Abstract
The goal of individualized and targeted treatment and precision medicine requires the assessment of potential therapeutic targets to direct treatment selection. The biomarkers used to direct precision medicine, often termed companion diagnostics, for highly targeted drugs have thus far been almost entirely based on in vitro assay of biopsy material. Molecular imaging companion diagnostics offer a number of features complementary to those from in vitro assay, including the ability to measure the heterogeneity of each patient’s cancer across the entire disease burden and to measure early changes in response to treatment. We discuss the use of molecular imaging methods as companion diagnostics for cancer therapy with the goal of predicting response to targeted therapy and measuring early (pharmacodynamic) response as an indication of whether the treatment has “hit” the target. We also discuss considerations for probe development for molecular imaging companion diagnostics, including both small-molecule probes and larger molecules such as labeled antibodies and related constructs. We then describe two examples where both predictive and pharmacodynamic molecular imaging markers have been tested in humans: endocrine therapy for breast cancer and human epidermal growth factor receptor type 2–targeted therapy. The review closes with a summary of the items needed to move molecular imaging companion diagnostics from early studies into multicenter trials and into the clinic.
Introduction
The goal of individualized and targeted treatment—often termed precision medicine—requires the assessment of potential therapeutic targets to direct patients to those treatments most likely to be effective.1 A closely related need is the ability to measure the effect of the drug on the target and the underlying disease process to determine whether the selected therapy is likely to be effective. Both types of indicators can be broadly classified as disease biomarkers.1,2 Biomarkers that are highly specific to a particular target or therapy are often called companion diagnostics and typically measure the therapeutic target itself or closely related partner molecules. Such markers fall under the general heading of predictive biomarkers.1,3 Biomarkers that measure the effect of the treatment on the disease process are often termed as response biomarkers, and the class of these markers apropos to measuring early drug action on the target is often termed as pharmacodynamic (PD) markers.1,3 PD markers measure downstream effects of the drug on the cancer cell and on the disease. In this review, we consider the application of molecular imaging to precision medicine—specifically to cancer treatment—as a companion diagnostic for selecting targeted cancer therapy. We provide an overview of molecular imaging as a companion diagnostic for targeted cancer therapy, discuss the approach to developing imaging probes for predictive and PD markers, and then highlight two examples of molecular imaging: endocrine therapy for breast cancer and human epidermal growth factor receptor type (HER2)-targeted treatments.
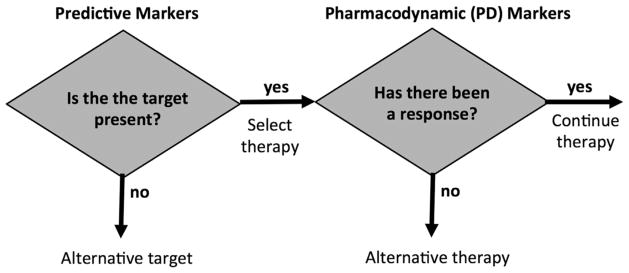
A model for using predictive and PD markers to guide targeted cancer therapy is illustrated in Figure 1. In this approach, individualized treatment selection is considered in two steps:
Figure 1.
Diagram illustrating potential roles for molecular imaging companion diagnostics as predictive markers and as pharmacodynamic (PD) markers.
What therapeutic targets are present?
Does a selected treatment directed to one or more of the therapeutic targets have an effect on the cancer?
How can imaging aid this approach? For cancer, the identification of therapeutic targets is typically done by in vitro assay of biopsy material. Advances in methods to assess tumor genomics, gene expression, and protein expression provide an increasingly comprehensive characterization of each patient’s cancer and the identification of possible therapeutic targets for each patient.4 Imaging is unlikely to replace biopsy and in vitro assay in the initial assessment for treatment targets for newly diagnosed cancer as imaging measures only up to a few therapeutic targets, whereas assay of biopsy material can screen for many targets at the same time. However, imaging has a unique ability to measure the regional heterogeneity of target expression, especially in patients with advanced disease where target expression may vary from site to site. In this case, biopsy of a single site may not be representative of the entire burden of disease. Thus imaging can play a complementary role to biopsy in assessing target expression.
Molecular imaging can play an even more important role as a PD marker and has some significant advantages over other existing approaches.5 The noninvasive nature of imaging facilitates the repeat measurements needed to assess response. Imaging avoids the challenges (sampling error, patient comfort, and risk of complications) associated with serial biopsy to assess response. Molecular imaging also has significant advantages over other forms of largely anatomically based imaging in that it can quantify specific molecular processes likely to be affected early after the initiation of drug treatment—for example, tumor proliferation—long before anatomical changes can be detected.6,7
The Approach to Probe Development for Imaging Companion Diagnostics
Predictive Markers
Predictive markers designed to measure the expression of a therapeutic target require molecular imaging probes that are highly specific to the target. Traditionally these probes have been small molecules that target receptors, transporters, or enzymes with high affinity and selectivity, while at the same time having sufficiently rapid clearance from tissue not expressing the target to allow visualization of binding at the target by PET or SPECT.8 Perhaps, the earliest example of a radionuclide imaging predictive marker is the use of radioiodine imaging as a companion diagnostic for 131I− therapy.9 Here, the uptake and retention of 123I− or a diagnostic dose of 131I− provides a reliable indicator of those patients who are likely to respond to 131I−, and perhaps more importantly the absence of uptake in disease of sufficient size to be visualized by diagnostic imaging indicates a low likelihood of therapeutic success with 131I−.9 More recent examples of predictive markers include the use of small-molecule ligands labeled with 18F for PET imaging of steroid receptor expression in breast and prostate cancer.10–12 Peptide ligands have also been successful, most notably using somatostatin analogs labeled with 111In or 68Ga to provide specific markers for neuroendocrine tumors and to guide therapy targeted to the somatostatin receptor, including radionuclide therapy.13 In addition to receptors, small-molecule predictive imaging agents may target enzymes (eg, 18F-5-fluorouracil as a measure of thymidylate synthase activity14) and transporters (eg, 99mTc-sestamibi and 11C-verapamil to measure activity of the P-glycoprotein drug efflux transporter15–17). Probes may even target the tumor microenvironment—for example, the use of PET hypoxia imaging agents to identify patients most likely to respond to hypoxia-selective chemotherapy.18
Although small-molecule predictive markers have many attractive features, the complex structure-function relationships for highly specific probes require considerable investment of time and effort in the development of labeled probes that meet the needs of PET and SPECT imaging for high-affinity and highly specific binding. Although some success has come with the approach of using labeled therapeutic drugs to image drug targets, desirable properties of therapeutic drugs often do not lead to good imaging agents. For example, prolonged circulation time is an attractive feature of a therapeutic drug to limit dosing frequency, but diagnostic imaging agents need rapid clearance to be able to separate sites of probe retention from nonspecific background uptake. As such, there are a relatively limited number of small-molecule predictive markers that have made it to human trials, let alone clinical practice.19 The increasing use of monoclonal antibodies and immune-base target recognition in both in vitro diagnostics (eg, immunohistochemistry [IHC]) and cancer therapy (eg, therapeutic antibody-based drugs such as trastuzumab) has led to renewed interest in imaging using labeled antibodies and related molecules.20 Early efforts using SPECT-labeled intact antibody probes provided a proof of principle, but required prolonged clearance times after administration, resulted in poor image contrast, and had highly variable diagnostic performance.20 Despite some early enthusiasm, only very limited use of SPECT antibody imaging survives in current clinical practice. Several recent factors have contributed to a resurgence of interest in immune-based diagnostic imaging probes using PET. These include the more widespread availability of PET isotopes with long half-lives that are suitable for PET antibody imaging such as 124I and 89Zr, providing the advantages of PET but also the longer half-life needed for clearance of labeled antibodies.20 This comes at the same time as that of significantly improved capabilities for PET instruments, especially those with time-of-flight capability, to provide good quality images at the relatively low imaging count rates21 that arise in labeled antibody imaging, which is constrained to lower administered activity for the probes (owing to their long half-life and increased radiation burden). In addition, the development of alternative molecules—such as antibody fragments, diabodies, and affibodies—offers more rapid probe clearance and the ability to use short-lived isotopes while retaining the specificity of antibody recognition.22 These factors make the use of PET-labeled antibodies and related constructs a rapidly advancing and increasingly investigated approach for the development of companion diagnostics.20,22
PD Markers
PD markers have the goal of measuring the effect of targeted therapy on the target and downstream processes in the cancer cell early after administration of the drug. A positive response, indicated by a significant change in the marker after the therapy, indicates an effect of the drug on the target. Perhaps, more importantly, the lack of a change in the marker implies no effect of the drug on the target and cancer cell, indicating likely therapeutic futility. Ideally, companion diagnostic therapeutic markers target processes that are tightly coupled to the therapeutic target—for example, the binding of a ligand to a targeted receptor to provide a PD marker that is highly specific to the target.23–25 In practice, however, the long pathway from bench to bedside for molecular imaging agents favors the development of a more limited set of PD markers that are more universally applicable for measuring early cellular and disease responses. To retain broad relevance to a variety of therapies, such markers generally target relatively downstream markers of cancer response such as cellular proliferation, cell death, or other key cellular process such as metabolism. The most successful agents to date have been small molecules labeled with relatively short-lived positron emitters that have sufficiently rapid clearance to provide high-quality quantitative imaging data at relatively low radiation burdens.
