Abstract
Introduction:
Electronic cigarettes (ECIGs) heat a nicotine-containing solution; the resulting aerosol is inhaled by the user. Nicotine delivery may be affected by users’ puffing behavior (puff topography), and little is known about the puff topography of ECIG users. Puff topography can be measured using mouthpiece-based computerized systems. However, the extent to which a mouthpiece influences nicotine delivery and subjective effects in ECIG users is unknown.
Methods:
Plasma nicotine concentration, heart rate, and subjective effects were measured in 13 experienced ECIG users who used their preferred ECIG and liquid (≥12mg/ml nicotine) during 2 sessions (with or without a mouthpiece). In both sessions, participants completed an ECIG use session in which they were instructed to take 10 puffs with 30-second inter-puff intervals. Puff topography was recorded in the mouthpiece condition.
Results:
Almost all measures of the effects of ECIG use were independent of topography measurement. Collapsed across session, mean plasma nicotine concentration increased by 16.8ng/ml, and mean heart rate increased by 8.5 bpm (ps < .05). Withdrawal symptoms decreased significantly after ECIG use. Participants reported that the mouthpiece affected awareness and made ECIG use more difficult. Relative to previously reported data for tobacco cigarette smokers using similar topography measurement equipment, ECIG-using participants took larger and longer puffs with lower flow rates.
Conclusions:
In experienced ECIG users, measuring ECIG topography did not influence ECIG-associated nicotine delivery or most measures of withdrawal suppression. Topography measurement systems will need to account for the low flow rates observed for ECIG users.
Introduction
Electronic cigarettes (ECIGs) are steadily increasing in popularity, with use rates increasing among youth and adults.1,2 Broadly, ECIGs are a class of products that operate by heating a nicotine-containing solution (e-liquid) resulting in the production of an inhalable aerosol.
ECIGs deliver nicotine with different effectiveness, depending on the device and user behavior. For example, first generation “cigalike” ECIGs, designed to resemble tobacco cigarettes, deliver less nicotine to the user than newer ECIG models.3 In addition, experienced ECIG users may be able to extract nicotine from ECIGs more effectively than naïve ECIG users: experienced users using their own devices are capable of obtaining plasma nicotine concentrations that approach those observed in tobacco cigarette smokers.4 Experienced users also are able to obtain nicotine using first generation “cigalike” devices,3,5 whereas inexperienced users using similar devices may be unable to obtain nicotine.6 Further complicating the matter, nicotine delivery varies substantially among experienced users using the same devices.4,5
Detailed analysis of puffing behavior (i.e., puff topography) may help explain why nicotine delivery differs among ECIG users. Puff topography is the quantitative measurement of puff behavior, including puff number, duration, volume, velocity (flow rate), and interpuff interval (IPI). Puff topography measurement has been critical to understanding the use of novel tobacco products. For example, puff topography analysis revealed that “full flavor” tobacco cigarette smokers switched to a so-called “low-yield” cigarette take larger, longer, and more frequent puffs, thus resulting in toxicant delivery similar to cigarettes that are not “low-yield.”7
Puff topography is typically recorded via observational methods (e.g., video recorders and trained video scorers8) or mouthpiece-based computerized systems.8–10 Extant mouthpiece-based computerized systems (e.g., CReSS; Borgwaldt) are more efficient than observational methods for measuring topography in cigarette smokers,8 but their ability to measure ECIG topography has not been reported previously and may pose several challenges. First, the mouthpieces used in computerized topography measurement systems may interfere with ECIG-associated nicotine delivery. That is, the aerosol produced by ECIGs may condense inside the mouthpiece, inhibiting nicotine delivery and/or altering user behavior and effects. Second, the results of the few studies that have examined puff topography in ECIG users using observational methods suggest that the topography of an experienced ECIG user may differ from that of a tobacco cigarette smoker, with ECIG users taking puffs that are approximately twice as long.11,12 If the longer puff durations observed in ECIG users are indicative of low flow rate puffs, the accuracy of puff duration and volume measured by a mouthpiece-based system may be reduced, as mouthpiece-based systems rely on flow rate to detect the start and end of a puff (e.g., the system that is capable of sensing a flow rate greater than 15ml/s will not measure whatever portion of the puff is below that threshold). Collectively, previous ECIG topography research suggests current computerized topography devices that were designed to measure cigarette topography may need to be altered to measure ECIG topography adequately.
The goals of the present study were to: (a) compare nicotine delivery, heart rate, and subjective effects of experienced ECIG users when they used an ECIG with and without a mouthpiece-based device and (b) measure ECIG topography in the mouthpiece condition, including puff duration, volume, and flow rate.
Methods
Participants
Thirteen experienced ECIG users (11 males, 10 White) were recruited by advertisement and word-of-mouth, provided informed consent, completed this institutional review board-approved study, and were included in the final analyses. Seven additional participants provided informed consent but did not complete the study: five withdrew prior to study completion, one was discontinued due to lack of venous access, and one was discontinued due to elevated blood pressure. Additionally, two participants (who used the same ECIG liquid) completed the study but their data were not included here because analysis detected no nicotine in their ECIG liquid, despite the liquid being advertised as containing 12mg/ml nicotine. Finally, because ECIGs are non-combustible and therefore do not produce CO under normal conditions, retrospective examination of baseline plasma nicotine concentration was also used to ensure overnight nicotine/tobacco abstinence. Two participants’ data were eliminated because a baseline plasma sample revealed a nicotine concentration >7ng/ml. All other participants were ≤6ng/ml at baseline in both conditions.
