Abstract
Background
Waterpipe tobacco smoking is a traditional method of tobacco use, especially in the Eastern Mediterranean Region (EMR), but its use is now spreading worldwide. Recent epidemiological data, for example, show that waterpipe smoking has become the most prevalent tobacco use method among adolescents in the EMR, and the second most prevalent in the US. Waterpipes are used socially, often being shared between friends or family at home, or in dedicated bars and cafes that provide waterpipes to patrons. Because the smoke passes through a reservoir of water, waterpipe tobacco smoking is perceived as being less harmful than other methods of tobacco use. At least in some cultures, women and girls are more likely to use a waterpipe than to use other forms of tobacco, and it is popular among younger smokers. Accumulating evidence suggests that some waterpipe smokers become addicted, have difficulty quitting, and experience similar health risks as cigarette smokers.
Objectives
To evaluate the effectiveness of tobacco cessation interventions for waterpipe users.
Search methods
We searched the Cochrane Tobacco Addiction Review Group specialized register in June 2015. We also searched MEDLINE, EMBASE, PsycINFO and CINAHL , using variant terms and spellings ('waterpipe' or 'narghile' or 'arghile' or 'shisha' or 'goza' or 'narkeela' or 'hookah' or 'hubble bubble'). We searched for trials, published or unpublished, in any language, and especially in regions where waterpipe use is widespread.
Selection criteria
We sought randomized, quasi‐randomized or cluster‐randomized controlled trials of smoking cessation interventions for waterpipe smokers of any age or gender. The primary outcome of interest was abstinence from tobacco use, measured at six months post‐cessation or longer, regardless of whether abstinence was biochemically verified. We included interventions that were pharmacological (for example, nicotine replacement therapy (NRT) or bupropion) or behavioural, or both, and could be directed at individual waterpipe users or at groups of users. We only included tobacco cessation interventions, and did not consider trials of prevention of uptake.
Data collection and analysis
Two review authors assessed abstracts of the studies retrieved by the search strategy, for possible inclusion in the review. We retrieved full‐text articles for all abstracts that any of the authors believed might be suitable. Two review authors then extracted data and assessed trial quality independently in accordance with standard Cochrane Collaboration methodologies. We aimed to pool groups of studies that we considered to be sufficiently similar, provided there was no evidence of substantial statistical heterogeneity, and aimed to estimate a pooled risk ratio (RR) using the Mantel‐Haenszel fixed‐effect method. Where meta‐analysis was not possible, we presented summary and descriptive statistics.
Main results
Our search retrieved 1311 unique citations, of which 1289 were excluded after title and abstract screening. Of the remaining 22, we excluded 19 because they were empirical studies that were not randomized, quasi‐randomized or cluster‐randomized controlled trials (n = 12), because they were review articles (n = 3), because they described protocols only (n = 2), they were conducted among cigarette smokers only (n = 1), or they had only a three‐month follow‐up (n = 1).
We identified three controlled trials which tested cessation interventions for waterpipe smokers. Studies were carried out in Egypt (Mohlman 2013), Pakistan (Dogar 2014), and the US (Lipkus 2011). One was a randomized controlled trial and two were cluster‐randomized trials. Two studies tested individual‐level interventions, and one tested a community‐level intervention. Two studies included only behavioural interventions, and one study (Dogar 2014) included two intervention groups: one behavioural, and the other behavioural with bupropion. The Lipkus and Mohlman studies delivered waterpipe‐specific interventions, and the Dogar study delivered a non‐specific tobacco intervention. Due to study variation we did not pool results, and intervention effects are reported descriptively. Compared to control groups, waterpipe smoking cessation rates were higher in the intervention groups in all three studies, with a significant difference in two studies. For the Dogar study, the RRs for waterpipe smoking abstinence at 25 weeks among waterpipe‐only smokers were 2.2 (95% confidence interval (CI) 1.3 to 3.8; 180 participants) in the behavioural group, and 2.5 (95% CI 1.3 to 4.7; 84 participants) in the behavioural plus bupropion group. In our analysis we have combined both groups, to give a RR of 2.28 (95% CI 1.36 to 3.83; 200 participants). The Mohlman study delivered a RR in male waterpipe‐smokers at one year in favour of the intervention of 3.25 (95% CI 1.19 to 8.89).
Authors' conclusions
Although the literature on waterpipe cessation interventions remains sparse, the reviewed studies provide a basis for developing interventions in this area. The lack of statistically significant effects in one of the three studies is not unexpected, given the small and pilot nature of the studies. The studies highlight important design and content issues that need to be considered for future cessation trials in waterpipe smokers. These include building on the vast experience developed in the study of smoking cessation interventions in cigarette smokers, whilst including components and assessment tools that address the specific aspects of waterpipe smoking, such as its social dimension, unique experiences, and cues.
Plain language summary
Can users of waterpipes be helped to quit through smoking cessation interventions?
Background
Waterpipe smoking is a traditional method of tobacco use, especially in the Eastern Mediterranean Region, but its use is now spreading worldwide. It is smoked socially and often shared between friends or family at home, or in bars and cafes that provide waterpipes to patrons. In the absence of relevant data, many waterpipe tobacco smokers believe this form of tobacco use is less lethal and addictive than other methods of tobacco smoking, because the smoke passes through water on its way to the user. At least in some cultures, women and girls are more likely to use a waterpipe than other forms of tobacco, and it is popular among younger smokers. Current evidence suggests that waterpipe smoking may be as addictive as other forms of tobacco use, that some users have difficulty quitting on their own and that they may experience similar risks to health as cigarette smokers.
Study characteristics
We searched for controlled trials in the Cochrane Tobacco Addiction Review Group specialized register, in June 2015. We also searched a number of electronic databases, including MEDLINE, EMBASE, PsycINFO and CINAHL, using a variety of names and spellings for waterpipe use ('waterpipe' or 'narghile' or 'arghile' or 'shisha' or 'goza' or 'narkeela' or 'hookah' or 'hubble bubble'). We searched for published and unpublished trials in any language, and especially in areas where waterpipe use is widespread. We identified three studies that tested behavioural methods to help waterpipe smokers to quit. Two were waterpipe‐specific interventions and one was a non‐specific tobacco intervention.One small, pilot study was set in the USA, and delivered a Powerpoint presentation online to 91 college students who were using waterpipe. One study was a secondary analysis of data from 264 waterpipe smokers who were part of a trial that enrolled people suspected of having tuberculosis from 33 healthcare clinics in Pakistan. Clinics were randomly assigned to deliver a behavioural intervention versus control (usual care), or a behavioural intervention plus medication (bupropion) versus control (usual care). The third study, set in Egypt, targeted both cigarette and waterpipe smokers, and was a community‐based programme.
Key results
In all three trials, the percentage of participants who stopped smoking waterpipe was higher in the intervention groups than in the control groups, although this was a statistically significant finding in only two of the trials. People who received either behavioural treatment or behavioural treatment plus buproprion were more likely to quit waterpipe smoking at six months follow‐up than those who received usual care. Men smoking waterpipe in the Egyptian study were more likely to have quit at one year follow‐up in the intervention villages than in the control villages. These studies provide support to suggest that cessation interventions may help waterpipe smokers to quit. However, further larger studies are needed to build on this.
