Abstract
Background:
Current screening guidelines for colorectal cancer (CRC) do not consider thyroid dysfunction as a risk factor for disease development. We sought to determine the risk of developing CRC in patients with thyroid dysfunction, with and without thyroid hormone replacement (THR).
Methods:
We conducted a nested case-control study using a large population-based medical records database from the United Kingdom. Study case patients were defined as those with any medical code of CRC. Subjects with familial colorectal cancer syndromes or inflammatory bowel disease (IBD) were excluded. For every case patient, four eligible control patients matched on age, sex, practice site, and duration of follow-up before index date were selected using incidence density sampling. Exposure was THR therapy before index date. We further divided the THR unexposed group into patients with hypothyroidism (TSH > 4mg/dl), patients with hyperthyroidism (TSH < 0.4mg/dl), and subjects without documented thyroid abnormality. The adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for CRC were estimated using conditional logistic regression. All statistical tests were two-sided.
Results:
We identified 20990 CRC patients and 82054 control patients. The adjusted odds ratio for CRC associated with THR was 0.88 (95% CI = 0.79 to 0.99, P = .03) and 0.68 (95% CI = 0.55 to 0.83, P < .001) for treatment initiated five to 10 years and more than 10 years before index date, respectively. This protective association increased with cumulative duration of therapy. In contrast, hyperthyroidism (adjusted OR = 1.21, 95% CI = 1.08 to 1.36, P = .001) or untreated hypothyroidism (adjusted OR = 1.16, 95% CI = 1.08 to 1.24, P < .001) were associated with increased risk of CRC.
Conclusion:
Long-term THR is associated with a decreased risk of CRC. Hyperthyroidism and untreated hypothyroidism are associated with modestly elevated risk of CRC.
Besides regulation of body metabolism, thyroid hormones play an important role in cell proliferation and differentiation. The various nuclear and cellular surface receptors of thyroid hormones have been shown to trigger several important genetic pathways with competing effects on cancer development (1–7). Additionally, thyroid hormones may also act through the estrogen receptor pathway to influence cancer risk(8).
Given the substantial prevalence of thyroid dysfunction and thyroid hormone replacement (THR) therapy in the adult population, it is of great public health importance to determine whether these mechanistic links between thyroid hormone and tumorigenic pathways translate into clinically important alterations in cancer risk. Previous epidemiological studies evaluating the association between thyroid hormone supplementation, thyroid dysfunction (both hypo and hyperthyroidism), and cancer risk have yielded conflicting results. Some studies have shown an increased risk for breast (9), prostate (10,11), ovarian (12), lung (13), and pancreatic cancers (14) in patients with hyperthyroidism or Grave’s disease, while other studies demonstrated a higher risk for breast cancers in patients with untreated hypothyroidism with low free T4 (15,16). By contrast, hypothyroidism related to the use of tyrosine kinase inhibitors (eg, sunitinib) in patients diagnosed with cancer (eg, renal cell carcinoma) has also been associated with improved outcome in this population (17). Furthermore, patients with hypothyroidism treated with thyroid hormone replacement (THR) may have reduced breast cancer risk (18).
Colorectal cancer (CRC) is the second leading cause of cancer mortality in the United States. To our knowledge, the effect of THR on CRC risk has only been evaluated in a single study to date (19), in which THR therapy for more than five years was associated with a statistically significantly reduced risk of CRC. Importantly, that study, conducted among Israeli Ashkenazi Jews, did not distinguish the effect of THR from the effects of underlying hypothyroidism (19). In the current nested case-control study, we sought to elucidate the association between thyroid dysfunction, THR, and the risk of CRC in a large population-representative cohort.
Methods
Study Design
We conducted a nested case-control study with incidence density sampling. Case-control studies with incidence density sampling of controls yield odds ratios (ORs) that are statistically unbiased estimates of the incidence rate ratio (or hazard ratio) from a corresponding cohort study with proportional hazard analysis (20). The study was approved by the Institutional Review Board at the University of Pennsylvania and by the Scientific Review Committee of The Health Improvement Network (THIN).
Data Source
THIN, a large population-based electronic research database from the United Kingdom, contains comprehensive medical records on approximately 10 million patients treated by general practitioners in 570 practices throughout the UK. The demographic and geographic distributions of the THIN population are broadly representative of those in the general UK population. All practices contributing data to THIN follow a standardized protocol of entering information and transmitting information to the central database. Each medical diagnosis is defined using Read diagnostic codes, which is the standard coding system used by general practices in the UK (21,22). Each medication is coded using multiplex codes, and data quality is monitored through routine analysis of the entered data (23). Numerous pharmaco-epidemiologic studies have been performed using the THIN and have shown excellent quality of information on prescriptions and medical diagnosis.