Participants were deemed eligible if they were healthy, aged 18–55 (M = 29.8, SD = 6.6), used ≤5 cigarettes daily (M = 0, SD = 0), used ≥1ml of ECIG liquid daily (M = 3.7, SD = 2.3), used ECIG liquid with nicotine concentration ≥12mg/ml, and used their ECIG for ≥3 months (M = 14.7, SD = 12.1). Exclusion criteria included: history of chronic disease or psychiatric condition, regular use of a prescription medication, marijuana use >10 and alcohol use >25 days in the past 30, and use of other illicit drugs (e.g., cocaine, opioids, benzodiazepines, and methamphetamine) in the past 30 days. Women were excluded if they tested positive for pregnancy (by urinalysis) at screening.
Materials
For each condition, participants used their preferred ECIG devices and liquids. Table 1 lists the devices, liquid nicotine concentrations, and flavors (based on product labeling) that were used. The humectants used in ECIG liquids may influence nicotine yield,13 so, when available, Table 1 also includes the advertised ratio of propylene glycol (PG) to vegetable glycerin (VG). The majority of participants’ ECIGs normally used a “tank” system (N =12). The remaining participant normally used a re-fillable “cartomizer” (i.e., cartridge that contains the “atomizer” or heating element14). None of the participants used disposable “cigalike” devices, and all participants’ ECIGs were powered by a rechargeable battery. Because the sizes of tank and cartridge systems may not be compatible with existing mouthpiece-based topography systems, all participants were required to use a 510 “cartomizer” with 1.5 ohms resistance, and dual heating-coils. All “cartomizers” were produced by SmokTech and purchased either online or locally in Richmond, Virginia. Participants provided their preferred ECIG battery (see Table 1) and laboratory staff purchased their e-liquid from their usual source (i.e., either internet or local ECIG shop).
Table 1.
ECIG Devices, Liquid Nicotine Concentrations, Flavors, and Humectant Ingredients Used in the Present Study Based on Product Labeling and Manufacturer Information
| Participant | ECIG model | Nicotine concentration (mg/ml) | Humectant ingredients: PG/VG ratio | Liquid flavor |
|---|---|---|---|---|
| 1 | e-Go | 24 | 100/0 | No flavor |
| 2 | MVP | 24 | 50/50 | Gargamel’s Curse |
| 3 | Halo G6 | 24 | Not available | Torque 56 |
| 4 | e-Go T | 18 | 70/30 | Watermelon |
| 5 | i-Taste | 24 | 50/50 | Peach |
| 6 | e-Go | 24 | 30/70 | Gold Rush |
| 7 | Smoke Tech | 24 | 80/20 | DK Blend |
| 8 | V2 Cigs | 24 | Not available | Menthol |
| 9 | Tsunami | 24 | 30/70 | Persian Winter |
| 10 | iTaste | 24 | 50/50 | Vanilla Dr. Pepper |
| 11 | e-Go | 18 | 30/70 | Gold Rush |
| 12 | e-Go Twist | 12 | 30/70 | Aztec |
| 13 | iTaste | 18 | 60/40 | Carolina Crush |
Procedures
Participants attended VCU’s Clinical Behavioral Pharmacology Laboratory on two days (separated by a minimum of 48hr) to complete two approximately 2.5-hr sessions (conditions): one in which a mouthpiece-based topography recording device was attached to their ECIG and one in which it was not. Session order was counter-balanced across participants. Prior to each condition, participants were asked to abstain from nicotine/tobacco for ≥12hr. At the beginning of each session, participants’ expired air carbon monoxide (CO) concentration was measured (BreathCO monitor; Vitalograph) to verify abstinence from combustible tobacco (≤10 ppm, as in Breland et al.9). After expired air CO was measured, physiological monitoring of heart rate (HR) and blood pressure (BP) commenced. Then, an intravenous catheter was inserted into a forearm vein and 7ml blood was sampled. Participants then completed computerized questionnaires (see below). Thirty minutes after session onset, participants were instructed to take 10 puffs from their ECIG, with each puff separated by 30 s; an observer instructed participants when to puff and verified compliance. Other than the puff number and IPI, puffs were ad libitum. Immediately following the tenth puff, an additional 7ml blood was sampled and participants completed the same questionnaires again. After 10 additional minutes, another 7ml blood sample was collected. Following this third sample, participants completed other procedures not reported here. At the end of each session, the catheter was removed, and participants were compensated (US$100 after first session, US$150 after second).
Outcome Measures
Physiological Measures
All blood samples were centrifuged, stored at −70 °C, and sent to Virginia Commonwealth University’s Bioanalytical Analysis Core Laboratories for analysis of nicotine concentration (limit of quantitation or LOQ = 2ng/ml; see Breland et al.15). HR was monitored every 20 s (Criticare Systems model 507, fitted with pulse oximeter).