Quality of the evidence
The trials were all rated at very low quality of evidence, as they were relatively small studies, with at least one high risk of bias.
Summary of findings
Background
Estimates suggest that by 2030 there will be more than 10 million tobacco‐related deaths a year worldwide, with 70% of them occurring in developing countries (Peto 2001). Patterns of tobacco usage and uptake are of increasing concern, as the tobacco industry concentrates its marketing in developing countries, paying particular attention to women and girls and to a wide range of tobacco products (GYTS 2003).
One traditional method of smoking tobacco, especially in the Eastern Mediterranean Region (EMR), is the waterpipe, in which smoke passes through a reservoir of water before inhalation by the smoker. The waterpipe, known as narjeela in formal Arabic, goes by various local names such as shisha, narghile, arghile, and hookah (Maziak 2004). Although waterpipe use was uncommon in most of the world before the 1990s, it has enjoyed a recent resurgence, and is now spreading into areas where there was no previous tradition of use (Ward 2015). In most countries of the EMR, waterpipe smoking has become the most common tobacco use method among youth, and the trend is spreading to other world regions such as the US, where waterpipe smoking became the second most popular tobacco use method among college, high‐, and middle‐school students (Arrazola 2015; Maziak 2015; Primack 2009). While solitary waterpipe use is quite common, waterpipe use is predominantly a social phenomenon, occurring among friends or family, and often in dedicated cafés and bars (Akl 2015; Martinasek 2011).
It is hard to establish all of the potential factors responsible for the global spread of an addictive behaviour such as waterpipe smoking. An addictive behaviour will tend to spread gradually unless it is countered by effective policies and regulations. It is believed that the resurgence in the popularity of waterpipe was sparked by the introduction of flavoured, sweetened tobacco called Maassel in the Middle East during the early 1990s, whilst the global economy, advancements in communication and social media, emigration and tourism have helped to spread the practice globally (Maziak 2015). The lack of effective policies to deal with this relatively new trend is certainly contributing to the vacuum within which this tobacco use method is allowed to thrive (Jawad 2015).
Many waterpipe smokers believe that waterpipe smoking is a safer alternative to cigarettes; an apparent misperception given the available evidence (Akl 2015; Asfar 2008; El‐Zaatari 2015; Martinasek 2011). This evidence demonstrates the wide‐ranging potential harm of waterpipe smoking, as well as its addictive nature (Aboaziza 2015; El‐Zaatari 2015; Shihadeh 2015). Many waterpipe users become dependent, evidenced by urges to smoke waterpipe and other withdrawal symptoms when they abstain, relief of these symptoms when they smoke waterpipe, and difficulty in quitting (Aboaziza 2015). Several studies have reported that between 25% and 64% of waterpipe users want to quit (Akl 2013; Anjum 2008; Ward 2005) and that at least 25% make a quit attempt in any given year (Anjum 2008; Ward 2005; Ward 2006). Quit rates, however, are very low (Ward 2006).
The most identified behavioural association with waterpipe smoking is cigarette smoking. Many studies from around the world have documented the salience of cigarette smoking among waterpipe smokers, and cigarette smoking has been shown to be a major predictor of waterpipe smoking among youth. In the US Monitoring the Future survey, waterpipe use among high‐school seniors was associated with current and former cigarette smoking (Maziak 2015). Dual smoking, however, tends to decrease with age, as older smokers are usually more loyal to a single tobacco use method. For example, in a study alluded to earlier, comparing novice with established waterpipe smokers in Syria, the prevalence of dual smoking was 47.7% for novice smokers compared to 26.5% for established ones (Maziak 2015).
Given the global increase in waterpipe smoking, and evidence that many users become dependent and have difficulty quitting on their own, waterpipe‐specific tobacco cessation interventions are required. This review aims to summarize the evidence available regarding smoking cessation interventions for waterpipe smokers.
Objectives
To evaluate the effectiveness of tobacco cessation interventions for waterpipe users.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials, quasi‐randomized controlled trials and cluster‐randomized controlled trials.
Types of participants
Current (past month) users of waterpipes for tobacco smoking, of any age and either gender.
Types of interventions
We included interventions directed at waterpipe users that were pharmacological (for example, nicotine replacement therapy (NRT) or bupropion) or behavioural, or both. These could be directed at individual users or groups of users. We only included cessation interventions, and did not consider trials of prevention of smoking uptake.
Types of outcome measures
The primary outcome was abstinence from any tobacco waterpipe use for six months or more from the beginning of intervention. We report abstinence at longest follow‐up, and prefer the strictest definition of abstinence (continuous or prolonged over point prevalence, as defined by Hughes 2003). We prefer biochemically‐validated abstinence over self‐reported abstinence.
Search methods for identification of studies
We searched the Cochrane Tobacco Addiction Review Group specialized register for trials, using the terms 'waterpipe' or 'narghile' or 'arghile' or 'shisha' or 'goza' or 'narkeela' or 'hookah' or 'hubble bubble', plus variant spellings of these terms, and 'smoking' in the title or abstract, or as keywords. This register was developed from electronic searching of MEDLINE, EMBASE and PsycINFO, together with handsearching of specialist journals, conference proceedings and reference lists of previous trials and overviews. We also searched MEDLINE (1946 to present), EMBASE (1980 to present), CINAHL (1981 to present) and PsycINFO (1806 to present), using the above free‐text terms combined with MeSH or free‐text smoking‐related terms (smok* or tobacco or cigar* or nicotine). We searched for trials, published or unpublished, in any language, and especially in regions where waterpipe use is widespread. We also used our existing bibliography, compiled from earlier exhaustive reviews of the literature on waterpipe smoking (e.g. Aboaziza 2015; El‐Zaatari 2015; Jawad 2015; Maziak 2004; Maziak 2015; Shihadeh 2015). The most recent search was completed on 19th June 2015.
Data collection and analysis
Two review authors (MJ and SJ) assessed the abstracts of studies retrieved by the search strategy, for possible inclusion in the review. We retrieved full‐text articles for all abstracts which either review author believed might be suitable.
Assessment of full articles
Two review authors (MJ and SJ) assessed each full‐text article independently, using the agreed inclusion criteria. Where there was ambiguity in trial reporting or a lack of data, we contacted investigators for clarification where possible. If we could not retrieve missing data we considered exclusion on that basis.
We rated the overall methodological quality of studies as being at low, moderate, or high risk of bias for each of the following criteria to assess risk of bias:
Random sequence generation
Concealment of allocation
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data (attrition rates and losses to follow‐up)
Biochemical verification of smoking status
We maintained a full list of excluded studies.
