Abstract
Apathy, described as impaired motivation and goal-directed behavior, is a common yet often overlooked multidimensional psychopathological state in schizophrenia. Its underlying cognitive processes remain largely unexplored. Data was drawn from a longitudinal hospital study of patients with a DSM-IV diagnosis of schizophrenia; 137 (82.5%) participated at the 1-month follow-up and 81 (59.1%) at the 1-year follow-up. Apathy was assessed with the Lille Apathy Rating Scale, validated in French and in schizophrenia. Severe apathy, overall (total score > −13) and on 4 previously identified distinct dimensions, was considered. Episodic verbal learning was assessed with the California Verbal Learning Test, executive functioning with the Trail Making Test, the Six Element Test and the Stop Signal Paradigm and working memory with the Letter-Number Sequencing Test. After controlling for confounding variables, only episodic verbal learning was associated with severe overall apathy in the cross-sectional study. At 1 year, working memory was associated with an increased risk of severe overall apathy, adjusting for baseline apathy. Using a dimensional approach to apathy, specific types of cognition were found to be associated with specific dimensions of apathy. Our findings confirm the need for a multidimensional approach of negative symptoms in schizophrenia. Moreover, cognitive functioning could be a risk factor for developing severe apathy. Cognitive remediation may thus be a useful non-pharmacological intervention for treating apathy in schizophrenia patients.
Key words: apathy, cognition, longitudinal study, negative symptoms
Introduction
Apathy is a transnosographic psychopathological state affecting 53% of individuals with a diagnosis of schizophrenia,1 and contributing to poorer functioning2 and subjective quality of life in both first episode and chronic schizophrenia.3–5 Apathy, considered as a core component of negative symptoms6,7 can be defined as a quantitative reduction of voluntary goal-directed behaviors,8 and outside the schizophrenia literature has been the subject of fundamental research interest in neurological disorders such as Parkinson disease, brain injury and Alzheimer Disease.1,9 In spite of the strong negative impact of apathy related symptoms in schizophrenia,7 treatment studies for this frequent behavioral condition have provided limited evidence of efficiency.10 One reason for this could be the simplistic conception of apathy, frequently considered as a unitary syndrome. However, there is now evidence that apathy can no longer be considered as a unique construct but rather as a multidimensional and multifaceted psychopathological state comprising cognitive, emotional, behavioral and self-initiation components, with different underlying psychological, biological or environmental processes.8,11
Among psychological processes frequently involved in apathy, cognitive impairment appears to be a crucial factor in the maintenance and the onset of apathy. Several studies have evaluated the association between different types of cognitive functions and apathy, such as in traumatic brain injury,12 disorders involving the basal ganglia,13,14 or Alzheimer’s disease.15
In contrast to neurological disorders, the underlying cognitive processes associated with apathy in schizophrenia are poorly understood. A previous small-scale study found that chronic patients with high levels of apathy had poorer visuomotor sequencing, verbal learning and memory and lower performance IQ.16 In this same study, both groups, with lower and higher levels of apathy respectively, performed poorly on psychomotor speed and naming, and had lower verbal and full-scale IQ scores. One study on first episode psychosis replicated this finding of a significant relationship between apathy and executive functions, despite using different measures both for apathy and executive functions.17 In 2011, Konstantakopoulos et al3 found a correlation between apathy and executive function impairments but no correlation with verbal memory in schizophrenia patients. Interestingly, these findings were unrelated to level of depression or overall severity of psychopathology. We must also mention that the relationship between amotivation and cognition has been explored in several past studies. Effort has been shown to be a mediator of the relationship between motivation and cognition18 adding evidence that cognition is affected by impairment in motivation in schizophrenia patients.19,20
However, previous studies that have explored the relationships between cognition and apathy have several important limitations. First, the different tools currently used to measure apathy in schizophrenia are either non specific such as the Positive and Negative Syndrome Scale,21 the Scale to Assessment of Negative Symptoms,22 the Brief Negative Symptom Scale23 or, although specific to measuring apathy in this multidimensional construct, have not been validated in schizophrenia (ie, the Apathy Evaluation Scale24). Second, these apathy scales do not have validated cut-off points representing clinically significant apathy. Finally, past studies are mainly cross-sectional, which limits inferences of causality.
Consequently, the primary objective of this study was to explore the relationships between the different facets of apathy and cognition by using a validated apathy scale for schizophrenia. The secondary objective was to describe the cognitive determinants associated with severe apathy over an 11-month follow-up.
To address these 2 issues, we conducted an 11-month longitudinal investigation of the relationship between cognition and apathy in schizophrenia patients. We used the Lille Apathy Rating Scale,25 recently validated in schizophrenia patients in French,26 which proposes composite subscores for 4 different domains of apathy and an empirically validated cut-off point for clinically significant overall apathy only. In addition we controlled for key variables shown to be associated with negative symptoms (ie, depression, psychotic symptoms, medication), and partly neglected in previous studies in schizophrenia.27
Materials and Methods
Study Design
Participants with schizophrenia diagnosis were recruited from full- and part-time hospitalization and ambulatory care services of the Departments of Adult Psychiatry in Montpellier, Marseille, and Nice. Diagnosis of schizophrenia was established via the Structured Clinical Interview for DSM-IV (SCID-I)28 by the treating psychiatrist. Patients were aged between 18 and 60 years old and had to understand, talk and read French. Exclusion criteria were: (a) known neurological disease, (b) brain injuries, or (c) Axis II diagnosis of developmental disorders. Participants were followed-up at 1, 3, 6, and 12 months. They were tested individually in 1-hour sessions at inclusion, 3 and 6 months and more extensively in 3-hour sessions at 1 and 12 months. Written informed consent was obtained from all participants and the local ethical committee approved the protocol.
