Abstract
SLC6A3, which encodes the primary regulator of extracellular dopamine (DA) concentration, the DA transporter, has been implicated in schizophrenia (SCZ). However, the details of its genetic effect on risk remain largely unknown. The purpose of this candidate gene study was to identify a specific SLC6A3 activity associated with SCZ by using functional genetic approaches. We first examined gene activity in DA neurons isolated from case-control postmortem nigral tissue and found that the average SLC6A3 mRNA level in controls was only 0.37-fold of that in cases (P = .0034). To understand this expression difference, we examined the association of 10 genetic markers, mostly located in the promoter region, with SCZ in 1717 subjects collected from Toronto and McLean cohorts, including 881 controls and 836 cases and identified the 5′ promoter SNP rs1478435 as having a significant association signal (uncorrected P value: .00462; adjusted P value: .0319) in unrelated Caucasians. Allele T was over-represented in controls (OR = .75); T-carrier controls had decreased mRNA levels in nigral DA neurons, contributing to the reduced activity in the controls. In vitro functional analysis confirmed that T carriers displayed attenuated enhancement of promoter activity. These findings collectively suggest that increased nigral SLC6A3 activity may be a risk factor for SCZ, and may help to explain high rates of comorbidity with substance abuse.
Key words: dopamine neurons, functional genetics, gene expression, human postmortem midbrain, promoter function
Introduction
Both clinical and preclinical findings implicate presynaptic dysregulation of dopamine (DA) transmission in the pathophysiology of schizophrenia (SCZ).1–5 Amelioration of positive symptoms (ie, hallucinations, delusions, thought disorder) with DA receptor blockers further implicates dysregulation of DA transmission.6,7 One potential source of DA dysregulation is SLC6A3, the gene that encodes the presynaptic, principal regulator (the DA transporter or hDAT) of DA transmission in the brain. The hDAT protein is expressed in brain regions (eg, prefrontal cortex and limbic) implicated in SCZ.8–14 DNA sequence variation at SLC6A3 regulatory sites may cause dysregulation of DA transmission in these brain regions by altering SLC6A3 activity and protein levels.15–18
The promoter, not the 3′ end, appears to carry SLC6A3-related genetic risk for SCZ. Previous association studies used a 40-bp variable number tandem repeat (VNTR) marker located in the last exon (50kb downstream of the promoter region) of SLC6A3, but none of these studies showed a significant association with SCZ (www.schizophreniaforum.org). Although this negative finding may seem inconsistent with the notion that dysregulation of DA transmission contributes to SCZ symptoms, such a conclusion would be premature, because 3′VNTR genotypes and in vivo hDAT protein levels are only weakly associated. In contrast, all 3 studies of the SLC6A3 promoter region showed positive and significant associations between single nucleotide polymorphisms (SNPs) and SCZ in 4 populations19–21 with different ancestral backgrounds. However, there is currently no direct evidence regarding whether SLC6A3 activity in DA neurons is altered in SCZ and which functionally distinct SLC6A3 promoter variants confer risks for SCZ. In this study, we characterize SLC6A3 activity in SCZ using 4 complementary approaches: (1) by measuring SLC6A3 mRNA levels in DA neurons isolated from control and case postmortem brain tissue; (2) by typing functional polymorphisms located mainly in the 5′ promoter regions in Caucasian controls and SCZ patients; (3) by correlating SCZ-associated promoter variants with SLC6A3 mRNA levels in DA neurons; and (4) by in vitro functional verification of significantly correlated variants (see supplementary figure 1 for study design).
Methods
Postmortem midbrain tissue was provided by the Harvard Brain Tissue Resource Center at McLean Hospital. Single nigral DA neurons were isolated randomly from nigral sections by laser capture microdissection (LCM). All subjects were recruited at both the Neurogenetics Laboratory at the Centre for Addiction and Mental Health (CAMH) in Toronto, Ontario, Canada and the Psychology Research Laboratory at McLean Hospital. All mRNA levels were assessed by standard quantitative reverse-transcription polymerase chain reaction (qRT-PCR) 17 where “β-actin” was used as an internal control because cases and controls did not differ in its expression and this internal control could address postmortem tissue-associated RNA quality issue in qRT-PCR.22,23 Extraction of DNA from blood, genotyping, ancestry ascertainment, quality controls, imputation, association and permutation analyses used standard protocols24–32 (see supplementary table 1 for primers and probes). Luciferase activity-based functional assay of rs1478435 allelic regulation of 2.5-kb SLC6A3 promoter activity followed a previous in vitro procedure.18 Association, ANOVA or Student’s t test results with P values of < .05 were considered as statistically significant, either uncorrected or adjusted (detailed methods are provided in supplementary information).
Results
Significant Reduction in SLC6A3 mRNA Levels in Controls Compared With Cases
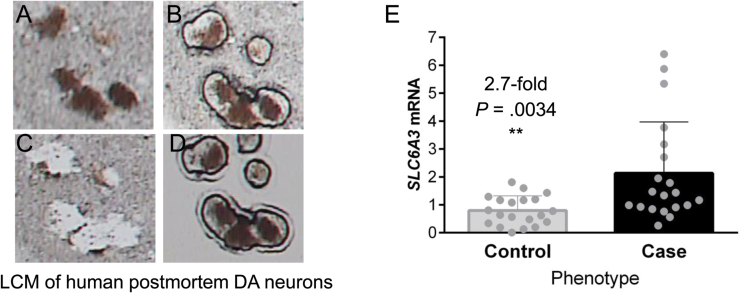
To measure SLC6A3 mRNA levels, we used LCM and DA neurons isolated randomly from human postmortem brain nigral blocks in 20 controls and 20 age- and gender-matched cases (18/20 cases had medical records available showing 29.6±4.5 years of exposure to antipsychotic (and often other psychotropics as well) medications with an average of 2.94±0.50 antipsychotic drugs per case; see table 2 for demographic information; see figures 1A–D for LCM), followed by qRT-PCR analysis of SLC6A3 mRNA levels in the isolated DA neurons. The average SLC6A3 mRNA level in controls was only 0.37-fold of that in cases (P = .0034 by Student’s t tests; figure 1E). Because it has been reported that haloperidol treatment reduced hDAT expression levels in SCZ patients,33 our data suggest that significantly elevated SLC6A3 activity was associated with SCZ and was not a medication effect. Supporting this conclusion, neither antipsychotic medication dose (R 2 = .0052, P = .7899) nor years of medication exposure (R 2 = .048, P = .3979) was correlated with SLC6A3 mRNA levels, ruling out medication effects. To identify the cause of the SLC6A3 elevation in cases, we took a genetic approach to delineate whether DNA sequence variation in the SLC6A3 gene contributed to altered SLC6A3 activity.
