Abstract
Despite the biological plausibility of an association between obstetric mode of delivery and psychosis in later life, studies to date have been inconclusive. We assessed the association between mode of delivery and later onset of psychosis in the offspring. A population-based cohort including data from the Swedish National Registers was used. All singleton live births between 1982 and 1995 were identified (n = 1 345 210) and followed-up to diagnosis at age 16 or later. Mode of delivery was categorized as: unassisted vaginal delivery (VD), assisted VD, elective Caesarean section (CS) (before onset of labor), and emergency CS (after onset of labor). Outcomes included any psychosis; nonaffective psychoses (including schizophrenia only) and affective psychoses (including bipolar disorder only and depression with psychosis only). Cox regression analysis was used reporting partially and fully adjusted hazard ratios (HR) with 95% confidence intervals (CI). Sibling-matched Cox regression was performed to adjust for familial confounding factors. In the fully adjusted analyses, elective CS was significantly associated with any psychosis (HR 1.13, 95% CI 1.03, 1.24). Similar findings were found for nonaffective psychoses (HR 1.13, 95% CI 0.99, 1.29) and affective psychoses (HR 1.17, 95% CI 1.05, 1.31) (χ2 for heterogeneity P = .69). In the sibling-matched Cox regression, this association disappeared (HR 1.03, 95% CI 0.78, 1.37). No association was found between assisted VD or emergency CS and psychosis. This study found that elective CS is associated with an increase in offspring psychosis. However, the association did not persist in the sibling-matched analysis, implying the association is likely due to familial confounding by unmeasured factors such as genetics or environment.
Key words: mode of delivery, Caesarean, psychosis, confounding, sibling control
Introduction
Psychosis commonly emerges early on in life, often in the late teens or twenties.1 A recent systematic review estimated the median prevalence of psychotic symptoms was 17% among children aged 9–12 years and 7.5% among adolescents aged 13–18 years.2 Another systematic review estimated the overall prevalence of psychotic symptoms to be 7.2%, but also determined that symptoms would not persist for roughly 80% of people reporting them.3 The etiology of psychosis is complex, most psychotic disorders are thought to be caused by a combination of genetic predisposition,4 neurodevelopmental abnormalities,5 and environmental stressors.6,7 One such environmental factor may be birth by Caesarean section (CS), which has previously been found to be associated with bipolar disorder.8
There are several hypotheses regarding a possible association between CS and psychosis. Disruptions to the gut microbiome by environmental factors in the initial months of life could lead to the subsequent emergence of adverse mental health outcomes later in life.9 Studies in germ-free animals and in animals exposed to pathogenic bacterial infections, probiotic bacteria or antibiotic drugs all suggest a role in general for the gut microbiota in the regulation of anxiety, mood, cognition, and pain.6 Infants born by elective (ie, pre labor/planned) CS are primarily colonized by bacteria from the hospital environment and maternal skin, whereas infants delivered vaginally are colonized by bacteria found in the birth canal, thus altering their microbiota.10 Other theories include “early-term” birth,11 or alterations in stress response.12 Alternatively, it is possible that any association is driven by factors independently associated with both CS and psychological development, and is not due to CS itself. Indeed, a recent sibling-control design concluded that the previously established relationship between birth by CS and autism spectrum disorder could be explained by shared family characteristics.13
Epidemiological studies bolster the hypothesis that risk of disease can be influenced by environmental experience.14–18 To our knowledge, the association between CS and overall psychosis has not been investigated. A systematic review on obstetric risk factors for schizophrenia found it to be associated with emergency CS, but not CS overall.19 Affective psychoses have been reported as not being associated with CS overall.20 However, a recent population-based study found bipolar disorder to be associated with elective, but not emergency CS.21
Therefore, the aim of the current study was to investigate the association between obstetric mode of delivery and onset of psychosis in offspring diagnosed at age 16 or later in a large Swedish population-based cohort.
Method
Study Design and Data Sources
The current study used a population-based cohort study design using the Swedish national registers (maintained by Statistics Sweden and the Swedish National Board of Health and Welfare). Through the use of a unique personal identification number (PIN)22 assigned to each Swedish citizen, record linkage was carried out linking the Medical Birth Register,23 the National Patient Register,24 and the Multigenerational Register.25 The PIN is recorded in all contacts within health, social, and administrative facilities enabling accurate and complete linkage between the different registers.
Study Population and Follow-up
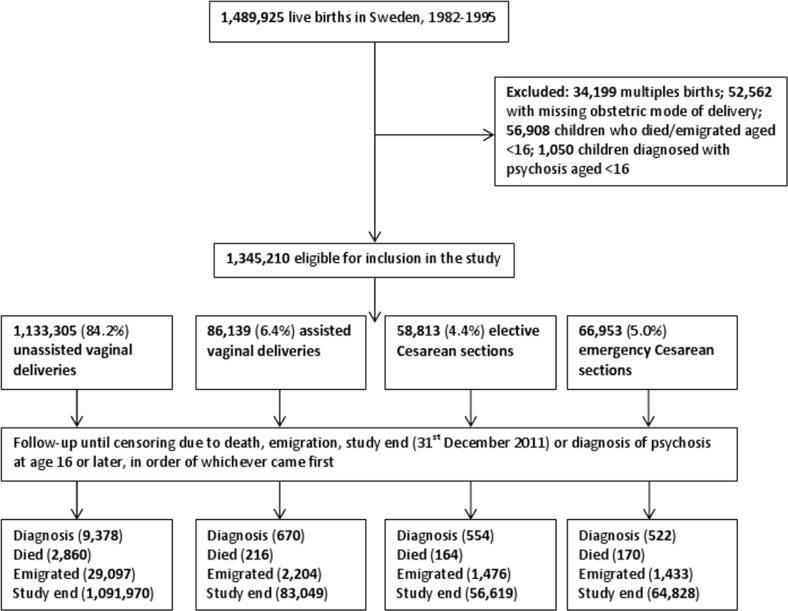
All live births in Sweden between January 1st 1982 and December 31st 1995 were identified (n = 1 489 925). We started follow-up at age 16 and censored at the date of first diagnosis of psychosis, death, emigration, or study end (31st December 2011), in order of whichever came first (figure 1).
