Abstract
Background:
Individuals with subclinical psychotic symptoms provide a unique window on the pathophysiology of psychotic experiences as these individuals are free of confounders such as hospitalization, negative and cognitive symptoms and medication use. Brain network disturbances of white matter connections are thought to play a central role in the pathophysiology of psychosis. Based on the structural network disconnection hypothesis in schizophrenia, we expect less and weaker connections, and altered brain network organization in individuals with clinical and those with subclinical psychotic symptoms.
Methods:
We used diffusion tensor imaging to study 35 patients with a psychotic disorder, 35 subjects with subclinical psychotic symptoms, and 36 healthy controls. The structural brain network was analyzed on 3 levels: connection density, white matter microstructure (fractional anisotropy, mean diffusivity, and magnetic transfer ratio), and network organization. Network organization was studied with minimum spanning tree analysis, a method to reconstruct a backbone of structural highways in the brain.
Results:
Decreased fractional anisotropy and increased mean diffusivity was found in both groups with psychotic symptoms, while their network topology showed decreased overlap with a healthy reference network. Decreased centrality was found in several brain regions, including parietal hubs and language areas, in both groups with psychotic symptoms. Deviation of network characteristics was more apparent in clinical subjects than in subclinical subjects.
Discussion:
Weaker connections and decreased centrality of parietal hubs characterize the structural brain network in subjects with psychotic symptoms. These differences are more notable in clinical than in subclinical subjects with psychotic experiences.
Key words: psychosis continuum, brain networks, minimum spanning tree, diffusion tensor imaging, hubs, fractional anisotropy
Introduction
Psychotic symptoms such as auditory verbal hallucinations (AVH) are present not only in patients with psychosis, but also in a minority of subclinical individuals, which suggests that there is a psychosis continuum (for a review see van Os et al.1). In this spectrum, individuals may function within normal limits while they have psychotic symptoms, as these symptoms remain below the threshold for a clinical disorder1 and are not associated with negative symptoms or cognitive dysfunction.2,3 Subjects with subclinical psychotic symptoms (SCP) in the general population take an intermediate place in the spectrum between schizophrenia patients and healthy subjects. Compared to healthy controls (HCs), nonclinical individuals with AVH have a lower level of global functioning in daily life, have an increased schizotypal and delusional tendency, and have more family members with psychotic disorders.3 However, these subjects have no clinically defined delusions, disorganization or negative symptoms, and they do not meet criteria for schizotypal personality disorder.3 They can thus be considered to experience psychotic features, in the relative absence of other symptoms, without an interrupted intellectual development, and, importantly, without medication effects. Neurobiological evidence for the psychosis continuum is scarce but promising.4,5 Cortical thickness in subjects with SCP was recently shown to be globally decreased compared to HCs, but higher than in patients with a psychotic disorder.6 Furthermore, white matter microstructure is known to be affected in first-degree relatives of patients with psychosis, which are unaffected by psychosis but tend to show higher rates of cluster A and B personality traits.7–9
Schizophrenia is thought to be a disconnection syndrome, where symptoms arise from disturbed connectivity between spatially remote brain regions.10 Structural connectivity disturbances in schizophrenia consist of a loss of physical connections, and of altered microstructure of white matter.11 A lower density of white matter connections has also been described in subjects with SCP.12 In addition, the higher level organization of the human brain network as characterized with graph theoretical analysis is also disturbed in schizophrenia.13 Alterations seem to specifically affect hub nodes (ie, nodes with a central role in the network), leading to decreased efficiency of communication between brain regions.14–16 Together these findings suggest altered connectivity and network organization in patients with psychosis on multiple levels. However, network analysis is hindered by a methodological problem when both the number of connections in the structural brain network and the topological organization are altered simultaneously. Graph characteristics such as efficiency are affected by differences in connection density, which makes it difficult to disentangle network organization alterations from the effects of global loss of measured connections.17 Indeed, a recent study in subjects with SCP described both decreased connection density and lower efficiency of network organization compared to controls.12 We propose that the minimum spanning tree (MST), a subnetwork containing only the strongest backbone connections from the original network, may be analyzed to overcome these issues (figure 1).18,19
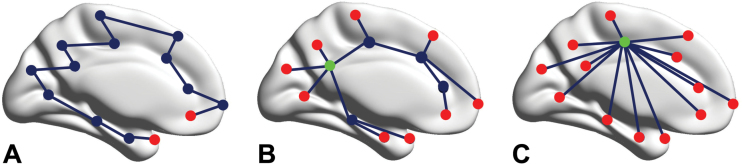
Fig. 1.