Table 2.
Demographic Information on Subjects Used in This Study
| Phenotype | Number of Subjects | Male | Female | Unknown | Average Age | AAO |
|---|---|---|---|---|---|---|
| American postmortem sample used in mRNA level analysis | ||||||
| Controls | 20a | 11 | 9 | 0 | 58.75±3.58 | |
| Cases | 20 | 11 | 9 | 0 | 58.30±3.62 | |
| Total | 40 | 22 | 18 | 0 | ||
| AIM-defined Caucasians of European ancestry used in association study | ||||||
| Controls | 796 | 201 | 592 | 3 | 55.28±0.40 | |
| Cases | 426 | 328 | 98 | 0 | 40.53±0.61c | 21.14±0.30 |
| Total | 1222b | 529 | 690 | 3 | ||
Note: AAO, age-at-onset; AIM, ancestry-informative marker.
aOne of the 20 cases was self-reported African American and all other 39 as self-reported US Caucasians.
bOut of 1717 subjects studied.
cRecruitment age.
Fig. 1.
SLC6A3 mRNA levels in isolated postmortem dopamine (DA) neurons of controls vs patients with schizophrenia (SCZ; case). (A) TH-positive DA neurons on section before LCM. (B) Laser-capturing of DA neurons. (C) Section after DA neuron capture. (D) Captured DA neurons for RNA isolation and quantitative polymerase chain reaction. (E) Increased expression levels in SCZ than in controls.
Selection of 10 Genetic Markers in SLC6A3
We selected 10 markers to type, including 6 that were previously reported to be associated with various mental disorders such as attention deficit hyperactivity disorder (ADHD), drug addiction, depression and SCZ (see table 1 17,21,34–44). Of these 6 markers, Int8VNTR and rs3756450 are functional based on in vitro assays and 3 (rs2455391, rs67175440 and rs3756450) were previously implicated in SCZ.21 The 5′VNTR site was included, because our previous study suggested that this marker was significantly correlated with mRNA levels in control midbrain.17 Three novel SNPs were included: rs11564751 (a core promoter SNP), rs12652860 and rs6860992; the latter 2 are located near the distal functional region of the SLC6A3 promoter.
Table 1.
Ten Markers Selected for Genotyping in This Study
| Marker | chr5bpa | Type | SLC6A3 Locationb | Supporting Evidence | Reference |
|---|---|---|---|---|---|
| rs3836790 | 1411855-6 | int8VNTRd | Intron 8 | ADHD, Drug addiction | Guindalini et al35 ; Laucht et al36 ; O’Gara et al37 ; Franke et al38 ; Maitra et al34 |
| Correlation with mRNA levels | Brookes et al39 | ||||
| In vitro functional | Guindalini et al35 ; Hill et al40 | ||||
| rs2455391 | 1443498 | SNP | Intron 1 (2051) | SCZ | Zheng et al41 |
| rs67175440 | 1443603c | SNP | Intron 1 (1945) | SCZ | Zheng et al41 |
| rs11564751 | 1447223 | SNP | −1675 | (novel core promoter region) | |
| rs11564750 | 1447762 | SNP | −2217 | ADHD | Doyle et al42 |
| rs3756450 | 1448148 | SNP | −2590 | SCZ | Talkowski et al21 |
| Protein binding in vitro | Bamne et al43 | ||||
| rs2550948 | 1450444 | SNP | −4896 | Depression | Huang et al44 |
| rs12652860 | 1453772 | SNP | −8223 | (novel) | |
| rs6860992 | 1455946 | SNP | −10 397 | (novel) | |
| rs70957367 | 1456666 | 5′VNTRe | −11 115 | Correlation with mRNA levels | Zhou et al17 |
Note: ADHD, attention deficit hyperactivity disorder; SCZ, schizophrenia; VNTR, variable number tandem repeat.
aPer GRCh37.p13.
bAssuming that TSS = 1.
cTogether with its adjacent SNP rs2975223 form a dinucleotide polymorphism (DNP, LD = 1), see Zhou et al17 .
d30bp repeat, n = 5–7.
e60bp repeats n = 6–8.
Ancestry-Informative Marker-Defined vs Self-Reported Caucasians
Ancestry-informative marker (AIM) was used to genetically verify Caucasians of European ancestry for inclusion in the data analyses. As a result of the AIM analysis, 15.2% of the total sample was excluded from the AIM-defined Caucasian group (supplementary figure 2). The AIM-defined Caucasian group overlapped with the self-reported Caucasian group (supplementary table 2). This was especially evident in the Toronto case sample, perhaps due to its great admixture. Out of 610 patients, 433 (71.0%) were AIM-defined Caucasians and 427 (70.0%) were self-reported Caucasians. However, only 388 (63.6%) subjects met both AIM and self-report criteria for being “Caucasians,” displaying a discordance of 10.4% in this subsample. The overall discordance rate was 3.9%. In this study, we classified Caucasians by AIM for the association analyses because it is more objective. Therefore, although association data on “Self-report” or “AIM+Self report” ethnicity were available (supplementary figure 4), we restricted our analyses to AIM-defined Caucasians to reduce ancestral heterogeneity and minimize false positive findings.
Linkage Disequilibrium Difference Between Controls and Cases
Among controls, rs11564751 (−1675bp) had lower linkage disequilibrium (LD) scores with 2 upstream SNPs, rs11564750 (−2214bp) and rs3756450 (−2600bp), compared with the other SNP pairs (supplementary figure 3, upper panel). In cases, the low levels of LD displayed by rs11564750 and rs3756450 were even weaker and extended to rs12652860 (−8224bp) (supplementary figure 3, lower panel). These LD data indicate a loss of LD in the SLC6A3 promoter of SCZ patients.