Subjective Questionnaires
Four questionnaires were administered using a computerized visual analog scale (VAS), consisting of a word or phrase centered on a horizontal line with “not at all” on the left and “extremely” on the right. Responses were recorded by participants moving a mouse cursor and clicking at any point on the horizontal line, with scores being expressed as a percentage of total line length. Nicotine withdrawal severity and abstinence symptom suppression were assessed using a modified version of the Hughes-Hasukami withdrawal scale (11 items, excluding the items “Increased eating,” and “Insomnia/Disturbed sleep”16). The direct effects of nicotine (10 items17) and ECIG use (10 items18,19) were also measured. Finally, in the topography mouthpiece condition, topography equipment effects were assessed using 6 VAS items (as in Blank et al.8). Where necessary, questionnaires were modified such that when the words “cigarette” or “smoking” appeared in the original, they were replaced by “e-cigarette” or “vaping” in this study.
Puff Topography
Puff topography measurements were made using an ECIG topography instrument developed and manufactured at the American University of Beirut (AUB). Similar to commercially available cigarette topography instruments, the instrument senses flow-induced pressure drop across an orifice that is incorporated into the mouthpiece. The pressure drop is sensed by a pressure transducer whose output voltage every 100ms is amplified, digitized, and sampled. Most importantly, the orifice dimensions and pressure-sensing transducer were chosen to provide sensitivity sufficient to ensure valid measurements at puff velocities as low as 3ml/s. We have previously reported that topography devices used to study cigarette smoking behavior may not be sensitive enough to measure ECIG topography accurately.20
Several mouthpieces were manufactured for the device, and each was individually calibrated using a custom built automatic digital flow calibrator. Calibration coefficients were used to relate puff velocity (ml/s) to the pressure transducer voltage signal. The instrument was calibrated prior to each session in which it was used.
Data Preparation and Analysis
For plasma nicotine data, any instances where the measurement was lower than the assay’s LOQ were replaced with the LOQ (2ng/ml) as in previous work (e.g., Vansickel et al.6). Prior to analysis, heart rate data were averaged for the 5min prior to and 5min during ECIG use. At the end of each ECIG use session with the mouthpiece, the topography instrument software integrated puff velocity data to produce the topography measures puff number, puff duration, puff volume, interpuff interval (IPI), and mean puff velocity (see Shihadeh et al.21 for details). Prior to analysis, the software performed a data cleaning procedure to correct for transducer noise. Data cleaning included combining into a single puff any two puffs that were separated by less than 100ms and deleting any puffs with a duration of less than 300ms. Remaining data for each measure were averaged for each participant.
Linear mixed-effect repeated measures ANOVA models were used to examine plasma nicotine, HR, and subjective questionnaire data using SAS (Version 9.3). For subjective measures given in both conditions (i.e., Hughes-Hatsukami, direct effects of ECIG use, and direct effects of nicotine) and heart rate, two by two factorial designs with two within-subject factors (Time [pre- and post-bout] and condition [with and without mouthpiece]) were used. Each questionnaire item was examined individually. A two by three factorial design was used to examine plasma nicotine data as there was an additional post-bout time point. The random effects define the three different experimental units in this model, allowing covariance to vary due to the participant, time measured, and condition. For the subjective measures analyses, an unstructured variance-covariance matrix was determined for the level of condition and a compound symmetry variance-covariance matrix was assigned for the level of time. For the heart rate analysis, the variance-covariance matrix was unstructured and compound symmetry for the level of condition and time point was used separately. For the plasma nicotine analysis, a first-order autoregressive variance-covariance matrix was assigned for the level of time and pairwise comparisons were examined using Tukey’s Honestly Significant Difference test (HSD). All reported summary statistics (i.e., means and SEMs) are from the observed data, and not those estimated from the linear mixed-effect repeated measures ANOVAs.
For the six items assessing the influence of topography equipment, administered in the mouthpiece condition only, post-ECIG use scores were averaged using SPSS (Version 22.0). Pre ECIG use scores were not relevant for this questionnaire, as participants could not judge the influence of the topography equipment prior to using it.
Independent samples t tests were conducted to compare topography data in the present study to topography data from 123 tobacco cigarette smokers, as described in Kleykamp et al.22 and Kleykamp,23 using SPSS (Version 22.0).
Results
Main effects and interactions for all ANOVA analyses (plasma nicotine, heart rate, and subjective measures) are displayed in Table 2. The main effect of time and the interaction between time and condition were of greatest interest, as the main effect shows the influence of ECIG use and the interaction shows the extent to which the effects of ECIG use over time were affected by the presence of a topography mouthpiece. As Table 2 shows, there were 17 measures for which a main effect of time was observed, two measures for which a main effect of condition was observed, and no measures on which an interaction of time and condition were observed.
Table 2.
