Abstract
Introduction:
Clinical laboratory work among intermittent and daily waterpipe tobacco smokers has revealed significant risks for tobacco dependence and disease associated with waterpipe tobacco smoking (WTS). No studies have compared these groups directly. This study examined whether WTS frequency was associated with differential puff topography, toxicant exposure, and subjective response using a placebo-control design.
Methods:
Eighty participants reporting WTS of 2–5 episodes (LOW; n = 63) or ≥20 episodes (HIGH; n = 17) per month for ≥6 months completed 2 double-blind, counterbalanced 2-hr sessions that were preceded by ≥12hr of tobacco abstinence. Sessions differed by product smoked ad libitum for 45+ min: preferred brand/flavor of waterpipe tobacco (active) or a flavor-matched tobacco-free waterpipe product (placebo). Outcomes included puff topography, plasma nicotine, carboxyhemoglobin (COHb), and subjective response.
Results:
HIGH users had more puffs, shorter inter-puff-intervals, and a higher total puff volume for placebo relative to active, as well as relative to LOW users during placebo. Plasma nicotine concentrations increased when smoking active (but not placebo) with no significant differences between groups at 25min post-product administration. COHb increased significantly during all conditions; the largest increase was for HIGH users when smoking placebo. There was some evidence of higher baseline scores for nicotine/tobacco nicotine abstinence symptomology.
Conclusions:
Higher frequency waterpipe users may be more sensitive to the effects of waterpipe smoke nicotine content. Among HIGH users, higher baseline nicotine/tobacco abstinence symptoms may indicate greater nicotine dependence. These data support continued surveillance of WTS and development of dependence measures specific to this product.
Introduction
Waterpipe tobacco smoking continues to increase in popularity in the United States, particularly among adolescent and young adult populations.1–3 A recent survey of students at a large, southeastern U.S. university revealed that rates of ever use (46.4% vs. 42.1%) and past year use (28.4% vs. 19.6%) for WTS surpassed those of cigarettes.4 These increases may be due, in part, to the continued belief among many smokers that WTS is less harmful and less addictive than cigarette smoking.5–8 For example, sampled WTS users have reported that waterpipe tobacco contains “less nicotine” and “less chemicals” than cigarette tobacco, that the “water filters (the) smoke,” and that “steam” or “water vapor” is being inhaled.9 In contrast, detailed examinations of the waterpipe smoke content reveal it contains nicotine that causes dependence, polycylic aromatic hydrocarbons that cause cancer, volatile aldehydes that cause lung disease, and carbon monoxide (CO) that contributes to cardiovascular disease.10,11
With respect to nicotine dependence, waterpipe smokers report symptoms commonly associated with nicotine/tobacco withdrawal following a period of overnight smoking abstinence: urges to smoke, craving, and restlessness.12,13 These same symptoms are suppressed by a subsequent bout of WTS.12,13 Moreover, WTS has shown to deliver pharmacologically active doses of nicotine to the user12,14,15 and peak plasma nicotine levels do not differ between a single WTS session and a cigarette.12 Taken together, existing evidence suggests that waterpipe users likely are at risk for many of the same diseases as cigarette smokers,16–19 including nicotine/tobacco dependence.20,21
Cross-sectional surveys reveal that the majority of sampled U.S. waterpipe smokers report less than daily use22,23 and most often smoke in a group setting with family and/or friends.4,7 Some of these waterpipe users, however, may eventually transition to more frequent WTS, consequently displaying a different pattern of use. For comparison, relative to daily cigarette smokers, intermittent cigarette smokers exhibit lower nicotine dependence scores,24,25 smoke fewer cigarettes on smoking days and more frequently on weekends,25 are more likely to engage in smoking during social situations and around other smokers,25 and report a lower desire to smoke following a period of abstinence.24 Moreover, the development of nicotine dependence may coincide with changes in the manner in which a cigarette is smoked (numbers of puffs, interpuff interval [IPI]) and the frequency of consumption (i.e., cigarettes per day [CPD];26). For instance, scores on one measure of nicotine dependence have shown to be positively correlated with cigarette topography parameters such as puff number and volume.27 In another study among cigarette smokers, increasing dependence level was associated with longer puff duration and shorter IPI as well as greater compensation when smoking a low nicotine-yield cigarette.28 When the influence of social stimuli (e.g., conversing with two individuals) were compared between heavy (≥22 CPD) and light (≤18 CPD) smokers, only light smokers’ puff topography was significantly influenced; under social conditions this group took longer and more frequent puffs.29 In waterpipe smokers, higher scores on the Lebanese Waterpipe Dependence Scale30 have also been associated with reporting more smoking episodes per week (men only), higher CO levels, and longer puff duration.31 In sum, data from cigarette smoking literature and preliminary findings among waterpipe users suggest differential profiles of use may exist between users based upon dependence level and consumption frequency.
To date, no study has examined whether WTS frequency (daily vs. intermittent) is associated with differential puff topography. An investigation of this type could lead to important information about the variability in response to nicotine delivered via WTS and user behavior profiles. Thus, individuals who self-reported WTS of 2–5 episodes or ≥20 episodes per month were recruited to participate in this study. Participants completed two double-blind, counter-balanced conditions: active waterpipe tobacco or tobacco-free waterpipe product. Both sessions were preceded by at least 12hr of nicotine/tobacco abstinence. Primary outcomes of interest were puff topography, toxicant exposure, and subjective response as compared between individuals with low and high frequency WTS history.
