Abstract
Psychosis is often characterized by paranoia and poor social functioning. Neurally, there is evidence of functional dysconnectivity including abnormalities when processing facial affect. We sought to establish whether these abnormalities are resolved by cognitive behavioral therapy for psychosis (CBTp). The study involved 38 outpatients with one or more persistent positive psychotic symptoms, and 20 healthy participants. All participants completed an implicit facial affect processing task during functional magnetic resonance imaging (fMRI). Subsequently, patients either continued to receive standard care only (SCO, n = 16) or received CBTp on top of standard care (+CBTp, n = 22), with fMRI repeated 6–8 months later. To examine the mechanisms underlying CBTp-led changes in threat processing and appraisal, functional connectivity during the social threat (angry faces) condition was assessed separately from left amygdala and right dorsolateral prefrontal cortex (DLPFC) seeds. At baseline, patients, compared with healthy participants, showed greater amygdala connectivity with the insula and visual areas, but less connectivity with somatosensory areas. These differences normalized following CBTp and, compared with the SCO group, the +CBTp group showed greater increases in amygdala connectivity with DLPFC and inferior parietal lobule, with the latter correlating with improvement in positive symptoms. From the DLPFC seed, the +CBTp (compared with SCO) group showed significantly greater increase in DLPFC connectivity with other prefrontal regions including dorsal anterior cingulate and ventromedial prefrontal cortex. These findings indicate that CBTp strengthens connectivity between higher-order cognitive systems and those involved in threat and salience, potentially facilitating reappraisal.
Key words: connectivity, CBT, therapy, psychosis, amygdala
Introduction
There has been a growing interest in understanding the neural effects of psychological interventions, to gain a better understanding of the maintaining mechanisms of disorders and to improve future therapies. There is now considerable evidence that interventions such as a cognitive behavioral therapy produce lasting neural activation changes in a range of disorders.1 However, few to date have examined changes in the widespread connections between distributed systems. This is surprising, given that cognitive behavioral therapy targets dysfunctional appraisals of distributed cognitive, affective, and physical inputs.2 Remediation of functional connectivity is likely to be of particular importance in psychosis, which has long been conceptualized as a disorder of network dysconnectivity.3,4 Yet, to date, none have examined changes in functional connectivity following psychological therapy in psychosis.
Psychological models of psychosis emphasize a hyper-vigilance for threat cues as well as a difficulty in disengaging threat-related affective content from conscious awareness.5,6 Neurobiological accounts are in accordance with this view7 and there is convincing evidence of a bias to perceive facial expressions as threatening.8 This social threat network includes limbic (amygdala, putamen, thalamus), visual (fusiform gyrus), and prefrontal regions, in addition to the insula.9 In healthy participants, cognitive reappraisal strategies have been shown to modulate activity in this network, through top-down connections with frontal areas such as the dorsolateral prefrontal cortex (DLPFC).10,11 However, in psychosis there is reduced amygdala connectivity with prefrontal regions when processing threatening faces,12–14 as well as a reduced ability to regulate insula.15–17 At the symptom level, these abnormalities in social threat perception are likely to be involved in the generation and maintenance of persecutory delusions and poor social functioning.18 Accordingly these are an important therapeutic target.
There is considerable evidence that psychological interventions target DLPFC and other prefrontal regions,1,19 perhaps linking to a role in reappraisal of affective inputs mentioned previously.10,11 However, there are no studies to date that have examined changes in functional connectivity following psychological intervention in psychosis. In studies of social threat processing in anxiety disorders, functional connectivity of the amygdala has been implicated both as a predictor of,20 and a responder to,21 CBT. Furthermore, in psychosis, connectivity changes following treatment with antipsychotic medication frequently involve the DLPFC and parietal lobule, as well as other frontal and temporal areas.22–24 The inferior parietal lobule (IPL) is involved in empathy,25 self-awareness,16,26 and self-other discrimination.27 Consistent with these functions, IPL has been linked to cognitive insight; the ability to reflect on one’s own beliefs and to entertain those of others16,28 which is a predictor of favorable CBTp outcome.29
We have previously shown that cognitive behavioral therapy for psychosis (CBTp) brings about significant changes in the processing of social threat. Specifically, there was a reduction in the activation of arousal and salience regions including insula, thalamus, putamen, and visual areas, and these changes correlated with improvement in positive symptoms.30 The present study sought to elaborate on the mechanism underlying these functional activation changes by examining, in the same patients, concomitant changes in functional connectivity with higher-order cognitive systems. A further aim was to establish whether CBTp-led changes result in normalization of connectivity by additionally comparing connectivity with healthy participants. We hypothesized that patients with psychosis would show greater recruitment of affective and salience regions with reduced regulation from higher cognitive areas. In line with this, we predicted greater amygdala connectivity with insula and reduced connectivity with DLPFC12–14 and the parietal lobule,22–24 compared with healthy participants. We further predicted that these abnormalities would normalize following CBTp and that CBTp-led changes in connectivity would correlate with improvement in psychotic symptoms.
