists and low doses of D2 receptor antagonists would also be a promising approach. Heterobivalent drugs with an A2A agonist pharmacophor linked to a D2 antagonist pharmacophor are presently being developed. They may preferentially target A2A–D2 like heteroreceptor complexes and are of high interest in view of their potential high specificity in targeting the A2A–D2 heteroreceptor complexes with high specificity and affinity. Their penetration into the brain can be an initial problem but their actions on the central nervous system (CNS) can be determined through intracerebroventricular (i.c.v.) microinjections or local infusions into a brain region via reverse microdialysis. Small inhibitory interface peptides targeting the A2A–D2 interface are given i.c.v. or locally to disrupt the A2A–D2 heteroreceptor complexes and determine their role versus A2A homomers in models of schizophrenia.
In summary, increasing the A2A protomer mediated allosteric brake on the D2 protomer signaling in the A2A–D2 heteroreceptor complex of the dorsal, and especially the ventral striato-pallidal GABA pathway, represents a highly promising novel strategy for treatment of schizophrenia. The dopaminergic basis of salience dysregulation in schizophrenia appear to be mainly linked to the mesolimbic DA neurons with relevance for both negative and positive symptoms of schizophrenia [Juckel et al. 2006; Schlagenhauf et al. 2009; Nielsen et al. 2012; Winton-Brown et al. 2014].
On the potential existence of higher order A2A–D2 heteroreceptor complexes
Putative A2A–D2–sigma-1 heteroreceptor complexes
The sigma-1 receptor (σ1R) is known as a transmembrane chaperone protein at the endoplasmic reticulum and the plasma membrane that participates in a number of protein complexes. It modulates calcium signaling, a number of voltage-gated channels, and GPCRs as well as NMDA receptors [Hayashi and Su, 2007; Maurice and Su, 2009].
Sigma1-D2 heteromers exist and form higher order oligomers in the mouse striatum and are specific for D2 versus D3 and D4 receptors using proximity ligation assay (PLA) and co-immunoprecipitation [Navarro et al. 2013]. Sigma1-D1 heteroreceptor complexes also exist [Navarro et al. 2010]. Recently it was shown with bioluminescence resonance energy transfer (BRET) analysis in cellular models that σ1R can form heteroreceptor complexes to the same degree with D2 short and D2 long [Pinton et al. 2015a, 2015b]. It was also found that σ1R competes to a very low degree with the formation of the sigma1-D2 heterocomplex unlike the D2 short and D2 long receptors. The explanation can be a higher plasticity in the interface for the σ1R. It may bind to many regions of the D2R and form higher order sigma1-D2 heteroreceptor complexes as indicated also in previous work.
A detailed in situ PLA analysis revealed that high densities of sigma1-D2 heteroreceptor complexes are present in the nucleus accumbens core and shell of the rat brain [Pinton et al. 2015a,b]. The rat dorsal striatum instead showed only moderate densities of these complexes. With CRE luciferase reporter gene assays, allosteric receptor–receptor interactions were indicated between A2A–D2–σ1Rs in the plasma membrane in cellular models [Pinton et al. 2015a, 2015b]. Therefore, it is likely that A2A–D2–sigma-1 heteroreceptor complexes exist in the CNS, especially in the nucleus accumbens where high densities of the three receptors exist.
Thus, these observations indicate that these higher order A2A–D2–sigma-1 complexes may exist in substantially higher densities in the nucleus accumbens versus the dorsal striatum. This can help explain the differential modulation of D2 receptor recognition by the A2A receptor agonist in the nucleus accumbens versus the dorsal striatum in the amphetamine model of schizophrenia [Pintsuk et al. 2016].
Putative A2A–D2–mGlu5 heteroreceptor complexes
The existence of antagonistic glutamate receptor–D2 receptor interactions were first observed in 1984 through the ability of glutamate to reduce the affinity of the high affinity D2 agonist binding sites in striatal membrane preparations [Fuxe et al. 1984]. Subsequently it was observed that combined incubation with an A2A agonist and a mGluR5 agonist may synergistically reduce the affinity of the striatal D2 agonist binding sites potentially linked to a reduction of D2 agonist induced turning behavior [Popoli et al. 2001]. These results indicated the possibility that A2A–D2–mGluR5 heteroreceptor complexes may exist in the D2 positive striato-pallidal GABA neurons, which was supported by co-immunoprecipitation of the three receptors in striatal preparations [Ferre et al. 2002]. Synergistic A2A–mGluR5 interactions were also observed on GABA release using microdialysis [Diaz-Cabiale et al. 2002] and on c-fos expression and extracellular-signal-regulated kinase (ERK) and DARPP-32 phosphorylation [Ferre et al. 2002; Fuxe et al. 2003; Nishi et al. 2003].
In 2008 it was proposed that combined treatment with A2A and mGluR5 agonists targeting A2A–D2–mGluR5 heterocomplex in the ventral striato-pallidal GABA pathway can represent a new strategy for treatment of schizophrenia [Fuxe et al. 2008]. The mechanism involves a strong reduction of D2 protomer recognition and signaling markedly increasing activity in this antireward pathway and the treatment can be combined with low doses of D2 receptor antagonist antipsychotics [Fuxe et al. 2008].
In 2009 evidence for the existence of the A2A–D2–mGluR5 heteroreceptor complex could for the first time be obtained by using living cells in combination with bimolecular fluorescence complementation [Cabello et al. 2009]. It is of substantial interest that these three receptors were found to be collocated in the extrasynaptic plasma membrane of spines receiving putative glutamate synapses [Ciruela et al. 2012]. Thus, all the three receptor protomers of this heteroreceptor complex are reached by extrasynaptic volume transmission signals, namely DA, glutamate and adenosine. Very recently Borroto-Escuela and colleagues have demonstrated with the PLA technique the codistribution of high densities of A2A–D2 and A2A–mGlu5 PLA positive complexes in nucleus accumbens core and shell, some of which may represent the A2A–D2–mGluR5 heterotrimeric complexes [Borroto-Escuela et al. 2015]. These novel findings give further strong indications for the existence of A2A–D2–mGluR5 heteroreceptor complexes in these regions of the nucleus accumbens. The dysregulation of the mesolimbic DA neurons and their postjunctional heteroreceptor targets may be involved in producing the symptoms of schizophrenia [Fuxe, 1970; Meltzer and Stahl, 1976; Deutch et al. 1992; Grace et al. 1997] through development of dysfunction in the ventral striatal-mediodorsal thalamic-prefrontal cortical circuit they regulate [Fuxe et al. 2008].