Data collection
We extracted and reported the following information, where available, concerning each study:
Country and study setting
Dates study was conducted
Theoretical framework (including a brief description of the intervention)
Focus of the intervention (e.g. any tobacco use, waterpipe smoking)
Type of intervention, its duration, intensity, delivery format
Length of follow‐up
Number of participants or number of clusters and participants
Age range, socio‐economic status, gender and ethnicity (if relevant) of participants
Definition of smoking status used (e.g. level of waterpipe use, concurrent use of other tobacco)
Definition of abstinence
Biochemical validation (if present)
Differential effects post‐intervention relating to age, gender, ethnicity and intensity of intervention
Adverse effects of the intervention
Sources of funding
We aimed to pool groups of studies that we considered to be sufficiently similar in their interventions, comparison groups, setting and participants, provided that there was no evidence of substantial statistical heterogeneity as assessed by the I² statistic (Higgins 2003). We aimed to estimate a pooled risk ratio (RR) using the Mantel‐Haenszel fixed‐effect method, based on the quit rates at longest follow‐up for trials with at least six months follow‐up from the start of the intervention. Where meta‐analysis was not possible, we present a descriptive summary and descriptive statistics.
We include a glossary of tobacco‐specific terms (Appendix 1) as an additional table in this review.
Results
Description of studies
Our search retrieved 1311 unique citations, of which 1289 were excluded after title and abstract screening. Of the remaining 22, we excluded 19 for the following reasons: they were non‐randomized studies (n = 12), review articles (n = 3), a protocol only (n = 2), conducted among cigarette smokers only (n = 1), or had only a three‐month follow‐up (n = 1). The flow of studies is illustrated in Figure 2.
2.
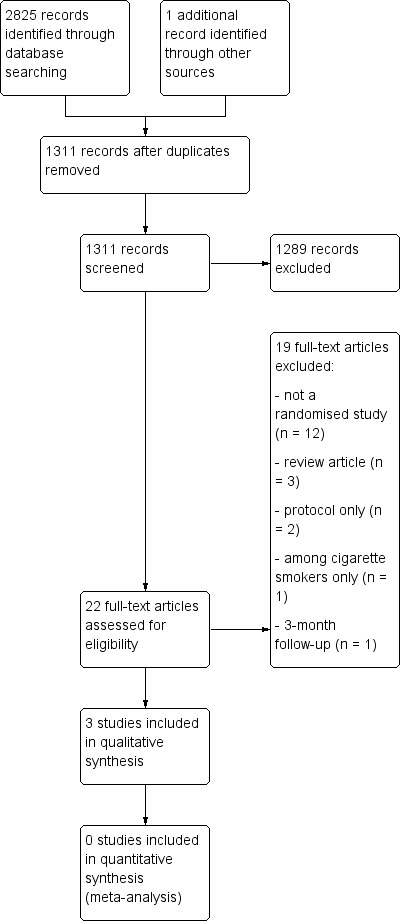
Study flow diagram.
We identified three eligible studies which tested cessation interventions for waterpipe smokers. One was a randomized controlled trial (Lipkus 2011) and two were cluster‐randomized trials (Dogar 2014; Mohlman 2013). Two were individual‐level interventions (Dogar 2014; Lipkus 2011) and one was a community‐level intervention (Mohlman 2013). Interventions were carried out in Egypt (Mohlman 2013), Pakistan (Dogar 2014), and the US (Lipkus 2011). All interventions were behavioural interventions, although one study included two intervention groups: one behavioural, and the other behavioural with bupropion (Dogar 2014). One study was based on a theoretical framework (Dogar 2014). Further characteristics of included studies can be found in Characteristics of included studies.
The first study took place in Pakistan between 2010 and 2011 and was a three‐arm, cluster‐randomized non‐inferiority trial among a mix of cigarette‐only smokers (n = 1181), waterpipe‐only smokers (n = 200) and mixed smokers (n = 460) (Dogar 2014). The three arms were standard care (control group), a brief behavioural intervention (BSS) and a brief behavioural intervention plus bupropion for seven weeks (BSS+). The behavioural intervention was adapted from evidence‐based treatments used for cigarette smokers and involved two structured sessions (the first 30 minutes long, the second 10 minutes long) one week apart. It was delivered by tuberculosis DOTS (directly observed treatment, short course) paramedics. The bupropion regimen was 75 mg/day for the first week and 150 mg/day for the next six weeks. The control group received a leaflet with standard health messages about the harms of tobacco. The clusters were primary and secondary healthcare centres registered as diagnostic centres by a tuberculosis programme. The study authors adjusted for the effect of clustering by conducting a multi‐level analysis. The study was funded by the International Development Research Centre, Canada.
The second study took place in the US between 2009 and 2010, and was a randomized controlled web‐based intervention among waterpipe smoking college/university students (Lipkus 2011). Ninety‐one students were randomized to non‐health‐related information about waterpipe (control group) or to both non‐health‐related and health‐related information about waterpipe (intervention group). The study was funded by grants from the US National Cancer Institute and National Institute on Drug Abuse.
The final study took place in Egypt between 2004 and 2005, and was a cluster‐randomized controlled community‐level intervention (Mohlman 2013). The clusters were villages in the Qalyubia governorate. Villages, with a total of 7657 participants, were randomised to receive a behavioural intervention through a variety of activities engaging school students, places of worship, and adult women, and delivered by teachers, religious leaders and female social‐change agents respectively. Primary school students partook in activities to prevent the initiation of tobacco use through its deglamorisation and teaching of health effects. Preparatory and secondary school students were taught social skills to handle peer pressure to smoke. Religious communities were informed of the health effects of tobacco use/secondhand smoke, and the sinful nature of smoking. Adult women at home were taught about the health effects of tobacco use/secondhand smoke and how to protect themselves and their children from it in a culture‐specific way. Control villages received no intervention but had access to Egypt's National Tobacco Control Program during the study. The study authors adjusted for the effect of clustering in the analysis. The study was funded by The Fogarty International Center of the US National Institutes of Health.
Studies were not comparable in terms of participants' smoking status, intervention type, and outcome measures. For example, participants in Dogar 2014 smoked a local form of unflavoured waterpipe tobacco a median of 10 times per day, participants in Mohlman 2013 smoked two to three times per day, and participants in Lipkus 2011 smoked flavoured Maassel monthly. With regards to the delivery method of interventions, one study provided a web‐based intervention (Lipkus 2011), one provided group intervention (Mohlman 2013), and one provided interventions aimed at individuals (Dogar 2014). With regards to outcomes, only one study (Dogar 2014) biochemically validated abstinence by expired carbon monoxide (CO < 9 ppm), while the remaining two trials relied on only self report of smoking status (Lipkus 2011; Mohlman 2013). Follow‐up length ranged from six months to one year post‐intervention.
Risk of bias in included studies
We considered two studies (Dogar 2014; Lipkus 2011) to be at low risk of bias for random sequence generation as they adequately described a simple randomization process. Mohlman 2013 used a randomized design but the details were not reported. We deemed only one study to be at low risk of bias for adequate allocation concealment (Dogar 2014) as it was concealed by a researcher blinded to centre identity. The remaining two studies did not provide information on allocation concealment (Lipkus 2011; Mohlman 2013). We rated none of the studies at low risk of bias for blinding of participants and personnel; Dogar 2014 was an open‐label trial, while in Lipkus 2011 and Mohlman 2013 blinding was not mentioned. Although none of the studies reported the presence of blinding, we considered one study (Dogar 2014) to be at low risk of bias for blinding of outcome assessment, as it was biochemically verified with a carbon monoxide measurement of 9 ppm or less. We rated all three at low risk of bias for completeness of data, as missing data ranged from 7% (Dogar 2014) to 23% (Lipkus 2011). Mohlman 2013 excluded women from the dataset because smoking prevalence was very low in this group. No studies were at low risk of bias for selective reporting. In Dogar 2014 outcomes at five weeks were not reported, whereas in Lipkus 2011 and Mohlman 2013 cessation data were not reported using appropriate effect estimates. A summary of the 'Risk of bias' assessment for the included studies can be found in Figure 1.
