Abstract
Introduction:
Some electronic cigarette (ECIG) users attain tobacco cigarette–like plasma nicotine concentrations while others do not. Understanding the factors that influence ECIG aerosol nicotine delivery is relevant to regulation, including product labeling and abuse liability. These factors may include user puff topography, ECIG liquid composition, and ECIG design features. This study addresses how these factors can influence ECIG nicotine yield.
Methods:
Aerosols were machine generated with 1 type of ECIG cartridge (V4L CoolCart) using 5 distinct puff profiles representing a tobacco cigarette smoker (2-s puff duration, 33-ml/s puff velocity), a slow average ECIG user (4 s, 17 ml/s), a fast average user (4 s, 33 ml/s), a slow extreme user (8 s, 17 ml/s), and a fast extreme user (8 s, 33 ml/s). Output voltage (3.3–5.2 V or 3.0–7.5 W) and e-liquid nicotine concentration (18–36 mg/ml labeled concentration) were varied. A theoretical model was also developed to simulate the ECIG aerosol production process and to provide insight into the empirical observations.
Results:
Nicotine yields from 15 puffs varied by more than 50-fold across conditions. Experienced ECIG user profiles (longer puffs) resulted in higher nicotine yields relative to the tobacco smoker (shorter puffs). Puff velocity had no effect on nicotine yield. Higher nicotine concentration and higher voltages resulted in higher nicotine yields. These results were predicted well by the theoretical model (R 2 = 0.99).
Conclusions:
Depending on puff conditions and product features, 15 puffs from an ECIG can provide far less or far more nicotine than a single tobacco cigarette. ECIG emissions can be predicted using physical principles, with knowledge of puff topography and a few ECIG device design parameters.
Introduction
Awareness and use of electronic cigarettes (ECIGs) is growing worldwide, as indexed by Internet searches1 and nationally representative surveys.2,3 Although ECIG designs vary widely, their common defining feature is an electrically powered heating element that vaporizes a liquid that usually contains nicotine. Ostensibly free of many of the toxicants associated with tobacco combustion, the resulting vapors condense to form an aerosol that is inhaled by the user through the mouth end of the device. Though the mixture exiting the ECIG mouthpiece is commonly referred to as a “vapor,” it is more correctly termed an “aerosol mist”—a system of liquid droplets suspended in a gas or gas mixture.4
The apparent popularity of these products likely is attributable to a variety of factors, including marketing,5 their availability in appealing flavors,6,7 perceptions that they are less lethal than tobacco cigarettes,8 and their ability to deliver nicotine,9,10 a psychomotor stimulant that supports dependence.11 Although some commentators speculate that ECIGs promise reduced tobacco-caused disease and death in cigarette smokers,12,13 others speculate that daily, long-term use of these novel products imperils users with adverse health consequences, including nicotine dependence in individuals who were not nicotine-experienced prior to their ECIG use.14
Critical to this debate is understanding nicotine delivery to ECIG users. Studies have reported mixed results with regard to the ability of ECIGs to deliver nicotine systemically. ECIG-naive cigarette smokers were found to attain negligible levels of plasma nicotine when using ECIGs,15–17 whereas experienced ECIG users were able to achieve plasma nicotine concentrations approaching those attained by tobacco smokers.10 Because plasma nicotine levels are related to the amount of nicotine inhaled, understanding the factors that influence mainstream nicotine emissions from ECIGs is important for understanding nicotine delivery, and ultimately, for addressing questions of safety and effectiveness.
One factor that likely influences ECIG nicotine and other toxicant emissions is the puffing behavior of the user. The study of “puff topography” parameters (e.g., puff volume, puff velocity, interpuff interval) has long been important in understanding the toxicant content of tobacco smoke18–24 and the toxicant delivery to tobacco smokers.11,25 With ECIGs, differences in topography may help explain the fact that sometimes these products deliver nicotine and sometimes they do not.9,10,16 Indeed, two studies have used observational methods to examine ECIG user puff topography, and both suggest that experienced ECIG users take longer puffs (e.g., approximately 4 s, on average) than tobacco cigarette smokers, approximately 2 s, on average.26,27 In extreme cases, puff durations as long as 8 s have been observed.27 Although puff duration is important, other parameters are also relevant, including puff velocity throughout the puff. Taken together with duration, puff velocity determines puff volume that predicts nicotine delivery in cigarette smokers.24 The average puff velocity of tobacco cigarette smokers is generally 29–38ml/s18,28,29 though experienced ECIG users may draw lower puff velocities.30 To date, no study has examined the combined influence of puff duration and velocity on nicotine yield in ECIG aerosol.