However, a recent highly interesting study [Kegeles et al. 2010] demonstrated an increased DA function in associative striatum in schizophrenia using positron emission tomography (PET) before and during DA depletion with α-methyl-p-tyrosine, a tyrosine hydroxylase inhibitor. The percentage increase in D2 receptor availability was particularly high in the precommisural dorsal caudate richly connected to the dorsolateral prefrontal cortex. It becomes important to validate these findings. Enhanced D2 mediated DA transmission appears to mainly mediate positive symptoms of schizophrenia [Davis et al. 1991].
The coming together at the molecular level of the DA and glutamate hypothesis of schizophrenia: integration in D2–NMDA and A2A–D2–mGlu5 heteroreceptor complexes in the striatum
The glutamate hypothesis states that a reduction of NMDA receptor function leads to the development of schizophrenia [Alagarsamy et al. 1999; Wieronska et al. 2015]. There is general agreement that mGlu5 interacts directly with NMDA receptors leading to allosteric facilitatory reciprocal interactions between them. One target for antipsychotic actions of mGluR5 agonist and positive allosteric modulators can therefore also be mGlu5–NMDA heterocomplexes mainly located in synapses. Instead mGlu5 antagonists and negative allosteric regulators like NMDA receptor antagonists produce propsychotic actions [Wieronska et al. 2015].
It is of great interest that Fang Liu and her colleagues have demonstrated D2–NMDA heteroreceptor complexes, mainly located in synapses, which involves the NR2B subunit [Liu et al. 2006]. The result of this interaction is a reduced ability of Ca2+/calmodulin dependent protein kinase II to bind to NR2B. Therefore, a reduced phosphorylation of the NR2B subunit is obtained with a reduction of NMDA receptor signaling, which can contribute to symptoms of schizophrenia.
It is proposed that a dynamic balance can exist in the glutamate synapses between mGlu5–NMDA and D2–NMDA heterocomplexes in which mGlu5–NMDA–D2 heterocomplexes may exist as an intermediate molecular complex. A dominance of the D2–NMDA complex can lead to propsychotic activity due to the development of an hypofunction of the NMDA receptor signaling in the glutamate synapses on the ventral striato-pallidal GABA neurons, reducing the activity in the glutamate projections from the mediodorsal thalamic nucleus to the prefrontal cortex. This glutamate projection may preferentially innervate the cortical GABA interneurons and thus maintain a necessary balanced inhibition of the glutamate neuronal networks in the prefrontal cortex. When this inhibition is reduced, an exaggerated and dysfunctional activity can develop in many cortical glutamate systems leading to psychosis development.
Another major site of integration of glutamate and DA signals at the molecular level is in the A2A–D2–mGluR5 heteroreceptor complex located extrasynaptically of the glutamate synapses on the ventral and dorsal striato-pallidal GABA neurons. As discussed, the A2A and mGluR5 protomers synergize to put a brake on D2 protomer recognition and signaling in this trimeric heteroreceptor complex. It may be that, in schizophrenia, the brake on D2 signaling in these neurons is reduced and overactive mesolimbic DA neurons in this disease can therefore induce excessive inhibition of the ventral striato-pallidal GABA antireward neurons. This may contribute to development of schizophrenic symptoms due to generalized salience which disturbs the interactions with the environment and may contribute to psychotic symptoms.
Therefore, combined treatment with A2A agonists and mGlu5 positive allosteric regulators is proposed to be a novel interesting strategy for treatment of schizophrenia by targeting this heterotrimeric receptor complex. It may be more effective than treatment with A2A agonists alone. This hypothesis also postulates that very low doses of D2 receptor antagonists can be added to enhance the brake on D2 signaling if necessary [Fuxe et al. 2008]. It should be noted that these A2A–D2–mGluR5 heterotrimeric complexes also exist in the dorsal striato-pallidal GABA neurons. Thus, this target is of relevance also for the dorsal striatum including its associative regions discussed above.
Putative FGFR1–A2A–D2 heteroreceptor complexes
The FGFR1–A2A heteromer was demonstrated with the yeast two-hybrid method [Flajolet et al. 2008] and by bioluminescent resonance energy transfer analysis [Borroto-Escuela et al. 2013a]. In neuronal cell lines and primary cultures, evidence was presented that co-activation of this heteromer with fibroblast growth factor (FGF) and A2A agonist produced marked increases in structural plasticity associated with the activation of the MAPK/ERK pathway. These results indicated the existence of FGFR1–A2A heteroreceptor complexes in the D2 positive dorsal striato-pallidal GABA neurons which also contain A2A–D2 heteroreceptor complexes [Trifilieff et al. 2011; Borroto-Escuela et al. 2013c]. It was therefore earlier suggested that FGFR1–A2A–D2 heterocomplexes exist in the striatum [Fuxe et al. 2014a]. The receptor interfaces involved may, for instance, be different from each other and therefore FGFR1 and D2 receptors may not compete for binding to the A2A receptor.
The potential existence of such a trimeric complex is of substantial interest. Adenosine is a volume transmission (VT) signal that acts as a feedback detector of neuronal activation [Ferre and Fuxe, 2000]. It produces motor depression and sleep under physiological conditions in part mediated via A2A receptors. Under pathological conditions, extracellular levels of adenosine levels increase leading to reduction of glutamate release and free radicals. In the FGFR1–A2A–D2 heterocomplex, adenosine-induced activation of A2A may then enhance signaling over FGFR1 via the allosteric receptor–receptor interaction, increasing structural plasticity and repair. At the same time the adenosine activation of A2A through its antagonistic A2A–D2 interaction can also bring about a reduction of Gi/o mediated inhibitory D2 signaling in these neurons of the indirect pathway which mediates motor inhibition. This is also physiologically relevant, since a reduction of behavioral arousal will develop so the animal can move into a state of rest with restoration of energy stores. It may illustrate the potential benefits of having higher order heterocomplexes formed containing both RTK and GPCR receptors [Fuxe et al. 2007; Flajolet et al. 2008; Borroto-Escuela et al. 2012].
5-HT2A–D2 heteroreceptor complexes and their relevance for schizophrenia and its treatment
5-HT2A–D2 heteroreceptor complexes were demonstrated in cellular models using the BRET2 method [Borroto-Escuela et al. 2010c]. The receptor interface appeared to involve four sets of triplet amino acid homologies that may be part of the receptor interface based on the triplet puzzle theory [Tarakanov and Fuxe, 2010; Fuxe et al. 2014a,b]. They give a kind of code that guides the receptors towards each other. In a subsequent paper using the PLA technique, 5-HT2A-D2 heteroreceptor complexes were found also in discrete regions of the dorsal and ventral striatum, especially in the dorsolateral part and fundus of the dorsal striatum and in the nucleus accumbens core and shell [Borroto-Escuela et al. 2014a, 2015b]. Thus, they are likely located in the D2 positive dorsal and ventral striato-pallidal GABA neurons and their distribution overlaps at least in part with that of the A2A–D2 heteroreceptor complexes. However, it is not known if they are present in the same neuron and in the same synapses present on these neurons.
