Abstract
Background
Vemurafenib, a novel selective small molecule inhibitor of BRAF, has recently been shown to be effective in the treatment of melanomas harboring the BRAF V600E mutation. Similar to the broad-spectrum RAF inhibitor sorafenib, vemurafenib induces development of squamous cell carcinomas and keratoacanthomas as a side effect of therapy.
Objective
We sought to detail additional cutaneous adverse effects of vemurafenib and a similar BRAF inhibitor, dabrafenib.
Methods
We evaluated the clinical and histologic feature of skin side effects developing on vemurafenib or dabrafenib therapy in 14 patients.
Results
Eight patients developed one or more squamous cell carcinomas, and 11 patients formed benign verrucous keratoses. Eight patients developed single lesions and/or widespread eruptions with histopathologic findings of acantholytic dyskeratosis, consistent with warty dyskeratomas and Darier- or Grover-like rashes, respectively. One patient developed palmoplantar hyperkeratosis, and darkening of existing nevi and new nevi within 2 months of starting vemurafenib. Side effects presented as early as 1 week after beginning therapy, with a mean time of onset of 12.6 weeks in our cohort.
Limitations
This study was limited by the small number of cases, all from a single institution.
Conclusion
Selective BRAF inhibitor therapy is associated with the development of malignant and benign growths, including keratoacanthoma-like squamous cell carcinomas, warty dyskeratomas, and verrucous keratoses, along with widespread eruptions with histologic features of acantholytic dyskeratosis. Given the potential for malignant lesions to develop on treatment, awareness of potential adverse effects of these agents is necessary, and a low threshold for biopsy of new growths is recommended.
Keywords: acantholytic dyskeratosis, BRAF, drug adverse effects, nevi, squamous cell carcinoma, verrucous keratosis, warty dyskeratoma
Vemurafenib is a recently developed small molecule inhibitor of the serine/threonine kinase BRAF, a component of the RAS–RAF–MEK–ERK (mitogen-activated protein kinase [MAPK]) signaling pathway that effects cellular processes including proliferation, survival, and differentiation.1 The drug specifically targets tumor cells that harbor activating mutations in BRAF, most commonly the substitution of glutamic acid for valine at codon 600 (V600E).2 Vemurafenib was approved by the Food and Drug Administration for treatment of metastatic melanoma in August 2011, after promising tumor responses to the medication in phase I, II, and III clinical trials.3–5 A similar selective BRAF inhibitor, dabrafenib (GSK2118436), is currently being tested in clinical studies for treatment of advanced stage and metastatic melanoma, as a single agent and in combination with a MEK inhibitor, trametinib (GSK1120212).1,6,7
Several cutaneous adverse effects of vemurafenib have been noted in earlier clinical studies.3,4 Photosensitivity, sometimes resulting in blistering reactions, has been documented.3,8 Palmar-plantar dysesthesia occurred in several patients.4 Keratosis pilaris–like eruptions occur in roughly one third of patients treated.3,9 Lastly, squamous cell carcinomas (SCCs) and keratoacanthomas (KAs) have been reported in 20% to 30% of patients treated with vemurafenib.3,4
Here, we describe in detail the cutaneous side effects observed in 14 patients after initiation of BRAF inhibitor therapy. These findings include the previously noted eruptive SCCs, and several additional skin reactions, which will be discussed in detail.
REPORT OF REPRESENTATIVE CASES
Case 1
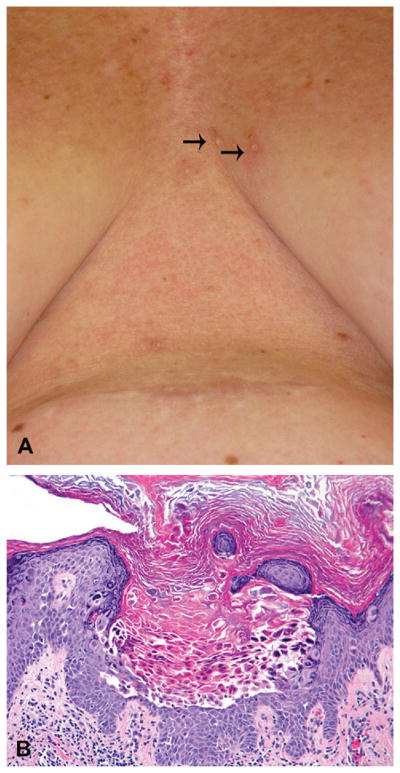
A 47-year-old Caucasian woman (patient 1, Table I) was diagnosed with stage IV melanoma with lung involvement, and was found to have V600E mutation in BRAF. Vemurafenib 960 mg twice daily was initiated, and 12 weeks after beginning therapy she noticed new scaly papules on the central aspect of her chest (Fig 1, A), upper aspect of her shoulders, back, and face. Several biopsies were performed at these sites. A biopsy from a 2-mm, pink, hyperkeratotic papule on the central aspect of her chest revealed an epidermal invagination featuring acantholysis and dyskeratosis and clefting of the suprabasilar epidermis, characteristic of warty dyskeratomas (Fig 1, B). Histopathologic examination of a papule on the patient’s face demonstrated a benign verrucous keratosis, and samples taken from the shoulder and back revealed SCCs (not shown). After 2 months of treatment with vemurafenib, the patient had a 60% reduction of her tumor burden based on computed tomography examination, and symptomatically experienced relief of her disease-related chronic cough and fatigue.