The most widely used molecular imaging PD agent thus far is 18F-FDG.26–28 It has a number of attractive properties that include the fact that glucose metabolism is a key process in cancer cells that is downstream of many therapeutic targets. FDG is widely available as a clinical imaging agent and is a key component of cancer staging and restaging for many cancer types29 and approaches to quantifying glucose metabolism using FDG-PET have been developed and widely implemented.30 A number of studies have indicated the ability of FDG to measure the response to a variety of treatments that include cytotoxic chemotherapy,28 endocrine therapy,31,32 and tyrosine kinase inhibitors.33 However, FDG has some disadvantages as a PD marker. Glycolysis is not specific to cancer; it is also associated with potentially confounding processes such as inflammation.27 For example, the early inflammatory and reparative response to radiation has been blamed for the failure of FDG-PET as an early PD marker for radiotherapy. In addition, not all cancers have elevated rates of glycolysis sufficient to enable FDG as a PD marker. Finally, the complex cellular regulation of glycolysis and the many cellular inputs that determine glycolytic rate can confound the interpretation of a change in FDG uptake with treatment. For example, a prior study using FDG as a PD marker for mTOR-directed therapy found that although the therapy seemed to affect FDG uptake, the change in FDG uptake did not predict subsequent clinical response.34 These limitations of FDG as a PD marker have led to a search for other markers that monitor other processes apropos to use as cancer PD markers.
A highly promising set of PET PD markers measure tumor proliferation.35 Aberrant cellular proliferation is a hallmark of cancer36 and a decline in cellular proliferation is an early event in successful anticancer therapy that occurs in both cytotoxic and cytostatic therapies.37 The use of cellular proliferation assays—specifically Ki-67—to measure early response in serial biopsy has been shown to be highly predictive of subsequent clinical response and outcomes.38 The most successful approach for proliferation imaging agents to date is to measure flux through the exogenous or salvage pathway of thymidine incorporation into DNA. This approach is based on early studies that used 3H- and 14C-labeled thymidine probes to measure cellular proliferation in vitro in efforts dating back to the 1950s.39 In the 1990s, work with 11C-thymidine PET demonstrated the potential of labeled thymidine as a cellular proliferation agent, demonstrating an even more robust ability to measure early response to chemotherapy than that of FDG-PET.6 Subsequent research focused on the development of less heavily metabolized, 18F-labeled thymidine analogs, of which the most successful to date has been 18F-fluorothymidine (FLT).35,40 Early studies have shown that FLT measures response to both cytotoxic and cytostatic agents early in the course of therapy,41,42 and these findings have been supported by recent multicenter studies.43 Alternative proliferation agents targeted to other measures of proliferation have also been developed and tested. For example, agents targeted to the sigma-2 receptor, a receptor that is upregulated in actively cycling cells, have shown promise for measuring cellular proliferation by a method independent of the thymidine analogs and may offer advantage in their ability to image both cellular proliferation and quiescence.44,45
Imaging agents can be targeted to other processes that are markers of cancer therapeutic response, and some of these processes have also been exploited for the development of imaging PD markers. Cellular death is an important outcome of cytotoxic treatments. Imaging agents targeted to molecules exposed or activated during apoptosis, a form of cellular death important in cancer therapeutic response, have been developed and undergone early testing. Examples include agents targeted to annexin V, an inner cellular membrane molecule associated with apoptosis46 and probes of the caspase system, which is activated in most processes leading to apoptosis.46,47 Diffusion MRI has also been touted as a marker of cell death; an increase in free water diffusion, as measured by diffusion-weighted imaging, has been shown to correlate with a decline in tumor cellularity in early responses to treatment.48 A variety of other imaging PD markers for cancer continue to be developed.
Example 1: Molecular Imaging Companion Diagnostics of Endocrine Therapy for Breast Cancer
The physiology of sex steroids, in particular estrogens and progestins, is important for mammary gland development and function, and is also a key component of breast cancer pathogenesis and growth. Interruption of steroid hormone growth signal, often termed endocrine therapy, is one of the most important therapeutic strategies for treating breast cancer. Determination of the status of hormone receptors, both the estrogen receptor (ER) and progesterone receptor (PR), in patients with breast cancer is an important and well-accepted companion diagnostic for endocrine therapy, as tumor expression of ER (or PR or both) is necessary for endocrine responsiveness.49,50 Most of the newly diagnosed breast cancers are ER positive.51 Currently, the most commonly used method to assess hormonal receptor status, including the ER, is assay of biopsy material using IHC.52,53 Imaging of the ER is an excellent example of how imaging companion diagnostics may be complementary to tissue-base diagnostics, including the potential for serial evaluation, the ability to measure receptor expression in the entire disease burden, to characterize heterogeneity, and to measure ER expression in sites that are challenging to biopsy and assay such as bone.54–56
Current PET ER imaging agents are analogs of estradiol. Important tracer properties include specific and high affinity for the ER as well as affinity for sex hormone-binding globulin (SHBG) (a sex steroid transport protein) that is close to estradiol.57 The most successful hormone receptor imaging agent to date is the ER imaging radiopharmaceutical 16α-[18F] fluoro-17β-estradiol (FES).58 The binding characteristics of this positron-emitting radiopharmaceutical are similar to estradiol for both the ER and SHBG,59 making it an excellent marker of ER expression. Like estradiol, most of FES within the blood is bound to protein, primarily SHBG and albumin, and the exact ratio depends on the concentration of SHBG.60–62 As with any other steroid, FES is metabolized by the liver.60 Studies in both animal models and humans demonstrate that blood clearance and metabolism of FES is very rapid60,61; after 2 hours, most of the remaining activity in both blood and nontarget tissues is due to metabolites. Human studies demonstrated a predominance of metabolites (mainly glucuronide and FES sulfate61) over unmetabolized FES by 20–60 minutes after injection. Although these radiolabeled blood metabolites persist, declining only slowly after 30 minutes, they do not readily bind to SHBG or penetrate the cell to bind to the nuclear receptor61,62; thus, they do not contribute significantly to target tissue uptake, and delayed imaging beyond 30 minutes offers good visualization of ER-rich tissues.61
Studies have validated FES-PET as a marker of ER expression in studies that compared FES uptake in patients to tumor assay of ER expression of biopsy material by both radioligand binding63 and IHC64 (Fig. 2). Using a minimum standard uptake value (SUV) of 1.1 as the cutoff for determining ER-positive tumors, a concordance rate of 94% was found between IHC results and FES uptake.64 These early studies established FES-PET as a quantitative measure of regional ER expression. Further studies demonstrated the ability of FES-PET to assess ER expression in multiple sites of disease, including axillary lymph nodes and distant metastases,10 and also demonstrated concordance between FES uptake and in vitro ER status.24
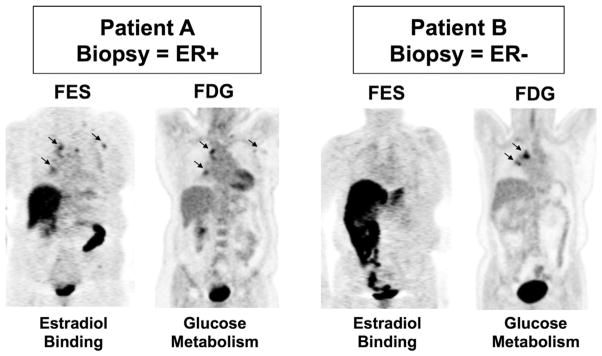
Figure 2.