1.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
for the main comparison.
| Waterpipe intervention compared with a control for waterpipe cessation | ||||
| Outcomes | Impact | Number of Participants (Studies) | Certainty of the evidence (GRADE)* | Comments |
| Prolonged Cessation | (Dogar 2014) RR 2.48 (95% CI: 1.36 to 3.83) for 25 weeks cessation (Lipkus 2011) RR 1.46 (95% CI: 0.81 to 2.62) for 6 months (Mohlman 2013) RR 3.25 (95% CI: 1.19 to 2.12) for 12 months cessation |
200 (1) 91 (1) 540 (1) |
⊕⊝⊝⊝ very low | The studies were not pooled as the interventions were not sufficiently similar in design and participant demographics. There is no pooled effect estimate. |
| CI: Confidence interval; RR: Risk Ratio | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
| *The certainty of the evidence was very low as all the studies had at least one high risk of bias in accordance with the GRADE framework (see Figure 1). | ||||
Due to the variation in studies outlined above, we did not pool the results of studies using meta‐analysis or conduct statistical tests for heterogeneity. We report the intervention effects descriptively and present abstinence data for individual trials in Figure 3.
3.
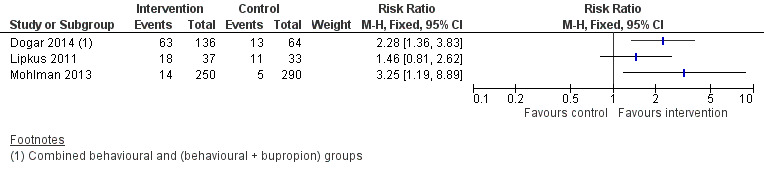
Forest plot of comparison: 1 Raw Data, outcome: 1.1 Prolonged Cessation.
For Dogar 2014 we considered the data provided by waterpipe smokers only (n = 200). Compared to the control group, the risk ratio (RR) of smoking abstinence at 25 weeks was 2.2 (95% CI 1.3 to 3.8) for the BSS group and 2.5 (95% CI 1.3 to 4.7) for the BSS+ group. In Figure 3 we have combined the BSS and BSS+ groups to create a single intervention group.
For Lipkus 2011, when comparing the intervention to the control group, the RR for waterpipe cessation at six months was 1.46 (95% CI 0.81 to 2.62; n = 70).
For the third study (Mohlman 2013), the RR of waterpipe cessation at one year was 3.25 (95% CI 1.19 to 8.89; n = 540) for the intervention group compared to the control group.
Discussion
Waterpipe use is a growing phenomenon associated with substantial toxicant exposure, numerous health risks, and development of dependence in a sizeable proportion of users (Aboaziza 2015; El‐Zaatari 2015; Maziak 2015; Shihadeh 2015). Despite these adverse consequences, development and evaluation of cessation interventions for the waterpipe are scarce. In our review, we found only three studies which met our inclusion criteria, covering 831 participants, that have examined interventions to help waterpipe users quit smoking. All three studies tested behavioural interventions, and one study also included a combined behavioural/pharmacological (bupropion) intervention group (Dogar 2014). Due to lack of comparability across the three studies in terms of participants’ smoking status, intervention type, and outcome assessment, we did not conduct statistical tests for heterogeneity and meta‐analysis, but present intervention effects descriptively. Two trials were conducted among adults in the Middle East (Dogar 2014; Mohlman 2013), and one study was conducted among young adults in the US (Lipkus 2011). This should be considered for the generalizability of findings from these studies. Compared to control groups, smoking cessation rates were higher in the intervention groups in all three studies; however, the difference was not statistically significant in one study (Lipkus 2011). These findings suggest that waterpipe smokers may be more likely to stop smoking successfully when using a community or a tailored smoking cessation intervention than usual care; however they should be treated with caution due to the paucity and limitations of the available data.
The lack of a statistically significant effect in the American trial is not unexpected, given the small, pilot nature of the study. A Cochrane review of individual behavioural interventions for cigarette smoking cessation demonstrated an RR of 1.39 (95% CI 1.2 to 1.57) (Lancaster 2005). At least two of the studies (Dogar 2014; Lipkus 2011) were unlikely to have the power to detect a comparable RR. Suboptimal length of follow‐up (less than one year) was another limitation for two of the three included studies, as well as a reliance on self‐reported data in all but one study (Dogar 2014). Abstinence verification methods should also be suitable for waterpipe smoking. Expired breath CO, which is good for the detection of smoking in the past 24 hours only, may not accurately verify abstinence in intermittent waterpipe smokers, but can be used as a 'bogus pipeline' (Asfar 2014; Murray 1987; Patrick 1994) to encourage truthful reporting of abstinence violations among intermittent users. The absence of standard definitions for waterpipe smoking status for inclusion in the trials was also an issue that affected comparability of the reviewed studies. For example, two of the reviewed studies (Lipkus 2011; Mohlman 2013) recruited current waterpipe smokers who smoked waterpipe in the past month, and one study (Dogar 2014) recruited regular waterpipe smokers who “smoke >= 1 waterpipe per day.” Cigarette cessation trials typically enrol daily smokers and outcome evaluation is often focused on whether participants have returned to daily smoking. We noted the same inconsistency for the definition and verification of abstinence in the three studies. In Dogar 2014, the primary outcome was continuous abstinence at six‐month follow‐up, while in Lipkus 2011 abstinence was defined as reporting no longer using waterpipe at the six‐month follow‐up, and finally, in Mohlman 2013 abstinence was defined as not smoking waterpipe in the last month before the 12‐month follow‐up. Not only are these outcomes hard to compare, they are not consistent with the relevant scientific recommendations for cessation trials and with common patterns of waterpipe smoking. Because many waterpipe smokers are cigarette smokers as well, cessation outcomes should be standardised to allow comparison with the cigarette literature as well as to accommodate waterpipe smokers’ usage patterns. For example, the definition of prolonged abstinence defined as; no smoking, not even a puff, after a grace period of two weeks after quit date, and relapse as smoking at least once a week on two consecutive weeks (SRNT 2002), are standard cigarette‐based definitions that would seem to be suitable for waterpipe.
