Abstract
Although the insula and temporal pole (TP) of paralimbic regions are important in both affective and cognitive processing, it is not well known whether gray matter volume (GMV) abnormalities in these regions show post-onset progression and differentially affect first-episode schizophrenia (FESZ) and first-episode affective psychosis (FEAFF) patients. To determine whether there are initial and progressive GMV deficits in insula and TP in FESZ and FEAFF (mainly manic) patients, their relative specificity to FESZ or FEAFF, and relationship to symptoms, we conducted a naturalistic study at first hospitalization for psychosis and follow-up ~1.5 years later. Initial 1.5T magnetic resonance imaging (MRI) scans and follow-up scans were on the same scanner. Twenty-two FESZ, 23 FEAFF, and 23 healthy control (HC) subjects were group matched for age, gender, parental socioeconomic status, and handedness. At first hospitalization, FESZ showed significantly smaller bilateral insular GMV compared with FEAFF, and smaller left TP GMV compared with FEAFF and HC. Moreover, on 1.5 years follow-up, FESZ showed progressive GMV decreases in bilateral insula compared with FEAFF and HC, and in TP GMV compared with HC. In contrast, FEAFF showed no progression. Progression of FESZ GMV in both insula and TP was inversely associated with changes in the overall Brief Psychiatric Rating Scale symptom score, indicating less improvement or worsening of symptoms.
Key words: schizophrenia, temporal pole, insula
Introduction
One of the persistent conundrums in psychiatry is whether (and to what extent) psychotic bipolar disorder and schizophrenia differ in their magnetic resonance imaging (MRI) neuroanatomic phenotypes. Some MRI studies in schizophrenia and bipolar disorder have suggested that there may be relative specificity to abnormalities of neuroanatomical regions for each disorder,1–3 although this conclusion remains controversial and in need of more data.3 Our group has addressed this issue by performing a series of manually delineated region of interest (ROI) MRI studies in first-episode (first hospitalization) patients with schizophrenia (FESZ) or affective psychosis (mainly manic, FEAFF). Cross-sectionally, these first episode and affective psychosis studies adopting identically processed subject ROI data indicated there were smaller gray matter volumes (GMV) in FESZ compared with FEAFF in the superior and middle temporal gyri,4,5 and in prefrontal cortex.6 In contrast, smaller volumes were found in both FESZ and FEAFF in the posterior inferior temporal gyrus.5
A longitudinal study approach is particularly relevant since the trajectory of volumetric changes over time may be an important element of phenotypic similarity or difference between FESZ and FEAFF psychosis. Documenting post-onset progressive volume changes would provide a neuroimaging biomarker for the effectiveness of treatment. Other researchers using manually delineated ROI7 and a meta-analysis8 reported that frontotemporal gray matter undergoes progressive reduction over time in schizophrenia.
Longitudinal ROI data from our patient cohorts do suggest the possibility of FESZ-FEAFF differences in progression. For example, the superior temporal gyrus and its components manifested a progressive post-onset loss of GMV in FESZ but none in FEAFF.9 Furthermore, overall neocortical GMV showed an initial reduction in both FEAFF and FESZ but post-onset progression occurred only in FESZ.10 Many subregions of the cingulate gyrus also showed progression in FESZ while progression in FEAFF was limited to the subgenual region linked to affective control.11
Insula and temporal pole (TP) are major components of what Mesulam12 in 2000 has termed the paralimbic cortex. Paralimbic cortex has functional links both to neocortical regions involved in complex processing and to regions involved in affective control.13,14 A review of the insula noted its key role in interoception of body sensations and movement, self-recognition, emotional awareness, attention and salience detection.15 Moreover, the TP and insula are strongly interconnected16; thus, both could be related to psychopathologies such as psychosis or mood regulation of both schizophrenia and bipolar disorder.2,17 Finally, our earlier cross-sectional study18 found bilaterally smaller insula in FESZ relative to FEAFF while both groups had smaller TP compared with healthy control (HC) suggesting that these differences might persist longitudinally and further prompting the current study.
Cross-sectional neuroanatomical neuroimaging studies have reported smaller GMV in the insula in schizophrenia7,8,18 and to a lesser extent in affective psychosis.18,19 Although an important component of the paralimbic system and prominent in functional MRI (fMRI) studies, the TP has been much less studied, with our group’s study reporting no differentiation of FESZ and FEAFF GMV, although both were smaller than HC. Other reports however, showed no significant reductions in insular and TP volumes in schizophrenia19and affective disorder.20 Longitudinally, no study has addressed either the presence of progression in insula or of TP in FESZ and FEAFF, much less the specificity of progression to either disorder. To our knowledge there is only one report of progression in either insula or TP; progressive volume loss in insular gray matter was reported in non-diagnostically specific first-episode psychosis.7
Based on our previous cross-sectional study, we predicted FESZ-FEAFF differentiation in insula but less differentiation in TP, both cross-sectionally and longitudinally. We here report cross-sectional and longitudinal GMV findings for insula and TP in patients with FESZ or FEAFF, compared with HC subjects. Finally our choice of using highly reliable manual ROI vs the frequently used voxel-based analysis such as Freesurfer automated program was based on Enigma data, showing Freesurfer 5.0 and later versions had low test-retest intraclass correlation coefficients for TP (mean = 0.41) and for insula (mean 0.63) in healthy subjects with 4T scans21 in contrast to the very high inter-rater correlation coefficients for our manual ROI (see Methods). Moreover using Freesurfer on the same images, ROI, and statistical processing as used in for our manual ROI did not yield any longitudinal group differences (see supplementary data), in contrast to our results.
Methods
Participants
Forty-five first-episode patients with psychosis, 22 with schizophrenia (3 women) and 23 (3 women) with affective psychosis, and 23 HC subjects (4 women) participated in this study (table 1). Only subjects who completed both baseline and the second scan at approximately 1.5 years later and showed the same diagnosis were included. Subjects common to our earlier cross sectional study were 11 FESZ, 9 FEAFF, and 9 HC.18 FEAFF patients were all psychotic and included 20 patients with bipolar disorder in a manic phase and 3 with major depressive (unipolar) disorder. Patients were recruited in order of presentation to the McLean inpatient wards subject to initial inclusion criteria, and those with 2 usable images were included in this study, subject to group matching on age and sex. HC were recruited through newspaper advertisements. This study was approved by the McLean Hospital and Harvard Medical School Institutional Review Boards. Written informed consent was obtained from all participants before study participation.
Table 1.
