Abstract
IMPORTANCE
Little is known of glutamic acid decarboxylase antibodies (GAD-abs) in the paraneoplastic context. Clinical recognition of such cases will lead to prompt tumor diagnosis and appropriate treatment.
OBJECTIVE
To report the clinical and immunological features of patients with paraneoplastic neurological syndromes (PNS) and GAD-abs.
DESIGN, SETTING, AND PARTICIPANTS
Retrospective case series study and immunological investigations conducted in February 2014 in a center for autoimmune neurological disorders. Fifteen cases with GAD65-abs evaluated between 1995 and 2013 who fulfilled criteria of definite or possible PNS without concomitant onconeural antibodies were included in this study.
MAIN OUTCOMES AND MEASURES
Analysis of the clinical records of 15 patients and review of 19 previously reported cases. Indirect immunofluorescence with rat hippocampal neuronal cultures and cell-based assays with known neuronal cell-surface antigens were used. One hundred six patients with GAD65-abs and no cancer served as control individuals.
RESULTS
Eight of the 15 patients with cancer presented as classic paraneoplastic syndromes (5 limbic encephalitis, 1 paraneoplastic encephalomyelitis, 1 paraneoplastic cerebellar degeneration, and 1 opsoclonus-myoclonus syndrome). When compared with the 106 non-PNS cases, those with PNS were older (median age, 60 years vs 48 years; P = .03), more frequently male (60% vs 13%; P < .001), and had more often coexisting neuronal cell-surface antibodies, mainly against γ-aminobutyric acid receptors (53%vs 11%; P < .001). The tumors more frequently involved were lung (n = 6) and thymic neoplasms (n = 4). The risk for an underlying tumor was higher if the presentation was a classic PNS, if it was different from stiff-person syndrome or cerebellar ataxia (odds ratio, 10.5; 95%CI, 3.2–34.5), or if the patient had coexisting neuronal cell-surface antibodies (odds ratio, 6.8; 95%CI, 1.1–40.5). Compared with the current series, the 19 previously reported cases had more frequent stiff-person syndrome (74%vs 13%; P = .001) and better responses to treatment (79% vs 27%; P = .005). Predictors of improvement in the 34 patients (current and previously reported) included presentation with stiff-person syndrome and the presence of a thymic tumor.
CONCLUSIONS AND RELEVANCE
Patients with GAD-abs must be screened for an underlying cancer if they have clinical presentations different from those typically associated with this autoimmunity or develop classic PNS. The risk for cancer increases with age, male sex, and the presence of coexisting neuronal cell-surface antibodies.
High serum levels of antibodies to the synaptic enzyme glutamic acid decarboxylase (GAD-abs) is a very sensitive biomarker of stiff-person syndrome (SPS) and have also been described in subgroups of patients with limbic encephalitis (LE),1 cerebellar ataxia,2 epilepsy, and isolated cases of palatal tremor, as well as downbeat or periodic alternating nystagmus.3 Patients with neurological syndromes associated with GAD-abs are not considered at risk for cancer and extensive search for a tumor is not indicated unless they harbor additional onconeural antibodies. However, there are case reports of patients with GAD-abs whose cancer was identified by the time of the neurological diagnosis, suggesting a paraneoplastic mechanism.4,5 Whether these cases represent a casual association or a true GAD-ab–associated paraneoplastic neurological syndrome (PNS) is unclear.
The discovery of antibodies against neuronal cell-surface receptors and synaptic antigens in patients with encephalitis adds complexity to the study of GAD-ab–associated neurological syndromes. Patients with LE may have coexistent GAD-abs and antibodies against the γ-aminobutyric acid (GABA) b receptor, and this association seems more frequent in patients with cancer.6 A systematic determination of neuronal cell-surface antibodies has not been done in patients with GAD-abs and suspected PNS.
In this study, we retrospectively examined a cohort of patients with clinical criteria of definite or possible PNS but without onconeural antibodies in whom GAD-abs were identified during investigations for a paraneoplastic etiology. In addition, we performed a systematic review of previously reported cases of GAD-ab–associated PNS. The aims of this study were to describe the PNS and tumor types associated with GAD-abs, the occurrence of additional neuronal cell-surface antibodies, and the neurological response to cancer treatment and immunotherapy, as well as to provide the more frequent GAD-abs clinical settings in which a tumor screening is warranted.
Methods
Patients
In February 2014, we retrospectively identified patients examined between 1995 and 2013 with definite or possible diagnosis of PNS according to the PNS Euronetwork criteria,7 whose serum samples were sent to our laboratory for the determination of onconeural antibodies but routine immunohistochemistry on paraformaldehyde-perfused brain tissue revealed GAD-ab reactivity (a positive brain tissue serum reactivity indicates high GAD-ab levels, usually >2000 U/mL when determined by radioimmunoassay).3 In all samples with evidence of GAD-ab reactivity, the presence of GAD-abs was subsequently confirmed by radioimmunoassay. All patients were seen by at least 1 of the authors. Basic clinical information was obtained from medical records and additional information was collected through a structured questionnaire focused on symptom presentation, type of tumor, and response to immunotherapy and tumor treatment. Neurological disability was measured by the modified Rankin Scale8; a change of 1 point was required to define improvement or symptom progression.
To compare the findings of our patients with those of previously reported cases, we performed a comprehensive PubMed search using the terms GAD antibodies AND cancer and identified all cases until January 1, 2015. Only cases published in English that included clinical information were included. Articles were also identified by searches of the authors’ files.