Table I.
Characteristic of patients on BRAF inhibitors
| Characteristic | Patient no.
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| Age, y | 46 | 64 | 47 | 60 | 54 | 53 | 55 | 58 | 69 | 77 | 68 | 64 | 83 | 57 |
| Sex | F | M | F | F | F | M | F | F | F | M | F | M | F | M |
| Targeted therapy | V | V | V | V | V | D + T | V | V | V | V | D | D + T; V | V | V |
| Time before first skin lesion, wk | 12 | 5 | 20 | 56 | 12 | 19 | 4 | 10 | 5 | 3 | 8 | 14 | 1 | 8 |
| History of SCC | + | + | − | − | − | − | + | − | − | − | − | − | − | − |
| Type of lesion | ||||||||||||||
| SCC | + | + | − | + | + | − | + | − | + | − | + | − | + | − |
| SCC, KA type | + | − | − | + | − | − | − | − | − | − | + | − | + | − |
| Verrucous keratosis | + | + | + | − | + | + | − | + | + | + | + | + | + | + |
| Acantholytic dyskeratosis | + | − | + | + | − | − | + | − | + | + | + | + | − | − |
| Eruptive nevi | − | − | − | − | − | − | − | − | − | − | − | − | − | + |
D, Dabrafenib (GSK2118436); F, female; KA, keratoacanthoma; M, male; SCC, squamous cell carcinoma; T, trametinib (GSK1120212); V, vemurafenib.
Fig 1.
Warty dyskeratoma (case 1). A, New keratotic papules on chest. B, Biopsy specimen demonstrating acantholysis and dyskeratosis, consistent with warty dyskeratoma. (Hematoxylin-eosin stain; original magnification: ×200.)
Case 2
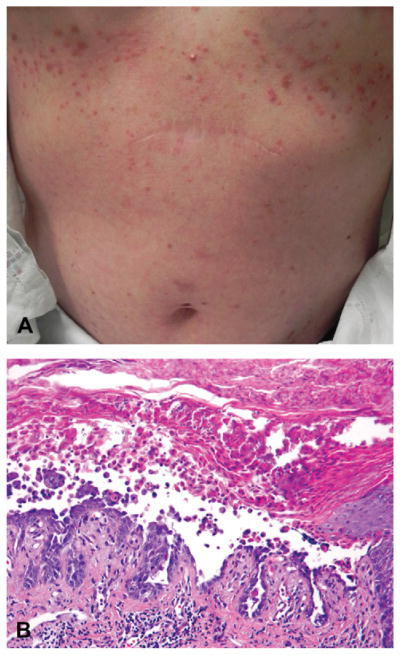
A 55-year-old Caucasian woman (patient 7, Table I) had a history of multiple primary melanomas on her abdomen, arm, and elbow and developed metastatic disease to her axillary lymph nodes and brain 1 month after detection of her third primary melanoma. Tumor genotyping revealed the BRAF V600E mutation. Vemurafenib was started at a dose of 960 mg twice a day, and she developed a new rash on her chest, face, and arms 4 weeks later. Of note, she had no history of nonmelanoma skin cancers. On physical examination, she had many erythematous, 2- to 3-mm scaly papules on her chest and abdomen (Fig 2, A), and a 1-cm dome-shaped hyperkeratotic, tender red papule on her arm. Histopathologic examination of the chest lesions demonstrated acantholysis and dyskeratosis consistent with Grover disease (also known as transient acantholytic dyskeratosis) or Darier disease (Fig 2, B). An additional biopsy specimen revealed a SCC on her arm (not shown). The acantholytic rash was responsive to treatment with triamcinolone 0.1% ointment, and the SCC was treated with wide local excision.
Fig 2.
Acantholytic dyskeratosis resembling Darier disease (case 2). A, Eruption of crusted papules on trunk. B, Biopsy specimen demonstrating prominent acantholytic dyskeratosis. (Hematoxylin-eosin stain; original magnification: ×200.)
Coincident with the development of her skin lesions, the patient noted a reduction in size of her subcutaneous lymph nodes. However, her brain disease later progressed on therapy, and she subsequently died.