Fig. 1.
Study flow diagram.
Obstetric Mode of Delivery
Obstetric mode of delivery (exposure) was categorized as follows: unassisted vaginal delivery (VD) (including both spontaneous and induced labor); assisted VD (vacuum or forceps instrumental deliveries); elective CS (planned before the onset of labor), and emergency CS (unplanned, after the onset of labor). Although the Medical Birth Register was established in 1973, detailed information for mode of delivery, including whether a CS was elective or emergency, was not recorded until 1982; hence the data for this study begins from 1982 onwards. Data on induction of labor was recorded from 1990 onwards.
Outcome Classification
Information on the outcomes of interest and date of first diagnosis were extracted from the National Patient Register based on the International Classification of Diseases (ICD) codes (version 10). Date of diagnosis was defined as the date of hospitalization that led to the first psychosis diagnosis. The National Patient Register includes inpatient data from 1964 and outpatient data from 2001 (which will capture outcome diagnoses made in day cases not requiring hospitalization).
Primary Outcome: Any Psychosis.
The primary outcome was defined as any psychosis diagnoses based on ICD-10 codes: Schizophrenia, Schizotypal disorder, Persistent delusional disorders, Acute and transient psychotic disorders, Induced delusional disorder, Schizoaffective disorders, Other nonorganic psychotic disorders, Unspecified nonorganic psychosis (F20-29), Mania with psychotic symptoms (F30.2), Bipolar affective disorder (F31), Severe depressive episode with psychotic symptoms (F32.3), and Recurrent depressive disorder, current episode severe with psychotic symptoms (F33.3).
Secondary Outcomes.
Nonaffective psychoses, affective psychoses (defined according to previous literature which used the Swedish registry data).26
Nonaffective Psychoses
Schizophrenia, schizotypal disorder, persistent delusional disorders, acute and transient psychotic disorders, induced delusional disorder, schizoaffective disorders, other nonorganic psychotic disorders, and unspecified nonorganic psychosis (F20-29).
A subgroup analysis including schizophrenia only was performed.
Affective psychoses
Bipolar affective disorder (F31), Mania with psychotic symptoms (F30.2), severe depressive episode with psychotic symptoms (F32.3), and recurrent depressive disorder, current episode severe with psychotic symptoms (F33.3).
Subgroup analyses including bipolar disorder only and depression with psychosis only were performed.
For the main psychosis analysis, the date of first psychosis diagnosis was used (if a person had multiple diagnoses, date of first diagnosis was used). If a person was ever diagnosed with nonaffective psychoses, they were included in the analysis with the date of diagnosis. Likewise, if a person was ever diagnosed with affective psychoses, they were included in the analysis with the date of diagnosis. Therefore a person could contribute separately to the nonaffective and affective psychoses subgroup analyses.
Potential Confounders.
Data on the following covariates were available for the adjusted analyses and categorized as presented in Table 1 and supplementary table 1: birth year (1982–1995), infant sex (male, female), maternal age in years (<20, 20–29, 30–39, 40+), gestational age in weeks (<37, 37, 38, 39, 40, >40), maternal and paternal citizenship (Swedish, Other Nordic country, Other country), large-for-gestational age (LGA), and small-for gestational age (SGA) (defined as birth weight for gestational age that differed from the mean by ± 2 standard deviations, respectively). Gestational age was estimated by the first or early second trimester ultrasound if available; otherwise, information from the last menstrual period was used; Apgar score at 5min (low [0.3], intermediate [4–6], high [7–10]), parity (primiparous/multiparous), receiving social welfare at time of birth (yes, no; data on social welfare available from 1983 onwards), family disposable income at time of birth (based on quintiles, first–fifth), parental highest education at time of birth (pre-high school, high school, post-high school) [data on education available from 1990 onwards], and maternal and paternal history of depression, bipolar disorder, and nonaffective psychosis (categorized as never, diagnosed before birth, diagnosed after birth).
Table 1.
