Abstract
Neurological soft signs (NSSs) bear the promise for early detection of schizophrenia spectrum disorders. Nonetheless, the sensitivity and specificity of NSSs in the psychosis continuum remains a topic of controversy. It is also unknown how NSSs reveal neurodevelopmental abnormality in schizophrenia. We investigated the effect sizes of NSSs in differentiating individuals with schizophrenia spectrum disorders from individuals with other psychiatric conditions and from covariate-matched healthy subjects. We also investigated the partitioned age-related variations of NSSs in both schizophrenia and healthy individuals. NSSs were assessed by the abridged version of the Cambridge Neurological Inventory (CNI) in 3105 participants, consisting of healthy individuals (n =1577), unaffected first-degree relatives of schizophrenia patients (n = 155), individuals with schizotypal personality disorder (n = 256), schizophrenia patients (n = 738), and other psychiatric patients (n = 379). Exact matching and propensity score matching procedures were performed to control for covariates. Multiple regression was used to partition age-related variations. Individuals along the schizophrenia continuum showed elevated levels of NSSs, with moderate effect sizes, in contrast to other psychiatric patients who had minimal NSSs, as well as matched healthy controls. Furthermore, the age-and-NSS relationship in schizophrenia patients was represented by a flat but overall elevated pattern, in contrast to a U-shaped pattern in healthy individuals. In sum, NSSs capture a moderate portion of psychosis proneness with reasonable specificity. Lifespan profiling reveals an abnormal developmental trajectory of NSSs in schizophrenia patients, which supports the endophenotype hypothesis of NSSs by associating it with the neurodevelopmental model of schizophrenia.
Key words: neurological soft sign, schizophrenia spectrum disorders, lifespan profiling, psychopathology, endophenotype
Introduction
Schizophrenia is a chronic, devastating, and complex neuropsychiatric disorder characterized by a range of cognitive,1 affective,2 and neurodevelopmental abnormalities.3,4 To foster optimal therapeutic interventions for these patients and reduce health care challenges that are faced by patients’ families and the society,5 researchers have been searching for target features, known as endophenotypes, that encompass the genetic and nongenetic processes underpinning the predisposition to schizophrenia.6 To qualify as an endophenotype, a marker must be reliably associated with the illness, demonstrates state-dependent cosegregation, and reveals heritable familial association.6,7 Neurological soft signs (NSSs), conventionally defined as nonlocalizable abnormality in neurological functions, such as failure in sensory integration, motor incoordination, and disinhibition,8–10 have been considered prominent candidates (ie, the endophenotype hypothesis of NSSs).6,7,11 Evidence has robustly shown significant associations between NSSs and a wide range of neurocognitive dysfunctions,6 which are often manifested in schizophrenia spectrum disorders12 and are sensitive to the development of psychosis.4,6 Recently, empirical findings from structural13,14 and functional15 neuroimaging studies have challenged the “nonlocalizable” view by showing a strong link between NSSs and neural abnormalities in schizophrenia-related brain regions,16 suggesting that NSSs may reveal the underlying neural dysfunctions of schizophrenia.15
Nonetheless, the sensitivity and specificity of NSSs in revealing schizophrenia-related conditions remain controversial. Previous meta-analytic studies suggested that individual differences in age, gender and intelligence might influence the prevalence of NSSs.10,17 Moreover, other psychiatric conditions, such as obsessive-compulsive disorder (OCD)18–20 and mood disorders,21,22 may also manifest NSSs. However, this association diminishes when individual differences, such as gender, age, and education, are matched between OCD patients and healthy controls,20 and when medications and other confounders, such as symptom profile, are controlled for between schizophrenia patients and individuals with mood disorders.22 Thus, a large-scale population-based study including clinical, subclinical, and healthy samples with a unified assessment of NSSs and careful matching procedure is needed.
Apart from sensitivity and specificity issues, little is known about how NSSs change over the human lifespan; let alone how schizophrenia patients differ from healthy individuals in the developmental trajectory of NSSs. Both the theory of psychosis continuum and the neurodevelopmental model of schizophrenia3,23 suggest that schizophrenia is not simply a binary phenotype, but the result of the interplay of multiple etiological factors.24 Concomitant evaluation of NSSs and schizophrenia would thus require profiling the trajectory of NSSs in individuals with psychosis compared with healthy individuals, since neurodegeneration may also affect NSSs.25 Lifespan information like this can integrate brain-behavior phenotypes in a developmental context and identify critical age-related characteristics of the vulnerability to schizophrenia to provide tools for early detection.4
Hence, the present study examined NSSs in a large number of participants (N = 3105) sampled from normal healthy individuals, schizophrenia patients, unaffected first-degree relatives of schizophrenia patients, SPD individuals, and other psychiatric patients (see table 1) with 2 specific goals. First, we aimed to clarify the sensitivity and specificity of NSSs in schizophrenia spectrum disorders after controlling for covariates between healthy and nonhealthy comparison groups (ie, schizophrenia, SPD, relatives, or other psychiatric groups) using both exact matching and propensity score matching (see Data Analysis).26,27 According to the endophenotype hypothesis,6 we hypothesized that NSSs would mostly manifest in individuals along the schizophrenia continuum, but not in patients with other psychiatric conditions, as compared to matched healthy controls.
Table 1.