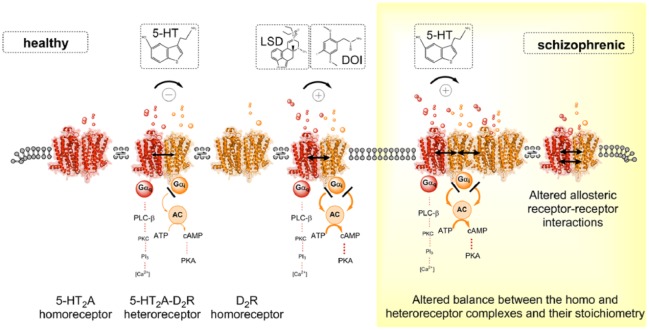
It is of particular interest that only the 5-HT2A hallucinogens lysergic acid diethylamide (LSD) and 2,5-dimethoxy-4-iodoamphetamine (DOI), but not a standard 5-HT2A agonist, enhance the D2 protomer signaling in this heteroreceptor complex via the allosteric receptor–receptor interaction (Figure 3) [Borroto-Escuela et al. 2014a, 2015b]. This action by the hallucinogens is blocked by the 5-HT2A receptor antagonist ketanserin and is not found in cells expressing D2 receptor alone. D2 protomer signaling was monitored using the CREB-Luc induction assay. The hallucinogenic drugs DOI and LSD increase the density and affinity of the D2-like receptors using the D2-like antagonist radioligand 3H-raclopride in cellular models and the ventral striatum, actions blocked by ketanserin. Thus, cryptic D2 receptors in the plasma membrane appear to become functional and bind the radioligand.
Figure 3.
5-HT2AR–D2R heteroreceptor complexes and their relevance for schizophrenia and its treatment. Left panel. Schematic representation of the agonist action of the endogenous ligand 5-HT and hallucinogenic 5-HT2AR agonists LSD and DOI at the 5-HT2AR protomer of the 5-HT2AR–D2R heteroreceptor complexes. Experimental evidence demonstrated that, in the nanomolar range, the hallucinogenic 5-HT2AR agonists LSD and DOI lead to enhanced Gi/o signaling over the D2R protomer producing an enhanced inhibition of the AC–PKA–CREB pathway in this heteroreceptor complexes. In contrast, the endogenous ligand 5-HT exerted an allosteric antagonistic action on D2R signaling in 5-HT2A–D2R heteroreceptor complexes. Right panel. In schizophrenia, alterations in the allosteric receptor–receptor interactions or a balance between 5-HT2A–D2 heteroreceptor complexes and their homoreceptor complexes may develop. This may make it possible for the endogenous 5-HT to change its action and also enhance D2 protomer signalling in this complex as observed for the hallucinogenic 5-HT2AR agonists LSD and DOI in the left panel.
5-HT, 5-hydroxytryptamine; AC, adenylyl cyclase; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; DOI, 2,5-dimethoxy-4-iodoamphetamine; LSD, lysergic acid diethylamide; PKA, protein kinase A; PLC, phospholipase C.
The psychotic like actions of the 5-HT2AR hallucinogens can therefore involve enhancement of D2R protomer signaling in the D2–5-HT2A heteroreceptor complex in the ventral striatum. It may be that, in schizophrenia, alterations in the allosteric receptor–receptor interaction may develop in this heteroreceptor complex which will make it possible for 5-HT to change its action and also enhance D2 protomer signaling in this complex. Such a pathological event can then increase the inhibition of the ventral striato-pallidal GABA neurons and contribute to psychotic symptoms as discussed above. Such pathological facilitatory receptor–receptor interactions in the D2–5-HT2A heteroreceptor complex leading to increased D2R signaling may at least develop in certain schizophrenic patients.
Such a pathological allosteric enhancement of D2 protomer signaling in this heterocomplex in schizophrenia through 5-HT2A protomer activation gives a novel understanding of the molecular mechanisms involved for the antipsychotic actions of atypical antipsychotic drugs. Thus, for example, risperidone, olanzapine and clozapine can block the 5-HT2A receptor with a high potency due to a high affinity for the 5-HT2A receptors [Meltzer et al. 1989, 2003; Ebdrup et al. 2011; Meltzer, 2012], but a 5-HT1A agonism also appears to play a role besides their D2 antagonist and 5-HT2A blocking activities [Oyamada et al. 2015].
One advantage of several atypical antipsychotics with high affinity for 5-HT2A receptors may therefore be that they can reduce an exaggerated enhancement of D2 protomer signaling at low doses. This action adds to their ability to reduce D2 protomer signaling which also takes place direct blockade of the D2 orthosteric binding site in the D2–5-HT2A heteroreceptor complex. This may help reduce the dose of many atypical antipsychotic drugs leading to diminished extrapyramidal and cognitive side effects.
It is of substantial interest that a serotonin receptor inverse agonist pimavanserin appears to have an antipsychotic potential in Parkinson’s disease psychosis [Meltzer et al. 2010]. A reversal of psychosis like behaviors was also observed in a rat model of Parkinson’s disease [McFarland et al. 2011]. According to our hypothesis, this action can at least in part take place in the ventral striatal 5-HT2A–D2 heteroreceptor complexes blocking the pathological transfer of facilitatory allosteric communication to the D2 protomer from the 5-HT2A protomer. However, 5-HT2A immunoreactive neuronal processes were observed in many parts in the forebrain, especially in the apical dendrites of pyramidal nerve cells [Jansson et al. 2001] and may also mediate the antipsychotic action of pimavanserin. It may involve a pimavanserin induced blockade of a 5-HT2A mediated activation of a cortical-ventral midbrain pathway increasing firing in the ventral tegmental DA neurons [Pehek et al. 2001]. It may be that Parkinson’s disease psychosis has a particularly high sensitivity to 5-HT2A inverse agonism, since levodopa and DA receptor agonist treatment to reduce the motor symptoms have produced marked changes in the composition and density of the 5-HT2A–D2 heteroreceptor complexes and their allosteric receptor–receptor interactions. It appears possible that a marked shift towards a facilitatory allosteric communication from the 5-HT2A protomer to the D2 protomer has developed which can be blocked by the inverse agonism of pimavanserin. This hypothesis will be tested in cellular models and in models of Parkinson’s disease.
NTS1–D2 heteroreceptor complexes and their relevance for schizophrenia and its treatment
Nemeroff introduced the hypothesis in 1980 that NT was an endogenous neuroleptic [Nemeroff, 1980]. We therefore studied the potential mechanism and found that NT reduced the affinity of the D2 agonist binding sites in the dorsal striatum and nucleus accumbens [Agnati et al. 1983; von Euler and Fuxe, 1987]. The D1 receptors were not affected by NT. In 1992 it was proposed that antagonistic pre and post synaptic NT–D2 receptor–receptor interactions produced a plasticity change in DA transmission with a switching towards a D1 mediated transmission [Fuxe et al. 1992a, 1992b]. This plasticity change was enhanced by the NTS1 mediated inhibition of the nerve terminal D2 autoreceptor signaling increasing DA release [Tanganelli et al. 1989].