Methods
Eighty-three participants recruited from the metropolitan area surrounding Richmond, VA provided informed consent and attended at least one session in this IRB-approved study between 2008–2010. Two withdrew and one was discontinued due to poor venous access. Thus, there were 80 completers of the current study. Participants were eligible if their WTS patterns for the past 6 months were low (2–5 waterpipes per month; n = 63) or high (20 waterpipes or more per month; n = 17) frequency. While equal recruitment of both frequency groups was attempted, as shown in Table 1 63 low frequency users (LOW; 3 Hispanic) and 17 high frequency users (HIGH; 1 Hispanic) ultimately completed the current study. Results from a subset of the LOW frequency users (n = 37) have been reported elsewhere.14 HIGH users were more likely than LOW users to be non-White, older, report a higher number of years of education, report smoking significantly more waterpipes per month, and have a higher expired air CO reading at screening (all ps < .05).
Table 1.
Statistical Analysis Results for Completers’ Demographic Data by Waterpipe Tobacco Smoking Frequency
| LOW (n = 63) | HIGH (n = 17) | p a | |
|---|---|---|---|
| Gender | |||
| Male (%) | 42 (67) | 13 (77) | n.s. |
| Race | |||
| Caucasian (%) | 38 (60) | 5 (29) | <.05 |
| Non-White (%) | 25 (40) | 12 (71) | |
| Age (years)b | 20.5 (2.2) | 21.7 (2.3) | <.05 |
| Student status (%) | 53 (84) | 14 (82) | n.s. |
| Education (years)b | 13.5 (1.4) | 14.6 (1.7) | <.01 |
| Waterpipe uses/monthb | 3.6 (1.2) | 23.8 (4.7) | <.001 |
| Duration of waterpipe use (months)b | 19.8 (13.9) | 22.4 (17.2) | n.s. |
| Screening expired air CO (ppm)b | 2.9 (3.0) | 6.6 (5.4) | <.01 |
| Past 30-day cigarette smoking (%) | 3 (5) | 1 (6) | n.s. |
| Past 30-day alcohol use (%) | 43 (68) | 12 (71) | n.s. |
| Past 30-day marijuana use (%) | 19 (30) | 2 (12) | NA |
WTS = waterpipe tobacco smoking. a Non-parametric techniques (Chi-square or Mann-Whitney U) used due to small sample sizes and lack of normality; NA indicates cell sizes were too small for analysis.
b Data presented as mean (standard deviation).
Exclusion criteria included self-reported history of chronic health problems or psychiatric conditions, regular use of prescription medications (other than vitamins or birth control), and current pregnancy (verified by urinalysis) or breastfeeding, as well as self-reported current use of >5 cigarettes/month, other tobacco products, marijuana (>5 days in past month) or other illicit drugs (past 30-day use of cocaine, benzodiazepines, opioids, or methamphetamine; confirmed by urinalysis).
Materials
The WTS apparatus and materials used in all sessions were identical to those described in previous work.12,14 Each session’s product was loaded into a glazed ceramic waterpipe head and was covered with a perforated circular sheet of aluminum foil. A quick-lighting charcoal briquette was lit by study staff and placed on top of the foil. The leather hose was fitted with puff topography measurement hardware (see puff topography section) and included a wooden mouthpiece capped with a sterile plastic tip.
During the active condition, participants smoked their preferred brand and flavor of waterpipe tobacco product. The most popular tobacco-based brand was Starbuzz (United States; n = 36), followed by Nakhla (Egypt; n = 15), Al Fakher (United Arab Emirates; n = 9) and Fumari (n = 1); Nakhla was used as the default brand for participants who did not report a preference (n = 19). The most popular flavors reported were fruit-based: strawberry (n = 15), apple/double apple (n = 13), mango (n = 10), watermelon/melon (n = 8), peach (n = 5), guava (n = 4), cherry (n = 3), mixed fruit (n = 3), orange (n = 2), and grape (n = 1). Non-fruit preferred flavors were mint (n = 12), rose (n = 2), and vanilla (n = 2). During the placebo condition, participants smoked a flavor-matched, tobacco-free herbal waterpipe product (SoeX; SoeX India Pvt. Ltd.). According to the manufacturer, SoeX is “0% nicotine, 0% tar, and above all 0% tobacco,” primarily composed of “non-tobacco herbal material”.32 We have previously verified that waterpipe smoke produced using SoeX products contains no nicotine.11,33
Procedure
Complete procedural details of the current study have been described previously,14 and thus abbreviated below where appropriate. Participants completed two counterbalanced, 2-hr sessions that differed by product used: active tobacco or flavor-matched, tobacco-free placebo. At each session, once overnight tobacco abstinence was verified (CO levels ≤ 10 ppm), a catheter was inserted into a forearm vein and physiological recording commenced. Thirty minutes later, baseline measures of physiological and subjective response were assessed. Participants then began a double-blind use period with 10g of session-specific product. Participants were given a minimum of 45min to smoke the waterpipe ad libitum, and puff topography was measured throughout the smoking period. Venous blood samples, for plasma nicotine and carboxhemoglobin (COHb; blood measure of CO exposure) concentration, were taken 5 and 25min after smoking onset, and again upon the completion of smoking (45min or later). Subjective measures and physiological measures were also assessed post-smoking. During each session, participants were permitted to watch a movie of their choice, except while completing the subjective measures. The laboratory room was ventilated; during session, mean peak ambient CO level was 4.0 ppm (SD = 1.0; collapsed across condition; data available for n = 128 sessions). Payment for completing both sessions was $175.
Puff Topography
Puff topography was measured via a venturi meter integrated into the waterpipe hose34 whereby inhalation-induced pressure changes are measured via a pressure transducer digitized and sampled. Previously calibrated software converted digital signals to air flow (milliliters per second) and processed these data to produce measures of puff volume, puff duration, number of puffs, IPI, and total volume inhaled.
