Abstract
Objectives:
Most clinicians refrain from trauma treatment for patients with psychosis because they fear symptom exacerbation and relapse. This study examined the negative side effects of trauma-focused (TF) treatment in patients with psychosis and posttraumatic stress disorder (PTSD).
Methods:
Analyses were conducted on data from a single-blind randomized controlled trial comparing TF treatment (N = 108; 8 sessions prolonged exposure or eye movement desensitization) and waiting list (WL; N = 47) among patients with a lifetime psychotic disorder and current chronic PTSD. Symptom exacerbation, adverse events, and revictimization were assessed posttreatment and at 6-month follow-up. Also investigated were symptom exacerbation after initiation of TF treatment and the relationship between symptom exacerbation and dropout.
Results:
Any symptom exacerbation (PTSD, paranoia, or depression) tended to occur more frequently in the WL condition. After the first TF treatment session, PTSD symptom exacerbation was uncommon. There was no increase of hallucinations, dissociation, or suicidality during the first 2 sessions. Paranoia decreased significantly during this period. Dropout was not associated with symptom exacerbation. Compared with the WL condition, fewer persons in the TF treatment condition reported an adverse event (OR = 0.48, P = .032). Surprisingly, participants receiving TF treatment were significantly less likely to be revictimized (OR = 0.40, P = .035).
Conclusions:
In these participants, TF treatment did not result in symptom exacerbation or adverse events. Moreover, TF treatment was associated with significantly less exacerbation, less adverse events, and reduced revictimization compared with the WL condition. This suggests that conventional TF treatment protocols can be safely used in patients with psychosis without negative side effects.
Key words: trauma, adversities, safety, schizophrenia
Introduction
Both childhood abuse1 and posttraumatic stress disorder (PTSD)2,3 are highly prevalent in individuals suffering from psychosis (CI 4.0%–20.8%). Comorbid PTSD is associated with higher symptom levels and poorer social functioning.4 Many patients with psychosis are stuck in a vicious cycle of “stable instability” as long as PTSD goes untreated.5 In clinical practice however, in this population 96.9% of the comorbid PTSD is missed.3 In the unlikely event that PTSD is diagnosed in patients with psychotic disorders, treatment is not offered.6–10 The most important harm expectancy of therapists is the fear that symptom exacerbation will destabilize the patient and result in adverse events, crisis, suicide attempts, hospitalization, revictimization, and dropout.6,11,12
Tarrier and colleagues13 reported symptom exacerbation in a proportion of trial participants who underwent imaginal exposure using the criterion of a 1-point increase in the Clinician-Administered PTSD Scale (CAPS) score; however, a later trial could not replicate these findings.14 A recent study using a reliable change index for exacerbation found that trauma-focused (TF) treatments have a significantly lower rate of exacerbation of symptoms (both PTSD and depression) than a waiting list (WL) condition.15 Moreover, besides symptom exacerbation, clinicians fear that TF treatments may cause dropout,6,12 albeit this does not appear to be the case in general PTSD samples.16 In addition, in several PTSD samples, treatment dropout was found to be unrelated to symptom exacerbation.11,15
Individuals with psychotic disorders are more often revictimized than people in the general population.17 PTSD symptoms are seen not only as a consequence of but also as a precursor of victimization and are reported to be a significant mediator in the relationship between childhood sexual abuse and revictimization.18 However, to date, no study has tested whether PTSD treatment influences revictimization rates.
Unfortunately, none of the above-described clinical concerns of harm-inducing TF treatments have been tested in samples with severe mental illness, such as PTSD patients with persistent psychosis, because psychosis is the most often applied exclusion criterion in PTSD trials.19
Therefore, the present study aimed to investigate the side effects of TF treatment in patients with psychosis and to test whether trauma treatment increases symptom exacerbation, adverse events, high dropout rates, and revictimization.
Methods
The present study is based on secondary analyses of a recently published multicenter single-blind randomized controlled trial investigating PTSD treatment in patients with a psychotic disorder.20 The trial design was approved by the Medical Ethics Committee of the VU University Medical Center (NL:36649.029.12) and was registered at Current Controlled Trials (ISRCTN 79584912). All participants gave written informed consent and were randomly allocated to prolonged exposure (PE) therapy (N = 53), eye movement desensitization and reprocessing (EMDR) therapy (N = 55), or WL. PE and EMDR were found to be significantly more effective than WL. Because no differences were found between the TF conditions PE and EMDR,20 for the present study, these 2 active treatment conditions were combined into one TF treatment condition (N = 108) that was tested against a WL condition (N = 47). Participants were assessed at baseline, at posttreatment, and at 6-month follow-up. Full details of the study procedures are published elsewhere.20,21
Participants
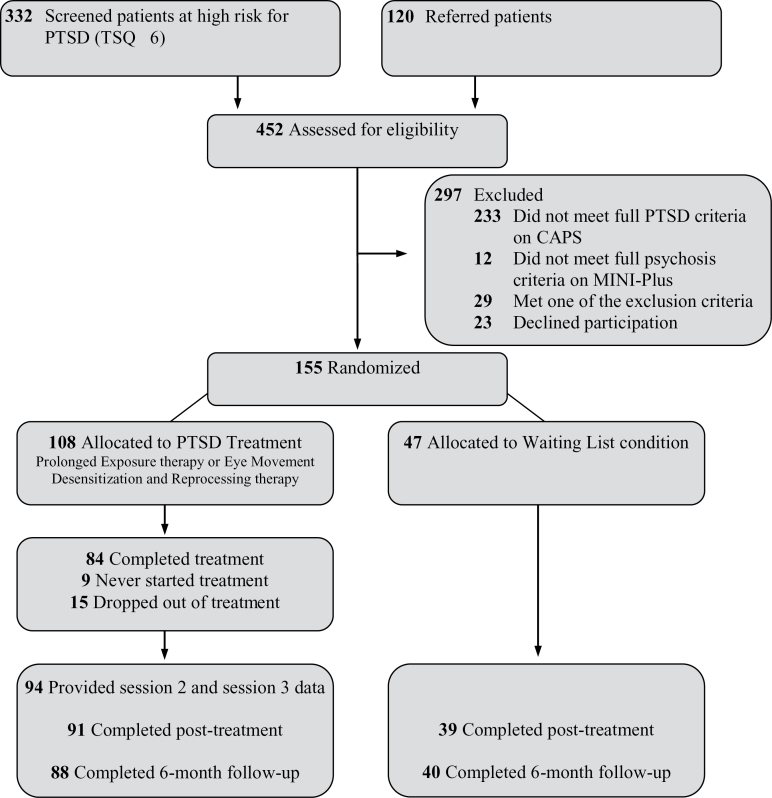
Figure 1 shows the flow of participants through the trial, the dropout, and loss to follow-up. The sample consisted of 155 participants recruited in 13 comparable outpatient services for patients with severe mental illnesses in the Netherlands. We adopted a minimum of exclusion criteria: (1) extremely high acute suicide risk, operationalized as meeting all 3 of the following criteria: high suicidality score on the MINI-International Neuropsychiatric Interview-Plus (MINI-Plus),22 a serious suicide attempt within the past 6 months, and a Beck Depression Inventory-II (BDI-II)23 score ≥ 35; (2) changes in antipsychotic or antidepressant medications ≤ 2 months (to control for medication effects); (3) insufficient competence in the Dutch language; (4) estimated IQ ≤ 70; e) not being able to travel (or be accompanied) to the outpatient service; and (5) involuntary admission in a closed ward. Current psychosis, personality disorder, substance dependence, and suicidal ideation were not considered as contraindications.