Study Cohort
All people receiving medical care from 1995 to 2013 from a THIN practitioner were potentially eligible for inclusion (Figure 1). A previous study conducted in a UK general practice database showed that there was substantial misclassification of prevalent CRC cases as incident cases within the first six months of database follow-up (24). Therefore, in order to avoid all prevalent cases, we excluded patients with a documented CRC diagnosis before the start of follow-up as well as those with a CRC diagnosis within the first 183 days of their follow-up. Additionally, our objective was to study risk factors for sporadic CRC. Therefore, we chose to exclude those who likely did not have sporadic CRCs, including those who were diagnosed with CRC at a young age (ie, <40 years), those who had a history of familial CRC syndromes, or those who had inflammatory bowel disease (IBD). The observational period (which corresponded to the at-risk period considered in incidence density sampling of control patients) of the eligible study cohort started at the later of either the date when the THIN practice started using the electronic medical record software or the date at which the patient registered with their general practitioner and ended on the earliest of CRC diagnosis date, date of death, transferring out of the database, or the end date of the database.
Figure 1.
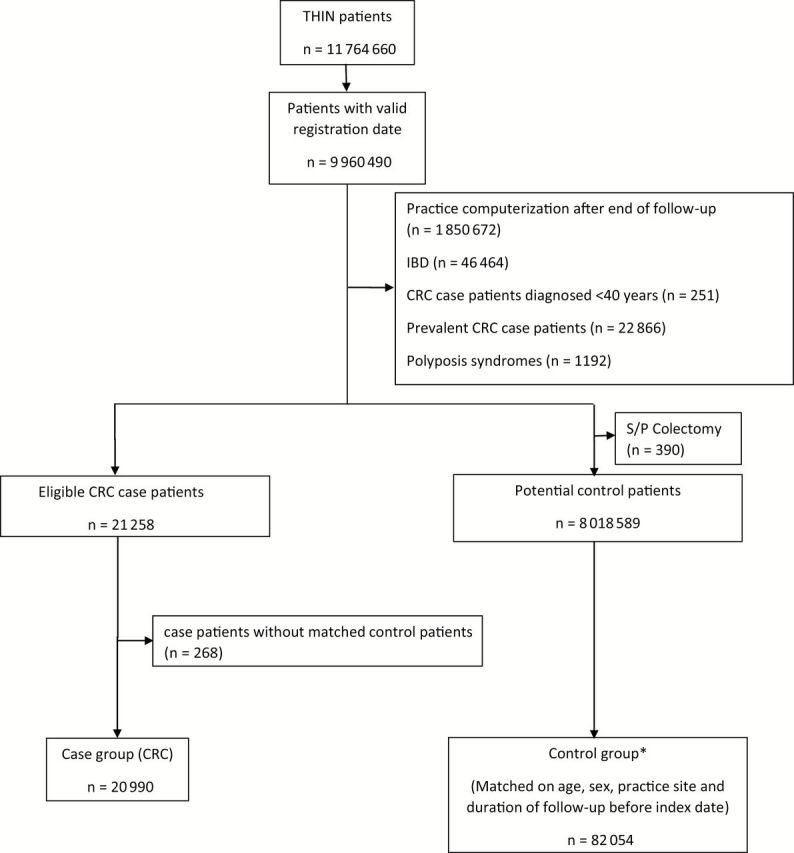
Patient selection flowchart. *Selected by incidence density sampling. Thus, some case patients were selected as control patients prior to their diagnosis of colorectal cancer (CRC) (n = 1105). IBD = inflammatory bowel disease; S/P colectomy = status postcolectomy; THIN = The Health Improvement Network.
Case Selection
Case patients were defined as all individuals in the cohort with at least one Read diagnosis code for CRC (see Supplementary Data, available online) during the follow-up period. Index date was defined as the date of first CRC diagnosis. Of note, a previous study demonstrated that the incidence of CRC in THIN was comparable with the incidence in the entire population of the UK as reported in cancer registry data (23,25). Furthermore, previous validation of the diagnostic codes for CRC in the UK general practices by us and others showed positive predictive values of 93% to 97% (26,27). The coding for CRC included all stages of disease from carcinoma in situ to metastatic disease.
Selection of Controls
Selection of the control group was based on incidence density sampling (20,28). The potentially eligible control pool for each case patient comprised all individuals from the THIN database who remained at risk for CRC on the calendar date when the case patient was first diagnosed with CRC. For each case patient, up to four eligible control patients were randomly selected who matched on age (using age group categories of five years), sex, practice site, and both calendar time and duration of follow-up. Control patients were assigned the same index date as their matched case patients.