Physiological Measures
Carboxyhemoglobin (COHb) concentration was analyzed within 2min after venous blood sampling (NPT7 blood gas analyzer, Radiometer America). The 10ml blood samples were then centrifuged, and the plasma stored at −70 °C for later analysis of nicotine level (limit of quantitation 2.0ng/ml using a modified LC–MS/MS version of that reported by Naidong et al.35(see Breland et al.36 for details). Heart rate (HR) was measured every 20 s and blood pressure/mean arterial pressure (BP/MAP) every 5min (Model 507E, Criticare Systems). Expired and ambient air CO levels were assessed with a BreathCO monitor (Vitalograph). Expired air nitric oxide (NO) was analyzed using a Nitric Oxide Analyzer (280i, Ionics Inst); the average of three satisfactory measurements for each time point was used in analyses. Pulmonary function testing (PFT) was performed with a spirometer (Vitalograph) to measure forced expiratory volume in 1 s (FEV1), forced expiratory vital capacity (FVC), and FEV1/FVC ratio.37 The better of two satisfactory PFT maneuvers for each time point (based on FEV1 results) was used in analyses.
Subjective Measures
Participants used a computer keyboard and mouse to respond to four subjective measures. Individual items for each measure are summarized below (see Blank et al.14 for more details). The adapted version of the Minnesota Nicotine Withdrawal Scale (MNWS)38 consists of 11 visual analog scale (VAS) items. Items are presented as a word or phrase centered above a horizontal line that ranges from 0 (“Not at all”) to 100 (“Extremely”). Participants used a computer mouse to place a vertical mark anywhere along the horizontal line, and the score is the distance of the vertical mark from the left anchor, expressed as a percentage of total line length. The Questionnaire of Smoking Urges (QSU): Brief Form39 consists of 10 smoking-related items (e.g., “I crave a cigarette right now”) that participants rate on a 7-point scale (“Strongly disagree” to “Strongly agree”). The items were collapsed into two previously-defined factors: “intention to smoke” (Factor 1) and “anticipation of relief from withdrawal” (Factor 2). QSU-Brief items were modified by replacing the word “cigarette” with “waterpipe.” The Direct Effects of Nicotine Scale (DENS) consists of 15 VAS items developed to assess the incidence of nicotine-related side effects.40 The Direct Effects of Tobacco Scale41 consists of 13 VAS items developed to assess commonly reported cigarette smoking effects (items modified so that the word “cigarette” was replaced by “waterpipe”).
Data Analysis
HR, BP, and MAP values were averaged into 5-min bins beginning with the 5min preceding product administration (missing values replaced with average of value before and after; <1% of data). Data preparation techniques were identical to those used previously.14 Due to human error or device malfunctioning, sample sizes are 76 for puff topography (LOW = 59; HIGH = 17) and plasma nicotine (LOW = 60; HIGH = 16), 78 for MAP (LOW = 61; HIGH = 17), 71 for NO (LOW = 56; HIGH = 15), 72 for pulmonary function measures (LOW = 57; HIGH = 15), and 79 for subjective outcomes (LOW = 63; HIGH = 16). Forty-five of the 80 completers chose to smoke for longer than the minimum 45-min smoking period during at least one session: the active condition only (n = 14), the placebo condition only (n = 19), or both conditions (n = 12). Across sessions during which smoking was greater than 45min, average time spent smoking during placebo was 55.6min (SD = 12.1; n = 31) and during active was 54.4min (SD = 9.6; n = 25; exact time for one session missing due to topography device malfunction). Participants who smoked for 45 or >45min did not differ significantly in terms of age, gender, race, months smoking waterpipe, or WTS frequency status (37/63 [59%] of LOW users, 8/17 [47%] of HIGH users; all ps > .05). While our previous analysis of LOW users did not reveal an effect of smoking duration on study outcomes,14 our initial analysis among this sample did indicate a potential influence of this factor on physiological and subjective outcomes. Thus, only time points that were measured at baseline and within the initial 45-min product administration period are included here for all measures. For topography measures, only data collected during the initial 45min of the smoking period were analyzed. Nicotine and COHb were truncated to baseline and the 25min time point measurements. HR, BP, and MAP were truncated to the 5-min bin preceding product administration and nine 5-min bins post-administration (up to 45min). Baseline data for expired air NO and pulmonary function measures were also analyzed. For subjective questionnaires, only baseline measures were included given that the post-smoking time points occurred after 45min for some participants. Consequently, the DETS subjective measure was excluded entirely from analyses as items from this scale ask participants to rate the product smoked (not relevant for baseline values).
These truncated data for all measures were analyzed using a mixed analysis of variance (ANOVA) where WTS frequency (LOW, HIGH) was the between-subjects factor and condition (active, placebo) and/or time (pre vs. post) were the within-subjects factors. For all ANOVA analyses, Huynh-Feldt corrections were used to adjust for violations of the sphericity assumption.42 Planned comparisons (paired or independent) using Student’s t test were used to examine differences between means and comparisons for which p < .05 are reported as significant.43 All analyses were performed in IBM SPSS Version 21.
Results
Puff Topography
Summary statistics and statistical analysis results from the truncated puff topography data are shown in Table 2. A significant frequency group by condition interaction was observed for IPI, puffs per session, and total inhaled volume [Fs (1, 74) > 4.5, ps < .05]. HIGH users had a significantly shorter average IPIs during placebo relative to active, and this average IPI was significantly shorter than that observed for LOW users during placebo (ps < .05). HIGH users also took significantly more puffs during placebo than active, and significantly more puffs than LOW users during placebo (ps < .05). This same pattern was observed for total puff volume during the placebo condition (ps < .05). There were no significant differences within frequency group or between LOW and HIGH users for either condition for puff duration or mean puff volume.
Table 2.
