Abstract
The biological effect of an inorganic particle (i.e., silica) can be associated with a disruption in cell iron homeostasis. Organic compounds included in particles originating from combustion processes can also complex sources of host cell iron to disrupt metal homeostasis. We tested the postulate that (1) wood smoke particle (WSP) sequesters host cell iron resulting in a disruption of metal homeostasis, (2) this loss of essential metal results in both an oxidative stress and biological effect in respiratory epithelial cells, and (3) humic-like substances (HULIS), a component of WSP, have a capacity to appropriate cell iron and initiate a biological effect. BEAS-2B cells exposed to WSP resulted in diminished concentrations of mitochondrial 57Fe, whereas preincubation with ferric ammonium citrate (FAC) prevented significant mitochondrial iron loss after such exposure. Cellular oxidant generation was increased after WSP exposure, but this signal was diminished by coincubation with FAC. Similarly, exposure of BEAS-2B cells to 100 μg/mL WSP activated mitogen-activated protein (MAP) kinases, elevated NF-E2-related factor 2/antioxidant responsive element (Nrf2 ARE) expression, and provoked interleukin (IL)-6 and IL-8 release, but all these changes were diminished by coincubation with FAC. The biological response to WSP was reproduced by exposure to 100 μg/mL humic acid, a polyphenol comparable to HULIS included in the WSP that complexes iron. We conclude that (1) the biological response following exposure to WSP is associated with sequestration of cell iron by the particle, (2) increasing available iron in the cell diminished the biological effects after particle exposure, and (3) HULIS included in WSP can sequester the metal initiating the cell response.
Graphical abstract
INTRODUCTION
The human lung is regularly exposed to a variety of particles, including those in air pollution, cigarette smoke, environmental tobacco smoke, forest fires, emissions from gas and wood stoves, burning of biomass other than wood, and mining/processing of coal and mineral oxides. Features of the clinical presentation and changes in human physiology and pathology following exposure to many of these diverse particles appear to be comparable to some extent and can include (1) respiratory symptoms of cough, wheezing, and shortness of breath, (2) an acute, reversible decrement in pulmonary function and elevation in bronchial hyperreactivity, (3) histopathological changes of acute neutrophilic inflammation, emphysema, and parenchymal fibrosis, (4) hemorrheological changes with elevations in white blood cell counts and increases in C-reactive protein, fibrinogen, and blood viscosity, and (5) an association with cardiovascular disease.1–7 This shared clinical, physiological, and pathological presentation after exposure to many disparate particles suggests a common mechanism for their biological effect. Therefore, a single pathway may exist for all particles through which a biological effect is generated. If this is true, a proposed explanation for the activity of a specific particle could be relevant to all.
One proposal that could explain the biological effect common to these particles is an altered iron homeostasis in the host cell following their exposure. Iron is an essential micronutrient utilized in almost every aspect of normal cell function. Consequently, life has evolved with a dependency on iron availability, and all living organisms require it. Unfortunately, those same chemical properties that allow iron to function as a catalyst in reactions involving molecular oxygen can make it a threat to life via the generation of O2-based free radicals. In an aqueous environment, oxides, oxide minerals, and incompletely combusted carbon include oxygen-containing functional groups at their surface (e.g., Si–OH, CHO, and CO–OH groups). The deposition of particles with surfaces comprised of oxygen-containing functional groups introduces an electronegative interface, following deprotonation at physiologic pH values, into the lower respiratory tract. As a result of its electropositivity, iron cation has a high affinity for such oxygen-donor ligands. Therefore, this metal will react with oxygen-containing functional groups at particle surfaces. In the lower respiratory tract, retained particles have consistently demonstrated the capacity to accumulate iron from available cell sources, reflecting the ability of the surface to complex host iron.8,9
The host responds to a loss of requisite metal with attempts to resequester so as to make it less available to the particle surface. This is accomplished, in some measure, through storage in a less catalytically reactive state within ferritin. Following the introduction of any particle (and fiber) into the lung, this disruption of iron homeostasis and the attempt to re-establish normal metal equilibrium in the host can result in the formation of a ferruginous body.9,10 The disruption of host iron homeostasis could contribute to the generation of oxidative stress and a biological effect following particle exposure.
The complexation of iron by the particle surface with consequent oxidant generation is especially pertinent to particles that contain organic compounds including a wood smoke particle (WSP). Following exposure to the particle, organic constituents (e.g., humic-like substances or HULIS) retained in the lung can complex available metal.11,12 This can be observed in vivo with the formation of ferruginous bodies in survivors of fires involving wooden structures.13 We tested the postulate that (1) WSP sequesters host cell iron resulting in a disruption of cell metal homeostasis, (2) this loss of essential metal results in both an oxidative stress and biological effect in respiratory epithelial cells, and (3) HULIS are a component of WSP with the capacity to appropriate host iron and initiate a biological effect.
EXPERIMENTAL PROCEDURES
Materials
All reagents were from Sigma Co. (St. Louis, MO) unless otherwise specified. Wood smoke was generated by heating red oak wood on an electric heating element (Brinkmann Corporation, Dallas, TX) in a Quadra-fire 3100 woodstove (Colville, WA). The log was heated slowly over 4 to 6 h to maximize particle release. WSP was obtained by its mechanical disruption from the chimney above the woodstove followed by sonication in water to disaggregate the particles. Prior to precipitation on the chimney, the number median diameter was 0.14 ± 0.01 μm. The ratio of elemental to total carbon in the particle was 0.005 ± 0.006 (n = 2). Ionizable metal in the WSP was quantified as that concentration displaced into 1 N HCl (1.0 mg/1.0 mL) after 1 h agitation. These metals were quantified in triplicate using inductively coupled plasma optical emission spectroscopy (ICPOES; Model Optima 4300D, PerkinElmer, Norwalk, CT) and were (in ppm): 0.31 ± 0.02 aluminum, 7.66 ± 0/09 calcium, 0.09 ± 0.00 chromium, 0.02 ± 0.00 copper, 0.76 ± 0.01 iron, 0.35 ± 0.01 magnesium, 0.06 ± 0.00 nickel, 0.00 ± 0.00 vanadium, and 0.10 ± 0.01 zinc. HULIS in the WSP was isolated as that fraction soluble in aqueous solution at pH 8.5 but insoluble at pH 2.0. After drying, this was weighed and demonstrated to be 21.2 ± 4.7% total particle mass (n = 2).