Association Analysis of Genotypes
Among 836 typed patients, we excluded 44 with DSM-IV diagnoses of nonschizophrenic psychotic disorders who would not meet a “narrow” definition of SCZ: 19 patients with a diagnosis of bipolar disorder, 1 with a personality disorder, and 24 with a diagnosis of psychosis not otherwise specified. Considering the 792 patients who met the “narrow” phenotype definition (excluding schizoaffective disorders), 1222 subjects met AIM criteria; the male:female ratios were 1:1.30 on average (1:2.95 for controls and 1:0.30 for cases, table 2). The average recruitment age was approximately 55.28 years old for controls and 40.53 years old for cases; average age-at-onset in cases was about 21.14 years old.
Significant association signals were found for 3 of the 10 typed markers: rs67175440, rs12652860, and 5′VNTR (independent uncorrected P values = .00830–.02272). Among the 2 typed VNTRs, 5′VNTR had the 509bp variant underrepresented in cases (OR = 0.7868), whereas Intron 8 VNTR did not differ at a statistically significant level in either allelic or genotypic frequency in controls and cases. rs12652860 survived correction for multiple testing with an adjusted P value of .0477 (table 3). For rs12652860, located in the distal promoter region (−8224bp), the A allele was under-represented in SCZ (OR = 0.7799). None of the other markers was significantly associated with SCZ.
Table 3.
Association of Genotyped SLC6A3 Markers With SCZ in Caucasians
| Marker | BP | SLC6A3 Location | A1 | Case | Control | ChiSq | OR (95% CI) | P-Value | Adjusted P a |
|---|---|---|---|---|---|---|---|---|---|
| rs67175440 | 1443602 | Intron 1 | G | 0.3873 | 0.4351 | 5.190 | 0.8209 (0.6927–0.9729) | .02272 | .1201 |
| rs12652860 | 1453772 | 5′ promoter | A | 0.2176 | 0.3190 | 6.969 | 0.7799 (0.6483–0.9382) | .00830 | .0477 |
| rs70957367 (5′VNTR)a | 1456666 | 5′ promoter | 509 | 0.2700 | 0.3197 | 6.520 | 0.7868 (0.6543–0.9460) | .0107 |
Note: aBoth EMP2 and SNPSpD agreed; bold, P-value is less than .05 and considered statistically significant.
Weak rs12652860 Correlation With mRNA Levels in DA Neurons
Based on these association findings (supplementary figure 4a), we next examined whether rs12652860 had any functional significance. To do so, we used qRT-PCR to measure SLC6A3 mRNA levels in DA neurons from controls and SCZ as described above. These postmortem analyses showed that the A allele was associated with reduced mRNA levels in controls, but this reduction was not statistically significant (0.80-fold, P = .3009) (supplementary figure 5). By genotype, A-carriers showed reduced mRNA levels in controls as well, but this reduction also did not reach statistical significance (0.69-fold, P = .1779). No statistically significant differences were found in cases or in case/control ratios either for alleles or for genotypes (supplementary figure 5). The lack of significant association between mRNA level and allele or genotype suggested that examining other markers using imputation might be more informative.
Association Analysis of Imputed Genotypes
Imputation with the 1000 Genome Project multiple population reference panel increased the number of SNPs from 8 to 17. This additional association analysis revealed 7 markers showing statistically significant association signals (table 4). Among the 7, four significant associations survived multiple testing, all residing beyond rs12652860 in distal promoter regions. The most significant association was with rs1478435, which is 840bp upstream of rs12652860. The T allele of rs1478435 was under-represented in SCZ, with an OR of 0.7500 (uncorrected P = .00444; adjusted P = .0356).
Table 4.
Association of Imputed SLC6A3 Markers With SCZ in Caucasians
| Marker | BP | SLC6A3 Location | Prot. Allele | Case | Control | ChiSq | OR (95% CI) | P-Value | Adjusted P b |
|---|---|---|---|---|---|---|---|---|---|
| rs2975223 | 1443603 | Intron 1 | A | 0.3873 | 0.4351 | 5.190 | 0.8209 (0.6927–0.9729) | .02272 | .4401 |
| rs67175440 | 1443604 | Intron 1 | G | 0.3849 | 0.4332 | 5.199 | 0.8187 (0.6893–0.9724) | .02260 | .1445 |
| rs12652860 | 1453772 | 5′ promoter | A | 0.2676 | 0.3190 | 6.969 | 0.7799 (0.6483–0.9382) | .00830 | .0622 |
| rs1478435 a | 1454612 | 5′ promoter | T | 0.2124 | 0.2645 | 8.094 | 0.7500 (0.6150–0.9147) | .00441 | .0356 |
| rs10061889 | 1456803 | 5′ promoter | A | 0.2222 | 0.2741 | 7.458 | 0.7566 (0.6192–0.9246) | .00632 | .0477 |
| rs748209 | 1457554 | 5′ promoter | A | 0.2175 | 0.2714 | 8.308 | 0.7463 (0.6114–0.9109) | .00395 | .0319 |
| rs2937650 | 1458018 | 5′ promoter | A | 0.2175 | 0.2714 | 8.308 | 0.7463 (0.6114–0.9109) | .00395 | .0319 |
Note: aSelected for functional assessments.
aBold, P-value is less than .05 and considered statistically significant.
Strong rs1478435 Correlation With mRNA Levels in DA Neurons
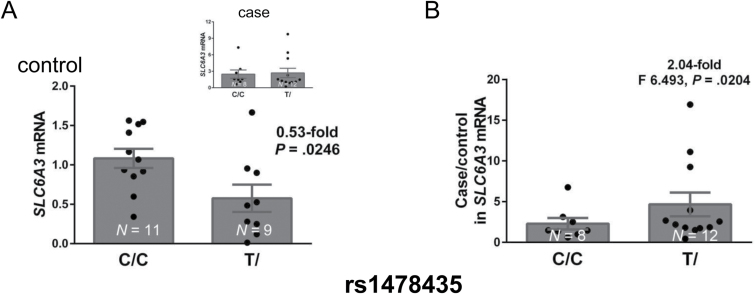
Based on these association findings (supplementary figure 4b), we next examined whether rs1478435 had any functional consequences. A comparison of SLC6A3 mRNA levels in isolated DA neurons between 20 controls and 20 cases carrying the T and C alleles was used for a variant-expression correlation analysis.