Participants’ Characteristics by Groups
| Characteristics | Healthy | SPD | Schizophreniaa | Relatives | OPb |
|---|---|---|---|---|---|
| Sample size | 1577 | 256 | 738 | 155 | 379 |
| Gender (% male) | 51% | 52% | 63% | 42% | 58% |
| Age | 29.53 (18.85) | 20.77 (4.17) | 30.40 (10.95) | 45.22 (18.59) | 30.30 (16.35) |
| Age group (%) | |||||
| ≤19 | 30.7 | 39.8 | 14.1 | 8.4 | 21.9 |
| 20–29 | 43.4 | 57.8 | 41.6 | 23.2 | 42.5 |
| 30–39 | 5.1 | 0.4 | 21.8 | 5.8 | 16.4 |
| 40–59 | 5.6 | 2.0 | 22.0 | 36.8 | 11.1 |
| ≥60 | 15.2 | 0.0 | 0.5 | 25.8 | 8.2 |
| Intellect | 2.72 (1.18) | 3.22 (0.73) | 2.37 (1.02) | 2.66 (1.03) | 2.49 (1.13) |
| Intellect (%) | |||||
| 1: ≤25% (bottom) | 23.0 | 3.1 | 23.7 | 16.1 | 27.2 |
| 2: 25–50% | 19.0 | 8.6 | 30.9 | 26.5 | 20.6 |
| 3: 50–75% | 21.4 | 51.2 | 29.4 | 32.3 | 28.8 |
| 4: ≥75% (top) | 36.7 | 37.1 | 16.0 | 25.2 | 23.5 |
Note: Means (SD) are displayed for age and intellect. The current study only included the subjects who have verifiable and clear diagnosis (88.6% of the recruited samples). OP, other psychiatric; SPD, schizotypal personality disorders.
aIn the schizophrenia group, 29% were first-episode and 71% were chronic; bIn the other psychiatric group, among all recruited participants, 31.3% suffered from OCD, 21.3% had bipolar disorder (BD), 6.5% had comorbid OCD and BD, 17.8% had major depression disorder (MDD), 6.8% had mild cognitive impairment (MCI), 3.0% had attention deficit hyperactive disorder (ADHD), 1.9% had anxiety disorder (AD), and 11.4% had psychopathological conditions with unknown or unclear diagnostic category.
Second, to investigate the neurodevelopmental abnormality of NSSs in schizophrenia, we partitioned the age-related variance of NSSs in both healthy and schizophrenia patient groups using multiple regression. Since NSS-related brain regions and their functions change significantly across the lifespan,28–31 most likely captured by a quadratic function,31 we hypothesized that in a typical developing situation, NSSs would decrease due to neural maturation but increase due to ageing or neural degeneration, reflected as a U-shaped quadratic relationship between NSSs and chronological age. However, schizophrenia patients, who have abnormalities in NSSs, may show relatively stable but elevated levels of NSSs across the lifespan, considering that both first-onset and chronic patients have been reported to have comparable levels of NSSs.32,33
Methods
Participants
The study included 3105 (out of 3976) participants recruited from 2 major urban areas in China (ie, Beijing and Hong Kong) from 2006 to 2014, stratified based on their psychopathological conditions, namely healthy individuals (n =1577), SPD individuals (n = 256), schizophrenia patients (n = 738), unaffected first-degree schizophrenia relatives (n = 155), and other psychiatric patients (n = 379). All participants (1) had a full assessment of NSSs, (2) provided complete demographic information, (3) had information concerning their intellectual level, such as estimated IQ or years of education, and (4) were free from any disorder that could markedly impair mobility or cognition (eg, paresis, intellectual disability), or any neurological or medical condition that might affect NSSs (eg, past encephalitis, seizures, substance use). Participants who had missing NSS scores and basic demographic information (eg, age or gender, or both IQ and years of education) or suffered from the physical conditions listed above were excluded from further analysis.
Clinically stable patients were diagnosed and referred by certificated psychiatrists, who performed the Structure Clinical Interview as per the DSM-IV criteria,34 in Anding Hospital (Beijing), the Institute of Mental Health of Peking University (Beijing), and Castle Peak Hospital (Hong Kong). Formally trained researchers conducted neuropsychological testing under the psychiatrists’ supervision. Nonschizophrenia patients meeting criteria for other psychiatric disorders with clear diagnosis, namely mood, anxiety, attention-deficit, and disruptive behaviors, were recruited to the other psychiatric patients group to address specificity issue4 (see table 1). The relative group included unaffected first-degree relatives of schizophrenia patients,17 who were mostly recruited from schizophrenia patients’ caregivers. Volunteers screened with a brief mental status questionnaire were recruited from the local communities for the healthy and SPD groups. Following the screening procedure, (1) healthy participants should not have a family or personal history of mental illness and should score lower than 35 on the Schizotypal Personality Questionnaire (SPQ)35; (2) SPD individuals were identified from community participants who scored within the top tenth percentile on the SPQ, without a diagnosis of any psychiatric disorder.36
Data collected from the majority of the samples have not been included in previous studies.12,36 Ethics Committees of all the participating institutes or hospitals approved the study protocol. Written informed consents were obtained from all participants and all received monetary compensation.
Measures
NSSs were evaluated with the abridged version of the Cambridge Neurological Inventory (CNI),12 which captured participants’ abnormalities in motor coordination (eg, finger opposition, rapid finger tapping; 9 items), sensory integration (eg, finger agnosia, extinction; 8 items), and disinhibition (eg, saccade blink, head movement, mirroring behaviors; 4 items plus another 4 items in the motor coordination subscales). All the items were rated on a dichotomized manner, “absent” (0) or “present” (1), by trained researchers. Item scores were summed up to subscale scores for motor coordination, sensory integration, disinhibition, and a total score of NSSs. A higher score indicates a higher level of abnormality. The inter-rater reliability (intraclass correlation coefficient) and internal consistency (Cronbach’s α) of the subscales and the full-scale were all above 0.85.12
In addition, the abbreviated Chinese version of the Wechsler Adult Intelligence Scale-Revised or the Wechsler Intelligence Scale for Children-Revised was administered to participants who were older or younger than 15 years of age, respectively.37,38 However, due to practical issues, IQ tests were not administered to participants aged over 65. In the healthy group, a large proportion of older adults aged above 55 years (222 out of 246 participants) completed the mini-mental state examination (MMSE, M = 26.11, SD = 3.12), according to established procedures.39 To ensure maximum data inclusion, we transformed the estimated IQ scores and years of education into a composite score of intellect for all participants (see Data Analysis). While we did not exclude participants based on MMSE scores (see Participants section), we analyzed the data with older adults who scored 24 or above in MMSE (less susceptible for cognitive impairment40) separately as summarized in supplementary table S5.