The concept of the minimum spanning tree. Three minimum spanning tree network types. Left a path tree is shown, where every node except the 2 end nodes or leafs (red) is connected to its 2 neighbors (low leaf number), but it takes a lot of steps to reach the other end of the network (large diameter). The right figure shows a star tree, which consists of a central node (high betweenness centrality, green) that is connected to all other nodes (high degree), which are all leaf nodes. This network is highly efficient (small diameter), but may result in an overload of information flow through the central hub node. The middle panel represents a hierarchical tree, which is a possible intermediate between the 2 extremes.
We aimed to study whether a “dose-effect” is present for network disturbances in the psychosis spectrum, where the level of network disturbance scales with the severity of psychotic symptoms. We hypothesized that subjects with SCP (ie, AVH, suspicion and magical beliefs) have intermediate structural brain network disturbances between patients with a clinical psychosis (CP) and HCs. Disturbances were hypothesized to be 3-fold: decreased connection density, altered white matter microstructure, and a less optimal topology of the backbone of the network with a decreased centrality of hub regions.
To test these hypotheses, we used diffusion weighted imaging (DWI) to study structural brain networks (figure 2). Firstly, we compared the connection density between the 3 groups. We then mapped the backbone of the network using MST analysis. In this network, white matter integrity was analyzed using fractional anisotropy (FA) and mean diffusivity (MD), and the magnetization transfer ratio (MTR) was used to characterize white matter myelination. Finally, we characterized the topology of the MST to study the organization structural backbone of the brain on a macroscopic level, which was the primary outcome measure of our study.
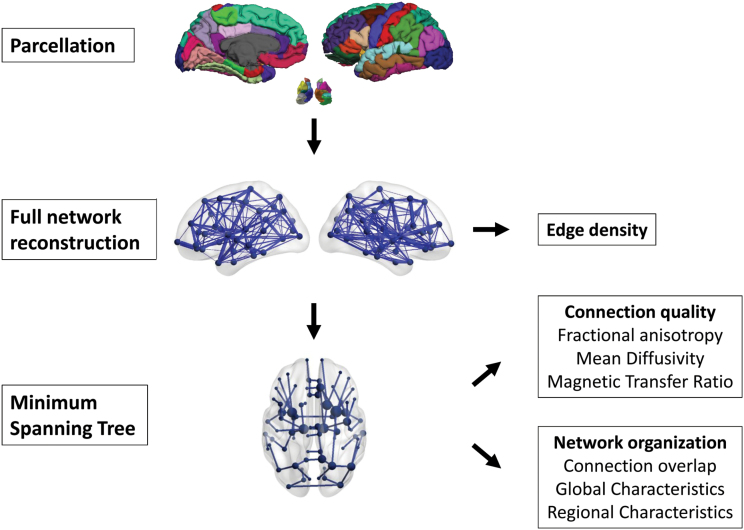
Fig. 2.
Data analysis pipeline. Overview of data processing steps. The cortex was parcelated in 68 cortical and 14 subcortical regions. Structural connections between these regions were reconstructed, and the connection density was calculated for each subject. Subsequently, the minimum spanning tree was used to obtain the backbone of the network. Connection quality measures were calculated of connections present in a reference network based on a sample of healthy control subjects. Furthermore, the overlap with this reference network was calculated for each subject (% of overlapping connections), and global and local network characteristics were analyzed.
