Abstract
Introduction:
Relatively few treatment programs have been developed specifically for smokeless tobacco (ST) users who want to quit. Their results suggest that self-help materials, telephone counseling, and nicotine lozenges are efficacious. This study provides the first direct examination of the separate and combined effects of telephone counseling and lozenges.
Methods:
We recruited ST users online (N = 1067) and randomly assigned them to 1 of 3 conditions: (a) a lozenge group (n = 356), who were mailed 4-mg nicotine lozenges; (b) a coach calls group (n = 354), who were offered 3 coaching phone calls; or (c) a lozenge + coach calls group (N = 357), who received both lozenges and coaching calls. Additionally, all participants were mailed self-help materials. Self-reported tobacco abstinence was assessed at 3 and 6 months after randomization.
Results:
Complete-case and intention-to-treat (ITT) analyses for all tobacco abstinence were performed at 3 months, 6 months, and both 3 and 6 months (repeated point prevalence). ITT analyses revealed a highly similar result: the lozenge + coach calls condition was significantly more successful in encouraging tobacco abstinence than either the lozenge group or the coach calls group, which did not differ.
Conclusions:
Combining nicotine lozenges and phone counseling significantly increased tobacco abstinence rates compared with either intervention alone, whereas coach calls and lozenges were equivalent. The study confirms the high tobacco abstinence rates for self-help ST cessation interventions and offers guidance to providing tobacco treatment to ST users.
Introduction
Smokeless tobacco (ST) is a known human carcinogen1,2 and poses significant risk to public health.3 Long-term use increases the risk for cardiovascular mortality.4–6 In contrast to the declining use of cigarettes in the United States, sales and consumption of ST have been increasing.7,8 As most ST users want to quit, programs with proven efficacy for the treatment of ST dependence are needed.
Despite this need, few effective treatments for ST users exist. We have previously reported that ST users receiving a mailed behavioral self-help ST cessation manual plus two “coach calls” (calls from a phone counselor/coach) have significantly higher tobacco abstinence rates than ST users receiving a self-help manual only.9,10
Currently, no nicotine replacement therapy (NRT) is U.S. Food and Drug Administration–approved for the treatment of tobacco dependence in ST users. Nicotine lozenges are available over-the-counter, have been shown to decrease tobacco withdrawal symptoms, and may increase short-term tobacco abstinence among ST users.11 Combining coaching calls with pharmacological therapy via lozenges may further increase abstinence rates.
We conducted a randomized clinical trial among ST users interested in quitting to evaluate the combined efficacy of interventions using nicotine lozenges and coach calls. Within the context that all participants received self-help materials for quitting, we made the following comparisons: (a) coach calls and nicotine lozenges versus a coach calls only; (b) coach calls and nicotine lozenges intervention versus nicotine lozenges only; and (c) coach calls only versus nicotine lozenges only.
Methods
Study Overview
We used a Google AdWords campaign to recruit an average of 15 study participants each week from July 2011 to October 2012. The study protocol was approved by Oregon Research Institute’s (ORI) Human Subjects Institutional Review Board (approval # FWA00005934) and registered with ClinicalTrials.gov (ID#NCTNCT01341938). To be eligible for participation, participants had to be at least 18 years of age, be using ST as primary tobacco product and using daily for at least 1 year, willing and motivated to quit in the next month, and have an e-mail and a U.S. mailing address. Exclusion criteria included the following: (a) past 30-day use of any type of NRT or prescription for tobacco cessation; (b) past 30-day participation in any other formal treatment for tobacco cessation or reduction; (c) other household member participating in this program; (d) history of phenylketonuria; (e) unstable angina, past 6-month history of heart attack or a coronary angioplasty; or (f) current pregnancy, breast feeding or likely to become pregnant within the next 6 months. Individuals not interested in participating or ineligible were able to use the freely available MyLastDip Web-based ST cessation program.12 Eligible ST users were mailed a packet that contained an informed consent document, a baseline assessment, and a stamped self-addressed return envelope. Upon return of a signed consent and completed baseline, individuals were randomized and promptly mailed another program packet that contained the self-help materials and condition-specific materials.