Parental and Infant Characteristics of the Study Population in Sweden 1982–1995 (Summary)
| Characteristic | Total Population 1 345 210 (100.0) | Unassisted VD 1 133 305 (84.2) | Assisted VD 86 139 (6.4) | Elective CS 58 813 (4.4) | Emergency CS 66 953 (5.0) |
|---|---|---|---|---|---|
| All psychosis | 11 124 (0.83) | 9378 (0.70) | 670 (0.78) | 554 (0.94) | 552 (0.82) |
| Affective psychosis | 7181 (0.53) | 6055 (0.53) | 430 (0.50) | 362 (0.62) | 334 (0.50) |
| Non-affective psychosis | 5030 (0.38) | 4213 (0.37) | 322 (0.37) | 262 (0.45) | 233 (0.35) |
| Maternal age in years | |||||
| <20 | 39 321 (2.92) | 33 753 (2.98) | 2972 (3.45) | 819 (1.39) | 1777 (2.65) |
| 20–29 | 809 708 (60.19) | 689 965 (60.88) | 55 056 (63.92) | 26 958 (45.84) | 37 739 (56.37) |
| 30–39 | 47 402 (35.12) | 391 919 (34.58) | 26 857 (31.18) | 28 161 (47.88) | 25 466 (38.04) |
| 40+ | 23 764 (1.77) | 17 663 (1.56) | 1254 (1.46) | 2876 (4.89) | 1971 (2.94) |
| Maternal country of birth | |||||
| Sweden | 1 187 482 (88.28) | 1 001 740 (88.39) | 76 410 (88.71) | 51 439 (87.46) | 57 893 (86.47) |
| Other Nordic | 52 274 (3.89) | 43 847 (3.87) | 3127 (3.63) | 2541 (4.31) | 2759 (4.12) |
| Other | 105 441 (7.84) | 87 707 (7.74) | 6600 (7.66) | 4833 (8.22) | 6301 (9.41) |
| Missing | 8 (0.00) | 6 (0.00) | 2 (0.00) | 0 (0.00) | 0(0.00) |
| Parental highest educationa | |||||
| Prehigh school | 50 592 (3.76) | 42 577 (3.76) | 2606 (3.03) | 2474 (4.21) | 2935 (4.38) |
| High school | 332 751 (24.74) | 277 949 (24.53) | 20 771 (24.11) | 14 962 (25.44) | 19 069 (28.48) |
| Posthigh school | 216 445 (16.09) | 177 774 (15.69) | 15 438 (17.92) | 10 545 (17.93) | 12 688 (18.95) |
| Missing | 12 197 (0.91) | 10 230 (0.90) | 765 (0.89) | 404 (0.69) | 798 (1.19) |
| Born prior to 1990 | 733 221 (54.51) | 624 771 (55.13) | 46 559 (54.05) | 30 428 (51.74) | 31 463 (46.99) |
| Parents receiving welfareb | |||||
| Yes | 120 477 (8.96) | 101 678 (8.97) | 6789 (7.88) | 5196 (8.83) | 6814 (10.18) |
| No | 1 119 909 (83.25) | 941 792 (83,10) | 72 285 (83.92) | 49 371 (84.56) | 56 101 (83.79) |
| Missing | 23 045 (1.71) | 19 517 (1.72) | 1435 (1.67) | 882 (1.50) | 1211 (1.81) |
| Born prior to 1983 | 81 775 (6.08) | 70 314 (6.20) | 5630 (6.54) | 3004 (5.11) | 2827 (4.22) |
| Disposable income (quintiles) | |||||
| First | 255 297 (18.98) | 224 435 (19.80) | 10 035 (11.65) | 10 331 (17.57) | 10 496 (15.68) |
| Second | 265 152 (19.71) | 231 811 (20.45) | 11 033 (12.81) | 11 502 (19.56) | 10 806 (16.14) |
| Third | 268 952 (19.92) | 229 276 (20.23) | 14 924 (17.33) | 11 588 (19.70) | 12 164 (18.17) |
| Fourth | 269 965 (20.07) | 222 421 (19.63) | 21 195 (24.61) | 11 591 (19.71) | 14 758 (22.01) |
| Fifth | 262 131 (19.56) | 205 278 (18.11) | 27 470 (31.89) | 12 895 (21.93) | 17 488 (26.12) |
| Missing | 23 708 (1.76) | 20 079 (1.77) | 1482 (1.72) | 906 (1.54) | 1241 (1.85) |
| Infant sex, male | 654 024 (48.62) | 558 504 (49.28) | 36 199 (42.02) | 29 063 (49.42) | 30 258 (45.19) |
| Gestational age | |||||
| <37 weeks | 65 431 (4.86) | 43 795 (3.86) | 2217 (2.57) | 7288 (12.39) | 12 131 (18.12) |
| 37 weeks | 68 003 (5.06) | 53 363 (4.71) | 2815 (3.27) | 6783 (11.53) | 5042 (7.53) |
| 38 weeks | 178 137 (13.24) | 134 529 (11.87) | 7375 (8.56) | 27 816 (47.30) | 8417 (12.57) |
| 39 weeks | 315 878 (23.48) | 278 614 (24.58) | 16 714 (19.40) | 11 003 (18.71) | 9547 (14.26) |
| 40 weeks | 380 661 (28.30) | 339 859 (29.99) | 25 299 (29.37) | 3057 (5.20) | 12 446 (18.59) |
| 40+ weeks | 333 645 (24.80) | 280 281 (24.73) | 31 494 (36.56) | 2710 (4.61) | 19 160 (29.62) |
| Missing | 3450 (0.26) | 2859 (0.25) | 225 (0.26) | 156 (0.27) | 210 (0.31) |
| SGA | 34 839 (2.60) | 22 948 (2.04) | 2448 (2.86) | 3677 (6.30) | 5766 (8.69) |
| LGA | 43 585 (3.26) | 34 633 (3.07) | 2509 (2.93) | 3454 (5.92) | 2989 (4.50) |
| Apgar score (5min) | |||||
| Low (0–3) | 2 658 (0.20) | 1683 (0.15) | 277 (0.32) | 167 (0.28) | 531 (0.79) |
| Intermediate (4–6) | 8374 (0.62) | 3852 (0.34) | 1700 (1.97) | 637 (1.08) | 2185 (3.26) |
| High (7–10) | 1 302 195 (96.80) | 1 099 991 (97.06) | 82 869 (92.20) | 56 531 (96.12) | 62 804 (93.80) |
| Missing | 31 979 (2.38) | 27 775 (2.45) | 1293 (1.50) | 1478 (2.51) | 1433 (2.14) |
| First born child | 552 003 (41.03) | 424 147 (37.43) | 69 549 (80.74) | 18 671 (31.75) | 39 636 (59.20) |
Notes: More than 1 diagnosis was possible and so the number of affective and non-affective psychosis do not add up to the total for all psychosis. Data are n (%) unless otherwise stated. CS, Caesarean section; VD, vaginal delivery; LGA, large for gestational age; SGA, small for gestational age.