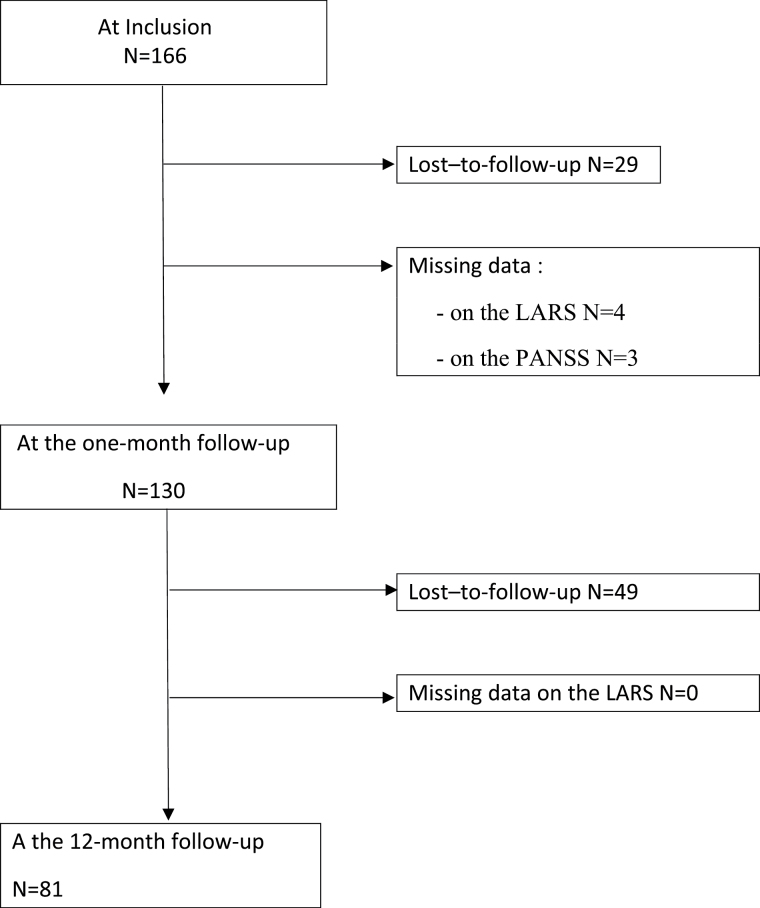
In all, 166 patients with schizophrenia (77% male) aged between 19 and 59 years (median age: 37) completed the study between January 2011 and December 2012. The current study was carried out on the 1-month and 12-month follow-up data; 137 (82.5%) participated at the 1-month follow-up and 81 patients (59.1%) at both follow-ups (see figure 1 flow chart of the recruitment and follow-up process).
Fig. 1.
Flow chart of the recruitment and follow-up process.
Apathy
The Lille Apathy Rating Scale25 (LARS) was administered at inclusion, at the 1-month and at the 1-year follow-up. The LARS is a subjective semi-structured interview assessing specific components of apathy, including 33 items, divided into 9 domains (ie, Everyday Productivity [EP], Interests [INT], Taking Initiative [INI], Novelty Seeking [NS], Voluntary Actions [VA], Emotional Responses [ER], Concern [C], Social Life [SL], and Self-Awareness [SA]). The LARS has been validated in schizophrenia by Yazbek et al.26 The factorial analysis revealed 4 distinct dimensions: Novelty and Social Life (NSL = NS + SL items), Behavioral Involvement (BI = INI + VA items), Emotional Involvement (EI = ER item) and Judgment Skills (JS = INT + SA items). As discussed elsewhere,26 the Novelty and Social Life dimension of the LARS corresponds to the cognitive dimension of apathy, while Behavioral Involvement refers to behavioral apathy, and Emotional Involvement to emotional apathy. The Judgment Skills dimension is specific to the LARS and is not a dimension of apathy considered by the neuroanatomical model of Levy & Dubois8 for example. This dimension refers to a particular manifestation of apathy highlighted by Stuss29 who considered self and social awareness as a metacognitive ability, necessary to mediate information from a personal, social past and current history with projections to the future.
The items are presented as positively worded questions to which the subject is expected to answer clearly “yes” or “no,” in order to reduce subjective interpretations as much as possible (eg, “When you have to go to an appointment, a meeting or a formal occasion, do you have to be told to get yourself ready?”). With the exception of the first 3 questions (which are coded on a 5-point Likert-type scale), responses are coded by the clinician on a binary (yes/no) scale, with an additional “NA” (not available) condition for non-classifiable answers or nonapplicable items. Finally, the scale was designed in such a way that each of the 9 domains can be evaluated through subscales, which contribute with equal weighting to the global score. Hence the global score ranges from −36 to +36, with a higher score representing a greater degree of apathy.
Cognitive Assessment
Cognitive functioning was assessed at the 1-month and 12-month follow-ups.
Episodic verbal learning was evaluated using the California Verbal Learning Test (CVLT).30 For the purpose of the study we used the total number of words recalled during the first trial of the words in list A as this is considered as a more dynamical assessment of learning potential.31 Higher number of words recalled reflects better memory.
Cognitive flexibility was evaluated using the Trail Making Test (TMT).32 The study variable was the TMT B-A score calculated as the difference between TMT-B and TMT-A times (in seconds), which is considered as a measure of cognitive flexibility relatively independently of manual dexterity.33 A higher score reflects poorer cognitive flexibility.
Multitasking was assessed with the Modified Six Elements Test (MSET)34 The MSET requires the individual to devise a simple plan, schedule the subtests efficiently, and keep track of the time. The dependent variable was the mean profile score, an index based on the number of tasks attempted and the number of rule breaks.35 A higher mean score reflects better multitasking ability.
Inhibition was assessed with the Stop Signal Paradigm (SSP).36 In the SSP, participants perform a go task, such as reporting the identity of a stimulus. Occasionally, the go stimulus is followed by a stop signal, which instructs subjects to withhold the response. Participants completed a total of 80 stop trials and 20 go trials. A stop-signal delay of 350ms was used, because typically the poorest response inhibition occurs at the 350-millisecond Stop Signal because the response initiation process has progressed to the point where a response will occur prior to being averted to the inhibition process. The duration of the task was approximately 15 minutes. A higher percentage inhibition reflects better inhibition capacity.36
Working memory was assessed using the Letter-Number Sequencing subtest (LNS) of the Wechsler Adult Intelligence Scale-III (WAIS-III).37 LNS constitutes a good predictor of fluid intelligence and strongly correlates with laboratory working memory measures.38 The mean score was retained for the analysis. A higher score reflects better working memory capacity.
Clinical Variables
Symptom severity was measured using the Positive and the Negative Syndrome Scale (PANSS),39 a 30-item rating scale Assessments are conducted on a 7-point rating scale (1 = absent, 7 = extreme). For this study, 4 of the analytically derived PANSS factor component scores were taken into account: Total, General Psychopathology, Positive and Negative.
Depression was evaluated at the 1-month follow-up using the Calgary Depression scale for Schizophrenia (CDSS).40 A cut-off score of 6 has been proposed to identify patients considered clinically depressed.41
Except for the SCID-I, patients were assessed by trained clinical psychologists who rated the LARS and other clinical scales after a unique clinical interview.
Statistical Analysis
Severe total apathy was defined according to Yazbek et al’s validated cut-off values26: patients with scores > −13 were considered as severely apathetic and compared to non-apathetic and moderately apathetic patients with scores ≤ −13. The threshold for severe apathy had been defined in order to exclude the healthy controls from this group.