Case 3
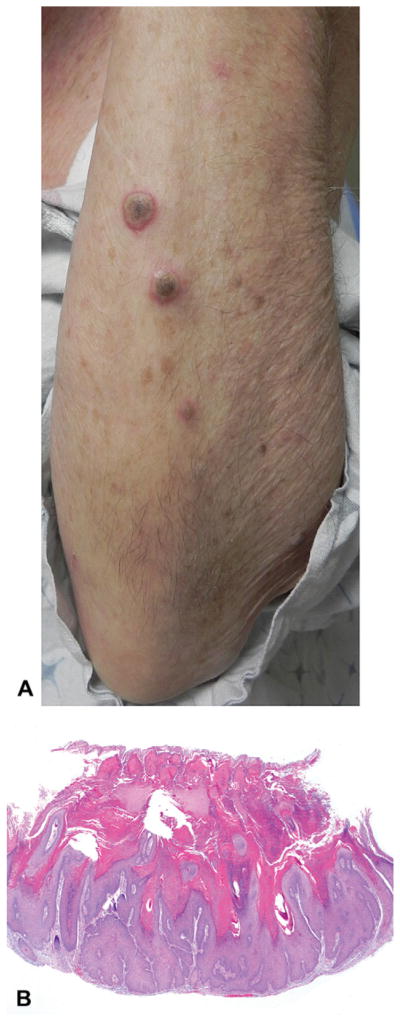
An 83-year-old Caucasian woman (patient 13, Table I) with a history of an aggressive melanoma on her scalp status-post excision was given the diagnosis of metastatic disease involving her lungs and left breast. She was initially treated with temozolomide and radiation therapy, with partial response. Vemurafenib was then started at 960 mg twice daily after detection of BRAF V600K mutation in her tumor. After 1 week on the medication, she noticed eruptive growths on her forehead, arms, legs, and back. She denied any history of non-melanoma skin cancers before starting vemurafenib. On physical examination, she had approximately 30 3- to 10-mm inflamed papules and nodules with keratotic cores on the face, back, arms, and legs (Fig 3, A), Biopsy specimens taken from multiple lesions demonstrated similar findings of large cup-shaped atypical proliferations of squamous epithelial cells, with central keratin-filled craters, diagnostic of SCC of the KA type (Fig 3, B). The patient’s SCCs were all well differentiated, and exhibited moderate solar elastosis in the surrounding dermis. The lesions ranged from 2 to 3.5 mm in thickness, extending from superficial to deep reticular dermis.
Fig 3.
Squamous cell carcinoma (SCC) (case 3). A, Multiple eruptive nodules on forearm. B, Histopathologic examination reveals well-differentiated SCC with keratoacanthoma-like features. (Hematoxylin-eosin stain; original magnification: ×20.)
Marked reduction in size of the patient’s scalp melanoma was observed after 4 weeks of treatment. Because of medication-related fatigue, the patient’s dose of vemurafenib was subsequently decreased to 720 mg twice daily, but she continued to develop additional SCCs. The patient was prescribed topical 5-fluorouracil to apply twice daily to SCCs indefinitely; several lesions have demonstrated regression clinically on therapy.
Case 4
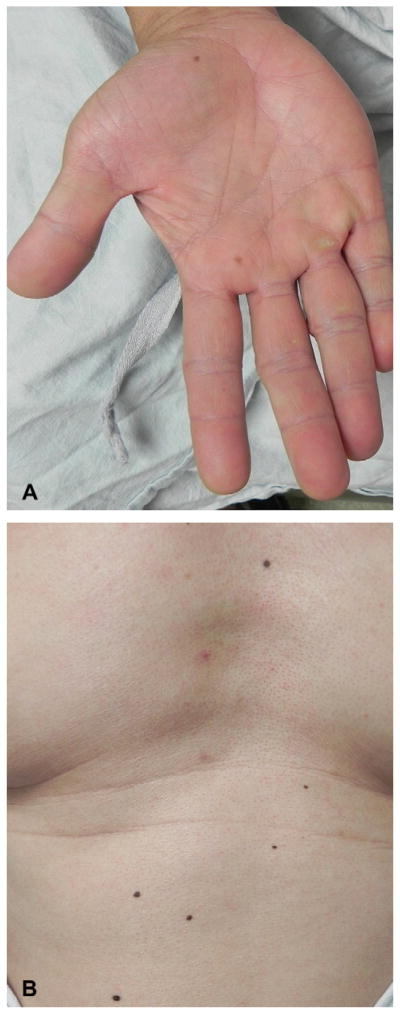
A 57-year-old Chinese man (patient 14, Table I) had a history of papillary thyroid cancer status-post total thyroidectomy, with involvement in his lymph nodes, jaw, and right parotid gland. He received but was resistant to radioactive iodine and was placed on a clinical trial of vemurafenib for his metastatic disease after confirmation of a BRAF V600E mutation. After 8 weeks of treatment, he had a 22% reduction of tumor burden on computed tomography scan. At the same time, he developed several new papules on his face. He also noticed new pigmented macules on his palms. He denied history of nonmelanoma skin cancers.