aParental highest education: data recorded from 1990 onwards.
bParents receiving welfare: data recorded from 1983 onwards.
Statistical Analysis
Using Cox regression, we estimated the partially and fully adjusted hazard ratios (HR), with 95% confidence intervals (CI). We report partially adjusted models controlling for birth year as crude models violated the proportional hazards assumption. The effects of all covariates based on previous literature were tested individually (supplementary figure 1)27. As no individual variable appeared to have a confounding effect, the fully adjusted model controlled for a priori defined variables gestational age, SGA, Apgar score, maternal age, and maternal psychiatric history.14,15,28 The relationship between mode of delivery and Apgar score is complex. Apgar score may have a confounding or mediating effect on the observed associations, however in the present study, Apgar score alone appeared to have no effect on the association. In the first set of analyses, we analyzed exposure to obstetric mode of delivery for the primary and secondary outcomes (any psychosis, nonaffective psychoses, and affective psychoses).
Additional Analyses.
We undertook further analyses as follows: among sub-populations (term infants only [37+ weeks], by infant sex [females only, males only], babies born in Stockholm county, and in those with and without a history of maternal depression, bipolar disorder or nonaffective disorder); according to birth order (among first born children, among only children, and among those not delivered by repeat CS); and by excluding those with low Apgar scores (0–3); repeating the analyses with follow-up starting from the year 2001 onwards (as outpatient data were available from this date onwards) and restricting the analysis to women who did not have induction of labor (data available from 1990 onwards). Additionally, we estimated the association between mode of delivery and any psychosis adjusted only for parental and sibling history of psychosis.
Sibling-Matched Cox Regression Study.
We used stratified Cox regression models with a separate stratum for each maternal ID. In the stratified Cox regression, rather than being compared to the entire cohort, people with psychosis were compared only to their siblings without psychosis. In this way, the potential effects of genetics, family environment, and other unmeasured factors shared between siblings, were accounted for. Analyses adjusted for the same covariates as in the main Cox regression models. Only families discordant for the exposure and outcome contributed information to the main estimates, though all families with at least 1 child with a diagnosis of psychosis were included in the analysis, as they contributed to covariate estimates. We repeated stratified analysis among those not delivered by repeat CS (excluding children born by CS whose mothers’ had previously delivered through CS).
Missing data.
Where data were missing, the missing indicator method29 was employed including the missing data as a separate category.
Analyses were conducted using the SAS software package, version 9.4, employing the PROC PHREG command.30
Results
Descriptive Statistics
There were 1 489 925 live births in Sweden between January 1st 1982 and December 31st 1995. After exclusion criteria were applied [multiple births (n = 34199); missing data on obstetric mode of delivery (n = 52 562); children who died or emigrated before age 16 (n = 56 908); had a diagnosis of psychosis before age 16 (n = 1050)], there were 1 345 210 children eligible for inclusion in the study (figure 1). The characteristics of the study population are summarized in table 1 (detailed version in supplementary table 1). Of these, 1 333 305 (84.2%) gave birth by unassisted VD; 86 139 (6.4%) by assisted VD; 58,813 (4.4%) by elective CS, and 66 953 (5.0%) by emergency CS. There were 11 124 (0.83%) diagnoses for any psychosis, 5030 (0.38%) nonaffective psychoses, and 7181 (0.53%) affective psychoses in the total population (table 1). Note that as more than 1 diagnosis was possible (ie, a person could have both nonaffective and affective psychoses diagnoses) the total numbers of nonaffective and affective psychoses diagnoses do not add up to the total for any psychosis.
Cohort Cox Regression
Any Psychosis.
Elective CS was associated with any psychosis in the partially (HR 1.19, 95% CI 1.07–1.32) and fully (HR 1.13, 95% CI 1.03–1.24) adjusted models compared to unassisted VD (table 2). No association between assisted VD (HR 0.95, 95% CI 0.87–1.03) or emergency CS (HR 0.97, 95% CI 0.88–1.06), and development of psychosis in offspring was found in the fully adjusted models.
Table 2.