Another factor that likely influences ECIG toxicant emissions involves device operating and design characteristics. ECIGs vary considerably in terms of power source voltage, heating element resistance, and other design features. For example, so-called variable voltage devices allow the user to control the power input, with marketed products ranging from 2.9 to 6.0 V (e.g., http://www.provape.com/provari-variable-voltage-ecig-s/36.htm#). The electrical power input—which is proportional to the square of the voltage and inversely proportional to the heater resistance—influences the temperature at which the aerosol is produced, and this in turn may influence nicotine and other toxicant emissions. Few studies have looked at the effect of varying puff topography and/or device output voltage on toxicant emissions. In one recent study, carbonyl compounds measured in ECIG aerosol from 13 different nicotine solutions at 3.2 and 4.8V were compared: solvent and output voltage significantly affect the amount of carbonyls in the aerosol.31 Prior studies have also explored the toxicant emissions produced from different brands of ECIGs but few have varied voltage systematically (see Kosmider et al31), and none have explored the influence of user topography. Goniewicz et al12 measured the nicotine yield in aerosols generated from 16 different ECIG brands using an average puffing condition based on topography measurements of 10 ECIG users (1.8-s puff duration, 70-ml puff volume) and found that the nicotine yield in 15 puffs ranged from 0.025 to 0.77mg, which is lower than the dose inhaled in one conventional cigarette.32 Using a 2-s puff duration, 100-ml/puff protocol, Trehy et al33 investigated three different brands of ECIGs and reported nicotine yields ranging from 0 to 43.2 μg of nicotine per 100ml of puff.
The purpose of this study was to examine the influence of user puff topography (duration and puff velocity) and device power source voltage on nicotine yield, and to demonstrate the feasibility of predicting the effects of these variables by modeling the underlying physical phenomena mathematically. Because liquids of varying nicotine concentrations are available, we also included an examination of this factor. Taken together, these data will be useful for informing test methods for regulatory action, and for developing a framework in which physical principles can be invoked to guide empirical investigation of ECIG performance and thereby facilitate evaluation of products in this rapidly evolving product category.
The ECIG Aerosol Production Process
A common ECIG configuration comprises an electrical heating element (a.k.a., “atomizer”) combined with a cartridge that contains nicotine liquid. This heater/cartridge combination is called a “cartomizer.” A cartomizer (Supplementary Figure S1) typically contains a metallic electrical heating coil that is wound around a central wick; the coil is activated either by pressing a button or by an automatic puff sensor. The wick is saturated with the so-called “juice” or “e-liquid,” which is typically composed of a solution of propylene glycol (PG), vegetable glycerin (VG), flavorants, and nicotine. Nicotine concentrations in commercially available products usually range from 0 to 36mg/ml. A textile sheath envelops the coil–wick assembly. The sheath in turn is surrounded by a fibrous wool-like material that is soaked in liquid and that serves as a reservoir for the wick. The wool, sheath, heating coil, and wick are all packaged in a cylindrical metal case with dimensions similar to those of a cigarette filter.
When a user draws a puff, air is drawn into the cartomizer through the bottom of the assembly. The air passes over the heated coil and carries away the e-liquid vapors as well as thermal energy from the coil–wick assembly. As the hot, vapor-laden air continues traveling through the cartridge beyond the heater assembly across a transfer tube, it cools and vapors begin to condense to form liquid droplets, likely with a diameter in the 120–165-nm range.34 As with tobacco smoke, the condensed droplets scatter light and thereby render the aerosol plume visible.
A battery usually powers the heater within the cartomizer, typically via an electric circuit that regulates the output voltage, allows recharging, and allows electrical current to flow to the cartomizer during a puff. ECIG batteries are available in a wide variety of energy storage and current draw capacities. Similarly, ECIG heater coils are available in a range of resistances and geometries. Combined with the fact that puff topography varies widely across individuals and devices, ECIG aerosols can be produced under a very wide range of conditions. These variations may result in nicotine yields that are far greater or far less than those of a typical cigarette.
Materials and methods
ECIG Cartridges
Forty-six V4L CoolCart cartomizers, of which 34 were labeled as having a nicotine concentration of 18mg/ml and 12 of 36mg/ml, were procured from an Internet vendor in the United States. The resistance of these cartomizers was measured using a standard laboratory Ohmmeter and found to be 3.6±0.16 Ω (M ± SD) at 22 °C. Four of the above cartridges exhibited erratic resistances indicating a faulty internal electrical connection and were therefore excluded from the study. Liquid from three randomly selected V4L CoolCart cartridges, for each of the two nicotine concentration mentioned previously, were extracted and analyzed for nicotine and found to be 8.53±0.71 and 15.73±1.21mg/ml, respectively.
