Summary
Background
Increasing evidence suggests that seizures and status epilepticus can be immune-mediated. We aimed to describe the clinical features of a new epileptic disorder, and to establish the target antigen and the effects of patients’ antibodies on neuronal cultures.
Methods
In this observational study, we selected serum and CSF samples for antigen characterisation from 140 patients with encephalitis, seizures or status epilepticus, and antibodies to unknown neuropil antigens. The samples were obtained from worldwide referrals of patients with disorders suspected to be autoimmune between April 28, 2006, and April 25, 2013. We used samples from 75 healthy individuals and 416 patients with a range of neurological diseases as controls. We assessed the samples using immunoprecipitation, mass spectrometry, cell-based assay, and analysis of antibody effects in cultured rat hippocampal neurons with confocal microscopy.
Findings
Neuronal cell-membrane immunoprecipitation with serum of two index patients revealed GABAA receptor sequences. Cell-based assay with HEK293 expressing α1/β3 subunits of the GABAA receptor showed high titre serum antibodies (>1:160) and CSF antibodies in six patients. All six patients (age 3–63 years, median 22 years; five male patients) developed refractory status epilepticus or epilepsia partialis continua along with extensive cortical-subcortical MRI abnormalities; four patients needed pharmacologically induced coma. 12 of 416 control patients with other diseases, but none of the healthy controls, had low-titre GABAA receptor antibodies detectable in only serum samples, five of them also had GAD-65 antibodies. These 12 patients (age 2–74 years, median 26·5 years; seven male patients) developed a broader spectrum of symptoms probably indicative of coexisting autoimmune disorders: six had encephalitis with seizures (one with status epilepticus needing pharmacologically induced coma; one with epilepsia partialis continua), four had stiff-person syndrome (one with seizures and limbic involvement), and two had opsoclonus-myoclonus. Overall, 12 of 15 patients for whom treatment and outcome were assessable had full (three patients) or partial (nine patients) response to immunotherapy or symptomatic treatment, and three died. Patients’ antibodies caused a selective reduction of GABAA receptor clusters at synapses, but not along dendrites, without altering NMDA receptors and gephyrin (a protein that anchors the GABAA receptor).
Interpretation
High titres of serum and CSF GABAA receptor antibodies are associated with a severe form of encephalitis with seizures, refractory status epilepticus, or both. The antibodies cause a selective reduction of synaptic GABAA receptors. The disorder often occurs with GABAergic and other coexisting autoimmune disorders and is potentially treatable.
Funding
The National Institutes of Health, the McKnight Neuroscience of Brain Disorders, the Fondo de Investigaciones Sanitarias, Fundació la Marató de TV3, the Netherlands Organisation for Scientific Research (Veni-incentive), the Dutch Epilepsy Foundation.
Introduction
Seizures and status epilepticus can result from immunological responses to excitatory or inhibitory synaptic receptors or associated cell-surface proteins.1–3 These include the N-methyl-D-aspartate receptor (NMDAR),4 the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR),5 the gamma-aminobutyric acid-B receptor (GABABR),6 leucine-rich glioma inactivated protein 1 (LGI1),7 contactin-associated protein-like 2 (Caspr2),8,9 dipeptidyl-peptidase-like protein-6 (DPPX),10 and the metabotropic glutamate receptor 5 (mGluR5).11 The seizures that accompany any of these disorders are often refractory to antiepileptic treatment unless the immune mechanism is identified and treated.6,12,13 In some patients, generalised seizures or status epilepticus can be the first manifestation of the disease, with patients needing heavy sedation or induced pharmacological coma.6,14–16 These treatments might conceal other symptoms such as dyskinesias or psychiatric alterations, delaying the recognition of the syndrome. Hitherto, the main epilepsy-related inhibitory receptor known to be a target of autoimmunity was the GABABR.9,16,17 Most patients with GABABR antibodies develop early seizures or status epilepticus as a component of limbic encephalitis. About 50% of these patients have an underlying small-cell lung cancer, and the neurological symptoms usually respond to immunotherapy and treatment of the cancer.9,16,17 Although the GABABR belongs to the category of metabotropic G protein-coupled receptors, the GABAA receptor (GABAAR) is a ligand-gated ion channel that modulates most of the fast inhibitory synaptic transmission in the brain and has not been previously recognised as a target of autoimmunity.
The identification of the above-mentioned disorders, all potentially treatable with immunotherapy,1–11 has enhanced aware ness of autoimmune mechanisms in patients with encephalitis associated with refractory seizures or status epilepticus, leading to an increased recognition of cases in which the antigens are unknown. Some patients might have several autoantibodies, suggesting that they have a propensity to autoimmunity, but also leading investigators to attribute the disorder to intracellular antigens that are not accessible to circulating antibodies, such as thyroid peroxidase or glutamic acid decarboxylase 65 (GAD65),5,6 and therefore of questionable pathogenic significance. In such patients, other more relevant, yet unknown cell-surface antigens can be overlooked, as occurred in previously reported patients who were eventually shown to have AMPAR or GABABR anti bodies.5,6 We aimed to establish the identity of a novel synaptic antigen in a subset of patients with encephalitis and refractory seizures or status epilepticus. We report the clinical features of this new syndrome, the identity of the antigen, and the effects of patients’ antibodies on neuronal cultures.
Methods
Study design and participants
Between Aug 20, 2012, and Dec 10, 2012, we identified two patients with encephalitis, refractory seizures, and serum and CSF antibodies with a similar pattern of reactivity against the neuropil of rat brain (appendix). The severity of the symptoms and unknown identity of the antigen prompted us to immunoprecipitate the antigen and to retrospectively review clinical and immunological information from patients with similar symptoms. We assessed serum and CSF samples, collected worldwide between April 28, 2006, and April 25, 2013, from 1134 patients with encephalitis and seizures that were suspected to be autoimmune. The samples had been sent to two referral centres (Department of Neurology, Hospital of the University of Pennsylvania, PA, USA, and Center of Neuro immunology, Institut d’Investigacions Biomediques August Pi i Sunyer [IDIBAPS], Hospital Clinic, University of Barcelona, Barcelona, Spain) for confirmation of the presence of cell-surface antibodies or investigation for novel antibodies after standard laboratory studies were negative. Serum and CSF samples were kept frozen at −80° C. For all patients, we obtained clinical information using a questionnaire completed by the treating physicians when the samples were sent to our centres. The treating physician also did subsequent clinical follow-up via email or phone.
Of these 1134 patients, 356 (44%) had antibodies that reacted with known cell-surface or synaptic antigens such as NMDAR, AMPAR, and LGI1, and 140 (including the two index patients) had the triad of encephalitis, seizures, and antibodies against unknown rat brain neuropil antigens. In all instances, the assessment of antibodies to brain neuropil antigens was done independently by two investigators (FG and JD), with results kept in a database. We then re-examined serum and CSF samples from these 140 patients with immunohistochemistry of rat brain, cultured live neurons, and a cell-based assay to establish whether they had similar antibodies to the two index patients. We also examined serum samples from 75 otherwise healthy individuals (blood donors) and serum or CSF samples from 416 patients with a range of neurological disorders (worldwide referrals). These 416 patients with diverse disorders were re-examined for neuropil antibodies and antibodies to α1/β3 subunits of the GABAAR. They included 41 seronegative patients with encephalitis and seizures or status epilepticus, 59 with opsoclonus-myoclonus, 20 with non-inflammatory degenerative ataxia, nine with herpes-simplex-virus encephalitis, 30 with multiple sclerosis, 101 with antibodies against GAD65 (16 limbic encephalitis, 33 epilepsy, 13 ataxia, 39 stiff-person syndrome), 90 with stiff-person syndrome without GAD65 antibodies, 30 with NMDAR antibodies, 19 with GABABR antibodies, and 17 with LGI1 antibodies. Only serum samples were available from 238 patients, and only CSF samples were available from 35 patients, with both types of samples available from 143 patients.