Methods
Subjects
Thirty-five patients diagnosed with a psychotic disorder, 35 subclinical individuals with AVH but without a psychiatric diagnosis,3 and 46 HC subjects without hallucinations or other psychotic features participated in the study. A reference network was based on data of all control subjects as described below. For group comparisons, 36 subjects were selected from the control group in order to obtain matched samples for age, gender, and handedness. Subjects were recruited via a website (www.verkenuwgeest.nl.). Diagnoses (or their absence) were assessed by an independent psychiatrist using the comprehensive assessment of symptoms and history interview (CASH) according to DSM-IV criteria.20 The positive and negative syndrome scale (PANSS) was used for the assessment of symptoms on the day of the magnetic resonance imaging (MRI) scan.21 The psychotic symptoms rating scales (PSYRATS) questionnaire was used to test hallucination severity in SCP.22 All HC and SCP subjects were confirmed to have no psychiatric disorder including substance abuse in the CASH interview and the SCID-II interview to screen for axis II pathology. Schizotypal tendency was assessed in these groups using the schizotypal personality questionnaire (SPQ).23 Handedness was assessed in all participants with the Edinburgh handedness inventory.24 The study was approved by the medical ethical committee of the University Medical Center and all participants signed a written informed consent. The majority of the MRI scans in this study were also reported on in an entirely different investigation, namely FA of the arcuate fasciculus in these 3 groups.25
Imaging Acquisition, Scan Processing, and Network Reconstruction
See supplementary materials for a detailed description of the imaging acquisition and processing steps. In brief, MRI scans were acquired on a 3 Tesla Philips Achieva as previously reported in.25 DWI scans, a T1-weighted scan, and an MTI scan were collected from each participant. The T1-weighted image was used for anatomical reference and for network node definition. Gray matter brain regions (network nodes) were selected automatically using the FreeSurfer segmentation pipeline (V5.1;http://surfer.nmr.mgh.harvard.edu).26 The brain was divided into 82 distinct anatomical regions using an automated segmentation procedure, consisting of 14 subcortical structures, and a parcellation of the neocortex into 68 regions.27 An individual mask was created containing all 82 regions for each subject. All possible tracts in the brain were reconstructed individually using the diffusion tensor images in native space using the fiber assignment by continuous tracking (FACT) algorithm.28,29 The presence of a white matter connection between 2 grey matter regions was determined by labeling each streamline with the grey matter region it connects based on the anatomical segmentation mask. The number of streamlines (NOS) was summed between all possible node pairs and represented in an 82×82 weighted structural connectivity matrix.
Connection Density and Microstructural Measurements
Connection density was calculated as the number of nonzero connections for each subject, based on the whole brain connectivity matrix. The reference MST (MSTref; see description below) was used as a template or mask of connections to characterize white matter structure, to ensure that this characterization was based on the same connections for all subjects. MD and FA and the MTR were calculated for each subject over all edges that participated in the MSTref to characterize the microstructure of white matter fibers (see supplementary methods for a detailed description). FA and MD are thought to reflect the coherence of the fiber orientation and free water concentrations, whereas MTR is sensitive to the proportion of macromolecules within a voxel and is thought to be sensitive to myelin.30,31 FA, MD, and MTR values over all connections in the MST matrix were respectively averaged to obtain 1 overall mean FA, MD, and MTR value for each subject.
Graph Analysis
Graph analysis was performed in Matlab R2011b (The Mathworks, Inc). The connectivity matrices were analyzed as graphs, consisting of nodes (ie, each gray matter region of the atlas is a node) and edges (ie, all connections between any pair of nodes). We used MST analysis to further characterize network topology from the weighted adjacency matrix using Kruskal’s algorithm.32 The MST is a subnetwork of the original network, which connects all nodes such that the connections with minimum cost (ie, maximum connectivity) are included, but without forming loops.33 Although the MST discards most connections, its characteristics can still be interpreted along the lines of conventional network metrics, with the advantage that the MST is insensitive to connection density of the underlying network.19 We applied this procedure to reconstruct the tree containing the connections with the maximum number of fibers, resulting in an MST for each subject containing 82 nodes and 81 edges. The unweighted MST (ie, binary graph containing edge weights of 0 and 1 only) was used to further characterize network topology.
In order to test the hypothesis that the MST in the (sub)clinical psychosis group differed from controls, a MSTref was calculated based on the average connectivity matrix of 46 control subjects. The overlap between the MST of each individual subject (MSTsubject) and MSTref was quantified by calculating the fraction of edges that was present in both MSTs (MSToverlap; ranging between 0 (no matching edges) and 1 (exact match); similar to the survival rate of an MST connection described by34). The value of MSToverlap was used to test whether there was a significant difference in the MST between the controls and the 2 hallucinating groups. Networks were visualized using BrainNet Viewer v1.43.35
MST Topology
Several measures can be used to characterize the topology of the MST (figure 1). When group differences were found for MSToverlap, these differences were further characterized with MST measures of network topology.19,36 Two measures were used to describe regional network characteristics (computed using the brain connectivity toolbox [https://sites.google.com/site/bctnet/]):
Degree, which is the number of links connected to node i. We used the degree fraction normalized for network size to characterize nodal degree, obtained by dividing the degree by the total number of links.