Demographic and Clinical Characteristics of Insula and Temporal Pole Gray Matter Volume Longitudinal Study Subjectsa
| FESZ Group (N = 22) | FEAFF Group (N = 23) | HC Group (N = 23) | Statistical Analysis | |||
|---|---|---|---|---|---|---|
| F or t Testb | df c | P Value | ||||
| Age, mean (SD [range]), y | 25.3 (8.3) [18–45] | 22.7 (5.1) [18–42] | 24.2 (3.9) [18–34] | 1.1 | 2,65 | .34 |
| Time between scans, mo | 17.1 (11.5) | 16.6 (6.1) | 16.1 (5.4) | 0.1 | 2,65 | .91 |
| Sex, No. M/Fd | 19/3 | 20/3 | 19/4 | |||
| Race, Caucacian/ African-Am, Asian/Hispanice | 35/4, 0/3 | 33/2, 4/5 | 30/7, 3/6 | |||
| Handednessf | 0.80 (0.2) | 0.76 (0.1) | 0.76 (0.2) | 0.2 | 2,63 | .82 |
| SESg | 3.3 (1.4) | 2.7 (1.2) | 2.3 (1.0) | 3.8 | 2,65 | .03g |
| Parental SES | 1.8 (0.6) | 1.5 (0.9) | 1.4 (0.6) | 1.6 | 2,65 | .21 |
| Years of educationh | 13.4 (2.2) | 14.1 (2.1) | 15.4 (1.9) | 5.6 | 2,65 | .006i |
| WAIS-R information, scaled baseline | 11.9 (3.1) | 13.9 (2.3) | 13.6 (2.3) | 3.7 | 2,62 | .30 |
| WAIS-R digit span, scaled baseline | 9.8 (2.3) | 11.0 (2.5) | 11.5 (2.9) | 2.6 | 2,62 | .08 |
| MMSEj | ||||||
| Baseline scan | 27.6 (2.5) | 29.1 (1.0) | 29.1 (1.1) | 5.9 | 2,64 | .005k |
| Second scan | 28.0 (2.4) | 29.6 (0.9) | 29.4 (0.8) | 6.7 | 2,64 | .002k |
| BPRS | ||||||
| Baseline scanl | 42.0 (12.1) | 33.8 (8.2) | NA | 2.6 | 41 | .01l |
| Second scan | 28.3 (7.2) | 33.8 (8.2) | NA | 1.1 | 41 | .25 |
| GAS | ||||||
| Baseline scan | 35.6 (7.5) | 40.7 (8.8) | NA | −2.0 | 42 | .04m |
| Second scan | 51.2 (13.5) | 64.4 (15.4) | NA | −2.9 | 40 | .005m |
| Medication dosage at baseline CPZ equiv, mg/d | 273.3 (194.5) | 227.1 (167.4) | NA | 0.8 | 42 | .40 |
| Duration of antipsychotic medication before baseline scan, median (range), wkn | 2 (0–14) | 3 (0–15) | NA | |||
| Medication usage, patient, N | ||||||
| Neuroleptics, TYP/ATYP/overlap | ||||||
| At baseline scan | 7/17/3 | 8/15/3 | ||||
| At second scan | 0/17/1 | 2/11/0 | ||||
| Mood stabilizer, lithium/VPA/overlap | ||||||
| At baseline scan | 1/2/0 | 6/7/0 | ||||
| At second scan | 2/1/0 | 9/6/0 | ||||
| Medication between scan 1 & 2 | ||||||
| AP-Treated/ AP-Free(>3 months) | 17/5 | N = 9/14 | ||||
| MS-Treated/ MS-Free(>3 months) | 7/15 | N = 14/9 | ||||
Note: AP, antipsychotics; ATYP, atypical neuroleptics; BPRS, Brief Psychiatric Rating Scale; CPZ equiv, chloropromazine equivalent: FEAFF, first-episode affective psychosis; FESZ, first-episode schizophrenia; GAS, Global Assessment Scale; HC, healthy control; MMSE, Mini-Mental State Examination; MS, mood stabilizer; NA, data not applicable; SES, socioeconomic status; TYP, typical neuroleptics; VPA, valproic acid; WAIS-R, Wechsler Adult Intelligence Scale-Revised. Before the second scan, duration of neuroleptic therapy was less than 3 months in 5 patients with FESZ and 13 patients with FEAFF and 3 months to 35 months in 17 patients with FESZ (all atypical antipsychotics) and 9 patients with FEAFF (9 atypical antipsychotics, [overlap in 1 patients]). For mood stabilizers (MS), including lithium carbonate and valproic acid (VPA), 3 of 22 patients with FESZ (13%) and 13 of 23 with FEAFF (56%) were treated at their first-episode hospitalization before the baseline MRI scan. Duration of MS therapy was less than 3 month in 15 patients with FESZ and 9 patients with FEAFF and 3 months to 29 months in 7 patients with FESZ (lithium carbonate in 4, VPA in 3) and 14 patients with FEAFF (lithium carbonate in 8, VPA in 6). There was no association between the duration of lithium treatment and gray matter volume changes in our subjects.
aOf 23 patients with FEAFF, 20 patients had bipolar disorder and were in manic phase, and 3 had unipolar psychotic depression. Unless otherwise indicated data are expressed as mean (SD).
bThe F tests (1-way analysis of variance) were performed among FESZ, FEAFF, and HC groups for age, time between magnetic resonance imaging (MRI) scans, handedness, SES, parental SES, WAIS-R information and Digit Span scaled score, and MMSE scores. The t tests were performed between FESZ and FEAFF groups for BPRS scores, GAS, duration of illness, and medication dosage.
cThe degrees of freedom differ among variables owing to unavailablility of data in some participants.
dχ2 test (χ2 = 0.2, P = .90) showed no difference in sex ratio among the 3 groups.
eχ2 test (χ2 = 7.8, P = .24) showed no difference in number of race among the 3 groups.
fEvaluated using the Edinburgh Handedness Inventory as ([right hand − left hand] × 100)/(right hand − left hand); scores >0 indicate right-handedness.
gHigher numbers represent lower SES, based on the Hollingshead 2-factor index of SES. The FESZ group showed a significantly lower SES than the HC group (Tukey Honestly Significant Difference [HSD] test, P = .021).
h P < .05.
iThe FESZ group showed significantly fewer years of education than the HC group in Tukey HSD tests (P = .01).
j P < .01.
kThe FESZ group showed significantly lower scores than the FEAFF group (P = .010) and the HC group(P = .013) in Tukey HSD tests at baseline scan, and the FESZ group also showed significantly lower scores than the FEAFF group (P = .004) and the HC group (P = .011) in Tukey HSD tests at second scan.
lThe t tests were performed between 2 groups. The FESZ group showed significantly higher BPRS total scores than the FEAFF group at baseline scan.
mThe t tests were performed between 2 groups. The FESZ group showed significantly lower GAS scores than the FEAFF group at baseline scan. The FESZ group also showed significantly lower GAS scores than the FEAFF group at second scan.
nPatients were not in a stable state and medication was being adjusted.
The protocols for diagnosis and clinical evaluations have previously been described in detail.22 Briefly, patients and controls were aged 18 to 45 years, had an estimated IQ (Wechsler Adult Intelligence Scale23) greater than 75, were right-handed (Edinburgh Inventory24), and had no history of seizures, head trauma with loss of consciousness, neurologic disorder, nor any lifetime history of substance dependence or abuse within the past 6 months. We also excluded substance abuse or dependence patients at follow up. Control subjects were screened using the Structured Clinical Interview for DSM-III-R,25 by trained interviewers (D.F.S. and M.E.S.). The HCs had no Axis I or Axis II disorder according to the Structured Clinical Interview for DSM-III-R Nonpatient Edition26 and Structured clinical Interview for DSM-IV Personality disorders,27 and no Axis I disorder in their first-degree relatives (self-report). The same trained interviewers diagnosed patients based on the DSM-IV criteria using the Structured Clinical Interview for DSM-III-R25 and information from the medical records. The Mini Mental State Examination (MMSE) was used to rule out any dementia or delirium and to evaluate cognitive function. First-episode was operationally defined as the first psychiatric hospitalization.4 Medication history before and during the first hospitalization, between scans and during any second hospitalization, if present, was assessed by the patients’ report and chart reviews. Median duration of psychotropic medication use before MRI was ≤ 3 weeks (table 1). Dosage of antipsychotics28 did not correlate with any volume or volume change (see Results). Baseline antipsychotic medication dosage did not differ between the FESZ and the FEAFF.