Cell Culture
The respiratory epithelial cells used in all studies were BEAS-2B cells (S6 subclone; obtained from Dr. Curtis Harris). This is an immortalized line of normal human bronchial epithelium derived by transfection of primary cells with SV40 early region genes. Cells were grown to 90–100% confluence on uncoated plastic 12-well plates in keratinocyte growth medium (KGM; Lonza Inc., Allendale, NJ). Iron homeostasis in BEAS-2B cells has been previously demonstrated to be comparable to that in primary human bronchial epithelial cells.14
Mitochondrial Iron Concentrations
BEAS-2B cells in 75 cm2 flasks were exposed to 1.0 μM 57Fe FAC for 4 h. The cells were washed with phospate buffered saline (PBS) and exposed to PBS, 100 μg/mL WSP (WSP 100), or 200 μg/mL WSP (WSP 200) for 15 min. Nuclear and mitochondrial fractions were isolated, hydrolyzed in 1.0 mL of 3 N HCl/10% TCA for 24 h, and nonheme 57Fe in the fraction supernatants were measured using inductively coupled plasma mass spectroscopy (ICPMS; Elan DRC II, PerkinElmer).15 BEAS-2B cells were grown in 75 cm2 flasks, incubated in media with 200 μM FAC for 4 h, and then treated with 1.0 μM 57Fe FAC for 4 h. The cells were washed with PBS and exposed to PBS, 100 μg/mL WSP, or 200 μg/mL WSP for 15 min. Nuclear and mitochondrial fractions were collected, hydrolyzed in 1 N HCl/10% TCA for 24 h, and 57Fe in the fraction supernatants was measured.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated using a Qiagen kit (Qiagen, Valencia, CA) and reverse transcribed to generate cDNA using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA). Oligonucleotide primer pairs and fluorescent probes for divalent metal transporter 1 (DMT1) and GAPDH were designed using a primer design program (Primer Express, Applied Biosystems) and obtained from Integrated DNA Technologies (Coralville, IA). Quantitative fluorogenic amplification of cDNA was performed using the ABI Prism 7500 Sequence Detection System (Applied Biosystems), primer/probe sets of interest, and TaqMan Universal PCR Master Mix (Applied Biosystems). The relative abundance of GAPDH mRNA was used to normalize mRNA levels.
Cell Iron Concentrations
BEAS-2B cells were exposed to PBS, 100 μg/mL WSP, or 200 μg/mL WSP with or without 200 μM FAC. After 4 h of incubation, the buffer and exposure were removed, and the cells were washed with PBS and scraped into 1.0 mL of 3 N HCl/10% trichloroacetic acid (TCA). After hydrolysis at 70 °C, iron (nonheme) concentration in the supernatant was determined using inductively coupled plasma optical emission spectroscopy (ICPOES; Model Optima 4300D, PerkinElmer, Norwalk, CT).
Cell Ferritin Concentrations
BEAS-2B exposures to PBS, 100 μg/mL WSP, or 200 μg/mL WSP with or without 200 μM FAC were repeated for 24 h. After the media was removed, cells were washed with PBS, scraped into 1.0 mL of PBS, and disrupted using five passes through a 25 gauge needle. The concentrations of ferritin in the lysates were quantified using an immunoturbidimetric assay (Kamiya Biomedical Company, Seattle, WA).
Cellular Oxidant Generation
Oxidant generation by BEAS-2B cells was determined using Amplex Red (Molecular Probes, Eugene, OR) fluorescence. Cells grown on 96 well Co-Star (Corning Corp., Corning, NY) white-walled tissue culture plates to confluence were preloaded with the dye prior to exposure for 20 min at 37 °C/5% CO2. BEAS-2B cells were pretreated with either PBS or 200 μM FAC and then exposed to PBS or 100 μg/mL WSP in PBS. The reported value is fold change over control cells that have been preloaded with dye and exposed to only PBS. In addition, the cells were pretreated with 1.0 μM rotenone for 15 min and exposed to 100 μg/mL WSP.
Mitogen-Activated Protein (MAP) Kinase Activation
Protein kinase phosphorylation was analyzed by Western blotting. Cells were pretreated with PBS or 200 μM FAC and then exposed to PBS or 100 μg/mL WSP in PBS for 4 h. After washing with PBS, the cells were lysed with RIPA lysis buffer. Following normalization for protein content, cell extracts were subjected to SDS-PAGE on 11% gradient PAGE gels with a Tris-glycine-SDS buffer. Proteins were then electroblotted onto nitrocellulose. The membranes were incubated with 1:1,000 phospho-specific extracellular signal-regulated kinase (ERK) 1/2 and p38 antibodies (Cell Signaling Technology, Danvers, MA) followed by incubation with 1:2000 horse radish peroxidase (HRP)-conjugated secondary antibody.
Transcription Factor Activation
For trans-activation of NF-E2-related factor 2/antioxidant responsive element (Nrf2/ARE) promoter to be measured, BEAS-2B cells were cotransfected with Nrf2/ARE-luciferase reporter plasmid along with 0.02 μg of thymidine kinase-Renilla luciferase using Fugene 6 (Roche) according to the manufacturer’s recommendations. After 24 h, cells were pretreated with PBS or without 200 μM FAC and then exposed to PBS or 100 μg/mL WSP in PBS. Cells were then pelleted and lysed in passive lysis buffer (Promega, Madison, WI). The trans-activation activity was measured as luciferase light units as described previously.16
Interleukin (IL)-6 and IL-8 Release
BEAS-2B cells were pretreated with PBS or without 200 μM FAC and then exposed to PBS or 100 μg/mL WSP in PBS for 24 h. IL-6 and IL-8 concentrations in cell media were measured using immunoassays (MesoScale Discovery, Rockville, MD).
Humic Acid Studies
BEAS-2B cell exposure to 57Fe FAC was repeated but followed by exposure to 100 μg/mL of humic acid for 15 min. Nuclear and mitochondrial fractions were isolated, and nonheme 57Fe was measured using ICPMS. Following incubation with 100 μg/mL of humic acid for 4 h, RT-PCR for DMT1 was quantified, and cell iron uptake was determined. Finally, Amplex Red fluorescence for oxidant generation and IL-6 and IL-8 release by cells exposed to 100 μg/mL humic acid were measured.
Statistics
Data are expressed as mean values ± standard error unless otherwise specified. The minimum number of replicates for all measurements was three. Differences between two and multiple groups were compared using t tests of independent means and one-way analysis of variance, respectively. The posthoc test employed was Duncan’s Multiple Range test. Two-tailed tests of significance were employed. Significance was assumed at p <0.05.