On physical examination, he had flesh-colored and slightly erythematous verrucous papules on the nose and left cheek (Fig 4, A). He was also noted to have two new brown macules on his palm (Fig 5, A), and one on the plantar aspect of his foot, on a background of new focal palmoplantar hyperkeratosis. He had multiple evenly pigmented nevi scattered on his trunk and arms (Fig 5, B), which had darkened after starting the medication. The darkening was marked, noted both by the patient and the clinician based on pretreatment and post-treatment evaluations. Histopathologic examination of a facial papule demonstrated hyperkeratosis, acanthosis, and papillomatosis without apparent koilocytic change, consistent with a verrucous keratosis (Fig 4, B). Biopsy specimen of a dark nevus on the trunk revealed a junctional dysplastic nevus with moderate atypia (not shown).
Fig 4.
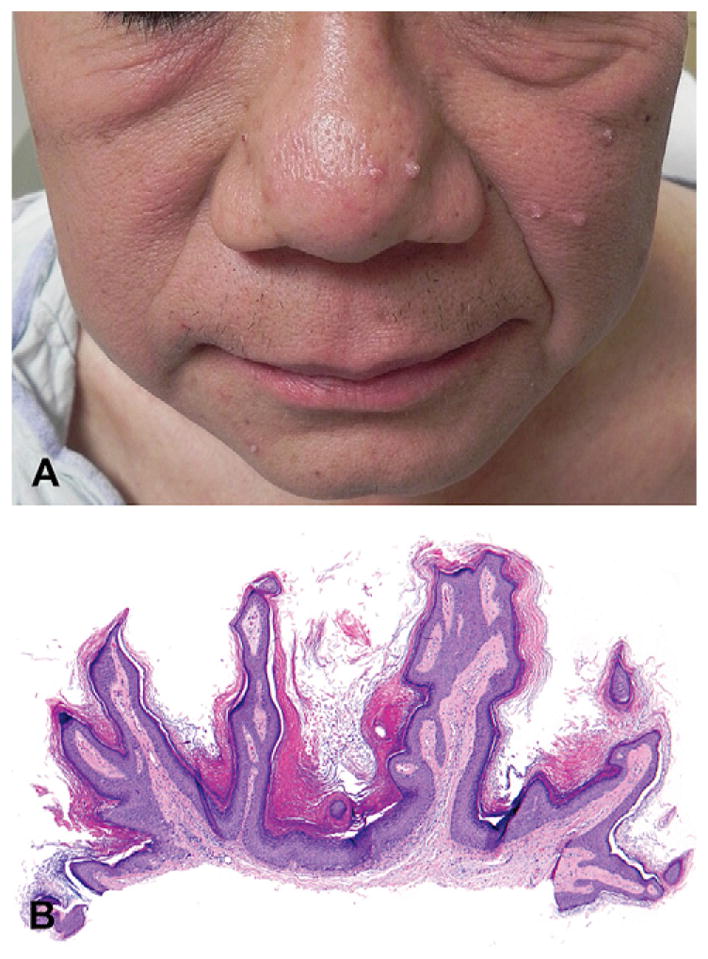
Verrucous keratosis (case 4). A, Multiple verrucous papules on face. B, Biopsy specimen of facial papule reveals hyperkeratosis, acanthosis, and papillomatosis in absence of viral changes, all features of verrucous keratosis. (Hematoxylin-eosin stain; original magnification: ×40.)
Fig 5.
Eruptive nevi and darkening nevi (case 4). A, New nevi on palm developing on treatment with vemurafenib, and focal palmar hyperkeratosis. B, Nevi on trunk, which darkened after initiation of vemurafenib.
DISCUSSION
Vemurafenib and dabrafenib commonly induce cutaneous reactions. Each of the 14 patients (13 with metastatic melanoma and one with metastatic thyroid cancer) reported here developed one or more skin side effects after initiation of vemurafenib or dabrafenib treatment, and 13 of the 14 exhibited at least two different types of skin reactions (Table I). Verrucous keratoses were most commonly observed, occurring in 12 of 14 patients. SCCs and acantholytic eruptions each appeared in 8 of the 14 patients (Table II). Patient 14, with metastatic papillary thyroid cancer, experienced darkening of his pre-existing nevi and eruption of several new nevi on acral sites.
Table II.
