Abstract
Introduction:
Glycerin (VG) and propylene glycol (PG) are the most common nicotine solvents used in e-cigarettes (ECs). It has been shown that at high temperatures both VG and PG undergo decomposition to low molecular carbonyl compounds, including the carcinogens formaldehyde and acetaldehyde. The aim of this study was to evaluate how various product characteristics, including nicotine solvent and battery output voltage, affect the levels of carbonyls in EC vapor.
Methods:
Twelve carbonyl compounds were measured in vapors from 10 commercially available nicotine solutions and from 3 control solutions composed of pure glycerin, pure propylene glycol, or a mixture of both solvents (50:50). EC battery output voltage was gradually modified from 3.2 to 4.8V. Carbonyl compounds were determined using the HPLC/DAD method.
Results:
Formaldehyde and acetaldehyde were found in 8 of 13 samples. The amounts of formaldehyde and acetaldehyde in vapors from lower voltage EC were on average 13- and 807-fold lower than in tobacco smoke, respectively. The highest levels of carbonyls were observed in vapors generated from PG-based solutions. Increasing voltage from 3.2 to 4.8V resulted in a 4 to more than 200 times increase in formaldehyde, acetaldehyde, and acetone levels. The levels of formaldehyde in vapors from high-voltage device were in the range of levels reported in tobacco smoke.
Conclusions:
Vapors from EC contain toxic and carcinogenic carbonyl compounds. Both solvent and battery output voltage significantly affect levels of carbonyl compounds in EC vapors. High-voltage EC may expose users to high levels of carbonyl compounds.
INTRODUCTION
Electronic cigarettes (e-cigarettes; ECs) have been gaining increasing popularity as nicotine delivery tools. It has been shown that number of EC users is growing rapidly (Ayers, Ribisl, & Brownstein, 2011; Kosmider, Knysak, Goniewicz, & Sobczak, 2012). Scientific evidence is urgently needed to develop the best regulatory approach to ECs. The U.S. Food and Drug Administration (FDA) has authority to regulate ECs as tobacco or medicinal products, and such regulation is expected to be announced soon (Benowitz & Goniewicz, 2013). Recently, the European Parliament has voted that ECs will be regulated as tobacco products, but the U.K. Medicines and Healthcare products Regulatory Agency (MHRA) has announced that EC will be regulated as medicinal devices in the United Kingdom by 2016 (Hajek, Foulds, Le Houezec, Sweanor, & Yach, 2013).
Studies are urgently needed to evaluate the presence of potentially toxic and hazardous compounds in vapors generated by ECs and which are inhaled by product users. Vapors are generated from solutions, commonly known as e-liquids or e-juices, which contain solvents (so-called e-liquid base), various concentrations of nicotine, water, additives, and flavorings. The most popular solvents used in e-liquids are glycerin (most commonly of vegetable origin, VG), propylene glycol (PG), or their mixture in various ratios. The “base” usually constitutes 70% to 80% of all components in the e-liquid.
When an EC user takes a puff, it activates heating element that vaporizes the e-liquid. This vaporization process occurs at various temperature ranges. It has been estimated that theoretical vaporization temperature of the heating element may reach up to 350oC (Balhas et al., 2014; Schripp, Markewitz, Uhde, & Salthammer, 2013). This temperature is sufficiently high to induce physical changes of e-liquids and chemical reactions between the constituents of e-liquids. At this temperature, solvents may undergo thermal decomposition leading to formation of potentially toxic compounds. Both VG and PG have been shown to decompose at high temperatures generating low molecular weight carbonyl compounds with established toxic properties (e.g., formaldehyde, acetaldehyde, acrolein, and acetone) (Paschke, Scherer, & Heller, 2002). Moreover, carbonyls such as formaldehyde and acetaldehyde may be present in the e-liquid (Farsalinos, Spyrou, Tsimopoulou, Romagna, & Voudris, 2014). Formaldehyde is classified by the International Agency for Research of Cancer (IARC) as a human carcinogen (Group 1), and acetaldehyde is classified as possibly carcinogenic to humans (Group 2B) (IARC, 2012). Acrolein causes irritation of the nasal cavity, damages the lining of the lung (U.S. EPA, 2003), and has been shown to contribute to cardiovascular disease (Park & Taniguchi, 2008). Acetone is a mucous membrane irritant that has been shown to induce damage on olfactory neuroepithelium in mice after inhalation (Buron, Hacquemand, Pourié, & Brand, 2009). It has been hypothesized that exposure to carbonyls may cause mouth and throat irritation, one of the most commonly reported side-effects of ECs (Bullen et al., 2010).