Methods
Participants and Design
Participants were 38 right-handed outpatients with a diagnosis of paranoid schizophrenia,31 and 20 healthy participants matched, on average, to patients for age, sex, handedness, and years of education (table 1). Patients were recruited from community services in the South London and Maudsley NHS Foundation Trust (SLaM). Creation of the study groups followed a cohort case-controlled design in which one inclusion criteria was that all included patients were deemed as suitable for CBTp by consultant psychiatrists. Hence receipt vs nonreceipt of CBTp was independent of the research team and resulted from clinical resource limitations of the NHS Trust, rather than patient characteristics. Participants receiving standard care only (SCO group) were matched to participants receiving CBTp on top of standard care (+CBTp group) on the basis of having similar clinical and demographic characteristics. All patients had been on stable doses of antipsychotic medication for at least 3 months at entry to the study (ie, at T1) and this was not changed during the study. Hence, only the +CBTp group received any additional intervention as part of the study. The study procedures were approved by the ethics committee of the joint research ethics committee of SLaM and Institute of Psychiatry, Psychology & Neuroscience in London (ref: 209/02). All participants provided written informed consent and were compensated for their time and travel.
Table 1.
Demographics, Task Performance, and Clinical Characteristics of Participants
| +CBTp Group (n = 22, 18 Male) | SCO Group (n = 16, 14 Male) | Healthy Participants (n = 20, 15 Male) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||||||
| Baseline | Follow-up | Baseline | Follow-up | Baseline | ||||
| Age (years) | 35.7 (7.82) | 39.2 (9.37) | 37.3 (12.5) | |||||
| Education (years) | 13.9 (3.26) | 13.6 (1.71) | 14.8 (3.0) | |||||
| Predicted IQa | 109.4 (9.68) | 106.6 (9.73) | 115 (8.0) | |||||
| Age at illness onset | 24.8 (8.38) | 25.8 (8.49) | ||||||
| Duration of illness (years) | 10.9 (7.70) | 13.4 (10.2) | ||||||
| Medication | Atypical antipsychotic (n = 20); combined atypical and typical (n = 2) | Atypical antipsychotic (n = 14); combined atypical and typical (n = 2) | ||||||
| Chlorpromazine equivalent (mg) | 543 (479.3) | 448.9 (338.8) | ||||||
| Gender Discrimination Accuracy (%) | Neutral | 92.6 (10.8) | 91.8 (13.1) | 88.3 (10.8) | 90.0 (14.6) | 92.5 (18.3) | ||
| Fear | 90.5 (14.4) | 91.4 (16.5) | 87.9 (20.9) | 87.9 (18.5) | 92.7 (15.8) | |||
| Anger | 88.6 (15.2) | 88.9 (14.2) | 84.8 (16.1) | 87.3 (19.1) | 91.3 (19.4) | |||
| Happy | 94.7 (8.48) | 93.3 (9.94) | 92.4 (7.82) | 90.8 (12.3) | 94.5 (18.7) | |||
| Detection (%) | No face | 93.4 (12.4) | 91.5 (16.4) | 93.8 (13.7) | 92.5 (18.1) | 93.8 (22.3) | ||
| PANSSb symptoms | ||||||||
| Positive symptoms | 18.1 (4.84) | 14.9 (4.10) | t(21) = 3.90* | 18.6 (3.20) | 18.1 (3.30)ns | |||
| Negative symptoms | 17.7 (4.23) | 15.6 (4.29) | t(21) = 2.61* | 19.1 (4.13) | 20.3 (4.38)ns | |||
| General Psychopathology | 33.5 (7.24) | 28.6 (7.40) | t(21) = 2.97* | 35.4 (4.41) | 35.4 (6.49)ns | |||
| Total symptoms | 69.3 (13.3) | 59.0 (14.7) | t(21) = 3.72* | 73.1 (9.28) | 73.8 (11.8)ns | |||
| BDIc symptoms | 16.2 (8.3)d | 11.5 (9.9)*d | t(19) = 2.19* | 15.9 (10.4) | 15.8 (12.1)ns | |||
aNational Adult Reading Test.32
bPositive and Negative Syndrome Scale.33
cBeck Depression Inventory.34
dMissing data for 1 participant.
*Significant symptom reduction (P < 0.05) at follow-up relative to baseline.
Diagnosis was confirmed by means of the Structured Clinical Interview for DSM-IV (SCID).31 Psychotic symptoms were assessed blindly at both time points using the Positive and Negative Syndrome Scale (PANSS).33 Mood symptoms were assessed using the Beck Depression Inventory-II.34 All participants underwent functional magnetic resonance imaging (fMRI) at baseline (T1). Patients were scanned a second time 6–8 months later (T2), following either CBTp in addition to standard care (+CBTp group, n = 22) or standard care only (SCO group, n = 16). Healthy participants were recruited from the local community using existing research databases. Exclusion criteria included a history of mental illness, drug and alcohol abuse or a regular medical prescription. The absence of a clinical diagnosis in healthy participants was confirmed using the SCID nonpatient edition.35
Task
In the scanner, participants performed an implicit facial affect task in which they were presented with monochrome faces depicting fear, anger, happiness, or neutral expressions.36 These 4 conditions were counterbalanced across 16 blocks lasting 30 s (8 faces per block, with 3.75 s per face). Each block was followed by a 15 s block containing 4 baseline trials in which an empty oval frame without the faces (but matched for luminance to the faces) was shown. On presentation of each face, participants had to indicate whether the face was male or female by pressing the left (female) or the right (male) button on a button box. During the baseline blocks, they were required to press either the left or the right button when the empty oval frame appeared. All participants practiced the gender discrimination task once on all identities on a laptop computer and an identical button box in advance of their scheduled fMRI scan.