Frequency of lesions observed in patients on BRAF inhibitors
| Type of lesion | Total patients affected (%) | Distribution of lesions (% of total biopsied)
|
||
|---|---|---|---|---|
| Face | Extremities | Trunk | ||
| Verrucous keratosis | 12 (86) | 12 (63) | 2 (11) | 5 (28) |
| SCC | 8 (57) | 4 (19) | 13 (62) | 4 (19) |
| KA type | 4 (29) | 3 (27) | 6 (55) | 2 (18) |
| Acantholytic dyskeratosis | 8 (57) | 0 (0) | 4 (27) | 11 (73) |
| Eruptive nevi | 1 (7) | 0 (0) | 1 (7) | 0 (0) |
KA, Keratoacanthoma; SCC, squamous cell carcinoma.
Interestingly, only 3 of the 8 patients who developed a SCC on therapy had been given the diagnosis of a SCC before starting vemurafenib (patients 1, 2, and 7). Histologically, the SCCs observed in our patients were well-differentiated lesions. The SCCs biopsied ranged from in situ carcinoma, to invasive SCCs with a greatest thickness of 3.5 mm (data not shown). The thickest lesions extended to the mid to deep reticular dermis, and none demonstrated per-ineural invasion. All lesions occurred on a background of solar elastosis, with most exhibiting moderate sun damage (CSD 2, per the grading scheme devised by Landi et al10). In 4 of 8 patients with SCCs, at least one of their tumors exhibited features of a KA, with cup-shaped architecture and central keratin-filled crater. Whereas KAs classically demonstrate microabscesses composed of neutrophils and/or eosinophils within epithelial nests,11 these features were not observed in the biopsy specimens from this cohort.
Recent evidence suggests that SCCs and KAs arise specifically in the setting of RAF inhibitor therapy.12,13 Sorafenib, a multikinase inhibitor with pan-RAF activity, has been demonstrated in multiple reports to induce SCCs and KAs.14–24 Sorafenib has a much broader spectrum of action than vemurafenib and dabrafenib, exhibiting activity against vascular endothelial growth factor receptor 1, 2, and 3; platelet-derived growth factor receptor-β; FMS-like tyrosine kinase 3; c-kit; RET receptor tyrosine kinase; and all isoforms of RAF.25 Significantly, the multi-kinase inhibitor sunitinib, which targets many of the same kinases as sorafenib (vascular endothelial growth factor receptor 1, 2, and 3; platelet-derived growth factor receptor; c-kit; FMS-like tyrosine kinase 3; and RET tyrosine kinase) but not RAF, does not result in development of SCCs or KAs.13,26 Sorafenib is a nonselective inhibitor of wild-type and mutated forms of RAF, with poor activity against BRAF V600E tumor cells, and has been demonstrated to be an ineffective treatment for melanoma.7,27 An estimated 6% to 7% of patients treated with sorafenib develop SCCs and KAs, which stands in contrast to 20% to 30% of patients taking vemurafenib.3,4
The mechanism underlying development of SCCs in patients treated with RAF inhibitors is actively being investigated. Recent data suggest that pharmacologic RAF blockade in cells harboring wild-type BRAF paradoxically increases signaling through CRAF, which then increases MAPK signaling overall.28–30 Arnault et al31 examined normal-appearing skin biopsy specimens from patients treated with sorafenib, finding increased Ki67 and phosphorylated ERK staining in keratinocytes on histologic sections, compared with normal-appearing skin taken from placebo-treated patients. This suggests that MAPK signaling is in fact increased, presumably leading to increased keratinocyte proliferation. Paradoxical activation of MAPK signaling by itself may not be sufficient to induce SCCs and KAs. To this end, Oberholzer et al12 determined that RAS activating mutations are more frequently found in SCCs and KAs from patients treated with vemurafenib (30%) and sorafenib (11%) compared with those from control patients (3.2%). Pre-existing RAS mutations in keratinocytes (possibly induced by sun exposure or viral infection) may therefore receive a “second hit” via RAF inhibitor–driven paradoxical activation of MAPK signaling, which would then be sufficient for tumor development.
A large number of the patients reported here developed either single lesions or widespread eruptions with histopathologic findings of acantholytic dyskeratosis, consistent with warty dyskeratomas and Darier- or Grover-like rashes, respectively. To our knowledge, such findings have not been previously associated with RAF inhibitor therapy. Paradoxical activation of the MAPK pathway in normal keratinocytes may account for the findings of acantholytic dyskeratosis, as it has previously been shown that transfection of rat cardiac myocytes with constitutively active Ras and Raf leads to decreased expression of sarco/endoplasmic reticulum Ca2+-ATPase type 2 isoform (SERCA2), the protein deficient in Darier disease.32 It remains to be determined whether SERCA2 expression is decreased in the warty dyskeratomas and Darier-like eruptions observed in our patients.