We previously evaluated 12 various brands of ECs and found that the generated vapors contained various carbonyls (Goniewicz et al., 2014). The limited literature to date described the presence of formaldehyde, acetaldehyde, acetone, acrolein, propanal, butanal, glyoxal, and methylglyoxal in EC vapors (Goniewicz et al., 2014; Laugesen, 2008; Schripp et al., 2013; Uchiyama, Inaba, & Kunugita, 2010). The studies reported that the levels of carbonyls in EC vapors are significantly lower than those found in tobacco smoke. However, these studies used early models of EC (also referred as “first generation”).
EC product categories have been evolving very rapidly and a “second generation” was recently introduced to the market. New products include “tank systems” that can be refilled by users with various e-liquids (Supplementary Figure 1). Some new EC models allow users to increase vaporization temperature by changing battery output voltage (Supplementary Figure 1). An EC generates vapor by heating an atomizing device normally containing a heater coil. To produce more heat, the device needs more power. Variable voltage EC are power control devices that allow the user to control the voltage that is applied to the atomizer. Variable voltage EC allows user to change the voltage of the device to increase the vapor production and nicotine delivery. There is also a huge variety of e-liquids on the market, which are manufactured and distributed by various companies. The aim of the study was to evaluate the extent to which nicotine solvent and battery output voltage affect the levels of carbonyls in the vapors of these second generation products.
MATERIALS AND METHODS
Electronic Cigarette
The most popular device available on the Polish market as on January 2013 was selected for the study. Because the Internet is currently the main distribution channel for EC, we searched google.pl web browser and tracked the number of EC sell offers on Allegro.pl, which is the most popular online auction service in Poland. Based on the number of search hits and sell offers, we chose and purchased the eGo-3 brand (Volish, Ltd, Poland). The device has controlled maximum time for single puff of 10 s. We chose a model composed of a Crystal 2 clearomizer (Supplementary Figure 1), with a heating element with resistance of 2.4 ohms, a 900 mAh battery with voltage of 3.4V, and a battery voltage stabilization system. All batteries were charged for 24hr before each test. Only fully charged batteries were used for liquid generation, and batteries were replaced when the devices indicated a decrease in charging level from 100%–50% (white diode color) to 50%–10% (light blue diode color).
In order to test the effect of battery output voltage on carbonyl levels delivered to vapor, we used eGo-3 Twist battery. This 900 mAh battery has a dial that allows for gradually changing its voltage from 3.2 to 4.8V with precision of ±0.07V (Supplementary Figure 1).
Nicotine Solutions (E-Liquids)
Ten kinds of commercially available e-liquids with nicotine concentration from 18 to 24mg/ml were used to fill up the clearomizer (tank). All products except one had the labels or inserts that provided information about source of manufacturing, name of distributor, and ingredients (A1–A10; Table 1). However, only half of the product labels showed the concentrations of solvents and flavorings. Based on the labeling information, we grouped the products into VG based (only VG; A1–A3), VG:PG based (both VG and PG mixed in various ratios; A4–A6), and PG based (only PG; A7–A10). We collected 1ml of each e-liquid and refilled 10 clearomizers of the same type 24hr before aerosol generation. Each clearomizer was used only for one e-liquid. We followed instructions in the user’s manual and stored the clearomizers at room temperature in a horizontal position to equally distribute the solution inside the clearomizer.
Table 1.