Example of 18F-fluorestradiol (FES) PET to image the expression of the therapeutic target (the estrogen receptor [ER]). Imaging examples are from 2 patients who underwent FES and FDG scans before therapy. Left: patient A had mediastinal lesions appreciated by both FES and FDG. Right: patient B also had mediastinal disease clearly seen by FDG-PET, not visible on FES-PET. The core biopsy of a metastatic axillary lesion from patient A showed ER-positive breast cancer, whereas the needle biopsy from a vertebral lesion from patient B showed ER-negative breast cancer. (Adapted with permission from Peterson et al.68)
A significant advantage of ER imaging over tissue biopsy is the ability to assess site-to-site heterogeneity of ER expression in patients with metastatic breast cancer and multiple sites of disease.10,56,65 Thus, imaging is able to shed some light on patterns of ER expression not possible in biopsy-based studies. For example, studies showed that in patients with metastatic disease from a documented ER-expressing primary, there was significant interpatient variation and more modest intrapatient variation of FES uptake.10,56 This implies that loss of ER expression may precede disease dissemination in many cases. The ability to identify significant intrapatient variation, when it occurs, by imaging can direct tissue sampling to better understand the determinants of ER loss in previously ER+ disease. Imaging may also help shed light on the functional characteristics of ER mutations, increasingly recognized in resistant breast cancer.66
The most clinically relevant role for FES-PET is as a predictive biomarker for breast cancer endocrine therapy responsiveness. Studies have supported the level of FES uptake as a predictive marker for response to endocrine therapy with selective ER modulators or aromatase inhibitors in both first-line and salvage therapy settings.25,31,56,67 Studies to date suggest that patients with baseline tumor FES SUV values less than 1.5 are unlikely to benefit from endocrine therapy, whereas a baseline SUV more than 1.5 predicts a clinical benefit in some patients, including endocrine-refractory breast cancer.25,56,67,68 A recent study showed similar results for endocrine-refractory patients treated with estradiol.69 Further prospective studies are needed to validate these early findings.70 Although the absence of FES uptake indicates a low likelihood of response, the presence of FES uptake does not guarantee endocrine responsiveness. This is analogous to tissue assay for ER where absent ER reliably predicts a lack of endocrine responsiveness, but a positive assay predicts response less reliably.
Serial FES-PET may serve as a PD marker for ER blockade in endocrine therapy.25 In a study of women undergoing tamoxifen treatment of locally advanced or metastatic breast cancer, serial FES demonstrated early ER blockade after 7–10 days of treatment with tamoxifen. The percentage decrease in FES uptake was greater in patients who ultimately had a clinical response than in those patients who did not have disease response.25 A later study compared FES uptake changes in patients treated with tamoxifen vs fulvestrant. Although both ER blocking therapies were effective in decreasing FES binding in both the tumor and uterus (as measured by percent SUV decrease), only tamoxifen reliably produced complete blockade by both qualitative and quantitative measures,23 consistent with later data indicating that higher doses of fulvestrant were more effective.71 Recent studies have also used serial FES-PET to determine the adequacy of receptor blockade in other trials of estrogen-blocking drugs.72
As illustrated in Figure 1, in addition to determining target expression, imaging companion diagnostics should also indicate whether drugs directed at the target have an effect on the cancer that is likely to produce a treatment response. Data suggest that early serial FDG-PET/CT may predict breast cancer response to endocrine therapy. The early study of Mortimer et al25 showed that presence of the early tamoxifen agonist “flare” effect, measured as an increase in FDG uptake 7–10 days after starting tamoxifen, predicted later response. A related study by Dehdashti et al31 used an estradiol challenge, where an increase in FDG uptake 24 hours following estradiol administration was predictive of response to either an aromatase inhibitor or fulvestrant. The metabolic response measured by FDG-PET was also associated with significantly longer overall survival, independent of the type of endocrine therapy.31 FDG-PET may also be able to indicate early response to endocrine drugs that work by lowering estrogen levels such as aromatase inhibitors. Kurland et al32 found that a significant decline in FDG SUV (greater than or equal to 20%) after 2 weeks of aromatase therapy was associated with low post-therapy proliferation, as measured by Ki-67 in tumor biopsy specimens.
Studies based on serial biopsy show that successful endocrine therapy results in a decline in tumor proliferation, a well-known downstream effect of blocking estrogen-ER binding in ER-expressing cancers, as measured by Ki-67 assay of biopsy material.73 The basis of increased FDG uptake in response to ER stimulation (or decreased FDG uptake with estrogen withdrawal) has therefore been assumed to also be on the basis of cellular proliferation. However, some preclinical experiments suggest that an alternative process may underlie the metabolic flare. For example, the results of Ko et al74 suggest that estradiol augments FDG uptake in ER+ breast cancer cells via increased glycolysis and hexokinase activity and is mediated by nongenomic membrane-initiated action. This has motivated the use of PET proliferation tracers such as FLT to measure early breast cancer response to endocrine therapy, with promising early results.75 Further study of this promising area is warranted, especially with the recent addition of cell-cycle-targeted drugs to the compendium of drugs used in ER-expressing breast cancer.76
Example 2: Molecular Imaging Companion Diagnostics for HER-2 Targeted therapy
HER2 is a member of the tyrosine kinase receptor family and has been recognized as a key driver of breast cancer growth in breast cancers that overexpress this protein, approximately 15%–25% of newly diagnosed invasive breast cancers.77 Besides conferring a more aggressive phenotype, studies have demonstrated that overexpression of HER2 results in impaired response to both hormonal therapy via crosstalk with the ER78–81 as well as some forms of cytotoxic chemotherapy regimens.81,82 The development of HER2-targeted therapies, first accomplished by a HER2-targeted antibody (trastuzuamab), was one of the more significant breakthroughs in breast cancer treatment,77,83–85 leading to a marked increased in therapeutic response and a decline in the mortality for patients with HER2-overexpressing tumors.86 The range of drugs targeting HER2 has increased and includes small molecules targeted to the downstream kinase (eg, lapatinib), antibody-drug conjugates (eg, trastuzumab emtansine), and drugs targeted to related HER-family receptors (eg, pertuzumab).87,88 Akin to ER and PR, measurement of HER2 expression by in vitro assay of biopsy or surgical specimens has become an important and routinely assessed companion diagnostic for breast cancer, typically by IHC or fluorescence in situ hybridization.77 As with imaging ER expression, imaging HER2 may offer some complementary advantages to biopsy-based assays. HER2-positive breast cancer is a heterogeneous disease, with intratumoral and intertumoral heterogeneity by IHC as high as 13% and 30%, respectively.89 A study found a therapeutically significant discordance of HER2 status between the primary tumor and metachronous recurrence or metastasis of 21.5%.90 These factors have supported research in HER2 PET imaging for breast cancer and other diseases.