Verrucous keratoses, which we define as benign wartlike growths without apparent viral cytopathic changes, were observed in nearly all cases presented here. HPV immunostaining was performed on several biopsy specimens that had architectural changes suggestive of true verrucae; however, in each case the staining was negative. Lacouture et al9 also describe the occurrence of wartlike proliferations arising in the setting of vemurafenib therapy. Intriguingly, cutaneous papillomas have been reported to occur in patients with Costello syndrome, which is most frequently caused by activating germline HRAS mutations,33,34 and in those with cardiofaciocutaneous (CFC) syndrome, in which activating germline mutations in BRAF, MEK1, MEK2, and KRAS are the most common underlying genetic alterations.35 Papillomas are more common in Costello syndrome, occurring most often on the nose and central aspect of the face.34 Histologically, the papillomas of Costello syndrome resemble the verrucous keratoses observed in our study, demonstrating verrucous epidermal hyperplasia without the koilocytes and clumped keratohyalin granules found in common verrucae.36 It is interesting to note that most of the verrucous keratoses observed in our study were biopsied from the face (63%).
Eruptive nevi have been described in patients treated with sorafenib, occurring on acral sites in one patient.37 The patient presented in case 4 not only developed new acral nevi, but also experienced darkening of pre-existing nevi. These findings again warrant comparison with the inherited CFC syndrome, in which a greater than average number of nevi is a characteristic finding. Nevi in patients with CFC syndrome are typically evenly distributed across all body sites, and individually are uniformly pigmented and medium to dark brown in color.35
Mild palmoplantar hyperkeratosis, accentuated at pressure points, affected the patient in case 4 after starting vemurafenib, and several other patients in this practice. Palmoplantar hyperkeratosis is a frequent side effect of sorafenib therapy, in the context of the hand-foot skin reaction.38 Drawing further parallels to Costello and CFC syndromes, focal palmoplantar hyperkeratosis appears in both conditions often, in 76% and 36% of patients, respectively.34
Clinical management of the keratotic lesions presents challenges. First, patients frequently present with such a high number of keratotic lesions that surgical management with excision may be impractical or intolerable. For example, our patient who had approximately 30 SCCs of the KA type (case 3) declined excision except for lesions that were either symptomatic or growing quickly. Second, clinical examination could not reliably distinguish benign from malignant lesions, therefore a low threshold for skin biopsy of new growths is recommended. Even under the microscope, distinction between benign and malignant lesions can be challenging. For example, one case demonstrated focal areas of transition to SCC in situ in what otherwise had the histologic appearance of a benign verrucous keratosis.
Practitioners may consider several surgical approaches to manage these keratotic growths. For lesions that are large, tender, growing rapidly, or located in critical anatomic locations, excision or Mohs micrographic surgery is indicated. For small and superficial lesions, destructive modalities such as curettage and electrodessication or cryosurgery may be sufficient. We have used cryotherapy for treatment of small SCCs in case 3, with good results. Before destruction, biopsy to confirm the diagnosis and remove the clinically visible lesion would be prudent.
In cases where surgical treatment is either impractical or undesirable, other strategies may be necessary. We have observed anecdotally regression of lesions after treatment with topical 5-fluorouracil (case 3). Reduction of vemurafenib dose is another potential management strategy, although this did not prevent development of new SCCs in case 3. Bexarotene was used with apparent success for treatment of KAs in a patient treated with sorafenib, raising the possibility that it and other systemic retinoids may be helpful for vemurafenib-associated SCCs and KAs.23 Another potential therapeutic modality is the use of a MEK inhibitor in combination with vemurafenib or dabrafenib, with the idea that the MEK inhibitor may block paradoxical MAPK signaling downstream of CRAF in keratinocytes.13 This is a particularly appealing possibility as combination therapy may also be useful in circumventing at least some forms of resistance to BRAF inhibitors that develop in melanomas.39–41 In a recent study, SCCs did not develop in any of 45 patients treated with combination BRAF and MEK inhibitor therapy.42 In our cohort, the two patients treated with dabrafenib and trametinib (patients 6 and 12) did not form SCCs, although they developed benign keratoses.
In summary, we have described several cutaneous side effects associated with selective BRAF inhibitor therapy for metastatic melanoma and thyroid cancer, many of which may be attributed to paradoxical activation the MAPK signaling pathway in nontumor cells. Further investigation will be necessary to elucidate the precise molecular mechanisms underlying these phenomena. Awareness on the part of dermatologists and oncologists of the potential side effects of selective BRAF inhibitors will become increasingly important as these agents are used more widely.
Acknowledgments
Supported by the Skin Disease Research Center at the University of Pennsylvania.
Abbreviations used
- CFC
cardiofaciocutaneous
- KA
keratoacanthoma
- MAPK
mitogen-activated protein kinase
- SCC
squamous cell carcinoma
Footnotes
Disclosure: Dr Amaravadi is a consultant for and has received honoraria from Genentech. Dr Fecher is an investigator for Roche, Genentech, and GlaxoSmithKline, and has received research funding from those sources. Dr Schuchter is an investigator for Roche and GlaxoSmithKline, and has received grant funding from both sources. Drs Chu, Wanat, Miller, Brose, Seykora, and Rosenbach, Ms McGettigan, and Ms Giles have no conflicts of interest to declare.