Betweenness centrality (BC) of a node u is defined as the number of shortest paths (of the MST) between any 2 nodes i and j in the network that are passing through u but not including u, divided by the total number of shortest paths. BC ranges between 0 (leaf node) and 1 (central node in a star-like network).
In addition, 4 measures were used to characterize global MST topology: leaf fraction (measure of centrality), diameter (measure of network efficiency), kappa (measure that characterizes the degree distribution, and tree hierarchy (measure of hierarchical network organization). See supplementary methods for formal definitions.
Statistical Analysis
Statistical analyses of group characteristics were performed in IBM SPSS Statistics 22. SCP and HC SPQ scores were compared using Mann-Whitney U tests. Jonckheere-Terpstra trend tests (Monte Carlo 2-sided test, 10 000 permutations, P < .05) were used to test the a priori hypothesized (ie, HC<SCP<CP or vice versa) ordered differences in connection density, FA, MD, MTR, and MST Overlap.37–39 Since 5 tests were performed of nonindependent variables, the false discovery rate was used to correct for inflated type I errors.40
When significant group effects were found, spearman correlation tests were performed to explore correlations between PANSS scores and outcome measures in CP, and between PSYRATS scores and SCP.
Regional characteristics were compared between groups using Matlab R2011b (The Mathworks, Inc). We used permutation tests corrected for multiple testing to compare regional MST characteristics between groups (P < .05).41 Nodal degree of all 82 regions tested for group differences between CP and HC, CP and SCP, and SCP and HC. The same design was used to test for group differences of nodal BC.
Results
Subject characteristics are shown in table 1. Groups were well matched for age, gender and handedness. As expected, SPQ scores were higher in SCP than in controls (Mann-Whitney U = 137; P < .001).
Table 1.
Subject Characteristics
| Clinical Psychosis (N = 35) | Subclinical Psychosis (N = 35) | Healthy Controls (N = 36) | |
|---|---|---|---|
| Gender (male/female) | 18/17 | 14/21 | 16/20 |
| Mean age (SD) | 36 (11) | 41 (13) | 39 (16) |
| Age range (min-max) | 20–61 | 20–65 | 20–61 |
| Handedness (right/left) | 31/4 | 29/6 | 30/6 |
| Diagnosis (schizophrenia/ schizoaffective disorder/ psychosis NOS) | 20/5/10 | ||
| PANSS score (total/positive/ negative/general [SD])a | 57 (13)/14 (4)/14 (5)/28 (7) | ||
| Antipsychotic medication (None/typical/atypical/ typical + atypical) | 8/6/20/1 | ||
| SPQ | 28 (13) | 7 (6) | |
| PSYRATS | 16 (3,7) | ||
| GAF | 82 (8) |
Note: NOS = not otherwise specified; SPQ = schizotypal personality questionnaire; PANSS = positive and negative symptoms scale; PSYRATS = psychotic symptoms rating scales; GAF = global assessment of functioning.
aPANSS scores were available of 31 patients.
Connection Density and Microstructure
Although connection density varied between subjects, no group differences were found (Std. J-T = −1,1; P = .250; supplementary table S1). White matter microstructure did differ between groups: FA was decreased in subjects with psychotic symptoms compared to HC, such that values were intermediate in SCP, and lowest in CP (Std. J-T = −2.5; P = .013). MD was increased in subjects with psychotic symptoms, and MD in SCP was intermediate between HC and CP (Std. J-T = 2.6; P = .011). No group effects were found for MTR (Std. J-T = 1.1; P = .270).
MST Topology
We tested alterations in network topology by comparing individual MSTs to a reference network based on a group of 46 HCs. MST overlap was lowest in CP, and for SCP, MST overlap was intermediate between HC and CP (Std. J-T = −2.6; P = .007; supplementary table S1). Post hoc tests showed no differences between groups for MST leaf fraction (Std. J-T = −1.1; P = .285), diameter (Std. J-T = 0.7; P = .494), tree hierarchy (Std. J-T = −1.1; P = .283), or kappa (Std. J-T = 0.4; P = .720).
No significant correlations were found between PANSS scores and any of the outcome measures in CP, nor between PSYRATS scores and outcome measures in SCP.