Exposures and Covariates
The primary exposure of interest was any THR before index date. Since colorectal tumorigenesis is a multistep process occurring over a period of 10 to 15 years, the effect was further evaluated based on the timing of THR initiation (0–6 months, 6–12 months, 1–5 years, 5–10 years, and >10 years before index date) and cumulative duration of treatment (according to the following percentiles: 0% to 25% [1–468 days], 25% to 50% [469–1049 days], 50% to 75% [1050–1904 days], 75% to 90% [1905–2741 days], 90% to 95% [2742–3283 days], and above 95% [more than 3283 days]). Secondary exposures of interest were clinical or subclinical hypothyroidism without THR, defined as any TSH levels above 4mg/dl before index date without THR, and clinical or subclinical hyperthyroidism, defined as any TSH below 0.4mg/dl before index date without documented TSH values consistent with hypothyroidism subsequently. The reference group consisted of individuals with no thyroid dysfunction and no THR.
Additionally, based on published literature, we first compiled a comprehensive list of potential confounders that are linked with thyroid dysfunction and/or are known or suspected risk factors for CRC such as lifestyle parameters, including body mass index (BMI), smoking history (current, past, or never), alcohol consumption (any use and alcoholism/alcohol dependence), previous CRC screening, comorbidities (including diabetes mellitus, ischemic heart disease, and connective tissue disease), and chronic use of aspirin/NSAIDs (first prescription at least 12 months before index date and last prescription within six months before index date). We then applied an adapted directed acyclic graph approach (29) to this preliminary list of variables and excluded ischemic heart disease and BMI as confounders, because both may be caused by the thyroid dysfunction or treatment and thus were regarded as intermediates. The remaining variables were included in the multivariable regression model.
Statistical Analysis
The baseline characteristics of case patients and control patients were compared using chi square tests for categorical variables and t tests for continuous variables. The primary analysis was a multivariable conditional logistic regression to estimate odds ratios and 95% confidence interval (CIs) for the association between CRC risk and hypothyroidism treated with THR, hypothyroidism not treated with THR, and hyperthyroidism. In order to evaluate differential effect of THR on disease stage, an analysis was performed among a subgroup of case patients who underwent colectomy at the time of diagnosis, which is a surrogate for invasive tumors. Additionally, we conducted stratified analyses by sex for the primary exposure (ie, THR) in order to assess possible estrogenic effect on cancer risk. Likelihood ratio tests were performed to assess statistical significance of interaction between sex and THR. All statistical analyses were performed using STATA 13 (STATA Corp, College Station, TX). All statistical tests were two-sided.
Results
The analysis included 20990 CRC patients and 82054 matched control patients. The mean duration of follow up before index date was 6.5 years (SD = 4.0 years). Two hundred sixty-eight case patients (1.3%) had no matched control patients and were excluded from the data analysis (Figure 1). Characteristics of case patients and control patients are presented in Table 1. As expected, case patients were more likely to have a medical history of diabetes mellitus and more likely to be former or current smokers and alcohol users.
Table 1.
Characteristics of case patients and control patients
| Characteristics | Case patients (n = 20 990) |
Control patients (n = 82 054) |
Unadjusted OR (95% CI) |
P |
|---|---|---|---|---|
| Age at index date, y, (SD) | 71.3 (11.1) | 71.0 (11.2) | NA | NA |
| Male sex, no. (%) | 11 574 (55.1) | 45 254 (55.1) | NA | NA |
| Duration of follow-up before index date, mean y (SD) | 6.5 (4.0) | 6.5 (4.0) | NA | NA |
| Diabetes mellitus, no. (%) | 2641 (12.6) | 8305 (10.1) | 1.28 (1.22 to 1.34) | <.001 |
| Connective tissue disease, no. (%) | 611 (2.9) | 2334 (2.8) | 1.03 (0.94 to 1.14) | .48 |
| Smoking (ever), no. (%) Current smokers, no. (%) |
10 151 (48.4) 1631 (7.8) |
35 099 (42.8) 5988 (7.3) |
1.30 (1.25 to 1.34) 1.22 (1.15 to 1.3) |
<.001 <.001 |
| Alcohol use, no. (%) Alcoholism, no. (%) |
11 112 (52.9) 162 (0.8) |
40 944 (49.9) 398 (0.5) |
1.18 (1.13 to 1.22) 1.80 (1.49 to 2.17) |
<.001 <.001 |
| Chronic NSAIDs/aspirin use, no. (%)* |
5519 (26.3) | 22 311 (27.2) | 1.00 (0.97 to 1.04) | .82 |
| Colonoscopy >2 years before index date, No. (%) | 319 (1.5) | 1400 (1.7) | 0.88 (0.78 to 0.99) | .04 |
* The odds ratio was 0.92 (95% CI = 0.88 to 0.96, P < .001) when adjusted to ischemic heart disease, diabetes mellitus, body mass index, smoking status, and alcohol consumption. CI = confidence interval; OR = odds ratio.