The T allele of rs1478435 was marginally associated with reduced mRNA levels in controls (0.61-fold, P = .0617 by t tests). Heterozygotes were present in both allelic groups. The genotypic comparisons, which completely separated the individuals into 2 groups on the basis of genotypes, showed that T-carrier controls had a significantly reduced mean mRNA level (0.53-fold, P = .0246) (figure 2A). This genotypic correlation was not found in cases (figure 2A, Insert), resulting significant phenotypic difference in genotypic expression (P = .0062). Furthermore, the case/control ratio for mRNA levels was 2.04-fold higher in T-carriers than in the non-T carriers (F (11,7) = 6.493, P = .0204; figure 2B), suggesting that the T-associated variant was associated with significantly upregulated SLC6A3 activity in cases. Consistent with this finding, the T allele was also associated with reduced gene activity in lung tissue from 123 subjects based on eQTL results in the GTEx database (P-value = 7×10−10, www.gtexportal.com). On the basis of these findings, we carried out an in vitro functional analysis of this SNP.
Fig. 2.
Correlation of rs1478435 genotypes with mRNA levels in dopamine (DA) neurons. (A) Reduced mRNA expression levels in rs1478435 T-carrying controls. Insert, no genotypic difference in cases. Regression analysis showed a significant phenotype difference (P = .0062, with no interaction between genotype and phenotype). (B) Case/control ratio in expression levels increased in rs1478435 T-carriers. mRNA levels were normalized by actb mRNA levels; *P < .05; **P < .01 by Student’s t tests (N = 20 for each phenotype).
rs1478435: Allelic Regulation of SLC6A3 Promoter Activity in Cultured DA Cells
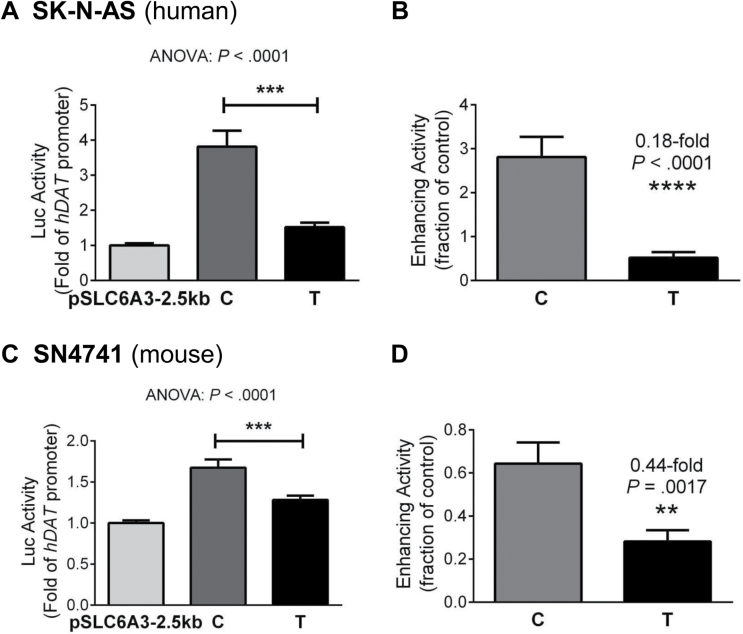
On the basis of the strong association between rs1478435 genotypes and mRNA levels in isolated DA neurons and lung tissue, we examined whether the observed correlation depended on linkage with another underlying functional polymorphism or whether rs1478435 was the underlying functional polymorphism; in the latter case, the 2 alleles of rs1478435 could differentially regulate promoter activity. To distinguish between these 2 possibilities, we carried out an in vitro functional analysis of the 2 alleles in 2 different DA cell lines, SK-N-AS derived from human and SN4741 from mouse substantia nigra DA neurons. Using exogenous DNAs for the expression analysis allowed well-defined conditions for better control of allelic activity. By Luc reporting of allelic regulations of the SLC6A3 promoter, we have observed that the C allele conferred higher promoter activity than the T allele (P < .0001 by ANOVA; figures 3A and 3C). This transcriptional enhancement was reduced consistently to 0.18- or 0.44-fold in the T allele compared with the C allele (P < .0001 by t tests, F (35, 35) = 12.72, P < .0001 in SK-N-AS; P = .0017 by t tests, F (42, 42) = 3.449, P = .0001 in SN4741; figures 3B and 3D). The consistency in the results between 2 independent cell lines validated the allelic difference in regulating promoter activity. Importantly, this allelic difference was consistent with the postmortem findings showing an association between the T allele and reduced SLC6A3 activity (low mRNA levels prevented obtaining reliable allelic expression information in the postmortem samples). Together, these new functional data also complement current tissue homogenate-based cis-eQTL information (http://www.braineac.org).45
Fig. 3.
In vitro functionality of rs1478435 in SK-N-AS (A, B) and SN4741 (C, D). (A, C) Lower 2.5-kb SLC6A3 promoter activity conferred by T than by C allele (by ANOVA tests, N = 3–5). (B, D) Reduced enhancement by T. Student’s t tests: **P < .01; ****P < .0001 (data in A, C).
Discussion
In isolated nigral DA neurons, we found increased SLC6A3 activity in schizophrenics compared to controls. This finding was not related to medication dose or years of exposure. There was no information on smoking history in the medical records so that we cannot rule out possible smoking effects. Furthermore, we identified a novel functional SNP, rs1478435, which is located −9064bp in the SLC6A3 distal promoter region and was associated with SCZ in our sample of unrelated Caucasians recruited in Toronto and at McLean Hospital. We also showed that the T allele of rs1478435, which is associated with reduced SLC6A3 transcription activity, is consistently under-represented in cases.