Aerosol Generation and Sampling
A custom-designed digital puff production machine at the American University of Beirut was used to generate ECIG aerosol. Puff durations were chosen to approximate that of a typical cigarette smoker (2 s),6,27 an experienced ECIG user taking an average length puff (4 s),6,27 and an experienced ECIG user taking a puff of the more extreme length observed (8 s).27 Puff velocity was chosen to approximate that observed in tobacco cigarette smokers (33ml/s),18,28,29 but because experienced ECIG users may puff more slowly,30 we also examined the effects of a lower velocity (17ml/s). As Table 1 shows, puff duration and velocity were combined to yield five distinct puff profiles representing a tobacco cigarette smoker (2 s, 33ml/s), an average ECIG user using low flow (4 s, 17ml/s), an average ECIG user using a high flow (4 s, 17ml/s), an extreme ECIG user using low flow (8 s, 17ml/s), and an extreme ECIG user using a high flow (8 s, 33ml/s). Each of these five combinations was tested at voltages representing lower (3.3V) and higher (5.2V) voltage devices available on the US market, resulting in 3.0- and 7.5-W electrical power input, respectively. All conditions described were tested using a medium strength e-liquid nicotine concentration (labeled as 18mg/ml). To examine the effect of nicotine concentration in the e-liquid, two of the above profiles (average and extreme) were repeated at a high nicotine level (labeled as 36mg/ml).
Table 1.
Measured Total Particulate Matter and Nicotine Yields in 15 ECIG Puffs (M ± SD) for Several User Puffing Profiles
| Profile | Puff duration (s) | Puff velocity (ml/s) | Puff volume (ml) | Voltage (V) | Measured nicotine concentration (mg/ml) | TPM (mg) | Nicotine yield (mg) | Nicotine flux (μg/s) |
|---|---|---|---|---|---|---|---|---|
| Tobacco cigarette smoker | 2 | 33 | 66 | 3.3 | 8.53 | 9.07±2.3 | 0.11±0.02 | 3.8±0.69 |
| Average experienced ECIG (slow) | 4 | 17 | 68 | 3.3 | 8.53 | 29.4±0.9 | 0.30±0.01 | 4.9±0.13 |
| Average experienced ECIG (fast) | 4 | 33 | 132 | 3.3 | 8.53 | 29.6±6.4 | 0.29±0.08 | 4.9±1.3 |
| Extreme experienced ECIG (slow) | 8 | 17 | 136 | 3.3 | 8.53 | 70.5±13.0 | 0.72±0.10 | 6.0±0.80 |
| Extreme experienced ECIG (fast) | 8 | 33 | 264 | 3.3 | 8.53 | 68.8±6.7 | 0.68±0.07 | 5.6±0.61 |
| Tobacco cigarette smoker | 2 | 33 | 66 | 5.2 | 8.53 | 64.9±9.8 | 0.64±0.10 | 21±3.2 |
| Average experienced ECIG (slow) | 4 | 17 | 68 | 5.2 | 8.53 | 128.3±23.1 | 1.18±0.28 | 20. ± 4.7 |
| Average experienced ECIG (fast) | 4 | 33 | 132 | 5.2 | 8.53 | 152.7±13.6 | 1.50±0.07 | 25±1.1 |
| Extreme experienced ECIG (slow) | 8 | 17 | 136 | 5.2 | 8.53 | 312.6±32.9 | 3.23±0.34 | 27±2.9 |
| Extreme experienced ECIG (fast) | 8 | 33 | 264 | 5.2 | 8.53 | 333.2±34.0 | 3.09±0.19 | 26±1.5 |
| Average experienced ECIG (slow) | 4 | 17 | 68 | 3.3 | 15.73 | 32.7±7.4 | 0.48±0.13 | 8.0±2.1 |
| Extreme experienced ECIG (slow) | 8 | 17 | 136 | 5.2 | 15.73 | 314.0±29.4 | 4.70±1.00 | 39±7.0 |
ECIG = electronic cigarette; TPM = total particulate matter.
The profiles represent a typical tobacco cigarette smoker and experienced ECIG users using 4 s (“average”) or 8 s (“extreme”) puff durations and slow (17ml/s) and fast (33ml/s). Each profile was tested under two voltage conditions (3.3 and 5.2V). All conditions were tested using an 8.53-mg/ml nicotine concentration liquid. The average and extreme (slow) conditions were also tested using a 15.73-mg/ml nicotine concentration liquid.
For each experimental condition, three sets of samples were generated for nicotine determinations. The conditions tested for the high nicotine concentration were generated from six sets of samples. All profiles tested at the lower voltage setting (3.3V and 3.0W), and both voltage settings for the tobacco smoker profile were generated by drawing 15 puffs from three different randomly selected cartomizers. However, to avoid overloading the filter pad (described in Methods), the profiles tested at the remaining conditions were generated by drawing five puffs; the results were thereafter multiplied by 3 for consistency.
For each experiment, the mouth end of the ECIG cartridge was connected by a 5-cm long Tygon® tube (ID) to a polycarbonate filter holder that contained a Gelman type A/E 47-mm glass fiber filter. The filter holder terminated in another 5-cm long Tygon® tube (ID). In preliminary experiments, we found that losses in the tubing connecting the ECIG cartridge to the filter pad were negligible.
For repeatability, the ECIG cartridge voltage was controlled using a regulated laboratory DC power supply (0.01-V resolution). We note that although regulated ECIG battery units are commonly used, many ECIG battery units do not employ voltage regulators and therefore allow the supplied voltage to decay as the battery is drained. This study did not attempt to investigate effects of battery drain with unregulated voltage ECIG designs.