Regions of Interest
We parcellated according to well-validated acceptable ROI definitions of insular cortex and TP ROIs provided in other studies7,29 and our previous cross-sectional paper18 (figure 1). For the delineation of the TP, the posterior border of the TP was defined as the coronal plane where there was no frontotemporal junction. The lateral, medial, superior, and inferior boundaries were defined simply by the natural limits of the temporal lobe, and the anterior boundary was the rostral end of the temporal lobe tissue adjacent to the sphenoid bone. Additional structural measures were obtained using Freesurfer (see supplementary data).
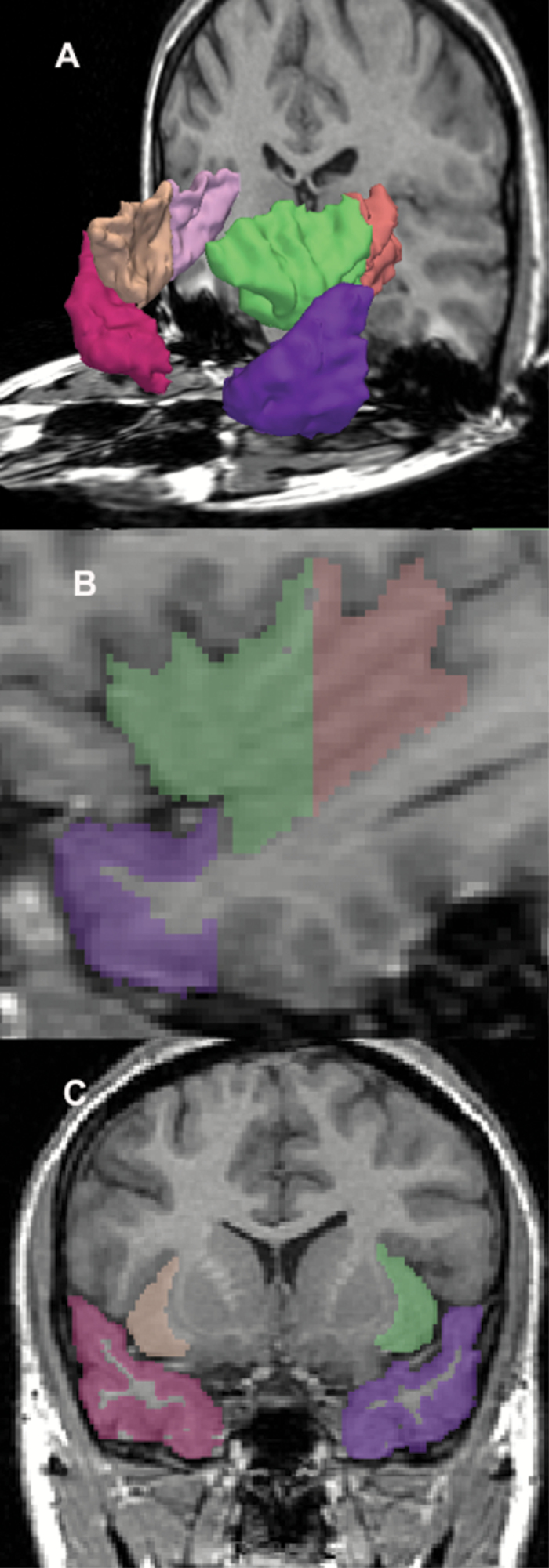
Fig. 1.
Insular gyrus and temporal pole gray matter region of interest (ROI). Three-dimensional reconstruction (A) of the insular gyrus and temporal pole gray matter. Insula anterior subdivision is green on subject left (beige on right); Posterior subdivision is orange on subject left (pink on right). Temporal pole is purple on left, red on right. (B) Left parasagittal view. (C) Coronal view.
MRI Processing
MRI images were acquired on the same 1.5-T scanner (General Electric Medical Systems) at baseline and follow-up, using the same acquisition sequences and without software upgrades. The MRI acquisition protocol and the post-processing of images have been described in detail elsewhere9 and in our supplementary text. The insular cortex and the TP ROIs were outlined manually on a workstation by individuals blinded to diagnosis. Baseline and follow-up scans were mixed and presented to raters in a blinded fashion. For interrater reliability, 3 raters (S-H.L., T.A., and T.O.) independently delineated the ROIs. Five cases were selected at random, and the raters evaluated every other slice. The intraclass correlation coefficients were 0.95 and 0.96 for left and right anterior insular gray matter, 0.99 and 0.98 for left and right posterior insular gray matter, 0.98 and 0.99 for left and right TP gray matter.
Statistical Analysis
We evaluated group differences in ROIs (insula or TP) volumes separately for baseline using a repeated-measures analysis of variance (RM-ANOVA). Group differences in insular or TP GMV were assessed using RM-ANOVA, with the diagnostic group as the between-subjects factor and the hemisphere (left or right) and subdivision (anterior and posterior) as the within-subjects factors. Relative volume was used to control for individual head size, calculated as 100 × (absolute volume)/(Volume of Intracranial Contents, ICC), and reported as a percentage. Groups did not differ significantly in ICC at baseline scan (P = .85) or in their ICC volume changes between imaging (P = .26). For the longitudinal volume comparison, the percentage of volume change was calculated with the following formula:
100 × (Relative Volume at Second Scan – Relative Volume at Baseline Scan)/(Relative Volume at Baseline Scan).
Group differences in percentage of volume change were analyzed by a RM-ANOVA model described above for cross-sectional comparison. To examine the associations between % volume change and clinical outcome, Spearman correlation coefficients were used in an exploratory analysis. Clinical outcome was evaluated as the percent of change in factor scores in Brief Psychiatric Rating Scale (BPRS)30,31 using the following equation:
100 × (Score at Second Scan – Score at Baseline Scan)/(Score at Baseline Scan).
Results
Groups were not significantly different in age, time between scans, sex, handedness, parental socioeconomic status (PSES),32 and medication dosage. FESZ, however, showed a significantly lower education level and lower socioeconomic status32 than HC. FESZ also showed significantly higher total BPRS scores, lower global assessment scale33 scores than FEAFF patients and lower MMSE scores than HC at the baseline scan, all consistent with reduced functioning due to the disorder (table 1). The statistical conclusions reported herein remained the same using the same statistical methods after exclusion of the female participants and also when only the manic FEAFF patients were included, and analyzing RM-ANOVA that incorporated time as a within-subject effect and even using linear mixed model.
Insular and TP GMVs at Baseline
A RM-ANOVA of insular gyrus GMV with group (FESZ, FEAFF, or HC) as the between-subjects factor and hemisphere (left vs right) and subdivision (anterior vs posterior insula) as the within-subjects factors revealed that groups differed in whole insular gyrus GMV (F 2,65 = 4.1, P = .02), with a significant interaction of group × subdivision (F 2,65 = 4.3, P= .02). All groups showed larger volumes in the anterior than in posterior insula (main effect, F = 834.0; P < .001).
Post hoc Tukey HSD showed the whole insular GMV of the FESZ group at baseline was smaller than that of the FEAFF group (P = .012), with no statistically significant differences in volumes between the FEAFF and HC (P = .324), and no statistically significant differences in volumes in the FESZ vs HC comparison (P = .113; table 2, supplementary figure S1). There were no significant effects of medication (typical or atypical antipsychotics, or presence or absence of mood stabilizers) on the insular volumes at baseline in the FESZ and the FEAFF group.
Table 2.
