Abstract
Diabetes mellitus and the coexisting conditions and complications, including hypo- and hyperglycemic events, obesity, high cholesterol levels, and many more, are devastating problems. Undoubtedly, there is a huge demand for treatment and prevention of these conditions that justifies the search for new approaches and concepts for better management of whole body metabolism. Emerging evidence demonstrates that the autonomic nervous system is largely involved in the regulation of glucose homeostasis; however, the underlying mechanisms are still under investigation. Within the hypothalamus, the paraventricular nucleus (PVN) is in a unique position to integrate neural and hormonal signals to command both the autonomic and neuroendocrine outflow. This minireview will provide a brief overview on the role of preautonomic PVN neurons and the importance of the PVN-liver pathway in the regulation of glucose homeostasis.
Keywords: paraventricular nucleus, liver-related neurons, glucose homeostasis
diabetes mellitus is the most common metabolic disorder. The National Diabetes Statistics Report from 2014 published by the Centers for Disease Control and Prevention (8a) estimated that 29.1 million people, which is 9.3% of the US population, suffer from diabetes mellitus. Even more alarming information is that the number of new diabetes cases showed an increase of 1.7 million within 2 yr, and in 2010 diabetes mellitus was listed as the seventh leading cause of death. The coexisting conditions and complications of diabetes mellitus are severe, including hypo- and hyperglycemic events, high blood pressure, obesity, high cholesterol levels, and many more. Undoubtedly, there is a huge demand for treatment and prevention of these conditions that justifies the search for new approaches and solutions for better management of whole body metabolism. One possibility, which has recently attracted more interest, is the role of the brain in the regulation of metabolism (46, 50), particularly the contribution of the autonomic nervous system (ANS) to metabolic control (51, 69, 73, 75).
The hypothalamus has been known as one of the critical brain structures that is involved in the regulation and coordination of homeostatic functions. Traditionally, the hypothalamus was appreciated for its neuroendocrine control system; however, its control over the ANS has also been recognized (17, 46, 51, 69, 75). Imbalance of the ANS predicts diabetes mellitus and cardiovascular diseases (67), and it has been revealed that there is a high risk of developing type 2 diabetes if autonomic dysfunction is present (8). In general, decreased activity of the parasympathetic nervous system and increased activity of the sympathetic nervous system is associated with metabolic syndrome (30), indicating that a critical balance between the sympathetic and parasympathetic branches of the ANS is necessary for normal glucose homeostasis.
Although the phrase “increased activity of the sympathetic nervous system” is used often, we have to point out that differential regulation of tissue-specific sympathetic output pathways is recognized (34). In human subjects, muscle sympathetic nerve activity was assessed from multiunit discharges and single units with defined vasoconstrictor properties (14). Single-unit muscle sympathetic nerve activity was significantly greater in subjects with metabolic syndrome with or without hypertension compared with control subjects, whereas the multiunit discharges showed a similar trend (14). Another study investigated whether during metabolic syndrome the sympathetic activation is generalized to the whole cardiovascular system or is compartmentalized by comparing muscle and skin sympathetic nerve activity (12). Multiunit recordings revealed increased muscle sympathetic nerve activity in obese individuals and in patients with metabolic syndrome compared with control subjects. In contrast, skin sympathetic nerve activity did not differ among the groups (12). Plasma norepinephrine levels were associated with muscle sympathetic nerve activity but not with skin sympathetic nerve activity. These data suggested that sympathetic overactivity observed in patients with metabolic syndrome is not distributed uniformly, and these authors speculate that insulin-induced sympathetic activation could be one of the mechanisms behind the differential regulation of sympathetic outflow (12). Furthermore, hypoglycemia leads to increase in sympathetic outflow to the liver and adrenal glands, whereas renal and cardiac sympathetic outflow are unaffected (4, 7, 33, 34). Studies conducted in rodents also support the differential control of sympathetic outflow, and a recent review summarized the findings in detail (34). Brown adipose tissue (BAT) is involved in thermoregulation and thus in energy homeostasis, and its function is controlled by the sympathetic nervous system. Selective activation of BAT sympathetic nerve activity by hypothermia, disinhibition of neurons in the rostral raphe pallidus, the differences in basal discharges, the characteristics of stimulated BAT vs. splanchnic sympathetic nerve activity, and the sensitivity of BAT and splanchnic sympathetic nerve activity to baroreceptor reflex all demonstrated the existence of a distinct subset of premotor neurons that regulate vasoconstriction and BAT thermogenesis (35). These findings revealed the existence of tissue-specific subsets of preautonomic neurons, which differentially control sympathetic outflow, in this case, the BAT. Moreover, it is highly likely that metabolically important organs (e.g., liver, pancreas) are also differentially controlled; however, the understanding of the neural circuits mediating the differential control of sympathetic and parasympathetic outflows remains an open area of investigation.