Summary Statistics and Statistical Analysis Results for Puff Topography Measures Truncated to the First 45min of Smoking
| Low (n = 59) | High (n = 17) | Condition (Cond) | Frequency (Freq) | Cond × Freq | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Active | Placebo | Active | F | p | F | p | F | p | |
| Puff duration (s) | 3.7 (1.5) | 3.7 (1.7) | 4.9 (2.4) | 4.4 (2.0) | 0.4 | n.s | 5.2 | <.05 | 0.6 | n.s |
| Interpuff interval (s) | 42.9 (24.6) | 43.2 (30.7) | 25.6 (12.2)#* | 45.2 (16.7) | 1.5 | n.s | 2.0 | n.s | 4.6 | <.05 |
| Puffs | 72.8 (44.8) | 78.4 (50.3) | 109.4 (70.9)#* | 57.0 (23.6) | 1.3 | n.s | 0.5 | n.s | 14.4 | <.001 |
| Total volume (l) | 56.6 (31.5) | 55.3 (44.5) | 90.9 (54.4)#* | 54.5 (35.6) | 2.6 | n.s | 3.7 | n.s | 6.6 | <.05 |
| Puff volume (l) | 0.8 (0.4) | 0.8 (0.5) | 1.0 (0.5) | 1.0 (0.6) | 0.2 | n.s | 3.4 | n.s | 0.3 | n.s |
Asterisks (*) indicate a significant difference between conditions within frequency group, and number signs (#) indicate a significant difference between LOW and HIGH users for that condition (ps < .05). Degrees of freedom: Cond = (1, 74); Freq = (1, 74); Cond × Freq = (1,74).
Physiological Measures
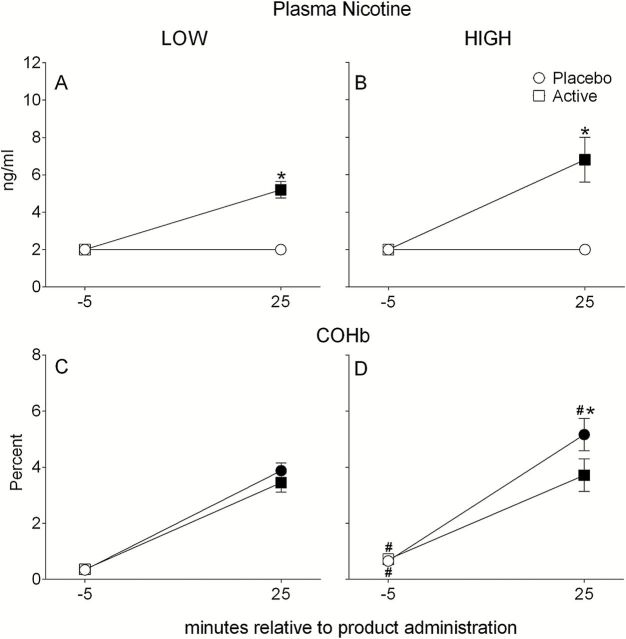
A significant interaction of condition by time was observed for plasma nicotine [F (1, 74) = 65.6, p < .001]. For both LOW and HIGH frequency users, mean plasma nicotine concentration increased significantly from baseline at 25min during the active condition (ps < .05; see Figures 1A and 1B) and was also significantly greater at 25min for active versus placebo conditions (ps < .01). However, no mean ± SEM significant difference was observed between LOW and HIGH users at 25min (LOW = 5.2±0.4ng/ml; HIGH = 6.8±1.2ng/ml; p = n.s.) during the active condition.
Figure 1.
Mean ± SEM by waterpipe use frequency group (LOW, HIGH) for plasma nicotine (panel A, n = 60; panel B, n = 16) and COHb (panel C, n = 63; panel D, n = 17) for the placebo and active conditions. Filled symbols indicate a significant difference relative to −5min; asterisks (*) indicate a significant difference between placebo and active conditions at that time point within frequency group; number signs (#) indicate a significant difference between LOW and HIGH users at that time point and same condition (ps < .05).
For COHb, a significant interaction of condition by time was observed [F (1, 78) = 8.6, p < .01] in that mean levels increased significantly relative to baseline at 25min during both conditions within frequency groups (ps < .001; see Figures 1B and 1C). While COHb did not differ between conditions for LOW users (Figure 1B), levels at 25min were significantly different between placebo and active conditions for HIGH users (p < .01; Figure 1C). Mean COHb at baseline during both conditions was significantly greater for HIGH users (placebo = 0.7±0.1%; active = 0.7±0.1%) compared to LOW users (placebo = 0.3±0.0%; active = 0.4±0.0%; ps < .05). During placebo, mean COHb during was significantly greater for HIGH versus LOW users at 25min (HIGH = 5.2±0.6%; LOW = 3.9±0.3%; p < .05).
Statistical analysis results for other physiological outcomes (HR, BP/MAP, expired air NO, pulmonary function) are included in the online Supplementary Appendix as they showed little difference between LOW and HIGH users, and were relatively equivalent to those from the previous report among LOW users only.14
Subjective Measures
Table 3 displays the statistical analysis results for three baseline subjective measures.
Table 3.