Characteristics of Nicotine Refill Solutions
| Code | Brand name | Manufacturer | Ingredients (as listed on labels) | Flavor | Nicotine (mg/ml) | Batch number |
|---|---|---|---|---|---|---|
| Commercially purchased refill solutions | ||||||
| A1 | E-Juice | Evaper Poland | Glycerin (VG), ethyl maltol, raspberry ketone, menthol, ethylvanillin, ethanol, purified water, nicotine | Island Tobacco | 18 | No data |
| A2 | DK-TAB | Changning Dekang Biotechnology | No data | Classic Tobacco | 18 | No data |
| A3 | Mild | Changning Dekang Biotechnology | Glycerin 80%, vanilla extract 10.2%, eleutheroside E1 4%, rose oil 1.5%, acetylpyrazine 1%, piperonal 0.8%, α-citronellol 0.3%, 2-hydroxy- 3-methyl-cyclopent-2-enon 0.2%, damascenones 0.2% | Mild Black | 18 | 2012524-1 |
| A4 | E-Juice | Evaper Poland | Propylene glycol (PG), glycerin, ethyl maltol, raspberry ketone, menthol, ethylvanillin, ethanol, purified water, nicotine | Island Tobacco | 18 | No data |
| A5 | E-Juice | Evaper Poland | Propylene glycol, glycerin, ethyl maltol, raspberry ketone, menthol, ethylvanillin, ethanol, purified water, nicotine | Island Tobacco | 18 | No data |
| A6 | LiQueen | Feelife Bioscience International | Polyethylene glycol 40%, propylene glycol 30%, glycerin 13.8%, sodium alginate 6%, enzymes 3%, ethyl maltol 2.5%, chamomile oil 0.5%, nicotine 2.4% | Sunny Banana | 24 | PI111014-2 |
| A7 | E-Juice | Evaper Poland | Propylene glycol, ethyl maltol, raspberry ketone, menthol, ethylvanillin, ethanol, purified water, nicotine | Island Tobacco | 18 | No data |
| A8 | E-Liquid | King E-Cigar Poland | Propylene glycol 69%, natural tobacco extract 27%, flavor extract 0.6%, linalool 0.6%, 2-acetylpyrazine 0.6%, 2,5-dimethylpyrazine 0.15%, nicotine 1.8% | Camel | 18 | No data |
| A9 | E-Liquid | King E-Cigar Poland | Propylene glycol, natural tobacco extract 27%, flavor extract 0.6%, linalool 0.6%, 2-acetylpyrazine 0.6%, 2,5-dimethylpyrazine 0.15%, nicotine 1.8% | Strong Hit | 18 | No data |
| A10 | Peleon PG | Changning Dekang Biotechnology | Propylene glycol, nicotine | Deluxe Tobacco | 18 | No data |
| Control refill solutions | ||||||
| C1 | Control 1 | Laboratory | Glycerin 88.2%, redistilled water 10%, nicotine 1.8% | No flavor | 18 | N/A |
| C2 | Control 2 | Laboratory | Glycerin 44.1%, propylene glycol 44.1%, redistilled water 10%, nicotine 1.8% | No flavor | 18 | N/A |
| C3 | Control 3 | Laboratory | Propylene glycol 88.2%, redistilled water 10%, nicotine 1.8% | No flavor | 18 | N/A |
Note. N/A = not applicable.
In addition to commercially available products, we prepared three sets of control e-liquids (C1–C3; Table 1). The control e-liquids were prepared by dissolving pure nicotine (>99%, Acros) in analytical-grade solvents and vortexing for 10min. The following control solutions were prepared: C1 with VG (88.2%), redistilled water (10.0%), and nicotine (1.8%); C2 with VG (44.1%), PG (44.1%), redistilled water (10.0%), and nicotine (1.8%); and C3 with PG (88.2%), redistilled water (10.0%), and nicotine (1.8%). None of the control e-liquid contained any flavorings or additives. These control e-liquids were used in experiments with adjustable battery voltage.
Generation of EC Vapors
Vapors from ECs were generated using the automatic smoking machine Palaczbot (University of Technology, Lodz, Poland) as described previously (Goniewicz, Kuma, Gawron, Knysak, & Kosmider, 2013). In the current study, all tests were performed with the following puffing conditions: puff duration 1.8 s, puff volume 70ml, and puff intervals 17 s as described previously (Goniewicz et al., 2013). A total of 30 puffs were taken from each EC in two series of 15 puffs with a 5-min interval between series. ECs were kept in a horizontal position in order to maintain natural conditions of puffing on EC. Because the device used in this study was manually activated, an operator of the smoking machine pressed the button manually 1 s before each puff was taken and released it immediately after the puff was completed. Vapors from each e-liquid were tested three times.