Statistical Analyses Results for Plasma Nicotine and Subjective Measures
| Outcome measures | Condition (C) (df), F value | p | Time (T) (df), F value | p | C × T (df), F value | p |
|---|---|---|---|---|---|---|
| Plasma nicotine | (1,36), 0.0 | ns | (2,24), 26.0 | <.001 | (2, 36), 0.0 | ns |
| Heart Rate | (1,36), 2.1 | ns | (1,36), 43.4 | <.001 | (1,36), 0.8 | ns |
| Subjective measures | ||||||
| Hughes-Hatsukami | ||||||
| Anxious | (1,24), 3.8 | ns | (1,12), 6.9 | <.05 | (1,24), 3.1 | ns |
| Craving | (1,24), 6.7 | <.05 | (1,12), 21.6 | <.001 | (1,24), 0.4 | ns |
| Depression | (1,36), 4.1 | ns | (1,36), 0.1 | ns | (1,36), 0.8 | ns |
| Difficulty Concentrating | (1,24), 1.8 | ns | (1,12), 0.7 | ns | (1,24), 0.4 | ns |
| Drowsy | (1,36), 0.0 | ns | (1,36), 3.9 | ns | (1,36), 0.4 | ns |
| Impatient | (1,24), 0.8 | ns | (1,12), 6.7 | <.05 | (1,24), 2.0 | ns |
| Irritable | (1,24), 3.9 | ns | (1,12), 4.2 | ns | (1,24), 0.0 | ns |
| Restless | (1,24), 0.5 | ns | (1,12), 3.9 | ns | (1,24), 0.8 | ns |
| Sweets | (1,36), 3.6 | ns | (1,36), 0.2 | ns | (1,36), 1.6 | ns |
| Urge to vape | (1,24), 2.0 | ns | (1,12), 28.0 | <.001 | (1,24), 0.6 | ns |
| Hunger | (1,36), 0.7 | ns | (1,36), 1.5 | ns | (1,36), 0.7 | ns |
| Direct effects of nicotine | ||||||
| Nauseous | (1,24), 1.1 | ns | (1,12), 0.3 | ns | (1,24), 1.8 | ns |
| Nervous | (1,24), 0.4 | ns | (1,12), 1.8 | ns | (1,24), 0.0 | ns |
| Salivation | (1,36), 1.4 | ns | (1,36), 0.3 | ns | (1,36), 1.0 | ns |
| Sweaty | (1,36), 0.0 | ns | (1,36), 2.7 | ns | (1,36), 0.2 | ns |
| Weak | (1,36), 7.1 | <.05 | (1,36), 0.1 | ns | (1,36), 0.2 | ns |
| Confused | (1,36), 2.0 | ns | (1,36), 0.3 | ns | (1,36), 0.0 | ns |
| Dizzy | (1,36), 0.2 | ns | (1,36), 4.4 | <.05 | (1,36), 0.5 | ns |
| Headache | (1,36), 3.6 | ns | (1,36), 0.1 | ns | (1,36), 0.2 | ns |
| Heart pound | (1,36), 0.5 | ns | (1,36), 0.0 | ns | (1,36), 1.2 | ns |
| Light-headed | (1,36), 0.2 | ns | (1,36), 8.8 | <.05 | (1,36), 1.0 | ns |
| Direct effects of vaping | ||||||
| Awake | (1,36), 0.7 | ns | (1,36), 12.9 | <.001 | (1,36), 2.4 | ns |
| Calm | (1,24), 0.6 | ns | (1,12), 24.3 | <.001 | (1,24), 3.2 | ns |
| Concentrate | (1,36), 0.1 | ns | (1,36), 9.9 | <.01 | (1,36), 0.9 | ns |
| Dizzy | (1,36), 0.1 | ns | (1,36), 10.3 | <.01 | (1,36), 0.8 | ns |
| Pleasant | (1,24), 0.6 | ns | (1,12), 61.8 | <.001 | (1,24), 2.7 | ns |
| Reduce hunger | (1,24), 0.4 | ns | (1,12), 6.0 | <.05 | (1,24), 2.1 | ns |
| Want ECIG right now | (1,24), 0.5 | ns | (1,12), 8.2 | <.05 | (1,24), 2.6 | ns |
| Satisfying | (1,24), 0.1 | ns | (1,12), 60.1 | <.001 | (1,24), 1.3 | ns |
| Sick | (1,24), 0.4 | ns | (1,12), 0.2 | ns | (1,24), 0.9 | ns |
| Taste good | (1,24), 0.0 | ns | (1,12), 121.2 | <.001 | (1,24), 0.6 | ns |
ns = non-significant.