Cognitive Behavioral Therapy for Psychosis and Standard Care Procedures
The +CBTp group received 6–8 months of manualized CBT for psychosis37 (see supplementary material).
Image Acquisition
Two hundred and forty T2*-weighted images were acquired using a 1.5 Tesla General Electric Signa system with the following parameters: echo time 40ms, repetition time 3 s, flip angle 90°, field of view 240mm, slice thickness 7.0mm, and interslice gap 0.7mm. In the same session, a high-resolution structural scan (T1-weighted images in the axial plane with 1.5mm contiguous sections) was acquired using a 3D inversion recovery prepared spoiled gradient recalled acquisition (echo time 5.1ms, repetition time 18ms, inversion time 450ms, flip angle 20 degrees with one data average, and 256×256×128 voxel matrix).
Data Analysis
Sample Characteristics and Task Performance.
Analysis of variance (ANOVA) was used to compare the patient and healthy participant groups on their age, predicted IQ,32 and years of education. For patient groups, duration of illness, age of onset, and number of episodes were also compared. For task performance, the percentage of correct trials for gender discrimination of facial expressions was compared across the 3 groups at baseline (T1) and across the 2 patient groups that completed the task a second time (at T2) using ANOVA. For T1, the factors were group (healthy participants, SCO, +CBT) and condition (anger, happy, fear, neutral). This ANOVA was repeated for T2 including only the SCO and +CBT groups.
CBTp-led Changes in Symptoms and Task-Related Activations.
The analysis of CBT-led changes in symptoms and task-related neural activation patterns have been reported in full elsewhere30 and as such are summarized in the results section.
Functional Connectivity Analysis.
We assessed functional amygdala and DLPFC connectivity through the psycho-physiological interaction (PPI) approach38—see supplementary methods for more information.
CBTp-led Changes in Connectivity.
The main analyses focused on direct social threat (angry faces) condition, as this yielded the highest neural activation and was responsive to CBTp in previous work.30 The prosocial affect (happy faces) condition was also deemed to be of clinical importance given that patients with psychosis often misread prosocial gestures,39 potentially in line with cognitive biases which are likely to be amenable to CBTp. These analyses are reported as supplementary material due to space constraints.
Separate voxel-wise PPI analyses yielded statistical parametric maps containing positive connectivity coefficients for healthy (T1 only) and patient groups (both T1 and T2). After establishing that the 2 psychosis groups did not differ from each other at baseline, we established baseline abnormalities by comparing healthy participants to the patients (collapsed across groups) by using independent-samples t-tests within a random-effects analysis. Next, we assessed longitudinal changes in the psychosis groups from T1 to T2 by entering the connectivity maps into a random-effects ANOVA with factors group (+CBT, SCO) and time (T1, T2). We focused on changes in the +CBT group and examined the group–time interaction to establish significant group differences over time. Finally, we assessed whether the baseline abnormalities (T1) were still present at T2 by comparing both clinical groups with the healthy group (reported as supplementary material). Based on previous reports of CBT effects in other disorders,20,40,41 we expected that connectivity changes would be relatively subtle. We thus initially applied a threshold of uncorrected P < .001 (≥7 contiguous voxels) for all contrasts of interest. We then examined which findings survived more conservative family-wise error correction.
Finally, we examined whether the connectivity abnormalities observed at T1 were still present at T2, by comparing the +CBTp and SCO groups separately to the healthy group. The F-contrast “patients different from healthy participants” from T1 was used to functionally mask the contrasts at T2 (“+CBTp T2 different from healthy participants”; “SCO T2 different from healthy participants”). To reduce false negatives a more lenient threshold (uncorrected P < .05) was used to define the T1 functional mask (with our standard threshold of uncorrected P < .001 kept for the T2 contrast).
Correlation with Symptoms and Assessing Normalization.
After identifying areas of connectivity that responded to CBTp, we examined associations with symptom change on the PANSS in the +CBTp group. To do this, we first defined functional regions of interest (ROI) as 2mm spheres around the +CBTp group peaks of the connectivity changes (within-group and interactive effects). Next, we used MarsBar42 to extract the mean connectivity coefficients within these ROIs for both time points. We then explored associations between the CBTp-led connectivity changes and symptom improvement. To reduce the risk of type 1 error due to multiple statistical tests and to test for effects at the multivariate level, we performed multivariate analysis of variance (MANOVA) instead of multiple univariate analyses.43 The CBTp-led connectivity changes were entered into a MANOVA as separate dependent variables and the changes on the positive and negative subscales of the PANSS were entered as additional regressors, separately for the 2 seeds. The direction of any significant effects was determined by partial correlation tests (uncorrected).