Regional Analysis
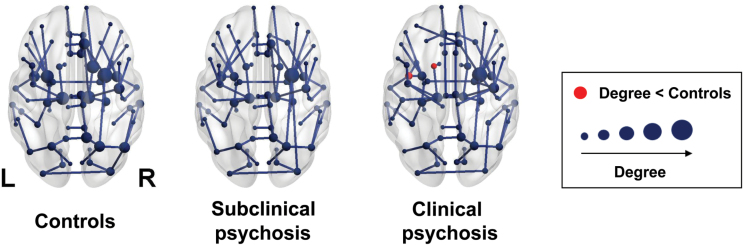
Using permutation analysis, we found that the degree of the left caudate and left insula was lower in CP than in HC (figure 3). No other group differences in degree were found.
Fig. 3.
Group averaged networks. Visualization of the mean minimum spanning tree for controls, subjects with subclinical psychosis, and patients with a psychotic disorder. The connections show the backbone connections of the network of each group. In the clinical psychosis group, red nodes mark regions with a significantly lower degree compared to the control group.
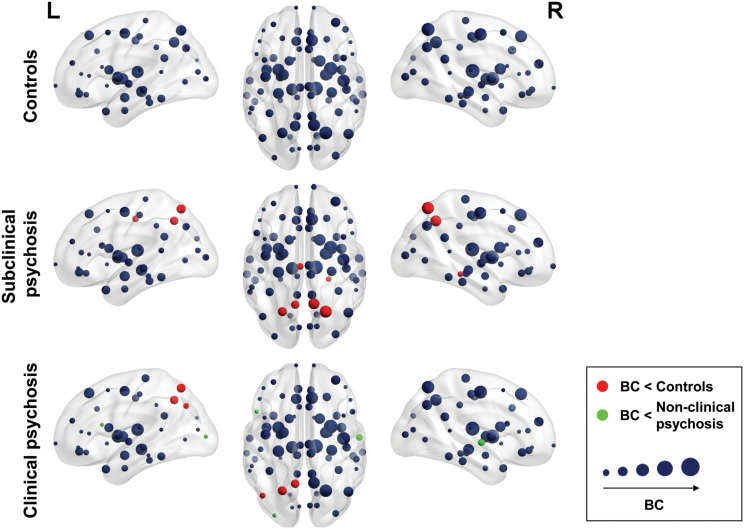
Several differences in regional BC were found between groups (figure 4). BC of the left precuneus and superior parietal lobule were lower in CP and SCP compared to controls. Left inferior parietal BC was lower in CP than in HC. Lower BC was found in CP than in SCP in the pars opercularis of the left inferior frontal gyrus and the left lateral occipital gyrus, and right superior temporal gyrus. BC of the left posterior cingulate, right parahippocampal gyrus, precuneus and superior parietal lobule was lower in SCP than in HC.
Fig. 4.
Betweenness centrality (BC). Visualization of the BC in the structural backbone controls, subjects with subclinical psychosis, and patients with a psychotic disorder. The size of the nodes corresponds to the BC. Red and green nodes mark regions with a significant group difference in BC.
Discussion
In this study, we analyzed the backbone of the structural brain network in patients with a clinical psychotic disorder (CP), subclinical subjects with psychotic symptoms (SCP), and HCs. We found that microstructure of white matter connections, as measured with FA and MD, and topology of the backbone differed between subjects with psychotic symptoms and controls. These differences were more notable in clinical than in subclinical subjects, and are in line with the hypothesis of a psychosis continuum.1 Regional network characteristics differed between the 3 groups with alterations in several central hub regions, and regions involved in conscious perception and language processing.
Microstructural Alterations
Connection density and structural network organization have not been studied in individuals with SCP. One previous study analyzed connection quality in the arcuate fasciculus in subjects who were also analyzed in the present study.25 FA was decreased in clinical but not in subclinical subjects with psychotic symptoms. We found a global decrease of FA and increase of MD, which shows that microstructural disturbances are not restricted to the arcuate fasciculus. White matter loss is found globally in the structural backbone as characterized by MST connections in patients with a psychotic disorder, while these alterations are less pronounced in subjects with SCP. Differences in MTR, which is sensitive to the proportion of macromolecules within a voxel and is thought to be sensitive to myelin, were not observed. Therefore, the pattern over all 3 white matter parameters may be interpreted to reflect a general loss of coherence of white matter fiber orientation or an increase of free water concentrations,30,31 as opposed to a loss of myelin.