The Association Between Mode of Delivery and Affective or Nonaffective Offspring Psychosis
| Outcome | Exposed Cases | Person-Years | Partially Adjusted HR (95% CI)a | Fully Adjusted HR (95% CI)b |
|---|---|---|---|---|
| Any psychosis | ||||
| Unassisted VD | 9,378 | 7 594 943 | Ref | Ref |
| Assisted VD | 674 | 574 390 | 0.95 (0.86, 1.05) | 0.95 (0.87, 1.03) |
| Elective CS | 554 | 380 397 | 1.19 (1.07, 1.32) | 1.13 (1.03, 1.24) |
| Emergency CS | 522 | 408 390 | 1.02 (0.92, 1.14) | 0.97 (0.88, 1.06) |
| Nonaffective psychosis | ||||
| Nonaffective psychosis including schizophrenia | ||||
| Unassisted VD | 4213 | 7 611 772 | Ref | Ref |
| Assisted VD | 322 | 575 842 | 1.01 (0.90, 1.13) | 1.01 (0.90, 1.14) |
| Elective CS | 262 | 381 518 | 1.24 (1.09, 1.40) | 1.13 (0.99, 1.29) |
| Emergency CS | 234 | 409 552 | 1.04 (0.91, 1.19) | 0.92 (0.80, 1.06) |
| Schizophrenia only | ||||
| Unassisted VD | 971 | 7 626 327 | Ref | Ref |
| Assisted VD | 80 | 576 632 | 1.08 (0.86, 1.36) | 1.09 (0.86, 1.38) |
| Elective CS | 56 | 382 250 | 1.16 (0.89, 1.52) | 1.02 (0.77, 1.35) |
| Emergency CS | 65 | 410 189 | 1.29 (1.00, 1.66) | 1.10 (0.85, 1.44) |
| Affective psychosis | ||||
| Affective psychosis including bipolar disorder and depression with psychosis | ||||
| Unassisted VD | 6055 | 7,610,601 | Ref | Ref |
| Assisted VD | 430 | 575,610 | 0.95 (0.86, 1.04) | 0.94 (0.85, 1.04) |
| Elective CS | 362 | 381,271 | 1.20 (1.08, 1.33) | 1.17 (1.05, 1.31) |
| Emergency CS | 334 | 409,367 | 1.02 (0.92, 1.14) | 0.77 (0.88, 1.10) |
| Bipolar disorder | ||||
| Unassisted VD | 5280 | 7 614 439 | Ref | Ref |
| Assisted VD | 377 | 575 842 | 0.95 (0.86, 1.06) | 0.94 (0.85, 1.05) |
| Elective CS | 312 | 381 518 | 1.18 (1.05, 1.33) | 1.16 (1.03, 1.31) |
| Emergency CS | 291 | 409 552 | 1.02 (0.91, 1.15) | 0.99 (0.88, 1.12) |
| Depression with psychosis | ||||
| Unassisted VD | 1096 | 7 625 928 | Ref | Ref |
| Assisted VD | 73 | 576 724 | 0.92 (0.71, 1.17) | 0.94 (0.73, 1.20) |
| Elective CS | 72 | 382 200 | 1.29 (1.00, 1.67) | 1.21 (0.92, 1.58) |
| Emergency CS | 53 | 410 231 | 0.91 (0.68, 1.21) | 0.84 (0.62, 1.14) |
Notes: Data are adjusted hazard ratios (HR) with 95% confidence intervals (CI). CS, Caesarean section, VD, vaginal delivery.
aPartially adjusted: for birth year.
bFully adjusted: for gestational age, small for gestational age, Apgar score at 5min, maternal age, and maternal psychiatric history.
Nonaffective Psychoses.
Schizophrenia Only (n = 1172)
In the partially adjusted model, an increased but insignificant association was found across all modes of delivery and development of schizophrenia (table 3): assisted VD (HR 1.08, 95% CI 0.86, 1.36); elective CS (HR 1.16, 95% CI 0.89–1.52) and emergency CS (HR 1.29, 95% CI 1.00–1.66). This insignificant association did not change in the fully adjusted model (table 3). When the association between obstetric mode of delivery and nonaffective psychosis including schizophrenia (n = 5031) was examined, elective CS was associated with an increased association in the partially adjusted model (HR 1.24, 95% CI 1.09–1.40) which became insignificant in the fully adjusted model (HR 1.13, 95% CI 0.99–1.29). No association was found between assisted VD or emergency CS and risk of nonaffective psychoses in both the partially and fully adjusted models (table 3).
Table 3.
The Association Between Mode of Delivery and Psychosis Using Cox Models Stratified on Family
| Outcome | Exposed Casesa | Person-Years | Partially Adjusted HR (95% CI)b | Fully Adjusted HR (95% CI)c |
|---|---|---|---|---|
| All psychosis | ||||
| Unassisted VD | 850 | 139 000 | Ref | Ref |
| Assisted VD | 437 | 6369 | 0.94 (0.79, 1.12) | 0.92 (0.77, 1.11) |
| Elective CS | 198 | 2758 | 1.05 (0.80, 1.38) | 1.03 (0.78, 1.37) |
| Emergency CS | 278 | 3936 | 1.07 (0.85, 1.35) | 1.07 (0.83, 1.36) |
Notes: Data are adjusted hazard ratios (HR) with 95% confidence intervals (CI). CS, caesarean section; VD, vaginal delivery.
aThough all families with at least 1 case of psychosis were included in analysis, numbers for exposed cases, and person-years reported here reflect only informative strata (discordant on both mode of delivery and psychosis).
bPartially adjusted: for birth year.
cFully adjusted: for gestational age, small for gestational age, Apgar score at 5min, maternal age, and maternal psychiatric history.
Affective Psychoses.
Bipolar Disorder Only (n = 6260)
A significantly increased hazard of bipolar disorder was found in the partially (HR 1.18, 95% CI 1.05–1.33) and fully (HR 1.16, 95% CI 1.03–1.31) adjusted models for elective CS only (table 3). There was no association between assisted VD or emergency CS and development of bipolar disorder in the partially or fully adjusted models (table 3).