For the 4 LARS dimensions, no validated cut-off values for severe apathy were provided given the limited number of response categories and the consequent overlap with scores of healthy control subjects. Given that all 4 distributions were skewed according to the Shapiro-Wilk test, we thus chose to divide scores for each dimension into terciles. We defined patients in the highest tercile as being apathetic for the given dimension. Although this cut-off threshold corresponded best in terms of proportion of apathetic patients to the validated threshold for severe total apathy, between 9.8% (for the Behavioral Involvement dimension) and 31.3% (for the Emotional Involvement dimension) of healthy control subjects are included in the highest tercile and would thus be considered apathetic.
The cognitive scores were analyzed as continuous variables, since linear relationships between scores and apathy were observed.
Firstly, a cross-sectional analysis was carried out at the 1-month follow-up at which cognitive functioning was assessed. Univariate logistic regression models were constructed showing the associations between the cognitive tests and apathy (total apathy and the 4 dimensions), adjusted for age entered as a continuous variable after testing for linearity (Model 0).
We then tested various known potential confounders of apathy with logistic regression analysis adjusted for age: gender, educational level at inclusion (university degree or less), current abuse or dependence for substance at inclusion (Yes/No), and at the 1-month follow-up: depression, Chlorpromazine equivalence (mg), and schizophrenia symptomatology (positive, negative, general psychopathology and total PANSS scores). Five sets of logistic regression analyses were run: for severe overall apathy and for each of the 4 subscales. Variables with a P-value < .10 for at least 1 of the 5 outcomes were retained for the multivariate analyses. Although chlorpromazine reached significance for 1 outcome (Novelty and Social Life dimension, P = .04), we decided not to include it in our multivariate models because of a high number of missing values (n = 15). We constructed models adjusting for age, educational level and PANSS total score (Model 1), and further adjusting for depression (Model 2). Bonferroni correction for multiple testing was not performed as this was not considered necessary.42
Secondly, a longitudinal logistic regression analysis was performed to study the effect of cognitive performance at 1-month on severe apathy (overall and for the 4 subscales) at the 11-month follow-up. The same approach with the same set of potential confounding variables was adopted as for the cross-sectional analysis. None of the variables reached the significance threshold. In addition, for severe overall apathy we adjusted for the 1-month overall apathy score and for each of the 4 subscales we adjusted for the 1-month subscale score (table 2). We adjusted for the 1-month apathy scores as continuous variables in order to control for the actual level of apathy rather than the classification as severe or not.
Table 2.
Cross-Sectional Analysis Between Apathy and Cognitive Performance at 1-Month Follow-up (Multivariate Logistic Regression)
| N/n | Model 0 | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|---|
| OR [95% CI] | P | OR [95% CI] | P | OR [95% CI] | P | ||
| Total apathy score ([−12; +36]) | |||||||
| MSET profile | 114/26 | 1.01 [0.76; 1.34] | .96 | 1.09 [0.80; 1.48] | .57 | 1.11 [0.81; 1.52] | .53 |
| CVLT: List A Trial 1 | 111/25 | 0.76 [0.60; 0.96] | .02 | 0.78 [0.61; 0.99] | .04 | 0.76 [0.59; 0.97] | .03 |
| TMT B–A | 109/27 | 1.11 [1.01; 1.20] | .02 | 1.07 [0.96; 1.06] | .22 | 1.05 [0.95; 1.17] | .35 |
| LNS | 111/26 | 0.93 [0.86; 1.01] | .07 | 0.95 [0.87; 1.04] | .30 | 0.95 [0.87; 1.05] | .31 |
| SSP | 100/20 | 0.98 [0.96; 1.01] | .17 | 0.98 [0.96; 1.01] | .15 | 0.99 [0.96; 1.01] | .23 |
| Novelty and social life (NSL) | |||||||
| MSET profile | 114/33 | 0.92 [0.70; 1.20] | .53 | 0.98 [0.74; 1.30] | .88 | 0.99 [0.74; 1.32] | .92 |
| CVLT: List A Trial 1 | 111/33 | 0.86 [0.71; 1.06] | .15 | 0.90 [0.73; 1.11] | .34 | 0.90 [0.73; 1.11] | .32 |
| TMT B–A | 109/32 | 1.11 [1.02; 1.21] | .01 | 1.10 [1.00; 1.21] | .06 | 1.09 [0.99; 1.21] | .08 |
| LNS | 111/34 | 0.89 [0.82; 0.96] | .005 | 0.88 [0.81; 0.98] | .013 | 0.89 [0.81; 0.98] | .014 |
| SSP | 100/26 | 0.99 [0.97; 1.01] | .39 | 0.99 [0.97; 1.01] | .39 | 0.99 [0.97; 1.01] | .45 |
| Behavioral involvement (BI) | |||||||
| MSET profile | 114/32 | 0.93 [0.71; 1.22] | .62 | 1.01 [0.76; 1.34] | .94 | 1.02 [0.76; 1.37] | .9 |
| CVLT: List A Trial 1 | 111/28 | 0.74 [0.59; 0.93] | .01 | 0.77 [0.61; 0.97] | .03 | 0.76 [0.59; 0.96] | .019 |
| TMT B–A | 109/33 | 1.18 [1.08; 1.29] | .0004 | 1.14 [1.03; 1.26] | .01 | 1.13 [1.02; 1.25] | .02 |
| LNS | 111/30 | 0.92 [0.85; 0.99] | .03 | 0.94 [0.86; 1.03] | .17 | 0.94 [0.86; 1.03] | .17 |
| SSP | 100/26 | 0.99 [0.98; 1.02] | .92 | 1.00 [0.98; 1.02] | .85 | 1.00 [0.98; 1.03] | .89 |
| Emotional involvement (EI) | |||||||
| MSET profile | 114/47 | 0.87 [0.68; 1.12] | .28 | 0.90 [0.70; 1.17] | .44 | 0.91 [0.70; 1.17] | .45 |
| CVLT: List A Trial 1 | 111/44 | 0.89 [0.74; 1.07] | .21 | 0.91 [0.76; 1.11] | .35 | 0.91 [0.75; 1.10] | .32 |
| TMT B–A | 109/44 | 1.03 [0.95; 1.11] | .52 | 1.00 [0.91; 1.09] | .94 | 1.00 [0.91; 1.09] | .91 |
| LNS | 111/45 | 1.07 [1.00; 1.14] | .06 | 1.12 [1.03; 1.22] | .006 | 1.13 [1.04; 1.23] | .005 |
| SSP | 100/37 | 1.01 [0.99; 1.03] | .61 | 1.01 [0.99; 1.03] | .62 | 1.01 [0.99; 1.03] | .46 |
| Judgment skills (JS) | |||||||
| MSET profile | 114/33 | 0.72 [0.54; 0.95] | .02 | 0.76 [0.57; 1.02] | .06 | 0.74 [0.55; 0.99] | .045 |
| CVLT: List A Trial 1 | 111/32 | 0.97 [0.80; 1.17] | .73 | 1.03 [0.84; 1.26] | .77 | 1.06 [0.86; 1.30] | .61 |
| TMT B–A | 109/32 | 1.13 [1.04; 1.23] | .006 | 1.09 [1.00; 1.20] | .08 | 1.11 [1.00; 1.22] | .049 |
| LNS | 111/31 | 0.94 [0.88; 1.02] | .13 | 0.99 [0.91; 1.08] | .88 | 0.99 [0.91; 1.08] | .84 |
| SSP | 100/30 | 1.00 [0.98; 1.02] | .85 | 1.00 [0.98; 1.02] | .9 | 1.00 [0.98; 1.02] | .81 |
Note: CI, confidence interval. Bold values: The level of significance was set to P < .05. Model 0: adjusting for age / Model 1: adjusting for age, educational level and PANSS total score /Model 2: adjusting for age, educational level and PANSS total score and depression.