Absolute and Relative Volumes of Insular and Temporal Pole (TP) Cortex Gray Matter (Baseline)
| FESZ Group (N = 22) | FEAFF Group (N = 23) | HC Group Mean (SD) (N = 23) | 1-Factor ANOVA | Post hoc Tukey HSD Testc | ||||
|---|---|---|---|---|---|---|---|---|
| Regions and Volume | Mean (SD) | Effect Sizea | Mean (SD) | Effect Size | F test | P Value | ||
| ICC, ml | 1509.8 (102.5) | 1492.5 (124.9) | 1508.8 (113.1) | 0.17 | .847 | NA | ||
| Insula | ||||||||
| Total | ||||||||
| Absolute, ml | 15.221 (1.125) | NA | 16.342 (1.891) | NA | 15.917 (1.197) | NA | NA | NA |
| Relative, % | 1.01 (0.071) | 0.31 (0.40)b | 1.10 (0.129) | −0.18 | 1.06 (0.080) | 4.70 | .012 | FESZ < FEAFF, FESZ = HC, FEAFF = HCd |
| Left | ||||||||
| Absolute, ml | 7.646 (0.547) | NA | 8.126 (0.834) | NA | 8.024 (0.605) | NA | NA | NA |
| Relative, % | 0.507 (0.042) | 0.30 (0.37) | 0.545 (0.051) | −0.13 | 0.533 (0.039) | 4.27 | .018 | FESZ < FEAFF, FESZ = HC, FEAFF = HC |
| Right | ||||||||
| Absolute, ml | 7.576 (0.695) | NA | 8.217 (1.408) | NA | 7.893 (0.660) | NA | NA | NA |
| Relative, % | 0.502 (0.038) | 0.25 (0.67) | 0.553 (0.010) | −0.41 | 0.524 (0.045) | 3.22 | .046 | FESZ < FEAFF, FESZ = HC, FEAFF = HC |
| TP | ||||||||
| Total | ||||||||
| Absolute, ml | 18.698 (2.359) | NA | 20.288 (3.484) | NA | 20.339 (2.850) | NA | NA | NA |
| Relative, % | 1.242 (0.159) | NA | 1.356 (0.172) | NA | 1.351 (0.038) | 3.180 | .048 | FESZ = FEAFF = HCe |
| Left | ||||||||
| Absolute, ml | 9.713 (1.220) | NA | 10.634 (1.550) | NA | 10.805 (1.629) | NA | NA | NA |
| Relative, % | 0.644 (0.076) | 0.37 | 0.712 (0.076) | NA | 0.717 (0.103) | 4.913 | .010 | FESZ < FEAFF, FESZ < HC, FEAFF = HC |
| Right | ||||||||
| Absolute, ml | 8.984 (1.360) | NA | 9.654 (2.140) | NA | 9.534 (1.393) | NA | NA | NA |
| Relative, % | 0.598 (0.096) | NA | 0.645 (0.113) | NA | 0.633 (0.088) | 1.36 | .263 | FESZ = FEAFF = HC |
Note: aCalculated based on relative volume between the FESZ and the HC or the FEAFF (parenthesis)b.
cTukey honestly significant difference tests were used for post hoc tests. The results reported herein are based on relative volumes. Using absolute volume as the dependent variable and intracranial content as the covariate did not alter the statistical conclusions.
dWhen we compared the total volume of insula in patients subgrouped by medication type (typical antipsychotics or atypical antipsychotics, presence or absence of mood stabilizer) and compared to HCs using repeated-measures ANOVA, neither FESZ or FEAFF in the whole insular volume at baseline showed significance (F 4,56 = 1.9, P = .11). Also, there were no significant interactions of total insular volume with region and hemisphere. There was no hemispheric difference in overall volume (F 2,65 = 3.7, P = .06), but a significant subdivision × hemisphere interaction (F 2,65 = 5.6, P = .021) indicated that the hemispheric lateralization differed for each subdivision for all groups. A separate analysis for each subdivision showed trend-level significance of asymmetry in the anterior subdivision (F 1,65 = 3.7, P = .06).
eWhen we compared the total volume of TP in patients subgrouped by medication type (typical antipsychotics or atypical antipsychotics, presence or absence of mood stabilizer) and compared to HCs using repeated-measures ANOVA, neither FESZ or FEAFF in the whole TP volume at baseline showed significance (F 4,56 = 1.4, P = .24). Also, there were no significant interactions of TP volume with hemisphere. All groups showed larger gray matter volume (GMV) in the left temporal pole than in the right (Main effect, F 1,65 = 52.5, P < .001; with no significant interactions of hemisphere × group [F 2,65 = 1.38, P = .26]).
A RM-ANOVA of TP GMV with group (FESZ, FEAFF, or HC) as the between-subjects factor and hemisphere (left vs right) as the within-subjects factors revealed that groups differed in whole TP GMV (F 2,65 = 3.18, P = .048). Post hoc Tukey HSD tests on the whole TP GMV showed trend-level significance for the FESZ vs FEAFF (P = .07) and the FESZ vs HC (P = .09) comparisons, and no statistical differences in volumes in the FEAFF vs HC (P = .918) comparison. In the left TP volume comparisons, the FESZ group had a smaller volume than the FEAFF (P = .030) and the HC (P = .017) groups (table 2; supplementary figure S1).
Longitudinal Volume Changes
Insular Gyrus and TP GMV Changes Over Time.
A RM-ANOVA of the relative volume (percentage change) with the groups as the between-subjects factor, and the hemisphere and subdivision (boundary of central insular sulcus) as the within-subjects factor revealed that groups differed in percentage of volume change in insular gyrus GMVs (F 2,65 = 11.2, P ≤ .001) with no significant interaction of group × subdivision (F 2,65 = 1.8, P = .17). Post hoc Tukey HSD test showed percentage of volume change in FESZ group was larger than those of both FEAFF (P < .001), and HC (P < 0.001) with no statistically significant differences in volume changes between the FEAFF vs HC (P = .99).
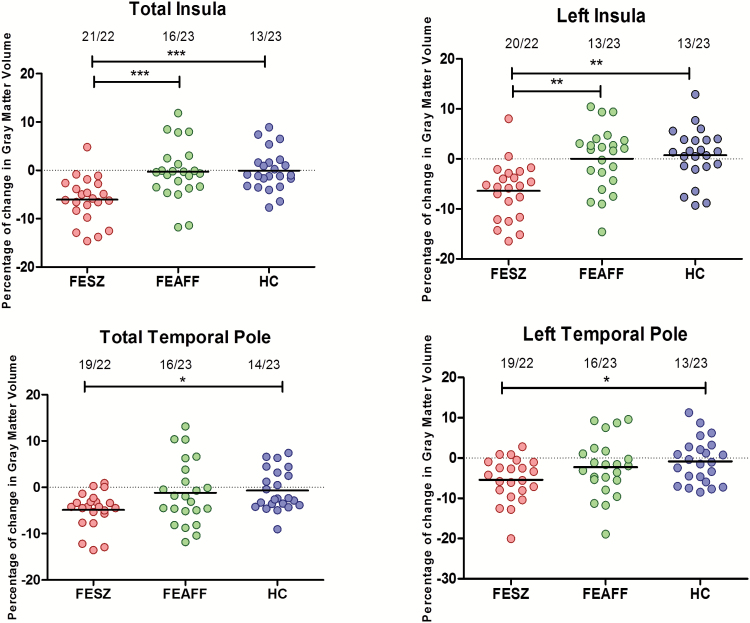
Of particular note was the high proportion of FESZ showing volume reduction over time for the insula (21/22) contrasted with less change in the FEAFF and HC groups (figure 2, supplementary figure S2).
Fig. 2.