Partial clinical information about two patients with coexisting GABABR antibodies (patients 4 and 6) has been reported elsewhere.9,18 Our final protocol was approved by the institutional review boards of the University of Pennsylvania and the Hospital Clinic, and written informed consent was obtained from all patients or representatives.
Laboratory procedures
All laboratory techniques are described in the appendix and elsewhere.5,19–22 Briefly, we did immunohistochemistry on rodent brain, immunocytochemistry of rodent neuronal cultures, immunoprecipitation, mass spectrometry, immunoabsorption and immune-competition studies, immunocyto chemistry on live or fixed HEK293 cells (cell-based assays), quantitative analysis of neuronal GABAAR immunoreactivity of patients’ antibodies, and analysis of the effects of these antibodies on GABAAR using confocal microscopy.
Role of the funding source
The study sponsors had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
On immunohistochemistry with rat brain, the serum and CSF samples of the two index patients produced a similar and intense pattern of neuropil reactivity (figure 1, appendix). This neuropil reactivity resembled that reported for GABABR antibodies (figure 1, appendix),6 but specific testing for these antibodies with a cell-based assay was negative in both index patients (data not shown). Findings from a subsequent assessment with cultured live rodent hippocampal neurons showed that the novel antigen was on the cell surface (figure 1). Immunoprecipitation of neuronal proteins reacting with antibodies from the two index patients, followed by electrophoretic protein separation and EZBlue gel staining, did not produce any specific band compared with serum samples from individuals in the healthy control group (data not shown). Mass spectrometry of all separated proteins showed that serum samples from the two index patients but not from otherwise healthy control individuals had precipitated protein fragments containing sequences of the β3 subunit of the GABAAR (sequences shown in appendix).
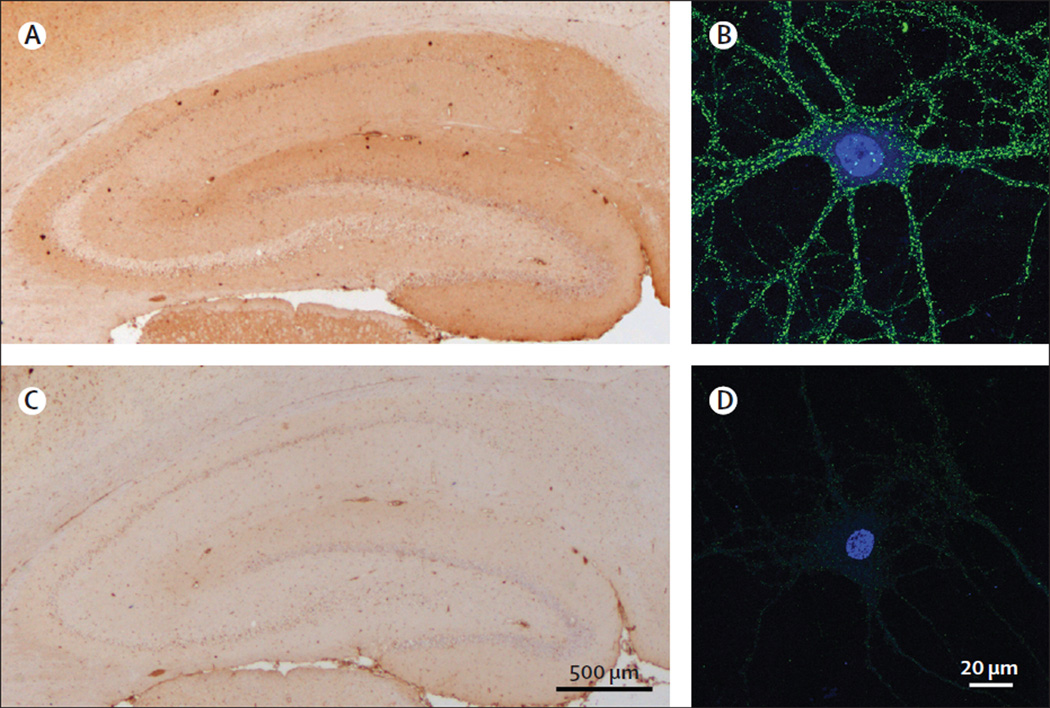
Figure 1. Reactivity with brain tissue and neuron cultures of the CSF of patients with GABAAR or GABABR antibodies.
The CSF of patient 2 showed extensive and diffuse immunostaining of the neuropil of cortical and subcortical regions (A; see the appendix for higher magnifications of selected brain regions). This pattern of brain and cerebellar staining is similar to that produced by the CSF of a patient with GABABR antibodies (C). However, patient 2 was negative for GABABR antibodies in a specific cell-based assay (data not shown). These findings suggested the presence of antibodies against a novel neuronal cell-surface antigen, which was confirmed in cultures of live rat hippocampal neurons (B). The CSF of the patient with GABABR antibodies also reacted with the neuronal cell surface, as expected (D). E and F show a similar study using CSF of a control patient without neuronal cell-surface antibodies. In B, D, and F the nucleus of the neurons was counterstained with DAPI. In A, C, and E the tissue was counterstained with haematoxylin.
Because the β3 subunit of the GABAAR forms complexes with the α1 subunit, we tested the reactivity of patients’ antibodies with HEK293 cells transfected with the human α1 or β3 subunits or both. This cell-based assay identified GABAAR antibodies in six patients, including four of the 140 patients with encephalitis, seizures or status epilepticus, and antibodies to unknown neuropil antigens, and two of the 19 patients with GABABR antibodies. All six patients’ serum and CSF samples reacted with cells coexpressing α1 and β3 subunits, but when the subunits were individually assessed, four patients’ samples reacted with both the α1 and β3 subunit, one patient’s sample reacted with only the α1 subunit, and another patient’s sample reacted with only the coexpression of α1 and β3 subunits. For this reason, we subsequently used α1 and β3 heteromers to assess antibody titres. To optimise the cell-based assay, we compared the sensitivity of the assay with live or fixed and permeabilised α1/β3 receptor-expressing HEK293 cells (live cell-based assay vs fixed cell-based assay). These studies showed that all patients’ CSF antibodies were detectable with either live or fixed cell-based assay, but serum antibodies were mostly visible with live cell-based assay (figure 2).
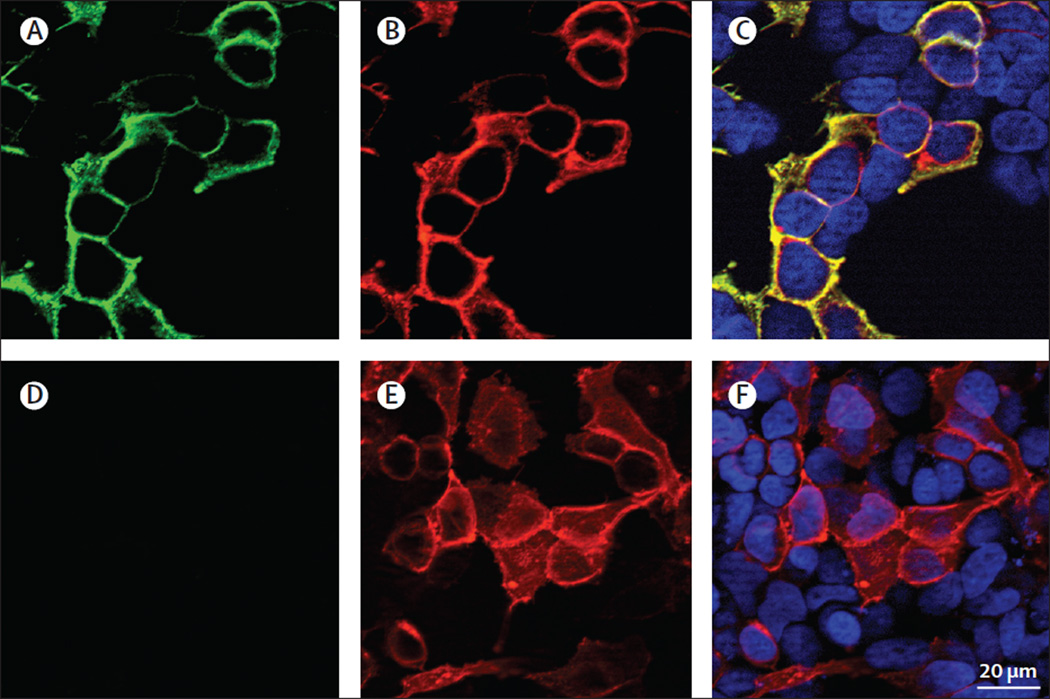
Figure 2. Reactivity of a patient’s serum with live HEK293 cells expressing GABAAR.
