Abstract
Electronic cigarettes (ECIGs) comprise an aerosolized nicotine delivery product category that provides consumers with probably unprecedented control over extensive features and operating conditions, allowing a wide range of nicotine yields to be obtained. Depending on the combination of such ECIG variables as electrical power input, geometry, liquid composition, and puff behavior, ECIG users can extract in a few puffs far more or far less nicotine than with a conventional combustible cigarette. These features of ECIG design and use present challenges for public health policy, central among which is the question of how to regulate nicotine delivery. In this commentary, we propose a conceptual framework intended to provide a convenient approach for evaluating and regulating the nicotine emitted from ECIGs. This framework employs nicotine flux to account for the total dose and rate at which nicotine reaches the user, 2 key factors in drug abuse liability. The nicotine flux is the nicotine emitted per puff second (e.g., mg/s) by a given ECIG design under given use conditions, and it can be predicted accurately using physical principles. We speculate that if the flux is too low, users likely will abandon the device and maintain conventional tobacco product use. Also, we speculate that if the flux is too high, individuals may suffer toxic side effects and/or the device may have higher-than-necessary abuse liability. By considering ECIG design, operation conditions, liquid composition, and puff behavior variables in combination, we illustrate how ECIG specifications can be realistically mandated to result in a target flux range.
Introduction
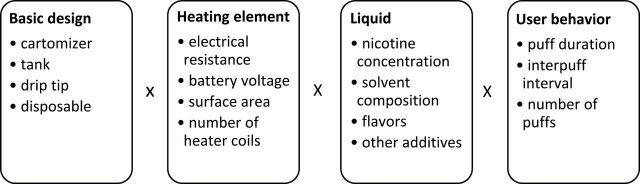
Electronic cigarettes (ECIGs) are a product category that encompasses a wide and rapidly evolving range of technologies and use methods.1 They are marketed as noncombusting nicotine delivery devices that use an electrically powered element to heat a liquid to form an inhalable aerosol. ECIG users can combine numerous basic designs, heating element features, liquids, and use behaviors that lead to thousands of possible configurations (Figure 1). Basic designs include products that are a single unit and are disposable, two-piece products that have a separate battery that screws onto a cartridge (or cartomizer) that stores the liquid and heating element in combination, or three (or more) piece products that position the liquid in a reservoir that screws onto the heating element that is then screwed onto the battery that powers it (so-called tank systems). Heating element features include the voltage it receives from the battery, its electrical resistance, and its surface area. Liquids come in a large number of flavors and nicotine concentrations that range from 0 to 36mg. User behavior refers primarily to the way an individual inhales from the mouth end of the ECIG (i.e., puff topography) and includes variables such as puff duration, number, and interpuff interval. Studies examining a small subset of these configurations indicate that, in a 5-min use session, an ECIG can produce aerosol with nicotine yields ranging from near zero to many times that of a single combustible tobacco cigarette.2–4
Figure 1.
Some electronic cigarette product designs, heating element features, liquid components, and user behaviors likely related to nicotine and other toxicant delivery. Each box lists a subset of many variables, and the product of the number of choices in each box conceptually represents the number of configurations available.
Consumer control over so many characteristics of a nicotine delivery device is a marked departure from the combustible tobacco cigarette that, by comparison, constrains the range of product choices, with a comparatively limited range of nicotine yields. ECIGs thus present a regulatory challenge, particularly with regard to one central issue: nicotine delivery to the user. To control nicotine delivery effectively, regulations will need to account for product design, element features, liquid composition, and user behavior in combination, rather than separately.3 In this commentary, we propose a conceptual framework intended to provide a convenient approach for evaluating from a drug administration perspective the multiplicity of possible ECIG/user behavior configurations. This framework centers on the concept of nicotine flux (), which is the rate at which nicotine flows from the mouth end of the ECIG per puff second (e.g., mg/s). We note that while this commentary focuses on nicotine administration, ECIG use may also be motivated by factors other than nicotine dose and delivery and that effective regulation of this product, therefore, will likely require that these factors be addressed in addition to the question of nicotine administration.
