Abstract
Background
Previous studies indicate that smoking affects the outcome of some infections and is a risk factor for Puumala virus (PUUV) infection. The aim of this study was to assess the effect of smoking on the clinical severity of PUUV infection and the prevalence of smoking in patients with PUUV infection.
Methods
A questionnaire on smoking habits was sent to 494 patients in 2012, who had been treated in Tampere University Hospital, Finland, for serologically confirmed PUUV infection during years 1982–2012.
Results
Of all patients, 357 (72%) participated. Maximum plasma creatinine level measured during acute illness was significantly higher in current smokers than in non-smokers (median: 273 versus 184 µmol/L, P < 0.001). Current smokers had a higher maximum blood leucocyte count than non-smokers (median: 10.8 versus 8.9 × 109/L, P < 0.001) and they were younger than non-smokers (38 versus 45 years, P < 0.001). There were no differences between current smokers and non-smokers in the other variables reflecting the severity of PUUV infection. Altogether 51% were current smokers at the time of onset of the illness, 57% of males and 36% of females. During these years in Finland, smoking among males in the same aged population has decreased from 33 to 22% and among females, smoking has varied between 14 and 20%.
Conclusions
Smoking is common in patients with PUUV infection. Current smokers suffer from more severe acute kidney injury (AKI) and they have higher leucocyte count than non-smokers in PUUV infection. Smoking cessation decreases the risk of severe AKI to the same level as observed in never-smokers.
Keywords: acute kidney injury, acute tubulointerstitial nephritis, hantavirus infection, Puumala virus, smoking
INTRODUCTION
Puumala virus (PUUV) is a member of Hantavirus genus and it is carried by the bank vole (Myodes glareolus) [1]. Transmission of PUUV to humans occurs by inhalation of aerosols from infectious rodent excreta [1]. Hantaviruses have been recognized to cause two kinds of clinical syndromes in humans, haemorrhagic fever with renal syndrome (HFRS) in Eurasia and hantavirus cardiopulmonary syndrome (HCPS) in the Americas [2]. However, increasing evidence suggests HFRS and HCPS to be the same disease, proposed to be called ‘hantavirus disease’ or ‘hantavirus fever’ [3–5]. Hantaviruses causing HFRS include PUUV, Dobrava, Hantaan and Seoul viruses [6]. PUUV-induced nephropathia epidemica, a mild form of HFRS, is common in Fennoscandia (Finland and Scandinavia) [2]. In Finland, ∼1000–3000 serological PUUV infection diagnoses are made annually and the seroprevalence in the population is 5% [7]. Large registry data from Finland have shown that 62% of cases are males, and the highest incidence is observed in the age group of 34–64 years [8].
The clinical course of PUUV infection varies from asymptomatic to fatal; the overall case fatality rate is low, ranging from 0.08% [8] to 0.4% [9]. Host genetics influence the clinical picture [10–12]. The most typical symptoms in PUUV infection are high fever, headache, nausea, abdominal pain and lumbalgiae [6, 13]. Haemorrhages are rare. Typical laboratory findings are thrombocytopenia, leukocytosis, anaemia and elevated plasma C-reactive protein (CRP) [6, 13]. Renal involvement includes transient, sometimes massive proteinuria, microscopic haematuria and acute kidney injury (AKI), which is followed by polyuria and spontaneous recovery. Up to 6% of hospitalized patients need transient haemodialysis treatment [6, 13]. The characteristic renal histopathological finding is acute tubulointerstitial nephritis [1].
Smoking is known to affect the severity of some bacterial and viral infections, such as pneumonia, influenza and tuberculosis [14, 15]. In the respiratory tract, smoking leads to structural and functional changes [14], which may alter both susceptibility to and the course of infection. In addition to smoking, other factors, such as age, gender, body mass index (BMI) and comorbid diseases, affect the incidence and severity of some infectious diseases [14, 16, 17].
Epidemiological studies have concluded that smoking is an independent risk factor for the development of proteinuria, progression of diabetic nephropathy, progression of chronic kidney disease (CKD) and graft failure after kidney transplantation [18, 19]. However, the role of cigarette smoking in development of AKI has been less studied. This may be an important and underestimated entity, probably commonly masked by other risk factors of AKI, such as advanced age, obesity and comorbid conditions. Recently, a novel mechanism by which cigarette smoking may be involved in the development of AKI has been reported [20]. Chronic nicotine exposure may increase production of reactive oxygen species (ROS) and mitochondrial depolarization resulting in an injury in proximal tubule cells [20].