The imbalance of the autonomic nervous system is due to altered neuronal activity in autonomic centers of the brain (75), and selective modulation of brain circuits may represent a possible means of both controlling glycemic balance and preventing complications. Within the hypothalamus, the paraventricular nucleus (PVN) is an integrative autonomic center, and this minireview will provide a brief overview of the role of preautonomic PVN neurons and the importance of the PVN-liver pathway in the regulation of glucose homeostasis.
Control of the Liver by Preautonomic PVN Neurons: In Vivo Findings
The first evidence of the brain being able to regulate glucose metabolism originated from Claude Bernard in 1854 (1). Bernard, who established the concept of homeostasis and was the founder of modern experimental physiology, demonstrated that puncturing the floor of the fourth ventricle resulted in increased peripheral glucose levels mimicking diabetic conditions. These early studies did not establish precise underlying mechanisms (e.g., insulin levels) or site of action (e.g., nuclei) but clearly showed that manipulating brainstem circuits influences peripheral function, in this case glucose levels. Then, with the discovery of insulin, the major focus of research shifted to the establishment of insulin-dependent mechanisms, mainly in the periphery. On the other hand, over the past few decades, with the discovery of new and more precise experimental approaches and the discovery of hormones such as leptin, which exerts its main effect through brain circuits, the original idea of regulation of glucose homeostasis via the brain has risen again (50).
Glucose production of the liver and modulation of glucose clearance via the actions of hormones, including insulin, which promotes glucose uptake in the skeletal muscle, are the major mechanisms controlling plasma glucose levels. The liver plays a crucial role in the maintenance of glucose homeostasis, and evidence supports a governing role for the autonomic nerves in the control of hepatic functions (5, 44). Activation of hepatic sympathetic innervation increases endogenous glucose production and glycogenolysis, whereas activation of the parasympathetic innervation decreases glucose production and promotes glucose storage (36, 52, 55, 56, 64). Hormones and nutrients, including glucose, leptin, and fatty acids, are also using the brain-liver pathway to regulate glucose homeostasis (28, 37, 40, 41, 46), and detailed information about the metabolic sensing, the neural innervation of the liver, or the autonomic control of hepatic lipid metabolism can be found in previous reviews (5, 44, 69, 72).
The autonomic nervous system consists of the sympathetic and parasympathetic branches and regulates the majority of organs in an opposite way; autonomic motor neurons for these systems are located in the spinal cord and brainstem, respectively, and convey information through the sympathetic and parasympathetic outflow. Neurons in higher brain areas projecting to these autonomic motor neurons, the so-called preautonomic neurons, are crucial for integration of brain signals, and establishing their cellular and molecular characteristics may help to understand how the central nervous system is able to control our peripheral organs and tissues (47, 48, 59, 61, 62). Remarkably, despite the fact that functions of the liver are governed by sympathetic and parasympathetic inputs, the exact location of the premotor inputs to the relevant sympathetic and parasympathetic motor neurons is not known.