Chemical Analysis
Total particulate matter (TPM) was determined gravimetrically by weighing the filter pad and holder before and after each sampling session. Nicotine was determined by sonicating the filter pad in 6ml of ethyl acetate for 30min at ambient temperature and analyzing an aliquot of the resulting solution by gas chromatography–mass spectrometry. An extracted calibration curve with concentrations ranging from 1 to 20 ppm was used to interpret the resulting chromatograms. Spiked filter assays of nicotine in PG solution showed recoveries of 90%.
Mathematical Modeling and Numerical Simulation
A mathematical model was developed to simulate the ECIG vaporization process under various conditions. The modeling effort was focused on determining the evaporated mass of nicotine and liquid in the vicinity of the cartomizer heating element. Because a fraction of these aerosols likely recondense on the internal surfaces of the cartomizer and therefore do not exit the mouthpiece, the evaporated mass represents a theoretical upper limit, or “potential mass” emitted from a given puffing session. Nonetheless, the potential nicotine mass can be a useful metric for regulators who need to understand how puffing behavior, ECIG liquid composition, and ECIG design parameters interact, and to predict the maximum amount of nicotine that theoretically could be obtained from a given ECIG design/puff topography/liquid composition combination.
Starting from first principles of energy and mass conservation, the relevant heat and mass transfer rate equations, ideal solution/ideal gas equations, and boundary layer approximations for the flow field in the vicinity of the ECIG heating element, we computed the transient temperature, evaporation rate, and nicotine concentration in the aerosol produced during each puff, accounting for cartomizer cooling during the interpuff intervals by natural convection to the surrounding air.
Other than puff topography (puff duration, velocity, interpuff interval, number of puffs) and electrical power input, the model requires specification of air flow tube geometry, heater element dimensions (diameter and length) and mass, and the geometric properties of the components of the atomizer (air inlet diameter of the atomizer and distance of inlet from heater coil), all of which were obtained readily by reverse engineering the V4L cartomizer. Thermodynamic and transport kinetic properties of air, PG, VG, and nicotine were taken from literature and are given in the online supplementary materials (Supplementary Table S1). The composition of the V4L liquid vehicle was assumed to be 80/20 PG/VG, in accordance with the manufacturer’s specifications. The nicotine concentration values input to the mathematical model were assumed to be equal to those measured by analysis of the cartomizer liquids.
The model results in a series of coupled differential equations, which are solved numerically in the Matlab® computing environment using a time-explicit algorithm, in increments of 0.01ms. Results were checked for independence of time increment.
Statistical Methods
Student’s t-test and analysis of variance were used for comparisons between TPM and nicotine values obtained by varying user puff topography (velocity and puff duration), ECIG design features (voltage), and liquid content (nicotine concentration).
Results
Measured Yields
TPM and nicotine yields (M ± SD) generated from the five ECIG user profiles are provided in Table 1. TPM yield ranged by more than 30-fold, whereas nicotine yield ranged by more than 50-fold across conditions. Voltage and puff duration had a significant impact on TPM and nicotine yield under all conditions, nicotine concentration had no significant effect on TPM, but had an effect on nicotine yield, whereas puff velocity had no effect under any condition, (p values obtained by varying the puff velocity, for the average experienced smoker at low and high voltages, are less than 0.97 and 0.12, respectively; for the case of the extreme experienced smoker, p < .58 at low voltage and .56, at high voltage).
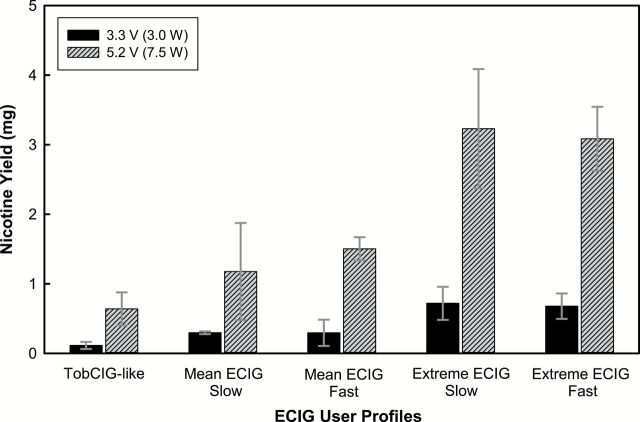
Figure 1 shows the effect of varying puff profile and device voltage on nicotine yield. Increasing the puff duration resulted in systematically higher nicotine delivery. Increasing the voltage resulted in higher nicotine yields across all conditions (p < .05). Increasing the e-liquid nicotine strength, from 8.53 to 15.73mg/ml, did not have an effect on TPM (p < .34, average experienced user and p < .95, extreme experienced user), but resulted in higher nicotine delivery for both average and extreme users (p < .05).
Figure 1.