Results
Sample Characteristics and Baseline Task Performance
Sample characteristics for the 2 patient groups and the healthy group are reported in table 1. At baseline, there were no differences between groups in terms of age, sex, or years of education (P ≥ .39). There were differences in predicted IQ [F(2, 54) = 4.69, P = .013], with the healthy participants showing higher scores than the SCO [t(54) = 2.22, P = .03] and +CBT [t(54) = 2.93, P = .005] groups but no difference between the 2 patient groups (P = .37). There were also no differences between the patient groups in terms of duration of illness, age of onset, number of episodes, or rates of drug use (P ≥ .19). Groups were also equivalent in terms of psychotic and depressive symptoms (P ≥ .32) and did not differ in their task performance for any condition at T1 (P ≥ .49) or at T2 (P ≥ .53).
CBTp-led Changes in Symptoms and Task-Related Activations
Only the +CBTp group showed a significant improvement of symptoms at T2, as measured by PANSS and Beck Depression Inventory-II (table 1). Neurally, compared with SCO, the +CBTp group showed significant reductions in activation (from T1 to T2) in left inferior frontal gyrus, left insula, bilateral putamen, and left occipital areas (table 3 in ref.30). In addition, these changes correlated with improvement in symptoms of psychosis (table 5 in ref.30).
Functional Connectivity Abnormalities in Patients at Baseline (T1)
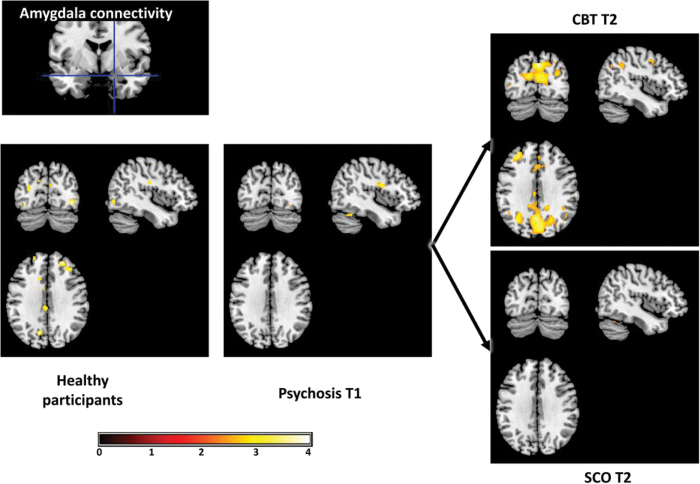
Healthy participants showed positive amygdala connectivity with several frontal areas, including right frontopolar cortex, right anterior cingulated, and left premotor cortex, as well as with left precuneus and thalamus (supplementary table 1). Compared to healthy participants, patients (collapsed across groups) showed greater amygdala connectivity with right lingual gyrus and left insula (straddling anterior and posterior segments), but less connectivity with left postcentral gyrus (table 2; see supplementary table 2 for network at T1 in psychosis participants).
Table 2.
Group Differences in Baseline (T1) Connectivity from Amygdala for Angry Faces Condition
| Contrast | MNI Coordinates | Z Score | Cluster Size | Brodmann Area | Region | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| AMYG | |||||||
| Healthy > Patients | 44 | −16 | 28 | 3.1 | 7 | 3 | Postcentral gyrus |
| Patients > Healthy | 32 | −62 | 10 | 3.12 | 10 | 19/37 | Lingual gyrus |
| −44 | −10 | 22 | 3.06 | 1 (10*) | 13 | Insula | |
*Uncorrected P < .005.
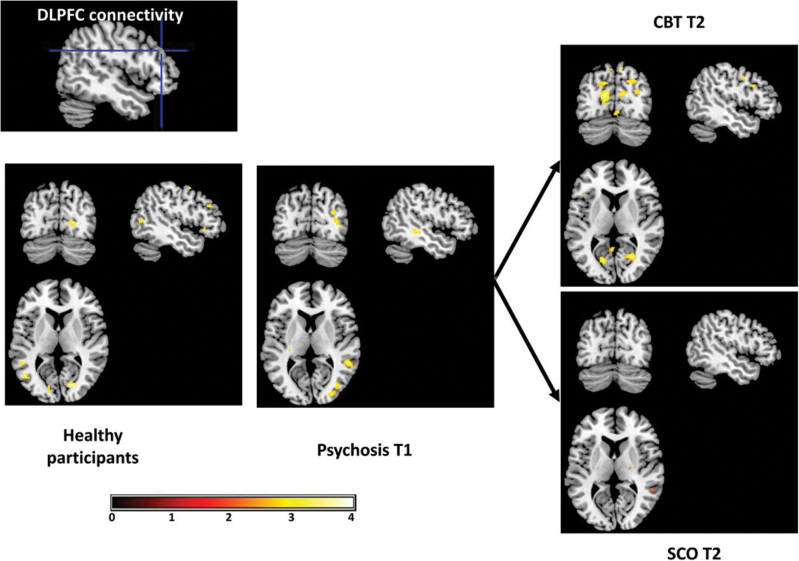
From the DLPFC seed, healthy participants showed widespread connectivity, including right insula, bilateral inferior frontal gyrus, left premotor cortex, right visual cortex, right superior parietal lobule, as well as mid- and superior- temporal areas bilaterally (supplementary table 1). Similar connections were observed in the psychosis participants, although there were no cingulate or precentral gyrus connections (supplementary table 2). However, differences between the healthy and psychosis participants did not reach significance at this time point.