Experimental Conditions
Participants were randomly assigned to one of three conditions: (a) lozenge; (b), coach calls; or (c) lozenge + coach calls. All participants received self-help materials comprised of a copy of the Enough Snuff self-help manual13 and companion DVD.14 The self-help manual and DVD contained content focused on assessing reasons for quitting, setting a quit date, assessing personal patterns of using ST, developing a quit plan, using quitting techniques, coping with withdrawal symptoms, and preventing relapse.
Lozenge Condition
Participants received three boxes of Nicorette® Mini-Lozenges (4mg; 81 lozenges/box) with a schedule that recommended tapered usage over time: weeks 1–6 = 1 lozenge every 1 to 2hr, weeks 7–9 = 1 lozenge every 2–4hr, and weeks 10–12 = 1 lozenge every 4–8hr. These instructions also included a toll-free phone number that could be used during weeks 1–12 to obtain additional lozenges from the research project, up to a maximum of 12 total boxes of lozenges in units of one to three boxes for each request.
Coach Calls Condition
Participants were scheduled to receive three brief proactive counseling calls intended to reinforce and personalize the procedures described in the self-help materials. Coaches were six female ORI research assistants experienced in motivational interviewing and tobacco research. Counseling adhered to a scripted protocol, and coaches were encouraged to build rapport and respond to questions and issues surfaced during each call. An effort was made to maintain the pairing between coach and study participant throughout the study. All calls were digitally recorded and selected tapes were reviewed during weekly supervisory sessions to facilitate call fidelity.
Three planned proactive calls were scheduled. The initial call was scheduled for approximately 1 week after randomization to allow shipment and receipt of materials mailed to participants. Call #2 was scheduled to occur 2–3 days following the participant’s quit date. If no quit date had been chosen, this call was scheduled for 7–10 days following call #1. Call #3 was scheduled for 14–21 days following call #2.
Lozenge + Coach Calls Condition
Participants received three boxes of Nicorette® Mini-Lozenges and proactive counseling calls. Protocols for the lozenge condition and coaching calls were maintained. In addition, calls encouraged participants to follow the schedule for taking nicotine lozenges.
Assessments
Participant demographics and tobacco history were assessed at screening and baseline. Tobacco dependence was assessed using the Severson Smokeless Tobacco Dependency Scale (SSTDS)15 with possible scores ranging from 0 to 19. Readiness to quit was assessed using the Contemplation Ladder16 adapted for ST cessation that used an 11-point scale with 1 = not ready to quit, 3 = should consider quitting someday, 5 = should quit but not quite ready, 7 = thinking about cutting down or quitting, 9 = have cut down and seriously considering quitting, and 11 = ready to quit now. Self-efficacy was assessed using a 5-point scale: How confident are you that you will not be using any tobacco a year from now? with answer options of 1 = not at all confident, 3 = somewhat confident, and 5 = completely confident. Anticipated support for quitting from spouse/romantic partner was assessed using a 4-point scale: 1 = not at all supportive, 2 = somewhat supportive, 3 = supportive, and 4 = very supportive.
Follow-up assessments were mailed at 3 and 6 months after randomization. If an assessment was not returned within 2 weeks, then another assessment was mailed and an automatic e-mail reminder sent. Failure to receive this assessment would prompt research staff to attempt to complete the assessment by phone and up to 25 attempts were made to collect this data. Any assessment not completed within a 45-day interval was coded as missing. Each completed assessment prompted a cash payment of $15 with an additional $25 provided when both assessments were completed.
Outcome Measures
Primary Tobacco Outcome Measures
Seven-day point prevalence tobacco use assessed at the 3- and 6-month follow-up assessments was the primary tobacco outcome. A parallel measure of ST use was also collected.
Secondary Tobacco Outcome Measures
In order to assess possible changes in the amount of ST use, participants who reported continued use were asked to describe the number of ST cans, pouches, or tins used per week they consumed.
Program Acceptability
At the 3-month assessment, participants rated the helpfulness of the overall program and their condition-specific treatments. All participants rated the helpfulness of the treatment program overall, self-help materials, coach calls, and lozenges on a 5-point Likert scale. Participants who received lozenges were asked to describe the symptoms they experienced (headache, nausea, flatulence [gas], hiccups, heartburn, sleep disturbances, diarrhea) using a 4-point severity rating scale: 0 = none, 1 = mild, 2 = moderate, and 3 = severe.