There are two previous reports of smoking as a risk factor to PUUV infection. Considerable odds ratios (ORs) of 9.1 and 3.6 were obtained in case–control studies performed in Belgium and Finland, respectively [21, 22]. However, there are no previous data on the effect of smoking on clinical course of PUUV infection or other hantavirus infections. The aim of this study was to investigate whether smoking affects the severity of the PUUV infection and to define the prevalence of smoking among Finnish patients with PUUV.
MATERIALS AND METHODS
Subjects
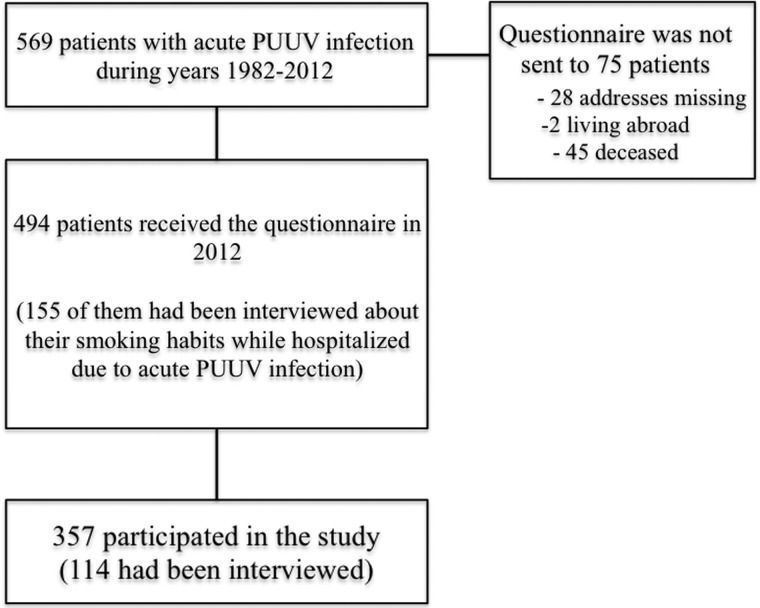
The study was carried out in Tampere University Hospital, Finland. All patients gave informed consent to participate and the study protocol was approved by the Ethics Committee of Tampere University Hospital. The study population consisted of 569 PUUV patients who had participated in our previous clinical studies [10–12, 23]. Patients had been treated at Tampere University Hospital with serologically confirmed PUUV infection [7, 24–26] during the years 1982–2012. Data on smoking habits were collected by a questionnaire sent to 494 patients from January to March 2012. Addresses could not be found in 28 patients, 2 were living abroad and 45 were deceased at the time of the study. Out of all patients, 357 (72%) participated in the study. The design of the study is described in Figure 1.
FIGURE 1:
Design of the study.
The mean age of participants was 41.3 (SD 12.2) years at the onset of disease and 257 (72%) of them were males. Forty patients had at least one of the following diseases before PUUV infection: hypertension, coronary artery disease, hypercholesterolaemia or diabetes. Hypertension was seen in 24 patients, coronary artery disease in 8 patients, diabetes in 7 patients and hypercholesterolaemia in 7 patients. Previous asthma had been diagnosed in eight patients and hypothyreosis in six patients. In addition, there were some separate chronic diseases in 24 patients. Otherwise the patients had been relatively healthy and 79% of them did not have any chronic diseases prior to PUUV infection. None of the patients had any known CKD prior to acute illness. Patients had not received non-steroidal anti-inflammatory drugs during their hospital stay.
Laboratory markers and clinical variables
Plasma creatinine and CRP concentrations, blood leucocyte and thrombocyte counts and blood haematocrit were determined by standard methods. The highest and lowest values of the various variables measured during hospitalization for each patient were designated as the maximum and minimum values, respectively. The clinical variables, such as length of hospitalization, systolic and diastolic blood pressure and change in weight (reflecting fluid retention during the oliguric phase) during acute illness, were obtained. Severe AKI was defined by plasma creatinine level equal to or more than 353.6 µmol/L or need of dialysis. This definition of severe AKI meets the criteria of KDIGO Clinical Practice Guidelines for AKI Stage 3 [27].