To define the frequency of a paraneoplastic etiology among different GAD-ab–associated neurological syndromes, we searched in our database for all cases diagnosed as having GAD-ab–associated neurological syndromes without cancer during the same period as the PNS cases were diagnosed. In total, 106 patients were identified, including 39 with cerebellar ataxia, 32 with SPS, 18 with isolated epilepsy, and 17 with LE (eFigure in the Supplement).
Patients’ serum and cerebrospinal fluid (CSF) samples are deposited in the collection of biological samples named Neuroinmunología registered in the Biobank of Institut d’Investigacions Biomediques August Pi i Sunyer (IDIBAPS). Written informed consent for the storage and use of the samples for research purposes was obtained from all patients. The study was approved by the ethics committee of the Hospital Clinic of Barcelona, Barcelona, Spain.
Autoantibody Assays
Levels of GAD65-abs were detected by enzyme-linked immunosorbent assay (RSR Limited) using a commercial kit following the manufacturer’s specifications. Because GAD65 titers in neurologic syndromes are high, serum and CSF samples were titrated to determine the optimal dilution factor. Briefly, enzyme-linked immunosorbent assay wells were seeded for 1 hour with patients’ serum samples diluted 1:10 000 or CSF diluted 1:200, followed by 1-hour incubation with GAD65 biotinylated protein, and 20-minute incubation with streptavidin peroxidase. In addition, serum and CSF samples were tested for antibodies to intracellular and neuronal cell-surface antigens using brain immunohistochemistry, as previously reported.9,10 Onconeural antibodies to Hu, Yo, Ri, CV2, amphiphysin, and Ma1/2 were determined with immunoblot assays and GAD67, gephyrin (cotransfected 1:1 with collybistin), and neuronal cell-surface antibodies were investigated using in-house cell-based assays, including leucine-rich, glioma-inactivated 1, contactin-associated protein-like 2, GluN1/2B subunits of theN-methyl-D-aspartate receptor, GluR1 and 2 subunits of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate, B1 and B2 subunits of the GABAbR, α1 and β3 subunits of the GABAaR, and the α1 subunit of the glycine receptor (GlyR), as reported.11 All DNA sequences were purchased from Origene except from those of GlyR, gephyrin, and collybistin (a gift of R. J. Harvey, PhD, Department of Pharmacology, University College London School of Pharmacy, London, England).
To demonstrate the expression of GAD65 and GAD67 in the tumor, paraffin sections were deparaffinized and the antigen retrieved, as reported.4 After inhibition of endogenous peroxidase with 0.3% hydrogen peroxide in phosphate-buffered saline for 15 minutes, sections were incubated with GAD65 (Hybrioma Bank) or GAD67 (Abcam) monoclonal antibodies (diluted 1:1000) overnight at 4°C, and developed with the avidin-biotin peroxidase technique (Vector Laboratories).
Statistical Analysis
The Fisher exact test was used to assess proportions when the expected frequencies were small (<5). For multivariate analysis of the probability of paraneoplastic origin in the present series, a logistic regression, including factors with a P value of .10 or less in the univariate analysis, was used. P ≤ .05 was considered statistically significant. The software used was Stata version 13.1 (StataCorp).
Results
Fifteen patients with PNS and GAD-abs were identified. None of them had onconeural antibodies (Table 1). Eight of them fulfilled the criteria of definite PNS and 7 of possible PNS. The mean age was 60 years (range, 29–80 years) and 6 (40%) were women. The most frequent clinical syndromes included encephalitis (6 patients; 5 of them had typical LE7) and cerebellar ataxia (4 patients; 1 of them had been previously reported).5 One patient fulfilled criteria of paraneoplastic cerebellar degeneration,7 whereas the remaining 3 had a slowly progressive course of the disease more suggestive of degenerative ataxia. Two additional patients developed SPS, 1 opsoclonus-myoclonus syndrome, 1 paraneoplastic encephalomyelitis (previously reported4), and 1 a syndrome that included vertigo, ataxia, axial rigidity, and dysautonomia (eTable 1 in the Supplement).
Table 1.
Clinical and Immunological Features of Patients With and Without PNS With GAD Antibodies
| Characteristic | PNS (n = 15) |
Non-PNS (n = 106) |
P Value |
|---|---|---|---|
| Age, mean (range), y | 60 (29–80) | 48 (5–79) | .03 |
| Female, No. (%) | 6 (40) | 88/102 (87) | <.001 |
| Diabetes mellitus at onset, No./total No. (%) | 2/13 (15) | 31/74 (42) | .12 |
| Other organ-specific autoimmune disorder, No./total No. (%) | 4/13 (31) | 43/75 (57) | .13 |
| Clinical syndrome | |||
| Encephalitis (LE, n = 5)a | 6 (26)b | 17 | <.001 |
| Cerebellar ataxia (PCD, n = 1)a | 4 (9)b | 39 | |
| Stiff-person syndrome | 2 (6)b | 32 | |
| Opsoclonus-myoclonus syndromea | 1 (100)b | 0 | |
| PEMa | 1 (100)b | 0 | |
| Otherc | 1 (100)b | 0 | |
| Isolated epilepsy | 0 (0)b | 18 | |
| GAD65-ab titer, median (IQR) | |||
| Serum, ×105 U/mL | 10.5 (1.2–31.9) | 5.9 (2.9–13.2) | .79 |
| CSF, ×103 U/mL | 3.5 (1.2–57.1) | 7.5 (1.7–17.2) | .86 |
| GAD67-ab, No./total No. (%) | |||
| Serum | 8/13 (62) | 93/106 (88) | .03 |
| CSF | 4/7 (57) | 61/61 (100) | <.001 |
| Neuronal cell-surface antibodies, No./total No. (%) | 8/15 (53)d | 12/106 (11)e | <.001 |
Abbreviations: CSF, cerebrospinal fluid; GAD, glutamic acid decarboxylase; IQR, interquartile range; LE, limbic encephalitis; PCD, paraneoplastic cerebellar degeneration; PEM, paraneoplastic encephalomyelitis; PNS, paraneoplastic neurological syndromes.