Program Usage
At the 3-month assessment, participants were asked how much they read the self-help guide (All, Some, None) and the number of times they watched the companion DVD. Participants who were provided lozenges were asked: “Since you received the nicotine lozenges, have you been using them (None of the days, Few days, Less than half of the days, More than half of the days, and Most days)” and “On the days that you used the lozenges, how much of each day did you use them? (I didn’t use lozenges, Little of the day, Less than half the day, More than half the day, Most of the day).” The number and duration of completed coach calls were tracked by coaches.
Statistical Analyses
Three pairwise comparisons were used to examine the impact of the treatment conditions on outcome: (a) coach calls group vs lozenge + coach calls group, (b) lozenge group vs lozenge + coach calls group, and (c) coach calls group vs lozenge group. The false discovery rate control procedure recommended by Benjamini and Hochberg17 was applied to multiple tests within a pairwise comparison. All analyses used IBM SPSS Statistics version 19.
Baseline Equivalence and Predictors of Attrition
Analysis of variance (ANOVA) and chi-square analysis were used to evaluate baseline equivalence across the three arms of the study. Attrition was analyzed by examining a priori interactions among the three pairwise comparisons and baseline sample characteristics, including age, gender, minority status, college degree, amount of daily ST use, years of ST use, ST dependence using the SSTDS,15 current use of cigarettes, one or more quit attempt in the last year, one or more friends using ST, readiness to quit, confidence, partner support, 13 or more alcoholic drinks a week, and depressive symptoms using the Patient Health Questionnaire Depression Scale (PHQ-8).18
Primary Tobacco Outcome Analyses
Primary tobacco outcomes focused on the intention-to-treat (ITT) imputation in which missing cases are assumed to be using tobacco.19 Complete cases analyses were also used in order to compare our results to the extant literature. As noted by Graham and colleagues,20 it is not possible to use a measure of continuous abstinence attached to a fixed date because participants were able to set their own quit dates. Instead, we used repeated point prevalence (RPP; 3- and 6-month assessments) measures of all tobacco abstinence and ST abstinence as a more stringent measure of continuous abstinence.
Secondary Tobacco Use Analyses
Regression models with covariate adjustment for baseline tobacco use were used to examine reduced ST usage among participants who continued to use tobacco (number of ST cans, pouches, or tins used per week).
Predictors and Moderators of Tobacco Outcomes
We used a two-step procedure to examine putative predictors of 6-month abstinence among complete cases. First, univariate binary logistic regression was used to examine participant baseline characteristics as potential predictors. Significant univariate predictors were then included in a multivariate binary logistic regression. To identify any differential effects of the intervention on the prediction of these outcomes, we included treatment condition as well as the interaction of the condition with each variable in these analyses for each pairwise comparison.
Program Usage and Reported Helpfulness
ANOVAs were used to evaluate these metrics of program usage by condition. Chi-square and logistic regressions were used to evaluate program usage by the measure of tobacco abstinence at the 6-month assessment. ANOVA analyses were conducted to examine program helpfulness ratings by condition.
Results
Participant Characteristics
Consistent with our prior research (e.g., Severson and colleagues21) and prevalent characteristics of ST users, participants were predominantly male and approximately 36 years of age (Table 1). No condition differences on baseline participant characteristics were found.
Table 1.