Scattergram of percentage of change for 1.5 years in the insular and the temporal pole gray matter volume in patients with first-episode schizophrenia (FESZ; n = 22) or first-episode affective psychosis (FEAFF; n = 23) and healthy control subjects (HCs; n = 23). Horizontal lines indicate means. Numbers at the top of the graphs indicated the proportion of subjects who showed volume reduction over time (number of subjects/total number of subjects). Total insula includes both hemispheres and subregions *P < .05; **P < .01; ***P < .001, by analysis of variance.
A RM-ANOVA of percentage of change in TP with group (FESZ, FEAFF, or HC) as the between-subjects factor and hemisphere as the within-subjects factor showed that groups differed in percentages of volume change in the entire TP GMV (F 2,65 = 5.5, P = .006). Post hoc Tukey HSD tests showed that percentage of volume reduction in the FESZ group was larger than those of the HC group (P = .025), with trend-level difference between the FESZ and FEAFF (P = .06) and no difference between the FEAFF and HC groups (P = .94; table 3). As observed in the insula, the proportion of FESZ showing TP reduction over time (19/22) was much higher than for the FEAFF and HC groups (figure 2, supplementary figure S2).
Table 3.
Absolute and Relative Volumes of Insular and Temporal Pole Gray Matter at Baseline (Time 1) and 1.5 Years Later (Time 2) and Percentage of Change in the FESZ, FEAFF, and HC Groups
| Regions | FESZ Group (n = 22) | FEAFF Group (n = 23) | HC Group (m = 23) | Overall 3 Group Comparison of Percentages of Change (1-Factor ANOVA by Group) | 2-Group comparison Tukey HSD Post hoc Tests | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time 1, Mean (SD) | Time 2, Mean (SD) | Change % (SE)b | Time 1, Mean (SD) | Time 2, Mean (SD) | Change % (SE) | Time 1 Mean (SD) | Time 2. Mean (SD) | Change % (SE) | F 2.65 | P Value | ||
| ICC, ml | 1509.8 (102.5) | 1508.4 (96.7) | −0.0006 (0.003) | 1492.5 (124.9) | 1486.9 (134.6) | −0.0039 (0.006) | 1508.8 (113.1) | 1517.1 (117.9) | 0.0053 (0.009) | 1.4 | .26 | FESZ = FEAFF = HC |
| Insula | ||||||||||||
| Total | ||||||||||||
| Absolute,ml | 15.221 (1.125) | 14.304 (1.413) | −6.13 (0.009) | 16.342 (1.891) | 15.995 (1.906) | −0.74 (0.010) | 15.917 (1.197) | 15.979 (1.215) | 0.48 (0.009) | 13.8 | <.001 | FESZ < HC, FESZ < FEAFF, FEAFF = HC |
| Relative, % | 1.010 (0.071) | 0.949 (0.090) | −6.05 (0.01) | 1.081 (0.129) | 1.078 (0.108) | −0.27 (0.01) | 1.057 (0.080) | 1.056 (0.084) | −0.05 (0.01) | 10.8 | <.001 | FESZ < HC, FESZ < FEAFF, FEAFF = HCa |
| Left | ||||||||||||
| Absolute,ml | 7.646 (0.547) | 7.162 (0.752) | −6.45 (1.16) | 8.126 (0.834) | 8.046 (0.941) | −0.96 (1.21) | 8.024 (0.605) | 7.997 (0.638) | −0.24 (1.07) | 8.6 | <.001 | FESZ < HC, FESZ < FEAFF, FEAFF = HC |
| Relative, % | 0.507 (0.042) | 0.475 (0.051) | −6.37 (1.15) | 0.545 (0.051) | 0.542 (0.054) | −0.48 (1.43) | 0.533 (0.039) | 0.529 (0.045) | −0.75 (1.07) | 6.9 | .002 | FESZ < HC, FESZ < FEAFF, FEAFF = HC |
| Right | ||||||||||||
| Absolute,ml | 7.576 (0.695) | 7.142 (0.741) | −5.75 (0.88) | 8.217 (1.408) | 7.949 (1.018) | −0.39 (5.90) | 7.893 (0.660) | 7.982 (0.660) | 1.29 (1.17) | 10.8 | <.001 | FESZ < HC, FESZ < FEAFF, FEAFF = HC |
| Relative, % | 0.502 (0.038) | 0.474 (0.045) | −5.66 (0.99) | 0.553 (0.010) | 0.536 (0.059) | 0.06 (1.31) | 0.524 (0.045) | 0.528 (0.044) | 0.75 (1.11) | 9.2 | <.001 | FESZ < HC, FESZ < FEAFF, FEAFF = HC |
| Left anterior insula | ||||||||||||
| Absolute, ml | 4.612 (0.442) | 4.330 (0.505) | −6.14 (1.40) | 5.101 (0.677) | 5.006 (0.674) | −1.70 (1.43) | 4.923 (0.464) | 4.926 (0.549) | 0.113 (1.79) | 5.8 | .005 | FESZ < HC, FESZ < FEAFF, FEAFF = HC |
| Relative, % | 0.306 (0.031) | 0.287 (0.032) | −6.06 (1.33) | 0.342 (0.039) | 0.337 (0.041) | −1.21 (1.62) | 0.327 (0.032) | 0.326 (0.038) | 0.41 (1.26) | 4.6 | .014 | FESZ < HC, FESZ = FEAFF, FEAFF = HC |
| Right anterior insula | ||||||||||||
| Absolute, ml | 4.531 (0.476) | 4.292 (0.561) | −5.35 (1.36) | 4.911 (0.610) | 4.888 (0.724) | −0.45 (1.74) | 4.746 (0.626) | 4.896 (0.576) | 3.86 (2.17) | 6.4 | .003 | FESZ < HC, FESZ = FEAFF, FEAFF = HC |
| Relative, % | 0.300 (0.026) | 0.284 (0.033) | −5.28 (1.43) | 0.331 (0.044) | 0.329 (0.042) | −0.02 (1.74) | 0.316 (0.044) | 0.324 (0.040) | 3.30 (2.10) | 5.9 | .005 | FESZ < HC, FESZ = FEAFF, FEAFF = HC |
| Left posterior insula | ||||||||||||
| Absolute, ml | 3.032 (0.242) | 2.832 (0.349) | −6.72 (1.67) | 3.024 (0.323) | 3.040 (0.394) | 0.76 (2.07) | 3.102 (0.315) | 3.071 (0.340) | −0.85 (1.49) | 4.9 | .010 | FESZ < FEAFF, FESZ = HC, FEAFF = HC |
| Relative, % | 0.202 (0.018) | 0.188 (0.024) | −6.65 (1.70) | 0.203 (0.023) | 0.205 (0.022) | 1.24 (2.18) | 0.206 (0.020) | 0.203 (0.023) | −1.35 (1.53) | 34.8 | .012 | FESZ < FEAFF, FESZ = HC, FEAFF = HC |
| Right posterior insula | ||||||||||||
| Absolute, ml | 3.045 (0.532) | 2.850 (0.301) | −6.15 (1.48) | 3.060 (0.357) | 3.061 (0.409) | 0.01 (1.35) | 3.147 (0.375) | 3.087 (0.263) | −1.25 (1.67) | 4.6 | .014 | FESZ < FEAFF, FESZ = HC, FEAFF = HC |
| Relative, % | 0.201 (0.020) | 0.190 (0.020) | −6.04 (1.61) | 0.205 (0.021) | 0.205 (0.025) | 0.49 (1.51) | 0.209 (0.024) | 0.205 (0.017) | −1.77 (1.67) | 1.0 | .018 | FESZ < FEAFF, FESZ = HC, FEAFF = HC |
| Whole TP | ||||||||||||
| Total | ||||||||||||
| Absolute, ml | 18.698 (2.359) | 17.775 (2.339) | −4.93 (0.85) | 20.288 (3.484) | 19.931 (3.454) | −0.17 (1.28) | 20.339 (2.850) | 20.304 (2.930) | −0.15 (0.91) | 5.4 | .007 | FESZ < HC, FESZ = FEAFF, FEAFF = HC |
| Relative, % | 1.242 (0.159) | 1.181 (0.159) | −4.87 (0.84) | 1.356 (0.172) | 1.339 (0.186) | −1.20 (1.43) | 1.351 (0.038) | 1.339 (0.181) | −0.67 (0.92) | 4.3 | .019 | FESZ < HC, FESZ = FEAFF, FEAFF = HC |
| Left | ||||||||||||
| Absolute, ml | 9.713 (1.220) | 9.164 (1.138) | −5.47 (1.16) | 10.634 (1.550) | 10.334 (1.603) | −2.71 (1.47) | 10.805 (1.629) | 10.759 (1.619) | −0.296 (1.18) | 4.0 | .022 | FESZ < HC, FESZ = FEAFF, FEAFF = HC |
| Relative, % | 0.644 (0.076) | 0.609 (0.073) | −5.41 (1.16) | 0.712 (0.076) | 0.695 (0.092) | −2.29 (1.48) | 0.717 (0.103) | 0.709 (0.095) | 0.81 (1.15) | 3.3 | .042 | FESZ < HC, FESZ = FEAFF, FEAFF = HC |
| Right | ||||||||||||
| Absolute, ml | 8.984 (1.360) | 8.611 (1.429) | −4.26 (0.88) | 9.654 (2.140) | 9.597 (1.993) | −0.12 (1.86) | 9.534 (1.393) | 9.544 (1.522) | 0.14 (1.48) | 2.8 | .071 | NA |
| Relative, % | 0.598 (0.096) | 0.573 (0.097) | −4.20 (0.86) | 0.645 (0.113) | 0.643 (0.107) | 0.40 (2.10) | 0.633 (0.088) | 0.630 (0.091) | −0.37 (1.50) | 2.4 | .101 | NA |
aPercentage of change is calculated as (volume at second scan − volume at baseline scan)/(volume at baseline scan × 100).