Classic PNS.
Percentage of patients in whom the syndrome was paraneoplastic.
Axial rigidity, vertigo, and dysautonomia (see the Results section).
γ-Aminobutyric acid bR antibody (n = 3), γ-aminobutyric acid aR antibody (n = 2), glycine receptor antibody (n = 1), and unknown antigen (n = 2).
γ-Aminobutyric acid aR antibody (n = 6) and glycine receptor antibody (n = 6).
Six patients had lung cancer (4 of them small-cell lung cancer [SCLC]), 4 had neuroendocrine tumors (2 pancreas and 2 thymic carcinoids), 2 had thymoma, 2 had breast cancer, and 1 had non-Hodgkin lymphoma. The neurological syndrome antedated the diagnosis of the cancer in 10 patients and led to the diagnosis of a tumor relapse after 10 years of remission in another patient. The median delay between the diagnosis of the neurological syndrome and cancer was 2.7 months (interquartile range, 1.2–4.5 months).
All patients received immunotherapy: 11 high-dose corticosteroids, associated with intravenous immunoglobulins in 6; 2 with isolated intravenous immunoglobulins; 1 with intravenous immunoglobulins combined with rituximab; and 1 with intravenous immunoglobulins followed by cyclophosphamide. In addition, 10 patients (71%) had oncological treatment (surgery, chemotherapy, and radiotherapy alone or combined). Clinical follow-up was available for all 15 patients: 8 (53%) had clinically progressed (5 had died owing to PNS); 4 were stable; and 3 had neurological improvement (all 3 with neoplasms of the thymus: 2 thymomas and 1 thymic carcinoid) (eTable 1 in the Supplement).
Immunological Studies
Serum and CSF GAD65-ab levels were similar in patients with or without PNS. The frequency of GAD67-abs was significantly lower in patients with PNS, particularly in CSF (Table 1). Gephyrin antibodies were not detected. Neuronal cell-surface antibodies were detected in 8 of 15 patients (53%). The antibodies, type of associated syndrome, and tumor were as follows: 3 patients with GABAbR antibodies (2 LE with SCLC and 1 paraneoplastic cerebellar degeneration with thymic carcinoma), 2 patients with GABAaR antibodies (2 LE with SCLC and thymoma), 1 patient with GlyR antibodies (cerebellar ataxia and thymic carcinoma), and 2 patients with antibodies against unknown neuronal cell-surface antigens (2 epidermoid lung cancer with opsoclonus-myoclonus syndrome and ataxia and myoclonus).
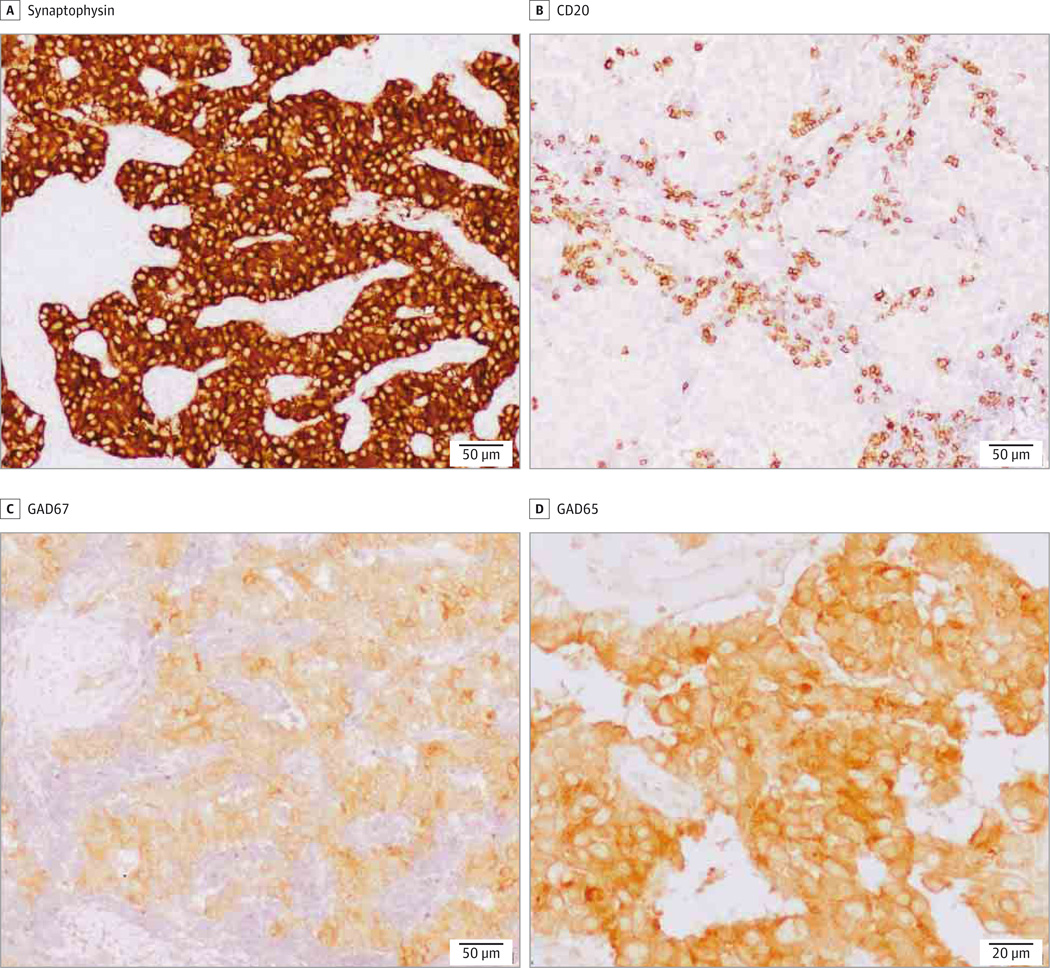
Tumor expression of GAD was assessed in 3 patients, 2 of them were previously reported.4,5 The 3 cases had a neuroendocrine tumor (2 pancreatic and 1 thymic carcinoma) that were found to express GAD65. Only one tumor was tested for expression of GAD67 and it was found to be positive (Figure 1).