Sample Characteristics by Groupa
| Coach calls (N = 354) | Lozenge (N = 356) | Lozenge + coach calls (N = 357) | Total (N = 1,067) | |
|---|---|---|---|---|
| Age, M (SD) | 35.8 (11.6) | 35.3 (10.2) | 36.2 (10.5) | 35.8 (10.8) |
| Male, n (%) | 344 (97.2) | 346 (97.2) | 351 (98.3) | 1,041 (97.6) |
| Hispanic ethnicity, n (%) | 13 (3.7) | 3 (0.8) | 5 (1.4) | 21 (2.0) |
| Race/ethnicity, n (%) | ||||
| White | 340 (96.3) | 341 (95.8) | 339 (95.2) | 1,020 (95.8) |
| Black | 2 (0.6) | 3 (0.8) | 5 (1.4) | 10 (0.9) |
| Native American | 4 (1.1) | 1 (0.3) | 4 (1.1) | 9 (0.8) |
| Asian | 0 (0.0) | 3 (0.8) | 2 (0.6) | 5 (0.5) |
| Pacific Islander | 1 (0.3) | 0 (0.0) | 2 (0.6) | 3 (0.3) |
| More than 1 race/ethnicity | 6 (1.7) | 8 (2.2) | 4 (1.1) | 18 (1.7) |
| Education, n (%) | ||||
| Not high school graduate | 12 (3.4) | 8 (2.2) | 12 (3.4) | 32 (3.0) |
| High school graduate | 197 (55.6) | 178 (50.0) | 171 (47.9) | 546 (51.2) |
| College graduate | 128 (36.2) | 148 (41.6) | 143 (40.1) | 419 (39.3) |
| Postcollege graduate | 16 (4.5) | 20 (5.6) | 31 (8.7) | 67 (6.3) |
| Number of years of using ST, M (SD) | 14.8 (9.9) | 15.5 (10.2) | 15.4 (9.2) | 15.2 (9.8) |
| Tobacco dependence, M (SD)b | 11.2 (3.4) | 11.3 (3.5) | 11.6 (3.7) | 11.4 (3.5) |
| Current smoking, n (%) | 25 (7.1) | 23 (6.5) | 19 (5.3) | 67 (6.3) |
| ≥ 1 ST quit attempt in last year, n (%) | 198 (55.9) | 199 (55.9) | 213 (59.7) | 610 (57.5) |
| Five best friends use ST, M (SD) | 1.9 (1.7) | 2.0 (1.6) | 2.1 (1.5) | 2.0 (1.6) |
| Readiness to quit, M (SD)c | 8.4 (1.9) | 8.6 (1.7) | 8.5 (1.9) | 8.5 (1.8) |
| Confidence not using tobacco in 1 year, M (SD)d | 2.5 (0.9) | 2.5 (0.9) | 2.4 (1.0) | 2.5 (0.9) |
| Expect support from partner, n (%) | 211 (72.3) | 207 (70.4) | 226 (75.3) | 644 (60.4) |
| ≥ 13 drinks/week, n (%) | 44 (12.4) | 37 (10.4) | 55 (15.4) | 136 (12.7) |
ST = smokeless tobacco.
aParticipants could refuse to answer any question. Sample for all data was 1,067 except for those who expect support from partner, which was based on n = 886, who answered and indicated that they had a partner who knew about their tobacco use.
bBased on the Severson Smokeless Tobacco Dependency Scale with possible scores ranging from 0 to 19.
cBased on the Contemplation Ladder adapted for ST cessation that used an 11-point scale with 1 = not ready to quit, 3 = should consider quitting someday, 5 = should quit but not quite ready, 7 = thinking about cutting down or quitting, 9 = have cut down and seriously considering quitting, and 11 = ready to quit now.
dItem asked “How confident are you that you will not be using any tobacco a year from now?” and used a 5-point scale: 1 = not at all confident, 3 = somewhat confident, and 5 = completely confident.
Attrition
Of the 1,067 ST users randomized, 895 (84%) completed the 3-month follow-up assessment (see Consolidated Standards of Reporting Trials (CONSORT) diagram, Figure 1), 898 (84%) completed the 6-month follow-up assessment, and 851 (80%) completed both assessments. Completion of a follow-up assessment was lower for participants in the coach calls group than in the other two conditions (lozenge group and lozenge + coach calls group) at 3 months (p < .001), 6 months (p < .001), and both 3 and 6 months (p < .001). RPP assessment completion results were 72%, 82%, and 86% for the coach calls group, lozenge + coach calls group, and lozenge group, respectively.
Figure 1.