RESULTS
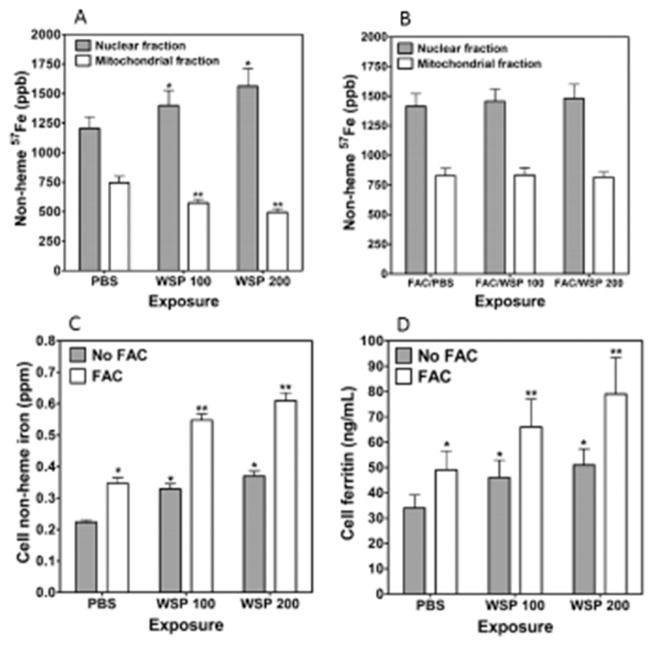
To determine if sequestration of host iron by WSP would immediately diminish intracellular iron concentrations, BEAS-2B cells were preloaded with 1.0 μM 57Fe FAC and then exposed to 100 or 200 μg/mL WSP for 15 min, and the nuclear and mitochondrial fractions were collected. Although concentrations of 57Fe decreased in the mitochondria, those in the nuclear fraction increased after exposure to WSP (Figure 1A). This supported the postulate that there is sequestration of host iron by the particle. BEAS-2B cells were then pre-exposed to 200 μM FAC for 4 h, augmenting intracellular iron levels. The cells were again incubated with 57Fe, and the exposure to WSP was repeated. There were no differences noted in 57Fe in either the nuclear or mitochondrial fractions in cells exposed to WSP (Figure 1B). These results established that making excess iron available in the cell diminished sequestration of host mitochondrial metal by the particle.
Figure 1.
Nonheme 57Fe concentrations in nuclear and mitochondrial fractions, cell iron, and ferritin concentrations after exposure to WSP. Cells were incubated with 57Fe FAC and exposed to 100 or 200 μg/mL WSP (WPS 100 and WPS 200, respectively) for 15 min. Collected fractions were hydrolyzed, and ICPMS was employed to measure 57Fe. After WSP exposure, 57Fe concentrations were increased in the nuclear fraction and decreased in the mitochondrial fraction (A). Cells were incubated with FAC followed by 57Fe FAC, and the exposures were repeated. Relative to PBS exposure, there were no differences in 57Fe in either the nuclear or the mitochondrial fraction after WSP (B). With exposure to 200 μM FAC, cells imported iron with increased nonheme iron concentrations at 4 h by ICPOES (C). Inclusion of 100 or 200 μg/mL WSP also elevated cell iron concentrations. However, coexposure to both FAC and WSP further increased cell metal levels to their highest levels. Exposures were repeated for 24 h, and ferritin was measured in cell lysate using an immunoturbidimetric assay. Exposures to FAC, 100 μg/mL, or 200 μg/mL WSP, or both increased cell ferritin concentration, but coexposure was associated with the greatest elevation in levels of the storage protein (D). In (A) and (B), * indicates a significant increase relative to the same fraction collected from BEAS-2B cells exposed to PBS, and ** indicates a significant decrease relative to the same fraction collected from BEAS-2B cells exposed to PBS. In (C) and (D), * indicates a significant increase relative to BEAS-2B cells exposed to PBS, and ** indicates a significant increase relative to BEAS-2B cells exposed to either FAC alone or WSP alone.
When an appropriation of host iron by the WSP immediately diminishes intracellular iron concentrations, metal import must increase accordingly for cell survival. Some portion of such intracellular transport is predicted to result in elevations in the storage protein ferritin and metal stored therein. After exposure to 100 μg/mL of WSP for 4 h, RNA for DMT1 (a major iron importer) significantly increased 1.8 ± 0.4 fold. Following exposure to either WSP or 200 μM FAC, cell nonheme iron increased relative to PBS (Figure 1C). However, cell incubations that included both WSP and FAC showed the greatest elevations in cell iron concentrations. This established that WSP significantly increased metal import, supporting an increased cell avidity for iron following exposure to this particle. Furthermore, cell concentration of the iron-storage protein ferritin was elevated following 24 h exposure of BEAS-2B cells to either FAC or WSP but was greatest when both were included (Figure 1D). This further supported that cell iron homeostasis was impacted by exposure to WSP.
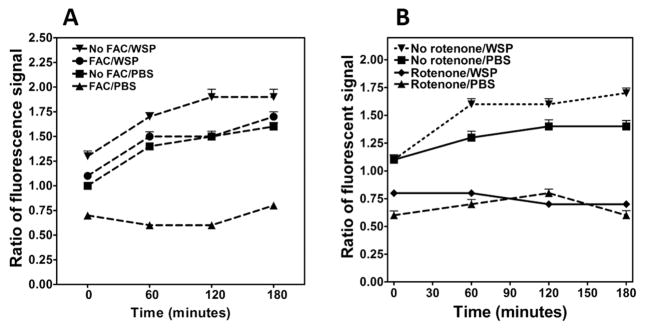
Cell oxidant generation after exposure to WSP was measured using Amplex Red. Pretreatment of cells with FAC diminished the oxidant generation following exposure to both PBS and 100 μg/mL WSP (Figure 2A), reflecting a decrease in the production of superoxide and dependent products. Cellular oxidant production corresponded to the disruption in iron homeostasis following WSP exposure with increased metal availability decreasing the fluorescence signal. Cell oxidant generation after exposure to WSP was again determined using Amplex Red fluorescence, but pretreatment of cells was with 1.0 μM rotenone, which interferes with the electron transport chain at Complex I in the mitochondria. Pretreatment with rotenone diminished oxidant generation following exposure to 100 μg/mL of WSP (Figure 2B). This implied that some portion of the oxidant generation after WSP exposure was mitochondrial in origin.
Figure 2.
Cell oxidant generation after exposure to wood stove particle. Oxidant generation was determined using Amplex Red fluorescence. The reported value is fold-change over control cells that were exposed to PBS at time zero. Oxidant generation by BEAS-2B cells was significantly increased by 100 μg/mL of WSP (A and B). Incubation of cells with 200 μM FAC diminished the oxidant generation following exposure to both PBS and WSP (A). Pretreatment of cells with 1.0 μM rotenone for 15 min decreased oxidant generation after WSP, suggesting a mitochondrial origin (B).