The patients with clinical or subclinical hypothyroidism and without THR had a modestly higher risk for CRC compared with individuals without documented thyroid dysfunction and no THR, with an adjusted odds ratio of 1.16 (95% CI = 1.08 to 1.24, P < .001). In contrast, patients with clinical or subclinical hypothyroidism treated with THR had lower risk of CRC risk with an adjusted odds ratio of 0.92 (95% CI = 0.86 to 0.98, P = .009). Subjects with hyperthyroidism also had an increased CRC risk with an adjusted odds ratio of 1.21 (95% CI = 1.08 to 1.36, P = .001) (Table 2).
Table 2.
Multivariable analysis for the association between thyroid dysfunction, with or without THR, and CRC risk
| Thyroid function | Case patients (n = 20 990), no. (%) |
Control patients (n = 82 054), no. (%) |
Unadjusted OR (95% CI, P) |
Adjusted OR* (95% CI, P) |
|---|---|---|---|---|
| No thyroid dysfunction and no THR therapy | 18 197 (86.7) | 71 763 (87.5) |
Ref. | Ref. |
| Subclinical/clinical hypothyroidism without THR | 1095 (5.2) | 3621 (4.4) | 1.19 (1.11 to 1.27, P < .001) | 1.16 (1.08 to 1.24, P < .001) |
| Subclinical/clinical hypothyroidism with THR | 1279 (6.1) | 5365 (6.5) | 0.94 (0.88 to 1.00, P = .05) | 0.92 (0.86 to 0.98, P = .009) |
| Hyperthyroidism | 419 (2.0) | 1305 (1.6) | 1.26 (1.13 to 1.41, P < .001) | 1.21 (1.08 to 1.36, P = .001) |
* Adjusted for diabetes mellitus, connective tissue diseases, smoking history, alcohol consumption, chronic use of aspirin/NSAIDs, and performance of screening colonoscopy. Analysis by conditional logistic regression. All statistical tests were two-sided. CI = confidence interval; OR = odds ratio; THR = thyroid hormone replacement.
The THR-exposed group was further analyzed according to the timing of the first observed THR prescription. In this analysis (Table 3), thyroid hormone replacement for more than one year was associated with a lower CRC risk. This protective association was stronger with increasing time since initiation of treatment to index date; the adjusted odds ratios for CRC associated with THR were 0.88 (95% CI = 0.79 to 0.99, P = .03) and 0.68 (95% CI = 0.55 to 0.83, P < .001) for treatment initiated 5 to 10 years and more than 10 years before index date, respectively. There was a borderline statistically significant suggestion of an increase in CRC risk with THR initiated within 6 months before index date (adjusted OR = 1.31, 95% CI = 1.01 to 1.71, P = .05) (Table 3). We also analyzed the cumulative duration of thyroid hormone replacement (Table 4). The multivariable analysis demonstrated a statistically significant association between cumulative duration of treatment above 1050 days and lower CRC risk (Table 5). For those above the 95th percentile of cumulative THR duration (ie, 3283 days), the adjusted odds ratio was 0.58 (95% CI = 0.42 to 0.8, P = .001) (Table 4).
Table 3.
Multivariable analysis of the association between timing of THR initiation and colorectal cancer risk
| Timing of THR initiation before index date | Case patients (n = 19 449), no. (%) |
Control patients (n = 71 808), no. (%) |
Unadjusted OR (95% CI, P) |
Adjusted OR (95% CI, P)* |
|---|---|---|---|---|
| No thyroid dysfunction and no THR therapy | 18 173 (93.4) | 66 943 (93.2) | Ref. | Ref. |
| 0–6 mo | 77 (0.4) | 213 (0.3) | 1.34 (1.03 to 1.74, P = .03) | 1.31 (1.01 to 1.71, P = .05) |
| 6–12 mo | 98 (0.5) | 323 (0.5) | 1.14 (0.91 to 1.45, P = .26) | 1.11 (0.88 to 1.41, P = .37) |
| 1–5 y | 586 (3.0) | 2,225 (3.1) | 0.95 (0.87 to 1.05, P = .33) | 0.93 (0.85 to 1.03, P = .15) |
| 5–10 y | 402 (2.1) | 1,550 (2.2) | 0.90 (0.80 to 1.01, P = .08) | 0.88 (0.79 to 0.99, P = .03) |
| >10 y | 113 (0.6) | 554 (0.8) | 0.69 (0.56 to 0.85, P = .001) | 0.68 (0.55 to 0.83, P < .001) |
* Adjusted for diabetes mellitus, connective tissue diseases, smoking history, alcohol consumption, chronic use of aspirin/NSAIDs, and performance of screening colonoscopy. Analysis by conditional logistic regression. All statistical tests were two-sided. CI = confidence interval; OR = odds ratio; THR = thyroid hormone replacement.
Table 4.