Depression with Psychosis (n = 1294)
An increased but insignificant hazard of depression with psychosis was found in children born through elective CS in the partially (HR 1.29, 95% CI 1.00–1.67) and fully (HR 1.21, 95% CI 0.92–1.58) adjusted models. There was no association between assisted VD or emergency CS and the development of depression with psychosis (table 3).
Affective Psychoses (n = 7,181)
When looking at all affective psychoses cases combined, elective CS was associated with affective psychosis in both the partially (HR 1.20, 95% CI 1.08–1.33) and fully (HR 1.17, 95% CI 1.05–1.31) adjusted models (table 3).
Additional Analyses
To further investigate the association between elective CS and the development of psychosis in offspring additional adjusted analyses across a range of subgroups were conducted (supplementary tables 2 and 3). Overall the results were not changed. Although female babies delivered by elective CS had a greater increased hazard of psychosis (HR 1.20, 95% CI 1.07–1.34) compared to male babies (HR 1.04, 95% CI 0.90–1.20), the difference was not statistically significant (P = .13). Adjusting for parental and sibling history of psychosis also had no effect on results (data not presented, available on request).
Sibling-Matched Cox Regression Study
To control for genetic and environmental confounding, a sibling control study using Cox regression models stratified on maternal ID was conducted. In the partially adjusted and fully adjusted analyses, there was no association between obstetric mode of delivery and development of any psychosis (Table 4). The previous association between elective CS offspring and psychosis disappeared (HR 1.03, 95% CI 0.78–1.37) and was no longer statistically significant. Results were similar among those not born through repeat CS (data not presented, available on request). Similarly, further analysis using Cox regression models stratified on maternal ID including affective psychoses only and bipolar disorder only showed no association (data not presented, available on request).
Discussion
Main Findings
In the current study, a large-population based cohort including all children born in Sweden between 1982 and 1995 was used to examine the association between obstetric mode of delivery and the development of psychosis at age 16 years or later. In the nonstratified Cox regression analyses, a small but statistically significantly increased association between delivery by elective CS and development of psychosis was found. This remained in extensive analyses by type of psychosis, and among different subgroups. In the sibling-matched Cox regression (stratified by maternal ID), which may control for the effects of environmental and familial confounding factors shared by siblings, the association between elective CS and psychosis in offspring disappeared. The increased association found in the Cox regression is most likely explained by residual confounding, for example underlying medical complications which necessitated an elective CS in the first place. The sibling-matched Cox regression study, which is a way of testing for causal associations in observational studies, negated the increased association found in the Cox models. Therefore, a causal relationship between elective CS and psychosis is not supported in this population.
Previous studies investigating obstetric mode of delivery and subsequent psychiatric illnesses in offspring have provided conflicting results. A recent study from Finland reported an increased OR of 2.51 (95% CI 1.32–4.78) for the development of bipolar disorder associated with elective CS only8 in contrast to a previous population-based study by Bain et al 31. In the current study, we found a 16% increased hazard of bipolar disorder among the elective CS group (HR 1.16, 95% CI 1.03–1.31), however age at diagnosis was 16 years or later in our study and age 10 years or later in the Finnish study.8 A large register-based study including over 500 000 children reported an increased but insignificant association between Caesarean delivery and odds of schizophrenia (OR 2.6, 95% CI 0.6–10.9).32 This was echoed in a case-control study which found that CS was not associated with schizophrenia in the offspring (OR 0.70, 95% CI 0.2–2.9). The main weaknesses of the studies conducted to date include lack of statistical power (ie, small sample sizes) to study rare outcomes such as psychosis.19 Also, lack of detailed information on obstetric confounders and variations in definitions recorded limit the ability to examine any association between obstetric mode of delivery and subsequent psychosis in the offspring.33 Furthermore, none has hitherto explored whether there is support or not for a causal relationship by sibling design.
Strengths and Limitations
The current study incorporated a large population-based cohort design using the Swedish National Registers, which are validated24 and include more than 99% of all births in Sweden. Therefore selection bias is not an issue. This study to our knowledge is the largest to date incorporating detailed data on obstetrical factors including the type of CS as well as reliable diagnoses of the various psychosis outcomes using ICD codes validated in previous studies as sufficiently sensitive and specific to be used in epidemiological studies.34,35 We were also able to adjust for many key potential confounders including maternal age and history of maternal psychosis. Rigorous additional analyses including specific subpopulations (term babies, male babies only, female babies only, babies born in Stockholm county, etc.) were also possible.