Effect of user profile and device voltage on aerosol nicotine yield from 15 puffs. The profiles represent a typical tobacco cigarette smoker (2-s puff duration, 33-ml/s puff velocity) and experienced ECIG users with 4-s (average) or 8-s (extreme) puff durations and slow (17ml/s) or fast (33 ml/s) puff velocities. Error bars indicate 95% confidence intervals. ECIG = electronic cigarette.
Mathematical Model
The mathematical model was used to predict potential nicotine mass emissions for the 12 experimental conditions listed in Table 1. The computed potential nicotine and the measured nicotine yield were strongly correlated, resulting in R 2 = 0.99, with a slope of 0.42 (Supplementary Figure S2).
Discussion
The primary purpose of this study was to explore systematically the influence of puff topography, ECIG device design, and liquid nicotine content on nicotine yield of the resulting ECIG aerosol, while also developing a mathematical model that would predict how other configurations of these variables might influence nicotine yield. Clearly, puff velocity does not influence nicotine yield, while puff duration, device voltage, and liquid nicotine concentration do. Moreover, the influence of these variables can be modeled effectively. Determining which factors do and do not influence the nicotine and other toxicant yield of existing ECIGs helps to understand ECIG user behavior and the ECIG marketplace today, and has clear regulatory implications for the future. Perhaps more important, the mathematical model presented here could, with further refinement, help predict the nicotine yield of ECIG designs that may not yet exist now but might in the future. Each of these issues is discussed below.
The observation that puff topography influences ECIG aerosol nicotine yield is relevant to understanding ECIG use as well as methods for regulating the nicotine intake of ECIG users. In terms of understanding ECIG use, previous reports suggest that experienced ECIG users take longer duration puffs than do cigarette smokers smoking cigarettes27 or using ECIGs for the first time.6 The current results help to explain these findings, as longer puffs lead to greater nicotine yield in ECIG aerosol. The fact that puff velocity does not influence nicotine yield may explain why ECIG user puff topography is associated with velocities that are less than those of cigarette smokers.30 That is, although a high-velocity puff increases combustion rate and therefore increases the rate at which nicotine is converted from the leaf to the smoke in a tobacco cigarette, it does not increase the rate at which nicotine is emitted from an ECIG. Experienced ECIG users may have learned with practice that the greater effort associated with higher velocity does not influence nicotine-mediated subjective effects. They, therefore, no longer expend the energy to produce the high-velocity puffs observed in cigarette smokers.
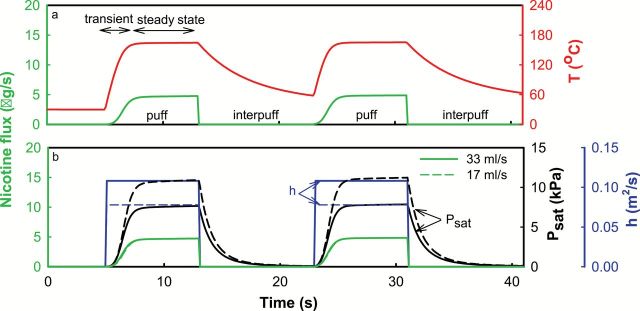
The influences of puff duration and velocity on nicotine yield can be understood by examining the structure of an ECIG puff, computed using our theoretical model. Figure 2 illustrates the computed V4L cartomizer heater coil temperature, mass transfer coefficient, and nicotine saturation vapor pressure (P sat) during two consecutive puffs, at two different puff velocities. As shown, when a puff commences and the heater coil is activated, the coil temperature (panel a) increases for some time (the “transient” phase), until it attains a steady-state temperature, at which time the electrical power input is balanced exactly by the thermal energy transferred out of the heater. Thus, increasing puff duration results in a larger proportion of the puffing time spent in the relatively high-temperature steady-state phase. Higher temperatures, in turn, result in a higher nicotine evaporation rate (panel b), due mainly to the higher P sat (panel b). This picture is corroborated by the measured nicotine emitted per puff second, the “nicotine flux.” In Table 1, it can be seen that longer puff durations lead to higher nicotine fluxes when all else is held constant. Therefore, longer puff durations result in greater nicotine yields and greater fluxes. The same will be true for yields of other volatile constituents of the liquid.
Figure 2.
Electronic cigarette (ECIG) temperature (T), nicotine flux, nicotine saturation pressure (P sat), and mass transfer coefficient (h) during and between puffs, as predicted by the mathematical model (condition shown for two 8-s puffs). Panel a illustrates the transient nature of the temperature during a puff. Panel b illustrates the effect of puff velocity on the computed variables.
The effect of puff velocity on nicotine yield is more complex and requires recognition of the fact that nicotine evaporation rate is proportional to the product of P sat and the mass transfer coefficient, h. The latter describes the ability of the flowing air to scavenge nicotine vapor from the heater surface, and this ability increases with puff velocity. Although h increases with puff velocity, heater temperature decreases with puff velocity, resulting in lower P sat. In the relevant flow regimes characteristic of ECIGs, it turns out that the increased h is offset almost exactly by the decreased P sat, resulting in a null effect of puff velocity on nicotine evaporation rate (panel b), and therefore a null effect on nicotine yield. Although nicotine yield is not affected by puff velocity, we caution that the same may not be true for other toxicants (e.g., formaldehyde) that form through temperature-dependent chemical reactions in the heater.