As the importance and nature of the binding ligand for HER2 is uncertain, most of the work to date to image HER2 expression is based on immune recognition. Labeled anti-HER2 immune-based agents tested for imaging include full immunoglobulins (trastuzumab and pertuzumab), immunoglobulin fragments, and novel constructs such as affibodies (Fig. 3).91–93 Some agents are radiolabeled with single-photon radionuclides (111In-labeled trastuzumab) whereas others are labeled with positron-emitting radionuclides for PET (64Cu-trastuzumab, 64Cu-DOTA-ZHER2:477, 68Ga-trastuzumab F (ab0)2 fragments, 68Ga-ABY-002, and 89Zr-trastuzumab).91–94
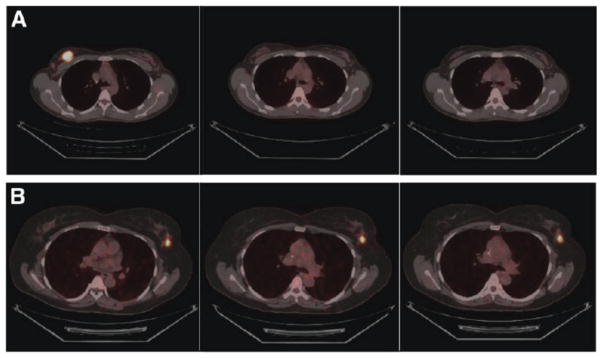
Figure 3.
Imaging HER2 expression in breast cancer. The figure shows three examples of patients with metastatic breast cancer arising from HER2-overexpressing tumors demonstrating the ability of 89Zr-trastuzumab to image regional HER2 expression. (Adapted with permission from Dijkers et al.93)
Many of the earliest studies used labeled intact antibodies, but success was quite variable for single-photon-emitting isotopes such as 131I or 111In. PET imaging using 89Z-trastuzumab-PET has shown more promise, offering good visualization of HER2-expressing sites in patients with meta-static disease.92 64Cu-DOTA-trastuzumab PET also showed promising results in early trials.95
The slow clearance of intact antibodies requires prolonged intervals from injection to scan and longer-lived labels, leading to logistic challenges and increased radiation doses. These considerations have spurred the development of HER2-imaging agents using smaller molecules that retain immune-recognition capabilities. These molecular probes include antibody fragments, and special constructs such as diabodies and affibodies.22,93 Smith-Jones et al96,97 demonstrated the ability to image HER2 expression in preclinical models using 68Ga-labeled Fab0 fragments. This was extended to patients in an early clinical study demonstrating the feasibility of the approach.98 Several groups have also tested affibody molecules to image HER2.91,99 In an example, a group at the NIH using an 18F-labeled affibody, N-[2-(4-18F-fluorobenzamido)ethyl] maleimide (18F-FBEM)–ZHER2:342,100 in mice xenografts found that tracer uptake correlated with HER2 receptor expression as assessed by IHC. Affibody-based HER2 imaging using a 68Ga-labeled or 111In-labeled construct (DOTA[0]-Z [HER2:342-pep2] [ABY-002])101 and another 111In-labeled construct (111In-ABY-025) have been extended to early human studies with promising early results.102
As with PET ER imaging, HER2-imaging agents can be used as PD markers for HER2-related therapy. This has been demonstrated in preclinical models using drugs targeted to HSP-90, a chaperone molecule important in mediating HER2 expression. Preclinical imaging demonstrated decreased HER2 expression in response to HSP-90-targeted drugs in studies using 68Ga-labeled Fab0 fragments and affibodies96 and 89Zr-trastuzumab.103 This has recently been extended to early human trials, demonstrating the ability to measure changes in HER2 expression by 89Zr-trastuzumab PET in response to an HSP90 inhibitor (NVP-AUY922).104 This is a promising future application of HER2 imaging that merits further study.
As in ER-targeted drugs, PD markers may play an important role as early response indicators, predicting the likelihood of success of drugs selected to target HER2-overexpressing breast cancers. As in endocrine therapy, FDG-PET has shown considerable promise for the early assessment of HER2-targeted therapy in breast cancer. Following initial studies that suggested utility of FDG in this patient population,105,106 the prospective Neo-ALTTO (Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimization) trial enrolled women with HER2 breast cancer and compared rates of pathologic complete response following anti-HER2 therapy to FDG metabolic response at 2 and 6 weeks after treatment (Fig. 4). Metabolic responses were evident in the primary tumors after 2 weeks of targeted therapy and were highly correlated with metabolic responses at 6 weeks. Pathologic complete responses were associated with greater declines in FDG uptake (as measured by maximum SUV) at both 2 and 6 weeks, indicating that FDG-PET/CT may identify patients with increased likelihood of complete response after neoadjuvant treatment with anti-HER2 therapy.107 Similar findings were found in other multi-center trials conducted in the United States and Europe.108–110 These studies, including the results of several multicenter trials, indicate excellent potential for serial FDG-PET as an integral marker to guide future HER2-targeted treatment studies and possibly to help direct clinical use of HER2-targeted drugs.
Figure 4.
FDG-PET is a pharmacodynamic (PD) marker for HER2-targeted therapy in breast cancer. Images of serial FDG-PET/CT scans performed as part of the Neo-ALTTO trial taken before therapy (left), and after 2 weeks (middle), and 6 weeks (right) of HER2-targeted therapy. Patient A (top row) had a metabolic response to treatment by FDG-PET and went on to achieve a complete pathologic response to therapy. Patient B’s tumor (bottom row) did not demonstrate a response by FDG-PET, and residual viable tumor was found at posttherapy surgery. (Adapted with permission from Gebhart et al.108)
A recent study also demonstrated the benefit of combining predictive and PD markers in directing targeted therapy.111 The ZEPHYR trials include a combination of HER2-imaging (89Z-trastuzumab-PET) and early serial FDG-PET as integrated markers in a study comparing HER2-directed therapies. Data presented at the 2014 ASCO meeting indicated that the presence or absence of HER2, as indicated by the predictive imaging marker (89Z-trastuzumab-PET), and a response, as indicated by the PD marker (decline in FDG uptake on serial PET), provided an essentially 100% accurate prediction of later clinical response to HER2-directed therapy. This study represents an important future paradigm for using imaging as cancer companion diagnostics and merits further study for drugs targeted to HER2 and other cancer targets.