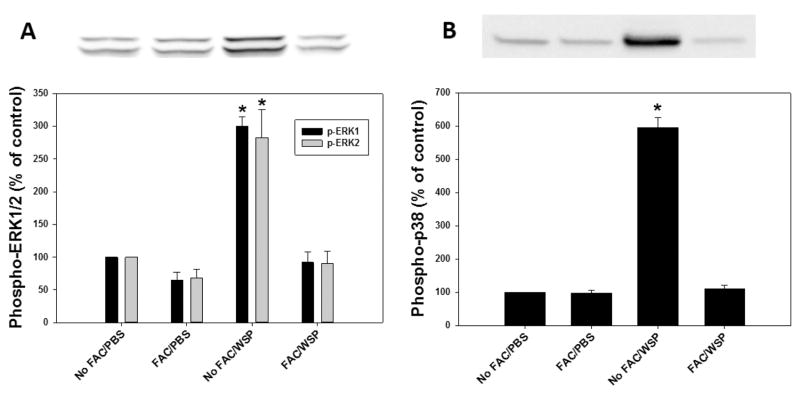
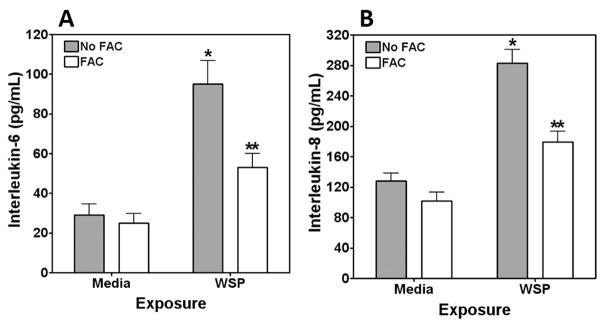
The biological effects of particles can include a cascade of events, such as MAP kinase activation, transcription factor activation, and release of inflammatory mediators. Using Western blotting, activation of both ERK 1/2 and p38 was observed (Figure 3A and B). Cell pretreatment with FAC diminished MAP kinase activation, supporting an association between MAP kinase activation and disruption of iron homeostasis by the particle. Activation of the transcription factor nrf2 in transfected cells by WSP was then quantified. Incubation of BEAS-2B cells with 100 μg/mL of WSP for 4 h activated Nrf2/ARE expression (Figure 4), whereas pretreatment of these cells with FAC diminished this response. Finally, the release of pro-inflammatory mediators after particle exposure was measured. Exposure to 100 μg/mL of WSP for 24 h increased concentrations of both IL-6 and IL-8 released into the media (Figure 5A and B). The inclusion of 200 μM FAC in the BEAS-2B cell incubation diminished interleukin release after particle exposure, supporting an association of pro-inflammatory effect with a disruption in iron homeostasis.
Figure 3.
Activation of MAP kinases after exposure to WSP. Bands were detected using enhanced chemiluminescence (ECL) detection reagents 1 and 2 and high-performance chemiluminescence ECL films (Amersham Pharmacia Biotech) with a model SRX-101 Konica medical film processor (Konica). BEAS-2B demonstrated greater phosphorylation of both ERK 1/2 (A) and p38 (B) after exposure to 100 μg/mL of WSP for 4 h. Co-incubation with 200 μM FAC diminished signals for p-ERK 1/2 and p-p38. * indicates a significant increase relative to all other exposures.
Figure 4.
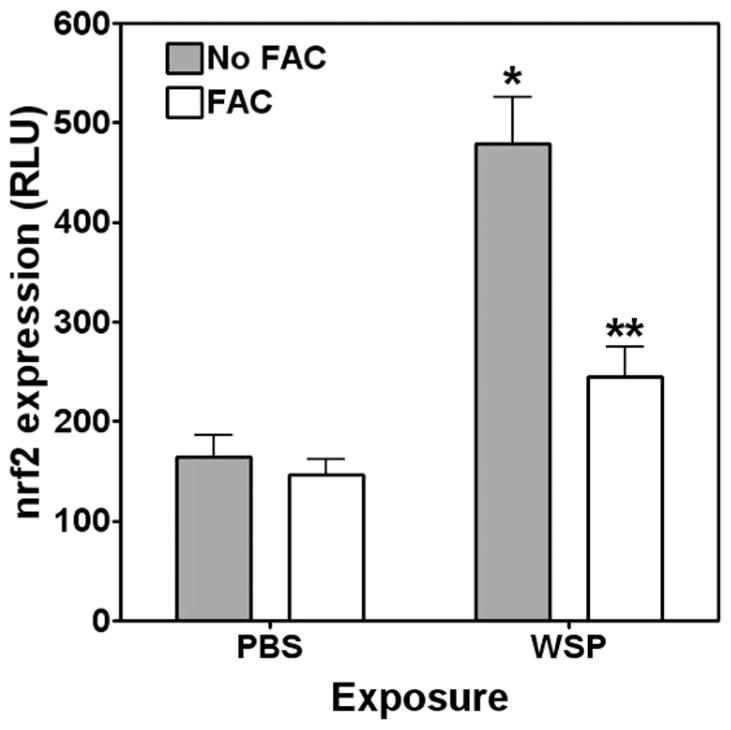
Activation of transcription factor after exposure to wood stove particle. Although exposure of BEAS-2B cells to 100 μg/mL of WSP for 4 h activated nrf2 ARE, treatment with 200 μM FAC diminished this response. * indicates a significant increase relative to all other exposures, and ** indicates a significant increase relative to PBS exposure.
Figure 5.
Release of pro-inflammatory mediators after exposure to WSP. Exposure to 100 μg/mL of WSP for 24 h increased the release of IL-6 (A) and IL-8 (B) by BEAS-2B cells. FAC treatment diminished this response to WSP. * indicates a significant increase relative to all other exposures, and ** indicates a significant increase relative to media exposure.