Statistical Analysis Results for Baseline Subjective Measures
| Condition (Cond) | Frequency (Freq) | Cond × Freq | ||||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Waterpipe-modified MWS | ||||||
| Urges to smoke a waterpipe | 0.7 | n.s | 12.7 | <.01 | <0.1 | n.s |
| Irritability/Frustration/Anger | 0.3 | n.s | 4.9 | <.05 | 1.8 | n.s |
| Anxious | 13.2 | <.001 | 10.7 | <.01 | 3.9 | n.s |
| Difficulty concentrating | 0.3 | n.s | <0.1 | n.s | 0.1 | n.s |
| Restlessness | 3.5 | n.s | 3.4 | n.s | 0.1 | n.s |
| Hunger | 0.7 | n.s | 1.1 | n.s | <0.1 | n.s |
| Impatient | 1.8 | n.s | 3.0 | n.s | 0.3 | n.s |
| Craving a waterpipe/Nicotine | 0.2 | n.s | 13.3 | <.001 | 1.5 | n.s |
| Drowsiness | 0.1 | n.s | 6.9 | <.05 | 0.1 | n.s |
| Depression/Feeling blue | 0.9 | n.s | 7.7 | <.01 | 1.6 | n.s |
| Desire for sweets | 0.1 | n.s | 5.7 | <.05 | 2.0 | n.s |
| Waterpipe-modified QSU-Brief | ||||||
| Factor 1 | 2.0 | n.s | 8.0 | <.01 | 0.3 | n.s |
| Factor 2 | 0.5 | n.s | 8.8 | <.01 | 0.1 | n.s |
| DENS | ||||||
| Nauseous | <0.1 | n.s | 2.9 | n.s | 0.3 | n.s |
| Dizzy | 0.5 | n.s | 2.6 | n.s | 0.3 | n.s |
| Lightheaded | 1.2 | n.s | 4.0 | <.05 | 0.3 | n.s |
| Nervous | 0.4 | n.s | 8.2 | <.01 | 1.4 | n.s |
| Sweaty | 3.1 | n.s | 0.8 | n.s | 0.1 | n.s |
| Headache | 1.4 | n.s | 0.9 | n.s | <0.1 | n.s |
| Excessive salivation | <0.1 | n.s | 5.7 | <.05 | 5.3 | <.05 |
| Heart pounding | <0.1 | n.s | 5.5 | <.05 | 2.4 | n.s |
| Confused | <0.1 | n.s | 2.6 | n.s | 3.0 | n.s |
| Weak | 1.6 | n.s | 7.0 | <.05 | 0.1 | n.s |
Degrees of freedom: Condition = (1, 77); Frequency = (1, 77); Condition × Frequency = (1, 77).
Nicotine/Tobacco Abstinence Symptoms
Across all baseline measures of the waterpipe-modified MWS, there were no interactions between condition and frequency group. One item, “Anxious,” revealed a main effect of condition, and nine items (“Urges to smoke a waterpipe,” “Irritability/Frustration/Anger,” “Anxious,” “Craving a waterpipe/Nicotine,” “Drowsiness,” “Depression/Feeling blue,” “Desire for sweets”) revealed a significant main effect of frequency group (see Table 3). Results for “Anxious” indicated that HIGH users’ mean baseline rating was significantly higher than that for LOW users’ during placebo (HIGH = 29.9±7.1, LOW = 11.1±2.1; p < .05), but not during active (HIGH = 16.4±6.2, LOW = 6.4±1.6, p = n.s.). Within frequency group, significantly greater baseline ratings of anxious were observed during the placebo condition for both HIGH and LOW users (ps < .05).
Two of the items from the waterpipe-modified MWS with the highest main effect F-values for frequency group were “Urges to smoke a waterpipe” and “Craving a waterpipe/Nicotine”. Mean baseline ratings for “Urges to smoke a waterpipe” during both conditions were twice as high for HIGH users (placebo = 45.4±7.7; active = 42.6±8.3) compared to LOW users (placebo = 22.4±2.8; active = 20.7±2.9) (ps < .05). There were no between condition differences within frequency groups. Mean baseline ratings for “Craving a waterpipe/Nicotine” also differed significantly between HIGH and LOW users during both conditions (ps < .05). Ratings were almost three times higher for HIGH users (placebo = 33.7±8.0; active = 30.1±7.8) compared to LOW users (placebo = 10.4±2.2; active = 12.5±2.4). There were no significant differences between conditions within frequency groups. Results for other waterpipe-modified MWS items followed a similar pattern with higher baseline ratings for the HIGH group during both conditions.
Factors 1 and 2 of the waterpipe-modified QSU-Brief had a significant main effect of frequency group (see Table 3). During both conditions, mean baseline ratings for Factor 1 of the waterpipe-modified QSU were significantly higher for HIGH users (placebo = 15.4±2.0; active = 15.7±2.3) relative to LOW users (placebo = 9.3±1.0; active = 10.4±0.9; ps < .05). Within frequency group there were no differences between conditions. Results for Factor 2 displayed an identical pattern of results with significantly higher ratings during both conditions for HIGH users and no between condition differences within frequency groups.
Direct Effects of Nicotine Scale
Across DENS baseline measures, one significant condition by frequency group interaction was observed for “Excessive Salivation” (see Table 3). Mean baseline ratings for “Excessive Salivation” differed significantly between HIGH and LOW users during placebo (HIGH = 11.9±4.2; LOW = 2.6±1.1; p < .05) but not during active (HIGH = 7.8±3.9, LOW = 3.6±1.2; p = n.s.). There were no significant differences between conditions within frequency groups.
A significant main effect of frequency group was observed five DENS items (“Lightheaded,” “Nervous,” “Excessive Salivation,” “Heart Pounding,” and “Weak” [see Table 3]). Nervous and Weak DENS items had the highest main effect F-values for frequency group. Baseline ratings for both items were higher among the HIGH users relative to LOW users but did not differ significantly. There were no significant differences between conditions within frequency groups.