Out of the three included studies, only one evaluated a combined intervention (behavioural plus bupropion), and did not show any apparent additional benefit of adding bupropion to behavioural support in achieving cessation (Dogar 2014), although these two conditions were not directly tested against one another. The efficacy of other pharmacological cessation modalities such as nicotine replacement therapy (NRT) or varenicline, which have been shown to be useful in dependent cigarette smokers, have not yet been tested in waterpipe smokers. Given that some waterpipe smokers exhibit signs and symptoms of dependence (Aboaziza 2015), pharmacotherapy may be useful during cessation. This can be particularly relevant to highly quit‐motivated dual waterpipe/cigarette users (Ward 2014). However, individuals who are less dependent, have smoked for shorter periods of times, and who cite social stigma (e.g. family disapproval) as a reason to stop smoking make up the majority of waterpipe smokers interested in quitting (Borgan 2013; Ward 2005). These individuals may be less likely to benefit from pharmacological treatments. Such considerations require having some standard, waterpipe‐specific measure of dependence that allows for the variability in both individual smoking habits and nicotine content of different waterpipe tobacco brands to be captured in a standardized manner. A recently developed scale (Lebanon Waterpipe Dependence Scale‐11) to characterize waterpipe dependence has shown that self‐reported dependence level correlates with measurements of nicotine metabolites in flavoured waterpipe tobacco users (Salameh 2008). This measure was developed based on cigarette smoking instruments and without input from waterpipe smokers, but could be the first step in accurately measuring dependence among waterpipe smokers.
Offering behavioural support adapted from a validated cigarette‐smoking cessation programme could be a useful starting point for waterpipe smokers who are interested in quitting. Of the three studies reviewed, two offered behavioural interventions utilizing similar strategies to those shown to be effective for cigarette smokers (Lipkus 2011; Mohlman 2013). Process evaluation data from one of our excluded studies (Asfar 2014) indicates that the methods they used, which were adapted from traditional smoking cessation methods, were acceptable to waterpipe smokers. However, such approaches will miss dealing with the strong social dimension of waterpipe use, as it shapes use patterns, cues for smoking, and the attitudes and preferences of waterpipe smokers (Aboaziza 2015; Jawad 2013; Maziak 2015).
Risks associated with the social use of waterpipe, such as the potential to contract infectious diseases through sharing the same waterpipe and using it repeatedly without proper sanitation in café settings, can also provide powerful drives for cessation. Future cessation efforts should consider introducing and examining new methods of cessation intervention, such as group smoking cessation. Results from the process evaluation in Asfar 2014 indicate that one‐third of waterpipe smokers were interested in participating in a group counselling intervention. As the social context of waterpipe smoking frequently involves family members (Akl 2015; Maziak 2015), family‐based cessation interventions could also be a promising avenue to pursue (Asfar 2014).
Despite the fact that the waterpipe epidemic is most pronounced among youth and young adults (such as college students), only one of the three reviewed studies was conducted among college students. The study provided preliminary evidence that minimally intensive interventions delivered online to educate college waterpipe smokers of the harm, addiction, and toxicant exposure associated with waterpipe smoking can increase understanding of the harms of waterpipe use, perceptions of risk, desire to quit, and eventually cessation (Lipkus 2011). Utilizing youth‐oriented technology such as smart phones, text messaging, social networks, or multimedia may provide promising cessation approaches for this at‐risk population.
Although the small number and methodological limitations of waterpipe cessation trials to date do not allow firm recommendations to be made on the comparative efficacy of various cessation methods, they do provide a new evidence base on which to build further. They highlight important design and content issues that need to be considered for future cessation trials in waterpipe smokers. These include building on the vast experience of cigarette smoking cessation interventions whilst introducing intervention components and assessment tools that address the specific aspects of waterpipe smoking. It also highlights some of the challenges of future waterpipe cessation trials that relate to their specific set‐up, usage patterns and context, and adaptability of cigarette‐based definitions and measures to the waterpipe.
Authors' conclusions
Implications for practice.
Waterpipe smoking is spreading globally, and carries considerable health risks. Due to its addictive nature, waterpipe users who want to quit find it difficult to do so. This review suggests that waterpipe smokers may be more likely to stop smoking successfully when using a smoking cessation intervention than usual care, but this needs to be treated with caution due to the paucity and limitations of the data. Relying on behavioural cessation approaches from the cigarette literature seems to be a good starting point, but these should be adapted to the specific nature of waterpipe smoking as a predominantly social and intermittent behaviour with prolonged sessions. Adding a pharmacological agent (bupropion) did not seem to have an additional benefit to behavioural support in achieving cessation, again based on the limited data available.
Implications for research.
Standard definitions and assessments of waterpipe use, dependence and cessation need to be adopted. For example, including smokers with “regular” waterpipe use, defined as smoking three or more waterpipes a week, in smoking cessation trials, will allow smoking cessation efforts to focus on those most in need (most dependent), and at the same time allow the use of standard cigarette‐based definitions and verification of abstinence (e.g. prolonged abstinence; saliva cotinine). Since cotinine offers a window of four to five days for detection of nicotine exposure, less frequent waterpipe smoking/abstinence can be hard to verify biochemically. Most of these definitions are already developed and need to be adopted by waterpipe cessation research (e.g. Jarvis 1988; SRNT 2002).
As waterpipe cessation trials are still in their infancy, it will be helpful to develop and adopt consistent standards for reporting outcomes to facilitate comparing study results (e.g. standard definition of regular smoker, definition of abstinence including duration, self report, and cut‐off point of various biochemical verification procedures).
Since many waterpipe smokers are cigarette smokers as well, such measures need to be consistent with the cigarette literature and at the same time accommodate waterpipe smokers’ intermittent usage patterns.
Waterpipe dependence measures need to be developed and adopted that can capture the common as well as unique (e.g. social dimension) aspects of tobacco dependence in waterpipe smokers.
Efforts to develop and test behavioural strategies that fit the unique features of waterpipe smoking (e.g. the social cues, and intermittent usage patterns) and address waterpipe‐specific facilitators and barriers to quitting are much needed (Maziak 2015).
Given how little is currently known about who will use cessation treatment, which treatments they will use, and what specific methods work, it is imperative that large‐scale, randomized controlled trials be conducted to rigorously test behavioural, pharmacological and combined cessation approaches.