In terms of regulation, nicotine yield, in addition to other variables such as nicotine delivery, liquid flavor, and aerosol production, may all be key features that determine the effects of ECIGs in tobacco cigarette smokers as well as in tobacco-naive individuals. This report is the first to address nicotine yield in a controlled and systematic manner; to our knowledge, controlled, systematic exploration of the importance of delivery, flavor, and aerosol production in ECIG effects has not yet been reported. In the absence of that information, we suggest here that controlling the nicotine yield in ECIG aerosol may be critical to limiting the likelihood that ECIGs are used by individuals who are not current tobacco cigarette users (e.g., tobacco-naive youth, tobacco-free former smokers). For these individuals, the availability of a device that provides a cigarette-like dose of nicotine easily may increase the chances of their becoming nicotine dependent when they otherwise would not. The current results suggest regulatory action that might limit this possibility: ECIGs might contain electronics that do not allow puffs that exceed a certain duration, and that require a pre-set interval of time to elapse between puffs so that the duration limit could not be easily overcome with a series of puffs that are performed rapidly in succession. Future study will help determine the range of duration values (in combination with other ECIG-specific factors) that might help limit abuse liability; this study suggests that puff duration, and not puff velocity, is a variable that could be regulated to limit ECIG aerosol nicotine yield. Further study is also necessary to relate nicotine yield in ECIG aerosol to nicotine delivery to the user, as indexed by plasma nicotine concentration, and this relationship may be critical in guiding ECIG regulation empirically.
The observation that device design characteristics (in this case, voltage) and the nicotine concentration of ECIG liquids influence ECIG aerosol nicotine yield also is relevant to understanding ECIG use as well as informing regulation. With respect to use, many experienced ECIG users report that they initiated ECIG use with so-called “cigalikes”: disposable ECIGs that are similar in appearance to a tobacco cigarette.35 These experienced users subsequently switched to a nondisposable product that, among other features, is often equipped with a higher voltage power source.35 This transition may reflect the failure of “cigalikes” to deliver nicotine in doses that resemble those delivered by a tobacco cigarette.17,36 Indeed, the availability of ECIG devices that allow the user to manipulate the voltage delivered to the heating element may indicate that users have learned that nicotine-induced effects are mediated by this device feature. Similarly, the availability of liquids with a wide range of nicotine concentrations (0–36mg/ml) suggests that ECIG users may be interested in manipulating nicotine intake. Regulators should be aware that ECIG aerosol nicotine yield is likely a function of electrical power, which in turn is a function of device voltage and resistance in addition to liquid nicotine concentration. Further study is necessary to determine how these and other variables interact to influence ECIG acceptability, abuse liability, and toxicity.
The mathematical model is perhaps the most important outcome of this study. This model demonstrates that the influence of product design and use characteristics on nicotine yield can be predicted remarkably well, as evidenced by the high correlation between measured yields and computed potential mass (Supplementary Figure S2) over a wide range of conditions. Mathematical modeling can thus provide an additional tool for ECIG product regulation, and can be used to help identify rapidly any potentially problematic products or product combinations currently on the market, as well as those proposed in the future.
This study has some important limitations. First, it was conducted using one ECIG model; other models and brands may use different design features that may alter the emissions. Moreover, this study did not examine the effect of varying the e-liquid PG/VG ratio; prior studies have shown that manipulating the ratio affects the nicotine and carbonyl yields.31,37 However, our main intention was not to investigate performance variation across products, but rather to illustrate the wide range of possible nicotine yields attainable even from a relatively constrained basis set. Our results show that even for a single ECIG brand, a single PG/VG ratio, and only five different user profiles, a 50-fold change in nicotine delivery is possible; a span that ranges from negligible amounts to several cigarettes worth. If a larger number of brands and products are examined, the span can only widen. From a regulatory perspective, this finding highlights the need for developing a robust mathematical model that reliably can predict nicotine yield for any circumstance.
A second limitation of this study is that we did not measure toxicant emissions other than nicotine. We, therefore, caution against extrapolating the current results to other toxicants. For example, although puff velocity did not influence nicotine yields, it did affect the predicted heater coil temperature, and therefore may influence in situ toxicant formation reactions and resulting yields (e.g., carbonyls). More research is needed to characterize effects of puff topography and device features on non-nicotine toxicant emissions.