Physiological Measures
Plasma Nicotine
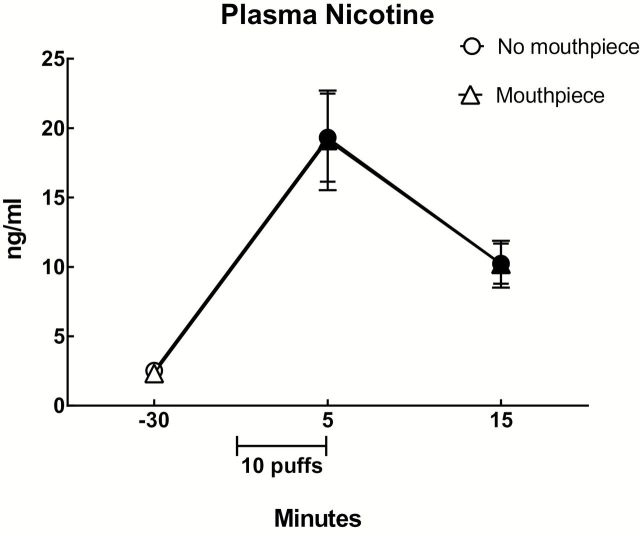
A significant main effect of time was observed for plasma nicotine (Table 2) and Figure 1 shows the mean data for each condition and time point. Collapsed across condition, mean (±SEM) plasma nicotine concentration immediately after ECIG use (19.2±2.3ng/ml) was significantly greater relative to baseline (2.4±0.2ng/ml) and 10min after ECIG use (10.2±1.1ng/ml; Tukey’s HSD). In addition, mean plasma concentration 10min after ECIG use was significantly different from baseline (Tukey’s HSD).
Figure 1.
Mean plasma nicotine concentrations (±SEM) for 13 participants who completed a 10-puff ECIG use bout (30 s interpuff interval) in two conditions: with no topography mouthpiece and with a topography mouthpiece. Filled symbols indicate a significant difference from before ECIG use (−30; the first value). All ps < .05; Tukey’s HSD.
Heart Rate
A significant main effect of time was observed for heart rate (Table 2). Collapsed across condition, mean (±SEM) heart rate after ECIG use (74.2±1.6) was significantly higher relative to baseline (65.7±1.5).
Subjective Measures
Hughes-Hatsukami Withdrawal Scale
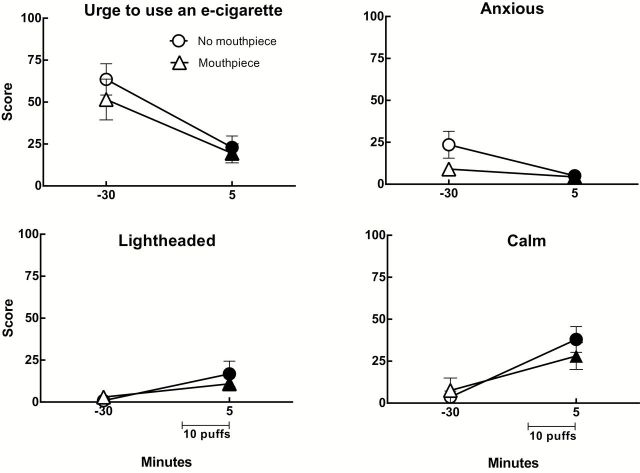
Significant main effects of time were observed for ratings of “Anxious,” “Urge to use an e-cigarette,” “Craving an e-cigarette,” and “Impatient” and Figure 2 displays the data for “Urge to use an e-cigarette” (top, left), and “Anxious” (top, right), two of the items with the largest F values. Collapsed across condition, mean ratings were: “Anxious” (Pre: 16.3±4.4; Post: 4.7±1.3), “Craving” (Pre: 56.1±7.9; Post: 21.4±4.9), “Impatient” (Pre: 8.4±3.0; Post: 2.2±1.5), and “Urge to use an e-cigarette” (Pre: 57.5±7.6; Post: 21.2±4.4). A significant main effect of condition was also observed for the item “Craving an e-cigarette,” and the mean ratings collapsed across time for this item were: (No Mouthpiece: 45.7±7.1; Mouthpiece: 31.8±7.4).
Figure 2.
Mean ratings (±SEM) for four visual analog scale items from 13 experienced ECIG users using their preferred device and strength/flavor in two sessions that differed by whether a mouthpiece-based topography system was attached to the ECIG. “Urge to Use an e-cigarette” (top left) and “Anxious” (top right) were from the Hughes-Hatsukami withdrawal scale. “Light-headed” (bottom left) was from the Direct Effects of Nicotine scale and “Calm” (bottom right) was from the Direct Effects of ECIG Use scale. Filled symbols indicate a significant difference from before ECIG use (i.e., −30). All ps < .05; Tukey’s HSD.
Direct Effects of Nicotine
Main effects of time were observed on VAS items evaluating “Dizzy” and “Light-headed,” and the data for “Light-headed” (the item with the largest F value) are displayed in Figure 2 (bottom, left). Collapsed across condition, mean (±SEM) post-ECIG use scores for “Dizzy” increased significantly relative to the pre-use scores (Pre: 0.9±0.5; Post: 7.3±3.1) while mean scores for the item “light-headed” also increased significantly after use (Pre: 1.9±1.1; Post: 13.8±3.9). A main effect of condition was observed on the VAS item evaluating “Weak.” Collapsed across time, the mean (±SEM) score for “Weak” was greater in the condition with a mouthpiece (4.9±1.9) than in the no mouthpiece condition (1.0±0.7).