Multivariable analysis of cumulative durations of THR and colorectal cancer risk
| Cumulative THR duration | Case patients (n = 19 449) , no. (%) |
Control patients (n = 71 808), no. (%) |
Unadjusted OR (95% CI, P) |
Adjusted OR* (95% CI, P) |
|---|---|---|---|---|
| No thyroid dysfunction and no THR therapy | 18 173 (93.4) | 66 943 (93.2) | Ref. | Ref. |
| 1–468 d | 325 (1.7) | 1095 (1.5) | 1.10 (0.97 to 1.26, P = .13) | 1.07 (0.94 to 1.22, P = .3) |
| 469–1049 d | 327 (1.7) | 1267 (1.8) | 0.94 (0.83 to 1.06, P = .31) | 0.93 (0.82 to 1.05, P = .24) |
| 1050–1904 d | 324 (1.7) | 1257 (1.8) | 0.91 (0.80 to 1.03, P = .13) | 0.89 (0.78 to 1.01, P = .07) |
| 1905–2741 d | 198 (1.0) | 736 (1.0) | 0.91 (0.78 to 1.07, P = .28) | 0.89 (0.76 to 1.05, P = .16) |
| 2742–3283 d | 56 (0.3) | 252 (0.4) | 0.75 (0.56 to 1.01, P = .06) | 0.73 (0.54 to 0.98, P = .04) |
| >3283 d | 46 (0.2) | 258 (0.4) | 0.60 (0.44 to 0.83, P = .002) | 0.58 (0.42 to 0.8, P = .001) |
* Adjusted for diabetes mellitus, connective tissue diseases, smoking history, alcohol consumption, chronic use of aspirin/NSAIDs, and performance of screening colonoscopy. Analysis by conditional logistic regression. All statistical tests were two-sided. CI = confidence interval; OR = odds ratio; THR = thyroid hormone replacement.
Table 5.
Multivariable analysis of cumulative durations of THR and CRC risk only among matched case-control clusters in which the case underwent colectomy after diagnosis of CRC
| Cumulative THR duration | Case patients (n = 6938), no. (%) |
Control patients (n = 25 438), no. (%) |
Unadjusted OR (95% CI, P) |
Adjusted OR* (95% CI, P) |
|---|---|---|---|---|
| No thyroid dysfunction and no THR therapy | 6470 (93.2) | 23 645 (93.0) | Ref. | Ref. |
| 1–468 d | 127 (1.8) | 416 (1.6) | 1.13 (0.92 to 1.39, P = .24) | 1.09 (0.88 to 1.34, P = .42) |
| 469–1049 d | 125 (1.8) | 471 (1.9) | 0.96 (0.79 to 1.18, P = .71) | 0.96 (0.79 to 1.18, P = .72) |
| 1050–1904 d | 120 (1.7) | 456 (1.8) | 0.93 (0.75 to 1.14, P = .47) | 0.92 (0.74 to 1.13, P = .42) |
| 1905–2741 d | 72 (1.0) | 264 (1.0) | 0.89 (0.68 to 1.17, P = .41) | 0.87 (0.66 to 1.15, P = .33) |
| 2742–3283 d | 15 (0.2) | 92 (0.4) | 0.53 (0.30 to 0.92, P = .03) | 0.51 (0.29 to 0.89, P = .02) |
| >3283 d | 9 (0.1) | 94 (0.4) | 0.31 (0.15 to 0.63, P = .001) | 0.29 (0.15 to 0.59, P = .001) |
* Adjusted for diabetes mellitus, connective tissue diseases, smoking history, alcohol consumption, chronic use of aspirin/NSAIDs, and performance of screening colonoscopy. Analysis by conditional logistic regression. All statistical tests were two-sided. CI = confidence interval; OR = odds ratio; THR = thyroid hormone replacement.
Additional analysis evaluated the association between thyroid dysfunction, THR, and CRC only among matched case-control clusters in which the case patient underwent colectomy after diagnosis of CRC (n = 7484). The modest risk increase seen in subjects with hypothyroidism without THR attenuated and was no longer statistically significant (adjusted OR = 1.11, 95% CI = 0.98 to 1.25, P = .1), and the risk increase among subjects with hyperthyroidism remained at the same magnitude but fell just below the threshold for statistical significance (adjusted OR = 1.21, 95% CI = 1.00 to 1.46, P = .05). There was no statistically significant association between any THR and CRC risk (adjusted OR = 0.91, 95% CI = 0.82 to 1.02, P = .1). However, longer durations of THR cumulative use was statistically significantly associated with CRC risk, with an adjusted odds ratio of 0.51 (95% CI = 0.29 to 0.89, P = .02) for 2742 to 3283 days and an adjusted odds ratio of 0.29 (95% CI = 0.15 to 0.59, P = .001) for more than 3283 days (Table 5).