Reactivity of live HEK293 cells expressing human a1/b3 subunits of the GABAAR with a patient’s serum (A) and a monoclonal antibody against the a1 subunit (B). Merged reactivities (C). A similar assay with serum from a control individual is shown in (D–F). The nuclei of the cells are shown with DAPI in C and F. Note the specific reactivity of patient’s antibodies with cells expressing GABAAR and the co-localisation with the reactivity of the commercial antibody.
Immunocompetition assays with serum antibodies of the six patients showed that all recognised the same epitopes of the GABAAR (appendix). Immunoabsorption of a representative serum sample with HEK293 cells expressing the α1/β3 subunits resulted in abrogation of reactivity in rat brain and culture neurons, further confirming the reactivity with the GABAAR (figure 3).
Figure 3. Abrogation of serum antibody reactivity with brain and cultures of neurons after GABAAR immunoabsorption.
Panels A and B show the reactivity of a patient’s serum after immunoabsorption with non-transfected HEK293 cells. C and D show that this reactivity is abolished after the serum has been immunoabsorbed with HEK293 cells expressing the a1/β3 subunits of the GABAAR.
Using live cell-based assay in cells coexpressing α1/β3, we identified two clinical-immunological groups of patients: the first comprised the six patients with GABAAR antibodies identified from the cohort of 140 patients with encephalitis and unknown neuropil antigens and from the group of 19 patients with GABABR antibodies; all six patients had a high titre (>1:160) of serum (when available) and CSF GABAAR antibodies (patients 1–6; table. The second group consisted of 12 patients from the disease control groups, but not from the group of healthy individuals. In these 12 patients, the serum antibody titre was always 1:160 or lower; CSF samples were available from three individuals (patients 7, 12, and 18) and all three were negative. Moreover, whereas in patients in the first group the antibodies were detectable with three techniques (immunohistochemistry with rat brain, cultured neurons, and live or fixed cell-based assay), in patients of the second group the antibodies were detectable only with live cell-based assay (all individuals) and cultured live neurons (patients 7–13).
Table.
Clinical characteristics of patients with GABAAR antibodies in serum and CSF samples
| Sex, age in years |
Presentation and main symptoms |
CSF | MRI | EEG e in CSF | History of autoimmunity or cancer |
Treatment | Outcome | Sample: subunit target (a1/β3 titres) |
|
|---|---|---|---|---|---|---|---|---|---|
| Patients with high titres of serum GABAAR anti bodies and with antibodies detectable in CSF | |||||||||
| 1 (index patient 1) | F, 16 | Memory, cognitive, and affective problems for several months. Developed headache and 9 days later tonic-clonic seizures progressing to status epilepticus |
23 WBC/µL; protein 60 mg/dL |
Multifocal increased T2/FLAIR signal with cortical-subcortical involvement |
Generalised slowing, bilateral temporal seizures. Generalised periodic discharges |
Hodgkin’s lymphoma 10 months before onset of encephalitis |
Anticonvulsants: LEV, TPM, MDZ, barbiturate coma. Immunosuppressants: MTP, IVIG, PEX, RTX, CPH |
Progressive neurological recovery after 12 weeks in hospital. At 15-month follow-up she had returned to school with mild cognitive deficit that is improving |
Serum: α1, β3 (>1/1280) CSF: α1 (>1/320) |
| 2 (index patient 2) | M, 51 | Behavioural change, depression, psychosis, and mutism for several weeks. Developed partial clonic seizures and epilepsia partialis progressing in 48 h to status epilepticus |
Normal WBC and protein concentration; OCB-positive |
Multifocal increased T2/FLAIR signal with extensive cortical- subcortical involvement |
Right temporal ictal activity, secondary generalisation. Generalised periodic discharges |
Idiopathic thrombocytopenic purpura; TPO and thyroglobulin antibodies |
Anticonvulsants: LEV, DZP, LCM, PHT, MDZ, PPF, barbiturate coma. Immunosuppressants: MTP, IVIG, PEX, CPH, RTX. |
After 10 weeks, status epilepticus persisted and the patient died of sepsis |
Serum: α1, β3 (1/1280) CSF: α1 (1/320) |
| 3 | M, 28 | Subacute presentation of behavioural and cognitive deficits followed 5 days later by complex partial seizures and status epilepticus |
Normal WBC and protein concentration |
Bilateral mesiotemporal high T2/FLAIR signal |
Ictal activity | TPO antibodies | Anticonvulsants: PPF, MDZ, LEV, PHT, TPM, CLB, barbiturate coma. Immunosuppressants: MTP with PDN taper. |
After 8 weeks in the intensive care unit he gradually returned to baseline function. At last follow-up (18 months) he was seizure free and back to work |
Serum: α1, β3 (1/640) CSF: α1, β3 (1/160) |
| 4 | M, 3 | Acute development of confusion, lethargy, dystonic tongue movements, chorea of limbs and trunk, opsoclonus, ataxia, evolving in 24 h to complex partial seizures and status epilepticus |
154 WBC/µL; protein 59 mg/dL |
Multifocal high T2/ FLAIR signal in brainstem and cerebellum with involvement of basal ganglia and hippocampi |
Generalised slowing and bioccipital ictal activity |
GABABR antibodies in serum and CSF |
Anticonvulsants: multiple, barbiturate coma. Decompressive posterior craniectomy due to cerebral oedema. Immunosuppressants: MTP, IVIG |
After 4 weeks, status epilepticus persisted and the patient died of sepsis |
Serum: NA CSF: α1, β3 (1/320) |
| 5 | M, 4 | Progressive right hemiparesis; 2 months later, partial seizures progressing to status epilepticus |
Increased WBC and protein concentration |
Abnormal FLAIR changes suggesting encephalitis |
Generalised slowing and ictal activity |
No | Anticonvulsants: LEV. Immunosuppressants: No |
Substantial recovery but, 2·5 years after symptom onset, still requires antiepileptics to prevent seizures. |
Serum: α1 (1/320) CSF: α1/β3 (1/40) |
| 6 | M, 63 | Subacute memory problems, gustatory and olfactory hallucinations, facial cramps, psychomotor agitation, tinnitus |
75 WBC/µL; increased protein concentration; OCB-positive |
Right temporal cortex high T2/ FLAIR signal |
Frontotemporal ictal activity |
GABABR, GAD65, TPO and thyroglobulin antibodies |
Anticonvulsants: VPA, LEV, barbiturate. Immunosuppressants: PDN |
Full recovery. 7 years later: diplopia and hemiataxia that spontaneously resolved (positive GAD65 but negative GABAAR and GABABR antibodies) |