MST Analysis of Structural Brain Networks
This is the first study to use MST analysis to compare the structural backbone of the brain between patients and controls. This is also the first study to measure structural network alterations in a specific population by quantifying overlap with a reference network. This method might provide additional information, as we found several topological differences between CP, NPC, and HC, while no group differences were found in global network characteristics.
Topological Network Alterations
Networks of CP deviated more from HC than from SCP, which is in line with the psychosis continuum hypothesis. Nodal network characteristics showed several group differences, and some findings are in line with previous work on network alterations related to psychosis and AVH.12,42,43 BC of the left precuneus and superior parietal gyrus were lower in CP and SCP compared to controls. These regions play a central role in the backbone structure of the MST, and are part of the so-called “rich club,” a subset of highly interconnected hub nodes, which is explicitly altered in schizophrenia.43 This finding provides further evidence that disruption of the backbone of the structural brain network is relevant for psychotic symptoms, and for the hypothesis that hub regions are generally implicated in brain disorders.12,16 The contralateral homologues of the left precuneus and superior parietal gyrus differed between SCP and HC, but not between CP and HC nor between HC and SCP. Qualitative analysis of the data suggests that a difference between CP and HC in these regions may be found in larger samples (supplementary figure S2).
We found that BC of the pars opercularis of the left inferior frontal gyrus (overlapping with Broca’s area) and right superior temporal gyrus (overlapping with the homologue of Wernicke’s area and the right primary auditory cortex) was lower in CP than in SCP. Previous studies showed that language lateralization is significantly decreased in schizophrenia patients but not in subclinical subjects with AVH.42 Interhemispheric connectivity in patients with long term auditory hallucinations was also decreased, which fits with our findings in CP.44 The decreased centrality in these regions seems to reflect an altered structural organization of the language system in CP but not in SCP. Lower BC in CP than in SCP was also found in the left lateral occipital gyrus, suggesting that structural connections with visual sensory areas may also be altered in CP. In previous work, decreased connectivity was found of the left frontal lobe in subjects at ultra-high risk for psychosis,45 which was not found in our SCP population.
We found lower BC of the left posterior cingulate, right parahippocampal gyrus, right precuneus and right superior parietal lobule in SCP than in HC. In an functional MRI study comparing SCP to controls, an increase in functional connectivity strength was found in right limbic regions and bilateral temporal regions, while BC was increased in the left superior temporal gyrus but decreased in the left paracentral lobule, right inferior temporal gyrus, and right amygdala.46 Although these findings are not entirely in line with ours, increased (contralateral) functional connectivity in regions with decreased centrality in the structural network fits with the hypothesis of a mismatch between functional and structural networks in subjects with psychotic symptoms, ie, that functional connectivity may both decrease and increase as a result of decreased structural connectivity.11
In summary, regional network alterations revealed decreased centrality in CP of language areas and contralateral homologues, as well as in hub regions in both CP and SCP, which suggest a global alteration of connections between association areas that facilitate global integration of information.
Limitations
This study has several limitations. Subclinical individuals were included on the basis of their experience of AVH, and may not be a representative sample of subjects with subclinical psychosis.6 Sixty percent of subjects in our SCP population were females, and CP and control subjects were included to match this population. This gender distribution is considered to be in line with previous reports on the SCP population.47,48 In patients with a psychotic disorder, medication effects may have influenced white matter connections, and qualitative analysis of our data suggests that group effects may be underestimated due to relatively small sample sizes. Based on the boxplots in supplementary figure S1, we do not expect effect sizes to be large enough for the DTI metrics to serve as a diagnostic marker. Rather, our results may be used to gain insight in the pathophysiology of psychotic symptoms.
Assessment scales for AVH differed between CP and SCP, and the use of the same scale would have had additional value for the interpretation of our findings in the psychosis continuum. Finally, a previous study of the SCP group showed significantly lower (but not clinically impaired) cognitive performance in the verbal domain and executive functioning domain.49 Future studies may elucidate whether DTI metrics are related to these cognitive performance deficits in the SCP group.
Conclusion
The backbone of the structural brain network differs between subjects with psychotic experiences and controls, and deviations from controls are more severe in patients with a clinical psychotic disorder than in subjects with SCP. These brain network alterations are present both in the microstructure and global topological organization of white matter pathways. Our findings are in line with the hypothesis of a psychosis continuum, ranging from healthy subjects to patients with a psychotic disorder.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Acknowledgment
The authors thank A. de Weijer for his efforts collecting the data. The authors declare that there are no conflicts of interest in relation to the subject of this study.