In addition, community participants completed the SPQ35 for further separation of the healthy and SPD groups. All participants also filled out a battery of questionnaires that included general health information, family and personal history of mental and physical disorders, and recent medication, which they (or patients’ caregivers) could choose not to respond. While general health information and history of mental and physical disorders had been used as subject exclusion criteria (see Participants section), lack of medication information was not used as a criterion to exclude subjects. Since we did not have full records of medication information or compliance information from the patients, we did not examine the effect of medication on NSSs (see Discussion for caveats), which could be inferred from first-onset medication-free cases and chronic patients as reported previously in a meta-analysis.10
Data Analysis
Estimated IQ scores and years of education were z-transformed independently and averaged into a composite score of intellect. For missing data in estimated IQ (mostly in elderly individuals), z-scored years of education were used to replace the missing values. The intellect scores were further broken down into quartiles (see table 1). This composite variable is highly correlated with both the raw estimated IQ scores (r = 0.79, P < .0001) and years of education (r = 0.81, P < .0001).
Matching procedures were implemented in Matching R package.41 In exact matching, participants from a non-healthy comparison group were matched with healthy participants who were identical on all covariates, namely age, gender, and intellect. In propensity score matching, participants were matched based on the probability of being in a comparison group conditioned on observed covariates using logistic regression.26,27 We identified an appropriate propensity model (consisting of age, age2, gender, intellect, intellect2, and age × intellect) using stepwise regression42 that included not only the covariates but also their quadratic effects and possible interactions42,43 to account for unobserved variances (see supplementary table S1–S3 for sensitivity analysis44,45 and discussion of potential differences between exact matching and propensity score matching). To avoid poor matches, matched samples were identified within a predefined propensity score radius that was adjusted on a case-by-case basis for each comparison until the 2 groups reached balance on all the covariates as indexed by the standardized difference.26
We considered one-to-one matching without replacement (ie, each healthy participant may be matched only once with another participant in the comparison group) to meet the independence assumption of significance testing.46 Since this approach is sensitive to matching order,45 matching was performed with random data sequences.43 We repeated the random matching procedure 10 001 times and identified the matched order with a median effect size as the representative estimation of group difference. As shown in supplementary figure S1, which summarizes density plots of propensity score distributions for the representative sample of the healthy control group and the comparison groups before and after one-to-one propensity score matching. Matching in general yielded good propensity score overlap between the 2 comparison groups. All matching samples between groups were balanced in predefined covariates, as shown in supplementary figure S2, with both exact matching and propensity score matching procedures. For outcome comparison, since matching might only reduce between-group variance, instead of making the matched samples truly correlated,47,48 the Mann–Whitney U test was used to assess the group difference, and effect size in terms of point-biserial correlation (r) was calculated based on the z values of the test.49,50 Results from paired-sample tests are also available in table 2.
Table 2.
Means (SD) of NSSs Before and After Matching
| Comparison Group | Healthy Group | Independent-Sample Testa | Paired-Sample Testb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | n | Mean | SD | n | P c | r [95% CI] | P c | r [95% CI] | |
| Before matching | ||||||||||
| Schi. | 5.14 | 3.52 | 738 | 4.05 | 3.24 | 1577 | 4.90E−13 | .15 [.11, .19] | ||
| SPD | 3.66 | 2.29 | 256 | .61 | −.01 [−.06, .03] | |||||
| Relatives | 4.35 | 2.96 | 155 | .085 | .04 [−.01, .09] | |||||
| OP | 4.08 | 3.01 | 379 | .46 | .02 [−.03, .06] | |||||
| After exact matching | ||||||||||
| Schi. | 4.89 | 3.37 | 373 | 3.49 | 2.80 | 373 | 3.40E−09 | .22 [.15, .28] | 5.40E−09 | .30 [.21, .39] |
| SPD | 3.59 | 2.23 | 220 | 2.81 | 2.39 | 220 | 8.00E−05 | .19 [.10, .28] | 4.70E−04 | .24 [.11, .36] |
| Relatives | 4.41 | 2.79 | 86 | 3.86 | 3.13 | 86 | .096 | .13 [−.02, .27] | .11 | .17 [−.04, .37] |
| OP | 4.01 | 2.99 | 251 | 3.96 | 3.19 | 251 | .59 | .02 [−.06, .11] | .93 | .01 [−.12, .13] |
| After Propensity Score matching | ||||||||||
| Schi. | 5.15 | 3.44 | 437 | 3.58 | 2.83 | 437 | 2.70E−12 | .24 [.17, .30] | 8.90E−13 | .34 [.26, .42] |
| SPD | 3.65 | 2.30 | 243 | 2.96 | 2.45 | 243 | 3.70E−04 | .16 [.07, .25] | 6.90E−04 | .22 [.09, .33] |
| Relatives | 4.36 | 2.96 | 148 | 3.77 | 2.98 | 148 | .054 | .11 [.00, .22] | .069 | .15 [−.01 .30] |
| OP | 4.03 | 3.09 | 293 | 4.02 | 3.17 | 293 | .77 | .01 [−.07 .09] | .69 | .02 [−.09 .14] |
Note: CI, confidence interval; NSS, neurological soft sign; OP, Other Psychiatric; SPD, schizotypal personality disorders.
aBased on Mann-Whitney U test (Wilcoxon rank-sum test); bBased on Wilcoxon signed-rank test; cThese P values were uncorrected for multiple comparisons due to these following reasons. First, because of 1:1 matching using random data sequence, each comparison might involve different samples. The reported samples are those found to have a medium effect size after 10 001 times of random matching. Second, the current procedure did not capitalize on chance; instead, it reported all results as planned comparison between healthy vs nonhealthy groups.