Serum: NA CSF: α1/β3 (1/20) |
| Patients with low titres of serum GABAAR antibodies and without antibodies detectable in CSF | |||||||||
| 7 | M, 2 | Subacute onset partial seizures; 4 months later, choreathetoid movements and status epilepticus. |
Normal WBC and protein concentration |
Cortical atrophy | Generalised slowing and right parietal ictal activity |
No | Anticonvulsants: CBZ, VPA, MDZ, LEV, ketogenic diet, barbiturate coma. Immunosuppressants: MTP with PDN taper |
Partial response, cognitive and motor skills improved. At last follow-up (2 years), partial seizures persist |
Serum: β3 (1/160) CSF: negative |
| 8 | M, 41 | Subacute onset generalised seizures with fever, epilepsia partialis continua, aphasia. 2 years later, status epilepticus |
Normal WBC and protein concentration |
Multifocal cortical- subcortical high T2/FLAIR signal in both hemispheres |
Bifrontal ictal activity |
GAD65 antibodies | Anticonvulsants: VPA, OXC, LEV. Immunosuppressants: MTP with PDN taper |
Partial response at initial presentation. Status epilepticus responded to anticonvulsants |
Serum: α1 (1/160) CSF: NA |
| 9 | F, 15 | Reduced verbal output and seizures |
8 WBC/µL; normal protein concentration |
Bilateral fronto- temporal increased T2/FLAIR signal, leptomeningeal enhancement |
Multifocal ictal activity |
GAD65 antibodies | NA | NA | Serum: α1, β3 (1/160) CSF: NA |
| 10 | F, 32 | Multifocal refractory seizures |
Normal WBC and protein concentration |
Normal | Bilateral temporal ictal activity and multifocal interictal epileptiform discharges |
Type 1 diabetes mellitus, Hashimoto’s thyroiditis. GAD65, TPO and thyroglobulin antibodies |
Anticonvulsants: OXC, CBZ, LCM, LEV, ZNS, TPM, CLB, PHT, LTG. Immunosuppressants: IVIG, PDN, ciclosporin |
After 7 years she still has uncontrolled seizures |
Serum: α1/β3 (1/40) CSF: NA |
| 11 | F, 74 | Subacute onset of lethargy and alternating changes in level of consciousness. Suspected temporal lobe seizures |
Normal WBC and protein concentration |
Normal | NA | Previous history of ovarian cancer |
NA | NA | Serum: α1/β3 (1/40) CSF: NA |
| 12 | F, 16 | Behavioural changes, insomnia, orofacial dyskinesia, decreased level of consciousness, brief seizure, dysautonomia |
17 WBC/µL; normal protein concentration |
Left temporal cortical-subcortical high T2/FLAIR signal |
Generalised Slowing |
NMDAR antibodies in serum and CSF samples |
Anticonvulsants: VPA Immunosuppressants: MTP, IVIG, PEX, RTX |
Full recovery, gradual improvement over many months |
Serum: α1/β3 (1/20) CSF: negative |
| 13 | M, 19 | stiff-person syndrome since age 14 years |
Normal WBC; protein 85 mg/dL |
Not done | Not done | Type 1 diabetes mellitus. GAD65 antibodies |
Clonazepam, baclofen. Immunosuppressants: No |
Marked improvement. At last follow-up (16 years) he is independent for all daily life activities |
Serum: α1/β3 (1/40) CSF: NA |
| 14 | M, 12 | stiff-person syndrome since age 5 years; brief episodes of seizures |
NA | Hippocampal high T2/FLAIR signal |
Right temporal seizures, bifrontal sharp waves |
GAD65 antibodies | Anticonvulsants: LEV. Immunosuppressants: IVIG, RTX |
Partial improvement of stiff-person symptoms, free of seizures |
Serum: α1/β3 (1/20) CSF: NA |
| 15 | M, 21 | stiff-person syndrome since age 16 years |
Normal WBC and protein concentration |
Normal | Normal | Antinuclear antibodies; anti-endomysial immunoglobulin A |
Anticonvulsants: CBZ, OXC. Immunosuppressants: IVIG |
Partial improvement | Serum: α1/β3 (1/20) CSF: NA |
| 16 | M, 46 | Stiff -limb syndrome | Not done | Not done | Not done | No | Baclofen Immunosuppressants: No |
Substantial improvement |
Serum: α1/β3 (1/20) CSF: NA |
| 17 | F, 34 | Opsoclonus-myoclonus syndrome |
NA | Normal | Not done | No | NA | NA | Serum: α1/β3 (1/40) CSF: NA |
| 18 | M, 65 | Opsoclonus-myoclonus syndrome |
Normal WBC and protein concentration |
Normal | Not done | Antinuclear antibodies |
Immunosuppressants: MTP |
No response, died few months after onset |
Serum: α1/β3 (1/20) CSF: negative |
CBZ=carbamazepine. CLB=clobazam. CPH=cyclophosphamide. DZP=diazepam. F=female. GABAR=gamma-aminobutyric acid receptor. GAD65=glutamic acid decarboxylase 65. IVIG=intravenous immunoglobulin. LCM=lacosamide. LEV=levetiracetam. LTG=lamotrigine. M=male. MDZ=midazolam. MTP=intravenous methylprednisolone. NA=not available. NMDAR=N-methyl-D-aspartate receptor. OCB=oligoclonal bands. OXC=oxcarbazepine. PDN=oral prednisone. PEX=plasma exchange. PHT=phenytoin. PPF=propofol. RTX=rituximab. TPM=topiramate. TPO=thyroid peroxidase. VPA=valproate. WBC=white blood cell count. ZNS=zonisamide.
Five of the six patients with high concentrations of GABAAR antibodies in serum and CSF samples were male; three were children and three were adults (age range 3–63 years). All developed a rapidly progressive encephalopathy that eventually resulted in refractory seizures, five of the six had status epilepticus and one of them (patient 2) also had epilepsia partialis continua (table). In all six patients, epileptic symptoms were preceded or associated with a change in behaviour or level of cognition. A 3-year-old child (patient 4), who also had GABABR antibodies, developed seizures along with confusion, opsoclonus, ataxia, and chorea. Another patient (patient 5) developed a progressive hemiparesis before seizures. One patient had normal CSF white-cell count and protein con centration and the other five had at least one abnormality, including pleocytosis in four of six patients, increased protein concentration in four of six patients, and oligoclonal bands in two of patients (patients 2 and 6 in the table). All six patients had abnormal brain MRI, often showing extensive abnormalities on FLAIR and T2 imaging, with multifocal or diffuse cortical involvement without contrast enhancement (figures 4 and 5); one patient had involvement of the basal ganglia. The EEG showed seizures in all patients, two of whom had periodic generalised discharges (appendix). In addition to GABAAR antibodies, three patients had thyroid peroxidase antibodies, one had GAD65 antibodies, and two had GABABR antibodies. Other findings suggestive of a propensity to autoimmunity or immune dys regulation included a past history of Hodgkin’s lymphoma in one patient, and idiopathic thrombocytopenic purpura in another.
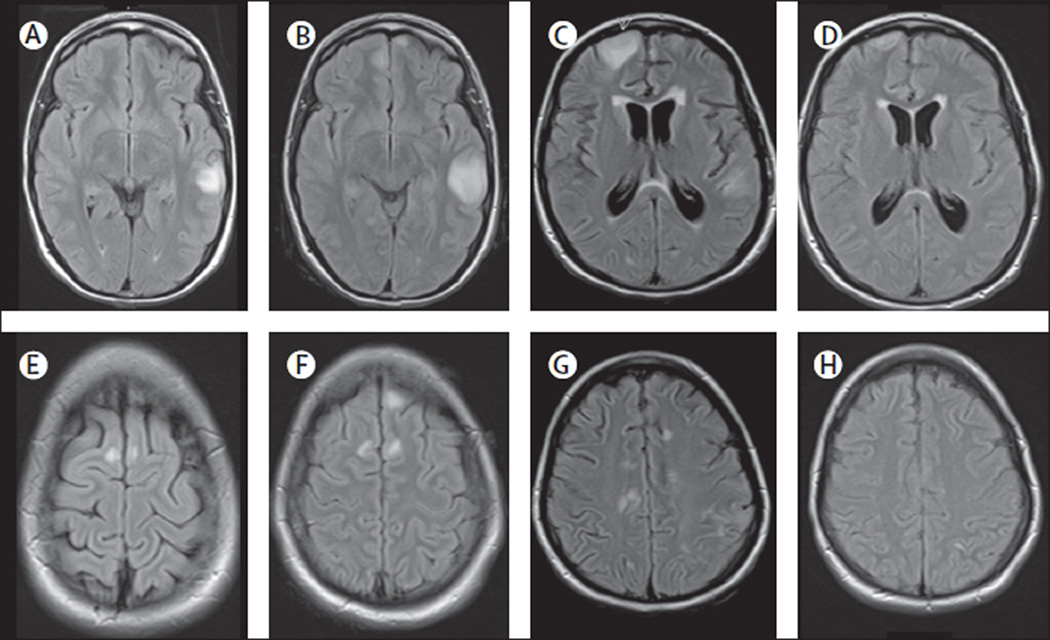
Figure 4. MRI findings in index patient 1.