Discussion
Tobacco use is maintained, in part, by dependence on nicotine.44 Defining features of nicotine dependence include increased use of tobacco over time, aversive withdrawal symptoms following tobacco cessation, and/or continued use of tobacco to avoid these withdrawal symptoms.45 For instance, these features are developed as cigarette smokers transition from light/intermittent use to regular use.26,46 Given that, like cigarette smoking, WTS also delivers nicotine to the user,21 similar features may be observed among waterpipe users (e.g., Alzoubi et al.31). The purpose of this study was to compare the smoking behavior, toxicant exposure, and subjective responses between regular and light/intermittent waterpipe smokers.
Puff topography parameters, and consequently plasma nicotine and COHb concentrations, did not differ between HIGH and LOW users when a nicotine-containing product was smoked. However, HIGH users took more puffs, more frequent puffs, and larger puffs than LOW users when a nicotine-free placebo product was smoked as well as compared within frequency group when HIGH users smoked a nicotine-containing product. LOW users’ puff topography did not differ between conditions consistent with the previous analysis.14 This difference in puffing behavior resulted in significantly greater CO exposure for HIGH during placebo relative to LOW users during placebo and to HIGH users during active. A similar effect has been observed among dependent cigarette smokers during initial bouts of low nicotine (i.e., de-nicotinized) cigarette administration. That is, puff volumes increase acutely as the nicotine content (or yield) of cigarettes is decreased among daily smokers.47,48 Such a behavioral response is hypothesized to be a compensatory mechanism for the reduced amount of nicotine extracted with each puff.49 In this study, we found that HIGH users were sensitive to the absence of nicotine and thus altered their puff topography in an attempt to extract more of the drug when using a placebo product. In this case, the alteration of puff topography is likely an index of greater dependence level in these users.
Consistent with a greater level of dependence in HIGH users, higher baseline scores of nicotine/tobacco withdrawal symptomatology were observed in this group relative to LOW users (as in Corrigall et al.24). These same symptoms (e.g., “irritability,” “craving”) are those commonly reported by dependent cigarette smokers following a period of nicotine/tobacco abstinence.38,50 Abstinence-induced withdrawal effects may also be seen as an index of dependence, and suppression and/or avoidance of these aversive effects may promote continued WTS.
The relationship between higher WTS frequency and dependence characteristics shown here, in combination with those from the Eastern Mediterranean Region where WTS has a longer history of use, highlights the need for a dependence measure that is specific to WTS.51,52 Unique features of WTS (e.g., social use, lack of product mobility relative to cigarettes/smokeless) suggest that modifying current dependence measures for cigarettes and/or smokeless tobacco may not be sufficient. With data from the United States suggesting increased rates of WTS among youth and young adult populations,4,53 more frequent use patterns may begin to emerge and there may be a greater risk for transition to nicotine/tobacco dependence. While waiting for a measure of WTS that has been validated in this population, state- and national-level tobacco surveillance efforts should add items that assess patterns of WTS use that provide finer detail than the relatively coarse measure of past 30-day use.
Limitations
Data from the current study must be considered in the context of several limitations. Waterpipe users in different geographical areas, or among adolescent or older adult populations, may differ in behavior and/or subjective response from the groups studied here. HIGH users differed in racial background from LOW users, possibly indicative of cultural influences on patterns of WTS. Importantly, the laboratory environment was standardized but WTS often occurs in a café or home environment.7,54 While efforts were made to approximate such an environment (e.g., reclining chairs, movies, etc), participants were not exposed to other likely important stimuli. For example, a waterpipe is often shared with family and friends in a group setting.54,55 Waterpipe users have also been observed to engage in a variety of other behaviors while smoking, including eating, drinking, and other tobacco use.56 In addition, some researchers have suggested that cigarette smoking history may influence WTS puff topography and toxicant exposure.15 While participants in the current study were not concurrent cigarette smokers, the extent to which they had ever smoked a cigarette is unclear. Exclusive waterpipe users, especially those reporting near daily use, may be even more dependent on this method of nicotine self-administration.
Conclusions
Relative to LOW users, HIGH users displayed a differential pattern of puffing behavior when smoking a placebo waterpipe product and also reported higher baseline nicotine/tobacco abstinence symptoms. These results provide evidence for a greater level of nicotine/tobacco dependence. Results support continued surveillance of WTS and research to understand the progression of use and role of nicotine in continued waterpipe use.
Supplementary Material
Supplementary Appendix can be found online at http://www.ntr.oxfordjournals.org
Funding
This work was supported by the National Institutes of Health (R01CA120142, R01DA025659, F31DA028102).
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
Portions of this work were presented at the 19th Annual Meeting of the Society for Research on Nicotine and Tobacco, Boston, MA, March 13–16, 2013. All work performed at Virginia Commonwealth University and the American University of Beirut.