Fig. 1.
Flow of participants through the trial. CAPS, Clinician-Administered PTSD Scale; PTSD, posttraumatic stress disorder; TSQ, Trauma Screening Questionnaire.
The mean age of the sample was 41.2 (SD = 10.5) years and 45.8% was male. The sample was characterized by long-standing psychotic disorders (duration M = 17.7, SD = 11.8 y). MINI-Plus Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)-TR diagnoses were: 61.3% schizophrenia, 29.0% schizoaffective disorder, 4.5% bipolar disorder with psychotic features, 2.6% psychotic disorder not otherwise specified, 1.9% depression with psychotic features, and 0.6% brief psychotic disorder (MINI-Plus). At baseline, participants reported current delusions (61.9%), current auditory verbal hallucinations (40.0%), a medium to high suicide risk (45.2%, MINI-Plus), moderate to severe depression (78.7%, BDI-II),23 and substance abuse or dependence (24.5%). Most participants experienced repeated and severe childhood traumatization and all met the full criteria for chronic PTSD on the CAPS.24 At baseline, there were no significant differences between participants randomized to TF or WL on any of the demographic and clinical variables.20
Assessments
Symptom Exacerbation.
Symptom exacerbation was based on change scores (from baseline to posttreatment, and from baseline to 6-mo follow-up).
The total severity score of the CAPS (range 0–136),24 based on the frequency and intensity ratings of each of the 17 DSM-IV-TR PTSD criteria, was used to determine exacerbation of PTSD symptoms. The CAPS has excellent psychometric properties in terms of reliability and validity.25,26 Cutoffs for reliable exacerbation were calculated using the following formula: Cutoff = SEdiff × 1.96 = SD1√2√(1 − r) × 1.96,27,28 where SD1 = the standard deviation of the baseline observations in our sample (see table 1) and r = the test-retest reliability of the measure. The test-retest reliability for the CAPS total severity score was found to be 0.89.29 Therefore, in our sample, the cutoff for reliable exacerbation was 14.89 points. In the present study, the assessors were successfully blinded for treatment allocation and the intraclass correlation coefficient for the CAPS was 0.81.
Table 1.
Baseline Clinical Characteristics of the Sample by Group Assignmenta
| Trauma-Focused Treatment (n = 108) | Waiting List (n = 47) | Total Sample (n = 155) | P Value (2-Tailed) | |
|---|---|---|---|---|
| CAPS, mean (SD) | 70.8 (16.3) | 68.1 (15.9) | 69.9 (16.2) | .330 |
| PSS-SR, mean (SD) | 29.4 (7.9) | 27.7 (8.9) | 28.9 (8.2) | .234 |
| GPTS, mean (SD) | 85.7 (33.6) | 83.8 (31.4) | 85.1 (32.8) | .749 |
| BDI-II, mean (SD) | 29.6 (11.5) | 29.7 (12.4) | 29.6 (11.7) | .927 |
| Adverse event in previous month, ratio (%)b | 34/108 (31.5) | 17/47 (36.2) | 51/155 (32.9) | .581 |
| Revictimization in previous month, ratio (%)c | 10/108 (9.3) | 2/47 (4.3) | 12/155 (7.7) | .349 |
Note: BDI-II, Beck Depression Inventory-II; CAPS, Clinician-Administered PTSD Scale; GPTS, Green et al. Paranoid Thought Scales; PSS-SR, Posttraumatic Stress Symptom Scale Self-Report.
aThe reference period for adversities and revictimization at baseline was 1 mo. Continuous variables were tested with the independent samples t test, categorical variables with the chi-square test, and number of adverse events with the Mann-Whitney U test.
bDefined as at least one incident of self-harm, suicide attempt, aggressive behavior, problematic alcohol abuse, problematic drug abuse, crisis contact with mental health care, or psychiatric hospitalization.
cDefined as experiencing at least one incident of sexual abuse, physical abuse, emotional abuse, or other traumatic events.