In 2013 it was demonstrated that a NTS1–D2 heteroreceptor complex exists in cellular models using BRET [Borroto-Escuela et al. 2013b]. It was found using the CRE luciferase gene assay that a marked inhibition is induced by the NTS1 agonist of the D2 inhibition of the AC–PKA–CREB pathway. A marked shift to the right was observed with NT of the D2 agonist induced concentration-response curve mediated by an allosteric receptor–receptor interaction. Instead the PLC signaling pathway was strongly enhanced by the NTS1 agonist likely through a synergistic PKC activation. The NTS1 antagonist blocked these actions of the NTS1 agonist.
Finally, with the PLA, the existence of the NTS1–D2 heteroreceptor complexes was demonstrated in the accumbens core and shell and especially in the dorsal striatum (Schäfer, Borroto-Escuela et al. unpublished data).
NTS1–D2 heteroreceptor complexes in local circuits of the nucleus accumbens
The major location of these heteroreceptor complexes is on the cortico-accumbens glutamate terminals. They are also found at the soma-dendritic level of the ventral striato-pallidal GABA neurons (the antireward system). No NTS1–D2 heteroreceptor complexes exist on the accumbens DA nerve terminals.
Thus, NTS1 agonists in the accumbens, through inhibitory allosteric receptor–receptor interactions, mainly reduce D2 receptor signaling of inhibitory D2 receptors on the cortico-accumbens glutamate terminals. In this way glutamate release is increased to activate the ventral striato-pallidal GABA neurons, which via the mediodorsal thalamic nucleus, restores the glutamate drive to the prefrontal cortex. The increased activity in the striato-pallidal GABA neurons also leads to enhancement of local GABA VT from soma and dendrites to produce reduced DA release via GABA receptors located on the DA terminals. In view of the previous discussion, such events may mediate the antipsychotic actions of NT peptides mediated via NTS1.
NTS1-D2 heteroreceptor complexes in local circuits of the ventral midbrain
NT mechanisms also exist in the ventral tegmental area and the substantia nigra where they appear to enhance DA neuron firing [Ferraro et al. 2014]. NT release from ventral midbrain NT terminals leads to activation of inhibitory NTS1–D2 autoreceptor interactions, increasing the firing of the ventral tegmental area (VTA) and substantia nigra DA nerve cells. Furthermore, facilitatory NTS1–NMDA (receptor–receptor and/or cytoplasmic) interactions exist in putative NTS1–NMDA heteroreceptor complexes on glutamate terminals and/or on nigral and VTA DA cells. Therefore, in contrast to the case in nucleus accumbens, at the midbrain level, NTS1 receptor agonists exert propsychotic actions and NTS1 antagonists should exert antipsychotic actions. It is not known which NT mechanism dominates in schizophrenia. It has therefore been difficult to develop antipsychotic drugs based on targeting the NTS1–D2 heteroreceptor complexes .It is not clear if NTS1 agonists or antagonists should be developed. Therefore, more work is highly warranted to determine the major NT mechanism disturbed in schizophrenia.
Future directions
Understanding how typical and atypical antipsychotics act on the different D2 heteroreceptor complexes. Which D2 protomers are the major targets for the therapeutic effects of antipsychotics and which mediate side effects.
Understanding the potential behavioral role of receptor–receptor interactions in distinct D2 heteroreceptor complexes and other monoamine receptor heteroreceptor complexes in key brain circuits like the ventral striatal-ventral pallidal-mediodorsal thalamic-prefrontal cortical circuit.
Understanding the location of the different D2 heteroreceptor complexes. Are they located in the same or different pathways? In the same or different neurons? In the same neuron but in different or the same synapses? Do they have a differential location with regard to prejunctional, postjunctional and soma-dendritic location?
Understanding the dynamic allosteric receptor–receptor interactions of the individual D2 heteroreceptor complexes.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors are supported by grants from the Swedish Medical Research Council (04X-715) to K.F. and by AFA Försäkring (130328) to K.F. and D.O.B.-E. D.O.B-E is a member of Academia de Biólogos Cubanos.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Dasiel O. Borroto-Escuela, Department of Neuroscience, Karolinska Institutet, Stockholm, Sweden Department of Biomolecular Science, Section of Physiology, University of Urbino, Italy
Julia Pintsuk, Department of Neuroscience, Karolinska Institutet, Stockholm, Sweden Institute of Biomedicine and Translational Medicine, University of Tartu, Estonia.
Thorsten Schäfer, Clinical and Molecular Pharmacy, Department of Chemistry and Pharmacy, Friedrich Alexander University, Erlangen-Nürnberg, Germany.
Kristina Friedland, Clinical and Molecular Pharmacy, Department of Chemistry and Pharmacy, Friedrich Alexander University, Erlangen-Nürnberg, Germany.
Luca Ferraro, Department of Life Sciences and Biotechnology, University of Ferrara, Italy.
Sergio Tanganelli, Department of Life Sciences and Biotechnology, University of Ferrara, Italy Department of Medical Sciences, University of Ferrara, Italy.
Fang Liu, Campbell Research Institute, Centre for Addiction and Mental Health, University of Toronto, Toronto, Ontario, Canada.
Kjell Fuxe, Department of Neuroscience, Karolinska Instituet, Retzius väg 8, 17177 Stockholm, Sweden.