What's new
| Date | Event | Description |
|---|---|---|
| 13 March 2015 | New citation required and conclusions have changed | Three new citations identified. Conclusions updated. |
| 14 December 2014 | New search has been performed | Searches updated, three studies identified and included. |
History
Protocol first published: Issue 4, 2005 Review first published: Issue 4, 2007
| Date | Event | Description |
|---|---|---|
| 4 August 2008 | Amended | Converted to new review format. |
| 9 August 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
None
Appendices
Appendix 1. Glossary of tobacco‐related terms
| Term | Definition |
| Abstinence | A period of being quit, i.e. stopping the use of cigarettes or other tobacco products, May be defined in various ways; see also: point prevalence abstinence; prolonged abstinence; continuous/sustained abstinence. |
| Biochemical verification | Also called 'biochemical validation' or 'biochemical confirmation': A procedure for checking a tobacco user's report that he or she has not smoked or used tobacco. It can be measured by testing levels of nicotine or cotinine or other chemicals in blood, urine, or saliva, or by measuring levels of carbon monoxide in exhaled breath or in blood. |
| Bupropion | A pharmaceutical drug originally developed as an antidepressant, but now also licensed for smoking cessation; trade names Zyban, Wellbutrin (when prescribed as an antidepressant) |
| Carbon monoxide (CO) | A colourless, odourless highly poisonous gas found in tobacco smoke and in the lungs of people who have recently smoked, or (in smaller amounts) in people who have been exposed to tobacco smoke. May be used for biochemical verification of abstinence. |
| Cessation | Also called 'quitting'. The goal of treatment to help people achieve abstinence from smoking or other tobacco use, also used to describe the process of changing the behaviour |
| Continuous abstinence | Also called 'sustained abstinence'. A measure of cessation often used in clinical trials involving avoidance of all tobacco use since the quit day until the time the assessment is made. The definition occasionally allows for lapses. This is the most rigorous measure of abstinence |
| 'Cold Turkey' | Quitting abruptly, and/or quitting without behavioural or pharmaceutical support. |
| Craving | A very intense urge or desire [to smoke]. See: Shiffman et al 'Recommendations for the assessment of tobacco craving and withdrawal in smoking cessation trials' Nicotine & Tobacco Research 2004: 6(4): 599‐614 |
| Dopamine | A neurotransmitter in the brain which regulates mood, attention, pleasure, reward, motivation and movement. |
| Efficacy | Also called 'treatment effect' or 'effect size': The difference in outcome between the experimental and control groups. |
| Harm reduction | Strategies to reduce harm caused by continued tobacco/nicotine use, such as reducing the number of cigarettes smoked, or switching to different brands or products, e.g. potentially reduced exposure products (PREPs), smokeless tobacco. |
| Lapse/slip | Terms sometimes used for a return to tobacco use after a period of abstinence. A lapse or slip might be defined as a puff or two on a cigarette. This may proceed to relapse, or abstinence may be regained. Some definitions of continuous, sustained or prolonged abstinence require complete abstinence, but some allow for a limited number or duration of slips. People who lapse are very likely to relapse, but some treatments may have their effect by helping people recover from a lapse. |
| nAChR | [neural nicotinic acetylcholine receptors]: Areas in the brain which are thought to respond to nicotine, forming the basis of nicotine addiction by stimulating the overflow of dopamine |
| Nicotine | An alkaloid derived from tobacco, responsible for the psychoactive and addictive effects of smoking. |
| Nicotine Replacement Therapy (NRT) | A smoking cessation treatment in which nicotine from tobacco is replaced for a limited period by pharmaceutical nicotine. This reduces the craving and withdrawal experienced during the initial period of abstinence while users are learning to be tobacco‐free The nicotine dose can be taken through the skin, using patches, by inhaling a spray, or by mouth using gum or lozenges. |
| Outcome | Often used to describe the result being measured in trials that is of relevance to the review. For example smoking cessation is the outcome used in reviews of ways to help smokers quit. The exact outcome in terms of the definition of abstinence and the length of time that has elapsed since the quit attempt was made may vary from trial to trial. |
| Pharmacotherapy | A treatment using pharmaceutical drugs, e.g. NRT, bupropion. |
| Point prevalence abstinence (PPA) | A measure of cessation based on behaviour at a particular point in time, or during a relatively brief specified period, e.g. 24 hours, 7 days. It may include a mixture of recent and long‐term quitters. cf. prolonged abstinence, continuous abstinence |
| Prolonged abstinence | A measure of cessation which typically allows a 'grace period' following the quit date (usually of about two weeks), to allow for slips/lapses during the first few days when the effect of treatment may still be emerging. |
| Relapse | A return to regular smoking after a period of abstinence. |
| Secondhand smoke | Also called passive smoking or environmental tobacco smoke [ETS]. A mixture of smoke exhaled by smokers and smoke released from smouldering cigarettes, cigars, pipes, bidis, etc. The smoke mixture contains gases and particulates, including nicotine, carcinogens and toxins. |
| Self‐efficacy | The belief that one will be able to change one's behaviour, e.g. to quit smoking. |
| SPC [Summary of Product Characteristics] | Advice from the manufacturers of a drug, agreed with the relevant licensing authority, to enable health professionals to prescribe and use the treatment safely and effectively. |
| Tapering | A gradual decrease in dose at the end of treatment, as an alternative to abruptly stopping treatment. |
| Titration | A technique of dosing at low levels at the beginning of treatment, and gradually increasing to full dose over a few days, to allow the body to get used to the drug. It is designed to limit side effects. |
| Withdrawal | A variety of behavioural, affective, cognitive and physiological symptoms, usually transient, which occur after use of an addictive drug is reduced or stopped. See: Shiffman et al 'Recommendations for the assessment of tobacco craving and withdrawal in smoking cessation trials' . Nicotine & Tobacco Research 2004: 6(4): 599‐614 |
Data and analyses
Comparison 1. Intervention versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prolonged Cessation | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
1.1. Analysis.
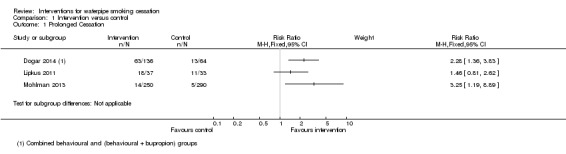
Comparison 1 Intervention versus control, Outcome 1 Prolonged Cessation.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dogar 2014.
| Methods | Year(s) of study: 2010 ‐ 2011 Study design: 3‐arm cluster‐randomized controlled non‐inferiority trial Country: Pakistan Region: Jhang and Sarghoda districts Setting: 33 primary and secondary health centres Theoretical framework: based on the World Health Organization’s '5As Approach' |
|
| Participants | Adults aged over 18 years with suspected tuberculosis (cough ≥ 3 weeks, of unknown cause) Excluded: those requiring hospitalization or urgent medical attention Recruitment method: patients attending primary and secondary healthcare centres registered as diagnostic centres by a tuberculosis program in 2 Pakistani districts 33 clusters, 200 adults Mean age 51.5 (SD 13.8), median household income USD 81.4 (IQR 69.8), 21% women Definition of smoking status: ≥ 1 waterpipe/day |
|
| Interventions | Focus of intervention: any smoking use Type of intervention: behavioural and pharmacological Description of the intervention: Control group: given a leaflet with standard health messages about the harms of tobacco Intervention group 1: 2 brief behavioural support cessations (1st visit 30 mins, 2nd on quit day 10 mins) Intervention group 2: 2 brief behavioural support cessations (as above) plus bupropion for 7 weeks (75 mg/day for 1st week, 150 mg/day for next 6 weeks) Intervention delivered by: tuberculosis DOTS (directly observed treatment, short course) paramedics |
|
| Outcomes | Continuous waterpipe smoking abstinence Length of follow‐up: six months Biochemical validation: CO verified (< 10 ppm) |
|
| Notes | Differential effects post‐intervention: none reported Adverse effects of intervention: none reported The study was funded by the International Development Research Centre, Canada |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not mentioned in sufficient detail in this paper; full trial methodology found in Siddiqi 2013: Quote: "a researcher who was blinded to center identity used computer‐generated random‐number lists to generate allocation sequence" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Not mentioned in sufficient detail in this paper; full trial methodology found in Siddiqi 2013: Quote: "a researcher who was blinded to center identity used computer‐generated random‐number lists to generate allocation sequence" Commment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned in this paper; full trial methodology found in Siddiqi 2013: Quote: "the lack of blinding also meant that a degree of observer bias was possible" Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not mentioned in this paper; full trial methodology found in Siddiqi 2013: Quote: "the lack of blinding also meant that a degree of observer bias was possible" Comment: outcome measurement is biochemically verified, and is unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data missing for 7.0% of waterpipe‐only smokers. Reasons for missing data unlikely to be related to true outcome. No exclusions reported. |
| Selective reporting (reporting bias) | High risk | Outcomes not reported at 5 weeks |
| Other bias | Low risk | Biochemical verification of outcome |
Lipkus 2011.