References
- 1.Flaherty KT, Yasothan U, Kirkpatrick P. Vemurafenib. Nat Rev Drug Discov. 2011;10:811–2. doi: 10.1038/nrd3579. [DOI] [PubMed] [Google Scholar]
- 2.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–9. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–14. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manousaridis I, Mavridou S, Goerdt S, Leverkus M, Utikal J. Cutaneous side effects of inhibitors of the RAS/RAF/MEK/ERK signalling pathway and their management. J Eur Acad Dermatol Venereol. doi: 10.1111/j.1468-3083.2012.04546.x. Published online April 28, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Ribas A, Flaherty KT. BRAF targeted therapy changes the treatment paradigm in melanoma. Nat Rev Clin Oncol. 2011;8:426–33. doi: 10.1038/nrclinonc.2011.69. [DOI] [PubMed] [Google Scholar]
- 8.Dummer R, Rinderknecht J, Goldinger SM. Ultraviolet A and photosensitivity during vemurafenib therapy. N Engl J Med. 2012;366:480–1. doi: 10.1056/NEJMc1113752. [DOI] [PubMed] [Google Scholar]
- 9.Lacouture ME, O’Reilly K, Rosen N, Solit DB. Induction of cutaneous squamous cell carcinomas by RAF inhibitors: cause for concern? J Clin Oncol. 2012;30:329–30. doi: 10.1200/JCO.2011.38.2895. [DOI] [PubMed] [Google Scholar]
- 10.Landi MT, Bauer J, Pfeiffer RM, Elder DE, Hulley B, Minghetti P, et al. MC1R germline variants confer risk for BRAF-mutant melanoma. Science. 2006;313:521–2. doi: 10.1126/science.1127515. [DOI] [PubMed] [Google Scholar]
- 11.Stoll DM, Ackerman AB. Subungual keratoacanthoma. Am J Dermatopathol. 1980;2:265–71. doi: 10.1097/00000372-198000230-00017. [DOI] [PubMed] [Google Scholar]
- 12.Oberholzer PA, Kee D, Dziunycz P, Sucker A, Kamsukom N, Jones R, et al. RAS mutations are associated with the development of cutaneous squamous cell tumors in patients treated with RAF inhibitors. J Clin Oncol. 2012;30:316–21. doi: 10.1200/JCO.2011.36.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robert C, Arnault JP, Mateus C. RAF inhibition and induction of cutaneous squamous cell carcinoma. Curr Opin Oncol. 2011;23:177–82. doi: 10.1097/CCO.0b013e3283436e8c. [DOI] [PubMed] [Google Scholar]
- 14.Adnot-Desanlis L, Bernard P, Reguiai Z. Squamous cell carcinoma in a patient receiving sorafenib [in French] Ann Dermatol Venereol. 2011;138:120–3. doi: 10.1016/j.annder.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 15.Arnault JP, Wechsler J, Escudier B, Spatz A, Tomasic G, Sibaud V, et al. Keratoacanthomas and squamous cell carcinomas in patients receiving sorafenib. J Clin Oncol. 2009;27:e59–61. doi: 10.1200/JCO.2009.23.4823. [DOI] [PubMed] [Google Scholar]
- 16.Donaldson MR, Stetson CL, Smith JL. Invasive squamous cell carcinoma and sorafenib in a black patient. Arch Dermatol. 2011;147:133–4. doi: 10.1001/archdermatol.2010.395. [DOI] [PubMed] [Google Scholar]
- 17.Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20–3. doi: 10.3816/CGC.2009.n.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong DS, Reddy SB, Prieto VG, Wright JJ, Tannir NM, Cohen PR, et al. Multiple squamous cell carcinomas of the skin after therapy with sorafenib combined with tipifarnib. Arch Dermatol. 2008;144:779–82. doi: 10.1001/archderm.144.6.779. [DOI] [PubMed] [Google Scholar]
- 19.Jantzem H, Dupre-Goetghebeur D, Spindler P, Merrer J. Sorafenib-induced multiple eruptive keratoacanthomas [in French] Ann Dermatol Venereol. 2009;136:894–7. doi: 10.1016/j.annder.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Kong HH, Cowen EW, Azad NS, Dahut W, Gutierrez M, Turner ML. Keratoacanthomas associated with sorafenib therapy. J Am Acad Dermatol. 2007;56:171–2. doi: 10.1016/j.jaad.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon EJ, Kish LS, Jaworsky C. The histologic spectrum of epithelial neoplasms induced by sorafenib. J Am Acad Dermatol. 2009;61:522–7. doi: 10.1016/j.jaad.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 22.Lynch MC, Straub R, Adams DR. Eruptive squamous cell carcinomas with keratoacanthoma-like features in a patient treated with sorafenib. J Drugs Dermatol. 2011;10:308–10. [PubMed] [Google Scholar]