References
- Agnati L., Fuxe K., Benfenati F., Battistini N. (1983) Neurotensin in vitro markedly reduces the affinity in subcortical limbic 3H-N-propylnorapomorphine binding sites. Acta Physiol Scand 119: 459–461. [DOI] [PubMed] [Google Scholar]
- Agnati L., Guidolin D., Leo G., Carone C., Genedani S., Fuxe K. (2009) Receptor–receptor interactions: a novel concept in brain integration. Prog Neurobiol 90: 157–175. [DOI] [PubMed] [Google Scholar]
- Alagarsamy S., Marino M., Rouse S., Gereau R., Heinemann S., Conn P. (1999) Activation of NMDA receptors reverses desensitization of mGluR5 in native and recombinant systems. Nat Neurosci 2: 234–240. [DOI] [PubMed] [Google Scholar]
- Anden N., Dahlstrom A., Fuxe K., Larsson K., Olson L., Ungerstedt U. (1966) Ascending monoamine neurons to the telencephalon and diencephalon. Acta Physiol Scand 67: 313–326. [Google Scholar]
- Andersen M., Fuxe K., Werge T., Gerlach J. (2002) The adenosine A2A receptor agonist CGS 21680 exhibits antipsychotic-like activity in Cebus apella monkeys. Behav Pharmacol 13: 639–644. [DOI] [PubMed] [Google Scholar]
- Azdad K., Gall D., Woods A., Ledent C., Ferre S., Schiffmann S. (2009) Dopamine D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2a-D2 receptor heteromerization. Neuropsychopharmacology 34: 972–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J., Espinoza S., Gainetdinov R. (2015) Dopamine receptors – IUPHAR review 13. Br J Pharmacol 172: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J., Marion S., Rodriguiz R., Medvedev I., Sotnikova T., Ghisi V., et al. (2008) A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell 132: 125–136. [DOI] [PubMed] [Google Scholar]
- Beaulieu J., Sotnikova T., Yao W., Kockeritz L., Woodgett J., Gainetdinov R., et al. (2004) Lithium antagonizes dopamine-dependent behaviors mediated by an Akt/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci U S A 101: 5099–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger B., Tassin J., Blanc G., Moyne M., Thierry A. (1974) Histochemical confirmation for dopaminergic innervation of the rat cerebral cortex after destruction of the noradrenergic ascending pathways. Brain Res 81: 332–337. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela D., Brito I., Di Palma M., Jiménez-Beristain A., Narváez M., Corrales F., et al. (2015a) On the role of the balance of GPCR homo/ heteroreceptor complexes in the brain. J Adv Neurosci Res 2: 36–44. [Google Scholar]
- Borroto-Escuela D., Brito I., Romero-Fernandez W., Di Palma M., Oflijan J., Skieterska K., et al. (2014a) The G protein-coupled receptor heterodimer network (GPCR-HETNET) and its hub components. Int J Mol Sci 15: 8570–8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroto-Escuela D., Flajolet M., Agnati L., Greengard P., Fuxe K. (2013a) Bioluminescence resonance energy transfer methods to study G protein-coupled receptor-receptor tyrosine kinase heteroreceptor complexes. Methods Cell Biol 117: 141–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroto-Escuela D., Marcellino D., Narvaez M., Flajolet M., Heintz N., Agnati L., et al. (2010a) A serine point mutation in the adenosine A2AR C-terminal tail reduces receptor heteromerization and allosteric modulation of the dopamine D2R. Biochem Biophys Res Commun 394: 222–227. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela D., Ravani A., Tarakanov A., Brito I., Narvaez M., Romero-Fernandez W., et al. (2013b) Dopamine D2 receptor signaling dynamics of dopamine D2-neurotensin 1 receptor heteromers. Biochem Biophys Res Commun 435: 140–146. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela D., Romero-Fernandez W., Garriga P., Ciruela F., Narvaez M., Tarakanov A., et al. (2013c) G protein-coupled receptor heterodimerization in the brain. Methods Enzymol 521: 281–294. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela D., Romero-Fernandez W., Mudo G., Perez-Alea M., Ciruela F., Tarakanov A., et al. (2012) Fibroblast growth factor receptor 1–5-hydroxytryptamine 1A heteroreceptor complexes and their enhancement of hippocampal plasticity. Biol Psychiatry 71: 84–91. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela D., Romero-Fernandez W., Narvaez M., Oflijan J., Agnati L., Fuxe K. (2014b) Hallucinogenic 5-HT2AR agonists LSD and DOI enhance dopamine D2R protomer recognition and signaling of D2–5-HT2A heteroreceptor complexes. Biochem Biophys Res Commun 443: 278–284. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela D., Romero-Fernandez W., Tarakanov A., Ciruela F., Agnati L., Fuxe K. (2011) On the existence of a possible A2A-D2-beta-arrestin2 complex: A2A agonist modulation of D2 agonist-induced beta-arrestin2 recruitment. J Mol Biol 406: 687–699. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela D., Romero-Fernandez W., Tarakanov A., Gomez-Soler M., Corrales F., Marcellino D., et al. (2010b) Characterization of the A2AR-D2R interface: focus on the role of the C-terminal tail and the transmembrane helices. Biochem Biophys Res Commun 402: 801–807. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela D., Romero-Fernandez W., Tarakanov A., Marcellino D., Ciruela F., Agnati L., et al. (2010c) Dopamine D2 and 5-Hydroxytryptamine 5-HT((2)A) receptors assemble into functionally interacting heteromers. Biochem Biophys Res Commun 401: 605–610. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela D., Wydra K., Ferraro L., Rivera A., Filip M., Fuxe K. (2015b) Role of D2-like heteroreceptor complexes in the effects of cocaine, morphine and hallucinogens. In: Preedy V. (ed.), Neurophatology of Drug Addictions and Substance Misuse. London: Elsevier. [Google Scholar]
- Bourgeron T. (2015) From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat Rev Neurosci 16: 551–563. [DOI] [PubMed] [Google Scholar]
- Bradshaw N., Porteous D. (2012) DISC1-binding proteins in neural development, signalling and schizophrenia. Neuropharmacology 62: 1230–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello N., Gandia J., Bertarelli D., Watanabe M., Lluis C., Franco R., et al. (2009) Metabotropic glutamate type 5, dopamine D2 and adenosine A2A receptors form higher-order oligomers in living cells. J Neurochem 109: 1497–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruela F., Fernandez-Duenas V., Llorente J., Borroto-Escuela D., Cuffi M., Carbonell L., et al. (2012) G protein-coupled receptor oligomerization and brain integration: focus on adenosinergic transmission. Brain Res 1476: 86–95. [DOI] [PubMed] [Google Scholar]
- Dahlstrom A., Fuxe K. (1964) Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand 232: 231–255. [PubMed] [Google Scholar]