Figure 1. Glutamic Acid Decarboxylase (GAD) Reactivity in a Pancreatic Tumor Sample.
Immunohistochemistry (of patient 9 in eTable 1 in the Supplement) revealed the tumoral expression of synaptophysin (A) and both isoforms of GAD (C and D). Contiguous section of the tumor immunostained with a monoclonal antibody against CD20 (B) shows infiltrates of B lymphocytes.
Comparison With Patients With GAD-abs Without Cancer
Compared with the 106 patients with nonparaneoplastic GAD-ab–associated disorders, patients with PNS were older (median age, 60 years vs 48 years; P = .03), were more frequently male (60% vs 13%; P < .001), had more often coexistent neuronal cell-surface autoantibodies (53% vs 11%; P < .001), and presented a different clinical profile (more classic PNS than SPS or cerebellar ataxia) (Table 1). Taking into account the clinical presentation, patients presenting with a syndrome different from SPS, cerebellar ataxia, or isolated epilepsy had a 10-fold increased risk for being paraneoplastic (odds ratio, 10.5; 95% CI, 3.2–34.5). Similarly, the detection of neuronal cell-surface antibodies carried a 7-fold increased risk for the presence of an underlying tumor (odds ratio, 6.8; 95%CI, 1.1–40.5). In a multivariate analysis that included age, sex, and the presence of neuronal cell-surface antibodies, the clinical presentation remained the most robust predictor of a paraneoplastic origin (odds ratio, 33.2; 95% CI, 5.0–220.2).
Comparison With Previously Reported GAD-ab–Associated PNS
A literature search identified 23 patients with PNS and isolated GAD-abs. Three patients12–14 were excluded from analysis because the period between the development of PNS and tumor diagnosis was unknown or longer than 2 years.7 Another patient was excluded because the neurological syndrome (SPS) occurred after autologous bone marrow transplantation for multiple myeloma, raising the possibility of an abnormal immune reconstitution rather than PNS as the cause of SPS.15 The clinical information of the remaining 19 patients is summarized in eTable 2 in the Supplement.16–34
Among these 19 patients, 53% were female. Stiff-person syndrome was the most common neurological syndrome (74%), with a remarkable frequency of focal forms (36%). By contrast, none of the patients developed LE, which was the most prevalent syndrome in the present series. The distribution of associated tumors was also different. Thymic tumors were the most frequent neoplasm (6 patients), followed by lung cancer (4 patients) and breast cancer (4 patients). Compared with the present series, the previously reported cases appeared to have better outcomes (79% vs 27%;P = .005;Table 2).
Table 2.
Clinical Features and Tumor Associations in Patients With PNS With GAD-abs From the Present Study and Previously Reported Cases16–34
| Feature | No. (%) | P Value | |
|---|---|---|---|
| Present Series (n = 15) |
Literature Review (n = 19) |
||
| Definite PNS | 8 | 4 | .05 |
| Age, mean (range), y | 60 (29–80) | 56 (31–85) | .43 |
| Sex | |||
| Male | 9 | 9 | .46 |
| Female | 6 | 10 | |
| Autoimmune diseasesa | 6 | 5 | .39 |
| PNS first, mo | 11 | 17 | .18 |
| Median delay to tumor diagnosis (IQR), mo | 2.7 (1.2–4.5) | 1 (1–3.9) | |
| Clinical syndrome | |||
| SPS (SLS) | 2 (0) | 14 (5) | .001 |
| Encephalitis (LE) | 6 (5) | 0 | |
| OMS | 1 | 2 | |
| CA (PCD) | 4 (1) | 1 | |
| Other | 2 (PEM and brainstem) | 2 (PEM and PERM) | |
| Tumors | |||
| Lung (SCLC) | 6 (4) | 4 (3) (Mesothelioma, n = 1) | .73 |
| Thymoma (malignant) | 4 (2) | 6 (1) | |
| Breast | 2 | 4 | |
| Hematological | 1 (NHL) | 3 (MM, NHL, and HD) | |
| Other | 2 (Pancreas) | 2 (Kidney and cavum) | |
| Clinical outcome | |||
| Improved | 4 | 15 | .005 |
| Stable | 3 | 0 | |
| Worse (death) | 8 (5) | 4 (4) | |
Abbreviations: CA, cerebellar ataxia; GAD-abs, glutamic acid decarboxylase antibodies; HD, Hodgkin disease; IQR, interquartile range; LE, limbic encephalitis; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; OMS, opsoclonus-myoclonus syndrome; PCD, paraneoplastic cerebellar degeneration; PEM, paraneoplastic encephalomyelitis; PERM, progressive encephalomyelitis, rigidity, and myoclonus; PNS, paraneoplastic neurological syndrome; SCLC, small-cell lung cancer; SLS, stiff-limb syndrome; SPS, stiff-person syndrome.