The analyses were performed using SAS software (version 9.2, SAS Institute Inc).
Results
Sample Description
The cross-sectional analysis was carried out on 130 patients with follow-up data at 1-month and no missing values for the main covariates. These patients did not differ significantly from those excluded (missing data or lost-to-follow-up) on the main sociodemographic, cognitive and clinical variables. Patients in the longitudinal analysis with data on apathy at the 1-year follow-up (N = 81) differed only from those lost to follow-up on the MSET, with a lower rank score (P = .008).
The sample (N = 130) is described in table 1, at inclusion for the main sociodemographic variables and at the 1-month follow-up for clinical variables.
Table 1.
Sample Description
| At Inclusion | N | %a | |
|---|---|---|---|
| Sex | Male | 130 | 76.92 |
| Age (median, min-max) | 130 | 37.18 (19–59) | |
| Age at 1st hospitalization (median, min-max) | 125 | 22.85 (15–57) | |
| Duration of illness (median, min-max) | 128 | 14.13 (0–41) | |
| Study center | Marseille | 31 | 23.85 |
| Nice | 20 | 15.38 | |
| Montpellier | 79 | 60.77 | |
| Educational level | Higher education | 130 | 21.54 |
| Marital status | Single | 130 | 91.54 |
| Live alone | Yes | 130 | 46.92 |
| Hospitalization | In-patient | 130 | 53.85 |
| Current smoker | Yes | 130 | 67.69 |
| Substance abuse or dependence | Yes | 129 | 49.61 |
| Daily chlorpromazine equivalent (CPZeq) drug dosage (mg/d) (median, min-max) | 111 | 612.5 (0–4250) | |
| Body mass index | Normal | 60 | 46.88 |
| Overweight | 40 | 31.25 | |
| Obesity | 28 | 21.88 | |
| At the 1-month follow-up | |||
| Depression (Calgary score) | ≥6 | 130 | 23.08 |
| PANSS scores (median, min-max) | |||
| Total score | 130 | 66 (37–106) | |
| Positive symptoms | 130 | 13 (7–31) | |
| Negative symptoms | 130 | 18 (8–35) | |
| General psychopathology | 130 | 34 (19–52) | |
| LARS apathy scores (median, min-max) | |||
| Total apathy | 130 | −19 (−33 to 5) | |
| Novelty and social life (NSL) | 130 | −5 (−8 to 5) | |
| Behavioral involvement (BI) | 130 | −4 (−8 to 6) | |
| Emotional involvement (ER) | 130 | −2 (−3 to 3) | |
| Judgment skills (JS) | 130 | −4 (−6 to 3) | |
| Apathy classification (Yazbek26) | |||
| None [−36; −24] | 31 | 23.85 | |
| Mild [−23; −19] | 40 | 30.77 | |
| Moderate [−18; −13] | 30 | 23.08 | |
| Severe [−12; +36] | 29 | 22.31 | |
| Cognitive tests (median, min-max) | |||
| MSET | 114 | 4 (1–6) | |
| CVLT: List A Trial 1 | 111 | 6 (0–14) | |
| TMT B-A | 109 | 60 (14–215) | |
| LNS | 111 | 32 (6–37) | |
| SSP | 100 | 70 (20–100) | |
Note: MSET, Modified Six Element Test; CVLT, California Verbal Learning Test; TMT, Trail Making Test; LNS, Letter-Number Sequencing subtest; SSP, Stop Signal Paradigm; PANSS, Positive and the Negative Syndrome Scale; LARS, Lille Apathy Rating Scale.
aOr median (min max) when specified.
Among the 81 patients with both the 1-month and 1-year follow-up examinations, 18 (22.22%) had severe overall apathy at baseline and 11 (13.58%) at the 1-year follow-up, of which 7 (63.63%) were ongoing or recurrent cases and 4 (36.36%) new cases of severe apathy.
Cross Sectional Association Between Cognition and Apathy
Whatever the level of adjustment, we found significant relationships between the CVLT score and severe overall apathy. The risk of overall severe apathy decreased with an increasingly good performance at the CVLT score (Model 2: OR 0.76, 95% CI: 0.59–0.97 per unit increase in score; P = .03).
Regarding the 4 different apathy dimensions, a significant relationship was observed between the LNS and Novelty and Social Life apathy in Model 2 with a decreased risk of apathy with better test scores (OR 0.89, 95% CI: 0.81–0.98; P = .014). For the Behavioral Involvement dimension, there was a significant association with CVLT, with a lower risk of apathy among patients with a higher score (Model 2: OR 0.76, 95% CI: 0.59–0.96; P = .019), and with TMT with the risk increasing as the time difference between the tests increased (Model 2: OR 1.13, 95% CI: 1.02–1.25; P = .02).
The Emotional Involvement dimension was significantly and positively associated with working memory (Model 2: OR 1.13, 95% CI: 1.04–1.23; P = .005). For the Judgment Skills dimension we found significant associations with Multitasking (Model 2: OR 0.74, 95% CI: 0.55–0.99; P = .045) and cognitive flexibility (Model 2: OR 1.11, 95% CI: 1.00–1.22; P = .049).
Longitudinal Association Between Cognition and Apathy
Adjusting for the 1-month apathy score the LNS was significantly associated with severe overall apathy at 1 year; the risk of severe apathy decreased with an increase in LNS score (OR 0.82, 95% CI: 0.70–0.98 per unit increase in score; P = .025; table 3).
Table 3.