- Davis K., Kahn R., Ko G., Davidson M. (1991) Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 148: 1474–1486. [DOI] [PubMed] [Google Scholar]
- De Bartolomeis A., Buonaguro E., Iasevoli F. (2013) Serotonin-glutamate and serotonin-dopamine reciprocal interactions as putative molecular targets for novel antipsychotic treatments: from receptor heterodimers to postsynaptic scaffolding and effector proteins. Psychopharmacology) 225: 1–19. [DOI] [PubMed] [Google Scholar]
- De Bartolomeis A., Latte G., Tomasetti C., Iasevoli F. (2014) Glutamatergic postsynaptic density protein dysfunctions in synaptic plasticity and dendritic spines morphology: relevance to schizophrenia and other behavioral disorders pathophysiology and implications for novel therapeutic approaches. Mol Neurobiol 49: 484–511. [DOI] [PubMed] [Google Scholar]
- Deutch A., Lee M., Iadarola M. (1992) Regionally specific effects of atypical antipsychotic drugs on striatal FOS expression: the nucleus accumbens shell as a locus of antipsychotic action. Mol Cell Neurosci 3: 332–341. [DOI] [PubMed] [Google Scholar]
- Diaz-Cabiale Z., Hurd Y., Guidolin D., Finnman U., Zoli M., Agnati L., et al. (2001) Adenosine A2A agonist CGS 21680 decreases the affinity of dopamine D2 receptors for dopamine in human striatum. Neuroreport 12: 1831–1834. [DOI] [PubMed] [Google Scholar]
- Diaz-Cabiale Z., Vivo M., Del Arco A., O’Connor W., Harte M., Muller C., et al. (2002) Metabotropic glutamate mGlu5 receptor-mediated modulation of the ventral striopallidal GABA pathway in rats. Interactions with adenosine A(2A) and dopamine D(2) receptors. Neurosci Lett 324: 154–158. [DOI] [PubMed] [Google Scholar]
- Ebdrup B., Rasmussen H., Arnt J., Glenthoj B. (2011) Serotonin 2A receptor antagonists for treatment of schizophrenia. Expert Opin Investig Drugs 20: 1211–1223. [DOI] [PubMed] [Google Scholar]
- Ferraro L., Beggiato S., Borroto-Escuela D., Ravani L., O’Connor W., Tomasini M., et al. (2014) Neurotensin NTS1-dopamine D2 receptor–receptor interactions in putative receptor heteromers: relevance for Parkinson’s disease and schizophrenia. Curr Protein Pept Sci 15: 681–690. [DOI] [PubMed] [Google Scholar]
- Ferre S., Fuxe K. (2000) Adenosine as a volume transmission signal. A feedback detector of neuronal activation. Prog Brain Res 125: 353–361. [DOI] [PubMed] [Google Scholar]
- Ferre S., Karcz-Kubicha M., Hope B., Popoli P., Burgueno J., Gutierrez M., et al. (2002) Synergistic interaction between adenosine A2A and glutamate mglu5 receptors: implications for striatal neuronal function. Proc Natl Acad Sci U S A 99: 11940–11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M., Zaniewska M., Frankowska M., Wydra K., Fuxe K. (2012) The importance of the adenosine A(2A) receptor-dopamine D(2) receptor interaction in drug addiction. Curr Med Chem 19: 317–355. [DOI] [PubMed] [Google Scholar]
- Flajolet M., Wang Z., Futter M., Shen W., Nuangchamnong N., Bendor J., et al. (2008) FGF acts as a co-transmitter through adenosine A(2A) receptor to regulate synaptic plasticity. Nat Neurosci 11: 1402–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K. (1965) Evidence for the existence of monoamine neurons in the central nervous system. IV. distribution of monoamine nerve terminals in the central nervous system. Acta Physiol Scand 64(Suppl. 247): 39–85. [PubMed] [Google Scholar]
- Fuxe K. (1970) Biological and pharmacological theories. Discussion. In: Bobon D., Janssen P., Bobon J. (eds), The Neuroleptics, Vol. 5 . Basel: Karger, 1–11. [Google Scholar]
- Fuxe K., Anden N. (1965) Studies on central monoamine neurons with special reference to the nigro-neostriatal dopamine neuron system. In: Costa E., Coté L., Yahr M. (eds), Biochemistry and Pharmacology of the Basal Ganglia. New York: Raven Press, 123–129. [Google Scholar]
- Fuxe K., Agnati L. (1987) Opening address. In: Fuxe K., Agnati L. (eds), Receptor–Receptor Interactions. London: McMillan, xiv–xviii. [Google Scholar]
- Fuxe K., Agnati L., Benfenati F., Celani M., Zini I., Zoli M., et al. (1983) Evidence for the existence of receptor-receptor interactions in the central nervous system. Studies on the regulation of monoamine receptors by neuropeptides. J Neural Transm 18: 165–179. [PubMed] [Google Scholar]
- Fuxe K., Agnati L., Jacobsen K., Hillion J., Canals M., Torvinen M., et al. (2003) Receptor heteromerization in adenosine A2A receptor signaling: relevance for striatal function and Parkinson’s disease. Neurology 61: S19–S23. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Borroto-Escuela D. (2016) Heteroreceptor complexes and their allosteric receptor-receptor interactions as a novel biological principle for integration of communication in the CNS: targets for drug development. Neuropsychopharmacology 41: 380–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K., Borroto-Escuela D., Romero-Fernandez W., Palkovits M., Tarakanov A., Ciruela F., et al. (2014a) Moonlighting proteins and protein-protein interactions as neurotherapeutic targets in the G protein-coupled receptor field. Neuropsychopharmacology 39: 131–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K., Borroto-Escuela D., Tarakanov A., Romero-Fernandez W., Ferraro L., Tanganelli S., et al. (2014b) Dopamine D2 heteroreceptor complexes and their receptor-receptor interactions in ventral striatum: novel targets for antipsychotic drugs. Prog Brain Res 211: 113–139. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Celani M., Martire M., Zini I., Zoli M., Agnati L. (1984) L-glutamate reduces the affinity of [3H]N-propylnorapomorphine binding sites in striatal membranes. Eur J Pharmacol 100: 127–130. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Dahlstrom A., Hoistad M., Marcellino D., Jansson A., Rivera A., et al. (2007) From the Golgi-Cajal mapping to the transmitter-based characterization of the neuronal networks leading to two modes of brain communication: wiring and volume transmission. Brain Res Rev 55: 17–54. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Dahlstrom A. (2009) Evidence for the Existence of Central Monoamine Neurons. Saarbrucken, Germany: VDM Verlag. [Google Scholar]
- Fuxe K., Dahlstrom A., Jonsson G., Marcellino D., Guescini M., Dam M., et al. (2010a) The discovery of central monoamine neurons gave volume transmission to the wired brain. Prog Neurobiol 90: 82–100. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Ferre S., Zoli M., Agnati L. (1998) Integrated events in central dopamine transmission as analyzed at multiple levels. Evidence for intramembrane adenosine A2A/dopamine D2 and adenosine A1/dopamine D1 receptor interactions in the basal ganglia. Brain Res Brain Res Rev 26: 258–273. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Hokfelt T., Jonsson G., Ungerstedt U. (1970) Fluorescence microscopy in neuroanatomy. In: Nauta W., Ebbesson S. (eds), Contemporany Research Methods in Neuroanatomy. Berlin: Springer-Verlag, 275–314. [Google Scholar]