There was no significant correlation between inter-scan interval and degree of volume change. For comparison with our previous study,18 we used the mammillary body landmark as the boundary of anterior and posterior insula, and found this method gave the essentially the same results as our present definition.
In summary, for the insular gyrus, group differences in percentage volume change existed between FESZ and both FEAFF and HC individuals. In contrast, for the TP, group differences in percentage volume change existed between FESZ and HC group but not between FESZ and FEAFF group.
Clinical Correlations With Volume Change Over Time.
In both patient groups, cross-sectional ROI volumes both at baseline and follow-up were not significantly associated with scores on clinical outcome measures. In FESZ, the GMV change percentage of total insula and the posterior insular subdivision were significantly negatively correlated with the change percentage in the withdrawal-retardation BPRS factor scores (total insula, ρ = −0.451, P = .040; posterior insula, ρ = −0.454, P = .039). Right insular GMV change was also negatively correlated with the change in the withdrawal-retardation BPRS factor score (ρ = −0.464, P = .034) (supplementary figure S3).Total TP and left TP GMV changes were negatively correlated with the anxiety-depression BPRS factor score at the follow-up scan (total TP, ρ = −0.456, P = .038; left TP, ρ = −0.468, P = .032, respectively).
Although the FEAFF group did not show significant GMV reduction over time, within the group, right TP GMV reduction was associated with worse or less improvement in the hostility-suspiciousness BPRS factor scores (Spearman’s ρ = −.481, P = .023).
After Bonferroni correction for multiple comparisons were run (6 ROIs × 5 BPRS factors × 2 groups), none of these BPRS factor correlations were significant. They are reported here as exploratory results so that future studies can use them as predictions.
Discussion
To our knowledge, this study is the first prospective study to demonstrate progressive GMV reduction both in insula and TP in FESZ as contrasted with FEAFF. Bilateral insular cortex GMV were significantly decreased over time in the FESZ group compared with both FEAFF and HC groups. In contrast, TP GMV were significantly decreased over time only in the FESZ vs HC group comparison.
Although the TP and insula paralimbic regions share certain emotional, auditory, visual, olfactory processing and cytoarchitectonic features, these results suggest that progressive volume reduction of bilateral insular cortex, but not TP, is relatively specific to schizophrenia vs affective (bipolar) psychosis.
With regard to the insular findings in the FESZ group, the cross-sectional findings were consistent with our previous report18 and previous research.8 The FESZ group showed baseline bilateral insular GMV reductions compared to the FEAFF group, consistent with our previous report,18 although the present larger sample showed no baseline difference compared to the HC group. We note that both the short median medication duration before the baseline scan in both FESZ and FEAFF (3wk) and the absence of significant mood stabilizer neurotrophic effects in both groups (shown in table 2) suggest the FESZ-FEAFF differences cannot be attributed to medication effects.
Whereas our study looked at the early stage of bipolar disorder, we note that the decreased insular GMV reported in other studies were on patients older and/or with longer duration of illnesses; metanalyses34,35 showed mean ages of 34.3 and 30.9 respectively, and mean illness duration was 12.3 years in another meta-analysis.8 We thus think our finding of early course differences between bipolar and schizophrenic psychosis remains valid. Most studies8 agree on decreased insular GMV in the early phase of schizophrenia. Moreover, reports indicate that insular GMV loss is present in schizophrenia high risk populations before the onset of psychosis, and may progress post onset,7,36 findings suggesting that insula cortex may be affected in the course of neural development.
Our TP cross-sectional findings are consistent with our previous report18 that left TP volumes of the FESZ group were smaller than that of HCs. Methodologic and sample differences may account for a previous VBM study showing right TP volume reduction in the FESZ group37 and for the Gur et al38 finding of significantly smaller bilateral TP GMV in patients with chronic schizophrenia. Our baseline cross-sectional findings suggest that the GMV of insula and TP paralimbic regions are smaller in the early phase of schizophrenia, but not in affective psychosis.
The GMV of insula and TP progressively decreased in the FESZ group. The percentage GMV reduction over 1.5 years in FESZ was 6.1% for insula and 4.9% for TP, insular percentages similar to Takahashi et al.7
Volumetric Changes Over Time and Symptom Relationships
That there were no consistent cross sectional associations with symptoms is a strong argument for longitudinal methods in the study of symptoms, since this method of looking at individual percentages of change uses each individual as his/her base line rather than a group mean, a less sensitive method.
The most striking change over time-clinical associations were found in FESZ. In the insula there was a significant association of percentage change over time of GMV in the total (bilateral) insula, in the total posterior volume and in the total right insular volume with the BPRS factor of withdrawal retardation. The more the volume reduction the higher the score on withdrawal retardation, with rho’s between −0.450 and −0.465 for these 3 regions. The BPRS withdrawal retardation factor used by us is comprised of emotional withdrawal + motor retardation + blunted affect39; this is the cluster of symptoms uniformly described as negative symptoms of schizophrenia (see review by Nicholson et al40).
The progressive loss of GMV is a possible substrate for the progression of negative symptoms. The posterior insula is especially tuned for interoception, for perception of body state.12 It thus is likely to be involved in perception of emotional experience and thus of emotional expression. Also of note, the anterior insula processes emotional responses.15 Hence, a defect in this system might thus be related to blunted affect and to emotional withdrawal. Motor retardation may be related to another function of the anterior insula, namely speech articulation, since slowness of speech is a primary symptom in the rating of motor retardation.