The Posttraumatic Stress Symptom Scale Self-Report (PSS-SR)30 was administered to assess exacerbation of self-reported PTSD symptoms (range 0–51). In addition to the assessments at baseline, posttreatment, and at 6-month follow-up, the PSS-SR was administered before each treatment session in the TF condition to assess exacerbation after the start of TF treatment and before treatment dropout. The PSS-SR has satisfactory internal consistency, high test-retest reliability, and good concurrent validity,30,31 also in a sample with a first episode of psychosis.32 The test-retest reliability was found to be 0.83;33 for our sample, this results in a cutoff for reliable exacerbation of 9.39 points.
The Green et al. Paranoid Thought Scales (GPTS)34 is a self-report measure that assesses paranoid ideation severity (range 32–160). The GPTS was found to be a valid measure with good internal consistency, sensitivity to clinical change, and a test-retest reliability of 0.90.34 This results in a cutoff for reliable exacerbation of 28.79 points.
Exacerbation in severity of depressive symptoms was indexed using the BDI-II. The BDI-II has good psychometric properties regarding validity and reliability.35 The test-retest reliability was found to be 0.96,36 resulting in a cutoff for reliable exacerbation in our sample of 6.50.
We could not calculate a reliable change index for our measure for hallucinations (ie, the Auditory Hallucination Rating Scale; AHRS)37 because the AHRS is not a continuous measure. Instead, we report the number of participants that did not hear voices at baseline but did report to hear voices at posttreatment and/or at 6-month follow-up.
Participants in the TF condition rated paranoid ideation, auditory verbal hallucinations, dissociative feelings, and suicidal ideation (0–10) before and after the first 2 active TF treatment sessions, to assess exacerbation of these symptoms after the initiation of TF treatment.
Adversities.
Adverse events were assessed at baseline (1-mo time frame), at posttreatment (3 mo), and at 6-month follow-up (3 mo) by self-report into 7 types of adversities: self-harm, suicide attempt, aggressive behavior, problematic alcohol abuse, problematic drug abuse, crisis contact with mental health care, and psychiatric hospitalization.
Revictimization.
Revictimization in the form of sexual abuse, physical abuse, emotional abuse (psychological maltreatment), and “other traumatic events” (eg, “In the preceding period did you experience sexual activities against your will?”)3 was assessed by self-report at baseline (1-mo time frame), at posttreatment (3 mo), and at 6-month follow-up (3 mo).
Intervention
In the TF condition, participants received 8 weekly 90-min sessions of either PE38 or EMDR39 within a 10-week time frame. Participants in the WL condition received no TF treatment. All participants received comparable treatment as usual for psychosis, delivered by multidisciplinary assertive outreach teams. No psychotherapeutic stabilization or skills training was applied. In the TF condition, session 1 comprised psycho-education and building a hierarchy of the most intrusive trauma memories. The active TF treatment started in session 2. Participants and caregivers were instructed not to start other forms of TF treatment during participation and to keep medications unchanged. At posttreatment, and at 6-month follow-up, patient files were reviewed regarding these factors. No subjects received other or additional TF treatments during the study period. There were no differences between TF and WL in prescribed medications or in additional support and therapy provided by the caregivers.20
Statistical Analysis
Statistical analyses were conducted with SPSS 22 (IBM SPSS). Symptom exacerbation on the CAPS, PSS-SR, GPTS, and BDI-II was analyzed over participants providing posttreatment or 6-month follow-up data by computing crosstabs with chi-square tests (continuity correction) for independence (2-tailed). Fisher’s exact test P values were reported in case of violation of the assumption of at least 5 observations per cell. The phi coefficient was reported as effect size statistic. We refrained from correcting for multiple testing because, for the present analyses, type II error is more undesirable than type I error. To test the robustness of our findings, we performed sensitivity analyses in which the last observation was carried forward (ie, no change) for missing follow-up data in the exacerbation variables.
Dichotomous outcomes on adverse events and revictimization were analyzed intention-to-treat (N = 155) with logistic generalized estimating equation (GEE) analyses with exchangeable correlation structure. Effects for TF vs WL were computed for “condition” (overall effect) and with interaction effects between time and condition for posttreatment (incidents during the treatment period) and for 6-month follow-up (incidents during the follow-up period). ORs from the GEE analyses are presented for all outcomes. The relative risk reduction was calculated for outcomes at posttreatment and at 6-month follow-up. Sensitivity analyses were performed in which we corrected for adverse events and revictimization at baseline.
Marked outliers with a z score ≥ 3.29 in the number of adverse events data were replaced by the following formula: mean + 3.29 × SD. Nevertheless, data were still overdispersed due to an excess of zeros (eg, 54.6% reported no adversities during the treatment period) making it impossible to perform reliable statistical analyses; therefore, only descriptives for the number of adverse events are reported.
Results
Table 1 presents the baseline clinical characteristics of the sample. There were no differences at baseline between the TF and the WL condition for the clinical characteristics, adversities, or revictimization.
Symptom Exacerbation
Compared with WL, half as many participants in the TF condition had any form of symptom exacerbation (table 2). This effect was significant during treatment (baseline to posttreatment) but not during the period baseline to 6-month follow-up. Participants receiving TF were particularly less likely to show exacerbation of self-reported PTSD symptoms during treatment. A trend in the same direction was found for exacerbation of paranoid ideation (P = .052). The results of the sensitivity analyses with last observation carried forward (data not reported) were similar to the results reported in table 2. At baseline, 93 participants reported not to hear voices (based on the AHRS). Of this subsample, 7.8% of the TF and 11.5% of the WL participants reported auditory verbal hallucinations at posttreatment, and 11.1% of the TF and 12.0% of the WL participants reported hearing voices at 6-month follow-up. No differences were found in the clinician-rated PTSD symptoms and depression.
Table 2.