Summary and Future Directions
Early experience with molecular imaging predictive and PD markers suggests considerable potential as companion diagnostics, complementary to diagnostics based on in vitro assay of biopsy material, for guiding targeted cancer therapy. Studies have demonstrated the potential for imaging agents to provide unique information as cancer biomarkers, including quantification of the heterogeneity of target expression, detection of changes in target expression with therapy, and facile measurement of early changes in processes downstream of the target as an early indicator of drug efficacy. Early clinical trials of predictive markers, PD markers, and combined predictive and PD markers all point to the great promise of molecular imaging as cancer companion diagnostics. What is needed to move from these early studies into larger clinical trials and clinical use? Key components of translation into the clinic require the following112,113:
Standardized methods for image acquisition and analysis —early studies should optimize approaches and develop standard methods for future use.
Determination of analytical validity—the accuracy and prediction of the imaging tests as predictive or PD markers need to be determined. This includes validating the relationship between imaging and target expression and measuring test and retest variability.
Development of supply chain for novel imaging probes —probes would need to be made available to other centers outside the few academic institutions that have done early research in novel probes. This supply is likely to come from a hybrid supply chain of both academic and commercial suppliers in early multicenter trials.
Use as integrated markers in therapeutic clinical trials—prospective integration in therapeutic clinical trials using strict entry criteria, well-defined treatment regimens, and preestablished clinical end points provides the optimal environment for confirming utility of molecular imaging methods as companion diagnostics. Such trials can also confirm quantitative interpretation criteria and measure performance expectations for the use of imaging markers in directing therapy in future clinical trials and clinical practice.
Rigorous testing as integral markers in therapeutic trials —the ultimate test of molecular imaging companion diagnostics—and the data needed to justify their clinical use and reimbursement— come from well-designed and properly powered studies testing the use of imaging as an integral marker that demonstrate an improvement in therapeutic outcome with the use of imaging markers.
These goals are challenging and lofty but are justified by the promise of molecular imaging studies as cancer therapy companion diagnostics and by early studies supporting their considerable potential.
Acknowledgments
This work was supported in part by Susan G. Komen Foundation Grant SAC140060, U.S. Department of Energy, United States Grant DE-SE0012476, U.S. Department of Defense, United States Grant W81XWH-13-1-0406, and National Institutes of Health, United States Grants U01CA148131 and P30CA016520.
References
- 1.Henry NL, Hayes DF. Cancer biomarkers. Mol Oncol. 2012;6:140–146. doi: 10.1016/j.molonc.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartwell L, Mankoff D, Paulovich A, et al. Cancer biomarkers: A systems approach. Nat Biotechnol. 2006;24:905–908. doi: 10.1038/nbt0806-905. [DOI] [PubMed] [Google Scholar]
- 3.Mankoff DA, O’Sullivan F, Barlow WE, et al. Molecular imaging research in the outcomes era: Measuring outcomes for individualized cancer therapy. Acad Radiol. 2007;14:398–405. doi: 10.1016/j.acra.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farwell MD, Clark AS, Mankoff DA. How imaging biomarkers can inform clinical trials and clinical practice in the era of targeted cancer therapy. JAMA Oncol. 2015;1:421–422. doi: 10.1001/jamaoncol.2015.0667. [DOI] [PubMed] [Google Scholar]
- 5.Mankoff DA. Imaging studies in anticancer drug development. In: Hidalo H, Eckhardt SG, Garrett-Meyer E, Clendeninn N, editors. Principles of Anticancer Drug Development. New York: Springer; 2011. pp. 275–304. [Google Scholar]
- 6.Shields AF, Mankoff DA, Link JM, et al. Carbon-11-thymidine and FDG to measure therapy response. J Nucl Med. 1998;39:1757–1762. [PubMed] [Google Scholar]
- 7.Weber WA. Positron emission tomography as an imaging biomarker. J Clin Oncol. 2006;24:3282–3292. doi: 10.1200/JCO.2006.06.6068. [DOI] [PubMed] [Google Scholar]
- 8.Mankoff DA, Link JM, Linden HM, et al. Tumor receptor imaging. J Nucl Med. 2008;49(suppl 2):149S–163S. doi: 10.2967/jnumed.107.045963. [DOI] [PubMed] [Google Scholar]
- 9.Pryma DA, Mandel SJ. Radioiodine therapy for thyroid cancer in the era of risk stratification and alternative targeted therapies. J Nucl Med. 2014;55:1485–1491. doi: 10.2967/jnumed.113.131508. [DOI] [PubMed] [Google Scholar]
- 10.Dehdashti F, Mortimer JE, Siegel BA, et al. Positron tomographic assessment of estrogen receptors in breast cancer: Comparison with FDG-PET and in vitro receptor assays. J Nucl Med. 1995;36:1766–1774. [PubMed] [Google Scholar]
- 11.Larson SM, Morris M, Gunther I, et al. Tumor localization of 16beta-18F-fluoro-5alpha-dihydrotestosterone versus 18F-FDG in patients with progressive, metastatic prostate cancer. J Nucl Med. 2004;45:366–373. [PubMed] [Google Scholar]
- 12.Clark AS, McDonald E, Lynch MC, et al. Using nuclear medicine imaging in clinical practice: Update on PET to guide treatment of patients with metastatic breast cancer. Oncology (Williston Park) 2014;28:424–430. [PubMed] [Google Scholar]
- 13.van Essen M, Sundin A, Krenning EP, et al. Neuroendocrine tumours: The role of imaging for diagnosis and therapy. Nat Rev Endocrinol. 2014;10:102–114. doi: 10.1038/nrendo.2013.246. [DOI] [PubMed] [Google Scholar]
- 14.Dimitrakopoulou-Strauss A, Strauss LG, Schlag P, et al. Fluorine-18-fluorouracil to predict therapy response in liver metastases from colorectal carcinoma. J Nucl Med. 1998;39:1197–1202. [PubMed] [Google Scholar]
- 15.Hendrikse NH, de Vries EG, Eriks-Fluks L, et al. A new in vivo method to study P-glycoprotein transport in tumors and the blood-brain barrier. Cancer Res. 1999;59:2411–2416. [PubMed] [Google Scholar]
- 16.Piwnica-Worms D, Chiu ML, Budding M, et al. Functional imaging of multidrug-resistant P-glycoprotein with an organotechnetium complex. Cancer Res. 1993;53:977–984. [PubMed] [Google Scholar]
- 17.Sasongko L, Link JM, Muzi M, et al. Imaging P-glycoprotein transport activity at the human blood-brain barrier with positron emission tomography. Clin Pharmacol Ther. 2005;77:503–514. doi: 10.1016/j.clpt.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Rischin D, Hicks RJ, Fisher R, et al. Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: A substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol. 2006;24:2098–2104. doi: 10.1200/JCO.2005.05.2878. [DOI] [PubMed] [Google Scholar]