On day 3 of admission, the MRI of this 16-year-old girl showed multiple cortical-subcortical abnormalities with increased FLAIR and T2 signal involving the left temporal lobe and frontal parasagittal regions (A, E). On day 10, a repeat MRI showed an increase of the size of the temporal lesion and a new cortical lesion in the left frontal lobe (B, F). Repeat MRIs on days 22 and 48 did not show substantial changes (data not shown). Another MRI done 4 months after disease onset showed many new multifocal abnormalities and diffuse atrophy and increase of the size of the ventricles (C, G). A repeat MRI 2 months later, 6 months after symptom onset, showed substantial improvement and resolution of the abnormalities as well as improvement of the ventricular dilatation (D, H).
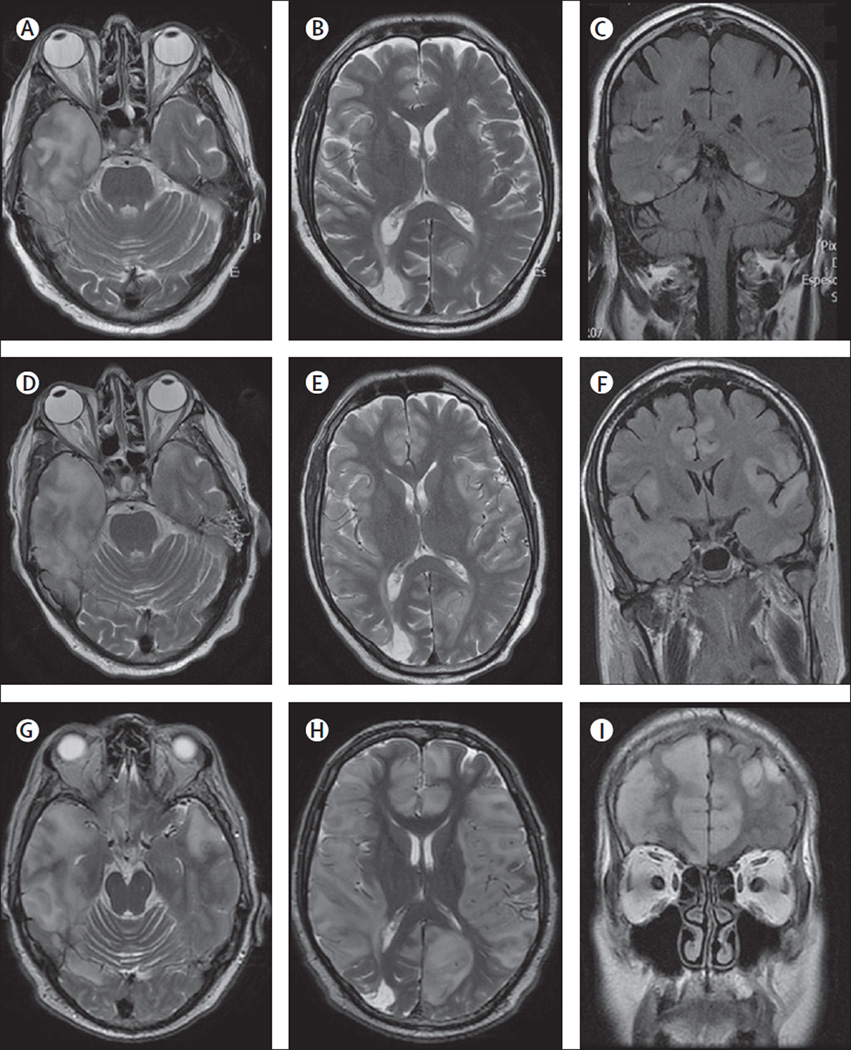
Figure 5. MRI findings in index patient 2.
On day 2 of admission, the MRI of this patient showed multiple areas of FLAIR and T2 signal abnormality predominantly involving cortical regions (A–C), without oedema, mass effect, or contrast enhancement (data not shown), but with blurring of the grey-white matter junction. On day 14, repeated MRI showed interval increase of the cortical-subcortical involvement, with oedema in the right temporal lobe (D–F). Subsequent MRIs showed a pronounced worsening of these abnormalities now extensively involving cortical and subcortical regions (G–I).
Treatment and follow-up were assessable in all six patients: one child received levetiracetam without immunotherapy and had substantial recovery, although 3 years after symptom onset he still requires antiepileptic treatment to avoid seizure recurrence. The other five received immunotherapy and multiple antiepileptic drugs, and four patients needed a pharmacologically induced coma. Three of these patients had total or partial recovery and two died as a result of sepsis during status epilepticus. One death was a child (patient 4) with concomitant GABABR antibodies indicated above (clinical and autopsy findings have been reported in detail elsewhere;18 the GABAAR antibodies were identified in archived serum and CSF samples). The oldest of the six patients (age 63 years) also had GABABR antibodies; the GABAAR antibodies were identified in samples that had been archived for 7 years. This patient fully recovered from the encephalopathy associated with antibodies against both GABARs, but 7 years later developed diplopia and hemiataxia with GAD65 antibodies (without antibodies to GABARs) from which he fully recovered.
12 patients (age 2–74 years, median 26·5 years; seven male patients) had low serum concentrations of GABAAR antibodies (table). Briefly, all six patients with encephalitis had seizures; one of them (patient 7; a 2-year-old boy) with refractory status epilepticus that needed a pharmacologically induced coma, and another patient (patient 8; a 41-year-old man) with epilepsia partialis continua. In the other six patients, four had stiff-person syndrome (one of them associated with seizures), and two had opsoclonus-myoclonus.
Six of the 12 patients had other neuronal antibodies in addition to GABAAR antibodies: five had GAD65 and one had NMDAR antibodies. Additional findings suggestive of a propensity to autoimmunity or immunological dysfunction included: Hashimoto’s thyroiditis with thyroid peroxidase antibodies in one patient, and type 1 diabetes mellitus in two patients. None of the patients with stiff-person syndrome had amphiphysin or glycine receptor (GlyR) antibodies. Treatment and follow-up were assessable in nine patients. Immunotherapy was used in seven patients: one had full recovery, five had partial recovery, and one died. The two patients who did not receive immunotherapy had stiff - person syndrome that was controlled symptomatically with clonazepam or baclofen.