References
- 1. van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39:179–195. [DOI] [PubMed] [Google Scholar]
- 2. Daalman K, van Zandvoort M, Bootsman F, Boks M, Kahn R, Sommer I. Auditory verbal hallucinations and cognitive functioning in healthy individuals. Schizophr Res. 2011;132:203–207. [DOI] [PubMed] [Google Scholar]
- 3. Sommer IE, Daalman K, Rietkerk T, et al. Healthy individuals with auditory verbal hallucinations; who are they? Psychiatric assessments of a selected sample of 103 subjects. Schizophr Bull. 2010;36:633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Daalman K, Boks MP, Diederen KM, et al. The same or different? A phenomenological comparison of auditory verbal hallucinations in healthy and psychotic individuals. J clin Psychiatry. 2011;72:320–325. [DOI] [PubMed] [Google Scholar]
- 5. Diederen KM, Daalman K, de Weijer AD, et al. Auditory hallucinations elicit similar brain activation in psychotic and nonpsychotic individuals. Schizophr Bull. 2012;38:1074–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Lutterveld R, van den Heuvel MP, Diederen KM, et al. Cortical thickness in individuals with non-clinical and clinical psychotic symptoms. Brain. 2014;137:2664–2669. [DOI] [PubMed] [Google Scholar]
- 7. Skudlarski P, Schretlen DJ, Thaker GK, et al. Diffusion tensor imaging white matter endophenotypes in patients with schizophrenia or psychotic bipolar disorder and their relatives. Am J Psychiatry. 2013;170:886–898. [DOI] [PubMed] [Google Scholar]
- 8. de Leeuw M, Bohlken MM, Mandl RC, Kahn RS, Vink M. Reduced fronto–striatal white matter integrity in schizophrenia patients and unaffected siblings: a DTI study. Schizophrenia. 2015;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moran ME, Luscher ZI, McAdams H, et al. Comparing fractional anisotropy in patients with childhood-onset schizophrenia, their healthy siblings, and normal volunteers through DTI. Schizophr Bull. 2014;41:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- 11. Fornito A, Bullmore ET. Reconciling abnormalities of brain network structure and function in schizophrenia. Curr Opin Neurobiol. 2015;30:44–50. [DOI] [PubMed] [Google Scholar]
- 12. Drakesmith M, Caeyenberghs K, Dutt A, et al. Schizophrenia-like topological changes in the structural connectome of individuals with subclinical psychotic experiences. Human Brain Map. 2015;36:2629–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fornito A, Zalesky A, Pantelis C, Bullmore ET. Schizophrenia, neuroimaging and connectomics. Neuroimage. 2012;62:2296–2314. [DOI] [PubMed] [Google Scholar]
- 14. van den Heuvel MP, Sporns O, Collin G, et al. Abnormal Rich Club Organization and Functional Brain Dynamics in Schizophrenia. JAMA Psychiatry. 2013;70:783–792. [DOI] [PubMed] [Google Scholar]
- 15. Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neurosci Biobehav Rev. 2011;35:1110–1124. [DOI] [PubMed] [Google Scholar]
- 16. Crossley NA, Mechelli A, Scott J, et al. The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain. 2014;137:2382–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Wijk BC, Stam CJ, Daffertshofer A. Comparing brain networks of different size and connectivity density using graph theory. PLoS One. 2010;5:e13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stam CJ, Tewarie P, Van Dellen E, van Straaten EC, Hillebrand A, Van Mieghem P. The trees and the forest: characterization of complex brain networks with minimum spanning trees. Int J Psychophysiol. 2014;92:129–138. [DOI] [PubMed] [Google Scholar]
- 19. Tewarie P, van Dellen E, Hillebrand A, Stam C. The minimum spanning tree: an unbiased method for brain network analysis. NeuroImage. 2014;104:177–188. [DOI] [PubMed] [Google Scholar]
- 20. Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–623. [DOI] [PubMed] [Google Scholar]
- 21. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 22. Haddock G, McCarron J, Tarrier N, Faragher E. Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS). Psychol Med. 1999;29:879–889. [DOI] [PubMed] [Google Scholar]
- 23. Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophre Bull. 1991;17:555. [DOI] [PubMed] [Google Scholar]