Direct Effects of ECIG Use
Main effects of time were observed for several items, all independent of condition. Figure 2 (bottom, right) displays the results for one representative measure, “Calm,” for both conditions and time points. Collapsed across condition, mean scores (±SEM) were: “Awake” (Pre: 5.4±4.0; Post: 26.5±5.9), “Calm” (Pre: 5.6±4.1; Post: 33.0±5.6), “Concentrate” (Pre: 5.3±4.0; Post: 17.0±4.9), “Dizzy” (Pre: 1.8±1.8; Post: 18.3±4.8), “Pleasant” (Pre: 5.8±4.2; Post: 68.1±6.1), “Reduce hunger” (Pre: 5.5±4.0; Post: 19.0±5.5), “Would like to use another ECIG right now” (Pre: 13.2±6.4; Post: 43.8±7.7), “Satisfying” (Pre: 5.7±4.1; Post: 66.8±6.2), and “Taste good” (Pre: 5.6±4.2; Post: 76.6±4.9).
Topography
As shown in Table 3, independent-samples t tests (equal variances not assumed) comparing various topography measures from the present study in which ECIG users used ECIGs with data from Kleykamp23; (see Kleykamp et al.22 for method) in which tobacco cigarette smokers used tobacco cigarettes revealed significant differences for puff volume: t (12.4) = 3.58, p < .01; puff duration: t (12.3) = 9.50, p < .001; and flow rate: t (14.2) = −4.48, p < .01. In the present study, participants using ECIGs took larger and longer puffs, with lower flow rates, compared to tobacco cigarette smokers using tobacco cigarettes.
Table 3.
Mean (SEM) Puff Parameters for the Present Study With ECIGs (N = 13) and for a Previously Published Study With Tobacco Cigarettes (Kleykamp, 23 N = 123; see also Kleykamp et al. 22 )
| Volume (ml) | Duration (s) | Flow rate (ml/s) | |
|---|---|---|---|
| ECIGs | 101.37a (50.01) | 4.16a (1.06) | 24.17a (10.66) |
| Tobacco cigarettes | 51.29 (19.23) | 1.36 (0.38) | 37.97 (9.66) |
Data from cigarette smokers were taken from the first cigarette of a session in which no other sources of nicotine were available. Independent-samples t tests (equal variances not assumed) were used to compare means for puff parameters between the present study and Kleykamp, 23 . All parameters were significantly different (p < .01).
a Indicates a significance difference between ECIGs and tobacco cigarettes on that measure (p < .01; independent samples t test, equal variances not assumed).
Topography Equipment Questionnaire
Mean post-ECIG use VAS values for the six topography equipment items were: “Affect taste”: (8.5, SD = 15.2), “Alter behavior”: (12.5, SD = 20.6): “Increase awareness”: (20.1, SD = 25.3), “Make using your ECIG more difficult”: (28.7, SD = 30.3), “Less likely to use your ECIG”: (13.0, SD = 26.9), and “Reduce ECIG use”: (11.1, SD = 22.4).
Discussion
The primary purpose of this study was to examine the influence of a mouthpiece-based topography measurement device on nicotine delivery, heart rate, and subjective effects in experienced ECIG users using their preferred device and liquid. Results of this study provide no evidence that mouthpiece-based topography measurement influences ECIG users’ nicotine delivery, heart rate, or most measures of the withdrawal suppression associated with ECIG use. Participants reported that the mouthpiece may influence their ECIG use, including increasing their awareness of use, and making ECIG use more difficult (similar to effects noted by Blank et al.8 with cigarette smokers using mouthpiece-based recording devices). Similar to previous research3–5 the present study also suggests that experienced ECIG users obtain physiologically active doses of nicotine and that some abstinence symptoms can be suppressed after short-term ECIG use; a placebo controlled study is necessary to determine unequivocally that these effects are nicotine-mediated. Also like previous research, participants in the present study were able to increase their plasma nicotine concentrations to levels approaching those observed in cigarette smokers.4
A secondary purpose of this study was to measure ECIG topography in experienced users, including variables that cannot be measured via observation (i.e., puff volume and flow rate). The mean puff duration reported here using mouthpiece-based equipment (4.16 s) is consistent with previous reports of 4.2 s11 and 4.3 s12 using video recordings. This report is the first to measure puff volume and flow rate in experienced ECIG users, and the data indicate that ECIG and tobacco cigarette puff topography differ significantly. Specifically, Table 3 compares the volume, duration, and flow reported here with data collected from 123 tobacco cigarette smokers who smoked a cigarette ad libitum in our laboratory using very similar recording equipment (note that, for these cigarette smokers puff number and IPI were not held constant23). As the table shows, ECIG users take puffs that are larger and longer than cigarette smokers, and also have a much slower flow rate. Recent data exploring factors that influence ECIG aerosol nicotine yield suggest that puff duration is critical, though flow rate is not (Talih et al., in press). Thus, we suggest that ECIG users learn to take longer puffs to increase nicotine delivery, and to take slower puffs simply because faster ones take more effort. However, the low flow rates observed here highlight the importance of using topography equipment that is sensitive to low flow rates in order to increase accuracy of ECIG topography measurement. Further study with larger samples and diverse populations (e.g., ECIG-naïve cigarette smokers) will be required to understand ECIG effects and to inform regulation. For example, for other tobacco products, there is a high correlation between the toxicant exposure of a user and the toxicant yield of product emissions when the machine that is “puffing” to produce the emissions is programmed to “puff” in a way that mimics human behavior precisely.24 If this same relationship holds for ECIGs, regulatory agencies can use puff topography data collected from ECIG users to understand ECIG aerosol toxicant content and estimate ECIG user toxicant exposure accurately.