Reduced SLC6A3 Activity Might Confer Protection Against SCZ
Davis et al46 proposed a model of DA contributions to the pathophysiology of SCZ, in which low DA activity contributes to negative symptoms (ie, blunted affect, lack of initiative, social withdrawal) whereas high DA concentration is associated with positive symptoms. Our findings provide direct genetic evidence to support the view that DA deficiency secondary to elevated SLC6A3 expression or excessive reuptake activity, may contribute to the pathophysiology of SCZ.47,48 Interestingly, these findings also support the possibility that antipsychotic medications may exert their therapeutic effects partly by attenuating elevated SLC6A3 activity. This possibility is consistent with a recent brain imaging finding that SCZ patients after 2 weeks of treatment with haloperidol displayed significantly reduced hDAT protein density compared with nontreated SCZ patients.33 The T allele of rs1478435 may provide a protective effect by reducing hDAT expression levels to permit sufficient DA signaling. This interpretation is consistent with human genetic studies on the DA-catabolizing COMT gene that have reported reduced DA signaling in the prefrontal cortex in SCZ49 and with imaging studies showing higher hDAT protein density in drug-naïve SCZ patients than in healthy controls.50
Increased DAT mRNA levels were recently found in the peripheral blood leukocytes (PBLs) of medicated SCZ patients (25 acute and 27 chronic, compared to 30 controls),51 consistent with our nigral results. However, other studies of protein levels in different tissue yielded inconsistent results. Imaging studies found either increased DAT binding in basal ganglia of schizophrenics, no difference in 1 cohort or reduced striatal DAT density in medicated patients in another cohort.52–54 Other postmortem studies reported reduced DAT density in cortex or amygdala in SCZ.55,56 The discrepancy with the protein findings reported here might be attributable to tissue dependence of protein expression or inhibitory effects of medication.33
Comparison With Previous Association Findings
Many genetic studies of SLC6A3 in SCZ used the 3′VNTR marker, located in the last exon of the gene, and none of them found a significant association with SCZ.57–67 These negative findings were inconsistent with a principal role for hDAT in DA transmission or dysregulation of DA activity in the pathophysiology of SCZ. However, these negative findings are likely attributable to the weak LD between 3′VNTR and the 5′ promoter, limiting the ability of the 3′VNTR to capture promoter association signals.17 This 3′ marker was only implicated in SCZ in 1 imaging study when considered in interaction with the COMT gene.15
More recently, promoter markers have been examined in association studies of SCZ. One core promoter SNP, rs2975226 (−68bp), was found to affect risk for SCZ in 2 independent Asian populations,19,68 but these results have not yet been replicated in other ethnic groups. SNP rs2652511 (−841bp) was associated with SCZ in Chinese, but not in Iranian samples.69 They are approximately 8kb downstream of rs1478435, but display high LD with each other (see SNPs # 7 and 8 vs #22 in supplementary figure 6), suggesting that the Chinese findings are likely to be LD-based signals. In another study, rs3756450 (−2600bp) was found to be significantly associated with SCZ,21 but that finding has not yet been replicated in other cohorts. In our entire cohort of mixed ancestries (1717 subjects), rs3756450 also displayed significant association signals (uncorrected P = .0006833; adjusted P = .0050, where G was the risk allele with an OR of 1.388) and was the smallest P-value among the examined markers.
For supporting information, we consulted available GWAS datasets in both dbGaP and the Psychiatric Genomics Consortium (PGC, http://www.med.unc.edu/pgc) databases. SLC6A3 genotype information was available for ancestry control from 2 dbGaP GWAS datasets, phs000021.v3.p2 and phs000167.v1.p1, on American SCZ. Phenotype QC was also performed to remove schizoaffective depression cases for phs000021.v3.p2. Neither GWAS was able to infer rs2975223, and phs000167.v1.p1 did not allow imputation of rs1478435, but both allowed the imputation of rs3756450. Meta-analysis of these studies (a Caucasian and an African American cohort from phs000021.v3.p2 and Caucasians only in phs000167.v1.p1) resulted in nonsignificant P values of .06009 for rs1478435 and .1126 for rs3756450 (see supplementary table 3 for more information; no rigorous phenotype control was performed in these GWAS). Meta-analysis of our data and the African American datasets showed a P value of .006122 for rs1478435 (the smallest P-value among 11 imputed SNPs) and 0.0848 for rs3756450. These results from the meta-analyses suggest that rs1478435 may have a stronger effect than rs3756450. PGC had 4 GWAS results available on SCZ (not included in the meta-analysis here because neither genotype nor phenotype information was available for the quality control). Two of these 4 PGC GWAS were imputed to the 1000 Genome Project templates and had rs1478435 marker information, but neither study implicated this marker in SCZ risk. One possible reason for the difference between our results and those of the PGC is that our cases underwent rigorous diagnostic assessments using standardized instruments, whereas the diagnostic methods were more varied in the PGC, resulting in a potentially more heterogeneous clinical phenotype. Another possibility is that the SNP-SCZ association finding reported here was a false-positive; replications in independent homogeneous cohorts are required (SLC6A3 was not reported in a more recent association study (Schizophrenia Working Group of the Psychiatric Genomics Consortium).70 Nevertheless, dbGaP and PGC together provided a total of 7 GWAS datasets on SCZ and 4 of the 7 had information on rs1478435. Among these 4 rs1478435-informative datasets, 3 (75%) showed ORs <1 for rs1478435, consistent with our association findings. For comparison purposes, we found that rs1478435 was not significantly associated with either bipolar disorder (P = .3914) or substance abuse (meta-analysis P = .9238) (no information was obtained for ADHD and Parkinson’s disease). Overall, these SNP-SCZ association findings from various populations implicate a genetic basis of our gene activity results.
Genetic Dissection of Comorbidity With Smoking
SCZ patients have significantly higher rates of cigarettes smoking than the general population,71,72 suggesting that common genetic risks may contribute to both phenotypes. Nicotine injection or exposure to smoke can increase SLC6A3 mRNA levels significantly in rat substantia nigra.73 Clinically, early exposure to secondhand smoke increased smoking risks in adulthood.74,75 These preclinical and clinical findings suggest that high SLC6A3 gene activity is associated with risks for substance abuse, including smoking. The high SLC6A3 activity in SCZ reported here may provide a genetic basis, together with the previous findings, for the high comorbidity of SCZ and cigarette smoking.