The results of sex-stratified analyses are presented in Supplementary Tables 1–8 (available online). The magnitude of the overall effect of THR was similar for men and women with odds ratios of 0.93 and 0.91, respectively. The magnitude of the effects for long-term THR and THR initiated more than 5 to 10 years were somewhat more pronounced among women than among men. However, statistical tests for interaction were not significant for any of the sex-stratified analyses (P interaction values according to exposure variable used: .7 for any THR, .86 for THR duration, and .9 for THR timing of initiation). Additionally, the odds ratios for CRC associated with long-term THR or THR initiated in the remote past were statistically significant only among women.
Discussion
In this nested case-control study conducted in a large UK population, a modestly increased CRC risk was noted in patients with untreated subclinical/clinical hypothyroidism and those with hyperthyroidism. THR appeared to have a protective effect against CRC if given more than one year prior to index date or for long durations. In sex-stratified analyses, the negative association between long-term THR and CRC was only statistically significant in women; the effect estimate was somewhat more pronounced among women than among men, but the sex-specific difference was not statistically significant. The negative association between THR and CRC was more prominent in a subgroup analysis that included case patients who underwent colectomy.
Our study has several important strengths. Under the UK National Health Services, 98% of the UK population receives healthcare through general practitioners; therefore, the data in THIN reflect the general healthcare delivery pattern for the entire UK population. THIN is a computerized medical records database that allows for an accurate assessment of drug prescriptions dosing and duration of treatment as well as laboratory results that capture thyroid function without recall bias. The diagnostic codes for CRC used in THIN have been previously validated, ensuring the high quality of case identification. The use of incidence density sampling ensured that the odds ratios generated are interpretable as incidence rate ratios (20,28).
Several potential limitations warrant consideration in this study. The THIN database lacks information regarding premalignant adenomas, tumor location, and staging. Thus, we were unable to evaluate an association between cancer stage and THR. We used colectomy following CRC diagnosis as a surrogate for invasive CRC, but this is an imperfect surrogate. It would have been useful to examine trends in thyroid function among patients treated with THR. However, there were insufficient longitudinal data on thyroid function in our dataset to meaningfully assess this issue.
The modest increase in CRC risk during the first year of THR therapy might be explained by surveillance bias. Patients with thyroid dysfunction likely had increased frequency of visits to their general providers during the first few months of being started on THR, leading to increased likelihood of being diagnosed with CRC. This risk increase associated with initiation of THR could also be partially because of confounding by indication (ie, the confounding because of residual effect of previously untreated hypothyroidism on CRC risk, which diminished after a period of THR treatment). However, such confounding by indication, if present, was likely limited to the first six months after initiation of THR and should not affect our conclusions based on long durations of THR or THR initiated in the remote past (ie, >5–10 years). We used matching and statistical adjustment of influential risk factors for CRC to control for potential confounding, but we could not completely exclude the possibility of residual confounding because of the observational nature of the study.
We assumed complete compliance with treatment. Never-theless, noncompliant patients would potentially bias the associations towards the null. Finally, compared with the 10 to 15 years required for CRC development, the mean duration of follow-up before index date in our cohort is somewhat short (mean: 6.5 years), limiting our ability to evaluate the influence of THR on tumor initiation.
The negative association between THR and CRC risk observed in our study is consistent with a prior report by Rennert et al. (19). We substantially extended the existing evidence regarding the role of thyroid hormones in CRC risk by elucidating the effect of the THR as well as the effects of untreated hypo- and hyperthyroidism on CRC risk. One prior study examined the association between hyperthyroidism and CRC and found a nonstatistically significant adjusted hazard ratio of 1.38 (95% CI = 0.70 to 2.73), but the statistical power might be limited in that study as there were only nine CRC case patients among hyperthyroid patients (13). Consistent with our observation of a positive association between hyperthyroidism and CRC, several previous studies have reported a similar association in other cancer types (9–14). The association between untreated hypothyroidism on CRC has not been studied previously, but the increased risk of CRC among the untreated hypothyroid group observed in our study is consistent with some prior reports of a positive association between untreated subclinical hypothyroidism and breast cancer risk (15,16).