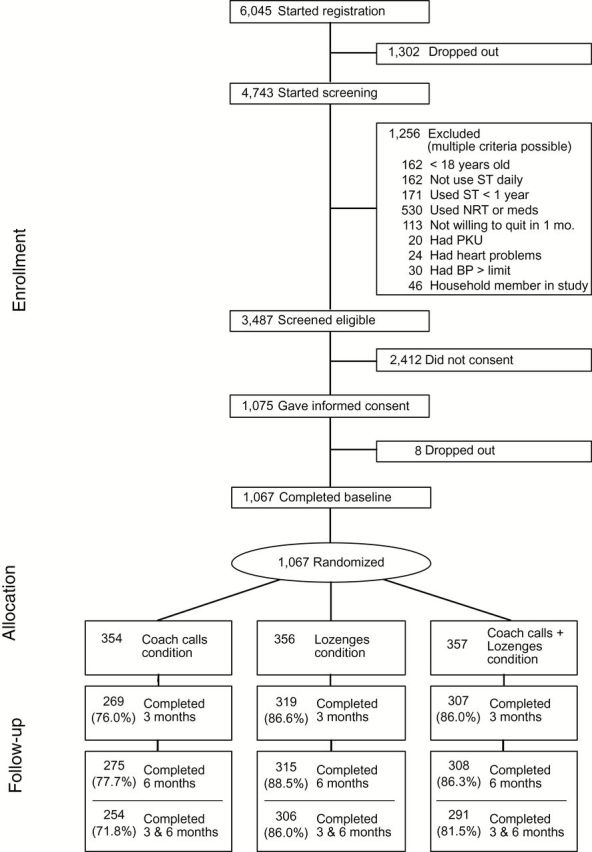
CONSORT diagram. BP = blood pressure; NRT = nicotine replacement therapy; ST = smokeless tobacco; PKU = phenylketonuria.
Primary Tobacco Outcomes
Seven-day all tobacco abstinence by conditions as well the results of the pairwise comparisons is presented in Table 2. The self-reported quit rates were quite high ranging from 37% to 57% at 3- and 6-month follow-up across all groups. For the ITT analysis, the lozenge + coach calls group was more likely to achieve tobacco abstinence than the coach calls group: at 3 months (odds ratio [OR] = 2.110; 95% confidence interval [CI] = 1.564, 2.846); 6 months (OR = 1.579; CI = 1.171, 2.128); and RPP (OR = 1.639; CI = 1.206, 2.227). Similarly, the lozenge + coach calls group significantly outperformed the lozenges condition: at 3 months (OR = 1.671; CI = 1.243, 2.247); 6 months (OR = 1.336; CI = 0.994, 1.795); and RPP (OR = 1.569; CI = 1.157, 2.129). No significant differences in abstinence were detected between the coach calls group and the lozenges group: at 3 months (OR = 1.263; CI = 0.937, 1.702); 6 months (OR = 1.182; CI = 0.875, 1.596); and RPP (OR = 1.044; CI = 0.762, 1.431). The results were similar for ST abstinence.
Table 2.
Self-Reported 7-Day Point Prevalence All Tobacco Abstinence Rates at Follow-Up
| Follow-up | Group | Pairwise comparisonsa | ||||
|---|---|---|---|---|---|---|
| CC (N = 354) | LZ (N = 356) | LZ + CC (N = 357) | LZ + CC versus CC | LZ + CC versus LZ | CC vs. LZ | |
| 3-month assessment | ||||||
| ITT, % | 39.3 | 44.9 | 57.7 | <.001 | .001 | .415 |
| Complete case, %, n | 51.7, 139/269 | 50.2, 160/319 | 67.1 206/307 | <.001 | <.001 | .795 |
| 6-Month assessment | ||||||
| ITT, % | 37.9 | 41.9 | 49.0 | .004 | .055 | .553 |
| Complete case, %, n | 48.7, 134/275 | 47.3, 149/315 | 56.8, 175/308 | .051 | .021 | .795 |
| RPP (3- and 6-month assessments) | ||||||
| ITT, % | 31.6 | 32.6 | 43.1 | .003 | .006 | .795 |
| Complete case, %, n | 44.1, 112/254 | 37.9 116/306 | 52.9 154/291 | .045 | .001 | .415 |
CC = coach calls group; ITT = intention-to-treat; LZ = lozenge group; LZ + CC = lozenge + coach calls group; RPP = repeated point prevalence.