In addition, we were able to conduct a sibling-matched Cox regression study which tested for causality and disproved the association which was found in the Cox regression analyses between elective CS and subsequent psychosis. The sibling-matched approach is probably the most widely used sibling design used to characterize designs based on stable family context.36 The advantage of this approach is its ability to vary one aspect of the environment while keeping so much else comparable and is particularly suitable for examining the relation of maternal exposures or conditions across pregnancies and provide a major advantage over population studies of unrelated individuals.37
Although we had information on whether obstetric mode of delivery was an emergency or elective CS, we did not have details on the medical indication for CS. In addition, the CS rate in Sweden (17%) is lower compared to other European countries (for example Cyprus has a rate of more than 52%).38 One implication of this is that there may be a very low rate of nonmedically indicated CS, due partly as a result of the unique healthcare system in Sweden and antenatal counseling offered to women which can reduce CS rates and maternal requests for a CS.39 The Swedish population may have limitations in its use for the current study as a result. Furthermore, information on outpatient cases was only recorded from 2001 onwards and so any cases of psychosis treated in the outpatient department before this may not have been recorded. We did conduct an additional analysis that restricted follow-up to 2001 or later however, and this did not change the overall findings. Furthermore, the offspring in the cohort born in 1995 are now only 20 years of age and some will still go on to develop schizophrenia at a later age and some subtypes of schizophrenia (eg, paranoid schizophrenia) more commonly develop in middle-aged people and bipolar illnesses also have a later onset. In addition, sibling control designs are not without limitations including the inability to determine whether any confounding is as a result of family environment, hereditary or a mixture of both.36,37,40 Another potential limitation of sibling control designs is the possibility that 1 child families may be meaningfully different than families with 2 or more children. Though we are unable to determine if controlling for genetics and family environment as we did with our sibling control design would have led to the same amelioration of the association in 1 child families, our subgroup analysis on 1 child families in the main cohort implies no difference in effect. As women are more likely to give birth by CS if they have had a previous CS,41 we cannot assume independence of exposure between siblings. Again, though we were able to conduct sensitivity analyses both in the main cohort (among first born and excluding repeat CS) and in the sibling control design (excluding repeat CS) which implied no effect on results, it is possible this relatedness of exposure may have affected the sibling control analysis findings.42 As with any observational epidemiological study, residual confounding cannot be ruled out, however, in the absence of clinical trials, population-based cohort studies remain the best way to test the association between mode of delivery and psychosis in offspring.
Conclusion
The current study found a small association between elective CS and psychosis, nonaffective psychoses and affective psychoses, which remained in extensive subgroup analyses. This positive association however, disappeared in the sibling-matched Cox regression analyses implying the relationship is not causal but most likely as a result of unknown familial confounding due to unmeasured factors such as genetics or environment. Further research using population-based data as in the current study is warranted to confirm the findings.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
The Irish Centre for Fetal and Neonatal Translational Research (INFANT) (Science Foundation Ireland (SFI) funded centre, 12|RC|2272). The APC Microbiome Institute is also funded by SFI (grant number SFI/12/RC/2273). G.C., T.G.D., and J.F.C. are supported by the Health Research Board (HRB) through Health Research Awards (grants no HRA_POR/2011/23; T.G.D., J.F.C. and G.C., and HRA_POR/2012/32; JFC, TGD). G.C. is supported by a NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation (Grant Number 20771). T.D. and J.F.C. are also funded by the European Community’s Seventh Framework Programme (MyNewGut, FFP7-KBBE/2013–2018, grant agreement no 613979). The APC has conducted studies in collaboration with several companies including GSK, Pfizer, Wyeth, and Mead Johnson. Funding for the cohort was from Swedish Research Council (Grant # 523-2010-1052). The funding source had no role in the study design, or collection, analysis and interpretation of data, writing of the report, or the decision to submit the article for publication. S.M.O.N. and E.A.C. had full access to the data and had final responsibility for the decision to submit for publication. The authors have no conflicts of interest to declare.
Supplementary Material
Acknowledgments
We thank Mr. Henrik Dal, Division of Public Health Epidemiology, Department of Public Health Sciences, Karolinska Institutet, Stockholm, Sweden, for providing data management support and advice.
References
- 1. Keshavan MS, Giedd J, Lau JY, et al. Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry. 2014;1:549–558. [DOI] [PubMed] [Google Scholar]
- 2. Kelleher I, Connor D, Clarke MC, et al. Prevalence of psychotic symptoms in childhood and adolescence: a systematic review and meta-analysis of population-based studies. Psychol Med. 2012;42:1857–1863. [DOI] [PubMed] [Google Scholar]
- 3. Linscott RJ, van Os J. An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med. 2013;43:1133–1149. [DOI] [PubMed] [Google Scholar]
- 4. Bruenig D, White MJ, Young RM, et al. Subclinical psychotic experiences in healthy young adults: associations with stress and genetic predisposition. Genet Test Mol Biomarkers. 2014;18:683–689. [DOI] [PubMed] [Google Scholar]
- 5. Eisenberger NI, Cole SW. Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat Neurosci. 2012;15:669–674. [DOI] [PubMed] [Google Scholar]
- 6. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. [DOI] [PubMed] [Google Scholar]