Questionnaire
The data of smoking history were collected by a questionnaire. The questionnaire contained six questions concerning smoking habits before and at the onset of PUUV infection. Previous and current smoking status was asked as well as the lifelong exposure to cigarette smoke at home or at work (passive smoking). The amount of daily smoking was divided into four groups: occasionally, under 10 cigarettes/cigars/pipes per day, 10–20 cigarettes/cigars/pipes per day and over 20 cigarettes/cigars/pipes per day.
According to the questionnaire, patients were divided into three groups: current smokers, ex-smokers and never-smokers. Current smokers smoked at the time of the onset the illness. Ex-smokers had quit smoking at some stage before acute PUUV infection, and never-smokers reported that they had never smoked. To evaluate the effect of current smoking on the severity of acute PUUV infection, the patients were divided into two groups: current smokers and non-smokers. The group of non-smokers included both never-smokers and ex-smokers.
Occupational status of the patients was considered as a potential confounder when studying the relationship between smoking and PUUV. The patients were divided into three groups in accordance with Statistics Finland's occupational categories (http://www.tilastokeskus.fi). More than half of the patients were blue-collar workers (54%), one-fourth were upper white-collar workers (24%) and the rest were lower white-collar workers (22%). Students and pensioned (n = 35) were excluded from the analysis, because there was no knowledge of their future or previous occupation.
The validity of the smoking information
The research nurse interviewed the patients during their hospital stay during the years 2005–12. The current smoking status at that time was asked. One hundred fifty-five (31%) of 494 patients to whom the questionnaire was sent had been interviewed (see Figure 1). According to this interview, smoking habits were known in 114 patients, who responded to the questionnaire, and in 41 of non-respondents. These interview data were compared with the data obtained from the questionnaire to test the validity of the questionnaire and the memory of respondents. There were differences in 2 (1.8%) out of 114 answers only between the questionnaire and the interview. Therefore, we consider the questionnaire data on smoking valid.
Analysis of non-respondents
Between respondents and non-respondents, there were no differences between gender distribution, BMI or clinical severity of PUUV infection (maximum creatinine level, maximum leucocyte count, minimum thrombocyte count, change of weight during acute illness or length of hospital stay). In this respect, respondents represented well the entire study population. Non-respondents were slightly younger than respondents at the time of the infection (mean age: 37 versus 41, P < 0.001). Based on the interview of the research nurse, non-respondents were more likely to be current smokers than respondents (73 versus 49%, P = 0.018).
Statistical analysis
The SPSS (version 20, IBM, Chicago, IL) statistical software package was used for statistical analyses. Data are presented as medians and ranges for continuous variables and numbers and percentages for categorical variables. Comparisons between the groups were made with Mann–Whitney U-test for continuous variables, while Pearson chi-square test or Fisher's exact test was used for categorical data. Correlations were calculated by the Spearman's rank correlation test. Logistic regression analysis was carried out to identify factors determining the severity of AKI. ORs were expressed with their 95% confidence intervals (CI). All tests were two sided and a P-value < 0.05 was considered as statistically significant.
RESULTS
Altogether 183 out of 357 (51%) patients were current smokers at the time of PUUV infection. Of non-smokers, 56 (16%) were ex-smokers and 118 (33%) had never smoked. Smoking was more prevalent in men than in women: 57% of men (147/257) and 36% of women (36/100) were current smokers (P < 0.001). Current smokers were younger than non-smokers (38 versus 45 years, P < 0.001), and they had fewer chronic diseases than the non-smokers (13 versus 29%, P < 0.001). Non-smokers had cardiovascular diseases and/or diabetes more often than current smokers (16 versus 6%, P = 0.001). Smoking was most prevalent among blue-collar workers, of which 62% were current smokers, while 53 and 38% of lower white-collar and upper white-collar workers were current smokers, respectively (P = 0.002).
As shown in Table 1, the median maximum plasma creatinine level was significantly higher in current smokers than in non-smokers (273 versus 184 µmol/L, P < 0.001). This difference was statistically significant in men only. Seventy-five of 183 (41%) current smokers developed severe AKI (plasma creatinine level equal to or higher than 353.6 µmol/L) compared with 46 out of 174 (26%) of non-smokers (P = 0.005).
Table 1.