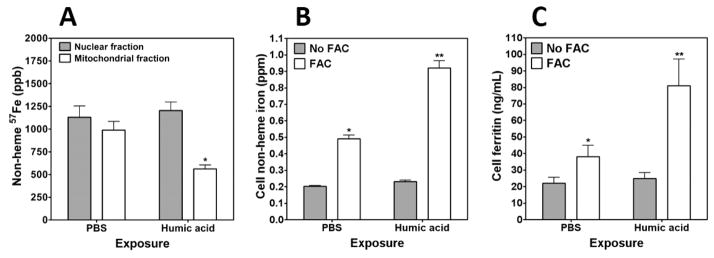
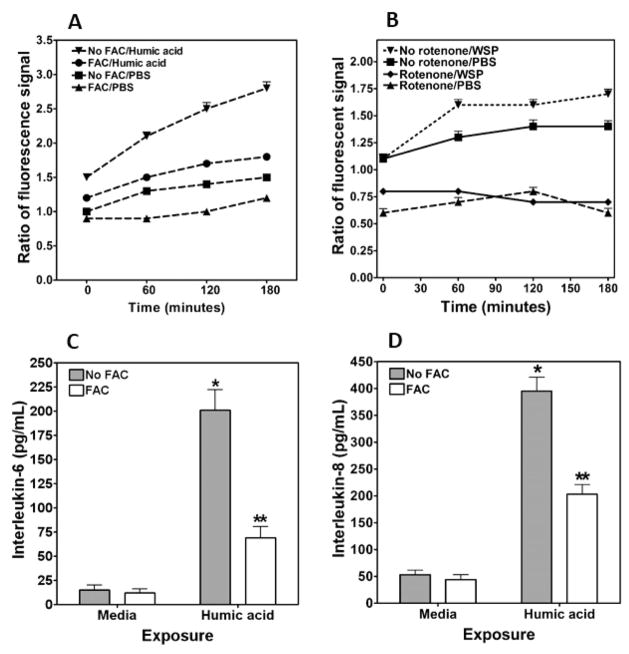
Humic acid, a compound chemically similar to HULIS previously demonstrated to be included in WSP, was then tested to determine its ability to disrupt iron homeostasis and impact biological effect following particle exposure.11 Fifteen minute exposure to 100 μg/mL humic acid decreased concentrations of 57Fe in the mitochondria, supporting the hypothesis of a sequestration of host iron by this component of WSP (Figure 6A). With exposure to 100 μg/mL humic acid, DMT1 RNA was increased 2.7 ± 0.9 fold. Comparable to WSP, 4 h coexposures to both FAC and humic acid demonstrated a greater impact on cell concentrations of both iron and ferritin relative to iron and humic acid alone (Figure 6B and C, respectively). Oxidant generation following exposure of BEAS-2B cells to 100 μg/mL humic acid was increased relative to PBS (Figure 7A). This production of superoxide-related products was significantly decreased with elevated availability of iron. Similar to the Amplex Red signal after WSP exposure, preincubation of cells with 1.0 μM rotenone diminished oxidant generation following exposure to 100 μg/mL humic acid (Figure 7B). The interaction between iron availability and biological effect of humic acid was determined by measuring mediator release. Concentrations of both IL-6 and IL-8 in the cell media were elevated following 24 h incubation with 100 μg/mL humic acid (Figure 7C and D). Inclusion of FAC in the cell incubation diminished interleukin release.
Figure 6.
Nonheme 57Fe concentrations in nuclear and mitochondrial fractions, cell iron, and ferritin concentrations after exposure to humic acid. Cells were incubated with 57Fe FAC and exposed to humic acid for 15 min. Collected fractions were hydrolyzed, and ICPMS was employed to measure 57Fe concentrations, which were decreased in the mitochondrial fraction following humic acid exposure (A). In (A), * indicates a significant decrease relative to the same fraction collected from BEAS-2B cells exposed to PBS. With exposure to 200 μM FAC, cells imported iron (B). Inclusion of both FAC and 100 μg/mL of humic acid increased metal levels further. Exposures were repeated for 24 h, and ferritin was measured in cell lysate using an immunoturbidimetric assay. Exposures to FAC and both FAC and humic acid increased cell ferritin concentration (C). Co-exposure to both FAC and humic acid resulted in the highest levels of cell ferritin. In (B) and (C), * indicates a significant increase relative to BEAS-2B cells exposed to PBS, and ** indicates a significant increase relative to BEAS-2B cells exposed to either FAC alone or humic acid alone.
Figure 7.
Cell oxidant generation and release of IL-6 and IL-8 after exposure to humic acid. Oxidant generation by BEAS-2B cells was increased following exposure to 100 μg/mL of humic acid (A). Incubation of cells with 200 μM FAC diminished the oxidant generation following exposure to PBS and humic acid. Pretreatment of cells with rotenone for 15 min inhibited oxidant generation after humic acid, suggesting a mitochondrial origin (B). Exposure of BEAS-2B cells to 100 μg/mL of humic acid for 24 h increased the release of IL-6 (C) and IL-8 (D), whereas the 200 μM FAC treatment diminished the pro-inflammatory response. In (C) and (D), * indicates a significant increase relative to all other exposures, and ** indicates a significant increase relative to media exposure.
DISCUSSION
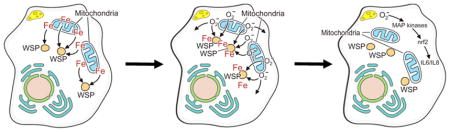
Following exposure to WSP, sources of iron in BEAS-2B cells were sequestered by the particle, reflecting a capacity to bind the metal via oxygen-containing functional groups.17 Mitochondrial concentrations of metal were shown to decrease following exposure to the particle; other intracellular sources of metal are likely to also be appropriated. Function in both the cell and specific individual organelles, including the mitochondria, will be compromised following such metal loss unless the normal intracellular iron concentration is re-established. The cell therefore responds to the WSP, its sequestration of host iron, and a relative iron deficiency associated with the immediate exposure by upregulating import of iron. DMT1 RNA was significantly elevated following exposure to WSP, reflecting insufficient iron concentrations available in the respiratory epithelial cells.18 Increased concentrations of imported iron were demonstrated following exposure of respiratory epithelial cells to WSP and FAC. Some portion of the newly imported metal affects an increased expression of storage protein in the cell and ferritin concentrations, which were also increased following WSP and FAC. A new iron homeostasis is determined by the interaction between the cell (including the mitochondria and probably other organelles) and the particle. This homeostasis includes increased total cell iron concentrations, elevated cell ferritin levels, and mitochondrial metal concentrations sufficient to meet requirements for continued function. In addition, some portion of cell metal will be complexed by functional groups on the surface of an intracellular particle. Accordingly, WSP residing in the cell binds some portion of the total metal but available iron concentrations can be adjusted and made to be adequate for continued cell survival.