Nicotine Flux
In the context of drug toxicity and abuse liability, both the total dose and the speed of delivery are key variables.5–7 For a combustible cigarette, nicotine yield, the mass of nicotine emitted per cigarette smoked (e.g., mg/cigarette), sometimes is used as a metric for characterizing the total dose of nicotine available from a single cigarette,8,9 though it has many limitations.10,11 Because tobacco cigarettes are standardized products with clearly defined use endpoints (i.e., the burning down of the tobacco rod), variation in the number of puffs and time of consumption of a single rod is relatively small across products. A single rod is typically consumed over 5min, during which 8–15 puffs are drawn.12 As a result, the nicotine yield, in addition to describing the total dose obtained per cigarette, is also an index of the speed of delivery (i.e., the yield is delivered in 5min).
In the case of ECIGs, the unit of consumption is not well defined in time. An ECIG may be used sporadically over several days, during which hundreds of puffs can be drawn. In addition, unlike combustible cigarettes, ECIGs have batteries that can be recharged, heating elements that can be replaced, and cartridges and tanks that can be refilled with liquid. In this respect, an ECIG is more like a motor vehicle, which can be driven indefinitely as long as fuel, spare parts, and other supplies are available. More pertinent to regulation, whether a vehicle is capable of attaining unacceptably high speeds or, conversely, whether it will move too slowly to be useful depends on a combination of factors, rather than any single variable. For example, a 400 horsepower engine mounted in a freight truck will result in very different vehicle performance than when mounted in a motorcycle. The latter combination, while potentially entertaining on a race track, would result in a vehicle with nearly uncontrollable acceleration and excessive maximum speed. The ratio of vehicle weight to engine power is a more relevant metric for safety and usefulness than either vehicle weight or power considered separately; indeed, power to weight ratio is used routinely as a metric in road vehicle regulations (e.g., EU Commission Directive 2012/36/EU).
Analogously, ECIG design, heating element features, liquid contents, and user behavior all, individually, have limited utility as metrics of inhalation-related nicotine exposure, toxicity, and effectiveness. The utility of these individually considered features is limited because no one feature alone determines the rate at which nicotine is emitted (i.e., the flux). For example, a high-voltage/low nicotine concentration combination can give the same or greater flux as a low-voltage/high nicotine concentration combination.3 The flux, in turn, determines the effect of a given nicotine dose, ranging from no effect to acute toxicity. If ECIG nicotine flux is low, users likely will abandon the device as they would a passenger vehicle that has a top speed of 3 km/hr. If the flux is high (e.g., exceeds levels characteristic of combustible cigarettes), users may accept the device despite the fact that it carries with it the potential for toxic side effects, like a motorcycle with a 400 horsepower engine.
Flux can be defined in numerous ways, such as the mass of nicotine per puff, per use episode duration, or per day. Here, we propose the nicotine mass obtained from a device per puff second. This definition of flux automatically accounts for some user-specific variables such as puff duration and number of puffs drawn, and it highlights the intrinsic performance of an ECIG in its role as a nicotine dosing device. With knowledge of the nicotine flux, the nicotine dose can be computed per puff, use episode, or day, by integrating the nicotine flux over any of those defined periods:
Nicotine flux depends on product characteristics (design, element, and liquid) and user puff topography in a predictable manner.3 For example, nicotine flux increases with longer puff duration and greater voltage and is unaffected by puff velocity.3 The effect of product characteristics on nicotine flux can be represented by a “design efficacy index” (Z d). Similarly, the ways that puff topography can influence nicotine flux can be represented by a puffing intensity parameter (Z p). Guided by dimensional analysis, Z d and Z p can be chosen in a manner that the nicotine fluxes from all ECIG design and operating condition combinations collapse onto a single universal surface relating flux, Z d and Z p. Such a surface would enable regulators to compute the possible nicotine flux span for a given product over a range of possible use scenarios.