Type 1 diabetes mellitus, thyroiditis, or myasthenia.
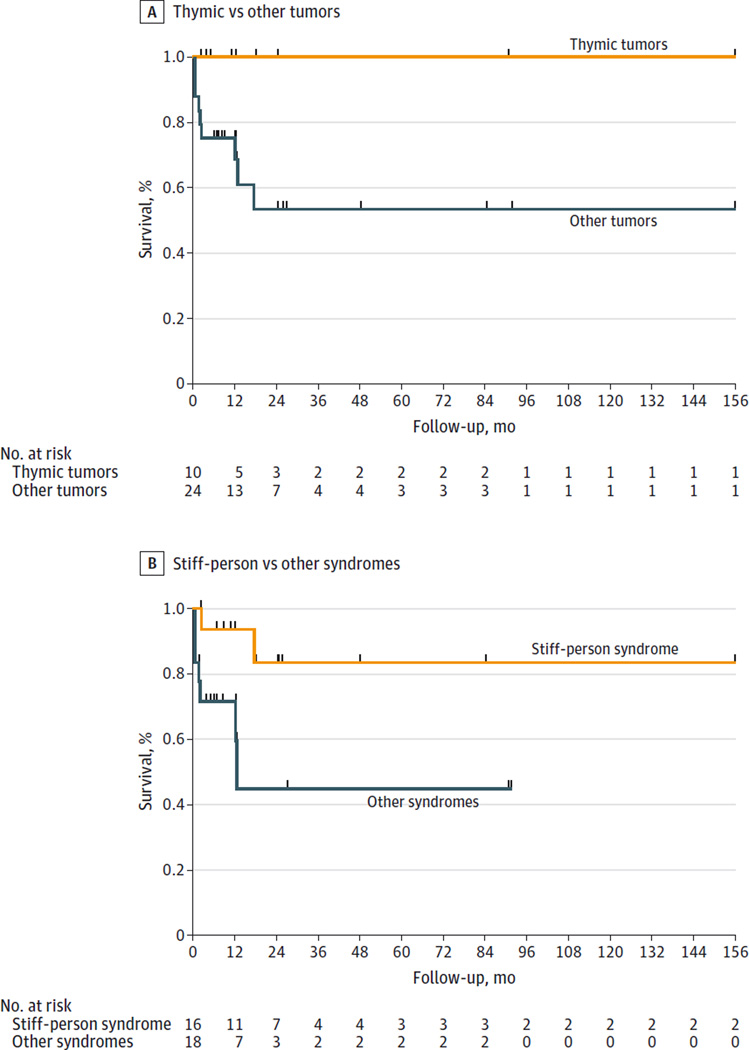
When considering together the 15 current PNS cases and the 19 previously reported, the probability of clinical improvement was greater in patients with thymic tumors, either benign or malignant (100% vs 38% other tumors; P = .001) and in those with SPS (75% vs 39% other syndromes; P = .03). Survival curves are shown in Figure 2.
Figure 2. Kaplan-Meyer Survival Curves of 34 Patients With Paraneoplastic Glutamic Acid Decarboxylase Syndromes by Tumor and Neurological Syndrome.
A, Survival curves for 10 patients with thymic tumors compared with 24 with other tumor types. B, Survival curves for 16 patients with stiff-person syndrome and 18 with other neurological syndromes.
Discussion
In some neurological syndromes with antibodies different from those strongly associated with cancer (onconeural antibodies), the immune response can be occasionally triggered by an underlying tumor and the syndrome is considered paraneoplastic. An example is LE, which, when it is associated with GABAbR-abs, may be idiopathic (autoimmune) or caused by an immune response against a tumor, usually SCLC, that expresses GABAbR.6,11 Patients with SPS, cerebellar ataxia, or other neurological syndromes that typically associate with GAD-abs rarely have cancer and therefore an aggressive or repeated tumor search is not indicated. However, our study showed that when GAD-abs occur in patients with LE or other classic PNS (paraneoplastic cerebellar degeneration, opsoclonus-myoclonus syndrome, or paraneoplastic encephalomyelitis)7 the risk for cancer is 10-fold higher than that in patients with SPS or cerebellar ataxia and the workup for a tumor is mandatory. The role of the tumor as a trigger of the immune response is supported by the demonstration of GAD65 in the tumors of these patients.4,5 The higher frequency of classic PNS in our series compared with the predominance of SPS in previously published cases probably reflects the fact that our laboratory receives samples from patients with a wide spectrum of neurological syndromes, not only from those typically related to GAD-abs.
A second important observation of our study was that the probability of an underlying cancer was 7 times higher in patients with GAD-abs and coexisting antibodies against neuronal cell-surface antigens. Therefore, the determination of these antibodies in patients with GAD-abs is indicated particularly in cases of LE or cerebellar ataxia. The neuronal cell-surface antibodies predominantly identified were against GABA receptors. γ-Aminobutyric acid bR antibodies usually occur in patients with LE and 58% of patients with these antibodies associate with cancer, mainly SCLC.35,36 γ-Aminobutyric acid aR and GlyR antibodies have been reported in a few patients with thymoma.37,38 However, it is important to keep in mind that with the exception of GABAbR-abs that are often detected in GAD-ab patients with cancer,6 the antibodies against GABAaR or GlyR are more frequently found in nonparaneoplastic patients with GAD-abs.39
The analysis of our series and the cases previously reported identified 3 subgroups of patients in whom GAD-ab–associated syndromes can be paraneoplastic. The first group included patients with classic PNS or neurological syndromes not usually associated with GAD-abs (38%; 13 of 34 patients). These patients had additional neuronal cell-surface antibodies (46%) and lung cancer was the most frequent tumor (46%), whereas thymomas were rare (15%); only 42% of patients in this group improved with treatment. Limbic encephalitis was the most frequent PNS. It is usually considered that LE associated with GAD-abs often occurs in young women with a predominant or isolated epileptic syndrome and it is not paraneoplastic.1 However, our study indicated that patients with LE and GAD-abs may have a tumor, usually an SCLC, and that this possibility is higher if LE occurs in older men.