To our knowledge, this is the first report that TP showed a progressive decrease of MRI GMV over time in FESZ patients. Although studies18,37 of TP in early schizophrenia have shown inconsistent results, studies on chronic schizophrenia showed significant reduction in TP GMV38 consistent with our longitudinal findings. TP has been implicated in emotional functions such as sad face affect processing,14 theory of mind,41 empathy,13 and neuroticism, which is closely related to depression and anxiety.14 In our study, percentage of change of total TP and left TP volumes were negatively correlated with anxiety-depression BPRS factor scores at the time of the follow-up scan in the FESZ group. These results suggest that progressive TP volume loss in FESZ patients can lead to anxiety-depressive symptoms, in contrast to the positive or disorganized symptoms found cross-sectionally in chronic schizophrenia.29
Correlational analysis in FEAFF also showed that volume changes of right TP were correlated with changes of the hostility-suspiciousness BPRS factor instead of the depression-anxiety factor. The presence of within FEAFF group correlations suggests that there might be small GMV changes over time too small to be detected in group statistical comparisons. It is clear, consistent with a previous VBM study,42 that FEAFF patients do not show progressive TP GMV reduction, in contrast with FESZ. However, in terms of association between volume change and clinical measures for both TP and insula, we emphasize that these symptom correlation analyses were exploratory in nature and therefore, confirmation will be needed in a future planned study.
This MRI study did not address the underlying pathophysiological mechanism of volume reduction. However, a postmortem study showed a 16% reduction in insular layer 2 neuronal volume in schizophrenia but not in affective disorder patients, a finding possibly linked to FESZ neuropil reduction.43 It has been speculated by many, including us, that hypofunction of the N-methyl–D-aspartate (NMDA) receptors on corticolimbic gamma-aminobutyric acid (GABA)-ergic interneurons and resulting lack of GABAergic inhibition may result in excessive glutamatergic excitation and neurotoxic effects in the early phase in schizophrenia, leading to progressive loss of neuronal dendrites and synapses (neuropil), appearing as GMV reduction on MRI.44,45 However, conclusive evidence must await further study.
Finally, this study used manual parcellation of ROI, the gold standard of MRI anatomical localization, since it allows cross-reference to basic cellular studies.1,12,46 This necessitated about 100 hours per subject, thus precluding the ability to look at other brain regions. The necessity of using manual longitudinal measures in the ROI studied here is highlighted in our supplementary data showing a failure of group difference detection by Freesurfer (supplementary figure S4).
Comparison and Context of Present Results With Our Group’s Previous Studies of FESZ, FEAFF, and HC From McLean Hospital.
In all the studies summarized in supplementary table S1, the 22 ROI were manually traced with very high interrater reliability (all > 0.92. most > 0.95). Unless stated otherwise all the following summary results were comparisons with HC (see supplementary table S2 for more detail).
-
1.
Nearly all of the ROI of FESZ showed a smaller volume at baseline (initial scan.) This smaller volume was left lateralized in nonmedial cortical ROI.
-
2.
FEAFF showed only one smaller volume at baseline, the subgenual cingulate.
-
3.
At baseline, FESZ showed smaller volumes than FEAFF in superior temporal gyrus, insula and left TP. (No ROI had FEAFF < FESZ)
-
4.
Almost all of the ROI of FESZ (left and/or right) showed a longitudinal progression of volume reduction with a mean reduction of 6.13 % (±0.14, SD) and a median of 5.88 % over the 1.5-year interscan interval. Progression was left lateralized except for the cingulate.
-
5.
In contrast to the FESZ longitudinal progression, FEAFF showed progression only in the anterior and subgenual cingulate.
-
6.
FESZ progression over time was greater than FEAFF in all ROI with the exception of the subgenual cingulate.
MRI-Electrophysiology Associations found in the McLean sample offered the important opportunity to relate structure with function, and we here briefly summarize. The P300 event-related potential (ERP) reduction showed a left lateralization (minimum at T3) that was highly correlated with GMV of the left Heschl gyrus and left planum temporale volume in FESZ but not in FEAFF or HC.47 In FESZ, Mismatch Negativity (MMN) at baseline was significantly correlated with left Heschl gyrus volume; moreover progressive MMN amplitude reduction was significantly correlated with the degree of progressive volume reduction in left Heschl gyrus, associations not present in HC or FEAFF.48 The gamma band auditory steady state response showed a left lateralized deficit in FESZ but not in FEAFF or HC, consistent with the left lateralization of cortical gray matter reduction in this FESZ subject pool.49
In conclusion, the present study adds to the strength of our previous studies in showing that longitudinal progression of volume loss in the first year or two of illness is a feature of FESZ but not regularly seen in FEAFF. Another strength is that it represents, to our knowledge, the largest longitudinal MRI study sample of insular gyrus and TP in patients with FESZ and FEAFF, and that longitudinal reductions in volume in FESZ were associated with worse symptom profiles at the time of rescan.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This study was supported by Department of Veterans Affairs Medical Research Awards (Schizophrenia Center, Merit Awards to R.W.M. and M.E.S.) and by grants K02 MH 01110 and R01MH50747 (M.E.S.), R01MH40799 and R01 MH 052807 (R.W.M.), CIDAR P50MH080272 (R.W.M. and M.E.S.), and R01 MH58704 (D.F.S.) from the National Institute of Mental Health and grants from the MIND (Mental Illness and Neuroscience Discovery) Foundation (R.W.M.) and National Alliance for Research on Schizophrenia and Depression (NARSAD) (D.F.S.).