Maximum plasma creatinine concentration and blood leucocyte count in current smokers and in non-smokers (ex-smokers and never-smokers)
| Current smokers |
n | Non-smokers |
n | P | |||
|---|---|---|---|---|---|---|---|
| Median | Range | Median | Range | ||||
| All patients | 183 | 174 | |||||
| Plasma creatinine max. (μmol/L) | 273 | 51–1645 | 184 | 52–1537 | <0.001 | ||
| Blood leucocyte count max (×109/L) | 10.8 | 4.1–44.7 | 8.9 | 3.8–50.3 | <0.001 | ||
| Males | 147 | 110 | |||||
| Plasma creatinine max. (μmol/L) | 292 | 71–1645 | 191 | 67–1537 | 0.005 | ||
| Blood leucocyte count max (×109/L) | 11.0 | 5.4–44.7 | 9.2 | 3.8–50.3 | 0.005 | ||
| Females | 36 | 64 | |||||
| Plasma creatinine max (μmol/L) | 227 | 51–1156 | 175 | 52–959 | 0.529 | ||
| Blood leucocyte count max (×109/L) | 9.7 | 4.1–43.3 | 8.6 | 4.2–31.2 | 0.030 | ||
Differences between genders are also shown. Values are expressed as median and ranges.
In logistic regression analysis with severe AKI as a dependent variable and smoking status (current smoker versus non-smoker), age, gender and occupational category (three categories) as independent variables, the only significant factor determining severe AKI turned out to be smoking status (OR: 1.8; 95% CI: 1.1–3.0). Previous hypertension, coronary artery disease, hypercholesterolaemia or diabetes did not influence the risk of severe AKI. There were no differences in maximum plasma creatinine level in patients with or without such comorbidities (172 versus 226 µmol/L, P = 0.360).
The amount of smoking did not affect the severity of AKI. Maximum creatinine level did not differ between heavy smokers, who smoked >20 cigarettes per day, and those who smoked less, i.e. 1–19 cigarettes per day (275 versus 271 µmol/L, P = 0.470). There were only 14 such never-smokers, who had been exposed to cigarette smoke at work or at home (passive smokers). There was no difference in median maximum creatinine level in 104 never-smokers, who had never been exposed to cigarette smoke compared with 14 passive smokers (223 versus 206 µmol/L, P = 0.973).
To evaluate the effect of previous smoking on the severity of acute PUUV-induced AKI, we compared a group of never-smokers with those who had smoked previously but stopped smoking at some stage before PUUV infection (ex-smokers). There were no significant differences in maximum plasma creatinine level between these two subgroups (163 versus 197 µmol/L, P = 0.563).
As shown in Table 1, median maximum blood leucocyte count was significantly higher in current smokers than in non-smokers. This difference was seen in both genders. Leucocyte count did not differ between heavy smokers (>20 cigarettes per day) and those who smoked 1–19 cigarettes per day (10.6 versus 10.8 × 109/L, P = 0.874). There was no difference in leucocyte count between ex-smokers and never-smokers (8.8 versus 9.0 × 109/L, P = 0.314). A correlation between maximum creatinine level and maximum leucocyte count was found (r = 0.432, P < 0.001).
There were no differences between current smokers and non-smokers in other markers of disease severity, i.e. minimum or maximum haematocrit level, minimum thrombocyte level, duration of hospital stay or change of weight during the acute illness (data not shown). Plasma CRP level, the lowest or highest systolic or diastolic blood pressure measured during hospital care or BMI did not differ between smokers or non-smokers (data not shown).
DISCUSSION
The present large cohort study showed that current smokers had more severe PUUV-induced AKI than non-smokers. Those who had quit smoking at some stage before acute infection did not have such an increased risk. The number of daily smoked cigarettes did not influence the severity of AKI. Current smokers also had significantly higher maximum blood leucocyte count than non-smokers. More than half of the patients were current smokers at the time of disease onset, which is clearly more than in the average population in Finland. This finding confirms previous observations of smoking as a risk factor for PUUV infection [21, 22].
There are studies indicating that smoking may affect the outcome of bacterial and viral infections, such as pneumococcal pneumonia and influenza [14, 15]. A recent study by Bello et al. [15] showed that active smoking increases the risk of death from pneumococcal pneumonia independently of age and comorbid conditions. Smoking has also been found to be a risk factor for fatal outcome of human avian influenza A (H7N9) [28]. To our knowledge, no previous studies have been published on smoking and the severity of PUUV or other hantaviral infections.