Lastly, we performed hierarchical multiple regression to delineate age-related characteristics of NSSs in schizophrenia patients and healthy participants. In particular, a negative binomial model was used to take into account over-dispersion of count data.51 In the first step, a linear effect of age was used to predict NSSs, followed by the second step in which we implemented a quadratic age effect model. The third step controlled for the variance contributed by gender, intellect and group category (ie, healthy vs schizophrenia). Lastly, in the fourth step, interaction effects of group category with age, gender, and intellect were included in the full model. All of the models were run with the centered values of age and intellect, whereas gender and group were dummy-coded.
Results
NSSs and Schizophrenia Spectrum Disorders
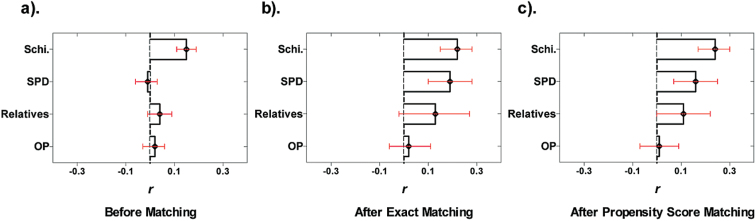
Before controlling for possible covariates, NSSs could already robustly differentiate schizophrenia patients from healthy participants with a moderate effect size (r = .15, 95% confidence interval: [.11, .19], P < .0001), even though they could not reliably differentiate SPD individuals (r = −.01 [−.06, .03], P = .61), unaffected relatives (r = .04 [−.01, .09], P = .085), and other psychiatric patients (r = .02 [−.03, .06], p = .46) from healthy participants (see figure 1). After exact matching on covariates, including age, gender, and intellectual levels, gradient differences of NSSs along the schizophrenia spectrum disorders from the healthy population emerged with r estimated as .22 [.15, .28] (P < .0001), .19 [.10, .28] (P < .0001), and .13 [−.02, .27] (P = .096), respectively, for the schizophrenia patient group, the SPD group, and the relative group, when each was compared to matched healthy controls (also see table 2). In contrast, NSSs could not differentiate other psychiatric patients from healthy participants (r = .02 [−.06, 11], P = .59), with its effect size significantly lower than that between schizophrenia patients and healthy controls (z = 3.52, P < .001). A similar pattern was observed using propensity score matched samples, in which schizophrenia patients, SPD individuals, and unaffected relatives differed from matched healthy controls in NSSs with r estimated as .24 [.17, .30] (P < .0001), .16 [.07, .25] (P < .001), and .11 [.00, .45] (P = .054), respectively. Again, other psychiatric patients did not differ from healthy controls in NSSs (r = .01 [−.07, 09], P = .77), with a significantly lower effect size than that between schizophrenia patients and healthy controls (z = 4.39, P < .0001).
Fig. 1.
Effect sizes (r) of group differences in NSSs in terms of nonhealthy vs healthy group comparisons before (a) and after exact matching (b) and propensity score matching (c). Greater value of r means that the nonhealthy comparison group shows more NSSs compared to the healthy group. Error bars denote 95% confidence intervals (CIs). NSS, neurological soft sign; OP, other psychiatric; Schi., schizophrenia; SPD, schizotypal personality disorders.
We further compared first-episode with chronic schizophrenia patients after controlling for covariates. It showed that the severity of NSSs was comparable between first-episode and chronic schizophrenia patients, for both exactly matched (first-episode: M = 4.68, SD = 3.67, n = 166; Chronic: M = 4.55, SD = 2.93, n = 166; z < 1) and propensity score matched samples (First-episode: M = 4.71, SD = 3.70, n = 214; Chronic: M = 4.73, SD = 2.93, n = 214; z < 1).
Age-Related and Other Individual Differences in NSSs
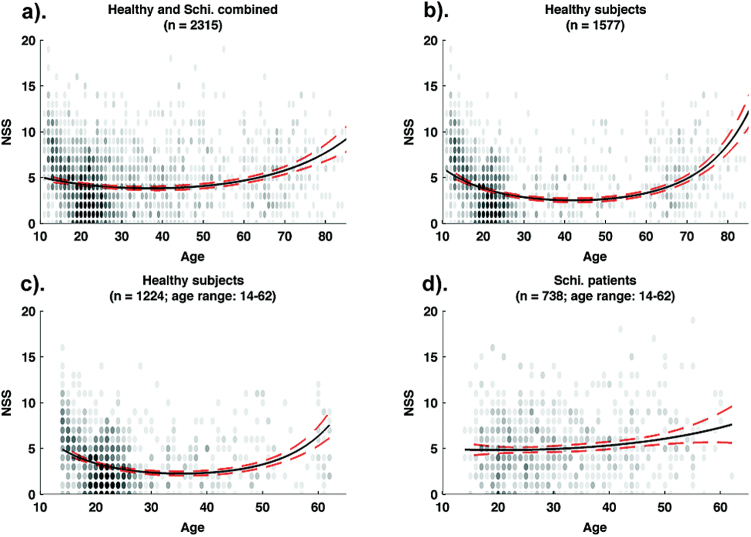
We then examined the scatter plots of chronological age and NSS total scores along with the age-and-NSS fitted curve for healthy and schizophrenia participants in figure 2. Both younger and older participants in the healthy group had more NSSs, which could be captured by a U-shaped function, in contrast to a relatively flat but elevated pattern of NSSs in the schizophrenia group across the lifespan. This observation was supported by the regression results (see table 3). When combining both the healthy and the schizophrenia populations, a quadratic function of age significantly increased model fit (χ2 (1) = 53.56, P < .0001), suggesting that a quadratic function could better describe the relationship between age and NSSs. Such a quadratic age effect remained robust even after controlling for gender, intellect and group effect (Step 3). In Step 4, when the interactions between group and other predictors were taken into account, there was a significant group and age interaction (z = 5.31, P < .0001), suggesting that the relationship between age and NSSs differed between healthy participants and schizophrenia patients. Further separate regression analyses in the healthy and the schizophrenia groups showed that the quadratic age effect only manifested in the healthy group (z = 8.39, P < .0001), but not in schizophrenia patients (|z| < 1). The quadratic age effect in the healthy group (r = .21) was significantly higher than that in the schizophrenia group (r = .03; z = 4.10, P < .0001). This pattern remained even when the age range of the healthy group and the schizophrenia group were matched (see supplementary table S5).