- Fuxe K., Marcellino D., Borroto-Escuela D., Guescini M., Fernandez-Duenas V., Tanganelli S., et al. (2010b) Adenosine-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther 16: e18–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K., Marcellino D., Rivera A., Diaz-Cabiale Z., Filip M., Gago B., et al. (2008) Receptor-receptor interactions within receptor mosaics. Impact on neuropsychopharmacology. Brain Res Rev 58: 415–452. [DOI] [PubMed] [Google Scholar]
- Fuxe K., O’Connor W., Antonelli T., Osborne P., Tanganelli S., Agnati L., et al. (1992a) Evidence for a substrate of neuronal plasticity based on pre- and postsynaptic neurotensin-dopamine receptor interactions in the neostriatum. Proc Natl Acad Sci U S A 89: 5591–5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K., Tarakanov A., Romero Fernandez W., Ferraro L., Tanganelli S., Filip M., et al. (2014) Diversity and bias through receptor-receptor interactions in GPCR heteroreceptor complexes. Focus on examples from dopamine D2 receptor heteromerization. Front Endocrinol 5: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K., von Euler G., Agnati L., Merlo Pich E., O’Connor W., Tanganelli S., et al. (1992b) Intramembrane interactions between neurotensin receptors and dopamine D2 receptors as a major mechanism for the neuroleptic-like action of neurotensin. Ann N Y Acad Sci 668: 186–204. [DOI] [PubMed] [Google Scholar]
- Ginovart N., Kapur S. (2012) Role of dopamine D(2) receptors for antipsychotic activity. Handb Exp Pharmacol: 27–52. [DOI] [PubMed] [Google Scholar]
- Grace A., Bunney B., Moore H., Todd C. (1997) Dopamine-cell depolarization block as a model for the therapeutic actions of antipsychotic drugs. Trends Neurosci 20: 31–37. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Su T. (2007) Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131: 596–610. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez S., Tkatch T., Perez-Garci E., Galarraga E., Bargas J., Hamm H., et al. (2000) D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC[Beta]1-IP3-calcineurin-signaling cascade. J Neurosci 20: 8987–8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillion J., Canals M., Torvinen M., Casado V., Scott R., Terasmaa A., et al. (2002) Coaggregation, cointernalization and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem 277: 18091–18097. [DOI] [PubMed] [Google Scholar]
- Jansson A., Tinner B., Bancila M., Verge D., Steinbusch H., Agnati L., et al. (2001) Relationships of 5-hydroxytryptamine immunoreactive terminal-like varicosities to 5-hydroxytryptamine-2a receptor-immunoreactive neuronal processes in the rat forebrain. J Chem Neuroanat 22: 185–203. [DOI] [PubMed] [Google Scholar]
- Juckel G., Schlagenhauf F., Koslowski M., Wustenberg T., Villringer A., Knutson B., et al. (2006) Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage 29: 409–416. [DOI] [PubMed] [Google Scholar]
- Kapur S. (2003) Psychosis as a state of aberrant salience: a framework linking biology, phenomenology and pharmacology in schizophrenia. Am J Psychiatry 160: 13–23. [DOI] [PubMed] [Google Scholar]
- Kegeles L., Abi-Dargham A., Frankle W., Gil R., Cooper T., Slifstein M., et al. (2010) Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry 67: 231–239. [DOI] [PubMed] [Google Scholar]
- Lee F., Pei L., Moszczynska A., Vukusic B., Fletcher P., Liu F. (2007) Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J 26: 2127–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Chu X., Mao L., Wang M., Lan H., Li M., et al. (2006) Modulation of D2R-NR2B interactions in response to cocaine. Neuron 52: 897–909. [DOI] [PubMed] [Google Scholar]
- Masri B., Salahpour A., Didriksen M., Ghisi V., Beaulieu J., Gainetdinov R., et al. (2008) Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc Natl Acad Sci U S A 105: 13656–13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T., Su T. (2009) The pharmacology of sigma-1 receptors. Pharmacol Ther 124: 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K., Price D., Bonhaus D. (2011) Pimavanserin, a 5-HT2A inverse agonist, reverses psychosis-like behaviors in a rodent model of Parkinson’s disease. Behav Pharmacol 22: 681–692. [DOI] [PubMed] [Google Scholar]
- Meltzer H. (2012) Serotonergic mechanisms as targets for existing and novel antipsychotics. Handb Exp Pharmacol: 87–124. [DOI] [PubMed] [Google Scholar]
- Meltzer H., Li Z., Kaneda Y., Ichikawa J. (2003) Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 27: 1159–1172. [DOI] [PubMed] [Google Scholar]
- Meltzer H., Matsubara S., Lee J. (1989) Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 PKI values. J Pharmacol Exp Ther 251: 238–246. [PubMed] [Google Scholar]
- Meltzer H., Mills R., Revell S., Williams H., Johnson A., Bahr D., et al. (2010) Pimavanserin, a serotonin(2A) receptor inverse agonist, for the treatment of Parkinson’s disease psychosis. Neuropsychopharmacology 35: 881–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer H., Stahl S. (1976) The dopamine hypothesis of schizophrenia: a review. Schizophr Bull 2: 19–76. [DOI] [PubMed] [Google Scholar]
- Navarro G., Moreno E., Aymerich M., Marcellino D., McCormick P., Mallol J., et al. (2010) Direct involvement of sigma-1 receptors in the dopamine D1 receptor-mediated effects of cocaine. Proc Natl Acad Sci U S A 107: 18676–18681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro G., Moreno E., Bonaventura J., Brugarolas M., Farre D., Aguinaga D., et al. (2013) Cocaine inhibits dopamine D2 receptor signaling via sigma-1-D2 receptor heteromers. PLoS One 8: e61245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff C. (1980) Neurotensin: perchance an endogenous neuroleptic? Biol Psychiatry 15: 283–302. [PubMed] [Google Scholar]
- Nielsen M., Rostrup E., Wulff S., Bak N., Lublin H., Kapur S., et al. (2012) Alterations of the brain reward system in antipsychotic naïve schizophrenia patients. Biol Psychiatry 71: 898–905. [DOI] [PubMed] [Google Scholar]