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Baumann B, Bogerts B. The pathomorphology of schizophrenia and mood disorders: similarities and differences. Schizophr Res. 1999;39:141–148; discussion 162. [DOI] [PubMed] [Google Scholar]
- 2. Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr Res. 2010;117:1–12. [DOI] [PubMed] [Google Scholar]
- 3. Ivleva EI, Bidesi AS, Keshavan MS, et al. Gray matter volume as an intermediate phenotype for psychosis: bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170:1285–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hirayasu Y, Shenton ME, Salisbury DF, et al. Lower left temporal lobe MRI volumes in patients with first-episode schizophrenia compared with psychotic patients with first-episode affective disorder and normal subjects. Am J Psychiatry. 1998;155:1384–1391. [DOI] [PubMed] [Google Scholar]
- 5. Kuroki N, Shenton ME, Salisbury DF, et al. Middle and inferior temporal gyrus gray matter volume abnormalities in first-episode schizophrenia: an MRI study. Am J Psychiatry. 2006;163:2103–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hirayasu Y, Tanaka S, Shenton ME, et al. Prefrontal gray matter volume reduction in first episode schizophrenia. Cereb Cortex. 2001;11:374–381. [DOI] [PubMed] [Google Scholar]
- 7. Takahashi T, Wood SJ, Soulsby B, et al. Follow-up MRI study of the insular cortex in first-episode psychosis and chronic schizophrenia. Schizophr Res. 2009;108:49–56. [DOI] [PubMed] [Google Scholar]
- 8. Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kasai K, Shenton ME, Salisbury DF, et al. Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am J Psychiatry. 2003;160:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nakamura M, Salisbury DF, Hirayasu Y, et al. Neocortical gray matter volume in first-episode schizophrenia and first-episode affective psychosis: a cross-sectional and longitudinal MRI study. Biol Psychiatry. 2007;62:773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koo MS, Levitt JJ, Salisbury DF, Nakamura M, Shenton ME, McCarley RW. A cross-sectional and longitudinal magnetic resonance imaging study of cingulate gyrus gray matter volume abnormalities in first-episode schizophrenia and first-episode affective psychosis. Arch Gen Psychiatry. 2008;65:746–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mesulam MM. Paralimbic (Mesocortical) Areas. New York, NY: Oxford University Press; 2000. [Google Scholar]
- 13. Völlm BA, Taylor AN, Richardson P, et al. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage. 2006;29:90–98. [DOI] [PubMed] [Google Scholar]
- 14. Jimura K, Konishi S, Miyashita Y. Temporal pole activity during perception of sad faces, but not happy faces, correlates with neuroticism trait. Neurosci Lett. 2009;453:45–48. [DOI] [PubMed] [Google Scholar]
- 15. Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. [DOI] [PubMed] [Google Scholar]
- 16. Mesulam MM, Mufson EJ. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol. 1982;212:1–22. [DOI] [PubMed] [Google Scholar]
- 17. McCrea SM. Bipolar disorder and neurophysiologic mechanisms. Neuropsychiatr Dis Treat. 2008;4:1129–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kasai K, Shenton ME, Salisbury DF, et al. Differences and similarities in insular and temporal pole MRI gray matter volume abnormalities in first-episode schizophrenia and affective psychosis. Arch Gen Psychiatry. 2003;60:1069–1077. [DOI] [PubMed] [Google Scholar]
- 19. Morgan KD, Dazzan P, Orr KG, et al. Grey matter abnormalities in first-episode schizophrenia and affective psychosis. Br J Psychiatry Suppl. 2007;51:s111–s116. [DOI] [PubMed] [Google Scholar]
- 20. McDonald C, Bullmore E, Sham P, et al. Regional volume deviations of brain structure in schizophrenia and psychotic bipolar disorder: computational morphometry study. Br J Psychiatry. 2005;186:369–377. [DOI] [PubMed] [Google Scholar]
- 21. Hibar D, Jahanshad N, Leonardo C, et al. Cortical thickness reliability measures evaluated with standardized protocols, the ENIGMA Consortium. The 20th Annual Meeting of the Organization for Human Brain Mapping; June 2014; Hamburg, Germany. [Google Scholar]
- 22. Hirayasu Y, McCarley RW, Salisbury DF, et al. Planum temporale and Heschl gyrus volume reduction in schizophrenia: a magnetic resonance imaging study of first-episode patients. Arch Gen Psychiatry. 2000;57:692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York, NY: Harcourt Brace Jovanovich Inc; 1981. [Google Scholar]
- 24. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 25. Spitzer RL, Williams JB, Gibbson M, First M. The Structured Clinical Interview for DSM-III-R (SCID). Washington, DC: American Psychiatric Association; 1990. [Google Scholar]
- 26. Spitzer RL, Williams JB, Gibbson M, First M. The Structured Clinical Interview for DSM-III-R-Non-Patient Edition (SCID-NP). Washington, DC: American Psychiatric Association; 1990. [Google Scholar]
- 27. First MB, Gibbson M, Spitzer RL, Williams JB, Benjamin L. Structured Clinical Interview for DSM-IV Personality Disorders (SCID-II): Interview and Questionnaire. Washington, DC: American Psychiatric Association; 1997. [Google Scholar]
- 28. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. [DOI] [PubMed] [Google Scholar]
- 29. Crespo-Facorro B, Nopoulos PC, Chemerinski E, Kim JJ, Andreasen NC, Magnotta V. Temporal pole morphology and psychopathology in males with schizophrenia. Psychiatry Res. 2004;132:107–115. [DOI] [PubMed] [Google Scholar]
- 30. Overall JE, Beller SA. The Brief Psychiatric Rating Scale (BPRS) in geropsychiatric research: I. Factor structure on an inpatient unit. J Gerontol. 1984;39:187–193. [DOI] [PubMed] [Google Scholar]
- 31. Overall JE, Hollister LE, Pichot P. Major psychiatric disorders. A four-dimensional model. Arch Gen Psychiatry. 1967;16:146–151. [DOI] [PubMed] [Google Scholar]
- 32. Hollingshead A. Two Factor Index of Social Position. New Haven, CN: Yale University Press; 1965. [Google Scholar]
- 33. Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–771. [DOI] [PubMed] [Google Scholar]
- 34. Bora E, Fornito A, Yücel M, Pantelis C. Voxelwise meta-analysis of gray matter abnormalities in bipolar disorder. Biol Psychiatry. 2010;67:1097–1105. [DOI] [PubMed] [Google Scholar]
- 35. Selvaraj S, Arnone D, Job D, et al. Grey matter differences in bipolar disorder: a meta-analysis of voxel-based morphometry studies. Bipolar Disord. 2012;14:135–145. [DOI] [PubMed] [Google Scholar]
- 36. Takahashi T, Wood SJ, Yung AR, et al. Insular cortex gray matter changes in individuals at ultra-high-risk of developing psychosis. Schizophr Res. 2009;111:94–102. [DOI] [PubMed] [Google Scholar]
- 37. Witthaus H, Kaufmann C, Bohner G, et al. Gray matter abnormalities in subjects at ultra-high risk for schizophrenia and first-episode schizophrenic patients compared to healthy controls. Psychiatry Res. 2009;173:163–169. [DOI] [PubMed] [Google Scholar]
- 38. Gur RE, Turetsky BI, Cowell PE, et al. Temporolimbic volume reductions in schizophrenia. Arch Gen Psychiatry. 2000;57:769–775. [DOI] [PubMed] [Google Scholar]
- 39. Overall JE, Klett CJ. Applied Multivariate Analysis. New York, NY: McGraw-Hill; 1972. [Google Scholar]
- 40. Nicholson IR, Chapman JE, Neufeld RW. Variability in BPRS definitions of positive and negative symptoms. Schizophr Res. 1995;17(2):177–185. [DOI] [PubMed] [Google Scholar]
- 41. Reniers RL, Völlm BA, Elliott R, Corcoran R. Empathy, ToM, and self-other differentiation: an fMRI study of internal states. Soc Neurosci. 2014;9:50–62. [DOI] [PubMed] [Google Scholar]
- 42. Farrow TF, Whitford TJ, Williams LM, Gomes L, Harris AW. Diagnosis-related regional gray matter loss over two years in first episode schizophrenia and bipolar disorder. Biol Psychiatry. 2005;58:713–723. [DOI] [PubMed] [Google Scholar]
- 43. Pennington K, Dicker P, Hudson L, Cotter DR. Evidence for reduced neuronal somal size within the insular cortex in schizophrenia, but not in affective disorders. Schizophr Res. 2008;106:164–171. [DOI] [PubMed] [Google Scholar]
- 44. Stone JM, Morrison PD, Pilowsky LS. Glutamate and dopamine dysregulation in schizophrenia–a synthesis and selective review. J Psychopharmacol. 2007;21:440–452. [DOI] [PubMed] [Google Scholar]
- 45. Grunze HC, Rainnie DG, Hasselmo ME, et al. NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci. 1996;16:2034–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J Psychiatry Neurosci. 2012;37:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McCarley RW, Salisbury DF, Hirayasu Y, et al. Association between smaller left posterior superior temporal gyrus volume on magnetic resonance imaging and smaller left temporal P300 amplitude in first-episode schizophrenia. Arch Gen Psychiatry. 2002;59:321–331. [DOI] [PubMed] [Google Scholar]
- 48. Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Spencer KM, Salisbury DF, Shenton ME, McCarley RW. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. 2008;64:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.