References
- 1. Centers for Disease Control and Prevention. Tobacco product use among middle and high school students - United States, 2011 and 2012. MMWR Morb Mortal Wkly Rep. 2013;62:893–897. [PMC free article] [PubMed] [Google Scholar]
- 2. Amrock SM, Gordon T, Zelikoff JT, Weitzman M. Hookah use among adolescents in the United States: results of a national survey. Nicotine Tob Res. 2014;16 (2):231–237. doi:10.1093/ntr/ntt160 [DOI] [PubMed] [Google Scholar]
- 3. Primack BA, Shensa A, Kim KH, et al. Waterpipe smoking among U.S. university students. Nicotine Tob Res. 2013; 15:29–35. 10.1093/ntr/nts076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barnett TE, Smith T, He Y, et al. Evidence of emerging hookah use among university students: a cross-sectional comparison between hookah and cigarette use. BMC Public Health. 2013;13:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aljarrah K, Ababneh ZQ, Al-Delaimy WK. Perceptions of hookah smoking harmfulness: predictors and characteristics among current hookah users. Tob Induc Dis. 2009;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cobb CO, Khader Y, Nasim A, Eissenberg T. A multiyear survey of waterpipe and cigarette smoking on a U.S. university campus. J Am Coll Health. 2012;60:521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sutfin EL, McCoy TP, Reboussin BA, Wagoner KG, Spangler J, Wolfson M. Prevalence and correlates of waterpipe tobacco smoking by college students in North Carolina. Drug Alcohol Depend. 2011;115:131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wray RJ, Jupka K, Berman S, Zellin S, Vijaykumar S. Young adults’ perceptions about established and emerging tobacco products: results from eight focus groups. Nicotine Tob Res. 2012;14:184–190. [DOI] [PubMed] [Google Scholar]
- 9. Smith JR, Novotny TE, Edland SD, Hofstetter CR, Lindsay SP, Al-Delaimy WK. Determinants of hookah use among high school students. Nicotine Tob Res. 2011;13:565–572. [DOI] [PubMed] [Google Scholar]
- 10. Katurji M, Daher N, Sheheitli H, Saleh R, Shihadeh A. Direct measurement of toxicants inhaled by water pipe users in the natural environment using a real-time in situ sampling technique. Inhal Toxicol. 2010;2:1101–1109. [DOI] [PubMed] [Google Scholar]
- 11. Shihadeh A, Salman R, Jaroudi E, et al. Does switching to a tobacco-free waterpipe product reduce toxicant intake? A crossover study comparing CO, NO, PAH, volatile aldehydes, “tar” and nicotine yields. Food Chem Toxicol. 2012;50:1494–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cobb CO, Shihadeh A, Weaver MF, Eissenberg T. Waterpipe tobacco smoking and cigarette smoking: a direct comparison of toxicant exposure and subjective effects. Nicotine Tob Res. 2011;13:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maziak W, Rastam S, Ibrahim I, Ward KD, Shihadeh A, Eissenberg T. CO exposure, puff topography, and subjective effects in waterpipe tobacco smokers. Nicotine Tob Res. 2009;11:806–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blank MD, Cobb CO, Kilgalen B, et al. Acute effects of waterpipe tobacco smoking: a double-blind, placebo-control study. Drug Alcohol Depend. 2011;116:102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jacob P, III, Abu Raddaha AH, Dempsey D, et al. Nicotine, carbon monoxide, and carcinogen exposure after a single use of a water pipe. Cancer Epidemiol Biomarkers Prev. 2011;20:2345–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Akl EA, Gaddam S, Gunukula SK, Honeine R, Jaoude PA, Irani J. The effects of waterpipe tobacco smoking on health outcomes: a systematic review. Int J Epidemiol. 2010;39:834–857. [DOI] [PubMed] [Google Scholar]
- 17. Khabour OF, Alzoubi KH, Bani-Ahmad M, Dodin A, Eissenberg T, Shihadeh A. Acute exposure to waterpipe tobacco smoke induces changes in the oxidative and inflammatory markers in mouse lung. Inhal Toxicol. 2012;24:667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rammah M, Dandachi F, Salman R, Shihadeh A, El-Sabban M. In vitro cytotoxicity and mutagenicity of mainstream waterpipe smoke and its functional consequences on alveolar type II derived cells. Toxicol Lett. 2012;211:220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rammah M, Dandachi F, Salman R, Shihadeh A, El-Sabban M. In vitro effects of waterpipe smoke condensate on endothelial cell function: a potential risk factor for vascular disease. Toxicol Lett. 2013;219:133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Auf RA, Radwan GN, Loffredo CA, El Setouhy M, Israel E, Mohamed MK. Assessment of tobacco dependence in waterpipe smokers in Egypt. Int J Tuberc Lung Dis. 2012;16:132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Neergaard J, Singh P, Job J, Montgomery S. Waterpipe smoking and nicotine exposure: a review of the current evidence. Nicotine Tob Res. 2007;9:987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heinz AJ, Giedgowd GE, Crane NA, et al. A comprehensive examination of hookah smoking in college students: use patterns and contexts, social norms and attitudes, harm perception, psychological correlates and co-occurring substance use. Addict Behav. 2013;38:2751–2760. [DOI] [PubMed] [Google Scholar]
- 23. Primack BA, Shensa A, Kim KH, et al. Waterpipe smoking among U.S. university students. Nicotine Tob Res. 2013;15:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Corrigall WA, Zack M, Eissenberg T, Belsito L, Scher R. Acute subjective and physiological responses to smoking in adolescents. Addiction. 2001;96:1409–1417. [DOI] [PubMed] [Google Scholar]
- 25. Shiffman S, Tindle H, Li X, Scholl S, Dunbar M, Mitchell-Miland C. Characteristics and smoking patterns of intermittent smokers. Exp Clin Psychopharmacol. 2012;20:264–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Caraballo RS, Novak SP, Asman K. Linking quantity and frequency profiles of cigarette smoking to the presence of nicotine dependence symptoms among adolescent smokers: findings from the 2004 National Youth Tobacco Survey. Nicotine Tob Res. 2009;11:49–57. [DOI] [PubMed] [Google Scholar]
- 27. Zielińska-Danch W, Goniewicz MŁ, Koszowski B, et al. [Relationship between nicotine dependence and smoking topography]. Przegl Lek. 2010;67:1033–1036. [PubMed] [Google Scholar]
- 28. Fagerstrom K, Bates S. Compensation and effective smoking by different nicotine-dependent smokers. Addict Behav. 1981;6:331–336. [Google Scholar]
- 29. Miller PM, Frederiksen LW, Hosford RL. Social interaction and smoking topography in heavy and light smokers. Addict Behav. 1979;4:147–153. [DOI] [PubMed] [Google Scholar]
- 30. Salameh P, Waked M, Aoun Z. Waterpipe smoking: construction and validation of the Lebanon Waterpipe Dependence Scale (LWDS-11). Nicotine Tob Res. 2008;10:149–158. [DOI] [PubMed] [Google Scholar]
- 31. Alzoubi KH, Khabour OF, Azab M, et al. CO exposure and puff topography are associated with Lebanese waterpipe dependence scale score. Nicotine Tob Res. 2013;15:1782–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. SoeX Herbal. 2013. SoeX Herbal. SOPARIWALA EXPORTS Pvt Ltd. Web site: http://www.soex.com/index.php?route=product/category&path=83_90 Accessed September 30, 2014.