In experiments with adjustable battery voltage, vapors were generated using three different battery voltages: 3.2, 4.0, and 4.8V. Three tests were conducted for each of nine solvent:voltage combinations. We used new clearomizers of the same type per each voltage setting. Because we did not use the same battery for all tests, differences in carbonyl levels in vapors generated at 3.2V were compared with the levels in vapors generated at 4.8V using a t test. For statistical analysis, results below lower limits of quantitation (LLOQ; see below) were estimated as LLOQ/√2.
Analysis of Carbonyl Compounds
The method recommended by the U.S. Environment Protection Agency (EPA) was applied for determination of carbonyl compounds (U.S. EPA, 2003). Briefly, it involves direct extraction of these compounds from aerosol to solid phase, that is, silica gel saturated with 2,4-dinitrophenylohydrazine (DNPH). The silica sorbent tubes (300/150mg; SKC Inc.) were placed between EC mouthpieces and smoking machine to trap carbonyls from freshly generated vapors. The sorbent tubes were placed directly behind the EC mouthpiece to avoid potential losses of analyzed compounds. DNPH derivatives of carbonyl compounds were desorbed from sorbent tubes using 1ml of acetonitrile. Ten microliters of the extract was analyzed using high-performance liquid chromatography (HPLC) with Eclipse PAH chromatographic column (4.5×250mm, 5 μm, Zorbax, Agilent Technologies) and a diode array detector (DAD; 365nm wavelength) (AT 1200, Agilent Technologies, USA). An elution gradient with acetonitrile:water mobile phase was used, and chromatographic separation was performed at a constant temperature of 40°C.
The method was calibrated and validated as per the International Conference on Harmonization guideline Q2 R1 (International Conference on Harmonization, 2005). All calibration and control samples were prepared by spiking the sorbent tubes with various amounts of stock solution of carbonyls and proceeding with whole analytical procedures. Blank samples were prepared by sampling air from the laboratory where all tests were performed. If any of the analyzed carbonyls were detected in blank samples, the background levels were subtracted from the levels detected in vapor samples. Precision and accuracy of the method varied from 4% to 12% and from 96% to 108%, respectively. In order to compare levels of carbonyls found in vapors with levels reported for tobacco smoke, results were recalculated per one series of 15 puffs from ECs. The LLOQ of the carbonyls were as follows: (ng/15 puffs): formaldehyde, 30; acetaldehyde, 15; acrolein, 30; acetone, 30; propionaldehyde, 20; crotonaldehyde, 40; butanal, 30; benzaldehyde, 40; isovaleric aldehyde, 20; valeric aldehyde, 20; o-methylbenzaldehyde, 35; and m-methylbenzaldehyde, 35.
RESULTS
Levels of Carbonyl Compounds Released From Commercially Available Refill Solutions
Table 2 shows amounts of each analyzed carbonyl compounds in 15 puffs of vapor from 10 commercially available e-liquids. The values presented in Table 2 are means with SD from three tests performed at the same voltage of 3.4V. All samples contained at least one carbonyl compound. Formaldehyde, acetaldehyde, acetone, and butanal were found in most of the analyzed samples. However, not all commercially available e-liquids emitted all these four carbonyls. Crotonaldehyde was detected in only one sample (A10), whereas acrolein was not detected in any sample.
Table 2.