The second group included patients with SPS and represented 47% (16 of 34) of all cases. Unlike patients of the first group, thymomas and breast cancer accounted for 53% of the tumors and 75% responded to therapy. Although patients with classic PNS and GAD-abs are more likely to be men, this is not the case in the subgroup of paraneoplastic SPS, where 69% of patients were women. The association of SPS with thymoma probably reflects the propensity of this tumor to induce a variety of autoimmune disorders and circulating autoantibodies.40,41 There is no evidence that thymoma cells express GAD65 or GAD67; therefore, the possible pathogenic mechanisms remain unclear. Patients with SPS and breast cancer usually harbor amphiphysin antibodies rather than GAD-abs.42,43 Some of the patients with SPS reported with GAD-abs and breast cancer also had type 1 diabetes mellitus and other organ-specific autoimmunities commonly seen in idiopathic SPS, therefore, the diagnosis of the breast cancer, a very frequent tumor, could be a coincidence. On the other hand, GAD65 is expressed in breast cancer cells and the GABAbR pathway is implicated in breast cancer cell invasion and migration.44 This observation supports the possibility that GAD may act as a tumor antigen and thus trigger, in some patients, an immune response leading to the development of SPS.
The third group included patients with cerebellar ataxia or progressive encephalomyelitis with rigidity and myoclonus that is usually associated with GAD-abs without cancer (15%; 5 of 34 patients). The most frequent tumors were lung and breast cancer, and 2 of the 5 patients improved. These patients, as those with classic PNS, were more frequently men (80%).
A previous study on patients who underwent extensive paraneoplastic screening, including also GAD autoimmunity, identified 62 patients with GAD-abs, none of them with cancer.45 Our experience also indicated that paraneoplastic GAD autoimmunity is infrequent2,3 but this possibility cannot be overlooked.
Conclusions
Patients with high levels of GAD-abs (in our setting >2000 U/mL by radioimmunoassay) and classic PNS or neurological syndromes not typically associated with GAD-abs should be screened for an underlying cancer. Considering the tumors identified in the current series and previous reported cases, the tumor workup should include mammogram and chest computed tomography or computed tomography–positron emission tomographic scan, depending on the clinical setting. The cancer risk increases with age, male sex, and presence of concomitant antibodies against neuronal cell-surface antigens.
Supplementary Material
Acknowledgments
Dr Dalmau has a research grant from Euroimmun and receives royalties from patents for the use of Ma2 and N-methyl-D-aspartate receptor as autoantibody tests.
Funding/Support: This work was supported by National Institutes of Health grants RO1NS077851 and RO1MH094741 (Dr Domingo); grants 11/01780 (Dr Domingo) and 12/00611 (Dr Graus) from the Fondo Investigaciones Sanitarias, Spain; and Fundació la Marató TV3 (Dr Domingo). Dr Ariño is supported by a postresidency grant from Hospital Clinic, Barcelona; Dr Armangue by a grant from Instituto Carlos III (CM14/00081); and Dr Martínez-Hernández by a grant from Instituto Carlos III (CD14/00155), Madrid, Spain.
Role of the Funder/Sponsor: The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: We thank all physicians who have contributed by providing clinical information of their patients and all patients for their generous contribution to research. We also thank Ellen Gelpi, MD (Institut d’Investigació Biomèdica August Pi i Sunyer brain bank), for her advice in the evaluation of the glutamic acid decarboxylase expression of tumors and Mercedes Alba and Eva Caballero (laboratory technicians, Institut d’Investigació Biomèdica August Pi i Sunyer) for technical support. They did not receive compensation from a funding sponsor for their contributions.
Footnotes
Author Contributions: Dr Graus had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs Ariño and Höftberger contributed equally to this article.
Study concept and design: Ariño, Höftberger, Dalmau, Graus.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Ariño, Dalmau, Graus.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Ariño.
Obtained funding: Ariño, Martínez-Hernandez, Dalmau, Graus.
Study supervision: Saiz, Dalmau, Graus.
Conflict of Interest Disclosures: No other disclosures were reported.