The effect of thyroid hormones on cell proliferation, differentiation, and tumorigenesis is complex and incompletely characterized. Thyroid hormones exert their biological effect primarily through their nuclear receptors, TRα1 and TRβ1, and a cell surface receptor, integrin αVβ3 (30). These receptors appear to mediate opposing effects with regard to CRC risk (31). Binding of thyroid hormones to TRα1 directly stimulates the expression of β-catenin (4,5), a major driver of intestinal cell proliferation. Furthermore, thyroid hormone binding of integrin αVβ3 activates PI3K and ERK1/2 pathways, leading to increased tumor cell proliferation and angiogenesis (6,7). On the other hand, TRβ1 mediates an antiproliferative effect of thyroid hormones. TRβ1 expression is associated with a more differentiated phenotype (2), consistent with the findings that loss of TRβ1 accompanies malignant transformation of human colon tumors (3). Apart from thyroid hormone receptors, there is evidence of crosstalk between thyroid hormones and the estrogen signaling pathway via estrogen receptors (8). Estrogen has been demonstrated in epidemiological studies to reduce the risk of CRC (32). It has been suggested that the reported protective effect of THR on CRC risk might be related to activation of the estrogen pathway by thyroid hormones (19). This might also explain the more definitive negative association between THR and CRC risk observed among women in our study. Thus, thyroid hormones may have pleotropic effects on CRC development, depending on the relative balance of the competing effects of the various signaling pathways they activate. Our results raise the possibility that this relative balance might be shifted toward a net increase in proliferation both in a state of untreated hypothyroidism or hyperthyroidism, while the mechanisms inhibiting proliferation might become more dominant in the setting of long-term THR. It is interesting to note that some of the epidemiological studies reported a similar nonlinear pattern of association between thyroid hormone status and breast cancer risk (9,15,16,18). However, we would like to emphasize that our study was not specifically designed to directly evaluate the biological mechanisms. Further studies are needed to delineate factors that regulate the interactions among the different cancer-related pathways activated by thyroid hormones.
In summary, we demonstrated a lower risk for CRC among users of THR and a higher risk among patients with hyperthyroidism or hypothyroidism not treated with thyroid hormones. The protective association of THR increased with increasing duration of treatment and cumulative dose and was higher for patients who underwent colectomy and more evident among women. If confirmed, results from this study may help health care providers decide between early vs late treatment with THR in asymptomatic subclinical hypothyroidism or alternatively increased colonic screening in those individuals. Further research evaluating the chemopreventive potential of THR and the exact biological mechanism for this effect might enable more specific chemopreventive drugs in the future.
Funding
The work was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1TR000003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary Material
Dr Yang and Dr Boursi had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. YX Yang, B. Boursi, K. Haynes, and R. Mamtani contributed to conception and design of the study; YX Yang and B. Boursi acquired the data; YX Yang, B. Boursi, K. Haynes, and R. Mamtani contributed to analysis and interpretation of data, drafting the article or revising it critically for important intellectual content, and final approval of the version to be published.
The authors would like to thank James D. Lewis, MD, MSCE, Anil K. Rustgi, MD, and Nadir Arber MD, MSc, MHA, for reviewing the manuscript. Dr Boursi would like to thank the Djerassi family for supporting his postdoctoral fellowship.
This work was performed in partial fulfillment of the requirements for a PhD degree of Ben Boursi, Sackler Faculty of Medicine, Tel-Aviv University, Israel.
None of the authors has any relevant conflict of interest to declare.
References
- 1. Bergh JJ, Lin HY, Lansing L, et al. Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology. 2005;146(7):2864–2871. [DOI] [PubMed] [Google Scholar]
- 2. Horkko TT, Tuppurainen K, George SM, et al. Thyroid hormone receptor beta1 in normal colon and colorectal cancer-association with differentiation, polypoid growth type and K-ras mutations. Int J Cancer. 2006;118(7):1653–1659. [DOI] [PubMed] [Google Scholar]
- 3. Markowitz S, Haut M, Stellato T, et al. Expression of the ErbA-beta class of thyroid hormone receptors is selectively lost in human colon carcinoma. J Clin Invest. 1989;84(5):1683–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Plateroti M, Kress E, Mori JI, et al. Thyroid hormone receptor alpha1 directly controls transcription of the beta-catenin gene in intestinal epithelial cells. Mol Cell Biol. 2006;26(8):3204–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kress E, Skah S, Sirakov M, et al. Cooperation between the thyroid hormone receptor TRalpha1 and the WNT pathway in the induction of intestinal tumorigenesis. Gastroenterology. 2010;138(5):1863–1874. [DOI] [PubMed] [Google Scholar]