Following sequestration of cell iron by a particle, an attempt to reacquire iron will necessitate ferrireduction to chemically reduce the metal to the ferrous state prior to transporting it across a membrane. Such chemical reduction can be accomplished by a superoxide generated by the cell and mitochondria (i.e., an oxidative stress). Immediate decrements (within 1 hr) in mitochondrial iron concentrations in the respiratory epithelial cells corresponded to increased generation of oxidants. An increased availability of iron (i.e., pretreatment with FAC) diminished both the relative iron deficiency in the organelle and the production of oxidants. The oxidative stress was generated by the cells (i.e., the host) and specifically by mitochondria because rotenone inhibited some portion of the oxidant generation by BEAS-2B cells. The mitochondrial electron transport chain is a major location of oxidant production; specific sites include complex I and complex III.19–21 Cellular oxidant generation, specifically superoxide, follows exposure to iron chelators.22–24 The evidence from this study suggests that decreased iron concentrations following exposure to WSP induce a mitochondrial response that includes cellular oxidant generation. It is proposed that this cellular and mitochondrial oxidant production after WSP exposure functions in a remedial response to iron loss following sequestration of the metal by the particle surface. Superoxide, produced by mitochondria in response to a metal deficit, may assist in the import of required iron. Ferrireduction is an essential, and frequently limiting, reaction in such iron import and can be accomplished in many cell types using superoxide. 25–27 Furthermore, such ferrireduction can be dependent on the electron transport chain with the mitochondria serving as a source of reducing equivalents.28–30
The biological effects of WSP were associated with a decreased concentration of cell and mitochondrial iron. The sequestration of host iron by the particle initiated a series of events that can culminate in a pro-inflammatory response. Comparable to numerous other particles, exposure of respiratory epithelial cells to WSP impacted an activation of MAP kinases.31–36 Activation of the MAP kinase cascade represents a signaling pathway by which exposure to particulate matter mediates specific biological effects.37 MAP kinase phosphorylation has also been demonstrated following reduction in cell iron levels.38 Phosphorylation of ERK 1/2 and p38 following WSP exposure was diminished by increasing the cell concentration of available iron. Comparable to MAP kinases, the transcription factor Nrf2 can be activated by particle exposure.39–42 Like the MAP kinases, the activation of this nuclear transcription factor was inhibited by elevating concentrations of cell iron. These nuclear transcription factors control the activity of genes involved in inflammation.43–46 Changes in protein expression for IL-6 and IL-8 after WSP exposure were diminished by cell treatment with iron. All these results demonstrate a relationship of the biological effect exerted by WSP with iron homeostasis.
Humic acid functioned to sequester host cell iron. Incomplete oxidation of carbon-based materials can produce HULIS. These heterogeneous, amorphous, organic materials are ubiquitous, occurring in all terrestrial and aqueous environments including soils, composts, sediments, peat bogs, coals, rivers, lakes, and oceans. HULIS in soil can include three different fractions: humic acids, fulvic acids, and humin. Humic acid is that fraction of these organic materials observed to be insoluble in water under acidic conditions (pH <2) but soluble at higher pH values. As a result of a variety of acidic functional groups, humic acid complexes metal cations to facilitate their mobilization and transport in soils and waters. These substances regulate the provision of trace metals to plants. However, such complexation of metals in cells and tissues by humic acid can impact oxidative stress and can consequently be associated with injury. Cells exposed to humic acid demonstrated decreased mitochondrial iron concentrations, elevated RNA for the major importer DMT1, and greater import of the metal. In addition, exposure of respiratory epithelial cells to humic acid resulted in greater oxidant generation and higher release of interleukins. Oxidant generation and release of interleukins after humic acid exposure were dependent on sequestration of cell iron and were inhibited by treatment with FAC. Oxidant generation by other organic compounds (e.g., quinones) can similarly be dependent on iron complexation.47 HULIS similar to humic acid in soil can be isolated not only from organic materials in natural environments but also in tobacco smoke particulate and both ambient and emission source particles.11,12 The instillation of such HULIS into a living system has been demonstrated to be associated with a disruption in iron homeostasis and metal accumulation, supporting the relevance of this same pathway following in vivo exposure.11 Oxalate and mineral oxides are also potentially included in emission and ambient air pollution particles and can similarly affect iron complexation at their surface. Accordingly, both could also contribute to the biological effects following exposure.
We conclude that the biological effect of WSP was dependent on sequestration of host iron by the particle. Following exposure to WSP, mitochondrial concentrations of metal decreased, reflecting the capacity of the particle to appropriate metal from cell sources. The cell responded to the WSP, its sequestration of host iron, and the relative iron deficiency associated with the immediate exposure by upregulating the import of iron. DMT1 RNA was significantly upregulated, reflecting insufficient iron concentrations available to the cells following exposure to WSP. Oxidant generation and indices of biological effect after exposure to WSP also showed a dependence on concentrations of available iron. Humic acid, similar to HULIS included in the WSP, supported participation in iron sequestration, oxidant generation, and biological effects.
Acknowledgments
Funding
Support was provided by the US Environmental Protection Agency.
ABBREVIATIONS
- DMT1
divalent metal transporter 1
- ERK
extracellular signal-regulated kinase
- FAC
ferric ammonium citrate
- HRP
horse radish peroxidase
- HULIS
humic-like substances
- ICPMS
inductively coupled plasma mass spectroscopy
- ICPOES
inductively coupled plasma optical emission spectroscopy
- IL
interleukin
- KGM
keratinocyte growth medium
- PBS
phosphate buffered saline
- RT-PCR
reverse transcription-polymerase chain reaction
- TCA
trichloroacetic acid
- WSP
wood smoke particle
Footnotes
The authors declare no competing financial interest.