| Methods | Year(s) of study: 2009 ‐ 2010 Study design: randomized controlled web‐based behavioural intervention Country: USA Region: North Carolina Setting: 6 college and university campuses Theoretical framework: none reported |
|
| Participants | Adults enrolled in a 4‐year college or university course Recruitment method: newspaper advertisements, flyers posted around campuses, Craig's list, campus‐wide Listserv 91 adults Mean age 20.4 (SD 2.0), 24.2% women, 76.7% white Definition of smoking status: past‐month waterpipe smoking |
|
| Interventions | Focus of intervention: waterpipe smoking Type of intervention: behavioural Description of the intervention: Control group: 8 MS PowerPoint slides on waterpipe mechanism of action, chemical composition, and epidemiology; average length of intervention 3.6 minutes Intervention group: 20 MS PowerPoint slides on waterpipe mechanisms of action, chemical composition, epidemiology, puff topography, toxicant exposure, and health outcomes; average length of intervention 7.5 minutes Intervention delivered by: online |
|
| Outcomes | Ticking the survey item: “no longer smoking waterpipe” Length of follow‐up: 6 months Biochemical validation: not present |
|
| Notes | Differential effects post‐intervention: none reported Adverse effects of intervention: none reported The study was funded by grants from the US National Cancer Institute and National Institute on Drug Abuse |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "those who logged on were randomized to either a control or an experimental group with equal probability by our program" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment Comment: insufficient confidence that allocation concealment was adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding Comment: insufficient confidence that blinding was adequate |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No information on blinding Comment: outcome measurement not biochemically verified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 23.1% of participants did not conduct the 6‐month follow‐up. Unlikely to be related to outcome. No reasons given for loss to follow‐up. 1 participant in the 6‐month follow‐up had missing data and was not analysed. No exclusions reported |
| Selective reporting (reporting bias) | High risk | Cessation data not appropriately presented as effect estimates |
| Other bias | High risk | No biochemical verification of outcome |
Mohlman 2013.
| Methods | Year(s) of study: 2004 ‐ 2005 Study design: cluster‐randomized controlled behavioural intervention Country: Egypt Region: Qalyubia governorate Setting: Villages Theoretical framework: None reported |
|
| Participants | All household members aged over 12 years old, although results pertain only to adult men (n of women for self‐reported smoking too small); Waterpipe smokers: Intervention villages 250, control villages: 290 Recruitment method: Systematic approach of households 6 clusters, 7657 residents Mean age 36.9, 41.8% illiterate, 87.3% employed, 55.3% women Definition of smoking status: Past‐month waterpipe smoking |
|
| Interventions | Focus of intervention: cigarettes and/or waterpipe smoking Type of intervention: behavioural Description of the intervention: educational approach for primary/preparatory/secondary school students, mosques and churches, and key female social change agents (raedat refeyat) Intervention delivered by: teachers, religious leaders, female social change agents Control group: no intervention |
|
| Outcomes | Waterpipe smoking prevalence Length of follow‐up: 12 months Biochemical validation: not present |
|
| Notes | Differential effects post‐intervention: none reported Adverse effects of intervention: none reported The study was funded by the Fogarty International Center of the US National Institutes of Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the remaining six villages were randomly allocated to either the control group or the intervention group" Comment: insufficient confidence that the allocation sequence was genuinely randomised |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation sequence Comment: insufficient confidence that allocation concealment was adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding Comment: insufficient confidence that blinding was adequate |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No information on blinding Comment: outcome measurement is not biochemically verified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 77.5% retention rate. Women excluded due to very low self‐reporting of tobacco use. All completed pre‐intervention survey but not all completed post‐intervention survey. No reasons given for loss to follow‐up. |
| Selective reporting (reporting bias) | High risk | Cessation data not appropriately presented as effect estimates |
| Other bias | High risk | No biochemical verification of outcome |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Asfar 2014 | 3‐month follow up |
Differences between protocol and review
None.
Contributions of authors
All authors contributed to the conceptualisation and preparation of the review, and approved the final text. MJ and SJ assessed the studies retrieved by the search strategy, and updated the Methods and Results sections accordingly. TA updated the Discussion according to the new results. WM and KW updated all the manuscript in light of new literature on the subject, and the reviewed articles. TE reviewed the updated version, edited and finalized it.
Sources of support
Internal sources
No sources of support supplied
External sources
US Public Health Service Grants TW05962, TW07233 USA; Initiative for Cardiovascular Health Research in the Developing Countries (IC‐Health), India.