Observed Outcomes of Symptom Exacerbation
| Trauma-Focused Treatment | Waiting List | df | χ2 | P Value | Phi | |||
|---|---|---|---|---|---|---|---|---|
| Ratio | % | Ratioa | % | |||||
| Any symptom exacerbationb,c | ||||||||
| Baseline to posttreatment | 13/91 | 14.3 | 12/39 | 30.8 | 1 | 3.78 | .050 | −0.19 |
| Baseline to follow-up | 10/88 | 11.4 | 9/39 | 23.1 | 1 | 3.07 | .108 | −0.15 |
| Clinician-rated PTSD symptoms (CAPS) | ||||||||
| Baseline to posttreatment | 3/91 | 3.3 | 2/39 | 5.1 | 1 | 0.00 | .636 | −0.04 |
| Baseline to follow-up | 2/88 | 2.3 | 2/40 | 5.0 | 1 | 0.08 | .589 | −0.07 |
| Self-reported PTSD symptoms (PSS-SR) | ||||||||
| Baseline to posttreatment | 0/91 | 0.0 | 3/39 | 7.7 | 1 | 4.16 | .026 | −0.23 |
| Baseline to follow-up | 3/88 | 3.4 | 2/40 | 5.0 | 1 | 0.00 | .647 | −0.04 |
| Paranoid ideation (GPTS) | ||||||||
| Baseline to posttreatment | 3/91 | 3.3 | 5/39 | 12.8 | 1 | 2.79 | .052 | −0.18 |
| Baseline to follow-up | 2/88 | 2.3 | 4/39 | 10.3 | 1 | 2.26 | .071 | −0.17 |
| Depressive symptoms (BDI-II) | ||||||||
| Baseline to posttreatment | 9/91 | 9.9 | 7/39 | 17.9 | 1 | 0.98 | .245 | −0.11 |
| Baseline to follow-up | 7/88 | 8.0 | 3/39 | 7.7 | 1 | 0.00 | 1.000 | 0.00 |
Note: Abbreviations are explained in the first footnote to table 1. χ2, chi-square test value; df, degrees of freedom; PTSD, posttraumatic stress disorder.
aOne participant withdrew from further participation after completing CAPS and PSS-SR at 6-mo follow-up. Therefore, at 6-mo follow-up N = 40 for CAPS and PSS-SR and N = 39 for the GPTS and BDI-II.
bAny exacerbation on CAPS, PSS-SR, GPTS, or BDI-II.
cWe repeated the analyses with lower cutoffs for exacerbation, ie, not multiplying symptom exacerbation by 1.96, because other studies used these lower cutoffs for exacerbation.(8,12) All the results for the analyses with lower cutoffs for exacerbation (not reported) were similar to the analyses reported here. The only difference is that there was significantly less lower cutoff exacerbation of paranoid ideations (GPTS) in the trauma-focused condition during the treatment period (P = .008; phi = −0.26).
The active TF treatment started in the second session. To investigate whether either the anticipation or initiation of active TF treatment was associated with an exacerbation of PTSD symptoms, we calculated PSS-SR change scores for the start of the first session to the start of the second session (anticipation) and from the second session to the third session (after initiation of TF treatment) in the participants with data for these sessions. Anticipating the start of TF treatment, 7 participants (7.1%) had reliable PTSD symptom exacerbation, 84 (85.7%) had stabile PTSD symptom severity, and 7 (7.1%) showed reliable improvement in PTSD symptoms. After the initiation of TF treatment, 1.1% showed symptom exacerbation, 87.2% had stabile symptom severity, and 11.7% showed an improvement in PTSD symptoms. Table 3 shows that there was no exacerbation of paranoid ideation, auditory verbal hallucinations, dissociative feelings, or suicidal ideation in the first active TF treatment sessions (sessions 2 and 3). Both paranoid ideation and suicidality decreased significantly during the first TF session, and paranoid ideation and dissociative feelings decreased during the second TF session.
Table 3.
Session Ratings of Paranoid Ideation, Auditory Verbal Hallucinations, Dissociative Feelings, and Suicidal Ideationa
| Before First TF Session (N = 98) | After First TF Session (N = 96) | P Value | Before Second TF Session (N = 94) | After Second TF Session (N = 90) | P Value | Week Before First TF Session (N = 98) | Week After First TF Session (N = 94) | P Value | |
|---|---|---|---|---|---|---|---|---|---|
| Paranoid ideation | 3.63 (3.4) | 2.78 (3.4) | .001 | 2.98 (3.2) | 2.49 (3.0) | .010 | 4.53 (3.3) | 3.85 (3.4) | .003 |
| Auditory verbal hallucinations | 1.89 (3.1) | 1.56 (3.0) | .089 | 1.84 (3.2) | 1.83 (3.2) | .901 | 2.71 (3.6) | 2.62 (3.5) | .475 |
| Dissociative feelings | 3.53 (3.2) | 2.92 (3.2) | .064 | 3.14 (3.2) | 2.30 (3.0) | .005 | 4.34 (3.3) | 4.22 (3.5) | .894 |
| Suicidal ideation | 2.12 (2.8) | 1.40 (2.6) | .001 | 1.80 (2.7) | 1.59 (2.9) | .356 | 2.78 (3.3) | 2.44 (3.1) | .206 |
Note: TF, trauma-focused.
aData are expressed as mean (SD). Scores range from 0 (no, not at all) to 10 (yes, very much). P values are based on paired samples t tests.
Dropout
The relationship between symptom exacerbation and treatment dropout was tested in the 91 (84.3%) participants in the TF condition that attended the posttreatment assessment. Nine of these participants (9.9%) dropped out of treatment. The 13 participants with reliable exacerbation of any symptoms (table 2) did not dropout more often than the 78 participants without exacerbation, χ2 (1, n = 91) = 0.05, P = .611, phi = −0.08. However, this analysis was based on data of only 9 of the 24 treatment dropouts (the remaining participants missed the posttreatment assessment). It is possible that several of the 15 dropouts that were lost to follow-up dropped out of treatment due to symptom exacerbation. Moreover, because causality is unclear in the previous analysis, we analyzed the PSS-SR session data of the 24 treatment dropouts of whom 9 never started treatment and 15 actually dropped out during treatment and provided session data. For these 15 participants, we compared the PSS-SR score for the last attended treatment session with the baseline PSS-SR score using the reliable change criterion of 9.39 points. Prior to dropout, 2 participants (13.3%) showed reliable exacerbation of PTSD symptoms, 7 (46.7%) showed no reliable change, and 6 participants (40.0%) showed reliable improvement in PTSD symptoms. These results show that, in this sample, dropout appeared to be unrelated to symptom exacerbation.