Strengths and Weaknesses of the Current Study
Two key requirements for an association study of unrelated individuals are controls for ancestry and phenotypic homogeneities. Less strict requirements mean that larger sample sizes would be needed to have adequate power to detect association signals.76 We used 2 methods, AIM and self-report (referring to parental ethnicities as well), to define Caucasian ethnicity and we used a “narrow” definition of the SCZ phenotype, excluding schizoaffective and non-SCZ-related psychiatric conditions in order to reduce phenotypic heterogeneity. The main limitation of this study is the modest sample size, which may be the reason for the relatively marginal adjusted P-values. Therefore, validation in larger samples is warranted. Another limitation is the relatively low marker density in the 18kb promoter regions. Because of this, the imputation inferred only a very small fraction (3.4%–6.4%) of known polymorphisms in the 18-kb promoter regions and was unable to capture a smoother signal curve (see supplementary figure 3b) to make sure that there were no additional signal peaks. Future studies would benefit from larger sample sizes and denser markers in the promoter regions.
In summary, we have identified reduced SLC6A3 activity and the T allele of rs1478435 as potential protective factors in SCZ. These findings support the role of reduced DA transmission in the pathophysiology of SCZ, particularly with respect to negative symptoms, which are thought to reflect reduced DA signaling in some areas of the SCZ brain and may help to explain the well-known comorbidity between SCZ and substance abuse.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This work was supported by research funding from a NARSAD Young Investigator Award and NIDA DA021409 (Z.L.), the Ellison Foundation, Team Daniel, the Carmela and Menachem Abraham Fund, and an Anonymous Foundation (D.L.), the Canadian Institutes of Health Research MOP-49525 (J.L.K.), and the Canada Brain Research Fund (J.G.P.).
Supplementary Material
Acknowledgments
We are grateful to the Harvard Brain Tissue Resource Center for providing the human brain tissue samples and associated medication information for these investigations, to Dr Ross Baldessarini for helping with the assessment of life-time medication exposure and to Dr Garrett Fitzmaurice for helping with the regression analyses. We thank dbGaP for granting Z.L. access to the GWAS datasets (Project# 1542). The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet. 2014;383:1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Egerton A, Chaddock CA, Winton-Brown TT, et al. Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biol Psychiatry. 2013;74:106–112. [DOI] [PubMed] [Google Scholar]
- 4. Matthews M, Bondi C, Torres G, Moghaddam B. Reduced presynaptic dopamine activity in adolescent dorsal striatum. Neuropsychopharmacology. 2013;38:1344–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McGowan S, Lawrence AD, Sales T, Quested D, Grasby P. Presynaptic dopaminergic dysfunction in schizophrenia: a positron emission tomographic [18F]fluorodopa study. Arch Gen Psychiatry. 2004;61:134–142. [DOI] [PubMed] [Google Scholar]
- 6. Daly EJ, Kent JM, Janssens L, et al. Metabolic and body mass parameters after treatment with JNJ-37822681, a novel fast-dissociating D2 receptor antagonist, vs olanzapine in patients with schizophrenia. Ann Clin Psychiatry. 2013;25:173–183. [PubMed] [Google Scholar]
- 7. Sakurai H, Bies RR, Stroup ST, et al. Dopamine D2 receptor occupancy and cognition in schizophrenia: analysis of the CATIE data. Schizophr Bull. 2013;39:564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kimoto S, Muraki K, Toritsuka M, et al. Selective overexpression of Comt in prefrontal cortex rescues schizophrenia-like phenotypes in a mouse model of 22q11 deletion syndrome. Transl Psychiatry. 2012;2:e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rolls ET, Loh M, Deco G, Winterer G. Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nat Rev Neurosci. 2008;9:696–709. [DOI] [PubMed] [Google Scholar]
- 10. Albert KA, Hemmings HC, Jr, Adamo AI, et al. Evidence for decreased DARPP-32 in the prefrontal cortex of patients with schizophrenia. Arch Gen Psychiatry. 2002;59:705–712. [DOI] [PubMed] [Google Scholar]
- 11. Meador-Woodruff JH, Haroutunian V, Powchik P, Davidson M, Davis KL, Watson SJ. Dopamine receptor transcript expression in striatum and prefrontal and occipital cortex. Focal abnormalities in orbitofrontal cortex in schizophrenia. Arch Gen Psychiatry. 1997;54:1089–1095. [DOI] [PubMed] [Google Scholar]
- 12. Fusar-Poli P, Meyer-Lindenberg A. Striatal presynaptic dopamine in schizophrenia, part II: meta-analysis of [(18)F/(11)C]-DOPA PET studies. Schizophr Bull. 2013;39:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sorg C, Manoliu A, Neufang S, et al. Increased intrinsic brain activity in the striatum reflects symptom dimensions in schizophrenia. Schizophr Bull. 2013;39:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howes OD, Williams M, Ibrahim K, et al. Midbrain dopamine function in schizophrenia and depression: a post-mortem and positron emission tomographic imaging study. Brain. 2013;136:3242–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prata DP, Mechelli A, Fu CH, et al. Epistasis between the DAT 3’ UTR VNTR and the COMT Val158Met SNP on cortical function in healthy subjects and patients with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:13600–13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prata DP, Mechelli A, Picchioni MM, et al. Altered effect of dopamine transporter 3’UTR VNTR genotype on prefrontal and striatal function in schizophrenia. Arch Gen Psychiatry. 2009;66:1162–1172. [DOI] [PubMed] [Google Scholar]