- 24. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 25. de Weijer AD, Neggers SF, Diederen KM, et al. Aberrations in the arcuate fasciculus are associated with auditory verbal hallucinations in psychotic and in non-psychotic individuals. Hum Brain Mapp. 2013;34:626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. [DOI] [PubMed] [Google Scholar]
- 27. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. [DOI] [PubMed] [Google Scholar]
- 28. Mandl RC, Schnack HG, Luigjes J, et al. Tract-based analysis of magnetization transfer ratio and diffusion tensor imaging of the frontal and frontotemporal connections in schizophrenia. Schizophr Bull. 2010;36:778–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mori S, Crain BJ, Chacko V, Van Zijl P. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. [DOI] [PubMed] [Google Scholar]
- 30. De Weijer A, Mandl R, Diederen K, Neggers S, Kahn R, Pol HH, Sommer I. Microstructural alterations of the arcuate fasciculus in schizophrenia patients with frequent auditory verbal hallucinations. Schizophr Res. 2011;130:68–77. [DOI] [PubMed] [Google Scholar]
- 31. Kubicki M, Park H, Westin C, et al. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage. 2005;26:1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kruskal JB. On the shortest spanning subtree of a graph and the traveling salesman problem. Proc Am Math Soc. 1956;7:48–50. [Google Scholar]
- 33. Wang H, Hernandez JM, Van Mieghem P. Betweenness centrality in a weighted network. Physical Review E. 2008;77:046105. [DOI] [PubMed] [Google Scholar]
- 34. Lee U, Kim S, Jung KY. Classification of epilepsy types through global network analysis of scalp electroencephalograms. Physical Review E. 2006;73:041920. [DOI] [PubMed] [Google Scholar]
- 35. Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8:e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boersma M, Smit DJ, Boomsma DI, Geus EJ, Delemarre-van de Waal HA, Stam C. Growing trees in child brains: graph theoretical analysis of EEG derived minimum spanning tree in 5 and 7 year old children reflects brain maturation. Brain Connectivity. 2012. [DOI] [PubMed] [Google Scholar]
- 37. Jonckheere AR. A distribution-free k-sample test against ordered alternatives. Biometrika. 1954;41:133–145. [Google Scholar]
- 38. Terpstra T. The asymptotic normality and consistency of Kendall’s test against trend, when ties are present in one ranking. Indagationes Mathematicae. 1952;14:327–333. [Google Scholar]
- 39. Bewick V, Cheek L, Ball J. Statistics review 10: further nonparametric methods. Crit Care. 2004;8:196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc. 1995;57:289–300. [Google Scholar]
- 41. Blair RC, Karniski W. An alternative method for significance testing of waveform difference potentials. Psychophysiology. 1993;30:518–524. [DOI] [PubMed] [Google Scholar]
- 42. Diederen KM, De Weijer AD, Daalman K, et al. Decreased language lateralization is characteristic of psychosis, not auditory hallucinations. Brain. 2010;133:3734–3744. [DOI] [PubMed] [Google Scholar]
- 43. van den Heuvel MP, Sporns O, Collin G, et al. Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry. 2013;70:783–792. [DOI] [PubMed] [Google Scholar]
- 44. Wigand M, Kubicki M, Clemm von Hohenberg C, et al. Auditory verbal hallucinations and the interhemispheric auditory pathway in chronic schizophrenia. World J Biol Psychiatry. 2015;16:31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carletti F, Woolley JB, Bhattacharyya S, et al. Alterations in white matter evident before the onset of psychosis. Schizophr Bull. 2012;38:1170–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van Lutterveld R, Diederen KM, Otte WM, Sommer IE. Network analysis of auditory hallucinations in nonpsychotic individuals. Hum Brain Mapp. 2014;35:1436–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tien AY. Distribution of hallucinations in the population. Soc Psychiatry Psychiatr Epidemiol. 1991;26:287–292. [DOI] [PubMed] [Google Scholar]
- 48. Sommer IE, Daalman K, Rietkerk T, et al. Healthy individuals with auditory verbal hallucinations; who are they? Psychiatric assessments of a selected sample of 103 subjects. Schizophr Bull. 2010;36:633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Daalman K, van Zandvoort M, Bootsman F, Boks M, Kahn R, Sommer I. Auditory verbal hallucinations and cognitive functioning in healthy individuals. Schizophr Res. 2011;132:203–207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.