Bold formatting indicates results reaching statistical significance.
a p values adjusted using the Benjamini and Hochberg false discovery rate control procedure. 17
Secondary Tobacco Outcomes
For continuing ST users, group differences in amount of ST use at 3 months were not detected. At 6 months, continuing ST users in the lozenge + coach calls group reported greater amounts of ST used than in the coach calls condition (β = −0.58; CI = −1.14, −0.01, t = −2.01, p < .05).
Predictors and Moderators of Tobacco Outcomes
Tobacco abstinence at 6 months was more likely to be reported by those who used less ST at baseline (β = −0.05; p = .010; OR = 0.95; CI = 0.91, 0.99), those with greater self-efficacy in their ability to quit (β = 0.22; p = .003; OR = 1.25; CI = 1.08, 1.44), and those experiencing less depressive symptoms (β = −0.03; p = .022; OR = 0.75; CI = 0.95, 0.99). No differential effects by condition were detected for these moderators. We assessed for differential abstinence rates for 67 participants (6.3% of sample) who reported using both ST and smoking (dual users), but there was no difference in the quit rates when compared with ST-only users.
Program Usability and Acceptability
Ninety-two percent of participants reported reading “some” or “all” of the guide and 63.7% reported watching the video. The amount of guide read did not vary by condition. The proportion of participants viewing the video varied by condition: coach calls group = 71%; lozenge + coach calls group = 68%; and lozenge group = 53% (χ2 [2, n = 890] = 23.39, p < .001). Neither reading the guide nor watching the DVD was significantly related to tobacco abstinence at 6 months.
Of the 711 participants with access to coaching calls, 14% (n = 103) did not have a single coach call (coach calls: 20%; lozenge + coach calls: 9%); 12% (n = 83) had one call; 14% (n = 97) had two calls; 60% (n = 428) had all three calls. The proportion of participants completing at least one counseling call in the lozenge + coach calls group (91%) was greater than in the coach calls group (80%): χ2 (1, n = 711) = 19.49, p < .001. Among participants who received at least one call, the average call duration was 30min (SD = 13.97). Call duration did not vary by condition. For completed cases, having at least one counseling call was significantly related to tobacco abstinence at 6 months, χ2 (1, n = 583) = 6.88, p = .009, OR = 0.46, 95% CI = 0.26, 0.83, with 55% of participants having coach calls and 36% of those not having a call achieving 6-month tobacco abstinence.
Forty-six percent of participants receiving nicotine lozenges used the lozenges on a “consistent basis.” This was defined as endorsing an assessment item indicating they used lozenges more than one half of the days and of each day more than one half of the day. Thirty-six percent requested a supplemental supply of lozenges. The proportion of participants requesting a supplemental supply of lozenge was greater in the lozenge + coach calls group (46%) than in the lozenge group (27%): χ2 (1, n = 713) = 27.79, p < .001. Consistent lozenge use did not differ by condition. Participants who requested supplemental lozenges were more likely to report tobacco abstinence at 6 months, χ2 (1, n = 623) = 16.39, p < .001, OR = 0.51, 95% CI = 0.37, 0.71). Participants who consistently used lozenges were more likely to report ST abstinence at 6 months, χ2 (1, n = 623) = 5.18, p = .022, OR = 0.69, 95% CI = 0.50, 0.95).
Seventy-one percent of the participants who responded (634 of 891) reported that the treatment program was very helpful or extremely helpful. These ratings significantly differed by treatment condition (F = 45.75, p < .001): 85% of the lozenge + coach calls group (M = 3.30, SD = 0.75); 72% of the lozenge group (M = 3.03; SD = 0.95), and 54% of the coach calls group (M = 2.57; SD = 1.05).
Participants who used/experienced the following intervention components reported that they were very helpful or extremely helpful: the quitting guide (51%), the quitting DVD (52%), counseling calls (66%), and lozenges (70%). These ratings did not differ by condition.
Very few participants reported moderate or severe symptoms associated with their lozenge use: 7.0% headache, 8.8% nausea, 6.8% flatulence, 12.0% hiccups, 18.7% heartburn, 11.8% sleep disturbance, and 3.1% diarrhea.