Longitudinal Changes in Functional Connectivity (from T1 to T2)
There were no differences between the +CBT and SCO groups at baseline for either the amygdala or DLPFC seeded connectivity analyses at our voxel threshold (uncorrected P < .001).
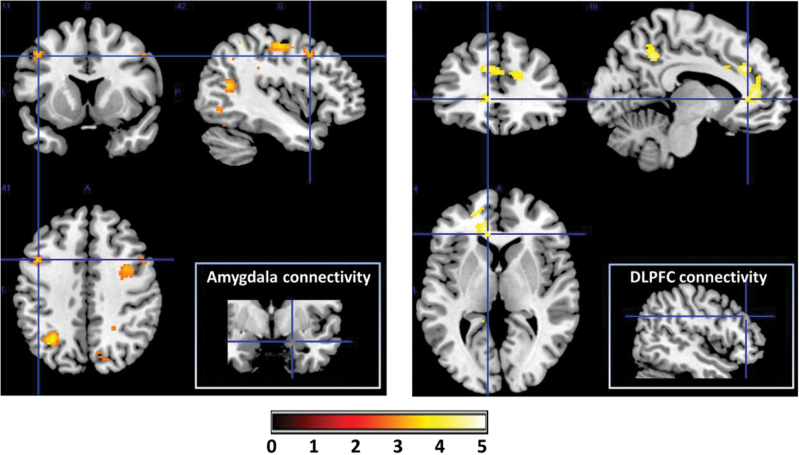
Within the +CBTp group, there were T1 to T2 increases in amygdala connectivity with left DLPFC and right IPL (figure 1; supplementary table 3) as well as bilateral premotor cortex and posterior cingulate gyrus. There were no reductions in connectivity in this group. To examine significant group differences in connectivity changes, we tested for group–time interaction effects. Compared with the SCO group, the +CBT group showed significantly greater increases in amygdala connectivity with right DLPFC, IPL, and posterior cingulate gyrus as well as left SPL, postcentral gyrus, and thalamus (figure 2, left pane). They also showed a greater reduction in connectivity with a cluster bordering left insula and inferior frontal gyrus.
Fig. 1.
Cognitive behavioral therapy for psychosis (CBTp) increases threat-related amygdala connectivity. At baseline (T1), the prefrontal and visual cortical connectivity evident in healthy participants (far left) were not evident in the psychosis participants. There were post-therapy increases in connectivity in these connections, as well as the inferior parietal lobule, in the +CBTp but not SCO group. Visualized with voxel threshold of P < .005 (uncorrected) around centre x = −43, y = −76, z = 33.
Fig. 2.
Cognitive behavioral therapy for psychosis (CBTp) increases threat-related dorsolateral prefrontal cortical connectivity. There were post-therapy increases in connectivity with visual, prefrontal, and cingulate cortices in the +CBTp but not SCO group. Visualized with voxel threshold of P < .005 (uncorrected) around centre x = −49, y = −73, z = 8.
From the DLPFC seed, the +CBTp group showed increased connectivity with left visual cortex (from T1 to T2; figure 3; supplementary table 4). The +CBT group showed significantly greater increases in DLPFC connectivity with dorsal and subgenual portions of the left anterior cingulate cortex and with left posterior cingulate, extending into the IPL (figure 2, right pane).
Fig. 3.
Cognitive behavioral therapy for psychosis (CBTp) increases functional connectivity for processing social threat. Compared with the standard care control group, the +CBTp group showed significantly greater increases in amygdala connectivity with a range of regions, including dorsolateral prefrontal cortex (DLPFC) and the inferior parietal lobule (left). Similarly the +CBTp group showed greater increases in DLPFC connectivity with other executive control regions, including dorsal (and ventral, not shown) portions of the anterior cingulate (right). Group-by-time interaction visualized with voxel threshold of P < .005 (uncorrected) around crosshair centres: amygdala (x = −43, y = 11, z = 41] and DLPFC (x = −10, y = 34, z = 4).
Finally, none of the above findings survived family-wise error correction.