- 23.Marquez CB, Smithberger EE, Bair SM, Wenham RM, Fenske NA, Glass LF, et al. Multiple keratoacanthomas arising in the setting of sorafenib therapy: novel chemoprophylaxis with bexarotene. Cancer Control. 2009;16:66–9. doi: 10.1177/107327480901600110. [DOI] [PubMed] [Google Scholar]
- 24.Smith KJ, Haley H, Hamza S, Skelton HG. Eruptive keratoacanthoma-type squamous cell carcinomas in patients taking sorafenib for the treatment of solid tumors. Dermatol Surg. 2009;35:1766–70. doi: 10.1111/j.1524-4725.2009.01289.x. [DOI] [PubMed] [Google Scholar]
- 25.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 26.Grimaldi AM, Guida T, D’Attino R, Perrotta E, Otero M, Masala A, et al. Sunitinib: bridging present and future cancer treatment. Ann Oncol. 2007;18(Suppl):vi31–4. doi: 10.1093/annonc/mdm221. [DOI] [PubMed] [Google Scholar]
- 27.Eisen T, Ahmad T, Flaherty KT, Gore M, Kaye S, Marais R, et al. Sorafenib in advanced melanoma: a phase II randomized discontinuation trial analysis. Br J Cancer. 2006;95:581–6. doi: 10.1038/sj.bjc.6603291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–5. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 29.Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–21. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signaling in cells with wild-type BRAF. Nature. 2010;464:427–30. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnault JP, Mateus C, Escudier B, Tomasic G, Wechsler J, Hollville E, et al. Skin tumors induced by sorafenib; paradoxical RAS-RAF pathway activation and oncogenic mutations of HRAS, TP53 and TGFBR1. Clin Cancer Res. 2012;18:263–72. doi: 10.1158/1078-0432.CCR-11-1344. [DOI] [PubMed] [Google Scholar]
- 32.Ho PD, Zechner DK, He H, Dillmann WH, Glembotski CC, McDonough PM. The Raf-MEK-ERK cascade represents a common pathway for alteration of intracellular calcium by Ras and protein kinase C in cardiac myocytes. J Biol Chem. 1998;273:21730–5. doi: 10.1074/jbc.273.34.21730. [DOI] [PubMed] [Google Scholar]
- 33.Rauen KA. HRAS and the Costello syndrome. Clin Genet. 2007;71:101–8. doi: 10.1111/j.1399-0004.2007.00743.x. [DOI] [PubMed] [Google Scholar]
- 34.Siegel DH, Mann JA, Krol AL, Rauen KA. Dermatological phenotype in Costello syndrome: consequences of Ras dysregulation in development. Br J Dermatol. 2012;166:601–7. doi: 10.1111/j.1365-2133.2011.10744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegel DH, McKenzie J, Frieden IJ, Rauen KA. Dermatological findings in 61 mutation-positive individuals with cardiofacio-cutaneous syndrome. Br J Dermatol. 2011;164:521–9. doi: 10.1111/j.1365-2133.2010.10122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez ME, Blanco FP, Garzon MC. Verrucous papules and plaques in a pediatric patient: cutaneous papillomas associated with Costello syndrome. Arch Dermatol. 2007;143:1201–6. doi: 10.1001/archderm.143.9.1201-b. [DOI] [PubMed] [Google Scholar]
- 37.Kong HH, Sibaud V, Chanco Turner ML, Fojo T, Hornyak TJ, Chevreau C. Sorafenib-induced eruptive melanocytic lesions. Arch Dermatol. 2008;144:820–2. doi: 10.1001/archderm.144.6.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robert C, Mateus C, Spatz A, Wechsler J, Escudier B. Dermatologic symptoms associated with the multikinase inhibitor sorafenib. J Am Acad Dermatol. 2009;60:299–305. doi: 10.1016/j.jaad.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 39.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS up-regulation. Nature. 2010;468:973–7. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nissan MH, Solit DB. The “SWOT” of BRAF inhibition in melanoma: RAF inhibitors, MEK inhibitors or both? Curr Oncol Rep. 2011;13:479–87. doi: 10.1007/s11912-011-0198-4. [DOI] [PubMed] [Google Scholar]
- 41.Poulikakos PI, Persaud Y, Janakiraman M, Kong X, Ng C, Moriceau G, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480:387–90. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Infante JR, Falchook GS, Lawrence DP, Weber JS, Kefford RF, Bendell JC, et al. Phase I/II study to assess safety, pharmacokinetics, and efficacy of the oral MEK 1/2 inhibitor GSK1120212 (GSK212) dosed in combination with the oral BRAF inhibitor GSK2118436 (GSK436). Presented at: 2011 ASCO Annual Meeting; Chicago, IL. [Google Scholar]