Adverse Events
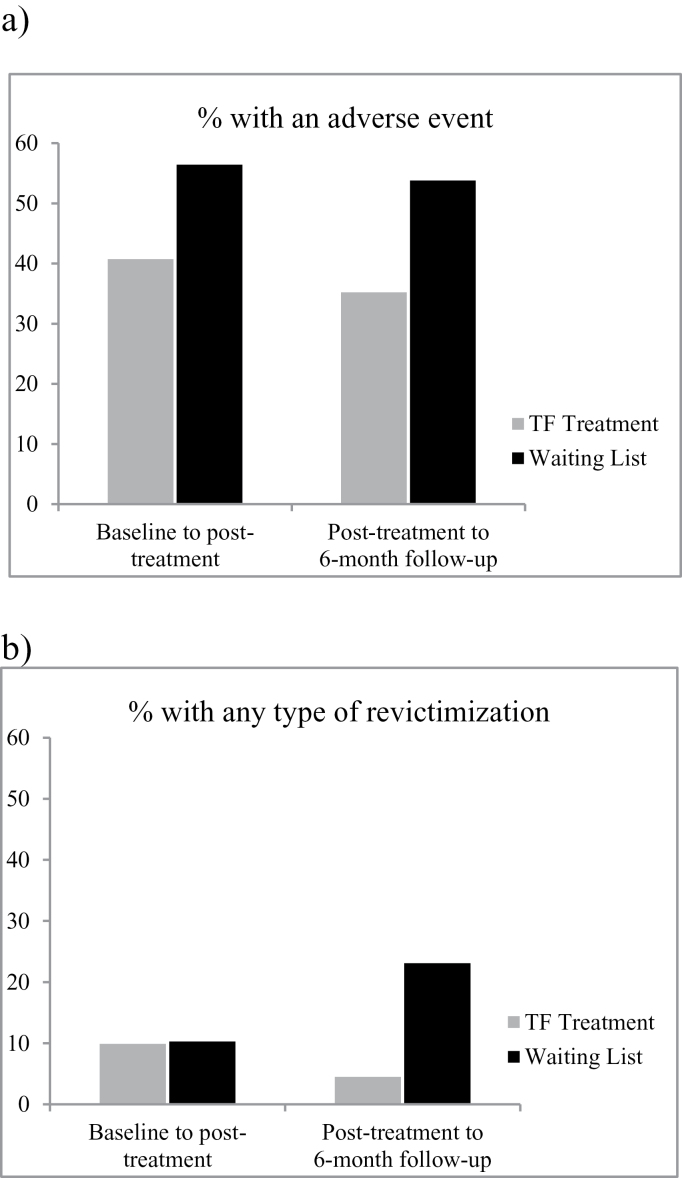
Figure 2a shows the percentage of participants with at least one adverse event during treatment and follow-up. Participants in TF were significantly less likely to experience adverse events than participants in WL (OR = 0.48, P = .032, 95% CI [0.25, 0.94]). This overall effect showed tendencies at the separate time points (posttreatment: OR = 0.49, P = .060, 95% CI [0.23, 1.03]; 6-mo follow-up: OR = 0.48, P = .052, 95% CI [0.22, 1.01]). The relative risk reduction during the treatment period was 27.9% and was 34.6% during follow-up. On average, participants in TF experienced 3.4 adverse events between baseline and posttreatment vs 7.2 in WL. Between posttreatment and 6-month follow-up, the mean number of adversities was 3.8 in TF vs 8.2 in WL. The results for the sensitivity intention-to-treat analyses with correction for baseline adversities were comparable with the intention-to-treat analyses, except that the overall effect for less participants with an adversity in TF lost significance (P = .066).
Fig. 2.
Adverse events and revictimization in TF treatment and waiting list during treatment and follow-up. (a) Defined as at least one incident of self-harm, suicide attempt, aggressive behavior, problematic alcohol abuse, problematic drug abuse, crisis contact with mental health care, or psychiatric hospitalization within that 3-mo time frame. (b) Defined as experiencing at least one incident of sexual abuse, physical abuse, emotional abuse, or other traumatic events within that 3-mo time-frame. TF treatment, trauma-focused treatment.
During the treatment period participants reported the following adversities in TF vs WL: self-harm (15.4% vs 10.3%), suicide attempt (2.2% vs 2.6%), aggressive behavior (1.1% vs 5.1%), problematic alcohol abuse (8.8% vs 23.1%), problematic drug abuse (5.5% vs 7.7%), crisis contact with mental health care (24.2% vs 28.2%), and psychiatric hospitalization (2.2% vs 10.3%). During follow-up participants reported (TF vs WL): self-harm (11.4% vs 10.3%), suicide attempt (3.4% vs 5.1%), aggressive behavior (3.4% vs 2.6%), problematic alcohol abuse (8.0% vs 25.6%), problematic drug abuse (3.4% vs 7.7%), crisis contact with mental health care (15.9% vs 30.8%), and psychiatric hospitalization (5.7% vs 10.3%).
Revictimization
Percentages of revictimization are presented in figure 2b. Participants that received TF treatment were significantly less likely to experience revictimization than participants in the WL condition (OR = 0.40, P = .035, 95% CI [0.17, 0.94]). This overall effect did not occur during treatment (baseline to posttreatment: OR = 1.0, P = .997, 95% CI [0.29, 3.43]), but during follow-up (posttreatment to 6-mo follow-up: OR = 0.16, P = .003, 95% CI [0.05, 0.56]). The relative risk reduction for revictimization was 3.6% during TF treatment and was 80.3% during follow-up. At 6-month follow-up, the TF group experienced less revictimization in all categories vs WL: sexual abuse (1.1% vs 2.6%), physical abuse (3.4% vs 5.1%), emotional abuse (1.1% vs 12.8%), and other traumatic events (0.0% vs 5.1%). The results for the sensitivity intention-to-treat analyses with correction for baseline were the same as the intention-to-treat analyses.