Interestingly, nicotine delivery varied considerably amongst the experienced ECIG users in the present study. One likely explanation for this variability is differences in puff topography (e.g., longer puff durations likely result in greater nicotine delivery). Unfortunately, the small sample size and many different ECIGs used make very challenging any examination of the association between puff topography and nicotine delivery from the present results. Nicotine delivery may also have varied due to the different liquids used. For example, an increase in plasma nicotine concentration of only 3.4ng/ml collapsed across condition was observed in one participant who used a liquid labeled as 18mg/ml, despite a mean puff duration greater than 4 s. In contrast, another participant used a different brand of liquid that was labeled as 12mg/ml, also averaged 4-s puffs, and a mean plasma nicotine concentration increase collapsed across condition of 18.0ng/ml was observed. For these two participants, the actual concentration of their liquid may have differed from the labeled concentration, as noted elsewhere.25
There were several limitations to the present study. First, participants were permitted to use their preferred ECIG battery and e-liquid, both of which varied considerably. Assessing the relationship between puff topography and plasma nicotine concentrations was difficult, given the variability in products and design features (e.g., battery voltage, PG/VG ratio, nicotine concentrations, etc.). Future research might include studies in which one of these design features is manipulated systematically while the others are held constant, in order to understand how each of them influences nicotine delivery, subjective effects, and other outcomes relevant to product effect and regulation. Second, standardization of the type of e-liquid heater system used (e.g., using one type of cartomizer for this study) was necessary to allow topography recording. Using this standardized system, as opposed to participants’ preferred one, may have influenced participants’ puff topography and/or nicotine delivery. Future research examining ECIG topography would benefit from mouthpieces that can accommodate “tank” and other systems so that participants can use their preferred products. In addition, the small sample and laboratory setting are limitations. Portable devices that enable the recording of ECIG puff topography in a non-laboratory setting could provide more naturalistic puff topography data, though these devices would need to be validated with the low flow rate puffs associated with ECIG use. Some ECIG models purportedly record certain puff topography variables (e.g., “Smokio” marketing materials indicate that it can record puff number and duration; “eVic” marketing materials indicate that it can record puff number), but the reliability and validity of these measures has not been assessed empirically. Finally, future laboratory research should examine puff topography in ECIG users using their device ad libitum, as this may aid in understanding ECIG users’ behaviors and/or toxicant exposure (including nicotine).
In summary, the present study demonstrated no influence of mouthpiece-based topography measurement on ECIG users’ nicotine delivery, heart rate, as well as many subjective effects. ECIG users clearly can obtain nicotine from their preferred device/liquid combinations, and the doses they receive can approach those observed in cigarette smokers, even after as few as 10 puffs. Experienced ECIG users’ puff topography differs markedly from that of cigarette smokers’, highlighting the importance of using ECIG-specific topography hardware and software, as well as the potential value of programming machines used to generate tobacco product emissions (i.e., puffing machines) with data that allow the machine to mimic the behavior of ECIG users.26 There is much more to be learned about factors that influence ECIG nicotine yield and delivery, and studies that manipulate these factors systematically are needed.
Funding
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number P50DA036105 and the Center for Tobacco Products of the US Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Declaration of Interests
None declared.
Acknowledgments
Portions of this work were presented at the 20th annual meeting of the Society for Research on Nicotine and Tobacco. We would like to thank B. Kilgalen, J. Austin, K. Pettaway, and K. Osei for their assistance in data collection and management, as well as M-S Yen and S. Sun from the VCU Department of Biostatistics for assistance with the statistical analysis.