- 17. Zhou Y, Michelhaugh SK, Schmidt CJ, Liu JS, Bannon MJ, Lin Z. Ventral midbrain correlation between genetic variation and expression of the dopamine transporter gene in cocaine-abusing versus non-abusing subjects. Addict Biol. 2014;19:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao Y, Xiong N, Liu Y, et al. Human dopamine transporter gene: differential regulation of 18-kb haplotypes. Pharmacogenomics. 2013;14:1481–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khodayari N, Garshasbi M, Fadai F, et al. Association of the dopamine transporter gene (DAT1) core promoter polymorphism -67T variant with schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2004;129B:10–12. [DOI] [PubMed] [Google Scholar]
- 20. Stöber G, Sprandel J, Jabs B, Pfuhlmann B, Möller-Ehrlich K, Knapp M. Family-based study of markers at the 5’-flanking region of the human dopamine transporter gene reveals potential association with schizophrenic psychoses. Eur Arch Psychiatry Clin Neurosci. 2006;256:422–427. [DOI] [PubMed] [Google Scholar]
- 21. Talkowski ME, Kirov G, Bamne M, et al. A network of dopaminergic gene variations implicated as risk factors for schizophrenia. Hum Mol Genet. 2008;17:747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hashimoto R, Straub RE, Weickert CS, Hyde TM, Kleinman JE, Weinberger DR. Expression analysis of neuregulin-1 in the dorsolateral prefrontal cortex in schizophrenia. Mol Psychiatry. 2004;9:299–307. [DOI] [PubMed] [Google Scholar]
- 23. Fleige S, Pfaffl MW. RNA integrity and the effect on the real-time qRT-PCR performance. Mol Aspects Med. 2006;27:126–139. [DOI] [PubMed] [Google Scholar]
- 24. Lahiri DK, Nurnberger JI., Jr A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 1991;19:5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;74:765–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kosoy R, Nassir R, Tian C, et al. Ancestry informative marker sets for determining continental origin and admixture proportions in common populations in America. Hum Mutat. 2009;30:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. International HapMap Consortium. The International HapMap Project. Nature. 2003;426:789–796. [DOI] [PubMed] [Google Scholar]
- 29. Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. [DOI] [PubMed] [Google Scholar]
- 30. Delaneau O, Marchini J, Zagury JF. A linear complexity phasing method for thousands of genomes. Nat Methods. 2012;9:179–181. [DOI] [PubMed] [Google Scholar]
- 31. Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anderson CA, Pettersson FH, Clarke GM, Cardon LR, Morris AP, Zondervan KT. Data quality control in genetic case-control association studies. Nat Protoc. 2010;5:1564–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schmitt GJ, Dresel S, Frodl T, et al. Dual-isotope SPECT imaging of striatal dopamine: a comparative study between never-treated and haloperidol-treated first-episode schizophrenic patients. Eur Arch Psychiatry Clin Neurosci. 2012;262:183–191. [DOI] [PubMed] [Google Scholar]
- 34. Maitra S, Sarkar K, Ghosh P, et al. Potential contribution of dopaminergic gene variants in ADHD core traits and co-morbidity: a study on eastern Indian probands. Cell Mol Neurobiol. 2014;34:549–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guindalini C, Howard M, Haddley K, et al. A dopamine transporter gene functional variant associated with cocaine abuse in a Brazilian sample. Proc Natl Acad Sci U S A. 2006;103:4552–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Laucht M, Skowronek MH, Becker K, et al. Interacting effects of the dopamine transporter gene and psychosocial adversity on attention-deficit/hyperactivity disorder symptoms among 15-year-olds from a high-risk community sample. Arch Gen Psychiatry. 2007;64:585–590. [DOI] [PubMed] [Google Scholar]
- 37. O’Gara C, Stapleton J, Sutherland G, et al. Dopamine transporter polymorphisms are associated with short-term response to smoking cessation treatment. Pharmacogenet Genomics. 2007;17:61–67. [DOI] [PubMed] [Google Scholar]
- 38. Franke B, Hoogman M, Arias Vasquez A, et al. Association of the dopamine transporter (SLC6A3/DAT1) gene 9-6 haplotype with adult ADHD. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1576–1579. [DOI] [PubMed] [Google Scholar]
- 39. Brookes KJ, Neale BM, Sugden K, Khan N, Asherson P, D’Souza UM. Relationship between VNTR polymorphisms of the human dopamine transporter gene and expression in post-mortem midbrain tissue. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:1070–1078. [DOI] [PubMed] [Google Scholar]
- 40. Hill M, Anney RJ, Gill M, Hawi Z. Functional analysis of intron 8 and 3’ UTR variable number of tandem repeats of SLC6A3: differential activity of intron 8 variants. Pharmacogenomics J. 2010;10:442–447. [DOI] [PubMed] [Google Scholar]
- 41. Zheng C, Shen Y, Xu Q. Association of intron 1 variants of the dopamine transporter gene with schizophrenia. Neurosci Lett. 2012;513:137–140. [DOI] [PubMed] [Google Scholar]
- 42. Doyle C, Brookes K, Simpson J, et al. Replication of an association of a promoter polymorphism of the dopamine transporter gene and Attention Deficit Hyperactivity Disorder. Neurosci Lett. 2009;462:179–181. [DOI] [PubMed] [Google Scholar]
- 43. Bamne MN, Talkowski ME, Chowdari KV, Nimgaonkar VL. Functional analysis of upstream common polymorphisms of the dopamine transporter gene. Schizophr Bull. 2010;36:977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang CC, Lu RB, Shih MC, Yen CH, Huang SY. The dopamine transporter gene possibly affects personality traits in patients with early-onset major depressive disorder. Acta Neuropsychiatr. 2013;25:227–234. [DOI] [PubMed] [Google Scholar]
- 45. Ramasamy A, Trabzuni D, Guelfi S, et al. Genetic variability in the regulation of gene expression in ten regions of the human brain. Nat Neurosci. 2014;17:1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. [DOI] [PubMed] [Google Scholar]
- 47. Chouinard G, Jones BD. Evidence of brain dopamine deficiency in schizophrenia. Can J Psychiatry. 1979;24:661–667. [DOI] [PubMed] [Google Scholar]
- 48. Cole DM, Oei NY, Soeter RP, et al. Dopamine-dependent architecture of cortico-subcortical network connectivity. Cereb Cortex. 2013;23:1509–1516. [DOI] [PubMed] [Google Scholar]