Nicotine Flux as a Regulatory Tool
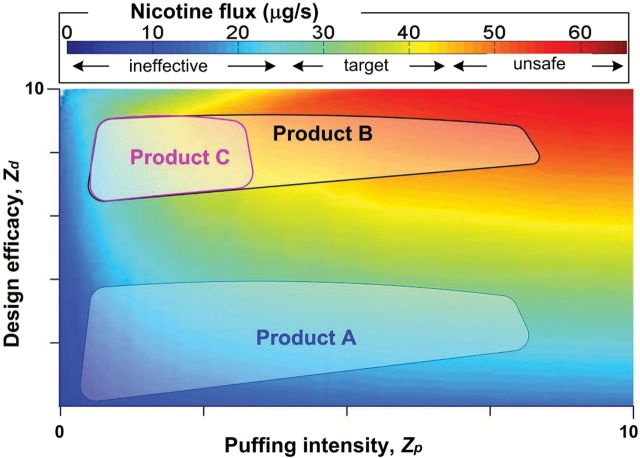
Figure 2 illustrates a nicotine flux surface plot computed over a range of plausible ECIG design, element, and liquid variables, as well as puff topography variables. Shown in the figure are hypothetical categories defining ineffective, target, and unsafe nicotine flux ranges that could be defined by a regulatory agency. We note that there are many other ways that flux ranges can be defined to suit a particular regulatory purpose and have here purposely simplified the categories in order to illustrate the utility of nicotine flux mapping. Also shown in Figure 2 are attainable nicotine fluxes for three hypothetical products labeled A, B, and C.
Figure 2.
Nicotine flux versus product design and topography parameters for three hypothetical products. Nicotine flux is indicated by color. A hypothetical regulatory target range for product effectiveness is given as 25–45 μg/s. The areas enclosed by each product box represent the ranges of possible nicotine fluxes given the possible product characteristics (e.g., design, element, and liquid) and puff topography (e.g., puff duration and interpuff interval).
Products A, B, and C span different ranges of Zd and Z p. Product A is a variable voltage device that the user can fill with their preferred liquid. Because Product A’s heater inherently is inefficient at aerosolizing the liquid, the target nicotine flux (yellow-green region) is achievable only at the highest available voltages and using liquids with the highest nicotine concentration. As a result, although its variable voltage and variable nicotine features allow Product A to occupy a large nicotine flux surface, most of the ways that Product A can be used will produce an unacceptably low nicotine flux. Product B is a fixed voltage device that is sold with nonrefillable disposable cartridges of fixed nicotine concentration. With long puff durations, Product B allows the user to obtain potentially unsafe nicotine fluxes (red region). Product C is identical to Product B. However, it is equipped with a microchip that automatically terminates power to the heater coil after a preset puff duration has been reached and that does not allow another puff to be executed prior to the passage of a minimum interpuff interval. As a result, the microchip constrains operation of the product to the desired range of nicotine flux.
From a hypothetical regulatory framework in which safety and effectiveness are defined by the target nicotine fluxes referred to in the figure, Products A and B would, in our opinion, raise cause for concern based on their design features and the knowledge of plausible ranges of user puff topography. The concern that might be raised regarding Product A is that, across a wide range of user behavior, its design features produce a level of nicotine flux that is so low as to fail to reinforce subsequent use in a tobacco cigarette smoker. That is, one primary motivator for continued cigarette use is negative reinforcement via suppression (or avoidance) of aversive tobacco/nicotine withdrawal symptoms.13 ECIGs are capable of partially suppressing these symptoms in tobacco cigarette smokers even when they do not deliver nicotine,14 and we speculate that they likely will provide even more withdrawal suppression and thus be even more likely to substitute for tobacco cigarettes, when they deliver nicotine effectively. Therefore, all other things being equal, a Product A-like ECIG, ineffective at nicotine delivery in many cases, is unlikely to maximize the public health promise of ECIG about which so much has been written.15–17
Product B produces a high level of nicotine flux across a wide range of user behavior and, therefore, raises two concerns. First, this high nicotine flux may produce toxicity, potentially limiting acceptability (a device that causes nicotine-induced nausea and vomiting is unlikely to be reinforcing) and, potentially, longer lasting harm. Second, assuming minimal toxicity, this high nicotine flux may produce positive subjective effects in nicotine-naive users who try an ECIG for the first time. If these positive subjective effects are reinforcing, they increase the likelihood of subsequent ECIG use and nicotine dependence: a product of unknown risk that induces drug dependence in previously drug-naive individuals is a legitimate public health concern.