- 19.Mankoff DA, Pryma DA, Clark AS. Molecular imaging biomarkers for oncology clinical trials. J Nucl Med. 2014;55:525–528. doi: 10.2967/jnumed.113.126128. [DOI] [PubMed] [Google Scholar]
- 20.Lapi SE, Welch MJ. A historical perspective on the specific activity of radiopharmaceuticals: What have we learned in the 35 years of the ISRC. Nucl Med Biol. 2013;40:314–320. doi: 10.1016/j.nucmedbio.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Surti S. Update on time-of-flight PET imaging. J Nucl Med. 2015;56:98–105. doi: 10.2967/jnumed.114.145029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olafsen T, Wu AM. Antibody vectors for imaging. Semin Nucl Med. 2010;40:167–181. doi: 10.1053/j.semnuclmed.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linden HM, Kurland BF, Peterson LM, et al. Fluoroestradiol positron emission tomography reveals differences in pharmacodynamics of aromatase inhibitors, tamoxifen, and fulvestrant in patients with metastatic breast cancer. Clin Cancer Res. 2011;17:4799–4805. doi: 10.1158/1078-0432.CCR-10-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGuire AH, Dehdashti F, Siegel BA, et al. Positron tomographic assessment of 16 alpha-[18F] fluoro-17 beta-estradiol uptake in meta-static breast carcinoma. J Nucl Med. 1991;32:1526–1531. [PubMed] [Google Scholar]
- 25.Mortimer JE, Dehdashti F, Siegel BA, et al. Metabolic flare: Indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol. 2001;19:2797–2803. doi: 10.1200/JCO.2001.19.11.2797. [DOI] [PubMed] [Google Scholar]
- 26.Farwell MD, Pryma DA, Mankoff DA. PET/CT imaging in cancer: Current applications and future directions. Cancer. 2014;120:3433–3445. doi: 10.1002/cncr.28860. [DOI] [PubMed] [Google Scholar]
- 27.Mankoff DA, Eary JF, Link JM, et al. Tumor-specific positron emission tomography imaging in patients: [18F] fluorodeoxyglucose and beyond. Clin Cancer Res. 2007;13:3460–3469. doi: 10.1158/1078-0432.CCR-07-0074. [DOI] [PubMed] [Google Scholar]
- 28.Weber WA. Assessing tumor response to therapy. J Nucl Med. 2009;50(suppl 1):1S–10S. doi: 10.2967/jnumed.108.057174. [DOI] [PubMed] [Google Scholar]
- 29.Kelloff GJ, Hoffman JM, Johnson B, et al. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res. 2005;11:2785–2808. doi: 10.1158/1078-0432.CCR-04-2626. [DOI] [PubMed] [Google Scholar]
- 30.Doot RK, McDonald ES, Mankoff DA. Role of PET quantitation in the monitoring of cancer response to treatment: Review of approaches and human clinical trials. Clin Transl Imaging. 2014;2:295–303. doi: 10.1007/s40336-014-0071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dehdashti F, Mortimer JE, Trinkaus K, et al. PET-based estradiol challenge as a predictive biomarker of response to endocrine therapy in women with estrogen-receptor-positive breast cancer. Breast Cancer Res Treat. 2009;113:509–517. doi: 10.1007/s10549-008-9953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurland BF, Gadi VK, Specht JM, et al. Feasibility study of FDG PET as an indicator of early response to aromatase inhibitors and trastuzumab in a heterogeneous group of breast cancer patients. EJNMMI Res. 2012;2:34. doi: 10.1186/2191-219X-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stroobants S, Goeminne J, Seegers M, et al. 18FDG-Positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec) Eur J Cancer. 2003;39:2012–2020. doi: 10.1016/s0959-8049(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 34.Ma WW, Jacene H, Song D, et al. [18F]fluorodeoxyglucose positron emission tomography correlates with Akt pathway activity but is not predictive of clinical outcome during mTOR inhibitor therapy. J Clin Oncol. 2009;27:2697–2704. doi: 10.1200/JCO.2008.18.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bading JR, Shields AF. Imaging of cell proliferation: Status and prospects. J Nucl Med. 2008;49(suppl 2):64S–80S. doi: 10.2967/jnumed.107.046391. [DOI] [PubMed] [Google Scholar]
- 36.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Dowsett M, Archer C, Assersohn L, et al. Clinical studies of apoptosis and proliferation in breast cancer. Endocr Relat Cancer. 1999;6:25–28. doi: 10.1677/erc.0.0060025. [DOI] [PubMed] [Google Scholar]
- 38.Dowsett M, Smith IE, Ebbs SR, et al. Proliferation and apoptosis as markers of benefit in neoadjuvant endocrine therapy of breast cancer. Clin Cancer Res. 2006;12:1024s–1030s. doi: 10.1158/1078-0432.CCR-05-2127. [DOI] [PubMed] [Google Scholar]
- 39.Krohn KA, Mankoff DA, Eary JF. Imaging cellular proliferation as a measure of response to therapy. J Clin Pharmacol. 2001;41:96S–103S. [PubMed] [Google Scholar]
- 40.Shields AF, Grierson JR, Dohmen BM, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998;4:1334–1336. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 41.Kenny L, Coombes RC, Vigushin DM, et al. Imaging early changes in proliferation at 1 week post chemotherapy: A pilot study in breast cancer patients with 30-deoxy-30-[18F]fluorothymidine positron emission tomography. Eur J Nucl Med Mol Imaging. 2007;34:1339–1347. doi: 10.1007/s00259-007-0379-4. [DOI] [PubMed] [Google Scholar]
- 42.Sohn HJ, Yang YJ, Ryu JS, et al. [18 F]Fluorothymidine positron emission tomography before and 7 days after gefitinib treatment predicts response in patients with advanced adenocarcinoma of the lung. Clin Cancer Res. 2008;14:7423–7429. doi: 10.1158/1078-0432.CCR-08-0312. [DOI] [PubMed] [Google Scholar]
- 43.Kostakoglu L, Duan F, Idowu MO, et al. A phase II study of [(18)F]-3 Deoxy-3′-fluorothymidine positron emission tomography (FLT-PET) in the assessment of early response of breast cancer to neoadjuvant chemotherapy: Results from ACRIN 6688. J Nucl Med. 2015;56:1681–1689. doi: 10.2967/jnumed.115.160663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dehdashti F, Laforest R, Gao F, et al. Assessment of cellular proliferation in tumors by PET using 18F-ISO-1. J Nucl Med. 2013;54:350–357. doi: 10.2967/jnumed.112.111948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sai KK, Jones LA, Mach RH. Development of (18)F-labeled PET probes for imaging cell proliferation. Curr Top Med Chem. 2013;13:892–908. doi: 10.2174/1568026611313080003. [DOI] [PubMed] [Google Scholar]
- 46.Blankenberg FG, Strauss HW. Recent advances in the molecular imaging of programmed cell death: Part I—Pathophysiology and radiotracers. J Nucl Med. 2012;53:1659–1662. doi: 10.2967/jnumed.112.108944. [DOI] [PubMed] [Google Scholar]