- 49. Roffman JL, Gollub RL, Calhoun VD, et al. MTHFR 677C –> T genotype disrupts prefrontal function in schizophrenia through an interaction with COMT 158Val –> Met. Proc Natl Acad Sci U S A. 2008;105:17573–17578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schmitt GJ, la Fougère C, Dresel S, et al. Dual-isotope SPECT imaging of striatal dopamine: first episode, drug naïve schizophrenic patients. Schizophr Res. 2008;101:133–141. [DOI] [PubMed] [Google Scholar]
- 51. Liu L, Yuan G, Cheng Z, Zhang G, Liu X, Zhang H. Identification of the mRNA expression status of the dopamine D2 receptor and dopamine transporter in peripheral blood lymphocytes of schizophrenia patients. PLoS One. 2013;8:e75259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mané A, Gallego J, Lomeña F, et al. A 4-year dopamine transporter (DAT) imaging study in neuroleptic-naive first episode schizophrenia patients. Psychiatry Res. 2011;194:79–84. [DOI] [PubMed] [Google Scholar]
- 53. Laakso A, Bergman J, Haaparanta M, et al. Decreased striatal dopamine transporter binding in vivo in chronic schizophrenia. Schizophr Res. 2001;52:115–120. [DOI] [PubMed] [Google Scholar]
- 54. Sjøholm H, Bratlid T, Sundsfjord J. 123I-beta-CIT SPECT demonstrates increased presynaptic dopamine transporter binding sites in basal ganglia in vivo in schizophrenia. Psychopharmacology (Berl). 2004;173:27–31. [DOI] [PubMed] [Google Scholar]
- 55. Rao JS, Kellom M, Reese EA, Rapoport SI, Kim HW. Dysregulated glutamate and dopamine transporters in postmortem frontal cortex from bipolar and schizophrenic patients. J Affect Disord. 2012;136:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56. Markota M, Sin J, Pantazopoulos H, Jonilionis R, Berretta S. Reduced dopamine transporter expression in the amygdala of subjects diagnosed with schizophrenia. Schizophr Bull. 2014;40:984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bodeau-Péan S, Laurent C, Campion D, et al. No evidence for linkage or association between the dopamine transporter gene and schizophrenia in a French population. Psychiatry Res. 1995;59:1–6. [DOI] [PubMed] [Google Scholar]
- 58. Maier W, Minges J, Eckstein N, et al. Genetic relationship between dopamine transporter gene and schizophrenia: linkage and association. Schizophr Res. 1996;20:175–180. [DOI] [PubMed] [Google Scholar]
- 59. Inada T, Sugita T, Dobashi I, et al. Dopamine transporter gene polymorphism and psychiatric symptoms seen in schizophrenic patients at their first episode. Am J Med Genet. 1996;67:406–408. [DOI] [PubMed] [Google Scholar]
- 60. Persico AM, Macciardi F. Genotypic association between dopamine transporter gene polymorphisms and schizophrenia. Am J Med Genet. 1997;74:53–57. [DOI] [PubMed] [Google Scholar]
- 61. King N, Bassett AS, Honer WG, Masellis M, Kennedy JL. Absence of linkage for schizophrenia on the short arm of chromosome 5 in multiplex Canadian families. Am J Med Genet. 1997;74:472–474. [PMC free article] [PubMed] [Google Scholar]
- 62. Georgieva L, Dimitrova A, Nikolov I, et al. Dopamine transporter gene (DAT1) VNTR polymorphism in major psychiatric disorders: family-based association study in the Bulgarian population. Acta Psychiatr Scand. 2002;105:396–399. [DOI] [PubMed] [Google Scholar]
- 63. Hauser J, Kapelski P, Czerski PM, et al. [Lack of association between VNTR polymorphism of DAT gene and schizophrenia]. Psychiatr Pol. 2002;36:403–412. [PubMed] [Google Scholar]
- 64. Szekeres G, Kéri S, Juhász A, et al. Role of dopamine D3 receptor (DRD3) and dopamine transporter (DAT) polymorphism in cognitive dysfunctions and therapeutic response to atypical antipsychotics in patients with schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2004;124B:1–5. [DOI] [PubMed] [Google Scholar]
- 65. Alvarez S, Mas S, Gassó P, Bernardo M, Parellada E, Lafuente A. Lack of association between schizophrenia and polymorphisms in dopamine metabolism and transport genes. Fundam Clin Pharmacol. 2010;24:741–747. [DOI] [PubMed] [Google Scholar]
- 66. Paweł K, Hauser J, Skibińska M, et al. [Family based association study of DRD1, DRD2, DRD3, DRD4, DAT, COMT gene polymorphism in schizophrenia]. Psychiatr Pol. 2010;44:405–413. [PubMed] [Google Scholar]
- 67. Pinsonneault JK, Han DD, Burdick KE, et al. Dopamine transporter gene variant affecting expression in human brain is associated with bipolar disorder. Neuropsychopharmacology. 2011;36(8):1644–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Huang SY, Chen HK, Ma KH, et al. Association of promoter variants of human dopamine transporter gene with schizophrenia in Han Chinese. Schizophr Res. 2010;116:68–74. [DOI] [PubMed] [Google Scholar]
- 69. Galehdari H, Hosseini S, Foroughmand AM, et al. Lack of association between the -839C/T polymorphism in the SLC6A3 gene promoter and schizophrenia in the Iranian population. J Genet. 2009;88:321–323. [DOI] [PubMed] [Google Scholar]
- 70. Lohr KM, Bernstein AI, Stout KA, et al. Increased vesicular monoamine transporter enhances dopamine release and opposes Parkinson disease-related neurodegeneration in vivo. Proc Natl Acad Sci U S A. 2014;111:9977–9982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005;76:135–157. [DOI] [PubMed] [Google Scholar]
- 72. Volkow ND. Substance use disorders in schizophrenia–clinical implications of comorbidity. Schizophr Bull. 2009;35:469–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li S, Kim KY, Kim JH, et al. Chronic nicotine and smoking treatment increases dopamine transporter mRNA expression in the rat midbrain. Neurosci Lett. 2004;363:29–32. [DOI] [PubMed] [Google Scholar]
- 74. Kandel ER, Kandel DB. Shattuck Lecture. A molecular basis for nicotine as a gateway drug. N Engl J Med. 2014;371:932–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. de la Pena JB, Ahsan HM, Tampus R, et al. Cigarette smoke exposure during adolescence enhances sensitivity to the rewarding effects of nicotine in adulthood, even after a long period of abstinence. Neuropharmacology. 2015;99:9–14. [DOI] [PubMed] [Google Scholar]
- 76. Manchia M, Cullis J, Turecki G, Rouleau GA, Uher R, Alda M. The impact of phenotypic and genetic heterogeneity on results of genome wide association studies of complex diseases. PLoS One. 2013;8:e76295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.