In contrast to Products A and B, Product C is designed to provide a middle ground. Its design allows users to move within a nicotine flux surface that has values that are high enough to provide complete suppression (or avoidance) of nicotine/tobacco withdrawal in tobacco cigarette smokers and that are low enough that it does not produces toxicity in any user and also does not reinforce subsequent use in tobacco-naive individuals who try it for the first time. Thus, Product C satisfies a hypothetical regulation concerning nicotine flux that is intended to maintain the putative promise of ECIGs as a tobacco cigarette substitute while also limiting at least some of their potential perils. As an aside, we note that this same approach—withdrawal suppression with lower abuse liability—is part of the driving force behind research investigating the effects of very low nicotine content cigarettes.18
Because the nicotine flux, Z p, and Z d can be related using computational models of the relevant physical phenomena (e.g., as in Talih et al.3), we suggest that the nicotine flux framework can be deployed efficiently to mandate acceptable ranges of Z p and Z d for any proposed product and to generate regulatory scenarios such as requiring a specific nicotine concentration for all marketed liquids or requiring incorporation of puff-limiting electronics.
While attending to variables in combination using nicotine flux offers regulatory advantages, the opposite case, failing to account for variables in combination, can lead to regulations that do not serve their intended purpose. For example, European Union Directive 2014/40/EU recently mandated a limit of 20mg/ml liquid nicotine concentration for the purpose of “allow(ing) for a delivery of nicotine that is comparable to the permitted dose of nicotine derived from a standard cigarette.” This regulation fails to account for the fact that nicotine concentration alone does not determine nicotine yield and, almost certainly, nicotine delivery to the user. That is, recent data and theoretical analyses suggest that, depending on user-selected battery voltage and heater resistance as well as user puff topography, a given ECIG loaded with 20mg/ml liquid can emit an aerosol that contains far more or far less nicotine than the smoke from a standard cigarette.3
For the concept of nicotine flux to guide regulation effectively, more research is needed to elucidate the functions Z p and Z d and to develop and validate empirically a flux model that is applicable to any ECIG design. Moreover, we have focused in here on nicotine emitted from the mouth end of an ECIG (i.e., yield) and acknowledge that many of the effects that will guide regulatory decision making (e.g., toxicity, withdrawal suppression) will likely require work that relates vapor nicotine flux to bloodstream nicotine flux (i.e., delivery/absorption) and, potentially, to the subjective effects produced (e.g., withdrawal suppression). Indeed, we have already elucidated one reliable method for determining the relationship between yield and delivery for another tobacco product19 and expect that future work will extend this method to ECIGs.
In summary, ECIGs present a level of complexity that is a challenge to regulation. There clearly is great variability in the marketplace today, with some ECIGs delivering little or no nicotine to the user20,21 and others, under certain conditions, yielding higher doses of nicotine in a few puffs than has been observed for an entire single combustible cigarette.3 With respect to nicotine yield, we suggest that the concept of flux and the critical functions Z p and Z d will allow regulators to navigate this complexity, charting the range of possible fluxes for various proposed rules. This notion becomes even more important as techniques are developed that allow prediction of nicotine delivery to the user (i.e., plasma nicotine concentration) when nicotine yield of product emissions has been determined based on actual user puff topography.19 Similar analyses for other toxicants may help regulators limit the total toxicant load of ECIG aerosol. On this empirical basis does the science of tobacco product regulation move forward.
Funding
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under award #P50DA036105 and the Center for Tobacco Products of the US Food and Drug Administration.
Declaration of Interests
None declared.
Acknowledgments
The authors thank Ms. Zainab Balhas and Mr. Nareg Karaoghlanian for their assistance in preparing the nicotine flux plot. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the US Food and Drug Administration.