Declarations of interest
KW has no known conflicts of interest MJ has no known conflicts of interest SJ has no known conflicts of interest TA has no known conflicts of interest WM is funded by National Institute on Drug Abuse (NIDA) grant R01 DA035160. WM's research in tobacco control, including the waterpipe, has been funded by the US National Institutes of Health since 2002. TE's research on waterpipe tobacco smoking and various other tobacco products, including electronic cigarettes, has been supported by grants from the U.S. National Institutes of Health and is currently supported by the U.S. National Institutes of Health's National Institute on Drug Abuse and the Center for Tobacco Products of the US Food and Drug Administration (P50DA036105). TE also occasionally receives honoraria for delivering invited lectures regarding tobacco product effects to a variety of university audiences. The content of the work published herein is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Dogar 2014 {published data only}
- Dogar O, Jawad M, Shah SK, Newell JN, Kanaan M, Khan MA, et al. Effect of cessation interventions on hookah smoking: post‐hoc analysis of a cluster‐randomized controlled trial. Nicotine & Tobacco Research 2014;16(6):682‐8. [DOI] [PubMed] [Google Scholar]
- Siddiqi K, Khan A, Ahmad M, Dogar O, Kanaan M, Newell JN, et al. Action to stop smoking in suspected tuberculosis (ASSIST)in Pakistan: a cluster randomized, controlled trial. Annals of Internal Medicine 2013;158(9):667‐75. [DOI] [PubMed] [Google Scholar]
Lipkus 2011 {published and unpublished data}
- Lipkus IM, Eissenberg T, Schwartz‐Bloom RD, Prokhorov AV, Levy J. Affecting perceptions of harm and addiction among college waterpipe tobacco smokers. Nicotine & Tobacco Research 2011;13(7):599‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohlman 2013 {published data only}
- Mohlman MK, Boulos DN, Setouhy M, Radwan G, Makambi K, Jillson I, et al. A randomized, controlled community‐wide intervention to reduce environmental tobacco smoke exposure. Nicotine & Tobacco Research 2013;15(8):1372‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Asfar 2014 {published data only}
- Asfar T, Al Ali R, Rastam S, Maziak W, Ward KD. Behavioral cessation treatment of waterpipe smoking: the first pilot randomized controlled trial. Addictive Behaviors 2014;39(6):1066‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Aboaziza 2015
- Aboaziza E, Eissenberg T. Waterpipe tobacco smoking: what is the evidence that it supports nicotine/tobacco dependence?. Tobacco Control 2015;24(Suppl):i44‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Akl 2013
- Akl EA, Jawad M, Lam WY, Co CN, Obeid R, Irani J. Motives, beliefs and attitudes towards waterpipe tobacco smoking: a systematic review. Harm Reduction Journal 2013;10(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Akl 2015
- Akl EA, Ward KD, Bteddini D, Khaliel R, Alexander AC, Loutfi T, Alaouie H, Afifi RA. The allure of the waterpipe: a narrative review of factors affecting the epidemic rise in waterpipe smoking among young persons globally. Tobacco Control 2015;24(Suppl):i13‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anjum 2008
- Anjum Q, Ahmed F, Ashfaq T. Knowledge, attitude and perception of water pipe smoking (Shisha) among adolescents aged 14‐19 years. The Journal of the Pakistan Medical Association 2008;58(6):312. [PubMed] [Google Scholar]
Arrazola 2015
- Arrazola RA, Singh T, Corey CG, Husten CG, Neff LJ, Apelberg BJ, et al. Tobacco use among middle and high school students ‐ United States, 2011‐2014. Morbidity and Mortality Weekly Report 2015;64(14):381‐5. [PMC free article] [PubMed] [Google Scholar]
Asfar 2008
- Asfar T, Weg MV, Maziak W, Hammal F, Eissenberg T, Ward KD. Outcomes and adherence in Syria's first smoking cessation trial. American Journal of Health Behavior 2008;32(2):146‐56. [DOI] [PubMed] [Google Scholar]
Borgan 2013
- Borgan SM, Marhoon ZA, Whitford DL. Beliefs and perceptions toward quitting waterpipe smoking among café waterpipe tobacco smokers in Bahrain. Nicotine & Tobacco Research 2013;15(11):1816‐21. [DOI] [PubMed] [Google Scholar]
El‐Zaatari 2015
- El‐Zaatari ZM, Chami HA, Zaatari GS. Health effects associated with waterpipe smoking. Tobacco Control 2015;24(Suppl):i31‐i43. [DOI] [PMC free article] [PubMed] [Google Scholar]
GYTS 2003
- Global Youth Tobacco Survey Collaborating Group. Differences in worldwide tobacco use by gender: findings from the global youth tobacco survey. Journal of School Health 2003;73(6):207‐15. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 2003
- Hughes JR, Keely JP, Niaura RS, Ossip‐Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine & Tobacco Research 2003;5(1):13‐25. [PubMed] [Google Scholar]
Jarvis 1988
- Jarvis MJ, Russell MA, Benowitz NL, Feyerabend, C. Elimination of cotinine from body fluids: implications for noninvasive measurement of tobacco smoke exposure. American Journal of Public Health 1988;78(6):696‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jawad 2013
- Jawad M, Jawad S, Mehdi A, Sardar A, Jawad AM, Hamilton FL. A qualitative analysis among regular waterpipe tobacco smokers in London universities. The International Journal of Tuberculosis and Lung Disease 2013;17(10):1364‐9. [DOI] [PubMed] [Google Scholar]
Jawad 2015
- Jawad M, Kadi L, Mugharbil S, Nakkash R. Waterpipe tobacco smoking legislation and policy enactment: a global analysis. Tobacco Control 2015;24(Suppl):i60‐i65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lancaster 2005
- Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001292.pub2] [DOI] [PubMed] [Google Scholar]
Martinasek 2011
- Martinasek MP, McDermott RJ, Martini L. Waterpipe (hookah) tobacco smoking among youth. Current Problems in Pediatric and Adolescent Health Care 2011;41(2):34‐57. [DOI] [PubMed] [Google Scholar]
Maziak 2004
- Maziak W, Ward KD, Afifi Soweid RA, Eissenberg T. Tobacco smoking using a waterpipe: a re‐emerging strain in a global epidemic. Tobacco Control 2004;13(4):327‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maziak 2015
- Maziak W, Taleb ZB, Bahelah R, Islam F, Jaber R, Auf R, et al. The global epidemiology of waterpipe smoking. Tobacco Control 2015;24(Suppl):i3‐i12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 1987
- Murray DM, O'Connell CM, Schmid LA, Perry CL. The validity of smoking self‐reports by adolescents: a reexamination of the bogus pipeline procedure. Addictive Behaviors 1987;12(1):7‐15. [DOI] [PubMed] [Google Scholar]
Patrick 1994
- Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self‐reported smoking: a review and meta‐analysis. American Journal of Public Health 1994;84(7):1086‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peto 2001
- Peto R, Lopez AD. Future worldwide health effects of current smoking patterns. In: Koop CE, Pearson CE, Schwarz MR editor(s). Critical Issues in Global Health. San Francisco CA: Jossey‐Bass, 2001. [Google Scholar]
Primack 2009
- Primack BA, Walsh M, Bryce C, Eissenberg T. Water‐pipe tobacco smoking among middle and high school students in Arizona. Pediatrics 2009;123(2):e282‐e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salameh 2008
- Salameh P, Waked M, Aoun Z. Waterpipe smoking: construction and validation of the Lebanon Waterpipe Dependence Scale (LWDS‐11). Nicotine and Tobacco Research 2008;10(1):149‐58. [DOI] [PubMed] [Google Scholar]
Shihadeh 2015
- Shihadeh A, Schubert J, Klaiany J, Sabban M, Luch A, Saliba NA. Toxicant content, physical properties and biological activity of waterpipe tobacco smoke and its tobacco‐free alternatives. Tobacco Control 2015;24(Suppl):i22‐i30. [DOI] [PMC free article] [PubMed] [Google Scholar]
SRNT 2002
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research 2002;4(2):149‐59. [DOI] [PubMed] [Google Scholar]
Ward 2005
- Ward KD, Hammal F, VanderWeg MW, Eissenberg T, Asfar T, Rastam S, et al. Are waterpipe users interested in quitting?. Nicotine & Tobacco Research 2005;7(1):149‐56. [DOI] [PubMed] [Google Scholar]
Ward 2006
- Ward KD, Eissenberg T, Rastam S, Asfar T, Mzayek F, Fouad MF, et al. The tobacco epidemic in Syria. Tobacco Control 2006;15(Suppl):24‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 2014
- Ward KD, Ahn S, Mzayek F, Al Ali R, Rastam S, Asfar T, et al. The relationship between waterpipe smoking and body weight: population‐based findings from Syria. Nicotine & Tobacco Research 2014;17(1):34‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 2015
- Ward KD. The waterpipe: an emerging global epidemic in need of action. Tobacco Control 2015;24(Suppl):i1‐i2. [DOI] [PMC free article] [PubMed] [Google Scholar]