References
- 1.Churg A, Zay K, Li K. Mechanisms of mineral dust-induced emphysema. Environ Health Perspect. 1997;105(Suppl 5):1215–1218. doi: 10.1289/ehp.97105s51215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grobbelaar JP, Bateman ED. Hut lung: a domestically acquired pneumoconiosis of mixed aetiology in rural women. Thorax. 1991;46:334–340. doi: 10.1136/thx.46.5.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozbay B, Uzun K, Arslan H, Zehir I. Functional and radiological impairment in women highly exposed to indoor biomass fuels. Respirology. 2001;6:255–258. doi: 10.1046/j.1440-1843.2001.00339.x. [DOI] [PubMed] [Google Scholar]
- 4.Pinkerton KE, Green FH, Saiki C, Vallyathan V, Plopper CG, Gopal V, Hung D, Bahne EB, Lin SS, Menache MG, Schenker MB. Distribution of particulate matter and tissue remodeling in the human lung. Environ Health Perspect. 2000;108:1063–1069. doi: 10.1289/ehp.001081063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pratt PC, Jutabha P, Klugh GA. The relationship between pigment deposits and lesions in normal and centrilobular emphysematous lungs. Am Rev Respir Dis. 1963;87:245–256. doi: 10.1164/arrd.1963.87.2.245. [DOI] [PubMed] [Google Scholar]
- 6.Rudell B, Blomberg A, Helleday R, Ledin MC, Lundback B, Stjernberg N, Horstedt P, Sandstrom T. Bronchoalveolar inflammation after exposure to diesel exhaust: comparison between unfiltered and particle trap filtered exhaust. Occup Environ Med. 1999;56:527–534. doi: 10.1136/oem.56.8.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss W. Cigarette smoking and diffuse pulmonary fibrosis. Am Rev Respir Dis. 1969;99:67–72. doi: 10.1164/arrd.1969.99.1.67. [DOI] [PubMed] [Google Scholar]
- 8.Ghio AJ, Jaskot RH, Hatch GE. Lung injury after silica instillation is associated with an accumulation of iron in rats. Am J Physiol. 1994;267:L686–692. doi: 10.1152/ajplung.1994.267.6.L686. [DOI] [PubMed] [Google Scholar]
- 9.Koerten HK, Brederoo P, Ginsel LA, Daems WT. The endocytosis of asbestos by mouse peritoneal macrophages and its long-term effect on iron accumulation and labyrinth formation. Eur J Cell Biol. 1986;40:25–36. [PubMed] [Google Scholar]
- 10.Ghio AJ, Churg A, Roggli VL. Ferruginous bodies: implications in the mechanism of fiber and particle toxicity. Toxicol Pathol. 2004;32:643–649. doi: 10.1080/01926230490885733. [DOI] [PubMed] [Google Scholar]
- 11.Ghio AJ, Stonehuerner J, Pritchard RJ, Piantadosi CA, Quigley DR, Dreher KL, Costa DL. Humic-like substances in air pollution particulates correlate with concentrations of transition metals and oxidant generation. Inhalation Toxicol. 1996;8:479–494. [Google Scholar]
- 12.Ghio AJ, Stonehuerner J, Quigley DR. Humiclike substances in cigarette smoke condensate and lung tissue of smokers. Am J Physiol. 1994;266:L382–388. doi: 10.1152/ajplung.1994.266.4.L382. [DOI] [PubMed] [Google Scholar]
- 13.Sporn TA, Roggli VL. Pneumoconioses, Mineral and Vegetable. In: Tomashefski JFJ, Cagle PT, Farver CF, Fraire AE, editors. Dail and Hammar’s Pulmonary Pathology. Vol. I. Nonneoplastic Lung Disease. Springer; 2008. p. 933. [Google Scholar]
- 14.Ghio AJ, Carter JD, Samet JM, Reed W, Quay J, Dailey LA, Richards JH, Devlin RB. Metal-dependent expression of ferritin and lactoferrin by respiratory epithelial cells. Am J Physiol. 1998;274:L728–736. doi: 10.1152/ajplung.1998.274.5.L728. [DOI] [PubMed] [Google Scholar]
- 15.Greenawalt JW. The isolation of outer and inner mitochondrial membranes. Methods Enzymol. 1974;31:310–323. doi: 10.1016/0076-6879(74)31033-6. [DOI] [PubMed] [Google Scholar]
- 16.Kesic M, Ward M, Semmes OJ, Green PL. Site-specific phosphorylation regulates human T-cell leukemia virus type 2 Rex function in vivo. J Virol. 2009;83:8859–8868. doi: 10.1128/JVI.00908-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghio AJ, Tong H, Soukup JM, Dailey LA, Cheng WY, Samet JM, Kesic MJ, Bromberg PA, Turi JL, Upadhyay D, Scott Budinger GR, Mutlu GM. Sequestration of mitochondrial iron by silica particle initiates a biological effect. Am J Physiol Lung Cell Mol Physiol. 2013;305:L712–724. doi: 10.1152/ajplung.00099.2013. [DOI] [PubMed] [Google Scholar]
- 18.Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 19.Moller IM. PLANT MITOCHONDRIA AND OXIDATIVE STRESS: Electron Transport, NADPH Turnover, and Metabolism of Reactive Oxygen Species. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:561–591. doi: 10.1146/annurev.arplant.52.1.561. [DOI] [PubMed] [Google Scholar]
- 20.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 21.Grivennikova VG, Vinogradov AD. Generation of superoxide by the mitochondrial Complex I. Biochim Biophys Acta, Bioenerg. 2006;1757:553–561. doi: 10.1016/j.bbabio.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Callens C, Coulon S, Naudin J, Radford-Weiss I, Boissel N, Raffoux E, Wang PH, Agarwal S, Tamouza H, Paubelle E, Asnafi V, Ribeil JA, Dessen P, Canioni D, Chandesris O, Rubio MT, Beaumont C, Benhamou M, Dombret H, Macintyre E, Monteiro RC, Moura IC, Hermine O. Targeting iron homeostasis induces cellular differentiation and synergizes with differentiating agents in acute myeloid leukemia. J Exp Med. 2010;207:731–750. doi: 10.1084/jem.20091488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaston TB, Watts RN, Yuan J, Richardson DR. Potent antitumor activity of novel iron chelators derived from di-2-pyridylketone isonicotinoyl hydrazone involves fenton-derived free radical generation. Clin Cancer Res. 2004;10:7365–7374. doi: 10.1158/1078-0432.CCR-04-0865. [DOI] [PubMed] [Google Scholar]
- 24.Dendorfer A, Heidbreder M, Hellwig-Burgel T, Johren O, Qadri F, Dominiak P. Deferoxamine induces prolonged cardiac preconditioning via accumulation of oxygen radicals. Free Radical Biol Med. 2005;38:117–124. doi: 10.1016/j.freeradbiomed.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Cakmak I, van de Wetering DA, Marschner H, Bienfait HF. Involvement of Superoxide Radical in Extracellular Ferric Reduction by Iron-Deficient Bean Roots. Plant Physiol. 1987;85:310–314. doi: 10.1104/pp.85.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rose AL, Salmon TP, Lukondeh T, Neilan BA, Waite TD. Use of superoxide as an electron shuttle for iron acquisition by the marine cyanobacterium Lyngbya majuscula. Environ Sci Technol. 2005;39:3708–3715. doi: 10.1021/es048766c. [DOI] [PubMed] [Google Scholar]