Longitudinal Analysis Between Apathy at 1 Year and Cognitive Performance at 1 Month (Multivariate Logistic Regression)
| N/n | OR [95% CI] | P | |
|---|---|---|---|
| Total apathy score (> −13) | |||
| MSET profile | 74/10 | 1.12[0.70; 1.78] | .64 |
| CVLT: List A Trial 1 | 77/10 | 0.90[0.65; 1.26] | .56 |
| TMT B–A | 72/9 | 1.02[0.86; 1.20] | .84 |
| LNS | 73/11 | 0.82[0.70; 0.98] | .025 |
| SSP | 70/8 | 0.97[0.94; 1.01] | .15 |
| Novelty and social life (NSL) | |||
| MSET profile | 74/21 | 1.06 [0.72; 1.57] | .75 |
| CVLT: List A Trial 1 | 77/22 | 0.81 [0.61; 1.07] | .14 |
| TMT B–A | 72/19 | 0.99 [0.98; 1.00] | .37 |
| LNS | 73/21 | 1.05 [0.94; 1.17] | .39 |
| SSP | 70/21 | 1.00 [0.97; 1.03] | .90 |
| Behavioral involvement (BI) | |||
| MSET profile | 74/13 | 0.74 [0.48; 1.14] | .17 |
| CVLT: List A Trial 1 | 77/14 | 0.82 [0.61; 1.11] | .20 |
| TMT B–A | 72/13 | 1.00 [0.99; 1.02] | .62 |
| LNS | 73/13 | 0.91 [0.79; 1.04] | .17 |
| SSP | 70/11 | 1.01 [0.98; 1.04] | .57 |
| Emotional involvement (EI) | |||
| MSET profile | 74/31 | 1.21 [0.88; 1.67] | .25 |
| CVLT: List A Trial 1 | 77/32 | 1.06 [0.87; 1.31] | .55 |
| TMT B–A | 72/30 | 1.00 [0.99; 1.01] | .41 |
| LNS | 73/30 | 1.09 [0.99; 1.20] | .08 |
| SSP | 70/32 | 1.02 [0.99; 1.04] | .15 |
| Judgment skills (JS) | |||
| MSET profile | 74/19 | 1.02 [0.72; 1.44] | .91 |
| CVLT: List A Trial 1 | 77/21 | 0.87 [0.69; 1.10] | .26 |
| TMT B–A | 72/19 | 1.00 [0.99; 1.01] | .73 |
| LNS | 73/19 | 0.99 [0.90; 1.09] | .83 |
| SSP | 70/19 | 1.01 [0.99; 1.04] | .30 |
Discussion
The 2 main aims of our study were to (a) explore the relationships between cognition and the different facets of apathy as measured by the Lille Apathy Rating Scale (LARS) in a relatively large sample of schizophrenia patients and (b) explore the association between cognition and severe apathy after an 11-month follow up.
First, using a dimensional approach of apathy, we found that the sub-domains of cognition used in our study were each associated with specific facets of apathy. To our knowledge, this is the first study to consider the apathy dimensions separately and explore their links with distinct cognitive domains in schizophrenia. If our results replicate the associations found in previous studies in schizophrenia patients between apathy, working memory, episodic verbal learning and executive functioning,16,17 they also shed new light on the specificity of these associations. Indeed, contrary to previous studies in schizophrenia that have considered apathy as a unitary syndrome, we found a differential influence of neuropsychological performance on the distinct dimensions of apathy symptoms in our schizophrenia group. More precisely, our results suggest that the cognitive dimension Novelty and Social Life of the LARS, representing low interest in novelty as well as a drop in the perceived need for knowledge, was negatively associated with working memory in schizophrenia patients. The pursuit of a new goal requires the effortful control of behavior, in which working memory is crucial notably in the setting of interfering processes34,43 and distractions and goal maintenance.44,45 Thus, impaired working memory could lead schizophrenia patients to a preference for routines with less effortful demand and consequently a reduction in the interest for new daily life activities. The dimension Behavioral Involvement corresponding to the behavioral facet of apathy (ie, low everyday productivity and lack of initiative) was specifically associated with episodic verbal learning and cognitive flexibility. Cognitive flexibility implies the ability to modify and adjust behavior while taking the environment and the consequences of actions into account.34,46,47 Most of our daily activities are complex and cognitively multidetermined, and take place in unstructured or novel contexts, in which the person him/herself must take the initiative to do something, carry out and modify a plan of action. Disturbance in cognitive flexibility could lead patients incapable of flexibly to modify intentions as a function of environmental stimuli and demands and “in fine” induce schizophrenia patients to drop their current action, leading to an increase of behavioral apathy.48
The Emotional Involvement dimension (ie, blunting of emotional responses and lack of concern) was associated specifically with working memory. Working memory can influence subjective emotional experience and evaluative response in healthy subjects and is involved in the maintenance of emotions to guide behavior.49 However its influence on motivation has been poorly studied in schizophrenia.50 Our result is unexpected and suggests that good working memory goes hand in hand with reduced emotional responses toward external emotional stimuli. This is further supported by a similar trend observed in the longitudinal analysis. However we must be cautious in interpreting this finding as no causal relationship can be established. It must be noted that the restricted sample size did not allow us to remove patients with apathy (here Emotional Involvement dimension) at baseline. Rather we adjusted for the apathy score for the given dimension. It is possible that Emotional Involvement apathy impacts positively on working memory as well as the opposite. One could imagine that a patient blunted to emotional stimuli could allocate more attentional resources to nonemotional stimuli such as those used in the letter-number sequencing tasks used in our study. However, this remains purely hypothetical and would need to be tested in further research.51
Finally, the Judgment Skills dimension, corresponding to an index of self-awareness was associated with multitasking and cognitive flexibility. These findings fit well with previous links found between insight and executive functioning, particularly cognitive flexibility in schizophrenia patients.52,53 Interestingly inhibition was not associated with any apathy dimension. Regarding the literature on executive functioning, there is actually a clear consensus to support the separation of executive functions into several key distinct processes (ie, Flexibility, inhibition, multitasking, updating).11,54 In our study we used several neuropsychological tests to assess these distinct executive processes in order to explore if specific processes were associated with specific dimensions of apathy. Thus, if we found that flexibility and multitasking were differentially associated with distinct dimension of apathy, inhibition was not associated with any apathy dimension. Thus, our findings strengthen the idea that executive functions are clearly separable from a cognitive approach but have also distinct influence on behavior.