Fig. 2.
Age-and-NSS associations in healthy and schizophrenia samples. (a) The overall relationship between age and NSSs in healthy and schizophrenia samples (n = 2315); (b) The relationship between age and NSSs in the healthy group (n = 1577); (c) the relationship between age and NSSs in the healthy group after matching the age range with the schizophrenia group (n = 1224); (d) the relationship between age and NSSs in schizophrenia group (n = 738). The transparent black dots are actual data points, with the less opacity representing more data points on certain values. The black solid lines represent the negative binomial regression predictions of the quadratic age effect on NSSs, and the surrounding broken red lines indicate 95% confidence intervals (CIs) of the estimations. NSS, neurological soft sign; OP, other psychiatric; SPD, schizotypal personality disorders.
Table 3.
Hierarchical Negative Binomial Regression of NSSs on Age, Gender, Intellect, and Group (Healthy Participants vs Schizophrenia Patients, n = 2135)
| Models and Variables | B | 95% Confidence Interval | e B | z | AIC χ2 (df) Change |
|
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Step1 | ||||||
| Intercept | 1.4777 | 1.4447 | 1.5108 | 4.3828 | 87.63**** | AIC = 11 638 |
| Age | 0.0042 | 0.0024 | 0.0061 | 1.0042 | 4.27**** | |
| Step 2 | ||||||
| Intercept | 1.3607 | 1.3159 | 1.4057 | 3.8990 | 58.83**** | AIC = 11 587 χ2 (1) = 53.56**** |
| Age | −0.0049 | −0.0080 | −0.0019 | 0.9920 | −2.92** | |
| Age2 | 0.0004 | 0.0003 | 0.0005 | 1.0003 | 7.11**** | |
| Step 3 | ||||||
| Intercept | 1.2167 | 1.1529 | 1.2805 | 3.3760 | 38.09**** | AIC = 11 185 χ2 (3) = 407.15**** |
| Age | −0.0087 | −0.0119 | −0.0056 | 0.9913 | −5.40**** | |
| Age2 | 0.0004 | 0.0003 | 0.0005 | 1.0004 | 7.43**** | |
| Gender | −0.0418 | −0.1024 | 0.0188 | 0.9591 | −1.36 | |
| Intellect | −0.2412 | −0.2693 | −0.2132 | 0.7857 | −16.87**** | |
| Group | 0.3247 | 0.2525 | 0.3970 | 1.3836 | 8.95**** | |
| Step 4 | ||||||
| Intercept | 1.1410 | 1.0709 | 1.2103 | 3.1289 | 32.50**** | AIC = 11 107 χ2 (4) = 86.00**** |
| Age | −0.0138 | −0.0175 | −0.0102 | 0.9863 | −7.29**** | |
| Age2 | 0.0005 | 0.0004 | 0.0006 | 1.0005 | 8.17**** | |
| Gender | 0.0223 | −0.0500 | 0.0946 | 1.0226 | 0.60 | |
| Intellect | −0.2977 | −0.3311 | −0.2645 | 0.7425 | −17.61**** | |
| Group | 0.4808 | 0.3781 | 0.5837 | 1.6174 | 9.19**** | |
| Group × age | 0.0188 | 0.0119 | 0.0257 | 1.0189 | 5.31**** | |
| Group × age2 | −0.0004 | −0.0008 | 0.0001 | 0.9996 | −1.69 | |
| Group × gender | −0.1391 | −0.2654 | −0.0128 | 0.8701 | −2.17* | |
| Group × intellect | 0.1932 | 0.1340 | 0.2523 | 1.2131 | 6.41**** | |
Note: The exponentiated coefficient, e B, is a rate ratio of the mean of the dependent variable (ie, ux0+1/ux0) corresponding to a one-unit change in the predictor while holding other predictors constant. That is, when the predictor changes in one unit, the dependent variable has a change rate as e B. When e B is greater (smaller) than 1, it indicates an increase (decrease) of the dependent variable when the predictor increases one unit. All coefficients here are based on the sample of healthy and schizophrenia groups (n = 2315). AIC, Akaike information criterion; NSS, neurological soft sign.
*P < .05; **P < .01; ***P < .001; ****P <. 0001.
As for other individual differences, while gender did not account for much of the variance in NSSs, there was a significant but modest group and gender interaction effect in Step 4 (z = −2.17, P = .030). Specifically, male and female participants in the healthy group seemed to have comparable levels of NSSs (|z| < 1), but male schizophrenia patients (M = 5.36, SD = 3.60) had higher levels of NSSs than female patients (M = 4.74, SD = 3.33). Similarly, there was also a significant interaction effect between group and intellect (z = 6.41, P < .0001). Higher intellect appeared to exert a protective effect in reducing NSSs, which was more apparent in healthy participants than in schizophrenia patients, since an increase of one unit of intellect with other covariates being controlled for would roughly lead to a drop of 26% (1 − e −0.2977) of mean total scores for NSSs in healthy participants, compared to a drop of 10% (1 − e −0.1045) in the schizophrenia group (supplementary table S5).
Discussion
Evidence from 2 different matching procedures supports the endophenotype hypothesis that NSSs are not only sensitive but also specific in distinguishing individuals along the psychosis continuum from healthy individuals. More importantly, consistent with the neurodevelopmental model of schizophrenia, predictions of the lifespan trajectories of NSSs are supported by the findings that schizophrenia patients exhibited elevated levels of NSSs that barely changed across the lifespan, in contrast to a U-shaped age-and-NSS relationship in healthy individuals. To the best of our knowledge, this is the first study that integrates the endophenotype hypothesis of NSSs with the neurodevelopmental model of schizophrenia, providing evidence that NSSs may be neurodevelopmental markers for schizophrenia spectrum disorders.