Functional Connectivity Associations with Symptom Change
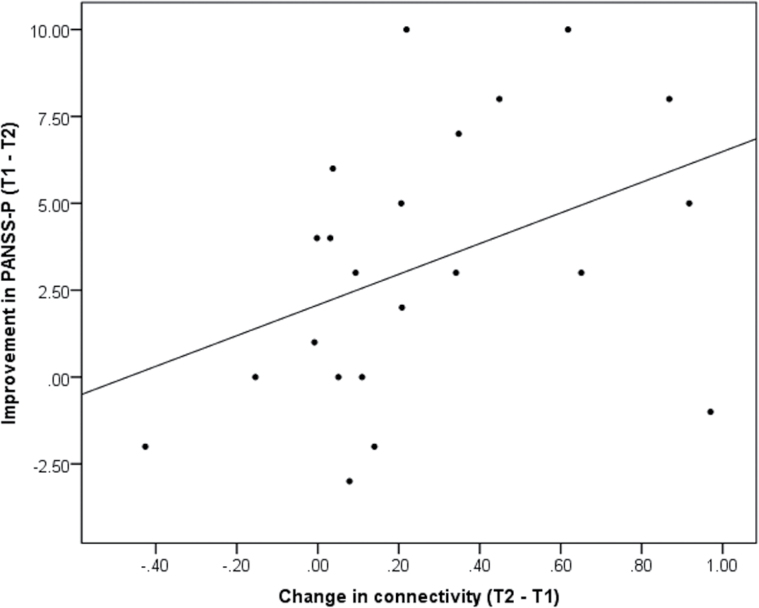
For the amygdala seed, the improvement in positive psychotic symptom (PANSS-P) marginally covaried with the change connectivity with IPL [F(1, 19) = 3.28, P = .08; positive correlation: r(22) = .43, P = .05; figure 4] and SPL [F(1, 19) = 3.66, P = .07; positive correlation: r(22) = .4, P = .07]. There were no other univariate effects for either the amygdala (P ≥ .1) or DLPFC (P ≥ .11) models.
Fig. 4.
Increase in amygdala connectivity with inferior parietal lobule following cognitive behavioral therapy for psychosis correlates with improvement in positive symptoms of psychosis (PANSS-P). Connectivity scale is in beta values.
To better understand the relationship with symptoms, these effects were followed up with correlations with improvement on individual PANSS-P items. The increase in connectivity with IPL was specifically and positively correlated with improvement on the perceived persecution (PANSS-P6) item [Spearman’s ρ(22) = .66, P = .001] but no other items, either for IPL (P ≥ .26) or for SPL (P ≥ .27) connections.
Functional Connectivity Abnormalities Revisited at T2
Further analyses examined whether the connectivity abnormalities observed at T1 were still present at T2, in the +CBTp and SCO groups separately. None of the 3 baseline amygdala abnormalities remained in the +CBTp group when compared again to the healthy group at T2. In contrast, the SCO group continued to show dysconnectivity with somatosensory cortex and, while dysconnectivity with insula was no longer significant at our a priori statistical threshold, it remained at a less conservative threshold (uncorrected P = .008). Furthermore, normalization of these abnormalities correlated with the CBTp-led changes in amygdala connectivity with DLPFC (see supplementary materials).
Discussion
This study found that CBTp resulted in reorganization of brain networks involved in processing social threat.
At baseline, psychosis patients showed amygdala hyperconnectivity with insula and visual occipital areas, compared with healthy participants. These findings are consistent with theoretical accounts of inappropriate activation of the salience network in psychosis, which may underlie hyper-vigilance to social threat and susceptibility to paranoia.17 The psychosis groups additionally showed reduced connectivity between amygdala and somatosensory areas. This finding is consistent with previous reports in psychosis of reduced activation in somatosensory areas when processing facial affect, which is associated with social anhedonia.44 Although not predicted, this is in line with previous reports of reduced activation in somatosensory areas when processing facial affect and can be explained by the role of the somatosensory cortex in supporting recognition of facial affect by providing a visuo-somatic reference template. 45 Hence, reduced coupling between amygdala and somatosensory cortex may indicate that when engaging in facial affect discrimination, people with psychosis do not optimally integrate affective and perceptual aspects, a finding which may explain misperception of affect.18 Importantly, there was evidence that these baseline differences normalized following CBTp, whereas the group receiving SCO continued to show amygdala dysconnectivity with somatosensory areas, compared with the healthy group. This CBTp-specific normalization tallies with reports that social skills training interventions result in increased somatosensory activation during facial affect recognition.46,47
In addition to normalization, there was additionally a widespread proliferation in connectivity post CBTp, as evidenced by changes in the number and strength of connections from amygdala and DLPFC (from T1 to T2). This diverse proliferation of functional connections is evidence that CBTp promotes integration of multiple and distributed neural systems, which may be particularly relevant for psychosis as a dysconnectivity syndrome.3
Many of these connections occurred within the threat network, including amygdala, inferior frontal gyrus, thalamus, and visual areas, which had, in previous analyses, shown reduced activation following CBTp.30 Together, these analyses better explicate a therapeutic mechanism in which aberrant threat network activation is reduced following CBTp, perhaps especially through improved top-down regulation from prefrontal cortical regions. There was also evidence of greater connectivity within prefrontal cortical networks, as evidenced by greater DLPFC connectivity with dorsal anterior cingulate cortex, involved in cognitive control,48 as well as ventromedial prefrontal cortex, which may act as a gateway for regulatory DLPFC projections to amygdala.49 Hence, CBTp may reduce threatening appraisals by integrating affective and salience signals with higher cognitive processes, such as reappraisal.11 Although not tested here, this explanation is in line with the aims of CBTp to shape cognitive appraisals of anomalous experiences and to regulate affective disturbances.6,37
Although no other studies have examined changes in connectivity following psychological therapy for psychosis, there is initial evidence of increased DLPFC regulation of amygdala during facial affective processing in social anxiety disorder.21 Further work will be needed to examine the possibility that this represents a transdiagnostic therapeutic mechanism for social threat.