Discussion
We observed that combination treatment with mailed 4-mg nicotine lozenges and counseling telephone calls significantly increased tobacco abstinence rates among ST users compared with lozenges or coaching calls only, which were essentially equivalent. Almost half of the participants receiving lozenges reported using them consistently with few side effects and they found them very or extremely helpful for quitting. Most participants who were offered coach calls completed at least one call and rated them as very or extremely helpful for quitting.
Overall abstinence rates for all groups were relatively high and consistent with levels reported in other low-intensity ST cessation studies. 9,10,21,22 The abstinence rates achieved are substantially higher than the observed rates of telephone quitline counseling for smoking cessation (13.9% at ≥6 months).23Higher abstinence rates among ST users are consistent with those observed in other studies of ST users involving pharmacotherapy11,24 and other low-intensity self-help interventions9,10 and may relate to the fact that few treatments are available for ST users and that motivated ST users may generally be more responsive to intervention.
Unlike these previous studies of ST cessation, we were able to detect a significant treatment effect with combination treatment at 6 months. Our findings support the U.S. Public Health Service Guideline Grade “A” recommendation that the combination of counseling and medication are more effective for smoking cessation than either medication or counseling alone. Although the guidelines have not provided specific recommendations for ST cessation, the results of this study would support a recommendation for combining counseling with nicotine lozenges as being more efficacious than either alone. Our study advances the science on the effectiveness of combination behavioral and pharmacological treatment for ST users and it informs current tobacco control efforts and future guidelines. We do not know why the combination of lozenges and coach calls were more effective, but it may be that the lozenges helped participants deal with withdrawal and the coach calls provided social support and this combination was more efficacious than either alone.
Our results are impressive in light of the fact that many participants did not, in fact, receive the phone coach calls, or report using the lozenges or make any request for additional medications (ITT 3- and 6-month cessation ranged from 32% to 43%). However, participants who received calls or reported greater use of the lozenges did achieve greater abstinence at 6-months follow-up, as were participants with baseline reports of lower ST use, greater self-efficacy, and fewer depressive symptoms.
Although previous investigators have argued that biochemical validation is sometimes not feasible and does not alter the conclusions of low-intensity intervention trials,25 we acknowledge that our results may overestimate true abstinence rates in our study population. However, we would not expect differential misreporting by study condition such that our conclusions would be altered.
Because we recruited a large number of participants from across the country (N = 1,067), our study may be considered an effectiveness trial and our results may generalize to existing real-world tobacco cessation services such as tobacco quitlines.26 Both phone coaching and mailed NRT would fit quitline service delivery procedures.
Funding
This work was supported by the National Cancer Institute of the National Institutes of Health (R01-CA142952).
Declaration of Interests
JOE has received personal fees from GlaxoSmithKline and has received research support from Pfizer, Orexigen, and JHP outside of this study.
GlaxoSmithKline provided the nicotine lozenges for the study, but it had no role in the conduct of the study (data collection, management, analysis, and interpretation) or in the preparation, review, approval of the manuscript, and decision to submit the manuscript for publication.
Acknowledgments
The authors wish to express their appreciation to InterVision (Eugene, OR) for their expertise in developing the technology for managing the research project screening and subsequent data, and to K. Danaher (Eugene, OR) for managing our Google AdWords analytics and online marketing campaign. We also acknowledge the contribution of our other research team members who contributed to this project: K. Newman, K. Clawson, and our phone coaches: N. Van Meter, M. S. Tyler, K. Newman, L. Marion, D. Blanchard, and Z. Brady. We also thank Google for its generous GoogleGrants subsidy to Oregon Research Institute for our AdWords campaign.
References
- 1. Lee PN, Hamling J. Systematic review of the relation between smokeless tobacco and cancer in Europe and North America. BMC Med. 2009;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Toxicology Program. 12th Report on Carcinogens (RoC). http://ntp.niehs.nih.gov/?objectid=03C9AF75-E1BF-FF40-DBA9EC0928DF8B15 Accessed September 24, 2013.