In the total sample, 119 participants provided both posttreatment and 6-month follow-up data. A reduction of CAPS PTSD symptom severity during the treatment period was associated with a significant reduction of revictimization during follow-up (P = .019) and explained between 5.3% (Cox and Snell R square) and 12.2% (Nagelkerke R squared) of the variance in revictimization. With the CAPS PTSD symptom subscales reexperiencing, avoidance, and arousal in one logistic regression model, only change in reexperiencing symptoms was significantly associated with revictimization (P = .017).
Discussion
In this study, treatment of PTSD in participants with psychosis was found to be safe and free of negative side effects. TF treatment did not cause symptom exacerbation, adverse events, or revictimization in this severely mentally ill population with long-standing and current psychotic symptoms and comorbidities, including substance abuse, depression, and suicidal ideation. In fact, a consistent pattern of the opposite emerged, ie, compared with TF, twice as many participants in the WL condition showed some form of symptom exacerbation. Exacerbation of self-rated PTSD (0.0%–3.4%) and paranoia symptoms (2.3%–3.3%) was extremely rare in TF. Exacerbation of depressive symptoms was more common in TF (8.0%–9.9%), but comparable with WL (7.7%–17.9%). In this sample, PTSD symptom exacerbation in anticipation of TF treatment was rare (7.1%) and after the initiation of TF treatment, PTSD symptom exacerbation was virtually absent (1.1%). Moreover, treatment dropout did not appear to be related to PTSD symptom exacerbation. In the first 2 TF treatment sessions, there was no exacerbation of auditory verbal hallucinations, dissociative feelings, or suicidal ideation, and especially paranoid ideation significantly decreased. Fewer participants in TF than in WL experienced an adverse event (relative risk reduction = 27.9%–34.6%). On average, patients in TF experienced half as many adverse incidents compared with those in WL. Participants receiving TF were also less likely to be revictimized during follow-up (relative risk reduction = 80.3%). The reduction of revictimization during follow-up was significantly associated with a reduction in PTSD symptoms (especially reexperiencing symptoms), during the treatment period.
Similar to studies in general PTSD samples,11,14,15 we found that guideline TF treatments with exposure to trauma memories did not induce symptom exacerbation. These findings are in contrast to the beliefs held by most clinicians about negative side effects of TF.6,7 The dropout rate in the present study (22.2%) was comparable with previous studies,16 and the fact that dropout was unrelated to symptom exacerbation is in line with studies in general PTSD samples.11,15
Adversities are common in this population. TF appears to reduce adversities; this is in line with previous findings that TF treatment is not only effective in reducing PTSD symptoms, but has additional value concerning reduction of related psychopathology, such as depression and social functioning (for a review, see40).
To our knowledge, this is the first study to report on the direct influence of TF treatment on revictimization, which is a significant problem in persons with persistent psychosis and other severe mental illnesses.5,17 The fact that a reduction in PTSD symptoms was associated with less revictimization suggests that effectively treating PTSD may safeguard individuals from revictimization. This is in line with studies reporting that PTSD mediates the relationship between childhood abuse and revictimization.18 It must, however, be noted that the effect appeared to be caused by both a decrease of revictimization in TF and an unexplained increase in revictimization in the WL. This last effect may be explained by the relatively small percentage of participants with an incident of revictimization. More research into this matter is necessary.
The present study has some limitations. First, adverse events and revictimization were self-rated; however, people with psychosis have been found to be reliable respondents on these matters.41 Second, “treatment as usual” for psychosis varies between countries. In the present trial, participants received multidisciplinary care for psychosis in community mental health teams. Although we included a large sample, some effects may not have reached significance due to the small numbers of events. Also, although replication of our findings is warranted, the direction of all outcomes is consistent, ie, negative incidents tended to occur less often in the TF condition.
The study also has several strengths. These include (1) the generalizability to clinical practice due to the use of standard treatment protocols in a representative sample of patients with severe symptoms (including comorbidities like depression, suicidal ideation, and substance abuse) in routine long-term care; (2) the controlled design with comparison with WL; (3) assessment of factors both during treatment and follow-up; (4) the limited loss to follow-up; (5) the use of statistically valid and clinically relevant operationalizations of symptom exacerbation; and (6) the fact that we studied a wide range of relevant harm expectancies. In our opinion, the results of this study clearly support the safety of TF treatment in psychosis. Future studies could use a similar design to investigate these issues in groups with other types of comorbidity profiles.
Concerning the underutilization of TF treatments in clinical practice, it is clear that the belief held by many clinicians that conventional guideline PTSD treatments have many negative side effects in patients with psychosis is at odds with the findings of both the present and earlier studies.20,42–44 Instead, withholding these patients from effective TF treatment may cause symptom exacerbations, adverse events, and revictimization. Effectively treating PTSD in these patients with severe and complex symptom patterns may be a highly efficient way to break the vicious cycles faced by many traumatized people and also reduce the need for mental health care.5
We conclude that patients with psychosis and PTSD can safely undergo TF treatment. Not only may PTSD symptoms be ameliorated but also adverse events and revictimization experiences might be prevented. TF treatment appears to have important positive side effects. Accordingly, both trauma and PTSD should be routinely assessed in patients with psychotic disorders and effective treatment should be available and offered.
Funding
Dutch Support Foundation “Stichting tot Steun VCVGZ” (awarded to Dr van der Gaag). Stichting tot Steun VCVGZ had no part in the design and conduct of the study or decisions about this report.