- 33. Hammal F, Chappell A, Wild TC, et al. Herbal’ but potentially hazardous: an analysis of the constituents and smoke emissions of tobacco-free waterpipe products and the air quality in the cafes where they are served. Tob Control. 2013. 10.1136/tobaccocontrol-2013–051169. [DOI] [PubMed] [Google Scholar]
- 34. Shihadeh A, Antonios C, Azar S. A portable, low-resistance puff topography instrument for pulsating, high-flow smoking devices. Behav Res Methods. 2005;37:186–191. [DOI] [PubMed] [Google Scholar]
- 35. Naidong W, Shou W, Chen Y-L, Jiang X. Novel liquid chromatographic-tandem mass spectrometric methods using silica columns and aqueous-organic mobile phases for quantitative analysis of polar ionic analytes in biological fluids. J Chromatogr B. 2001;754:387–399. [DOI] [PubMed] [Google Scholar]
- 36. Breland AB, Kleykamp BA, Eissenberg T. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nicotine Tob Res. 2006;8 (6):727–738. [DOI] [PubMed] [Google Scholar]
- 37. National Collaborating Centre for Chronic Conditions. Chronic obstructive pulmonary disease. National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax. 2004;59(Suppl 1):1–232. [PMC free article] [PubMed] [Google Scholar]
- 38. Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. [DOI] [PubMed] [Google Scholar]
- 39. Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. [DOI] [PubMed] [Google Scholar]
- 40. Evans SE, Blank M, Sams C, Weaver MF, Eissenberg T. Transdermal nicotine-induced tobacco abstinence symptom suppression: nicotine dose and smokers’ gender. Exp Clin Psychopharmacol. 2006;14:121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Foulds J, Stapleton J, Feyerabend C, Vesey C, Jarvis M, Russell MA. Effect of transdermal nicotine patches on cigarette smoking: a double blind crossover study. Psychopharmacology (Berl). 1992;106:421–427. [DOI] [PubMed] [Google Scholar]
- 42. Huynh H, Feldt LS. Estimation of the box correction for degrees of freedom from sample data in a randomized block and split-plot designs. J Educ Stat. 1976;1:69–82. [Google Scholar]
- 43. Keppel G. Design and Analysis: A Researcher’s Handbook. Englewood Cliffs, NJ: Prentice Hall; 1991. [Google Scholar]
- 44. Benowitz NL. Clinical pharmacology of nicotine. Annu Rev Med. 1986;37:21–32. [DOI] [PubMed] [Google Scholar]
- 45. American Psychological Association. Diagnostic and statistical manual of mental disorders: DSM-V. Washington, DC: American Psychological Association; 2013. [Google Scholar]
- 46. Zhan W, Dierker LC, Rose JS, Selya A, Mermelstein RJ. The natural course of nicotine dependence symptoms among adolescent smokers. Nicotine Tob Res. 2012;14:1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Macqueen DA, Heckman BW, Blank MD, Janse Van Rensburg K, Evans DE, Drobes DJ. Transient compensatory smoking in response to placebo cigarettes. Psychopharmacology (Berl). 2012;223:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Strasser AA, Lerman C, Sanborn PM, Pickworth WB, Feldman EA. New lower nicotine cigarettes can produce compensatory smoking and increased carbon monoxide exposure. Drug Alcohol Depend. 2007;86:294–300. [DOI] [PubMed] [Google Scholar]
- 49. Scherer G. Smoking behaviour and compensation: a review of the literature. Psychopharmacology (Berl). 1999;145:1–20. [DOI] [PubMed] [Google Scholar]
- 50. Buchhalter AR, Schrinel L, Eissenberg T. Withdrawal-suppressing effects of a novel smoking system: comparison with own brand, not own brand, and de-nicotinized cigarettes. Nicotine Tob Res. 2001;3:111–118. [DOI] [PubMed] [Google Scholar]
- 51. Akl EA, Aleem S, Gunukula SK, Honeine R, Abou Jaoude P, Irani J. Survey instruments used in clinical and epidemiological research on waterpipe tobacco smoking: a systematic review. BMC Public Health. 2010;10:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fagerström K, Eissenberg T. Dependence on tobacco and nicotine products: a case for product-specific assessment. Nicotine Tob Res. 2012;14:1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Barnett TE, Forrest JR, Porter L, Curbow BA. A multiyear assessment of hookah use prevalence among Florida high school students. Nicotine Tob Res. 2014; 16(3):373–377. 10.1093/ntr/ntt188 [DOI] [PubMed] [Google Scholar]
- 54. Ward KD, Eissenberg T, Gray JN, Srinivas V, Wilson N, Maziak W. Characteristics of U.S. waterpipe users: a preliminary report. Nicotine Tob Res. 2007;9:1339–1346. [DOI] [PubMed] [Google Scholar]
- 55. Asfar T, Ward KD, Eissenberg T, Maziak W. Comparison of patterns of use, beliefs, and attitudes related to waterpipe between beginning and established smokers. BMC Public Health. 2005;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Blank MD, Brown KW, Goodman RJ, Eissenberg T. An observational study of group waterpipe use in a natural environment. Nicotine Tob Res. 2014;16:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.