Also of clinical importance were the CBTp-specific increases in amygdala connectivity with IPL, which correlated with improvement in persecutory beliefs (measured by the positive symptoms scale of the PANSS). This may be attributable to the role of the IPL in self-other discrimination generally27 as well as clinical and cognitive insight in psychosis.16,28 There is evidence of increased (superior) parietal lobule connectivity following pharmacotherapy with antipsychotics,22 highlighting a common outcome irrespective of treatment modality. Further work will be needed to probe the potential overlap in neural mechanisms.
A limitation of the current study design is that the groups were not randomized and so we cannot rule out effects of selection bias for take-up of CBTp. In addition, healthy participants were only tested once and so our analyses to determine normalization did not take into account potential practice effects that could have occurred in the healthy participants had they been tested a second time. The present results did not survive family-wise error correction, increasing the possibility of type 1 errors. It should be noted however that the statistical threshold we applied is at least equal to, if not more conservative than, all extant reports of connectivity changes following CBT in other disorders.20,40,41 Collectively, the research indicates that the changes are relatively subtle or not well captured by existing data capture and/or analysis methods.
In summary, this study provides evidence that CBTp leads to reorganization of amygdala and prefrontal cortex connections mediating social threat processing. These changes included, but go beyond, normalization of the pathophysiology detected at baseline and include additional, compensatory changes. Only a single (prefronto-parietal cortical) connection correlated with improvement in psychotic symptoms, highlighting that additional, more sophisticated clinical psychometrics are needed to better capture the psychoneurobiological changes following CBTp.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
The Wellcome Trust (067427/z/02/z SRF to V.K.); Biomedical Research Centre for Mental Health at the Institute of Psychiatry, Psychology & Neuroscience (King’s College London) and South London and Maudsley NHS Foundation Trust, UK (partial support to V.K.).
Supplementary Material
Acknowledgments
We express our appreciation to the Psychological Interventions Clinic for outpatients with Psychosis (PICuP; South London and Maudsley NHS Foundation Trust), for providing CBTp for this study. All authors report that they have no biomedical financial interests or potential conflicts of interest.
References
- 1. Barsaglini A, Sartori G, Benetti S, Pettersson-Yeo W, Mechelli A. The effects of psychotherapy on brain function: a systematic and critical review. Prog Neurobiol. 2014;114:1–14. [DOI] [PubMed] [Google Scholar]
- 2. Beck AT. Cognitive Therapy and the Emotional Disorders. New York: International University Press; 1976. [Google Scholar]
- 3. Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome. Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- 4. Woodward ND, Waldie B, Rogers B, Tibbo P, Seres P, Purdon SE. Abnormal prefrontal cortical activity and connectivity during response selection in first episode psychosis, chronic schizophrenia, and unaffected siblings of individuals with schizophrenia. Schizophr Res. 2009;109:182–190. [DOI] [PubMed] [Google Scholar]
- 5. Green MJ, Phillips ML. Social threat perception and the evolution of paranoia. Neurosci Biobehav Rev. 2004;28:333–342. [DOI] [PubMed] [Google Scholar]
- 6. Garety PA, Kuipers E, Fowler D, Freeman D, Bebbington PE. A cognitive model of the positive symptoms of psychosis. Psychol Med. 2001;31:189–195. [DOI] [PubMed] [Google Scholar]
- 7. Corlett PR, Taylor JR, Wang XJ, Fletcher PC, Krystal JH. Toward a neurobiology of delusions. Prog Neurobiol. 2010;92:345–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taylor SF, Kang J, Brege IS, Tso IF, Hosanagar A, Johnson TD. Meta-analysis of functional neuroimaging studies of emotion perception and experience in schizophrenia. Biol Psychiatry. 2012;71:136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fusar-Poli P, Placentino A, Carletti F, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- 10. Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. [DOI] [PubMed] [Google Scholar]
- 12. Das P, Kemp AH, Flynn G, et al. Functional disconnections in the direct and indirect amygdala pathways for fear processing in schizophrenia. Schizophr Res. 2007;90:284–294. [DOI] [PubMed] [Google Scholar]
- 13. Fakra E, Salgado-Pineda P, Delaveau P, Hariri AR, Blin O. Neural bases of different cognitive strategies for facial affect processing in schizophrenia. Schizophr Res. 2008;100:191–205. [DOI] [PubMed] [Google Scholar]
- 14. Leitman DI, Loughead J, Wolf DH, et al. Abnormal superior temporal connectivity during fear perception in schizophrenia. Schizophr Bull. 2008;34:673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ruiz S, Lee S, Soekadar SR, et al. Acquired self-control of insula cortex modulates emotion recognition and brain network connectivity in schizophrenia. Hum Brain Mapp. 2013;34:200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van der Meer L, de Vos AE, Stiekema AP, et al. Insight in schizophrenia: involvement of self-reflection networks? Schizophr Bull. 2013;39:1288–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J Psychiatry Neurosci. 2012;37:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2006;32(suppl 1):S44–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kumari V. Do psychotherapies produce neurobiological effects? Acta Neuropsychiatrica. 2006;18:61–70. [DOI] [PubMed] [Google Scholar]