Acknowledgments
The authors thank all participants, therapists, research assistants, and all others who contributed to this study. M.G. and D.B. receive income for published books on psychotic disorders and for the training of postdoctoral professionals in the treatment of psychotic disorders. A.J. receives income for published books on EMDR therapy and for the training of postdoctoral professionals in this method. A.M. receives income for published book chapters on PTSD and for the training of postdoctoral professionals in Prolonged Exposure. C.R. receives income for the training of postdoctoral professionals in EMDR therapy. The other authors declare that they have nothing to disclose.
References
- 1. Matheson SL, Shepherd AM, Pinchbeck RM, Laurens KR, Carr VJ. Childhood adversity in schizophrenia: a systematic meta-analysis. Psychol Med. 2013;43:225–238. doi:10.1017/S0033291712000785. [DOI] [PubMed] [Google Scholar]
- 2. Achim AM, Maziade M, Raymond E, Olivier D, Mérette C, Roy MA. How prevalent are anxiety disorders in schizophrenia? A meta-analysis and critical review on a significant association. Schizophr Bull. 2011;37:811–821. doi:10.1093/schbul/sbp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Bont PA, van den Berg DP, van der Vleugel BM, et al. Predictive validity of the Trauma Screening Questionnaire in detecting post-traumatic stress disorder in patients with psychotic disorders. Br J Psychiatry. 2015;206:408–416. doi:10.1192/bjp.bp.114.148486. [DOI] [PubMed] [Google Scholar]
- 4. Mueser KT, Lu W, Rosenberg SD, Wolfe R. The trauma of psychosis: posttraumatic stress disorder and recent onset psychosis. Schizophr Res. 2010;116:217–227. doi:10.1016/j.schres.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 5. Mueser KT, Rosenberg SD, Goodman LA, Trumbetta SL. Trauma, PTSD, and the course of severe mental illness: an interactive model. Schizophr Res. 2002;53:123–143. [DOI] [PubMed] [Google Scholar]
- 6. Becker CB, Zayfert C, Anderson E. A survey of psychologists’ attitudes towards and utilization of exposure therapy for PTSD. Behav Res Ther. 2004;42:277–292. doi:10.1016/s0005-7967(03)00138-4. [DOI] [PubMed] [Google Scholar]
- 7. Meyer JM, Farrell NR, Kemp JJ, Blakey SM, Deacon BJ. Why do clinicians exclude anxious clients from exposure therapy? Behav Res Ther. 2014;54:49–53. doi:10.1016/j.brat.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 8. Salyers MP, Evans LJ, Bond GR, Meyer PS. Barriers to assessment and treatment of posttraumatic stress disorder and other trauma-related problems in people with severe mental illness: clinician perspectives. Community Ment Health J. 2004;40:17–31. [DOI] [PubMed] [Google Scholar]
- 9. Frueh BC, Cusack KJ, Grubaugh AL, Sauvageot JA, Wells C. Clinicians’ perspectives on cognitive-behavioral treatment for PTSD among persons with severe mental illness. Psychiatr Serv. 2006;57:1027–1031. doi:10.1176/appi.ps.57.7.1027. [DOI] [PubMed] [Google Scholar]
- 10. Gairns S, Alvarez-Jimenez M, Hulbert C, McGorry P, Bendall S. Perceptions of clinicians treating young people with first-episode psychosis for post-traumatic stress disorder. Early Interv Psychiatry. 2015;9:12–20. doi:10.1111/eip.12065. [DOI] [PubMed] [Google Scholar]
- 11. Foa EB, Zoellner LA, Feeny NC, Hembree EA, Alvarez-Conrad J. Does imaginal exposure exacerbate PTSD symptoms? J Consult Clin Psychol. 2002;70:1022–1028. doi:10.1037//0022-006X.70.4.1022. [DOI] [PubMed] [Google Scholar]
- 12. van Minnen A, Hendriks L, Olff M. When do trauma experts choose exposure therapy for PTSD patients? A controlled study of therapist and patient factors. Behav Res Ther. 2010;48:312–320. [DOI] [PubMed] [Google Scholar]
- 13. Tarrier N, Pilgrim H, Sommerfield C, et al. A randomized trial of cognitive therapy and imaginal exposure in the treatment of chronic posttraumatic stress disorder. J Consult Clin Psychol. 1999;67:13–18. [DOI] [PubMed] [Google Scholar]
- 14. Taylor S, Thordarson DS, Maxfield L, Fedoroff IC, Lovell K, Ogrodniczuk J. Comparative efficacy, speed, and adverse effects of three PTSD treatments: exposure therapy, EMDR, and relaxation training. J Consult Clin Psychol. 2003;71:330–338. doi:10.1037/0022-006X.71.2.330. [DOI] [PubMed] [Google Scholar]
- 15. Jayawickreme N, Cahill SP, Riggs DS, et al. Primum non nocere (first do no harm): symptom worsening and improvement in female assault victims after prolonged exposure for PTSD. Depress Anxiety. 2014;31:412–419. doi:10.1002/da.22225. [DOI] [PubMed] [Google Scholar]
- 16. Hembree EA, Foa EB, Dorfan NM, Street GP, Kowalski J, Tu X. Do patients drop out prematurely from exposure therapy for PTSD? J Trauma Stress. 2003;16:555–562. doi:10.1023/B:JOTS.0000004078.93012.7d. [DOI] [PubMed] [Google Scholar]
- 17. Maniglio R. Severe mental illness and criminal victimization: a systematic review. Acta Psychiatr Scand. 2009;119:180–191. doi:10.1111/j.1600-0447.2008.01300.x. [DOI] [PubMed] [Google Scholar]
- 18. Kuijpers KF, van der Knaap LM, Winkel FW. PTSD symptoms as risk factors for intimate partner violence revictimization and the mediating role of victims’ violent behavior. J Trauma Stress. 2012;25:179–186. doi:10.1002/jts.21676. [DOI] [PubMed] [Google Scholar]
- 19. Ronconi JM, Shiner B, Watts BV. Inclusion and exclusion criteria in randomized controlled trials of psychotherapy for PTSD. J Psychiatr Pract. 2014;20:25–37. doi:10.1097/01.pra.0000442936.23457.5b. [DOI] [PubMed] [Google Scholar]