In the present study, current smokers had higher maximum plasma creatinine level than non-smokers. This difference was statistically significant in males only. However, a similar trend was seen in females. The number of female smokers was small (n = 36), which might have affected the results. Interestingly, ex-smokers did not have more severe AKI than never-smokers, reflecting the reversibility of adverse effects of cigarette smoking after cessation.
The data of smoking history were collected by a questionnaire. We recognize the limitations of this kind of study design. There are, however, studies indicating that the validity of self-reported smoking is high and smokers very rarely claim to be non-smokers when self-reporting their smoking habits [29, 30]. Further, there was no inconsistency between the results of the questionnaire and the interviewed data of smoking.
Advanced age is a known risk factor for AKI. In the present study, current smokers were younger than non-smokers. The odds for developing severe AKI among the current smokers was 1.8 compared with the non-smokers. According to logistic regression analysis, when smoking status, age, gender and occupational category were chosen as independent variables, the only significant factor determining severe AKI turned out to be smoking status. Pre-existing CKD is a well-known risk factor for AKI. None of our patients had any known CKD prior to PUUV infection. In this previously relatively healthy population, vascular comorbidities or diabetes diagnosed prior to PUUV infection did not predispose to severe AKI, either.
Smokers had less chronic diseases than non-smokers. Smoking is known to predispose to cardiovascular diseases, but the effects of smoking are not usually seen before the age of 40 years. In the present study, smokers were younger than non-smokers (38 versus 45 years), which may explain the unexpected difference in the prevalence of chronic diseases between smokers and non-smokers.
In the present study, severe AKI was defined according to recent criteria of KDIGO Clinical Practice Guidelines for AKI (plasma creatinine level equal to or more than 353.6 µmol/L, or need of dialysis therapy). We did not use estimated glomerular filtration rate equations in our analysis, since the Modification of Diet in Renal Disease equation and other formulas have been developed in patients with stable CKD, and are inappropriate for use in AKI [31]. We are aware that all creatinine-based definitions of AKI can be misleading in patients whose creatinine kinetics and volume of distribution are variable [31].
The knowledge of association between smoking and development of AKI is limited. The suggested mechanisms of smoking-induced renal damage include non-haemodynamic and haemodynamic factors [18]. Smoking is known to increase blood pressure and heart rate, mainly because of the effects of nicotine [32]. Smoking also activates the renin–angiotensin system, which is thought to be one element in the pathophysiology of smoking-induced renal damage in CKD [33]. In the present study, there were no differences in acute-phase blood pressure levels between current smokers and non-smokers. That might indicate that smoking-related non-haemodynamic rather than haemodynamic mechanisms are involved in the development of PUUV-induced AKI.
Epidemiological reports have shown that smoking exacerbates the progression of CKD [19, 34]. Potential non-haemodynamic mechanisms affecting the progression of CKD are heavy metals, hypoxia, prothrombotic factors, oxidative stress, proinflammatory cytokines and the activation of nicotine acetylcholine receptors [18]. The knowledge about those mechanisms leading to AKI is limited. Smoking elevates oxidative stress/production of ROS in various organs including kidneys. The mitochondria are known to be affected by cigarette smoke. Arany et al. [20] have recently shown in a mouse model that chronic nicotine exposure increases protein p66shc expression resulting in mitochondrial ROS production and depolarization of mitochondria, consequently leading to injury in cultured proximal tubule cells.
Smoking can also lead to endothelial dysfunction [32]. Hantaviruses replicate in the endothelial cells without visible cytopathic effects [1, 6]. Hantavirus infection of endothelial cells leads to dysfunction of the normal barrier function of the endothelium causing capillary leakage [1, 6]. In the present study, patients who were current smokers were at increased risk to get severe AKI, but the amount of daily smoked cigarettes or history of ex-smoking did not influence the severity. There might, thus, be differences in the function of endothelium just at the time of PUUV infection between smokers and non-smokers leading to differences in the outcome.
A characteristic histopathological renal finding in acute PUUV infection is acute tubulointerstitial nephritis without specific glomerular changes [1]. Morphological abnormalities have been observed in the proximal tubular epithelium after exposure to chronic cigarette smoking [35] and low-grade damage of proximal tubules has also been found among smokers in the general population [35]. These pre-existing morphologic alterations in tubular cells among smokers might influence the severity of PUUV-induced acute tubulointerstitial nephritis. It is also theoretically possible that smoking may influence the infiltration of macrophages and T cells into interstitium during acute interstitial nephritis.