Taken together our findings highlights the fundamental importance of treating apathy as a multidimensional syndrome in order to accurately determine the distinct neuropsychological processes associated with the distinct facets of apathy. Indeed, as argued by some authors,11,12 the risk of considering apathy as a unitary syndrome is to be unable to capture the diversity and complexity of apathetic manifestations, which may hide important correlates of this state that become visible only when the disorder is considered as a multidimensional concept. We must however considerer instances where there is value in considering apathy as a singular construct such as clinical trials designating apathy as a primary outcome variable for example.
Second, using a categorical approach based on the existing clinical cut-offs for the LARS, multiple regression analyses revealed that episodic verbal learning was the only cognitive test associated with severe overall apathy in our schizophrenia sample. Memory for future goal, close to the concept of episodic future thinking,55 has been shown to be implicated in Gollwitzer’s notion of “implementation intentions,”56 which specify the “when, where, and how” of responses leading to goal attainment. Difficulties in learning precise information related to the phenomenological characteristics associated with goal directed behavior (ie, when, where, and what) could thus constitute a key cognitive process that contributes to the presence of severe apathetic manifestations in schizophrenia. Of course this study interpretation remains largely hypothetic and needs to be tested in a future study.
Another important finding of our study was that the risk of severe overall apathy at 1 year increased with a decrease in working memory ability at 1 month, adjusting for apathy score. Importantly, results are expressed per 1 unit increase in score. As the LNS scores ranges from 6 to 37, a 1-unit increase is actually quite considerable and can be considered clinically meaningful. This result is of major importance as contrary to our cross-sectional results described above and past studies on this topic, this suggests that a low working memory performance is a risk factor for severe apathy. Working memory is one of the core cognitive deficits of schizophrenia patients,57 and there are convincing arguments to support that working memory ability is crucial for the successful maintenance, manipulation, and/or monitoring of representations for goal-directed behavior.58
As written above several other aspects of executive functioning such as set-shifting are fundamental aspects of goal-oriented behaviors.59 In light of our results, working memory appears to be as a crucial process underlying overall severe apathy in schizophrenia. This result warrants further discussion. The emotional involvement dimension of the LARS concerns blunting of emotional responses (eg, “do you feel happy when you hear some good news,” or “do you feel sad when you hear some bad news”). There is robust evidence in the literature that schizophrenia patients have reduced working memory capacity and deficits in active maintenance of nonemotional information in working memory. If working memory is a critical resource for many other aspects of cognition there is now evidence that working memory contributes to the voluntary regulation of emotional expression and emotional experience.60 The evidence indicates that people with schizophrenia may have difficulty maintaining an emotional experience over time.61 Consequently, due to working memory deficits, it should not be surprising that people with schizophrenia have difficulty holding on to prior emotional experiences, leading to blunting of emotional responses. This hypothesis is in accordance with previous work by Burbridge and Barch62 who found that working memory moderated the relationship between the symptom of anhedonia and self-reported experience of enjoyment of pleasurable stimuli. However, our finding with regard to the Emotional Involvement dimension does not support this hypothesis and suggests the opposite association to that found for total severe apathy, with a trend (P = .08) for working memory increasing the risk of Emotional Involvement apathy. This should be interpreted with caution for several reasons: first, contrary to the other dimensions, Emotional Involvement is based on only one (Emotional Responses) of the 9 LARS domains, contributing minimally to overall severe apathy. Second, it is weakly correlated to other apathy dimensions.26 Also, the cut-off values for the dimensions are conceptually different to the cut-off threshold for severe apathy, identifying patients with moderate to severe apathy relative to the overall sample, rather than previously validated severe apathy.
Importantly, the cross-sectional associations found in our study between apathy and cognition cannot be explained by depression, psychotic symptoms, medication, substance abuse, or other demographical variables. Our findings suggest that the links between executive and working memory functioning and apathy are primary rather than secondary and represent core processes underlying apathy manifestation in schizophrenia patients.27
From a clinical perspective, our results support the use of cognitive remediation (CR) as an effective psychological intervention for treating apathy in schizophrenia patients. However if some studies have shown the effectiveness of CR to reduce negative symptoms,63,64 most of the therapies designed to enhance cognition do not seem to be effective in improving negative symptoms.65 This apparent contradiction can be partly explained by differences in study design. First, none of these studies have specifically targeted apathy. Secondly, these studies do not take into account the different facets of apathy separately. Targeting specific cognitive process (ie, working memory, cognitive flexibility, or episodic learning memory) with targeted and individualized cognitive remediation techniques, as a function of clinical apathetic profile of each patient could be promising in the treatment of apathy.
Our study has some limitations. First, we have a relatively high number of patients lost-to follow-up between the 1-month and 1-year follow-up (40.1%). This limitation is counterbalanced by the fact that patients in the longitudinal analysis differed only from those lost to follow-up on the MSET performance, indicating that we have not under or overestimated the level of apathy in our follow-up sample. However the limited number of patients did not allow us to exclude patients with apathy at 1 month in order to investigate risk factors for becoming apathetic at 1 year. We did however adjust for the 1-month apathy score in our longitudinal analysis. We choose to adjust for apathy as a continuous variable in order to gain in precision. However the results were largely unchanged when entering 1-month apathy as a binary variable. The absence of validated cut-offs for severe apathy for the 4 subtypes is a further limitation. Establishing a satisfying cut-off was difficult given the limited number of response categories. Using the highest terciles to define severe apathy may be over-inclusive and may explain the absence of significant findings in the longitudinal analysis. Indeed when applying the tercile cut-off values to the healthy control subjects, between 9.8% and 31.3% of the controls were classified as apathetic depending on the dimension. As opposed to the definition of total severe apathy, this approach is conceptually different enabling us to identify for each dimension patients with relatively moderate to high levels of apathy compared to the overall level in the sample.
Second, our study was a naturalistic, observational, longitudinal study which included patients consecutively admitted to 3 psychiatric services. The inclusion criteria implied a heterogeneous group of patients with respect to age, type of care received (ambulatory or inpatient care) and the evolution of their disorder. A more homogeneous group of patients with regard to the stage of their disease may no doubt have led to more specific results. Furthermore, we do not have agreement rates for symptom ratings between the 3 centers but all raters participated in a same training session on PANSS rating. Finally other possible key cognitive processes not assessed in our study, such as the ability to direct attention between self-generated thoughts and external information could also underlie apathetic manifestations in schizophrenia.66 Future studies are needed to test such a hypothesis. Our study has several strengths: (a) the relatively large sample size in comparison to the existing literature on the study of apathy in schizophrenia; (b) The control of potential confounding variables in the cross-sectional analysis such as antipsychotic medication, depression, psychotic symptomatology, and substance use; (c) a validated cut-off score for severe overall apathy; (d) a longitudinal design that allows us to draw conclusions regarding the direction of the relationship between cognition and apathy. (e) The use of routinely used clinical neuropsychological tools makes our results valid for clinical practice in standard psychiatric care.