- Nishi A., Liu F., Matsuyama S., Hamada M., Higashi H., Nairn A., et al. (2003) Metabotropic mGlu5 receptors regulate adenosine A2A receptor signaling. Proc Natl Acad Sci U S A 100: 1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintsuk J., Borroto-Escuela D., Lai T., Liu F., Fuxe K. (2016) Alterations in ventral and dorsal striatal A2A-D2 receptor-receptor interactions after amphetamine challenge: relevance for schizophrenia. Pharmacology Biochemistry and Behavior. [DOI] [PubMed] [Google Scholar]
- Oyamada Y., Horiguchi M., Rajagopal L., Miyauchi M., Meltzer H. (2015) Combined serotonin (5-HT)1A Agonism, 5-HT(2A) and dopamine D(2) receptor antagonism reproduces atypical antipsychotic drug effects on phencyclidine-impaired novel object recognition in rats. Behav Brain Res 285: 165–175. [DOI] [PubMed] [Google Scholar]
- Pehek E., McFarlane H., Maguschak K., Price B., Pluto C. (2001) M100,907, a selective 5-HT(2A) antagonist, attenuates dopamine release in the rat medial prefrontal cortex. Brain Res 888: 51–59. [DOI] [PubMed] [Google Scholar]
- Perreault M., Hasbi A., O’Dowd B., George S. (2014) Heteromeric dopamine receptor signaling complexes: emerging neurobiology and disease relevance. Neuropsychopharmacology 39: 156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton L., Borroto-Escuela D., Narváez M., Jiménez-Beristain A., Oflijan J., Ferraro L., et al. (2015a) Dopamine D2 receptor dynamic and modulation in the D2R-Sigma 1heteroreceptor complexes: role in cocaine action. Eur Neuropsychopharmacol 25(Suppl. 2): S609–s610. [Google Scholar]
- Pinton L., Borroto-Escuela D., Narváez M., Oflijan J., Agnati L., Fuxe K. (2015b) Evidence for the existence of dopamine D2R and sigma 1 allosteric receptor-receptor interaction in the rat brain: role in brain plasticity and cocaine action. SpringerPlus 4(Suppl. 1): P37. [Google Scholar]
- Popoli P., Pezzola A., Torvinen M., Reggio R., Pintor A., Scarchilli L., et al. (2001) The selective mGlu(5) receptor agonist CHPG inhibits quinpirole-induced turning in 6-hydroxydopamine-lesioned rats and modulates the binding characteristics of dopamine D(2) receptors in the rat striatum: interactions with adenosine A(2A) receptors. Neuropsychopharmacology 25: 505–513. [DOI] [PubMed] [Google Scholar]
- Portoghese P. (2001) From models to molecules: opioid receptor dimers, bivalent ligands and selective opioid receptor probes. J Med Chem 44: 2259–2269. [DOI] [PubMed] [Google Scholar]
- Rimondini R., Ferre S., Ogren S., Fuxe K. (1997) Adenosine A2A agonists: a potential new type of atypical antipsychotic. Neuropsychopharmacology 17: 82–91. [DOI] [PubMed] [Google Scholar]
- Sahlholm K., Marcellino D., Nilsson J., Ogren S., Fuxe K., Arhem P. (2014) Typical and atypical antipsychotics do not differ markedly in their reversibility of antagonism of the dopamine D2 receptor. Int J Neuropsychopharmacol 17: 149–155. [DOI] [PubMed] [Google Scholar]
- Salim H., Ferre S., Dalal A., Peterfreund R., Fuxe K., Vincent J., et al. (2000) Activation of adenosine A1 and A2A receptors modulates dopamine D2 receptor-induced responses in stably transfected human neuroblastoma cells. J Neurochem 74: 432–439. [DOI] [PubMed] [Google Scholar]
- Schlagenhauf F., Sterzer P., Schmack K., Ballmaier M., Rapp M., Wrase J., et al. (2009) Reward feedback alterations in unmedicated schizophrenia patients: relevance for delusions. Biol Psychiatry 65: 1032–1039. [DOI] [PubMed] [Google Scholar]
- Seeman P. (2010) Historical overview. Introduction to the dopamine receptors. In: Neve K. (ed.), The Dopamine Receptors. New York: Humana Press, 1–21. [Google Scholar]
- Seeman P., Tallerico T., Ko F., Tenn C., Kapur S. (2002) Amphetamine-sensitized animals show a marked increase in dopamine D2 high receptors occupied by endogenous dopamine, even in the absence of acute challenges. Synapse 46: 235–239. [DOI] [PubMed] [Google Scholar]
- Stromberg I., Popoli P., Muller C., Ferre S., Fuxe K. (2000) Electrophysiological and behavioural evidence for an antagonistic modulatory role of adenosine A2A receptors in dopamine D2 receptor regulation in the rat dopamine-denervated striatum. Eur J Neurosci 12: 4033–4037. [DOI] [PubMed] [Google Scholar]
- Su P., Li S., Chen S., Lipina T., Wang M., Lai T., et al. (2014) A Dopamine D2 receptor-DISC1 protein complex may contribute to antipsychotic-like effects. Neuron 84: 1302–1316. [DOI] [PubMed] [Google Scholar]
- Surmeier D., Ding J., Day M., Wang Z., Shen W. (2007) D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci 30: 228–235. [DOI] [PubMed] [Google Scholar]
- Tanganelli S., Von Euler G., Fuxe K., Agnati L., Ungerstedt U. (1989) Neurotensin counteracts apomorphine-induced inhibition of dopamine release as studied by microdialysis in rat neostriatum. Brain Res 502: 319–324. [DOI] [PubMed] [Google Scholar]
- Tarakanov A., Fuxe K. (2010) Triplet puzzle: homologies of receptor heteromers. J Mol Neurosci 41: 294–303. [DOI] [PubMed] [Google Scholar]
- Thierry A., Blanc G., Sobel A., Stinus L., Glowinski J. (1973) Dopaminergic terminals in the rat cortex. Science 182: 499–501. [DOI] [PubMed] [Google Scholar]
- Trifilieff P., Rives M., Urizar E., Piskorowski R., Vishwasrao H., Castrillon J., et al. (2011) Detection of antigen interactions ex vivo by proximity ligation assay: endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. Biotechniques 51: 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Euler G., Fuxe K. (1987) Neurotensin reduces the affinity of D-2 dopamine receptors in rat striatal membranes. Acta Physiol Scand 131: 625–626. [DOI] [PubMed] [Google Scholar]
- Wang C., Buck D., Yang R., Macey T., Neve K. (2005) Dopamine D2 receptor stimulation of mitogen-activated protein kinases mediated by cell type-dependent transactivation of receptor tyrosine kinases. J Neurochem 93: 899–909. [DOI] [PubMed] [Google Scholar]
- Wang M., Pei L., Fletcher P., Kapur S., Seeman P., Liu F. (2010) Schizophrenia, amphetamine-induced sensitized state and acute amphetamine exposure all show a common alteration: increased dopamine D2 receptor dimerization. Mol Brain 3: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieronska J., Zorn S., Doller D., Pilc A. (2015) Metabotropic glutamate receptors as targets for new antipsychotic drugs. Pharmacol Ther 157: 10–27. [DOI] [PubMed] [Google Scholar]
- Winton-Brown T., Fusar-Poli P., Ungless M., Howes O. (2014) Dopaminergic basis of salience dysregulation in psychosis. Trends Neurosci 37: 85–94. [DOI] [PubMed] [Google Scholar]
- Yang S., Dasgupta S., Lledo P., Vincent J., Fuxe K. (1995) Reduction of dopamine D2 receptor transduction by activation of adenosine A2A receptors in stably A2A/D2 (long-form) receptor co-transfected mouse fibroblast cell lines: studies on intracellular calcium levels. Neuroscience 68: 729–736. [DOI] [PubMed] [Google Scholar]