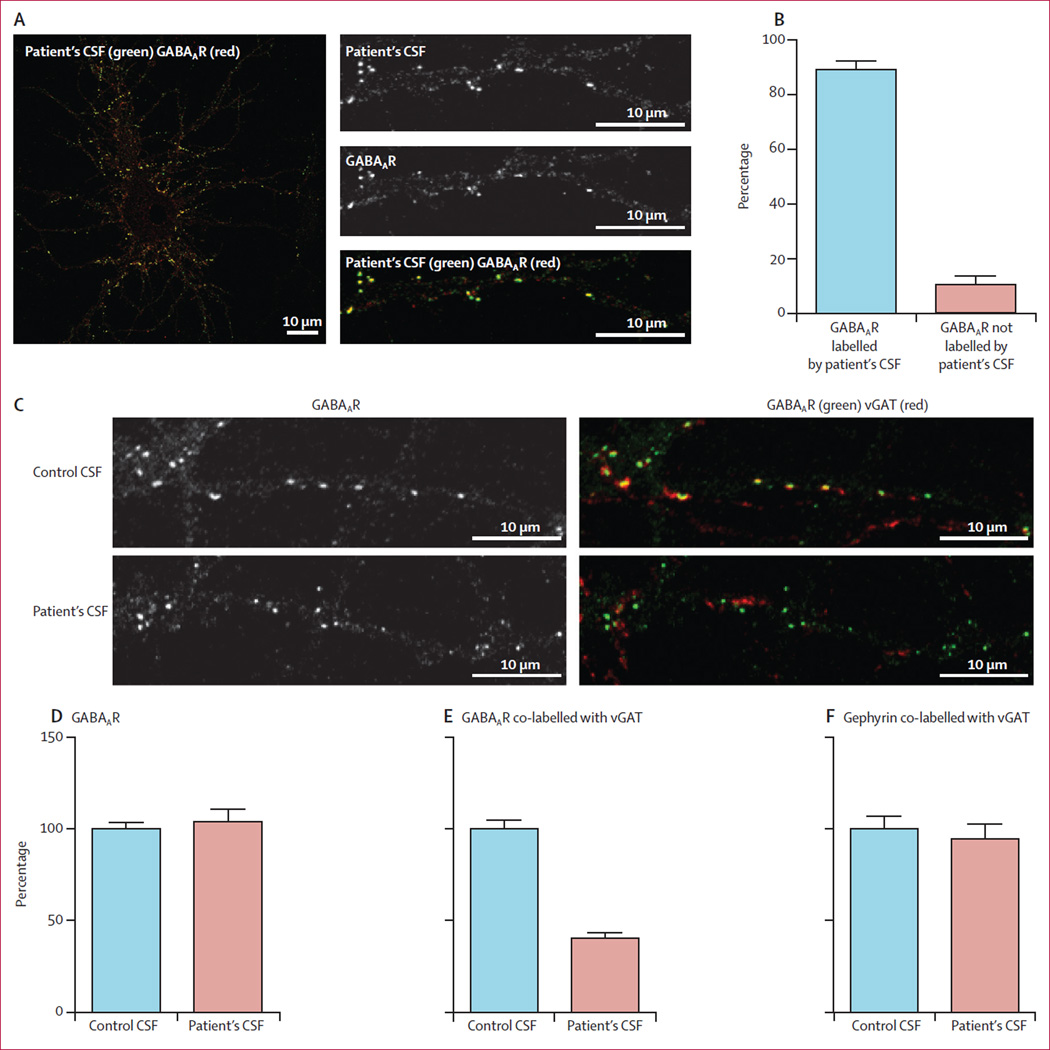
We did the following studies with CSF of a representative patient (index patient 1) with high titre serum and CSF antibodies reacting with only GABAAR. The reactivity was abrogated by pre-absorption with HEK293 cells expressing GABAAR (figure 3), and by immunocompetition assays with antibodies from the other five patients with high antibody titres, indicating that all patients’ antibodies targeted the same GABAAR epitopes (appendix). To examine the extent of recognition of GABAAR by patients’ CSF antibodies, we quantified GABAAR immunelabelling by confocal microscopy (figure 6). These results showed that 89% of patient’s antibodies labelled GABAAR-containing clusters (figure 6). To examine the effects of the antibodies on inhibitory synapses containing GABAAR, neurons were treated with patient’s CSF antibodies or a control CSF for 48 h. These studies showed that the density of GABAAR clusters along dendrites was not affected (figure 6), but the clusters of GABAAR at synapses, measured as cluster density co-labelled by the presynaptic marker vGAT (vesicular GABA transporter), were substantially reduced (figure 6). This finding suggests that the antibodies in the patient’s CSF, but not control CSF, removed GABAAR from synapses. The effect was specific for GABAAR because the cluster density of other synaptic markers such as gephyrin (figure 6) and the GluN1 subunit of the NMDAR (data not shown) were not affected.
Figure 6. Effect of patient’s antibodies on the density of GABAAR clusters in cultures of hippocampal neurons.
Live 14-day-in-vitro cultures of dissociated rat hippocampal neurons were stained with patient’s CSF containing GABAAR antibodies (green), then fixed and stained with commercial GABAAR antibodies (red; A). Quantification of colocalisation between patient’s CSF antibodies and the commercial GABAAR antibody shows that 89% (SE 3%) of receptors labelled by patient’s antibodies were colabelled with the commercial antibody against GABAAR (B). In a similar assay, neurons were incubated with patient’s CSF for 48 h and subsequently stained for postsynaptic GABAAR (green) and presynaptic vesicular GABA transporter (vGAT) (red; C). The synaptic GABAARs (shown as yellow clusters in control conditions) were greatly reduced after treatment with patient’s CSF (C). The number of GABAAR clusters along dendrites of neurons treated with patient’s CSF is not different from neurons treated with control CSF (Mann-Whitney test p=0·6; D). The number of GABAARs localised in synapses, however, decreased significantly in neurons treated with a patient’s CSF compared with neurons treated with control CSF (40% [3%] compared with control as 100%; p<0·0001; E). Patients’ CSF did not affect the clusters of post-synaptic gephyrin colabelled with presynaptic vGAT when compared with the effects of control CSF (p=0·5; F).
Discussion
We report the identification of high titre serum and CSF antibodies against the GABAAR in a subset of patients with encephalitis and refractory seizures or status epilepticus, who often needed pharmacologically induced coma. This finding is important because the disorder is potentially treatable. However, because of the rapid development of seizures and frequent presence of coexisting autoimmune disorders, recognition of the disorder might be difficult. Findings from the four following sets of experiments establish GABAAR as a relevant autoantigen: direct immunoprecipitation of the receptor by patients’ antibodies, specific immunostaining of HEK293 cells expressing α1/β3 subunits of the receptor, competition of patients’ antibodies for the same GABAAR epitopes, and demonstration that patients’ antibodies selectively remove GABAAR from synapses without affecting NMDAR or gephyrin (a scaffold protein that anchors the receptor at post-synaptic sites).
Most fast inhibitory neurotransmission in the adult brain is mediated by ligand-gated GABAAR.23 These receptors are regulated by many positive (barbiturates, benzodiazepines) and negative (picrotoxin, bicuculline) allosteric modulators, providing several models of GABAAR-antagonist induced seizures.24,25 The GABAARs are pentamers, the five subunits of which originate from eight gene families that encode different isoforms ((α1–6, β1–3, γ1–3, δ, ε, α, π, and α1–3). The subunit composition of the receptor governs the intrinsic properties of the channel, such as affinity for GABA, receptor conductance, kinetics, and modulation.26 These 19 subunits combine in different ways to form functional receptors, but at synaptic sites most receptors contain two α subunits (α1–3 isoforms), two β subunits, and a γ subunit arranged in the order γ-β-α-β-α. By contrast with receptors at synaptic sites, those at perisynaptic or extrasynaptic sites are mainly composed of α4 or α6 subunits combined with β and δ subunits.27 The antibodies of our patients reacted with the α1, β3, or both subunits (we did not assess other subunits), and when the reactivity with each subunit was individually assessed, the α1 subunit was always recognised by patients’ CSF. Therefore, that the main effects of patients’ antibodies occurred at synaptic sites, where the α1 receptors are enriched, is not surprising. Indeed, using cultures of rat hippocampal neurons, patients’ antibodies caused a decrease in the density of GABAAR at synaptic sites. The total density of GABAARs, including synaptic and extrasynaptic receptors, was not affected, suggesting a relocation of receptors from synaptic to extrasynaptic sites. This finding contrasts with the effects of antibodies identified in other autoimmune encephalitis, such as anti-NMDAR or anti-AMPAR, in which the decrease of the cor responding receptors occurs at both synaptic and extrasynaptic sites.5 22,28
At least four mutations in the α1 subunit of the GABAAR are associated with generalised epilepsy.27 Findings from in-vitro studies have shown that each of these mutations results in a substantial loss of α1-subunit function or level of expression.27 Additionally, mutations of the β3 subunit have been reported in children with absence epilepsy.29 In line with these findings, in our study, all patients with high titres of serum and CSF α1/β3 receptor antibodies developed seizures, status epilepticus, or epilepsia partialis continua. Most of these patients had an abnormal EEG with multifocal seizures and, in two cases, generalised periodic discharges. These findings were associated with extensive cortical and subcortical brain MRI abnormalities in all six patients with high serum antibody titres (all with CSF antibodies) and in three (25%) of 12 patients with low serum titres. We do not know if the MRI findings were caused by the immune response or resulted from the lengthy seizures. However, the multifocal and extensive brain MRI abnormalities were different from those seen in other autoimmune encephalitis, in which the MRI is often normal (NMDAR)30 or shows predominant involvement of the hippocampus (AMPAR, GABABR, LGI1).5,7,16 The comparison with other autoimmune encephalitis shows other differences: 39% of patients with GABAAR antibodies are younger than 18 years, whereas most patients with other encephalitis (except anti-NMDAR) are adults.31 Patients with GABAAR antibodies do not seem to frequently have an underlying tumour (similar to LGI1 autoimmunity), whereas about 30–60% of patients with other antibodies (Caspr2, GABABR, or AMPAR) have a tumour32 and, for patients with NMDAR antibodies, the frequency of tumours varies with age, sex, and ethnicity.30 Since the end of this study, we have identified a patient with a malignant thymoma and encephalitis, seizures, multifocal cortical FLAIR MRI abnormalities, and LGI1 and GABAAR antibodies, suggesting that patients with thymoma and seizures should be tested for GABAAR antibodies (data not shown).