In conclusion our results strengthen the hypothesis of a potential link between cognition and apathy in schizophrenia and support the need to adopt a multifactorial and integrative approach, focusing on the precise identification of the various mechanisms (ie, cognitive, but also emotional, environmental) underlying negative symptoms in schizophrenia. Research focusing on deconstruction of broadly defined negative symptoms is needed to better understand the underlying mechanisms and to develop new perspectives for effective treatments.
Funding
The study was financed by a University Hospital Clinical Research Grant, Montpellier (#UF8641), 2010. The University Hospital had no further role in the study design, in the collection, analysis and interpretation of data, in the writing of the report and in the decision to submit the article for publication.
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Mulin E, Leone E, Dujardin K, et al. Diagnostic criteria for apathy in clinical practice. Int J Geriatr Psychiatry. 2011;26:158–165. [DOI] [PubMed] [Google Scholar]
- 2. Fervaha G, Foussias G, Agid O, Remington G. Motivational and neurocognitive deficits are central to the prediction of longitudinal functional outcome in schizophrenia. Acta Psychiatr Scand. 2014;130:290–299. [DOI] [PubMed] [Google Scholar]
- 3. Konstantakopoulos G, Ploumpidis D, Oulis P, et al. Apathy, cognitive deficits and functional impairment in schizophrenia. Schizophr Res. 2011;133:193–198. [DOI] [PubMed] [Google Scholar]
- 4. Evensen J, Røssberg JI, Barder H, et al. Apathy in first episode psychosis patients: a ten year longitudinal follow-up study. Schizophr Res. 2012;136:19–24. [DOI] [PubMed] [Google Scholar]
- 5. Faerden A, Barrett EA, Nesvåg R, et al. Apathy, poor verbal memory and male gender predict lower psychosocial functioning one year after the first treatment of psychosis. Psychiatry Res. 2013;210:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kirkpatrick B, Fenton WS, Carpenter WT, Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32:214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strauss GP, Horan WP, Kirkpatrick B, et al. Deconstructing negative symptoms of schizophrenia: avolition-apathy and diminished expression clusters predict clinical presentation and functional outcome. J Psychiatr Res. 2013;47:783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex. 2006;16:916–928. [DOI] [PubMed] [Google Scholar]
- 9. Pagonabarraga J, Kulisevsky J, Strafella AP, Krack P. Apathy in Parkinson’s disease: clinical features, neural substrates, diagnosis, and treatment. Lancet Neurol. 2015;14:518–531. [DOI] [PubMed] [Google Scholar]
- 10. Fusar-Poli P, Papanastasiou E, Stahl D, et al. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. 2015;41:892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arnould A, Rochat L, Azouvi P. A multidimensional approach to apathy after 413 traumatic brain injury. Neuropsychol Rev. 2013;23:210–233. [DOI] [PubMed] [Google Scholar]
- 12. Njomboro P, Deb S. Distinct neuropsychological correlates of cognitive, behavioral, and affective apathy sub-domains in acquired brain injury. Front Neurol. 2014;5:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stuss DT, Levine B, Alexander MP, et al. Wisconsin Card Sorting Test performance in patients with focal frontal and posterior brain damage: effects of lesion location and test structure on separable cognitive processes. Neuropsychologia. 2000;38:388–402. [DOI] [PubMed] [Google Scholar]
- 14. Brown RG, Pluck G. Negative symptoms: the ‘pathology’ of motivation and goal-directed behaviour. Trends Neurosci. 2000;23:412–417. [DOI] [PubMed] [Google Scholar]
- 15. Stella F, Radanovic M, Aprahamian I, Canineu PR, de Andrade LP, Forlenza OV. Neurobiological correlates of apathy in Alzheimer’s disease and mild cognitive impairment: a critical review. J Alzheimers Dis. 2014;39:633–648. [DOI] [PubMed] [Google Scholar]
- 16. Roth RM, Flashman LA, Saykin AJ, McAllister TW, Vidaver R. Apathy in schizophrenia: reduced frontal lobe volume and neuropsychological deficits. Am J Psychiatry. 2004;161:157–159. [DOI] [PubMed] [Google Scholar]
- 17. Faerden A, Vaskinn A, Finset A, et al. Apathy is associated with executive functioning in first episode psychosis. BMC Psychiatry. 2009;8;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Foussias G, Siddiqui I, Fervaha G, et al. Motivated to do well: An examination of the relationships between motivation, effort, and cognitive performance in schizophrenia. Schizophr Res. 2015;166:276–182. [DOI] [PubMed] [Google Scholar]
- 19. Fervaha G, Zakzanis KK, Foussias G, Graff-Guerrero A, Agid O, Remington G. Motivational deficits and cognitive test performance in schizophrenia. JAMA Psychiatry. 2014;71:1058–1065. [DOI] [PubMed] [Google Scholar]
- 20. Tas C, Brown EC, Esen-Danaci A, Lysaker PH, Brüne M. Intrinsic motivation and metacognition as predictors of learning potential in patients with remitted schizophrenia. J Psychiatr Res. 2012;46:1086–1092. [DOI] [PubMed] [Google Scholar]
- 21. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 22. Andreasen NC. Scale for the assessment of negative symptoms (SANS). Br J Psychiatry. 1989;155:53–58. [PubMed] [Google Scholar]
- 23. Kirkpatrick B, Strauss GP, Nguyen L, et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. 2011;37:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38:143–162. [DOI] [PubMed] [Google Scholar]
- 25. Sockeel P, Dujardin K, Devos D, Denève C, Destée A, Defebvre L. The Lille apathy rating scale (LARS), a new instrument for detecting and quantifying apathy: validation in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2006;77:579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yazbek H, Norton J, Capdevielle D, et al. The Lille Apathy Rating Scale (LARS): exploring its psychometric properties in schizophrenia. Schizophr Res. 2014;157:278–284. [DOI] [PubMed] [Google Scholar]
- 27. Kirkpatrick B. Developing concepts in negative symptoms: primary vs secondary and apathy vs expression. J Clin Psychiatry. 2014;75:3–7. [DOI] [PubMed] [Google Scholar]
- 28. First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM- IV Axis Disorders (SCID), Clinician Version: User’s Guide. Washington, DC: Am Psychiatr Press; 1997. [Google Scholar]
- 29. Stuss DT, Van Reekum R, Murphy KJ. Differentiation of states and causes of apathy. In: Borod J, ed. The Neuropsychology of Emotion. New York, NY: Oxford University Press; 2000:340–363. [Google Scholar]