Levels of Carbonyl Compounds in Vapors Generated From EC Refilled With Commercially Available (A1–A10) and Control (C1–C3) Nicotine Solutions (ng/15 puffs; mean ± SD; N = 3)
| Carbonyl compounds | Levels in EC vapor (ng/15 puffs) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VG based | VG:PG based | PG based | |||||||||||
| A1 | A2 | A3 | C1 | A4 | A5 | A6a | C2 | A7 | A8 | A9 | A10 | C3 | |
| Formaldehyde | BLQ | 49±2 | BLQ | BLQ | 51±28 | 55±7 | ND | ND | BLQ | BLQ | 59±6 | BLQ | BLQ |
| Acetaldehyde | BLQ | 20±4 | 27±5 | ND | 104±74 | 107±24 | ND | ND | 60±12 | 40±5 | 41±9 | 54±11 | BLQ |
| Acetone | 59±12 | 62±5 | 64±4 | BLQ | 181±50 | 296±91 | ND | ND | 213±193 | 181±31 | ND | 127±34 | ND |
| Butanal | 15±4 | 35±28 | 49±7 | 16±4 | 35±1 | 41±16 | 104±96 | 222±85 | ND | 15±5 | BLQ | 185±77 | 152±185 |
| Propanal | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Acrolein | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | BLQ | ND |
| Benzaldehyde | ND | ND | ND | ND | 21±9 | BLQ | ND | ND | BLQ | BLQ | 46±15 | 27±6 | ND |
| Crotonaldehyde | ND | BLQ | BLQ | ND | BLQ | BLQ | ND | ND | ND | ND | ND | 53 | ND |
| Valeric aldehyde | BLQ | BLQ | BLQ | ND | BLQ | ND | ND | ND | ND | ND | BLQ | BLQ | ND |
| Isovaleric aldehyde | ND | ND | ND | ND | 47±17 | 40±3 | ND | ND | 33±10 | ND | ND | ND | ND |
| m-Methylbenzaldehyde | BLQ | BLQ | BLQ | BLQ | 94±51 | 78±25 | BLQ | BLQ | 39±19 | 39±18 | 54±14 | BLQ | BLQ |
| o-Methylbenzaldehyde | ND | ND | ND | ND | BLQ | ND | ND | ND | ND | ND | ND | BLQ | ND |
Note. VG = vegetable glycerin; PG = propylene glycol; BLQ = below limit of quantitation; ND = not detected.
aIn addition to VG (13.8%) and PG (30%), solution A6 contained PEG (polyethylene glycol; 40%).
Effect of Solvent and Battery Output Voltage on Carbonyl Yields Released to Vapors
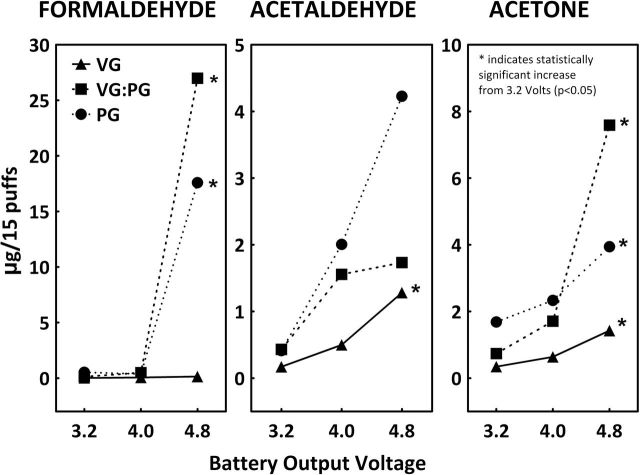
Figure 1 shows the effect of solvent and battery output voltage on amounts of formaldehyde, acetaldehyde, and acetone released to vapors with 15 puffs from EC refilled with three different control solutions (C1–C3). In general, PG-based e-liquids generated significantly higher levels of carbonyls than VG-based e-liquids (p < .05). Increased battery output voltage resulted in the higher levels of carbonyls in vapor. When low battery output voltage (3.2V) was used, the average amounts of formaldehyde released with 15 puffs from VG, VG/PG, and PG were (mean ± SD) 0.02±0.02, 0.13±0.11, and 0.53±0.19 µg, respectively. When battery output voltage was increased to 4.8V, the amounts of formaldehyde were 0.15±0.06 (p = .03), 27.0±7.9 (p < .01), and 17.6±19.7 µg (p = .21), respectively. When low battery output voltage (3.2V) was used, the average amounts of acetaldehyde released with 15 puffs from VG, VG/PG, and PG were 0.17±0.09, 0.43±0.50, and 0.41±0.28 µg, respectively. However, when the battery output voltage was increased to 4.8V, the amounts of acetaldehyde increased to 1.24±0.12 (p < .01), 1.73±1.21 (p = .16), and 4.23±3.23 µg (p = .11), respectively. Levels of acetone also increased with increased battery output voltage (from 0.34±0.09, 0.73±0.52, 1.68±0.30 to 1.43±0.14 [p < .01], 7.59±2.14 [p = .01], 3.94±0.47 [p < .01] µg/15 puffs, respectively, for VG, VG/PG, and PG-based solutions).
Figure 1.
Effects of nicotine solvent and battery output voltage on levels of carbonyl compounds released from ECs (µg/15 puffs; N = 3; puff duration 1.8 s, puff volume 70ml, puff intervals 17 s).