REFERENCES
- 1.Malter MP, Helmstaedter C, Urbach H, Vincent A, Bien CG. Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann Neurol. 2010;67(4):470–478. doi: 10.1002/ana.21917. [DOI] [PubMed] [Google Scholar]
- 2.Ariño H, Gresa-Arribas N, Blanco Y, et al. Cerebellar ataxia and glutamic acid decarboxylase antibodies: immunologic profile and long-term effect of immunotherapy. JAMA Neurol. 2014;71(8):1009–1016. doi: 10.1001/jamaneurol.2014.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saiz A, Blanco Y, Sabater L, et al. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain. 2008;131(pt 10):2553–2563. doi: 10.1093/brain/awn183. [DOI] [PubMed] [Google Scholar]
- 4.Hernández-Echebarría L, Saiz A, Arés A, et al. Paraneoplastic encephalomyelitis associated with pancreatic tumor and anti-GAD antibodies. Neurology. 2006;66(3):450–451. doi: 10.1212/01.wnl.0000196488.87746.7b. [DOI] [PubMed] [Google Scholar]
- 5.Bataller L, Valero C, Díaz R, et al. Cerebellar ataxia associated with neuroendocrine thymic carcinoma and GAD antibodies. J Neurol Neurosurg Psychiatry. 2009;80(6):696–697. doi: 10.1136/jnnp.2008.161042. [DOI] [PubMed] [Google Scholar]
- 6.Boronat A, Sabater L, Saiz A, Dalmau J, Graus F. GABA(B) receptor antibodies in limbic encephalitis and anti-GAD-associated neurologic disorders. Neurology. 2011;76(9):795–800. doi: 10.1212/WNL.0b013e31820e7b8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75(8):1135–1140. doi: 10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 9.Saiz A, Arpa J, Sagasta A, et al. Autoantibodies to glutamic acid decarboxylase in three patients with cerebellar ataxia, late-onset insulin-dependent diabetes mellitus, and polyendocrine autoimmunity. Neurology. 1997;49(4):1026–1030. doi: 10.1212/wnl.49.4.1026. [DOI] [PubMed] [Google Scholar]
- 10.Ances BM, Vitaliani R, Taylor RA, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128(pt 8):1764–1777. doi: 10.1093/brain/awh526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9(1):67–76. doi: 10.1016/S1474-4422(09)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai T, McGrath R. Lymphoma, thymoma and the wooden man: stiff-person syndrome post-thymoma excision and non-Hodgkin lymphoma remission. Intern Med J. 2012;42(2):205–207. doi: 10.1111/j.1445-5994.2011.02688.x. [DOI] [PubMed] [Google Scholar]
- 13.Piccolo G, Tavazzi E, Cavallaro T, Romani A, Scelsi R, Martino G. Clinico-pathological findings in a patient with progressive cerebellar ataxia, autoimmune polyendocrine syndrome, hepatocellular carcinoma and anti-GAD autoantibodies. J Neurol Sci. 2010;290(1–2):148–149. doi: 10.1016/j.jns.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Knudsen A, Bredholt G, Storstein A, et al. Antibodies to CRMP3–4 associated with limbic encephalitis and thymoma. Clin Exp Immunol. 2007;149(1):16–22. doi: 10.1111/j.1365-2249.2007.03379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clow EC, Couban S, Grant IA. Stiff-person syndrome associated with multiple myeloma following autologous bone marrow transplantation. Muscle Nerve. 2008;38(6):1649–1652. doi: 10.1002/mus.21153. [DOI] [PubMed] [Google Scholar]
- 16.Ferrari P, Federico M, Grimaldi LM, Silingardi V. Stiff-man syndrome in a patient with Hodgkin’s disease: an unusual paraneoplastic syndrome. Haematologica. 1990;75(6):570–572. [PubMed] [Google Scholar]
- 17.Silverman IE. Paraneoplastic stiff limb syndrome. J Neurol Neurosurg Psychiatry. 1999;67(1):126–127. doi: 10.1136/jnnp.67.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagiwara H, Enomoto-Nakatani S, Sakai K, Ugawa Y, Kusunoki S, Kanazawa I. Stiff-person syndrome associated with invasive thymoma: a case report. J Neurol Sci. 2001;193(1):59–62. doi: 10.1016/s0022-510x(01)00602-5. [DOI] [PubMed] [Google Scholar]
- 19.Sinnreich M, Assal F, Hefft S, et al. Anti-GAD antibodies and breast cancer in a patient with stiff-person syndrome: a puzzling association. Eur Neurol. 2001;46(1):51–52. doi: 10.1159/000050758. [DOI] [PubMed] [Google Scholar]
- 20.Schiff D, Dalmau J, Myers DJ. Anti-GAD antibody positive stiff-limb syndrome in multiple myeloma. J Neurooncol. 2003;65(2):173–175. doi: 10.1023/b:neon.0000003754.34527.f2. [DOI] [PubMed] [Google Scholar]
- 21.Thomas S, Critchley P, Lawden M, et al. Stiff person syndrome with eye movement abnormality, myasthenia gravis, and thymoma. J Neurol Neurosurg Psychiatry. 2005;76(1):141–142. doi: 10.1136/jnnp.2004.036558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka H, Matsumura A, Okumura M, Kitaguchi M, Yamamoto S, Iuchi K. Stiff man syndrome with thymoma. Ann Thorac Surg. 2005;80(2):739–741. doi: 10.1016/j.athoracsur.2004.02.076. [DOI] [PubMed] [Google Scholar]
- 23.Iwata T, Inoue K, Mizuguchi S, Morita R, Tsukioka T, Suehiro S. Thymectomy for paraneoplastic stiff-person syndrome associated with invasive thymoma. J Thorac Cardiovasc Surg. 2006;132(1):196–197. doi: 10.1016/j.jtcvs.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 24.McHugh JC, Murray B, Renganathan R, Connolly S, Lynch T. GAD antibody positive paraneoplastic stiff person syndrome in a patient with renal cell carcinoma. Mov Disord. 2007;22(9):1343–1346. doi: 10.1002/mds.21374. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal PA, Ichaporia NR. Glutamic acid decarboxylase antibody-positive paraneoplastic stiff limb syndrome associated with carcinoma of the breast. Neurol India. 2010;58(3):449–451. doi: 10.4103/0028-3886.65704. [DOI] [PubMed] [Google Scholar]