Third, we did not vary the nicotine concentration systematically. For the two cases examined, we found that increasing the nicotine concentration increased nicotine yield, as predicted by the theoretical model (Figure S2). Theoretically, for the highly dilute nicotine concentration conditions relevant to ECIG liquids, nicotine yield will always be directly proportional to the nicotine concentration, all else being equal; in this study, we found that increasing the nicotine concentration by a factor of 1.8 resulted in an increase in yield of 1.5±0.5 (mean ± 95% confidence interval). On the other hand, we also note that by manipulating puff duration and/or battery voltage, a user can obtain a nicotine yield in 15 puffs similar to that obtained from a conventional cigarette, for either nicotine concentration tested in this study. Indeed, in a study that did not control for these factors, nicotine concentration had little relation to nicotine yield.38 This observation highlights the importance of accounting for the overlapping influences of the many factors underlying nicotine yield in ECIG aerosol when measures are taken to minimize abuse liability and potential toxicity.
Conclusions
Previous reports on ECIG nicotine delivery to blood have mixed results. Some reports suggest that ECIGs deliver a considerable amount of nicotine,10 whereas other reports do not.15,16,17 It has been hypothesized that these mixed results derived from variations in user experience and device type,10,39 factors which, as this study has shown, likely affect the amount of nicotine obtained from the mouth end of an ECIG. Depending on user puff topography and operating conditions, we have found that a given ECIG product can provide far less or far more nicotine than a single combustible cigarette. Experienced ECIG users may extract higher nicotine doses by drawing relatively low velocity, long duration puffs in comparison to conventional tobacco cigarette smokers. ECIG design features also affect nicotine yield; increasing the battery voltage output and liquid nicotine concentration increase the nicotine yield. That these influences are predicted well by a mathematical model of the relevant physics highlights how engineering analysis can inform our understanding of human behavior in the self-administration of nicotine using an ECIG. It also indicates that mathematical modeling may provide a practical way for regulators to identify combinations of factors that would result in a mandated nicotine yield. In addition, it may help identify combinations that would produce ineffective or unsafe levels of nicotine, and regulators could instruct manufacturers accordingly.
Supplementary Material
Supplementary Table S1 and Figures S1 and S2 can be found online at http://www.ntr.oxfordjournals.org
Funding
This study was supported by the National Institute on Drug Abuse of the National Institutes of Health (P50DA036105) and the Center for Tobacco Products of the US Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the US Food and Drug Administration.
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
Portions of this work were presented at the 20th Annual Meeting of the Society for Research on Nicotine and Tobacco. The authors thank Rachel El Hage for her assistance with the nicotine assay.
References
- 1. Ayers JW, Ribisl KM, Brownstein JS. Tracking the rise in popularity of electronic nicotine delivery systems (electronic cigarettes) using search query surveillance. Am J Prev Med. 2011;40:448–453. [DOI] [PubMed] [Google Scholar]
- 2. Adkison SE, O’Connor RJ, Bansal-Travers M, et al. Electronic nicotine delivery systems: international tobacco control four-country survey. Am J Prev Med. 2013;44:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. CDC. About one in five US adult cigarette smokers have tried an electronic cigarette http://www.cdc.gov/media/releases/2013/p0228_electronic_cigarettes.html Accessed May 14, 2014.
- 4. Hinds W. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles. 2nd ed. Hoboken, NJ: Wiley-Interscience; 1999. [Google Scholar]
- 5. Noel JK, Rees VW, Connolly GN. Electronic cigarettes: a new ‘tobacco’ industry? Tob Control. 2011;20:81. [DOI] [PubMed] [Google Scholar]
- 6. Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Spyrou A, Voudris V. Impact of flavour variability on electronic cigarette use experience: an internet survey. Int J Environ Res Public Health. 2013;10:7272–7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grana RA, Ling PM. “Smoking revolution”: a content analysis of electronic cigarette retail websites. Am J Prev Med. 2014;46:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Henningfield JE, Zaatari GS. Electronic nicotine delivery systems: emerging science foundation for policy. Tob Control. 2010;19:89–90. [DOI] [PubMed] [Google Scholar]
- 9. Dawkins L, Corcoran O. Acute electronic cigarette use: nicotine delivery and subjective effects in regular users. Psychopharmacology (Berl). 2014;231:401–407. [DOI] [PubMed] [Google Scholar]
- 10. Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob Res. 2013;15:267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. USDHHS, ed. The health consequences of smoking: Nicotine addiction: a report of the Surgeon General ((CDC) 88–8406 ed.). Center for Health Promotion and Education. Office on Smoking and Health: USDHHS. http://profiles.nlm.nih.gov/NN/B/B/Z/D/ Accessed May 15, 2014.
- 12. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2013;23:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Westenberger BJ. Evaluation of e-cigarettes - Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Division of Pharmaceutical Analysis http://www.fda.gov/downloads/drugs/scienceresearch/ucm173250.pdf Accessed May 15, 2014.