DISCUSSION
We present novel findings on levels of carcinogenic and toxic carbonyl compounds in vapors from second generation of EC. Our findings show that vapors generated from various commercial and reference solutions expose EC users to toxic carbonyls, including the carcinogens formaldehyde and acetaldehyde. Our findings are consistent with previously published reports reporting presence of formaldehyde, acetaldehyde, acrolein, propanal, acetone, and butanal in EC vapors (Goniewicz et al., 2014; Laugesen, 2008; McAuley, Hopke, Zhao, & Babaian, 2012; Schripp et al., 2013).
Our study found that the amounts of formaldehyde and acetaldehyde in vapors from lower voltage tank system ECs were on average 13- and 807-fold lower than in tobacco smoke, respectively. We previously reported that levels of these toxicants in vapors from the first generation of EC were 9- and 450-fold lower than in tobacco smoke, respectively (Goniewicz et al., 2014). Schripp et al. (2013) found that the levels were 7- and 59-fold lower compared with tobacco smoke. Our findings suggest only a slight reduction in toxicant emission from the second generation low-voltage EC compared with first generation ECs. Despite findings from chemical analysis, in vitro studies of the effects of EC vapor on cultured cells have shown that cell survival was not associated with the nicotine solvent (Farsalinos Romagna, Allifranchini, et al., 2013). Therefore, clinical studies are needed in order to determine whether such levels of carbonyls may have the potential to cause disease to EC users.
We also showed that levels of carbonyl compounds in EC vapors are strongly affected by product characteristics, like type of nicotine solvent and battery voltage. In general, the highest levels of carbonyls were observed in vapors generated from PG-based solutions. This finding suggests that PG in ECs is more susceptible to thermal decomposition than VG. The presence of carbonyls in flavor-free control solutions indicates that the primary sources of these toxicants are nicotine solvents. An interesting finding of our study is that no toxic carbonyls were detected in a single sample with reduced content of VG and PG. In this product (A6), the primary solvent was polyethylene glycol (PEG). It would suggest that PEG-based e-liquids might have reduced toxicity from decomposition products. Further research should explore this hypothesis.
The striking finding of our study is that levels of carbonyls rapidly increase with increased battery output voltage. Increasing battery output voltage leads to higher temperature of the heating element inside EC. In addition, the increased battery output voltage results in more e-liquid consumed per puff. Our findings show that increasing voltage from 3.2 to 4.8V resulted in 4 to over 200 times increase in formaldehyde, acetaldehyde, and acetone levels. The levels of formaldehyde in vapors from high-voltage devices were in the range of levels reported in tobacco smoke (1.6–52 µg/cigarette; Counts, Morton, Laffoon, Cox, & Lipowicz, 2005). This finding suggests that in certain conditions ECs might expose their users to the same or even higher levels of carcinogenic formaldehyde than tobacco smoke. This finding is essential for the product safety and in the light of forthcoming regulation of the devices.
We also noted some inconsistency in results related to acrolein presence in vapor with previously published findings. In our study, we did not find acrolein in any products. However, our previous research as well as research published by other authors suggest the presence of acrolein in EC vapor. However, in current study, we measured carbonyls only in two series of 15 puffs, whereas in previous report, we used much larger samples (150 puffs). Thus, this inconsistency might be attributed to differences in detection limits. The other explanation would be that generation of acrolein increases with the duration of EC use. Extensive puff-by-puff analysis would facilitate verification of this hypothesis.
The present study have some important limitations. We only looked at two factors that might affect toxicity of EC, namely nicotine solvent and battery output voltage. More research is needed to describe how other product characteristics affect toxicity of ECs. Future studies should examine the types of heating elements, flavorings and additives, and product storage conditions. Secondly, recent studies showed significant variations in puffing topography among users of various EC models (Edmiston et al., 2014; Farsalinos, Romagna, Tsiapras, Kyrzopoulos, & Voudris, 2013; Vansickel et al., 2014). Puffing topography may affect levels of carbonyls released from different ECs. There are some discrepancies between puffing regime used in our study and the results of clinical studies (Farsalinos, Romagna, Tsiapras, et al., 2013). Future studies should examine the effect of puffing on carbonyl levels released to EC vapors. The other limitation of this study is that we used the SKC sorbent tubes to trap carbonyl compounds. These tubes are meant to capture gas-phase, rather than particle-phase carbonyls. It is likely that at least some of the carbonyls (e.g., formaldehyde) are partitioned between the gas and particle phase in EC aerosol and may not have been trapped efficiently in the sorbent tubes. It is possible that what was measured actually represents a lower bound of what could have been emitted by the ECs.