Within the hypothalamus, the PVN is a crucial, integrative center that incorporates signals from numerous brain areas, including sites known for controlling energy and glucose homeostasis, and contributes largely to the regulation of the sympathetic and parasympathetic nervous system and thus modulates autonomic functions (69, 75). Studies using electrical stimulation of the PVN have demonstrated direct connections between the PVN and parasympathetic brainstem neurons or sympathetic neurons in the spinal cord and thus demonstrated the ability of PVN to relay information through both autonomic pathways (29, 45, 68). These studies also suggest that the PVN is likely to be one of the locations of the premotor inputs governing sympathetic and parasympathetic motor neurons. Unilateral norepinephrine injection into the PVN evoked an immediate hyperglycemia that reached the peak ∼10 min following injection (15). The high glucose levels were associated with inhibition of insulin regardless of glucose levels (15), and the norepinephrine-induced hyperglycemia was largely reduced by a ganglionic blocker and was independent of corticosterone levels (15). These findings demonstrated that the hyperglycemia accompanied with inhibition of insulin is due to sympathetic activation (15). Furthermore, the revealed effects were similar to those observed following electrical stimulation of the splanchnic nerve, which caused hyperglycemia due to direct neural effects on the liver, secretion of glucagon, and catecholamine release (3, 13, 19, 20, 55).
In vivo administration of N-methyl-d-aspartate and the GABAA antagonist bicuculline with bilateral microdialysis probes into the PVN increased plasma glucose levels significantly, which was accompanied with increased glucagon levels, without change in plasma insulin (18). Moreover, the glucose increase was absent in rats following sympathectomy, indicating that the increased plasma glucose levels were attained via the sympathetic nervous system (18). This study also suggested that GABAergic inhibition plays important role in the PVN-dependent regulation of glucose levels and raised the question about the origin of the GABAergic inputs. The suprachiasmatic nucleus (SCN) has been proposed as one of the sources of GABAergic inputs that also could explain the daily rise in blood glucose levels with the withdrawal of the GABAergic inhibition of presympathetic PVN neurons by the biological clock; however, further evidence is needed to prove this hypothesis (18).
Insulin through hypothalamic insulin receptors has been shown to inhibit hepatic glucose production (38), and one proposed mechanism is the inhibition of neuropeptide Y (NPY) neurons in the arcuate nucleus (49). NPY neurons send dense projections to the PVN, and it has been demonstrated that hepatic sympathectomy abolishes the NPY/insulin effect on liver glucose production, suggesting that the hypothalamic insulin effect may depend on modulation of presympathetic PVN neurons (65). On the other hand, Pocai et al. (39) revealed that the parasympathetic outflow to the liver is involved in the inhibitory effect of hypothalamic insulin on hepatic glucose production. Similarly, the effect of leptin on insulin sensitivity of the liver was prevented by hepatic vagotomy (11), whereas orexin-dependent control of hepatic glucose production was blocked by sympathectomy of the liver (70). Taken together, these findings suggest that preautonomic PVN neurons are important integrating points in converging information to control hepatic glucose metabolism, as suggested before (17).
Certain pathophysiological conditions, including thyrotoxicosis, are linked to increased hepatic glucose production, hepatic insulin resistance, and hyperglycemia (9, 22). The hyperglycemia and increased hepatic glucose production was attenuated following hepatic sympathetic denervation (22). Bilateral infusion of triiodothyronine into the PVN elevated hepatic glucose production and plasma glucose levels, and hepatic sympathectomy prevented this effect. These findings demonstrated that the thyroid hormone-dependent hyperglycemia is mediated through the sympathetic nervous system without plasma glucoregulatory hormone concentrations being altered (21).
The importance of the ANS and in particular the PVN in the regulation of hepatic glucose production was further demonstrated with intracerebroventricular and PVN infusions of pituitary adenylyl cyclase-activating polypeptide (PACAP) (71). PACAP administration into the PVN resulted in hyperglycemia and increased hepatic glucose production, and this effect was largely but not fully mediated via the sympathetic innervation of the liver. The combination of brain injections, retrograde tracing from the thoracic spinal cord, hepatic denervation, and immunostaining showed that PACAP via sympathetic-related preautonomic PVN neurons largely controls hepatic glucose production and thus plasma glucose levels (71).