The maximum blood leucocyte count was significantly higher among current smokers compared with non-smokers. Chronic cigarette smoking produces 20–25% increase in the number of peripheral blood leucocytes [36]. The increase may be due to generalized stimulation of the bone marrow with the higher turnover of all leucocyte lines [36]. Leukocytosis is considered to be one marker of PUUV infection severity [37]. Also in this study, there was a correlation between the maximum leucocyte count and creatinine level.
In the Belgian pioneer study by Van Loock et al. [21], smoking was found to be a risk factor for PUUV infection with a high OR of 9.1. This finding was confirmed in the previous Finnish study [22] as well as in the present study. A Belgian study also showed that the amount of daily smoked cigarettes influenced the risk of getting PUUV infection [21].
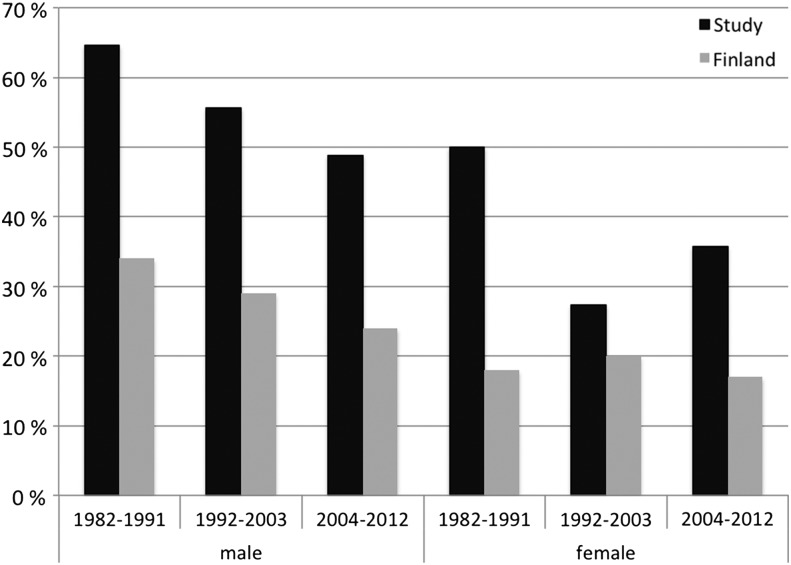
In our study, more than half of the patients were current smokers at the time of onset of the illness. Among men 57% and among women 37% smoked at that time. During the years 1982–2012, smoking in Finland among males decreased from 33 to 22% and among females, it has varied between 14 and 20% (www.thl.fi). A similar trend was found among the study populations, but during every decade, the study populations smoked clearly more than the average population in Finland (Figure 2).
FIGURE 2:
Percentage of daily smokers in 357 patients with acute PUUV infection (dark columns) and in the 15- to 64-year-old Finnish population (light columns) during three time periods in both genders (http://www.julkari.fi/bitstream/handle/10024/110537/URN_ISBN_978-952-245-931-2.pdf?sequence=1). The bar represents the average value within the presented time period.
Active smoking is known to increase the risk of developing influenza, community-acquired pneumonia and invasive pneumococcal disease, all transmitted through inhalation [14]. Also transmission of PUUV to humans occurs through inhalation of aerosols of infectious rodent excreta. It has been hypothesized that the condition of respiratory tract influences whether the infectious aerosol enters the alveoli and stays there long enough to cause the infection [22]. Smoking is known to lead to structural and functional changes in the respiratory tract [14]. In addition, smoking has effects on the local and systemic immune system [14]. These changes may alter both susceptibility to and the course of infection. One possible mechanism explaining smoking as a risk factor for severe PUUV infection could be higher local viral load due to effects of smoking to the respiratory tract. In fact, an early high viral load is thought to predict unfavourable outcome both in HCPS and in PUUV-induced HFRS [1].
In conclusion, current smokers are at clearly increased risk to suffer severe PUUV-induced AKI. The pathogenic mechanisms need further studies. Smoking cessation decreases the risk of severe AKI to the same level as never-smokers. These findings could be useful for clinicians to encourage the patients for smoking cessation.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this paper have not been published previously in whole or part, except in abstract format.