- 20. van den Berg DP, de Bont PA, van der Vleugel BM, et al. Prolonged exposure vs eye movement desensitization and reprocessing vs waiting list for posttraumatic stress disorder in patients with a psychotic disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72:259–267. doi:10.1001/jamapsychiatry.2014.2637. [DOI] [PubMed] [Google Scholar]
- 21. de Bont PA, van den Berg DP, van der Vleugel BM, et al. A multi-site single blind clinical study to compare the effects of prolonged exposure, eye movement desensitization and reprocessing and waiting list on patients with a current diagnosis of psychosis and co morbid post traumatic stress disorder: study protocol for the randomized controlled trial treating trauma in psychosis. Trials. 2013;14:151. doi:10.1186/1745-6215-14-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sheehan DV, Lecrubier Y, Harnett Sheehan K, et al. The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. Eur Psychiatry. 1997;12:232–241. [Google Scholar]
- 23. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 24. Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. [DOI] [PubMed] [Google Scholar]
- 25. Weathers FW, Keane TM, Davidson JR. Clinician-Administered PTSD Scale: a review of the first ten years of research. Depress Anxiety. 2001;13:132–156. [DOI] [PubMed] [Google Scholar]
- 26. Mueser KT, Rosenberg SD, Fox L, Salyers MP, Ford JD, Carty P. Psychometric evaluation of trauma and posttraumatic stress disorder assessments in persons with severe mental illness. Psychol Assess. 2001;13:110–117. doi:10.1037//1040-3590.13.1.110. [DOI] [PubMed] [Google Scholar]
- 27. Evans C, Margison F, Barkham M. The contribution of reliable and clinically significant change methods to evidence-based mental health. Evid Based Ment Health. 1998;1:70–72. [Google Scholar]
- 28. Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12. [DOI] [PubMed] [Google Scholar]
- 29. McDevitt-Murphy ME, Weathers FW, Adkins JW, Daniels JB. Use of the personality assessment inventory in assessment of posttraumatic stress disorder in women. J Psychopathol Behav Assess. 2005;27:57–65. doi:10.1007/s10862-005-5380-2. [Google Scholar]
- 30. Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J Trauma Stress. 1993;6:459–473. [Google Scholar]
- 31. Wohlfarth TD, van den Brink W, Winkel FW, ter Smitten M. Screening for posttraumatic stress disorder: an evaluation of two self-report scales among crime victims. Psychol Assess. 2003;15:101–109. [DOI] [PubMed] [Google Scholar]
- 32. Sin GL, Abdin E, Lee J. The PSS-SR as a screening tool for PTSD in first-episode psychosis patients. Early Interv Psychiatry. 2012;6:191–194. doi:10.1111/j.1751-7893.2011.00327.x. [DOI] [PubMed] [Google Scholar]
- 33. Foa EB, Cashman L, Jaycox L, Perry K. The validation of a self-report measure of posttraumatic stress disorder: the Posttraumatic Diagnostic Scale. Psychol Assess. 1997;9:445–451. [Google Scholar]
- 34. Green CE, Freeman D, Kuipers E, et al. Measuring ideas of persecution and social reference: the Green et al. Paranoid Thought Scales (GPTS). Psychol Med. 2008;38:101–111. doi:10.1017/S0033291707001638. [DOI] [PubMed] [Google Scholar]
- 35. Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and-II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. [DOI] [PubMed] [Google Scholar]
- 36. Sprinkle SD, Lurie D, Insko SL, et al. Criterion validity, severity cut scores, and test-retest reliability of the Beck Depression Inventory-II in a university counseling center sample. J Couns Psychol. 2002;49:381–385. doi:10.1037//0022-0167.49.3.381. [Google Scholar]
- 37. Haddock G, McCarron J, Tarrier N, Faragher EB. Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS). Psychol Med. 1999;29:879–889. [DOI] [PubMed] [Google Scholar]
- 38. Foa EB, Hembree EA, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences: Therapist Guide. Oxford: Oxford University Press; 2007. [Google Scholar]
- 39. Shapiro F. Eye Movement Desensitization and Reprocessing (EMDR): Basic Principles, Protocols, and Procedures. New York, NY: Guilford Press; 2001. [Google Scholar]
- 40. van Minnen A, Zoellner LA, Harned MS, Mills K. Changes in comorbid conditions after prolonged exposure for PTSD: a literature review. Curr Psychiatry Rep. 2015;17:549. doi:10.1007/s11920-015-0549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fisher HL, Craig TK, Fearon P, et al. Reliability and comparability of psychosis patients’ retrospective reports of childhood abuse. Schizophr Bull. 2011;37:546–553. doi:10.1093/schbul/sbp103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Frueh BC, Grubaugh AL, Cusack KJ, Kimble MO, Elhai JD, Knapp RG. Exposure-based cognitive-behavioral treatment of PTSD in adults with schizophrenia or schizoaffective disorder: a pilot study. J Anxiety Disord. 2009;23:665–675. doi:10.1016/j.janxdis.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van den Berg DP, van der Gaag M. Treating trauma in psychosis with EMDR: a pilot study. J Behav Ther Exp Psychiatry. 2012;43:664–671. doi:10.1016/j.jbtep.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 44. de Bont PA, van Minnen A, de Jongh A. Treating PTSD in patients with psychosis: a within-group controlled feasibility study examining the efficacy and safety of evidence-based PE and EMDR protocols. Behav Ther. 2013;44:717–730. doi:10.1016/j.beth.2013.07.002. [DOI] [PubMed] [Google Scholar]