CONCLUSIONS
Vapors from ECs contain toxic and carcinogenic carbonyl compounds. Both solvent and battery output voltage significantly affect levels of carbonyl compounds in EC vapors. Levels of carbonyls rapidly increase with increased battery output voltage. New generation of high-voltage ECs may put their users in increased health risk from exposure to high levels of carbonyl compounds although the risk will still probably be much lower compared with smoking.
SUPPLEMENTARY MATERIAL
Supplementary Figure 1 can be found online at http://www.ntr.oxfordjournals.org.
FUNDING
This work was supported by institutional internal funding from the Institute of Occupational Medicine and Environmental Health, Poland (ZSChiTG5), and Medical University of Silesia, Poland (KNW-1–031/N/3/0). The study sponsors had no involvement in the study design, collection, analysis, data interpretation, the writing of the manuscript, or the decision to submit the manuscript for publication.
DECLARATION OF INTERESTS
MLG received research funding from Pfizer, manufacturer of stop smoking medication. AS received research funds and travel expenses from Chic Group LTD, manufacturer of electronic cigarettes in Poland.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. R. O’Connor for valuable comments and C. Steger for editorial assistance with the manuscript.
REFERENCES
- Ayers J. W., Ribisl K. M., Brownstein J. S. (2011). Tracking the rise in popularity of electronic nicotine delivery systems (electronic cigarettes) using search query surveillance. American Journal of Preventive Medicine, 40, 448–453. 10.1016/j.amepre.2010.12.007 [DOI] [PubMed] [Google Scholar]
- Balhas Z., Talih S., Eissenberg T., Salman R., Karaoghlanian N., Shihadeh A. (2014). Effects of user puff topography and device characteristics on electronic cigarette nicotine yield. Presented at the 20th Annual Meeting of the Society for Research on Nicotine and Tobacco (SRNT), February 5–8, 2014, Seattle, WA. POS4-57. [Google Scholar]
- Benowitz N. L., Goniewicz M. L. (2013). The regulatory challenge of electronic cigarettes. The Journal of the American Medical Association (JAMA), 310, 685–686. 10.1001/jama.2013.109501 [DOI] [PubMed] [Google Scholar]
- Bullen C., McRobbie H., Thornley S., Glover M., Lin R., Laugesen M. (2010). Effect of an electronic nicotine delivery device (e-cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: Randomized cross-over trial. Tobacco Control, 19, 98–103. 10.1136/tc.2009.031567 [DOI] [PubMed] [Google Scholar]
- Buron G., Hacquemand R., Pourié G., Brand G. (2009). Inhalation exposure to acetone induces selective damage on olfactory neuroepithelium in mice. Neurotoxicology, 30, 114–120. 10.1016/j.neuro.2008.11.005 [DOI] [PubMed] [Google Scholar]
- Counts M. E., Morton M. J., Laffoon S. W., Cox R. H., Lipowicz P. J. (2005). Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regulatory Toxicology and Pharmacology, 41, 185–227. 10.1016/j.yrtph.2004.12.002 [DOI] [PubMed] [Google Scholar]
- Edmiston J., Vansickel A., Liang Q., Duhon C., Liu J., Sarkar M. (2014). The influence of menthol and water content of e-liquid on electronic cigarette puff volumes under specified puffing conditions in adult cigarette smokers. Presented at the 20th Annual Meeting of the Society for Research on Nicotine and Tobacco (SRNT), February 5–8, 2014, Seattle, WA. POS3-11. [Google Scholar]
- Farsalinos K. E., Romagna G., Allifranchini E., Ripamonti E., Bocchietto E., Todeschi S., … Voudris V. (2013). Comparison of the cytotoxic potential of cigarette smoke and electronic cigarette vapour extract on cultured myocardial cells. International Journal of Environmental Research and Public Health, 10, 5146–5162. 10.3390/ijerph10105146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K. E., Romagna G., Tsiapras D., Kyrzopoulos S., Voudris V. (2013). Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: Implications for research protocol standards definition and for public health authorities’ regulation. International Journal of Environmental Research and Public Health, 10, 2500–2514. 