ACKNOWLEDGEMENTS
The skilful technical assistance of Ms Katriina Ylinikkilä and Ms Mirja Ikonen is greatly appreciated. This study was financially supported by the Competitive State Research Financing of the Expert Responsibility Area of Tampere University Hospital (9P031), European Commission Project ‘Diagnosis and control of rodent-borne viral zoonoses in Europe’ (QLK2-CT-2002-01358), Tampere Tuberculosis Foundation and by grants from the National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID) (U19 AI57319), the Academy of Finland, the Sigrid Jusélius Foundation and the Finnish Kidney Foundation.
REFERENCES
- 1.Mustonen J, Mäkelä S, Outinen T, et al. The pathogenesis of nephropathia epidemica: new knowledge and unanswered questions. Antiviral Res 2013; 100: 589–604 [DOI] [PubMed] [Google Scholar]
- 2.Vapalahti O, Mustonen J, Lundkvist A, et al. Hantavirus infections in Europe. Lancet Infect Dis 2003; 3: 653–661 [DOI] [PubMed] [Google Scholar]
- 3.Desmyter J, van Ypersele de Strihou C, van der Groen G. Hantavirus disease. Lancet 1984; 2: 158. [DOI] [PubMed] [Google Scholar]
- 4.Clement J, Maes P, Lagrou K, et al. A unifying hypothesis and a single name for a complex globally emerging infection: hantavirus disease. Eur J Clin Microbiol Infect Dis 2012; 31: 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clement J, Maes P, Van Ranst M. Hemorrhagic Fever with Renal Syndrome in the New, and Hantavirus Pulmonary Syndrome in the Old World: paradi(se)gm lost or regained? Virus Res 2014; 187: 55–58 [DOI] [PubMed] [Google Scholar]
- 6.Vaheri A, Strandin T, Hepojoki J, et al. Uncovering the mysteries of hantavirus infections. Nat Rev Microbiol 2013; 11: 539–550 [DOI] [PubMed] [Google Scholar]
- 7.Brummer-Korvenkontio M, Vapalahti O, Henttonen H, et al. Epidemiological study of nephropathia epidemica in Finland 1989–96. Scand J Infect Dis 1999; 31: 427–435 [DOI] [PubMed] [Google Scholar]
- 8.Makary P, Kanerva M, Ollgren J, et al. Disease burden of Puumala virus infections, 1995–2008. Epidemiol Infect 2010; 138: 1484–1492 [DOI] [PubMed] [Google Scholar]
- 9.Hjertqvist M, Klein SL, Ahlm C, et al. Mortality rate patterns for hemorrhagic fever with renal syndrome caused by Puumala virus. Emerg Infect Dis 2010; 16: 1584–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mustonen J, Partanen J, Kanerva M, et al. Genetic susceptibility to severe course of nephropathia epidemica caused by Puumala hantavirus. Kidney Int 1996; 49: 217–221 [DOI] [PubMed] [Google Scholar]
- 11.Mäkelä S, Mustonen J, Ala-Houhala I, et al. Human leukocyte antigen-B8-DR3 is a more important risk factor for severe Puumala hantavirus infection than the tumor necrosis factor-alpha(-308) G/A polymorphism. J Infect Dis 2002; 186: 843–846 [DOI] [PubMed] [Google Scholar]
- 12.Laine O, Joutsi-Korhonen L, Mäkelä S, et al. Polymorphisms of PAI-1 and platelet GP Ia may associate with impairment of renal function and thrombocytopenia in Puumala hantavirus infection. Thromb Res 2012; 129: 611–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mustonen J, Brummer-Korvenkontio M, Hedman K, et al. Nephropathia epidemica in Finland: a retrospective study of 126 cases. Scand J Infect Dis 1994; 26: 7–13 [DOI] [PubMed] [Google Scholar]
- 14.Huttunen R, Heikkinen T, Syrjänen J. Smoking and the outcome of infection. J Intern Med 2011; 269: 258–269 [DOI] [PubMed] [Google Scholar]
- 15.Bello S, Menendez R, Torres A, et al. Tobacco smoking increases the risk of death from pneumococcal pneumonia. Chest 2014; 146: 1029–1037 [DOI] [PubMed] [Google Scholar]
- 16.Huttunen R, Laine J, Lumio J, et al. Obesity and smoking are factors associated with poor prognosis in patients with bacteraemia. BMC Infect Dis 2007; 7: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein SL, Marks MA, Li W, et al. Sex differences in the incidence and case fatality rates from hemorrhagic fever with renal syndrome in China, 2004–2008. Clin Infect Dis 2011; 52: 1414–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orth SR, Hallan SI. Smoking: a risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients—absence of evidence or evidence of absence? Clin J Am Soc Nephrol 2008; 3: 226–236 [DOI] [PubMed] [Google Scholar]