The first unique contribution of our findings is that it addresses the sensitivity and specificity issues of NSSs in schizophrenia spectrum disorders, which had remained unclear due to methodological issues in previous studies. The effect sizes (r) of NSS abnormalities in schizophrenia spectrum disorders were estimated to be “small-to-medium” based on Cohen’s criteria (.10, .30, and .50, respectively for small, medium, and large effect).52 Such an effect was markedly attenuated in patients with other psychiatric conditions. While NSSs may also manifest in other psychiatric conditions, such as OCD18–20 and mood disorders,21,22 they appear to be able to discriminate schizophrenia patients from healthy individuals, as demonstrated in supplementary table S4. In fact, very often, only patients with co-morbid psychotic disorders exhibited elevated NSSs.53 Although these effects seem modest, our results are consistent with 2 available meta-analyses on NSSs,10,17 which reported r ranging from .19 to .48 for schizophrenia patients vs. healthy controls, and from .24 to .41 for nonpsychotic first-degree relatives vs healthy controls. The fact that these meta-analyses included studies using heterogeneous assessments of NSSs with potential “file drawer” problem54 might have affected their effect size estimation. The present study, using a large sample recruited with the same criteria and assessed with the same NSS instrument, can address these limitations.
The “small-to-medium” effect size52 reported here is important when one considers how many affected individuals may be detected by clinicians based on the relatively simple tool (the CNI) that assesses NSSs, as compared to the more complex measure of IQ scores, especially across the lifespan (see supplementary figure S3). In a binomial effect size display framework,50,55 with r = .15 (Cohen’s d = 0.30, before matching), the success rate of solely using NSSs to detect an individual with psychosis tendency from healthy individuals increases from 42.5% to 57.5%, which is moderate, but not trivial in medical research (see Table 11.8 in Rosenthal and Rosnow,50eg, the effect size (r) of aspirin in treating heart attacks was .03). This success rate would increase further after taking into account covariates such as age, gender, and intellect, as the effect size would increase from .15 to .22 after matching these covariates. It is thus necessary to develop a normative guideline that includes distributions of NSSs conditioned on different demographic variables to enhance the potential clinical utility of NSSs in translational settings complementary to other face-valid symptom assessments.
Another novel contribution of our findings is the delineation of the lifespan trajectory of NSSs in schizophrenia patients and healthy participants. Our findings showed that schizophrenia patients exhibited a flat but overall elevated level of NSSs across the lifespan in contrast to the U-shaped pattern observed in healthy participants (see figure 2). The U-shaped pattern in healthy participants is consistent with the general developmental pattern of cognitive functions and physical development in humans.56,57 Most importantly, these findings support the endophenotype hypothesis of NSSs by linking it with the neurodevelopment hypothesis of schizophrenia3,58,59 that emphasizes the relative stability of neurocognitive functions after illness onset based on both cross sectional60 and longitudinal data.61 Longitudinal epidemiological studies of schizophrenia have shown early developmental adversity imparting increased risk of psychosis in adults with the occurrence of psychotic symptoms preceded by subtle motor and cognitive impairments in childhood and adolescence.62,63 A 30-year longitudinal prospective study further showed that people who developed schizophrenia in their adulthood had relatively delayed infant motor development and impaired executive function, suggesting that disruption of frontocerebellar structures may underlie both the early developmental and adult cognitive abnormalities in schizophrenia.63 Hence, developmental abnormality of this system and its related behavioral manifestation, such as NSSs, may be an endophenotype for schizophrenia.6,15
Our study could also resolve some issues that were less clear in the literature. First, it is not clear whether NSSs become worse as a result of chronicity. In our data, we further compared first-episode with chronic schizophrenia patients matched in age, gender, and intellectual level. We found no significant difference between these 2 groups, which is consistent with previous longitudinal studies32,33 and a recent meta-analysis64 that reported minimal changes in NSSs in chronic patients, in comparison to remitted patients.65 However, the role of antipsychotic medication on NSSs remains unclear, although a previous meta-analysis10 has suggested minimal impact of antipsychotic medications on NSSs. Future research with a trajectory-based neuroimaging approach may reveal a more subtle association between the effect of antipsychotic medication on brain structural alterations66 and the subsequent effect on the behavioral manifestations of NSSs.
Second, our results also clarified the impact of gender and intellect (ie, a combined variable of IQ and education) on NSSs in schizophrenia patients. The data confirm that intellect is negatively associated with NSSs.10 The significant interaction effect of group and intellect suggests that higher intellectual levels may have a protective effect in reducing NSSs, which is more pronounced in healthy participants. In addition, gender differences were found in schizophrenia patients but not in healthy participants, even though the effect was small. This finding is consistent with the cognitive literature,67 but it seems to be at odds with the NSS literature,68 which however tends to be limited by small sample sizes.
One of the caveats of this study is that the difference in NSSs between schizophrenia relatives and healthy participants was less robust compared to other comparison groups. Although first-degree relatives of schizophrenia patients may carry genetic vulnerability towards psychosis, the expression of these genes may be affected by various factors along the course of development.3,24 Thus, relatives may inherently have more variability in the manifestation of schizophrenia-related vulnerability markers, compared with SPD individuals. It is possible that a more reliable effect in the relative group would require a larger sample size. Future research should consider the use of better-controlled twin-study design with a reasonable sample size to investigate the genetic predisposition to the illness. Furthermore, the cross sectional nature of this study precluded the possibility of separating progression from cohort effects, as noted in most large-scale population-based studies,4 even though assessments of NSSs were based on third-person ratings that were less likely to be affected by sociocultural factors. While future longitudinal study is desirable, the unique contribution of a cross sectional study may outweigh its limitations by allowing for recruitment of a larger sample size to gain sufficient statistical power.4
In conclusion, our findings demonstrate the sensitivity and specificity of NSSs in schizophrenia spectrum disorders. Life span profiling reveals an abnormal developmental trajectory of NSSs in schizophrenia patients, relative to healthy participants. Future study should further adopt a combination of behavioral and trajectory-based neuroimaging measures to examine how the variations of NSSs manifest as structural and functional neural abnormalities in schizophrenia patients, from a lifespan perspective, thereby linking behavioral endophenotypes with neurodevelopmental biomarkers to gain a better understanding of the pathogenesis and to permit early detection of schizophrenia spectrum disorders.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Outstanding Young Investigator Award of the National Science Fund China (81088001); the Beijing Training Project for the Leading Talents in S & T (Z151100000315020); the National Basic Research Programme (973 Programme No. 2007CB512302); the Knowledge Innovation Project of the Chinese Academy of Sciences (KSCX2-EW-J-8); the key Laboratory of Mental Health, Institute of Psychology, and the CAS/SAFEA International Partnership Programme for Creative Research Teams (Y2CX131003).