- 26.Rakocevic G, Hussain A. Stiff person syndrome improvement with chemotherapy in a patient with cutaneous T cell lymphoma. Muscle Nerve. 2013;47(6):938–939. doi: 10.1002/mus.23706. [DOI] [PubMed] [Google Scholar]
- 27.Aghajanzadeh M, Alavi A, Aghajanzadeh G, Massahania S. Stiff man syndrome with invasive thymic carcinoma. Arch Iran Med. 2013;16(3):195–196. [PubMed] [Google Scholar]
- 28.Koca I, Ucar M, Kalender ME, Alkan S. The horses are the first thought but one must not forget the zebras even if they are rare: stiff person syndrome associated with malignant mesothelioma. BMJ Case Rep. doi: 10.1136/bcr-2013-203455. [published online April 7, 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi R, Kaji M, Horiuchi S, Miyahara N, Hino Y, Suemasu K. Recurrent thymoma with stiff-person syndrome and pure red blood cell aplasia. Ann Thorac Surg. 2014;97(5):1802–1804. doi: 10.1016/j.athoracsur.2013.07.103. [DOI] [PubMed] [Google Scholar]
- 30.Spitz M, Ferraz HB, Barsottini OGP, Gabbai AA. Progressive encephalomyelitis with rigidity: a paraneoplastic presentation of oat cell carcinoma of the lung: case report. Arq Neuropsiquiatr. 2004;62(2B):547–549. doi: 10.1590/s0004-282x2004000300033. [DOI] [PubMed] [Google Scholar]
- 31.Venker C, Krämer M, Berlit P. GAD-ab-associated movement disorder in a male patient with breast cancer. J Neurol. 2011;258(7):1356–1357. doi: 10.1007/s00415-011-5925-0. [DOI] [PubMed] [Google Scholar]
- 32.Carra-Dalliere C, Thouvenot E, Bonafé A, Ducray F, Touchon J, Charif M. Anti-GAD antibodies in paraneoplastic cerebellar ataxia associated with limbic encephalitis and autonomic dysfunction [in French] Rev Neurol (Paris) 2012;168(4):363–366. doi: 10.1016/j.neurol.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 33.Laroumagne S, Elharrar X, Coiffard B, et al. “Dancing eye syndrome” secondary to opsoclonus-myoclonus syndrome in small-cell lung cancer. Case Rep Med. doi: 10.1155/2014/545490. [published online March 23, 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamotte G, Danaila TC, Jaillon-Rivière V, Hitier M, Defer GL. Paraneoplastic opsoclonus myoclonus with autoantibodies to glutamic acid decarboxylase. Rev Neurol (Paris) 2014;170(1):50–51. doi: 10.1016/j.neurol.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Jeffery OJ, Lennon VA, Pittock SJ, Gregory JK, Britton JW, McKeon A. GABAB receptor autoantibody frequency in service serologic evaluation. Neurology. 2013;81(10):882–887. doi: 10.1212/WNL.0b013e3182a35271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Höftberger R, Titulaer MJ, Sabater L, et al. Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology. 2013;81(17):1500–1506. doi: 10.1212/WNL.0b013e3182a9585f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohkawa T, Satake S, Yokoi N, et al. Identification and characterization of GABA(A) receptor autoantibodies in autoimmune encephalitis. J Neurosci. 2014;34(24):8151–8163. doi: 10.1523/JNEUROSCI.4415-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carvajal-González A, Leite MI, Waters P, et al. Glycine receptor antibodies in PERM and related syndromes: characteristics, clinical features and outcomes. Brain. 2014;137(pt 8):2178–2192. doi: 10.1093/brain/awu142. [published correction appears in Brain. 2014;137(pt 12):e315] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alexopoulos H, Akrivou S, Dalakas MC. Glycine receptor antibodies in stiff-person syndrome and other GAD-positive CNS disorders. Neurology. 2013;81(22):1962–1964. doi: 10.1212/01.wnl.0000436617.40779.65. [DOI] [PubMed] [Google Scholar]
- 40.Evoli A, Lancaster E. Paraneoplastic disorders in thymoma patients. J Thorac Oncol. 2014;9(9 suppl 2):S143–S147. doi: 10.1097/JTO.0000000000000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vernino S, Lennon VA. Autoantibody profiles and neurological correlations of thymoma. Clin Cancer Res. 2004;10(21):7270–7275. doi: 10.1158/1078-0432.CCR-04-0735. [DOI] [PubMed] [Google Scholar]
- 42.Folli F, Solimena M, Cofiell R, et al. Autoantibodies to a 128-kd synaptic protein in three women with the stiff-man syndrome and breast cancer. N Engl J Med. 1993;328(8):546–551. doi: 10.1056/NEJM199302253280805. [DOI] [PubMed] [Google Scholar]
- 43.Pittock SJ, Lucchinetti CF, Parisi JE, et al. Amphiphysin autoimmunity: paraneoplastic accompaniments. Ann Neurol. 2005;58(1):96–107. doi: 10.1002/ana.20529. [DOI] [PubMed] [Google Scholar]
- 44.Zhang D, Li X, Yao Z, Wei C, Ning N, Li J. GABAergic signaling facilitates breast cancer metastasis by promoting ERK1/2-dependent phosphorylation. Cancer Lett. 2014;348(1–2):100–108. doi: 10.1016/j.canlet.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Pittock SJ, Yoshikawa H, Ahlskog JE, et al. Glutamic acid decarboxylase autoimmunity with brainstem, extrapyramidal, and spinal cord dysfunction. Mayo Clin Proc. 2006;81(9):1207–1214. doi: 10.4065/81.9.1207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.