- 27.Turi JL, Jaspers I, Dailey LA, Madden MC, Brighton LE, Carter JD, Nozik-Grayck E, Piantadosi CA, Ghio AJ. Oxidative stress activates anion exchange protein 2 and AP-1 in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2002;283:L791–798. doi: 10.1152/ajplung.00398.2001. [DOI] [PubMed] [Google Scholar]
- 28.Barnes R, Connelly JL, Jones OT. The utilization of iron and its complexes by mammalian mitochondria. Biochem J. 1972;128:1043–1055. doi: 10.1042/bj1281043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romslo I, Flatmark T. Energy-dependent accumulation of iron by isolated rat liver mitochondria. II Relationship to the active transport of Ca2+ Biochim Biophys Acta, Bioenerg. 1973;325:38–46. doi: 10.1016/0005-2728(73)90148-5. [DOI] [PubMed] [Google Scholar]
- 30.Tsuda A, Takeda S, Saito H, Nishioka J, Nojiri Y, Kudo I, Kiyosawa H, Shiomoto A, Imai K, Ono T, Shimamoto A, Tsumune D, Yoshimura T, Aono T, Hinuma A, Kinugasa M, Suzuki K, Sohrin Y, Noiri Y, Tani H, Deguchi Y, Tsurushima N, Ogawa H, Fukami K, Kuma K, Saino T. A mesoscale iron enrichment in the western subarctic Pacific induces a large centric diatom bloom. Science. 2003;300:958–961. doi: 10.1126/science.1082000. [DOI] [PubMed] [Google Scholar]
- 31.Wang T, Chiang ET, Moreno-Vinasco L, Lang GD, Pendyala S, Samet JM, Geyh AS, Breysse PN, Chillrud SN, Natarajan V, Garcia JG. Particulate matter disrupts human lung endothelial barrier integrity via ROS- and p38 MAPK-dependent pathways. Am J Respir Cell Mol Biol. 2010;42:442–449. doi: 10.1165/rcmb.2008-0402OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perng DW, Chang TM, Wang JY, Lee CC, Lu SH, Shyue SK, Lee TS, Kou YR. Inflammatory role of AMP-activated protein kinase signaling in an experimental model of toxic smoke inhalation injury. Crit Care Med. 2013;41:120–132. doi: 10.1097/CCM.0b013e318265f653. [DOI] [PubMed] [Google Scholar]
- 33.Li R, Ning Z, Cui J, Khalsa B, Ai L, Takabe W, Beebe T, Majumdar R, Sioutas C, Hsiai T. Ultrafine particles from diesel engines induce vascular oxidative stress via JNK activation. Free Radical Biol Med. 2009;46:775–782. doi: 10.1016/j.freeradbiomed.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li CJ, Ning W, Matthay MA, Feghali-Bostwick CA, Choi AM. MAPK pathway mediates EGR-1-HSP70-dependent cigarette smoke-induced chemokine production. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1297–1303. doi: 10.1152/ajplung.00194.2006. [DOI] [PubMed] [Google Scholar]
- 35.Lee TS, Liu YJ, Tang GJ, Yien HW, Wu YL, Kou YR. Wood smoke extract promotes both apoptosis and proliferation in rat alveolar epithelial type II cells: the role of oxidative stress and heme oxygenase-1. Crit Care Med. 2008;36:2597–2606. doi: 10.1097/CCM.0b013e318184979c. [DOI] [PubMed] [Google Scholar]
- 36.Albrecht C, Borm PJ, Adolf B, Timblin CR, Mossman BT. In vitro and in vivo activation of extracellular signal-regulated kinases by coal dusts and quartz silica. Toxicol Appl Pharmacol. 2002;184:37–45. [PubMed] [Google Scholar]
- 37.Samet JM, Stonehuerner J, Reed W, Devlin RB, Dailey LA, Kennedy TP, Bromberg PA, Ghio AJ. Disruption of protein tyrosine phosphate homeostasis in bronchial epithelial cells exposed to oil fly ash. Am J Physiol. 1997;272:L426–432. doi: 10.1152/ajplung.1997.272.3.L426. [DOI] [PubMed] [Google Scholar]
- 38.Yu Y, Richardson DR. Cellular iron depletion stimulates the JNK and p38 MAPK signaling transduction pathways, dissociation of ASK1-thioredoxin, and activation of ASK1. J Biol Chem. 2011;286:15413–15427. doi: 10.1074/jbc.M111.225946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knorr-Wittmann C, Hengstermann A, Gebel S, Alam J, Muller T. Characterization of Nrf2 activation and heme oxygenase-1 expression in NIH3T3 cells exposed to aqueous extracts of cigarette smoke. Free Radical Biol Med. 2005;39:1438–1448. doi: 10.1016/j.freeradbiomed.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Deng X, Rui W, Zhang F, Ding W. PM2.5 induces Nrf2-mediated defense mechanisms against oxidative stress by activating PIK3/AKT signaling pathway in human lung alveolar epithelial A549 cells. Cell Biol Toxicol. 2013;29:143–157. doi: 10.1007/s10565-013-9242-5. [DOI] [PubMed] [Google Scholar]
- 41.Cheng SE, Lee IT, Lin CC, Kou YR, Yang CM. Cigarette smoke particle-phase extract induces HO-1 expression in human tracheal smooth muscle cells: role of the c-Src/NADPH oxidase/MAPK/Nrf2 signaling pathway. Free Radical Biol Med. 2010;48:1410–1422. doi: 10.1016/j.freeradbiomed.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 42.Chan JK, Charrier JG, Kodani SD, Vogel CF, Kado SY, Anderson DS, Anastasio C, Van Winkle LS. Combustion-derived flame generated ultrafine soot generates reactive oxygen species and activates Nrf2 antioxidants differently in neonatal and adult rat lungs. Part Fibre Toxicol. 2013;10:34. doi: 10.1186/1743-8977-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen F, Sun S, Kuhn DC, Gaydos LJ, Shi X, Lu Y, Demers LM. Involvement of NF-kappaB in silica-induced cyclooxygenase II gene expression in rat alveolar macrophages. Am J Physiol. 1997;272:L779–786. doi: 10.1152/ajplung.1997.272.4.L779. [DOI] [PubMed] [Google Scholar]
- 44.Li N, Kim S, Wang M, Froines J, Sioutas C, Nel A. Use of a stratified oxidative stress model to study the biological effects of ambient concentrated and diesel exhaust particulate matter. Inhalation Toxicol. 2002;14:459–486. doi: 10.1080/089583701753678571. [DOI] [PubMed] [Google Scholar]
- 45.Su B, Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol. 1996;8:402–411. doi: 10.1016/s0952-7915(96)80131-2. [DOI] [PubMed] [Google Scholar]
- 46.Xiao GG, Wang M, Li N, Loo JA, Nel AE. Use of proteomics to demonstrate a hierarchical oxidative stress response to diesel exhaust particle chemicals in a macrophage cell line. J Biol Chem. 2003;278:50781–50790. doi: 10.1074/jbc.M306423200. [DOI] [PubMed] [Google Scholar]
- 47.Dikalov S, Alov P, Rangelova D. Role of iron ion chelation by quinones in their reduction, OH-radical generation and lipid peroxidation. Biochem Biophys Res Commun. 1993;195:113–119. doi: 10.1006/bbrc.1993.2017. [DOI] [PubMed] [Google Scholar]