- 14. Grana RA, Ling PM, Benowitz N, Glantz S. Electronic cigarettes. Circulation. 2014;129:e490–e492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bullen C, McRobbie H, Thornley S, Glover M, Lin R, Laugesen M. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tob Control. 2010;19:98–103. [DOI] [PubMed] [Google Scholar]
- 16. Eissenberg T. Electronic nicotine delivery devices: ineffective nicotine delivery and craving suppression after acute administration. Tob Control. 2010;19:87–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol Biomarkers Prev. 2010;19:1945–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Djordjevic MV, Stellman SD, Zang E. Doses of nicotine and lung carcinogens delivered to cigarette smokers. J Natl Cancer Inst. 2000;92:106–111. [DOI] [PubMed] [Google Scholar]
- 19. Katurji M, Daher N, Sheheitli H, Saleh R, Shihadeh A. Direct measurement of toxicants inhaled by water pipe users in the natural environment using a real-time in situ sampling technique. Inhal Toxicol. 2010;22:1101–1109. [DOI] [PubMed] [Google Scholar]
- 20. Maziak W, Rastam S, Shihadeh AL, et al. Nicotine exposure in daily waterpipe smokers and its relation to puff topography. Addict Behav. 2011;36:397–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shihadeh A. Investigation of mainstream smoke aerosol of the argileh water pipe. Food Chem Toxicol. 2003;41:143–152. [DOI] [PubMed] [Google Scholar]
- 22. Shihadeh A, Saleh R. Polycyclic aromatic hydrocarbons, carbon monoxide, “tar”, and nicotine in the mainstream smoke aerosol of the narghile water pipe. Food Chem Toxicol. 2005;43:655–661. [DOI] [PubMed] [Google Scholar]
- 23. Shihadeh A, Azar S. A closed-loop control “playback” smoking machine for generating mainstream smoke aerosols. J Aerosol Med. 2006;19:137–147. [DOI] [PubMed] [Google Scholar]
- 24. Zacny JP, Stitzer ML. Monograph 7: the FTC cigarette test method for determining tar, nicotine, and carbon monoxide yields of US cigarettes, National Cancer Institute. http://cancercontrol.cancer.gov/Brp/tcrb/monographs/7/index.html Accessed May 15, 2014.
- 25. Shihadeh AL, Eissenberg TE. Significance of smoking machine toxicant yields to blood-level exposure in water pipe tobacco smokers. Cancer Epidemiol Biomarkers Prev. 2011;20:2457–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities’ regulation. Int J Environ Res Public Health. 2013;10:2500–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hua M, Yip H, Talbot P. Mining data on usage of electronic nicotine delivery systems (ENDS) from YouTube videos. Tob Control. 2013;22:103–106. [DOI] [PubMed] [Google Scholar]
- 28. Eissenberg T, Adams C, Riggins EC, III, Likness M. Smokers’ sex and the effects of tobacco cigarettes: subject-rated and physiological measures. Nicotine Tob Res. 1999;1:317–324. [DOI] [PubMed] [Google Scholar]
- 29. Kleykamp BA, Jennings JM, Sams C, Weaver MF, Eissenberg T. The influence of transdermal nicotine on tobacco/nicotine abstinence and the effects of a concurrently administered cigarette in women and men. Exp Clin Psychopharmacol. 2008;16:99–112. [DOI] [PubMed] [Google Scholar]
- 30. Spindle T, Breland A, Shihadeh AL, Eissenberg T. Examination of electronic cigarette user puff topography: the effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects. Nicotine Tob Res. 2014; under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kosmider L, Sobczak A, Fik M, et al. Carbonyl compounds in electronic cigarette vapors-effects of nicotine solvent and battery output voltage. Nicotine Tob Res. 2014;16:1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15:158–166. [DOI] [PubMed] [Google Scholar]
- 33. Trehy ML, Ye W, Hadwiger ME, et al. Analysis of electronic cigarette cartridges, refill solutions, and smoke for nicotine and nicotine related impurities. J Liq Chromatogr R T. 2011;34:1442–1458. [Google Scholar]
- 34. Fuoco FC, Buonanno G, Stabile L, Vigo P. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ Pollut. 2014;184:523–529. [DOI] [PubMed] [Google Scholar]
- 35. McQueen A, Tower S, Sumner W. Interviews with “vapers”: implications for future research with electronic cigarettes. Nicotine Tob Res. 2011;13:860–867. [DOI] [PubMed] [Google Scholar]
- 36. Nides MA, Leischow SJ, Bhatter M, Simmons M. Nicotine blood levels and short-term smoking reduction with an electronic nicotine delivery system. Am J Health Behav. 2014;38:265–274. [DOI] [PubMed] [Google Scholar]
- 37. Kosmider L, Sobczak A, Knysak J, Goniewicz M. Effect of solvent and battery output voltage on nicotine levels released from electronic cigarette. Presented at: SRNT 20th Annual Meeting; February 2014; Seattle, WA. [Google Scholar]
- 38. Goniewicz ML, Hajek P, McRobbie H. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications. Addiction. 2014; 109:500–507. [DOI] [PubMed] [Google Scholar]
- 39. Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, Voudris V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep. 2014;4:4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.