Supplementary Material
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. [DOI] [PubMed] [Google Scholar]
- 2. Kring AM, Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophr Bull. 2008;34:819–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. [DOI] [PubMed] [Google Scholar]
- 4. Gur RC, Calkins ME, Satterthwaite TD, et al. Neurocognitive growth charting in psychosis spectrum youths. JAMA Psychiatry. 2014;71:366–374 [DOI] [PubMed] [Google Scholar]
- 5. Lieberman JA, Dixon LB, Goldman HH. Early detection and intervention in schizophrenia: a new therapeutic model. JAMA. 2013;310:689–690. [DOI] [PubMed] [Google Scholar]
- 6. Chan RC, Gottesman II. Neurological soft signs as candidate endophenotypes for schizophrenia: a shooting star or a Northern star? Neurosci Biobehav Rev. 2008;32:957–971. [DOI] [PubMed] [Google Scholar]
- 7. Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. [DOI] [PubMed] [Google Scholar]
- 8. Heinrichs DW, Buchanan RW. Significance and meaning of neurological signs in schizophrenia. Am J Psychiatry. 1988;145:11–18. [DOI] [PubMed] [Google Scholar]
- 9. Bombin I, Arango C, Buchanan RW. Significance and meaning of neurological signs in schizophrenia: two decades later. Schizophr Bull. 2005;31:962–977. [DOI] [PubMed] [Google Scholar]
- 10. Chan RC, Xu T, Heinrichs RW, Yu Y, Wang Y. Neurological soft signs in schizophrenia: a meta-analysis. Schizophr Bull. 2010;36:1089–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prasad KM, Keshavan MS. Structural cerebral variations as useful endophenotypes in schizophrenia: do they help construct “extended endophenotypes”? Schizophr Bull. 2008;34:774–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chan RC, Wang Y, Wang L, et al. Neurological soft signs and their relationships to neurocognitive functions: a re-visit with the structural equation modeling design. PLoS One. 2009;4:e8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dazzan P, Morgan KD, Chitnis X, et al. The structural brain correlates of neurological soft signs in healthy individuals. Cereb Cortex. 2006;16:1225–1231. [DOI] [PubMed] [Google Scholar]
- 14. Dazzan P, Morgan KD, Orr KG, et al. The structural brain correlates of neurological soft signs in AESOP first-episode psychoses study. Brain. 2004;127:143–153. [DOI] [PubMed] [Google Scholar]
- 15. Zhao Q, Li Z, Huang J, et al. Neurological soft signs are not “soft” in brain structure and functional networks: evidence from ALE meta-analysis. Schizophr Bull. 2014;40:626–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bora E, Fornito A, Radua J, et al. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res. 2011;127:46–57. [DOI] [PubMed] [Google Scholar]
- 17. Chan RC, Xu T, Heinrichs RW, Yu Y, Gong QY. Neurological soft signs in non-psychotic first-degree relatives of patients with schizophrenia: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2010;34:889–896. [DOI] [PubMed] [Google Scholar]
- 18. Bolton D, Gibb W, Lees A, et al. Neurological soft signs in obsessive compulsive disorder: Standardised assessment and comparison with schizophrenia. Behav Neurol. 1998;11:197–204. [DOI] [PubMed] [Google Scholar]
- 19. Hollander E, Kaplan A, Schmeidler J, et al. Neurological soft signs as predictors of treatment response to selective serotonin reuptake inhibitors in obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci. 2005;17:472–477. [DOI] [PubMed] [Google Scholar]
- 20. Jaafari N, Baup N, Bourdel MC, et al. Neurological soft signs in OCD patients with early age at onset, versus patients with schizophrenia and healthy subjects. J Neuropsychiatry Clin Neurosci. 2011;23:409–416. [DOI] [PubMed] [Google Scholar]
- 21. Boks MP, Russo S, Knegtering R, van den Bosch RJ. The specificity of neurological signs in schizophrenia: a review. Schizophr Res. 2000;43:109–116. [DOI] [PubMed] [Google Scholar]
- 22. Boks MP, Liddle PF, Burgerhof JG, Knegtering R, van den Bosch RJ. Neurological soft signs discriminating mood disorders from first episode schizophrenia. Acta Psychiatr Scand. 2004;110:29–35. [DOI] [PubMed] [Google Scholar]
- 23. Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. [DOI] [PubMed] [Google Scholar]
- 24. Bigdeli TB, Neale BM, Neale MC. Statistical properties of single-marker tests for rare variants. Twin Res Hum Genet. 2014;17:143–150. [DOI] [PubMed] [Google Scholar]
- 25. Urbanowitsch N, Degen C, Toro P, Schröder J. Neurological soft signs in aging, mild cognitive impairment, and Alzheimer’s disease - the impact of cognitive decline and cognitive reserve. Front Psychiatry. 2015;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosenbaum PR, Rubin DB. Constructing a control-group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–38. [Google Scholar]
- 27. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 28. Dosenbach NU, Nardos B, Cohen AL, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. [DOI] [PubMed] [Google Scholar]
- 30. Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. [DOI] [PubMed] [Google Scholar]
- 32. Smith RC, Hussain MI, Chowdhury SA, Stearns A. Stability of neurological soft signs in chronically hospitalized schizophrenic patients. J Neuropsychiatry Clin Neurosci. 1999;11:91–96. [DOI] [PubMed] [Google Scholar]
- 33. Chen EY-H, Hui CL-M, Chan RC-K, et al. A 3-year prospective study of neurological soft signs in first-episode schizophrenia. Schizophr Res. 2005;75:45–54. [DOI] [PubMed] [Google Scholar]