- 7. Kavanagh D, Tansey K, O’Donovan M, et al. Schizophrenia genetics: emerging themes for a complex disorder. Mol Psychiatry. 2014;20:72–76. [DOI] [PubMed] [Google Scholar]
- 8. Chudal R, Sourander A, Polo-Kantola P, et al. Perinatal factors and the risk of bipolar disorder in Finland. J Affect Disord. 2014;155:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Borre YE, O’Keeffe GW, Clarke G, et al. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. 2014;20:509–518. [DOI] [PubMed] [Google Scholar]
- 10. Stilling RM, Dinan TG, Cryan JF. Microbial genes, brain & behaviour - epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014;13:69–86. [DOI] [PubMed] [Google Scholar]
- 11. Mackay DF, Smith GC, Dobbie R, et al. Obstetric factors and different causes of special educational need: retrospective cohort study of 407,503 schoolchildren. BJOG. 2013;120:297–307. [DOI] [PubMed] [Google Scholar]
- 12. Cho CE, Norman M. Cesarean section and development of the immune system in the offspring. Am J Obstet Gynecol. 2013;208:249–254. [DOI] [PubMed] [Google Scholar]
- 13. Curran EA, Dalman C, Kearney PM, et al. Association between obstetric mode of delivery and autism spectrum disorder: a population-based sibling design study. JAMA Psychiatry. 2015;72:935–942. [DOI] [PubMed] [Google Scholar]
- 14. Rosso IM, Cannon TD, Huttunen T, et al. Obstetric risk factors for early-onset schizophrenia in a Finnish birth cohort. Am J Psychiatry. 2000;157:801–807. [DOI] [PubMed] [Google Scholar]
- 15. Clarke MC, Tanskanen A, Huttunen M, et al. Increased risk of schizophrenia from additive interaction between infant motor developmental delay and obstetric complications: evidence from a population-based longitudinal study. Am J Psychiatry. 2011;168:1295–1302. [DOI] [PubMed] [Google Scholar]
- 16. McNeil TF, Cantor-Graae E, Weinberger DR. Relationship of obstetric complications and differences in size of brain structures in monozygotic twin pairs discordant for schizophrenia. Am J Psychiatry. 2000;157:203–212. [DOI] [PubMed] [Google Scholar]
- 17. Rubio-Abadal E, Ochoa S, Barajas A, et al. Birth weight and obstetric complications determine age at onset in first episode of psychosis. J Psychiatr Res. 2015;65:108–114. [DOI] [PubMed] [Google Scholar]
- 18. Abel KM, Heuvelman HP, Jörgensen L, et al. Severe bereavement stress during the prenatal and childhood periods and risk of psychosis in later life: population based cohort study. BMJ. 2014;348:f7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002;159:1080–1092. [DOI] [PubMed] [Google Scholar]
- 20. Bain M, Juszczak E, McInneny K, Kendell RE. Obstetric complications and affective psychoses. Two case-control studies based on structured obstetric records. Br J Psychiatry. 2000;176:523–526. [DOI] [PubMed] [Google Scholar]
- 21. Chudal R, Sourander A, Polo-Kantola P, et al. Perinatal factors and the risk of bipolar disorder in Finland. J Affect Disord. 2014;155:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Axelsson O. The Swedish medical birth register. Acta Obstet Gynecol Scand. 2003;82:491–492. [DOI] [PubMed] [Google Scholar]
- 24. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ekbom A. The Swedish multi-generation register. Methods Biobank. 2011:215–220. [DOI] [PubMed] [Google Scholar]
- 26. Nosarti C, Reichenberg A, Murray RM, et al. Preterm birth and psychiatric disorders in young adult life. Arch Gen Psychiary. 2012;69:610–617. [DOI] [PubMed] [Google Scholar]
- 27. Textor J, Hardt J, Knüppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22:745. [DOI] [PubMed] [Google Scholar]
- 28. Fazel S, Bakiyeva L, Cnattingius S, et al. Perinatal risk factors in offenders with severe personality disorder: a population-based investigation. J Pers Disord. 2012;26:737–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Groenwold RH, White IR, Donders ART, et al. Missing covariate data in clinical research: when and when not to use the missing-indicator method for analysis. Cand Med Assoc. 2012;184:1265–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Allison P. Estimating Cox regression models with PROC PHREG. Survival analysis using the SAS system. 2000:111–184.
- 31. Bain M, Juszczak E, McInneny K, et al. Obstetric complications and affective psychoses. Two case-control studies based on structured obstetric records. Br J Psychiatry. 2000;176:523–526. [DOI] [PubMed] [Google Scholar]
- 32. Dalman C, Allebeck P, Cullberg J, et al. Obstetric complications and the risk of schizophrenia: a longitudinal study of a national birth cohort. Arch Gen Psychiatry. 1999;56:234–240. [DOI] [PubMed] [Google Scholar]
- 33. Verdoux H, Geddes JR, Takei N, et al. Obstetric complications and age at onset in schizophrenia: an international collaborative meta-analysis of individual patient data. Am J Psychiatry. 1997;154:1220–1227. [DOI] [PubMed] [Google Scholar]
- 34. Sellgren C, Landén M, Lichtenstein P, et al. Validity of bipolar disorder hospital discharge diagnoses: file review and multiple register linkage in Sweden. Acta Psychiatr Scand. 2011;124:447–453. [DOI] [PubMed] [Google Scholar]
- 35. Ekholm B, Ekholm A, Adolfsson R, et al. Evaluation of diagnostic procedures in Swedish patients with schizophrenia and related psychoses. Nord J Psychiatry. 2005;59:457–464. [DOI] [PubMed] [Google Scholar]
- 36. Frisell T, Öberg S, Kuja-Halkola R, et al. Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology. 2012;23:713–720. [DOI] [PubMed] [Google Scholar]
- 37. Donovan SJ, Susser E. Commentary: advent of sibling designs. Int J Epidemiol. 2011;40:345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Macfarlane A, Blondel B, Mohangoo A, et al. Wide differences in mode of delivery within Europe: risk‐stratified analyses of aggregated routine data from the Euro‐Peristat study. BJOG. [DOI] [PubMed] [Google Scholar]
- 39. Fagerberg MC, Maršál K, Källén K. Predicting the chance of vaginal delivery after one cesarean section: validation and elaboration of a published prediction model. Eur J Obstet Gynecol Reprod Biol. 2015;188:88–94. [DOI] [PubMed] [Google Scholar]
- 40. Keyes KM, Smith GD, Susser E. On sibling designs. Epidemiology. 2013;24:473–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lavender T, Hofmeyr GJ, Neilson JP, et al. Caesarean section for non-medical reasons at term. Cochrane Database Syst Rev. 2012. [DOI] [PubMed] [Google Scholar]
- 42. Frisell T, Öberg S, Kuja-Halkola R, et al. Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology. 2012;23:713–720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.