- 30. Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test - Second Edition. Adult Version. Manual. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- 31. Vaskinn A, Sundet K, Friis S, et al. Can learning potential in schizophrenia be assessed with the standard CVLT-II? An exploratory study. Scand J Psychol. 2008;49:179–186. [DOI] [PubMed] [Google Scholar]
- 32. Reitan RM. TMT, Trail Making Test A & B. South Tucson, AR: Reitan Neuropsychology Laboratory; 1992. [Google Scholar]
- 33. Drane DL, Yuspeh RL, Huthwaite JS, Klingler LK. Demographic characteristics and normative observations for derived-trail making test indices. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15:39–43. [PubMed] [Google Scholar]
- 34. Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114:727–741. [DOI] [PubMed] [Google Scholar]
- 35. Wilson BA, Alderman N, Burgess PW, Emslie H, Evans JJ. The Behavioural Assessment for the Dysexecutive Syndrome Manual. Bury St. Edmunds, UK: Thames Valley Test Company; 1996. [Google Scholar]
- 36. Logan GD. On the ability to inhibit thought and action: a user’s guide to the stop-signal paradigm. In: Dagenbach D, Carr TH, eds. Inhibitory Processes in Attention, Memory, and Language. San Diego, CA: Academic Press; 1994:189–239. [Google Scholar]
- 37. Wechsler D. Wechsler Adult Intelligence Scale-3rd Edition (WAIS-3). San Antonio, TX: Harcourt Assessment; 1997. [Google Scholar]
- 38. Shelton JT, Elliott EM, Hill BD, Calamia MR, Gouvier WD. A comparison of laboratory and clinical working memory tests and their prediction of fluid intelligence. Intelligence. 2009;37:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 40. Addington D, Addington J, Atkinson M. A psychometric comparison of the Calgary Depression Scale for Schizophrenia and the Hamilton Depression Rating Scale. Schizophr Res. 1996;9:205–212. [DOI] [PubMed] [Google Scholar]
- 41. Addington D, Addington J, Maticka-Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry. 1993;22:39–44. [PubMed] [Google Scholar]
- 42. Nakagawa S. A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav Ecol. 2004;15:1044–1045. [Google Scholar]
- 43. Prabhakar J, Hudson JA. The development of future thinking: young children’s ability to construct event sequences to achieve future goals. J Exp Child Psychol. 2014;127:95–109. [DOI] [PubMed] [Google Scholar]
- 44. Heerey EA, Matveeva TM, Gold JM. Imagining the future: degraded representations of future rewards and events in schizophrenia. J Abnorm Psychol. 2011;120:483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Strauss GP, Waltz JA, Gold JM. A review of reward processing and motivational impairment in schizophrenia. Schizophr Bull. 2014;40:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cañas J, Quesada JF, Antolí A, Fajardo I. Cognitive flexibility and adaptability to environmental changes in dynamic complex problem-solving tasks. Ergonomics. 2003;46:482–501. [DOI] [PubMed] [Google Scholar]
- 47. Ardila A. On the evolutionary origins of executive functions. Brain Cogn. 2008;68:92–99. [DOI] [PubMed] [Google Scholar]
- 48. Barch DM, Pagliaccio D, Luking K. Mechanisms underlying motivational deficits in psychopathology: similarities and differences in depression and schizophrenia. Curr Top Behav Neurosci. In press. [DOI] [PubMed] [Google Scholar]
- 49. Mikels JA, Reuter-Lorenz PA, Beyer JA, Fredrickson BL. Emotion and working memory: evidence for domain-specific processes for affective maintenance. Emotion. 2008;8:256–266. [DOI] [PubMed] [Google Scholar]
- 50. Gard DE, Cooper S, Fisher M, Genevsky A, Mikels JA, Vinogradov S. Evidence for an emotion maintenance deficit in schizophrenia. Psychiatry Res. 2011;187:24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Burbridge JA, Barch DM. Anhedonia and the experience of emotion in individuals with schizophrenia. J Abnorm Psychol. 2007;116:30–42. [DOI] [PubMed] [Google Scholar]
- 52. Nair A, Palmer EC, Aleman A, David AS. Relationship between cognition, clinical and cognitive insight in psychotic disorders: a review and meta-analysis. Schizophr Res. 2014;152:191–200. [DOI] [PubMed] [Google Scholar]
- 53. Raffard S, Bayard S, Gely-Nargeot MC, et al. Insight and executive functioning in schizophrenia: a multidimensional approach. Psychiatry Res. 2009;167:239–250. [DOI] [PubMed] [Google Scholar]
- 54. Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. [DOI] [PubMed] [Google Scholar]
- 55. Atance CM, O’Neill DK. Episodic future thinking. Trends Cogn Sci. 2001;5:533–539. [DOI] [PubMed] [Google Scholar]
- 56. Gollwitzer PM, Schaal B. Metacognition in action: the importance of implementation intentions. Pers Soc Psychol Rev. 1998;2:124–136. [DOI] [PubMed] [Google Scholar]
- 57. Barch DM, Smith E. The cognitive neuroscience of working memory: relevance to CNTRICS and schizophrenia. Biol Psychiatry. 2008;64:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5:184–194. [DOI] [PubMed] [Google Scholar]
- 59. Hussein S, Johnston K, Belbeck B, Lomber SG, Everling S. Functional specialization within macaque dorsolateral prefrontal cortex for the maintenance of task rules and cognitive control. J Cogn Neurosci. 2014;26:1918–1927. [DOI] [PubMed] [Google Scholar]
- 60. Schmeichel BJ, Volokhov RN, Demaree HA. Working memory capacity and the self-regulation of emotional expression and experience. J Pers Soc Psychol. 2008;95:1526–1540. [DOI] [PubMed] [Google Scholar]
- 61. Kring AM, Germans Gard M, Gard DE. Emotion deficits in schizophrenia: timing matters. J Abnorm Psychol. 2011;120:79–87. [DOI] [PubMed] [Google Scholar]
- 62. Burbridge JA, Barch DM. Anhedonia and the experience of emotion in individuals with schizophrenia. J Abnorm Psychol. 2007;116:30–42. [DOI] [PubMed] [Google Scholar]
- 63. Klingberg S, Wölwer W, Engel C, et al. Negative symptoms of schizophrenia as primary target of cognitive behavioral therapy: results of the randomized clinical TONES study. Schizophr Bull. 2011;37:98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sánchez P, Peña J, Bengoetxea E, et al. Improvements in negative symptoms and functional outcome after a new generation cognitive remediation program: a randomized controlled trial.Schizophr Bull. 2014;40:707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164:1791–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Burgess PW, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn Sci. 2007;11:290–298. [DOI] [PubMed] [Google Scholar]