The in vivo findings reviewed in this section contain complex experimental approaches, including delivery of drugs into specific brain areas, in this case to the PVN; therefore, certain methodological concerns have to be pointed out. In general, the correct placement of the microdialysis probe or the injection cannula into the targeted brain area always has to be verified, and results should be analyzed and interpreted following verification of the correct site. Negative responses following placement outside of the target area also give important information and could be used as negative control sites. In addition to the verification of the correct site, the spread of the administered drug is always a concern. The distribution of the drug could be assessed by coadministering a colored dye; however, despite the overlap between the coadministered drug and dye, the distribution is most likely not equal. Furthermore, the spread of the drug also depends on its concentration. Based on these methodological issues, we cannot entirely exclude that the administered drugs affected only the PVN or that the neighboring nuclei were unaffected. On the other hand, Kalsbeek et al. (18) estimated the spread of the administered drugs to be able to address this concern, and they demonstrated that the PVN is the key area. In their study, plasma glucose and corticosterone levels were analyzed following infusion of drugs into four different brain areas. Comparing the effects of drugs in the PVN with the effects in the neighboring brain nuclei (dorsomedial, ventromedial, and suprachiasmatic nuclei) was used to calculate the effective radius of the drug infusion, and the study found that the hyperglycemic effect of the drug was delayed when the drug was applied at the border of PVN compared with injection within the PVN. Furthermore, administration outside of the PVN had no effect on plasma glucose levels. Based on these data, these authors suggested that the PVN is responsible for the observed effect on glucose levels, and in their study, preautonomic PVN neurons are key structures (18). Because of experimental limitations, not every study uses or can use this type of evaluation; therefore, there is a chance that the neighboring nuclei could contribute to the demonstrated effects; however, Kalsbeek et al. (18) addressed this issue convincingly in one of their studies.
Together, the above-mentioned in vivo findings support the existence of the brain-dependent regulation of glucose metabolism and demonstrate the importance of the PVN in the brain-liver pathway; however, more detailed studies are necessary to understand the underlying mechanisms, including the neural circuits, molecular mechanisms, neurotransmitters, neuromodulators, and receptors regulating preautonomic neurons and thereby modulating glucose production of the liver.
Control of the Liver By Preautonomic PVN Neurons: Anatomic and Cellular Studies
Anatomic studies using anterograde and retrograde tracers in combination with immunohistochemistry were used to investigate the connections between the brain and liver. However, for a long time, there has been limited amount of information about the location, phenotype, and connectivity of preautonomic neurons. The development of neural tract tracing methods, including the use of transsynaptic retrograde viral tracers, allowed the identification of specific brain nuclei and a more accurate distribution of organ-related neurons and led to the description of detailed neural pathways connecting the hypothalamus and visceral organs, including the brain-liver pathways (6, 10, 18).
It has been well accepted that many hypothalamic nuclei, including the ventromedial hypothalamus (VMH), dorsomedial hypothalamus (DMH), lateral hypothalamus (LH), and PVN, contribute to the autonomic outflow and modulate metabolic activity in a variety of tissues (17, 64). The PVN as a critical command component of the autonomic pathway integrates signals from a variety of brain areas, including the above-mentioned hypothalamic nuclei, and it is in a unique position to integrate neuronal and humoral signals to organize the autonomic and neuroendocrine outflow, as mentioned above (48, 59, 61, 69, 75).
Early studies by Shimazu (53, 54) postulated that the LH directly relays information through the parasympathetic nuclei, which builds the vagal hepatic branch of the liver, whereas the VMH is involved in the control of the sympathetic pathway to the liver. Then, studies with retrograde transneuronal viral tracers revealed more detailed and refined pathways demonstrating that the PVN projects to the liver through both sympathetic and parasympathetic pathways (6, 27). Labeling with pseudorabies virus in rats resulted in a reproducible infection pattern and revealed polysynaptic neural connection between the liver and brain (26). Short-time (3 days) survival demonstrated pseudorabies labeling in the intermediolateral column of the spinal cord without labeling in parasympathetic cell groups. Intermediate survival time (4–5 days) revealed viral labeling in parasympathetic and sympathetic cell groups of the brainstem and also in the PVN (26). Longer survival time revealed labeling in hypothalamic areas connected to the PVN, including the medial preoptic area, DMH, arcuate nucleus, LH, circumventricular organs, and SCN (26). This study demonstrated the presence of polysynaptic autonomic pathways to the liver and also demonstrated that the PVN is able to control both sympathetic and parasympathetic pathways and integrate information from other nuclei (6, 26, 64).