- 19.Speeckaert MM, Delanghe JR, Vanholder RC. Chronic nicotine exposure and acute kidney injury: new concepts and experimental evidence. Nephrol Dial Transplant 2013; 28: 1329–1331 [DOI] [PubMed] [Google Scholar]
- 20.Arany I, Clark J, Reed DK, et al. Chronic nicotine exposure augments renal oxidative stress and injury through transcriptional activation of p66shc. Nephrol Dial Transplant 2013; 28: 1417–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Loock F, Thomas I, Clement J, et al. A case-control study after a hantavirus infection outbreak in the south of Belgium: who is at risk? Clin Infect Dis 1999; 28: 834–839 [DOI] [PubMed] [Google Scholar]
- 22.Vapalahti K, Virtala AM, Vaheri A, et al. Case-control study on Puumala virus infection: smoking is a risk factor. Epidemiol Infect 2010; 138: 576–584 [DOI] [PubMed] [Google Scholar]
- 23.Outinen TK, Tervo L, Mäkelä S, et al. Plasma levels of soluble urokinase-type plasminogen activator receptor associate with the clinical severity of acute Puumala hantavirus infection. PLoS One 2013; 8: e71335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hedman K, Vaheri A, Brummer-Korvenkontio M. Rapid diagnosis of hantavirus disease with an IgG-avidity assay. Lancet 1991; 338: 1353–1356 [DOI] [PubMed] [Google Scholar]
- 25.Vapalahti O, Kallio-Kokko H, Närvänen A, et al. Human B-cell epitopes of Puumala virus nucleocapsid protein, the major antigen in early serological response. J Med Virol 1995; 46: 293–303 [DOI] [PubMed] [Google Scholar]
- 26.Vapalahti O, Lundkvist A, Kallio-Kokko H, et al. Antigenic properties and diagnostic potential of Puumala virus nucleocapsid protein expressed in insect cells. J Clin Microbiol 1996; 34: 119–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khwaja A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin Pract 2012; 120: 179–184 [DOI] [PubMed] [Google Scholar]
- 28.Liu S, Sun J, Cai J, et al. Epidemiological, clinical and viral characteristics of fatal cases of human avian influenza A (H7N9) virus in Zhejiang Province, China. J Infect 2013; 67: 595–605 [DOI] [PubMed] [Google Scholar]
- 29.Prochazka M, Hall P, Granath F, et al. Validation of smoking history in cancer patients. Acta Oncol 2008; 47: 1004–1008 [DOI] [PubMed] [Google Scholar]
- 30.Vartiainen E, Seppälä T, Lillsunde P, et al. Validation of self reported smoking by serum cotinine measurement in a community-based study. J Epidemiol Community Health 2002; 56: 167–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen MT, Maynard SE, Kimmel PL. Misapplications of commonly used kidney equations: renal physiology in practice. Clin J Am Soc Nephrol 2009; 4: 528–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orth SR. Effects of smoking on systemic and intrarenal hemodynamics: influence on renal function. J Am Soc Nephrol 2004; 15 (Suppl 1): S58–S63 [DOI] [PubMed] [Google Scholar]
- 33.Orth SR. Smoking and the kidney. J Am Soc Nephrol 2002; 13: 1663–1672 [DOI] [PubMed] [Google Scholar]
- 34.Orth SR, Ritz E. The renal risks of smoking: an update. Curr Opin Nephrol Hypertens 2002; 11: 483–488 [DOI] [PubMed] [Google Scholar]
- 35.Arany I, Grifoni S, Clark JS, et al. Chronic nicotine exposure exacerbates acute renal ischemic injury. Am J Physiol Renal Physiol 2011; 301: F125–F133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terashima T, Wiggs B, English D, et al. The effect of cigarette smoking on the bone marrow. Am J Respir Crit Care Med 1997; 155: 1021–1026 [DOI] [PubMed] [Google Scholar]
- 37.Libraty DH, Mäkelä S, Vlk J, et al. The degree of leukocytosis and urine GATA-3 mRNA levels are risk factors for severe acute kidney injury in Puumala virus nephropathia epidemica. PLoS One 2012; 7: e35402. [DOI] [PMC free article] [PubMed] [Google Scholar]