- 3. USDHHS. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2012. [Google Scholar]
- 4. Gupta R, Gurm H, Bartholomew JR. Smokeless tobacco and cardiovascular risk. Arch Intern Med. 2004;164:1845–1849. [DOI] [PubMed] [Google Scholar]
- 5. Henley SJ, Thun MJ, Connell C, Calle EE. Two large prospective studies of mortality among men who use snuff or chewing tobacco (United States). Cancer Causes Control. 2005;16:347–358. [DOI] [PubMed] [Google Scholar]
- 6. Winn DM. Epidemiology of cancer and other systemic effects associated with the use of smokeless tobacco. Adv Dent Res. 1997;11:313–321. [DOI] [PubMed] [Google Scholar]
- 7. Delnevo CD, Wackowski OA, Giovenco DP, Manderski MT, Hrywna M, Ling PM. Examining market trends in the United States smokeless tobacco use: 2005–2011. Tob Control. 2012;23:107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. FTC. Federal Trade Commission Smokeless Tobacco Report for the Years 2009 and 2010. http://www.ftc.gov/os/2012/09/120921tobaccoreport.pdf Accessed September 24, 2013.
- 9. Severson HH, Andrews JA, Lichtenstein E, Danaher BG, Akers L. Self-help cessation programs for smokeless tobacco users: long-term follow-up of a randomized trial. Nicotine Tob Res. 2007;9:281–289. [DOI] [PubMed] [Google Scholar]
- 10. Severson HH, Andrews JA, Lichtenstein E, et al. A self-help cessation program for smokeless tobacco users: comparison of two interventions. Nicotine Tob Res. 2000;2:363–370. [DOI] [PubMed] [Google Scholar]
- 11. Ebbert JO, Severson HH, Croghan IT, Danaher BG, Schroeder DR. A pilot study of mailed nicotine lozenges with assisted self-help for the treatment of smokeless tobacco users. Addict Behav. 2010;35:522–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Danaher BG, Severson HH, Andrews JA, et al. Randomized controlled trial of MyLastDip: a Web-based smokeless tobacco cessation program for chewers ages 14–25. Nicotine Tob Res. 2013;15:1502–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Severson HH, Gordon JS. Enough Snuff: A Guide for Quitting Smokeless Tobacco. 8th ed. Eugene, OR: Applied Behavior Science Press; 2008. [Google Scholar]
- 14. Severson HH, Akers L, Christiansen S.Enough Snuff: A Video Program to Help You Quit Spit Tobacco. Eugene, OR: Applied Behavior Science Press; 2011. [Google Scholar]
- 15. Ebbert JO, Severson HH, Danaher BG, Schroeder DR, Glover ED. A comparison of three smokeless tobacco dependence measures. Addict Behav. 2012;37:1271–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Biener L, Abrams DB. The contemplation ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991;10:360–365. [DOI] [PubMed] [Google Scholar]
- 17. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B. 1995;57:289–300. [Google Scholar]
- 18. Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–173. [DOI] [PubMed] [Google Scholar]
- 19. Smolkowski K, Danaher BG, Seeley JR, Kosty DB, Severson HH. Modeling missing binary outcome data in a successful Web-based smokeless tobacco cessation program. Addiction. 2010;105:1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Graham AL, Cobb NK, Papandonatos GD, et al. A randomized trial of Internet and telephone treatment for smoking cessation. Arch Intern Med. 2011;171:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Severson HH, Gordon JS, Danaher BG, Akers L. ChewFree.com: evaluation of a Web-based cessation program for smokeless tobacco users. Nicotine Tob Res. 2008;10:381–391. [DOI] [PubMed] [Google Scholar]
- 22. Ebbert JO, Severson HH, Croghan IT, Danaher BG, Schroeder DR. A randomized clinical trial of nicotine lozenge for smokeless tobacco use. Nicotine Tob Res. 2009;11:1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- 24. Dale LC, Ebbert JO, Glover ED, et al. Bupropion SR for the treatment of smokeless tobacco use. Drug Alcohol Depend. 2007;90:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Glasgow RE, Mullooly JP, Vogt TM, et al. Biochemical validation of smoking status: pros, cons, and data from four low-intensity intervention trials. Addict Behav. 1993;18:511–527. [DOI] [PubMed] [Google Scholar]
- 26. Boyle RG, Enstad C, Asche SE, et al. A randomized controlled trial of telephone counseling with smokeless tobacco users: the ChewFree Minnesota study. Nicotine Tob Res. 2008;10:1433–1440. [DOI] [PubMed] [Google Scholar]