- 47.Chen DL, Engle JT, Griffin EA, et al. Imaging caspase-3 activation as a marker of apoptosis-targeted treatment response in cancer. Mol Imaging Biol. 2015;17:384–393. doi: 10.1007/s11307-014-0802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Padhani AR, Liu G, Koh DM, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations. Neoplasia. 2009;11:102–125. doi: 10.1593/neo.81328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schiavon G, Smith IE. Status of adjuvant endocrine therapy for breast cancer. Breast Cancer Res. 2014;16:206. doi: 10.1186/bcr3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puhalla S, Bhattacharya S, Davidson NE. Hormonal therapy in breast cancer: A model disease for the personalization of cancer care. Mol Oncol. 2012;6:222–236. doi: 10.1016/j.molonc.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 52.Allred DC, Harvey JM, Berardo M, et al. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 53.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spataro V, Price K, Goldhirsch A, et al. Sequential estrogen receptor determinations from primary breast cancer and at relapse: Prognostic and therapeutic relevance. The International Breast Cancer Study Group (formerly Ludwig Group) Ann Oncol. 1992;3:733–740. doi: 10.1093/oxfordjournals.annonc.a058330. [DOI] [PubMed] [Google Scholar]
- 55.Kuukasjarvi T, Kononen J, Helin H, et al. Loss of estrogen receptor in recurrent breast cancer is associated with poor response to endocrine therapy. J Clin Oncol. 1996;14:2584–2589. doi: 10.1200/JCO.1996.14.9.2584. [DOI] [PubMed] [Google Scholar]
- 56.Linden HM, Stekhova SA, Link JM, et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J Clin Oncol. 2006;24:2793–2799. doi: 10.1200/JCO.2005.04.3810. [DOI] [PubMed] [Google Scholar]
- 57.Jonson SD, Bonasera TA, Dehdashti F, et al. Comparative breast tumor imaging and comparative in vitro metabolism of 16alpha-[18F]fluoroestradiol-17beta and 16beta-[18F]fluoromoxestrol in isolated hepatocytes. Nucl Med Biol. 1999;26:123–130. doi: 10.1016/s0969-8051(98)00079-1. [DOI] [PubMed] [Google Scholar]
- 58.Sundararajan L, Linden HM, Link JM, et al. 18F-fluoroestradiol. Semin Nucl Med. 2007;37:470–476. doi: 10.1053/j.semnuclmed.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 59.Kiesewetter DO, Kilbourn MR, Landvatter SW, et al. Preparation of four fluorine-18-labeled estrogens and their selective uptakes in target tissues of immature rats. J Nucl Med. 1984;25:1212–1221. [PubMed] [Google Scholar]
- 60.Mathias CJ, Welch MJ, Katzenellenbogen JA, et al. Characterization of the uptake of 16 alpha-([18F]fluoro)-17 beta-estradiol in DMBA-induced mammary tumors. Int J Rad Appl Instrum B. 1987;14:15–25. doi: 10.1016/0883-2897(87)90156-5. [DOI] [PubMed] [Google Scholar]
- 61.Mankoff DA, Tewson TJ, Eary JF. Analysis of blood clearance and labeled metabolites for the estrogen receptor tracer [F-18]-16 alpha-fluoroestradiol (FES) Nucl Med Biol. 1997;24:341–348. doi: 10.1016/s0969-8051(97)00002-4. [DOI] [PubMed] [Google Scholar]
- 62.Tewson TJ, Mankoff DA, Peterson LM, et al. Interactions of 16alpha-[18F]-fluoroestradiol (FES) with sex steroid binding protein (SBP) Nucl Med Biol. 1999;26:905–913. doi: 10.1016/s0969-8051(99)00072-4. [DOI] [PubMed] [Google Scholar]
- 63.Mintun MA, Welch MJ, Siegel BA, et al. Breast cancer: PET imaging of estrogen receptors. Radiology. 1988;169:45–48. doi: 10.1148/radiology.169.1.3262228. [DOI] [PubMed] [Google Scholar]
- 64.Peterson LM, Mankoff DA, Lawton T, et al. Quantitative imaging of estrogen receptor expression in breast cancer with PET and 18F-fluoroestradiol. J Nucl Med. 2008;49:367–374. doi: 10.2967/jnumed.107.047506. [DOI] [PubMed] [Google Scholar]
- 65.Kurland BF, Peterson LM, Lee JH, et al. Between-patient and within-patient (site-to-site) variability in estrogen receptor binding, measured in vivo by 18F-fluoroestradiol PET. J Nucl Med. 2011;52:1541–1549. doi: 10.2967/jnumed.111.091439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jeselsohn R, Buchwalter G, Angelis C, et al. ESR1 mutations-a mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol. 2015;12:573–583. doi: 10.1038/nrclinonc.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peterson LM, Kurland BF, Schubert EK, et al. A phase 2 study of 16alpha-[18F]-fluoro-17beta-estradiol positron emission tomography (FES-PET) as a marker of hormone sensitivity in metastatic breast cancer (MBC) Mol Imaging Biol. 2014;16:431–440. doi: 10.1007/s11307-013-0699-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Kruchten M, de Vries EG, Brown M, et al. PET imaging of oestrogen receptors in patients with breast cancer. Lancet Oncol. 2013;14:e465–e475. doi: 10.1016/S1470-2045(13)70292-4. [DOI] [PubMed] [Google Scholar]
- 69.van Kruchten M, Glaudemans AW, de Vries EF, et al. Positron emission tomography of tumour [18F] fluoroestradiol uptake in patients with acquired hormone-resistant metastatic breast cancer prior to oestradiol therapy. Eur J Nucl Med Mol Imaging. 2015;42:1674–1681. doi: 10.1007/s00259-015-3107-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Humbert O, Cochet A, Coudert B, et al. Role of positron emission tomography for the monitoring of response to therapy in breast cancer. Oncologist. 2015;20:94–104. doi: 10.1634/theoncologist.2014-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28:4594–4600. doi: 10.1200/JCO.2010.28.8415. [DOI] [PubMed] [Google Scholar]
- 72.van Kruchten M, de Vries EG, Glaudemans AW, et al. Measuring residual estrogen receptor availability during fulvestrant therapy in patients with metastatic breast cancer. Cancer Discov. 2015;5:72–81. doi: 10.1158/2159-8290.CD-14-0697. [DOI] [PubMed] [Google Scholar]
- 73.Dowsett M, Smith IE, Ebbs SR, et al. Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clin Cancer Res. 2005;11:951s–958s. [PubMed] [Google Scholar]
- 74.Ko BH, Paik JY, Jung KH, et al. 17beta-estradiol augments 18F-FDG uptake and glycolysis of T47D breast cancer cells via membrane-initiated rapid PI3K-Akt activation. J Nucl Med. 2010;51:1740–1747. doi: 10.2967/jnumed.110.074708. [DOI] [PubMed] [Google Scholar]
- 75.Linden HM, Kurland BF, Link JM, et al. The role of FLT PET early assessment of response to endocrine therapy for early stage breast cancer. Presented a the San Antonio Breast Cancer Symposium (SABCS); San Antonio, TX. 2013. [Google Scholar]
- 76.Turner NC, Ro J, Andre F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 77.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 78.Carlomagno C, Perrone F, Gallo C, et al. c-erb B2 overexpression decreases the benefit of adjuvant tamoxifen in early-stage breast cancer without axillary lymph node metastases. J Clin Oncol. 1996;14:2702–2708. doi: 10.1200/JCO.1996.14.10.2702. [DOI] [PubMed] [Google Scholar]
- 79.Kurokawa H, Arteaga CL. ErbB (HER) receptors can abrogate antiestrogen action in human breast cancer by multiple signaling mechanisms. Clin Cancer Res. 2003;9:511S–515S. [PubMed] [Google Scholar]
- 80.Schiff R, Massarweh S, Shou J, et al. Breast cancer endocrine resistance: How growth factor signaling and estrogen receptor coregulators modulate response. Clin Cancer Res. 2003;9:447S–454S. [PubMed] [Google Scholar]
- 81.Ferretti G, Felici A, Papaldo P, et al. HER2/neu role in breast cancer: From a prognostic foe to a predictive friend. Curr Opin Obstet Gynecol. 2007;19:56–62. doi: 10.1097/GCO.0b013e328012980a. [DOI] [PubMed] [Google Scholar]
- 82.Gusterson BA, Gelber RD, Goldhirsch A, et al. Prognostic importance of c-erbB-2 expression in breast cancer. International (Ludwig) Breast Cancer Study Group. J Clin Oncol. 1992;10:1049–1056. doi: 10.1200/JCO.1992.10.7.1049. [DOI] [PubMed] [Google Scholar]