- 34. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4 ed Washington, DC: Author; 2000. [Google Scholar]
- 35. Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17:555–564. [DOI] [PubMed] [Google Scholar]
- 36. Chan RC, Wang Y, Zhao Q, et al. Neurological soft signs in individuals with schizotypal personality features. Aust N Z J Psychiatry. 2010;44:800–804. [DOI] [PubMed] [Google Scholar]
- 37. Gong YX, Cai TS. The Handbook of Chinese-Wechsler Intelligence Scale for Children. Changsha, China: Hunan Map Publishing Company; 1993. [Google Scholar]
- 38. Gong YX. Manual of Wechsler Adult Intelligence Scale - Chinese Version. Changsha, China: Chinese Map Press; 1992. [Google Scholar]
- 39. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiat Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 40. Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–935. [DOI] [PubMed] [Google Scholar]
- 41. Sekhon JS. Multivariate and Propensity Score Matching Software with automated balance optimization: the matching package for R. J Stat Softw. 2011;42:1–52. [Google Scholar]
- 42. Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516–524. [Google Scholar]
- 43. Dehejia RH, Wahba S. Propensity score-matching methods for nonexperimental causal studies. Rev Econ Stat. 2002;84:151–161. [Google Scholar]
- 44. Keele L.An overview of rbounds: An R package for Rosenbaum bounds sensitivity analysis with matched data. http://www.personal.psu.edu/ljk20/rbounds%20vignette.pdf Published August 20, 2010. Accessed March 29, 2015.
- 45. Rosenbaum PR. Observational Studies. 2nd ed New York, NY: Springer; 2002. [Google Scholar]
- 46. Pan W, Bai H. Propensity Score Analysis: Fundamentals and Developments. New York, NY: The Guilford Press; 2015. [Google Scholar]
- 47. Stuart EA. Matching methods for causal inference: A review and a look forward. Stat Sci. 2010;25:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schafer JL, Kang J. Average causal effects from nonrandomized studies: a practical guide and simulated example. Psychol Methods. 2008;13:279–313. [DOI] [PubMed] [Google Scholar]
- 49. Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141:2–18. [DOI] [PubMed] [Google Scholar]
- 50. Rosenthal R, Rosnow RL. Essentials of Behavioral Research: Methods and Data Analysis. New York, NY: McGraw-Hill; 2008. [Google Scholar]
- 51. Gardner W, Mulvey EP, Shaw EC. Regression analyses of counts and rates: Poisson, overdispersed Poisson, and negative binomial models. Psychol Bull. 1995;118:392–404. [DOI] [PubMed] [Google Scholar]
- 52. Cohen J. A power primer. Psychol Bull. 1992;112:155–159. [DOI] [PubMed] [Google Scholar]
- 53. Zhao Q, Ma YT, Lui SS, et al. Neurological soft signs discriminate schizophrenia from major depression but not bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:72–78. [DOI] [PubMed] [Google Scholar]
- 54. Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull. 1979;86:638–641. [Google Scholar]
- 55. Rosenthal R. How are we doing in soft psychology? Am Psychol. 1990;45:775–777. [Google Scholar]
- 56. Craik FI, Bialystok E. Cognition through the lifespan: mechanisms of change. Trends Cogn Sci. 2006;10:131–138. [DOI] [PubMed] [Google Scholar]
- 57. Huizinga M, Dolan CV, van der Molen MW. Age-related change in executive function: developmental trends and a latent variable analysis. Neuropsychologia. 2006;44:2017–2036. [DOI] [PubMed] [Google Scholar]
- 58. Weinberger DR. On the plausibility of “the neurodevelopmental hypothesis” of schizophrenia. Neuropsychopharmacol. 1996;14(3 suppl):1S–11S. [DOI] [PubMed] [Google Scholar]
- 59. Murray RM, O’Callaghan E, Castle DJ, Lewis SW. A neurodevelopmental approach to the classification of schizophrenia. Schizophr Bull. 1992;18:319–332. [DOI] [PubMed] [Google Scholar]
- 60. Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218. [DOI] [PubMed] [Google Scholar]
- 61. Censits DM, Ragland JD, Gur RC, Gur RE. Neuropsychological evidence supporting a neurodevelopmental model of schizophrenia: a longitudinal study. Schizophr Res. 1997;24:289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344:1398–1402. [DOI] [PubMed] [Google Scholar]
- 63. Ridler K, Veijola JM, Tanskanen P, et al. Fronto-cerebellar systems are associated with infant motor and adult executive functions in healthy adults but not in schizophrenia. Proc Natl Acad Sci USA. 2006;103:15651–15656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chen EY, Kwok CL, Au JW, Chen RY, Lau BS. Progressive deterioration of soft neurological signs in chronic schizophrenic patients. Acta Psychiatr Scand. 2000;102:342–349. [DOI] [PubMed] [Google Scholar]
- 65. Bachmann S, Degen C, Geider FJ, Schrà der J. Neurological soft signs in the clinical course of schizophrenia: results of a meta-analysis. Front Psychiatry. 2014;5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lesh TA, Tanase C, Geib BR, et al. A multimodal analysis of antipsychotic effects on brain structure and function in first-episode schizophrenia. JAMA Psychiatry. 2015;72:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shtasel DL, Gur RE, Gallacher F, Heimberg C, Gur RC. Gender differences in the clinical expression of schizophrenia. Schizophr Res. 1992;7:225–231. [DOI] [PubMed] [Google Scholar]
- 68. Chen EY-H, Chan RCK. The Cambridge neurological inventory: Clinical, demographic, and ethnic correlates. Psychiatric Ann. 2003;33:202–210. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.