10.3390/ijerph10062500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K. E., Spyrou A., Tsimopoulou K., Romagna G., Voudris V. (2014). Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Scientific Reports, 4, 4133. 10.1038/srep04133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz M. L., Knysak J., Gawron M., Kosmider L., Sobczak A., Kurek J., … Benowitz N. L. (2014). Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tobacco Control, 23, 133–139. 10.1136/tobacocontrol-2012–050859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz M. L., Kuma T., Gawron M., Knysak J., Kosmider L. (2013). Nicotine levels in electronic cigarettes. Nicotine & Tobacco Research, 15, 158–166. 10.1093/ntr/nts103 [DOI] [PubMed] [Google Scholar]
- Hajek P., Foulds J., Le Houezec J., Sweanor D., Yach D. (2013). Should e-cigarettes be regulated as a medicinal device? The Lancet Respiratory Medicine, 1, 429–431. 10.1016/S2213-2600(13)70124-3 [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC). (2012). Agents classified by the IARC (Monographs, Volumes 1–105). Geneva, Switzerland: International Agency for Research on Cancer: Retrieved from http://monographs.iarc.fr/ENG/Classification/index.php [Google Scholar]
- International Conference on Harmonization. (2005). Technical requirements for registration of pharmaceuticals for human use, Topic Q2 (R1): Validation of analytical procedures: Text and Methodology. Geneva, Switzerland: International Conference on Harmonization: Retrieved from www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf [Google Scholar]
- Kosmider L., Knysak J., Goniewicz M. L., Sobczak A. (2012). Electronic cigarette—a safe substitute for tobacco cigarette or a new threat? (in Polish). Przeglad Lekarski, 69, 1084–1089. [PubMed] [Google Scholar]
- Laugesen M. (2008). Safety report on the Ruyan® e-cigarete cartridge and inhaled aerosol. Christchurch, New Zeland: Health New Zeland Ltd; Retrieved from www.healthnz.co.nz/RuyanCartridgeReport30-Oct-08.pdf [Google Scholar]
- McAuley T. R., Hopke P. K., Zhao J., Babaian S. (2012). Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhalation Toxicology, 24, 850–857. 10.3109/08958378.2012.724728 [DOI] [PubMed] [Google Scholar]
- Park Y. S., Taniguchi N. (2008). Acrolein induces inflammatory response underlying endothelial dysfunction. A risk factor for atherosclerosis. Annals of the New York Academy of Sciences, 1126, 185–189. 10.1196/annals.1433.034 [DOI] [PubMed] [Google Scholar]
- Paschke T., Scherer G., Heller W. D. (2002). Effects of ingredients on cigarette smoke composition and biological activity: A literature overview. Beiträge zur Tabakforschung International/Contributions to Tobacco Research, 20, 107–247. [Google Scholar]
- Schripp T., Markewitz D., Uhde E., Salthammer T. (2013). Does e-cigarette consumption cause passive vaping? Indoor Air, 23, 25–31. 10.1111/j.1600-0668.2012.00792.x [DOI] [PubMed] [Google Scholar]
- Uchiyama S., Inaba Y., Kunugita N. (2010). Determination of acrolein and other carbonyls in cigarette smoke using coupled silica cartridges impregnated with hydroquinone and 2,4-dinitrophenyl-hydrazine. Journal of Chromatography A, 1217, 4383–4388. 10.1016/j.chroma.2010.04.056 [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (U.S. EPA). (1999). Compendium of methods for the determination of toxic organic compounds in ambient air Method TO-11A. Cincinnati, OH: U.S. Environmental Protection Agency. Retrieved from www.epa.gov/ttnamti1/files/ambient/airtox/to-11ar.pdf
- U.S. Environmental Protection Agency (U.S. EPA). (2003). Toxicological review of acrolein. Washington, DC: Retrieved from www.epa.gov/iris/toxreviews/0364tr.pdf [Google Scholar]
- Vansickel A., Edmiston J., Liang Q., Duhon C., Liu J., Sarkar M. (2014). Characterization of electronic cigarette prototype puff topography in adult exclusive cigarette smokers and adult exclusive electronic cigarette users. Presented at the 20th Annual Meeting of the Society for Research on Nicotine and Tobacco (SRNT), February 5–8, 2014, Seattle, WA. POS3-65. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.