- 20. Lueken U, Straube B, Konrad C, et al. Neural substrates of treatment response to cognitive-behavioral therapy in panic disorder with agoraphobia. Am J Psychiatry. 2013;170:1345–1355. [DOI] [PubMed] [Google Scholar]
- 21. Månsson KN, Carlbring P, Frick A, et al. Altered neural correlates of affective processing after internet-delivered cognitive behavior therapy for social anxiety disorder. Psychiatry Res. 2013;214:229–237. [DOI] [PubMed] [Google Scholar]
- 22. Schmidt A, Smieskova R, Aston J, et al. Brain connectivity abnormalities predating the onset of psychosis: correlation with the effect of medication. JAMA Psychiatry. 2013;70:903–912. [DOI] [PubMed] [Google Scholar]
- 23. Lui S, Li T, Deng W, et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67:783–792. [DOI] [PubMed] [Google Scholar]
- 24. Sarpal DK, Robinson DG, Lencz T, et al. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry. 2015;72:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iacoboni M. Neural mechanisms of imitation. Curr Opin Neurobiol. 2005;15:632–637. [DOI] [PubMed] [Google Scholar]
- 26. Raij TT, Riekki TJ, Hari R. Association of poor insight in schizophrenia with structure and function of cortical midline structures and frontopolar cortex. Schizophr Res. 2012;139:27–32. [DOI] [PubMed] [Google Scholar]
- 27. Uddin LQ, Molnar-Szakacs I, Zaidel E, Iacoboni M. rTMS to the right inferior parietal lobule disrupts self-other discrimination. Soc Cogn Affect Neurosci. 2006;1:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gerretsen P, Menon M, Mamo DC, et al. Impaired insight into illness and cognitive insight in schizophrenia spectrum disorders: resting state functional connectivity. Schizophr Res. 2014;160:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perivoliotis D, Grant PM, Peters ER, Ison R, Kuipers E, Beck AT. Cognitive insight predicts favorable outcome in cognitive behavioral therapy for psychosis. Psychosis 2010;2:23–33. [Google Scholar]
- 30. Kumari V, Fannon D, Peters ER, et al. Neural changes following cognitive behaviour therapy for psychosis: a longitudinal study. Brain. 2011;134:2396–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. First M, Spitzer R, Williams J, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) Washington. DC: American Psychiatric Association; 1995. [Google Scholar]
- 32. Nelson HE, Willison J. National Adult Reading Test (NART). Windsor, UK: Nfer-Nelson; 1991. [Google Scholar]
- 33. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 34. Beck AT, Steer RA, Brown GK. Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 35. First M, Spitzer R, Williams J, Gibbon M. Structured Clinical Interview for DSM-IV-Non-Patient Edition (SCID-NP, Version 1.0). Washington, DC: American Psychiatric Association; 1995. [Google Scholar]
- 36. Ekman P, Friesen WV, Press CP. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press; 1975. [Google Scholar]
- 37. Fowler D, Garety P, Kuipers E. Cognitive behaviour therapy for psychosis: theory and practice, Vol 68 Chichester, UK: Wiley; 1995. [Google Scholar]
- 38. Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. [DOI] [PubMed] [Google Scholar]
- 39. Hooker C, Park S. Emotion processing and its relationship to social functioning in schizophrenia patients. Psychiatry Res. 2002;112:41–50. [DOI] [PubMed] [Google Scholar]
- 40. Goldin PR, Ziv M, Jazaieri H, Hahn K, Heimberg R, Gross JJ. Impact of cognitive behavioral therapy for social anxiety disorder on the neural dynamics of cognitive reappraisal of negative self-beliefs: randomized clinical trial. JAMA Psychiatry. 2013;70:1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kircher T, Arolt V, Jansen A, et al. Effect of cognitive-behavioral therapy on neural correlates of fear conditioning in panic disorder. Biol Psychiatry. 2013;73:93–101. [DOI] [PubMed] [Google Scholar]
- 42. Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage. 2002;16:S497. [Google Scholar]
- 43. Huberty CJ, Morris JD. Multivariate analysis versus multiple univariate analyses. Psychol Bull. 1989;105:302. [Google Scholar]
- 44. Germine LT, Garrido L, Bruce L, Hooker C. Social anhedonia is associated with neural abnormalities during face emotion processing. Neuroimage. 2011;58:935–945. [DOI] [PubMed] [Google Scholar]
- 45. Pourtois G, Sander D, Andres M. et al. Dissociable roles of the human somatosensory and superior temporal cortices for processing social face signals. Eur J Neurosci. 2004;20:3507–3515. [DOI] [PubMed] [Google Scholar]
- 46. Habel U, Koch K, Kellermann T, et al. Training of affect recognition in schizophrenia: Neurobiological correlates. Soc Neurosci. 2010;5:92–104. [DOI] [PubMed] [Google Scholar]
- 47. Hooker CI, Bruce L, Fisher M, Verosky SC, Miyakawa A, Vinogradov S. Neural activity during emotion recognition after combined cognitive plus social cognitive training in schizophrenia. Schizophr Res. 2012;139:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. [DOI] [PubMed] [Google Scholar]
- 49. Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.