- 6. Lin HY, Sun M, Tang HY, et al. L-Thyroxine vs. 3,5,3’-triiodo-L-thyronine and cell proliferation: activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Am J Physiol Cell Physiol. 2009;296(5):C980–C991. [DOI] [PubMed] [Google Scholar]
- 7. Davis FB, Mousa SA, O’Connor L, et al. Proangiogenic action of thyroid hormone is fibroblast growth factor-dependent and is initiated at the cell surface. Circ Res. 2004;94(11):1500–1506. [DOI] [PubMed] [Google Scholar]
- 8. Vasudevan N, Ogawa S, Pfaff D. Estrogen and thyroid hormone receptor interactions: physiological flexibility by molecular specificity. Physiol Rev. 2002;82(4):923–944. [DOI] [PubMed] [Google Scholar]
- 9. Chen YK, Lin CL, Chang YJ, et al. Cancer risk in patients with Graves’ disease: a nationwide cohort study. Thyroid. 2013;23(7):879–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lehrer S, Diamond EJ, Bajwa AM, et al. Association between serum triiodothyronine (t3) level and risk of disease recurrence in men with localized prostate cancer. Prostate Cancer Prostatic Dis. 2001;4(4):232–234. [DOI] [PubMed] [Google Scholar]
- 11. Lehrer S, Diamond EJ, Stone NN, et al. Serum triiodothyronine is increased in men with prostate cancer and benign prostatic hyperplasia. J Urol. 2002;168(6):2431–2433. [DOI] [PubMed] [Google Scholar]
- 12. Ness RB, Grisso JA, Cottreau C, et al. Factors related to inflammation of the ovarian epithelium and risk of ovarian cancer. Epidemiology. 2000;11(2):111–117. [DOI] [PubMed] [Google Scholar]
- 13. Hellevik AI, Asvold BO, Bjoro T, et al. Thyroid function and cancer risk: a prospective population study. Cancer Epidemiol Biomarkers Prev. 2009;18(2):570–574. [DOI] [PubMed] [Google Scholar]
- 14. Ko AH, Wang F, Holly EA. Pancreatic cancer and medical history in a population-based case-control study in the San Francisco Bay Area, California. Cancer Causes Control. 2007;18(8):809–819. [DOI] [PubMed] [Google Scholar]
- 15. Kuijpens JL, Nyklictek I, Louwman MW, et al. Hypothyroidism might be related to breast cancer in post-menopausal women. Thyroid. 2005;15(11):1253–1259. [DOI] [PubMed] [Google Scholar]
- 16. Morabia A, Szklo M, Stewart W, et al. Thyroid hormones and duration of ovulatory activity in the etiology of breast cancer. Cancer Epidemiol Biomarkers Prev. 1992;1(5):389–393. [PubMed] [Google Scholar]
- 17. Schmidinger M, Vogl UM, Bojic M, et al. Hypothyroidism in patients with renal cell carcinoma: blessing or curse? Cancer. 2011;117(3):534–544. [DOI] [PubMed] [Google Scholar]
- 18. Cristofanilli M, Yamamura Y, Kau SW, et al. Thyroid hormone and breast carcinoma. Primary hypothyroidism is associated with a reduced incidence of primary breast carcinoma. Cancer. 2005;103(6):1122–1128. [DOI] [PubMed] [Google Scholar]
- 19. Rennert G, Rennert HS, Pinchev M, et al. A case-control study of levothyroxine and the risk of colorectal cancer. J Natl Cancer Inst. 2010;102(8):568–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pearce N. What does the odds ratio estimate in a case-control study? Int J Epidemiol. 1993;22(6):1189–1192. [DOI] [PubMed] [Google Scholar]
- 21. Chisholm J. The Read clinical classification. BMJ. 1990;300(6732):1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benson T. The history of the Read Codes: the inaugural James Read Memorial Lecture 2011. Inform Prim Care. 2011;19(3):173–182. [DOI] [PubMed] [Google Scholar]
- 23. Lewis JD, Schinnar R, Bilker WB, et al. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2007;16(4):393–401. [DOI] [PubMed] [Google Scholar]
- 24. Lewis JD, Bilker WB, Weinstein RB, et al. The relationship between time since registration and measured incidence rates in the General Practice Research Database. Pharmacoepidemiol Drug Saf. 2005;14(7):443–451. [DOI] [PubMed] [Google Scholar]
- 25. Haynes K, Forde KA, Schinnar R, et al. Cancer incidence in The Health Improvement Network. Pharmacoepidemiol Drug Saf. 2009;18(8):730–736. [DOI] [PubMed] [Google Scholar]
- 26. Garcia-Rodriguez LA, Huerta-Alvarez C. Reduced risk of colorectal cancer among long-term users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs. Epidemiology. 2001;12(1):88–93. [DOI] [PubMed] [Google Scholar]
- 27. Yang YX, Hennessy S, Lewis JD. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology. 2004;127(4):1044–1050. [DOI] [PubMed] [Google Scholar]
- 28. Greenland S, Thomas DC. On the need for the rare disease assumption in case-control studies. Am J Epidemiol. 1982;116(3):547–553. [DOI] [PubMed] [Google Scholar]
- 29. Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. 2008;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moeller LC, Fuhrer D. Thyroid hormone, thyroid hormone receptors, and cancer: a clinical perspective. Endocr Relat Cancer. 2013;20(2):R19–R29. [DOI] [PubMed] [Google Scholar]
- 31. Brown AR, Simmen RC, Simmen FA. The role of thyroid hormone signaling in the prevention of digestive system cancers. Int J Mol Sci. 2013;14(8):16240–16257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Simon MS, Chlebowski RT, Wactawski-Wende J, et al. Estrogen plus progestin and colorectal cancer incidence and mortality. J Clin Oncol. 2012;30(32):3983–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