References
- 1. Centers for Disease Control and Prevention (CDC). Notes from the field: electronic cigarette use among middle and high school students-United States, 2011–2012. MMWR. 2013;62:729 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6235a6.htm. Accessed November 17, 2014. [PMC free article] [PubMed] [Google Scholar]
- 2. King BA, Alam S, Promoff G, Arrazola R, Dube SR. Awareness and ever-use of electronic cigarettes among US adults, 2010-2011. Nicotine Tob Res. 2013;15:1623–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, Voudris V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep. 2014;4:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob Res. 2013;15:267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dawkins L, Corcoran O. Acute electronic cigarette use: nicotine delivery and subjective effects in regular users. Psychopharmacology. 2013;231:401–407. [DOI] [PubMed] [Google Scholar]
- 6. Vansickel AR, Cobb CO, Weaver MF, Eissenberg T. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol Biomarkers Prev. 2010;19:1945–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herning RI, Jones RT, Bachman J, Mines AH. Puff volume increases when low-nicotine cigarettes are smoked. BMJ. 1981;283:187 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1506678/pdf/bmjcred00668-0023.pdf. Accessed September 17, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blank MD, Disharoon S, Eissenberg T. Comparison of methods for measurement of smoking behavior: mouthpiece-based computerized devices versus direct observation. Nicotine Tob Res. 2009;11:896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Breland AB, Buchhalter AR, Evans SE, Eissenberg T. Evaluating acute effects of potential reduced-exposure products for smokers: clinical laboratory methodology. Nicotine Tob Res. 2002;4:131–140. [DOI] [PubMed] [Google Scholar]
- 10. Puustinen P, Olkkonen H, Kolonen S, Tuomisto J. Microcomputer-aided measurement of puff parameters during smoking of low- and medium-tar cigarettes. Scand J Clin Lab Invest. 1987;47:655–660. [DOI] [PubMed] [Google Scholar]
- 11. Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities’ regulation. Int J Environ Res Public Health. 2013;10:2500–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hua M, Yip H, Talbot P. Mining data on usage of electronic nicotine delivery systems (ENDS) from YouTube videos. Tob Control. 2013;22:723–728. [DOI] [PubMed] [Google Scholar]
- 13. Kosmider L, Sobczak A, Knysak J, Goniewicz ML. Effects of solvent and battery output voltage on nicotine levels released from electronic cigarette. Paper presented at the 20th Annual Meeting of the Socieity for Resarch on Nicotine and Tobacco; February 2014; Seattle, WA. [Google Scholar]
- 14. Etter JF. The electronic cigarette: an alternative to tobacco? Geneva, Switzerland: Jean-François Etter; 2012. [Google Scholar]
- 15. Breland AB, Kleykamp BA, Eissenberg T. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nicotine Tob Res. 2006;8:727–738. [DOI] [PubMed] [Google Scholar]
- 16. Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. http://file:///C:/Users/Tory/Downloads/archpsyc_43_3_013%20(1).pdf. Accessed September 17, 2014. [DOI] [PubMed] [Google Scholar]
- 17. Evans SE, Blank M, Sams C, Weaver MF, Eissenberg T. Transdermal nicotine-induced tobacco abstinence symptom suppression: nicotine dose and smokers’ gender. Exp Clin Psychopharmacol. 2006;14:121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Foulds J, Stapleton J, Feyerabend C, Vesey C, Jarvis M, Russell MA. Effect of transdermal nicotine patches on cigarette smoking: a double blind crossover study. Psychopharmacology. 1992;106:421–427. [DOI] [PubMed] [Google Scholar]
- 19. Pickworth WB, Bunker EB, Henningfield JE. Transdermal nicotine: reduction of smoking with minimal abuse liability. Psychopharmacology. 1994;115:9–14. [DOI] [PubMed] [Google Scholar]
- 20. Eissenberg T. Acute effects of electronic cigarettes in adults: nicotine delivery and abuse liability. Oral presentation in the Symposium entitled “Electronic Cigarettes: current state of the knowledge and future research” Chaired by Dr. Suchitra Krishnan-Sarin. 20th Annual Meeting of the Society for Research on Nicotine and Tobacco (SRNT); February 2014; Seattle, WA. [Google Scholar]
- 21. Shihadeh A, Azar S, Antonios C, Haddad H. Towards a topographical model of narghile water-pipe café smoking. Pharmacol Biochem Behav. 2004;79:75–82. [DOI] [PubMed] [Google Scholar]
- 22. Kleykamp BA, Jennings JM, Sams C, Weaver MF, Eissenberg T. The influence of transdermal nicotine on tobacco/nicotine abstinence and the effects of a concurrently administered cigarette in women and men. Exp Clin Psychopharmacol. 2008;16:99–112. [DOI] [PubMed] [Google Scholar]
- 23. Talih S., Balhas Z., Eissenberg T., Salman R., Karaoghlanian N., El-Hellani A. … & Shihadeh A. (in press). Effects of User Puff Topography, Device Voltage, and Liquid Nicotine Concentration on Electronic Cigarette Nicotine Yield: Measurements and Model Predictions. Nicotine & Tobacco Research. [DOI] [PMC free article] [PubMed]
- 24. Kleykamp BA. The influence of transdermal nicotine on tobacco/nicotine withdrawal and the effects of a concurrently administered cigarette in women and men. (Doctoral dissertation). 2007. http://search.proquest.com/docview/304705084/fulltextPDF/C39E816FB2C943B7PQ/1?accountid=14780. Accessed September 17, 2014. [Google Scholar]
- 25. Shihadeh AL, Eissenberg TE. Significance of smoking machine toxicant yields to blood-level exposure in water pipe tobacco smokers. Cancer Epidemiol Biomarkers Prev. 2011;20:2457–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trehy ML, Ye W, Hadwiger ME, et al. Analysis of electronic cigarette cartridges, refill solutions, and smoke for nicotine and nicotine related impurities. J Liq Chromatogr R T. 2011;34:1442–1458. [Google Scholar]
- 27. Evans SE, Hoffman AC. Electronic cigarettes: abuse liability, topography and subjective effects. Tob Control. 2014;23:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]