In the group with low serum titres of antibodies and absent CSF antibodies, all patients with encephalitis developed seizures; the youngest patient, a 2-year-old child, also required pharmacologically induced coma for status epilepticus. The frequent presence of other relevant autoimmunities could explain the broader spectrum of symptoms in this group. Indeed, two of the four patients with stiff-person syndrome had coexisting GAD65 antibodies, and another patient with GABAAR antibodies only detected in serum had high titres of NMDAR antibodies in serum and CSF samples that were responsible for most of the clinical features (anti-NMDAR encephalitis).
Findings from this and previous studies suggest that patients with encephalitis or seizures attributed to GAD65 antibodies should be examined for other relevant antibodies against cell-surface antigens, such as GABAARs and other synaptic receptors (panel).6,33–35 Additionally, the increasing recognition of autoimmune encephalitis with neuronal cell-surface antibodies and concurrent thyroid peroxidase antibodies (as in four of 18 patients in this study) suggests that Hashimoto’s encephalitis should be a diagnosis of exclusion—that is, the detection of thyroid peroxidase antibodies and symptom response to steroids are not sufficient criteria to establish the diagnosis of Hashimoto’s encephalitis.9,35,36
Evidence suggests that status epilepticus can lead to chronic epilepsy. The development of epilepsy is usually preceded by a silent period during which there is increasing hyperexcitability and a progressive decrease of synaptic GABAAR.26 This effect has been attributed in part to a disruption of the GABAAR-anchoring protein, gephyrin.26,37 Additionally, lengthy seizures reduce GABAAR inhibition, which might lead to the development of status epilepticus.38 These findings and the antibody-mediated decrease of synaptic GABAAR seen in neuronal cultures exposed to patients’ antibodies suggest a model whereby the GABAARs are removed from synapses leading to status epilepticus, which in turn causes a further decrease of receptors along with reduced GABAAR inhibition, resulting in a pathogenic reinforcement. This would explain the severity and refractory nature of the seizures associated with high concentrations of GABAAR antibodies, and why this disorder seems to be more difficult to treat than the syndromes associated with NMDAR, GABABR, AMPAR, or LGI1 antibodies, emphasising the importance of prompt diagnosis and treatment. Despite the difficulties in treatment, 12 of 15 patients had partial or complete response to immunotherapy (nine patients), symptomatic therapy (three patients), and extended intensive care support (all patients with encephalitis).
Our study has several limitations, including the retrospective assessment of most patients (except the two index patients), and the absence of CSF samples from nine of the 12 patients with low serum titres of GABAAR antibodies. Therefore, the clinical implications of low serum antibody titres should be interpreted with caution, especially because in some patients (eg, the patients with NMDAR antibodies) other coexisting immunological mechanisms could have contributed to the patients’ symptoms. Future studies should establish, in a prospective manner, the incidence of serum and CSF GABAAR antibodies in patients with seizures or status epilepticus, opsoclonus-myoclonus, and stiff-person syndrome with or without GAD65 autoimmunity, and whether the presence of antibodies in CSF always associates with seizures or status epilepticus.
Findings from this study have several clinical implications. The presence of GABAAR antibodies should be tested in patients with severe seizures or status epilepticus in the context of encephalitis of unclear cause with MRI and CSF abnormalities suggestive of an inflammatory process, patients with opsoclonus-myoclonus or stiff-person syndrome, and any patients with GAD65 or thyroid peroxidase antibodies and other clinical features suggesting a propensity to autoimmunity.
In addition to the clinical implications, the identification of a disorder in which patients’ antibodies specifically eliminate GABAARs from synapses provides a useful reagent (purified patients’ antibodies) to understand how selective disruption of these receptors leads to neuronal hyperexcitability, seizures, or status epilepticus.
Supplementary Material
Panel: Research in context.
Systematic review
We searched Medline and Embase up to Nov 1, 2013, for articles published in English with the search terms “gamma-aminobutyric acid-A receptor”, “GABAA”, “GABAB”, “GABA”, “receptors”, “antibodies” and “encephalitis” [MeSH terms]. We restricted searches to studies in human beings. We also reviewed the reference lists of the papers identified by this search. We identified 36 papers, of which none was related to encephalitis associated with GABAAR antibodies; of the papers identified, three were series of patients with GABABR antibodies, 18 were reviews of autoimmune encephalitis, 12 were original research articles, two were editorials, and one was a letter.
Interpretation
Our findings suggest that the GABAA receptor is a novel target antigen of autoimmune encephalitis, and provides an unambiguous test for the detection of patients’ antibodies in serum and CSF samples. Although we found high titres of serum and CSF GABAAR antibodies in patients with seizures, refractory status epilepticus, or epilepsia partialis continua, low titre serum antibodies were associated with a wider spectrum of symptoms, probably due to the high prevlalence of coexisting autoimmunities. We also show that patients’ GABAAR antibodies cause a selective decrease of the clusters of GABAAR at synapses, but not along dendrites, without altering other post-synaptic proteins such as the NMDAR or gephyrin. These findings are important for three reasons: they define a novel form of autoimmune epileptic disorder, usually non-paraneoplastic, that affects children and adults and is severe but potentially treatable; that the severity of the seizures (often needing pharmacologically induced coma) and frequent presence of other less relevant antibodies against intracellular antigens (eg, thyroid peroxidase, GAD65) can mislead diagnosis (eg, Hashimoto’s encephalitis, anti-GAD65 encephalitis or seizures); and that patients’ antibodies have a direct effect on the GABAAR receptors, which provides a useful reagent (purified patients’ antibodies) to understand how selective disruption of GABAAR leads to neuronal hyperexcitability, seizures, or status epilepticus.
Acknowledgments
This study was supported by Instituto Carlos III (TA, FI12/00366; EMH, CM12/00055, JD, FIS PI11/01780; and FG, PI12/00611), the National Institutes of Health (JD, RO1NS077851; and RB-G and JD, MH094741), Fundació la Marató TV3 (JD, 101530), the Netherlands Organisation for Scientific Research (MT), the Dutch Epilepsy Foundations (MT, project 14–19), and a ErasmusMC fellowship (MT). We thank Maria Rodés, Mercè Alba, Eva Caballero, and Esther Aguilar for excellent technical support and the patients and their families for volunteering to participate in this study. We thank Miquel Raspall-Chaure, Marcelo Malakooti, Mark Wainwright, Adam Kirton, Michael Esser, Albert Quilez-Martínez, Verónica González-Álvarez, Federico Ramos, Francesc Xavier Sanmartí, Jordi Bruna, Jong Woo Lee, Stacy A Rudnicki, and Alma R Bicknese for providing patients’ samples and clinical information.