References
- 1. Breland AB, Spindle TR, Weaver M, Eissenberg T. Science and electronic cigarettes: current data, future needs. J Addict Med. 2014;8:223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2012;15:158–166. [DOI] [PubMed] [Google Scholar]
- 3. Talih S, Balhas Z, Eissenberg T, et al. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions [published online ahead of print September 03, 2014]. Nicotine Tob Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Westenberger BJ. Evaluation of E-cigarettes. Rockville, MD: Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Division of Pharmaceutical Analysis; 2009. www.fda.gov/downloads/drugs/scienceresearch/ucm173250.pdf Accessed May 15, 2014. [Google Scholar]
- 5. Balster RL, Schuster CR. Fixed-interval schedule of cocaine reinforcement: effect of dose and infusion duration1. J Exp Anal Behav. 1973;20:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carter LP, Stitzer ML, Henningfield JE, O’Connor RJ, Cummings KM, Hatsukami DK. Abuse liability assessment of tobacco products including potential reduced exposure products. Cancer Epidemiol Biomarkers Prev. 2009;18:3241–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. USDHHS. The Health Consequences of Smoking—50 Years of Progress: Nicotine Addiction: A Report of the Surgeon General. Rockville, MD: Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, US Department of Health and Human Services; 2014. [Google Scholar]
- 8. Connolly GN, Alpert HR, Wayne GF, Koh H. Trends in nicotine yield in smoke and its relationship with design characteristics among popular US cigarette brands, 1997–2005. Tob Control. 2007;16:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Land T, Keithly L, Kane K, et al. Recent increases in efficiency in cigarette nicotine delivery: implications for tobacco control. Nicotine Tob Res. 2014;16:753–758. [DOI] [PubMed] [Google Scholar]
- 10. Coultas DB, Stidley CA, Samet JM. Cigarette yields of tar and nicotine and markers of exposure to tobacco smoke. Am Rev Respir Dis. 1993;148:435–440. [DOI] [PubMed] [Google Scholar]
- 11. Djordjevic MV, Stellman SD, Zang E. Doses of nicotine and lung carcinogens delivered to cigarette smokers. J Natl Cancer Inst. 2000;92:106–111. [DOI] [PubMed] [Google Scholar]
- 12. Kleykamp BA, Jennings JM, Sams C, Weaver MF, Eissenberg T. The influence of transdermal nicotine on tobacco/nicotine abstinence and the effects of a concurrently administered cigarette in women and men. Exp Clin Psychopharmacol. 2008;16:99–112. [DOI] [PubMed] [Google Scholar]
- 13. Eissenberg T. Measuring the emergence of tobacco dependence: the contribution of negative reinforcement models. Addiction. 2004;99:5–29. [DOI] [PubMed] [Google Scholar]
- 14. Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol Biomarkers Prev. 2010;19:1945–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hajek P. Electronic cigarettes for smoking cessation. Lancet. 2013;382:1614–1616. [DOI] [PubMed] [Google Scholar]
- 16. Wagener TL, Siegel M, Borrelli B. Electronic cigarettes: achieving a balanced perspective. Addiction. 2012;107:1545–1548. [DOI] [PubMed] [Google Scholar]
- 17. Abrams DB. Promise and peril of e-cigarettes: can disruptive technology make cigarettes obsolete? J Am Med Assoc . 2014;311:135–136. [DOI] [PubMed] [Google Scholar]
- 18. Donny EC, Hatsukami DK, Benowitz NL, Sved AF, Tidey JW, Cassidy RN. Reduced nicotine product standards for combustible tobacco: building an empirical basis for effective regulation [published online ahead of print June 23, 2014]. Prev Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shihadeh AL, Eissenberg TE. Significance of smoking machine toxicant yields to blood-level exposure in water pipe tobacco smokers. Cancer Epidemiol Biomarkers Prev. 2011;20:2457–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bullen C, McRobbie H, Thornley S, Glover M, Lin R, Laugesen M. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tob Control. 2010;19:98–103. [DOI] [PubMed] [Google Scholar]
- 21. Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol Biomarkers Prev. 2010;19:1945–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]