- 83.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 84.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 85.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 86.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 87.Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Potts SJ, Krueger JS, Landis ND, et al. Evaluating tumor heterogeneity in immunohistochemistry-stained breast cancer tissue. Lab Invest. 2012;92:1342–1357. doi: 10.1038/labinvest.2012.91. [DOI] [PubMed] [Google Scholar]
- 90.Santinelli A, Pisa E, Stramazzotti D, et al. HER-2 status discrepancy between primary breast cancer and metastatic sites. Impact on target therapy. Int J Cancer. 2008;122:999–1004. doi: 10.1002/ijc.23051. [DOI] [PubMed] [Google Scholar]
- 91.Capala J, Bouchelouche K. Molecular imaging of HER2-positive breast cancer: A step toward an individualized ‘image and treat’ strategy. Curr Opin Oncol. 2010;22:559–566. doi: 10.1097/CCO.0b013e32833f8c3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dijkers EC, Oude Munnink TH, Kosterink JG, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther. 2010;87:586–592. doi: 10.1038/clpt.2010.12. [DOI] [PubMed] [Google Scholar]
- 93.Tolmachev V, Tran TA, Rosik D, et al. Tumor targeting using affibody molecules: Interplay of affinity, target expression level, and binding site composition. J Nucl Med. 2012;53:953–960. doi: 10.2967/jnumed.111.101527. [DOI] [PubMed] [Google Scholar]
- 94.Linden HM, Dehdashti F. Novel methods and tracers for breast cancer imaging. Semin Nucl Med. 2013;43:324–329. doi: 10.1053/j.semnuclmed.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 95.Tamura K, Kurihara H, Yonemori K, et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J Nucl Med. 2013;54:1869–1875. doi: 10.2967/jnumed.112.118612. [DOI] [PubMed] [Google Scholar]
- 96.Smith-Jones PM, Solit D, Afroze F, et al. Early tumor response to Hsp90 therapy using HER2 PET: Comparison with 18F-FDG PET. J Nucl Med. 2006;47:793–796. [PMC free article] [PubMed] [Google Scholar]
- 97.Smith-Jones PM, Solit DB, Akhurst T, et al. Imaging the pharmacodynamics of HER2 degradation in response to Hsp90 inhibitors. Nat Biotechnol. 2004;22:701–706. doi: 10.1038/nbt968. [DOI] [PubMed] [Google Scholar]
- 98.Beylergil V, Morris PG, Smith-Jones PM, et al. Pilot study of 68Ga-DOTA-F(ab0)2-trastuzumab in patients with breast cancer. Nucl Med Commun. 2013;34:1157–1165. doi: 10.1097/MNM.0b013e328365d99b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Orlova A, Jonsson A, Rosik D, et al. Site-specific radiometal labeling and improved biodistribution using ABY-027, a novel HER2-targeting affibody molecule-albumin-binding domain fusion protein. J Nucl Med. 2013;54:961–968. doi: 10.2967/jnumed.112.110700. [DOI] [PubMed] [Google Scholar]
- 100.Kiesewetter DO, Kramer-Marek G, Ma Y, et al. Radiolabeling of HER2 specific Affibody(R) molecule with F-18. J Fluor Chem. 2008;129:799–805. doi: 10.1016/j.jfluchem.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baum RP, Prasad V, Muller D, et al. Molecular imaging of HER2-expressing malignant tumors in breast cancer patients using synthetic 111In- or 68Ga-labeled affibody molecules. J Nucl Med. 2010;51:892–897. doi: 10.2967/jnumed.109.073239. [DOI] [PubMed] [Google Scholar]
- 102.Sorensen J, Sandberg D, Sandstrom M, et al. First-in-human molecular imaging of HER2 expression in breast cancer metastases using the 111In-ABY-025 affibody molecule. J Nucl Med. 2014;55:730–735. doi: 10.2967/jnumed.113.131243. [DOI] [PubMed] [Google Scholar]
- 103.Oude Munnink TH, Korte MA, Nagengast WB, et al. (89)Zr-trastuzumab PET visualises HER2 downregulation by the HSP90 inhibitor NVP-AUY922 in a human tumour xenograft. Eur J Cancer. 2010;46:678–684. doi: 10.1016/j.ejca.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 104.Gaykema SB, Schroder CP, Vitfell-Rasmussen J, et al. 89Zr-trastuzumab and 89Zr-bevacizumab PET to evaluate the effect of the HSP90 inhibitor NVP-AUY922 in metastatic breast cancer patients. Clin Cancer Res. 2014;20:3945–3954. doi: 10.1158/1078-0432.CCR-14-0491. [DOI] [PubMed] [Google Scholar]
- 105.Groheux D, Giacchetti S, Hatt M, et al. HER2-overexpressing breast cancer: FDG uptake after two cycles of chemotherapy predicts the outcome of neoadjuvant treatment. Br J Cancer. 2013;109:1157–1164. doi: 10.1038/bjc.2013.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Humbert O, Cochet A, Riedinger JM, et al. HER2-positive breast cancer: (1)(8)F-FDG PET for early prediction of response to trastuzumab plus taxane-based neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2014;41:1525–1533. doi: 10.1007/s00259-014-2739-1. [DOI] [PubMed] [Google Scholar]
- 107.Gebhart G, Gamez C, Holmes E, et al. 18F-FDG PET/CT for early prediction of response to neoadjuvant lapatinib, trastuzumab, and their combination in HER2-positive breast cancer: Results from Neo-ALTTO. J Nucl Med. 2013;54:1862–1868. doi: 10.2967/jnumed.112.119271. [DOI] [PubMed] [Google Scholar]
- 108.Connolly RM, Leal JP, Goetz MP, et al. TBCRC 008: Early change in 18F-FDG uptake on PET predicts response to preoperative systemic therapy in human epidermal growth factor receptor 2-negative primary operable breast cancer. J Nucl Med. 2015;56:31–37. doi: 10.2967/jnumed.114.144741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Coudert B, Pierga JY, Mouret-Reynier MA, et al. Use of [(18)F]-FDG PET to predict response to neoadjuvant trastuzumab and docetaxel in patients with HER2-positive breast cancer, and addition of bevacizumab to neoadjuvant trastuzumab and docetaxel in [(18)F]-FDG PET-predicted non-responders (AVATAXHER): An open-label, randomised phase 2 trial. Lancet Oncol. 2014;15:1493–1502. doi: 10.1016/S1470-2045(14)70475-9. [DOI] [PubMed] [Google Scholar]
- 110.Lin NU, Guo H, Yap JT, et al. Phase II study of lapatinib in combination with trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: Clinical outcomes and predictive value of early [18F]fluorodeoxyglucose positron emission tomography imaging (TBCRC 003) J Clin Oncol. 2015;33:2623–2631. doi: 10.1200/JCO.2014.60.0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gebhart G, Lamberts LE, Garcia C, et al. PET/CT with 89Zr-trastuzumab and 18F-FDG to individualize treatment with trastuzumab emtansine (T-DM1) inmetastatic HER2-positive breast cancer (mBC) J Clin Oncol. 2014;32(suppl):5s. [Google Scholar]
- 112.Mankoff DA, Farwell MD, Clark AS, et al. How imaging can impact clinical trial design: Molecular imaging as a biomarker for targeted cancer therapy. Cancer J. 2015;21:218–224. doi: 10.1097/PPO.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 113.McShane LM, Hayes DF. Publication of tumor marker research results: The necessity for complete and transparent reporting. J Clin Oncol. 2012;30:4223–4232. doi: 10.1200/JCO.2012.42.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]