Footnotes
See Online for appendix
Contributors
M-PP, TA, and XP did the literature search, study design, data collection, data analysis, writing, and critical approval of the final paper. LB, TC, RD, LM, WM, MK, DR, WG, BM, CA, PSS, and MJT did the data collection and critical approval of the final paper. EM-H did the data collection, data analysis, interpretation, and critical approval of the paper. RBG did the data interpretation, critical approval of the final paper, and obtained funding. FG did the data collection, data analysis, data interpretation, critical approval of the final manuscript, and obtained funding. JD did the figures, study design, data collection, data analysis, data interpretation, writing, critical approval of the final paper, and obtained funding.
Conflicts of interest
JD holds patents for the use of Ma2 and NMDAR as autoantibody tests, and has filed patents for the use of DPPX, GABAAR, and GABABR as diagnostic tests. JD and PS-S receive research grant support from Euroimmun. PS-S has filed a patent for the use of DNER as diagnostic test. MJT received a travel grant for Lecturing in India from Sun Pharma, India. The rest of the authors have no conflicts of interest.
References
- 1.Dalmau J. Status epilepticus due to paraneoplastic and nonparaneoplastic encephalitides. Epilepsia. 2009;50(suppl 12):58–60. doi: 10.1111/j.1528-1167.2009.02352.x. [DOI] [PubMed] [Google Scholar]
- 2.Wong-Kisiel LC, McKeon A, Wirrell EC. Autoimmune encephalopathies and epilepsies in children and teenagers. Can J Neurol Sci. 2012;39:134–144. doi: 10.1017/s0317167100013147. [DOI] [PubMed] [Google Scholar]
- 3.Quek AM, Britton JW, McKeon A, et al. Autoimmune epilepsy: clinical characteristics and response to immunotherapy. Arch Neurol. 2012;69:582–593. doi: 10.1001/archneurol.2011.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalmau J, Tuzun E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai M, Hughes EG, Peng X, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol. 2009;65:424–434. doi: 10.1002/ana.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9:67–76. doi: 10.1016/S1474-4422(09)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai M, Huijbers MG, Lancaster E, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010;9:776–785. doi: 10.1016/S1474-4422(10)70137-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain. 2010;133:2734–2748. doi: 10.1093/brain/awq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lancaster E, Huijbers MG, Bar V, et al. Investigations of caspr2, an autoantigen of encephalitis and neuromyotonia. Ann Neurol. 2011;69:303–311. doi: 10.1002/ana.22297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boronat A, Gelfand JM, Gresa-Arribas N, et al. Encephalitis and antibodies to dipeptidyl-peptidase-like protein-6, a subunit of Kv4.2 potassium channels. Ann Neurol. 2013;73:120–128. doi: 10.1002/ana.23756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lancaster E, Martinez-Hernandez E, Titulaer MJ, et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology. 2011;77:1698–1701. doi: 10.1212/WNL.0b013e3182364a44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrade DM, Tai P, Dalmau J, Wennberg R. Tonic seizures: a diagnostic clue of anti-LGI1 encephalitis? Neurology. 2011;76:1355–1357. doi: 10.1212/WNL.0b013e3182152808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irani SR, Michell AW, Lang B, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol. 2011;69:892–900. doi: 10.1002/ana.22307. [DOI] [PubMed] [Google Scholar]
- 14.Johnson N, Henry C, Fessler AJ, Dalmau J. Anti-NMDA receptor encephalitis causing prolonged nonconvulsive status epilepticus. Neurology. 2010;75:1480–1482. doi: 10.1212/WNL.0b013e3181f8831a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bayreuther C, Bourg V, Dellamonica J, Borg M, Bernardin G, Thomas P. Complex partial status epilepticus revealing anti-NMDA receptor encephalitis. Epileptic Disord. 2009;11:261–265. doi: 10.1684/epd.2009.0266. [DOI] [PubMed] [Google Scholar]
- 16.Höftberger R, Titulaer MJ, Sabater L, et al. Encephalitis and GABA(B) receptor antibodies: Novel findings in a new case series of 20 patients. Neurology. 2013;81:1500–1506. doi: 10.1212/WNL.0b013e3182a9585f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeffery OJ, Lennon VA, Pittock SJ, Gregory JK, Britton JW, McKeon A. GABAB receptor autoantibody frequency in service serologic evaluation. Neurology. 2013;81:882–887. doi: 10.1212/WNL.0b013e3182a35271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kruer MC, Lim KY, Hoftberger R, Svoboda MD, Woltjer RL, Dalmau J. Aggressive course in encephalitis with opsoclonus, ataxia, chorea, and seizures. JAMA Neurol. in press doi: 10.1001/jamaneurol.2013.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ances BM, Vitaliani R, Taylor RA, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128:1764–1777. doi: 10.1093/brain/awh526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchhalter JR, Dichter MA. Electrophysiological comparison of pyramidal and stellate nonpyramidal neurons in dissociated cell culture of rat hippocampus. Brain Res Bull. 1991;26:333–338. doi: 10.1016/0361-9230(91)90003-3. [DOI] [PubMed] [Google Scholar]
- 21.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes EG, Peng X, Gleichman AJ, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30:5866–5875. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tretter V, Moss SJ. GABA(A) Receptor Dynamics and Constructing GABAergic Synapses. Front Mol Neurosci. 2008;1:1–13. doi: 10.3389/neuro.02.007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapur J, Macdonald RL. Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors. J Neurosci. 1997;17:7532–7540. doi: 10.1523/JNEUROSCI.17-19-07532.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vazquez-Lopez A, Sierra-Paredes G, Sierra-Marcuno G. Anticonvulsant effect of the calcineurin inhibitor ascomycin on seizures induced by picrotoxin microperfusion in the rat hippocampus. Pharmacol Biochem Behav. 2006;84:511–516. doi: 10.1016/j.pbb.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez MI. The possible role of GABAA receptors and gephyrin in epileptogenesis. Front Cell Neurosci. 2013;7:1–7. doi: 10.3389/fncel.2013.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou C, Huang Z, Ding L, et al. Altered cortical GABAA receptor composition, physiology, and endocytosis in a mouse model of a human genetic absence epilepsy syndrome. J Biol Chem. 2013;288:21458–21472. doi: 10.1074/jbc.M112.444372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mikasova L, De Rossi P, Bouchet D, et al. Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain. 2012;135:1606–1621. doi: 10.1093/brain/aws092. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka M, Olsen RW, Medina MT, et al. Hyperglycosylation and reduced GABA currents of mutated GABRB3 polypeptide in remitting childhood absence epilepsy. Am J Hum Genet. 2008;82:1249–1261. doi: 10.1016/j.ajhg.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lancaster E, Dalmau J. Neuronal autoantigens-pathogenesis, associated disorders and antibody testing. Nat Rev Neurol. 2012;8:380–390. doi: 10.1038/nrneurol.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lancaster E, Martinez-Hernandez E, Dalmau J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology. 2011;77:179–189. doi: 10.1212/WNL.0b013e318224afde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malter MP, Helmstaedter C, Urbach H, Vincent A, Bien CG. Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann Neurol. 2010;67:470–478. doi: 10.1002/ana.21917. [DOI] [PubMed] [Google Scholar]
- 34.Blanc F, Ruppert E, Kleitz C, et al. Acute limbic encephalitis and glutamic acid decarboxylase antibodies: a reality? J Neurol Sci. 2009;287:69–71. doi: 10.1016/j.jns.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Florance NR, Davis RL, Lam C. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009;66:11–18. doi: 10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tuzun E, Erdag E, Durmus H, et al. Autoantibodies to neuronal surface antigens in thyroid antibody-positive and -negative limbic encephalitis. Neurol India. 2011;59:47–50. doi: 10.4103/0028-3886.76857. [DOI] [PubMed] [Google Scholar]
- 37.Mukherjee J, Kretschmannova K, Gouzer G, et al. The residence time of GABA(A)Rs at inhibitory synapses is determined by direct binding of the receptor alpha1 subunit to gephyrin. J Neurosci. 2011;31:14677–14687. doi: 10.1523/JNEUROSCI.2001-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kapur J, Lothman EW, DeLorenzo RJ. Loss of GABAA receptors during partial status epilepticus. Neurology. 1994;44:2407–2408. doi: 10.1212/wnl.44.12.2407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.