Retrograde viral labeling following hepatic parasympathectomy revealed presympathetic liver-related neurons in the PVN and in the areas that project to the PVN, including medial preoptic area (MPO), anterior hypothalamic area, DMH, arcuate nucleus, VMH, circumventricular organs, and the SCN (18). Hepatic sympathectomy in combination with the retrograde viral labeling identified preparasympathetic liver-related neurons in the PVN and in the connected areas, including DMH, MPO, VMH, and SCN (18). It has to be mentioned that the studies revealing presympathetic liver-related neurons in the hypothalamus also identified neurons in the rostral ventrolateral, ventromedial medulla, raphe pallidus, and A5 region. These brain areas are known to project to sympathetic vasomotor preganglionic neurons that innervate the entire body, and thus it is likely that they contribute to the vasomotor innervation of the liver. This does not exclude the possibility that the labeled presympathetic neurons contribute to the nonvasomotor sympathetic innervation of the liver, but with the retrograde transsynaptic viral labeling, the neurons contributing to vasomotor and nonvasomotor sympathetic innervation of the liver cannot be distinguished. On the other hand, studies identifying preparasympathetic inputs to the liver are likely to identify the premotor parasympathetic neurons because of the lack of parasympathetic innervation of the blood vessels.
Labeling with this pseudorabies virus approach was also used to investigate segregation between sympathetic- and parasympathetic-projecting liver-related neurons (6). The analysis demonstrated a complete separation between presympathetic and preparasympathetic liver-related neurons in the hypothalamus (6) and suggested that preautonomic PVN neurons project through either the sympathetic or parasympathetic pathway. Interestingly, both presympathetic and preparasympathetic neurons were immunopositive for oxytocin, which suggest that both groups of preautonomic neurons can contain the same neuropeptide (Fig. 1) (6). Stanley et al. (57) revealed time-dependent expression of preautonomic neurons in hypothalamic nuclei following pseudorabies inoculation of the liver in mice. Then, using immunostaining, their study determined that a subset of liver-related neurons colocalized with neurons expressing oxytocin and corticotropin-releasing hormone but not vasopressin. These studies are consistent with previous findings indicating that the hypothalamic PVN is important in hepatic autonomic innervation and in the control of hepatic glucose metabolism (18, 64). Furthermore, demonstrating the distribution of liver-related PVN neurons laid down the anatomic background for physiological/functional studies.
Fig. 1.
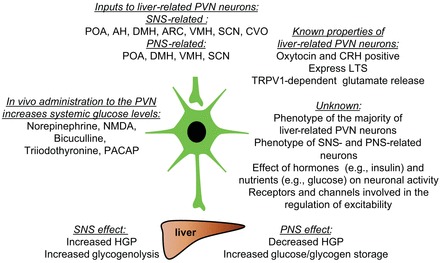
Overview of brain-liver connections. Liver-related preautonomic neurons in the paraventricular nucleus (PVN) of the hypothalamus receive inputs from a variety of hypothalamic nuclei and via the sympathetic (SNS) and parasympathetic nervous systems (PNS) modulate glucose levels. Some of the liver-related neurons express corticotropin-releasing hormone (CRH) and oxytocin, whereas the phenotype of the majority of liver-related PVN neurons is unknown. In vivo administration of neurotransmitters and modulators influences glucose levels, whereas the exact underlying mechanisms (e.g., receptors) are unclear. POA, preoptic area; AH, anterior hypothalamus; DMH, dorsomedial hypothalamus; VMH, ventromedial hypothalamus; SCN, suprachiasmatic nucleus; CVO, circumventricular organs; LTS, low-threshold spikes; TRPV1, transient receptor potential vanilloid type 1; HGP, hepatic glucose production.
Interestingly, “command” preautonomic PVN neurons were identified following simultaneous injections of the liver and epididymal white adipose tissue (57). Identification of these dual-labeled central command neurons supports the theory of coordinated response to more than one organ, which was suggested for sympathetic activation of the heart and adrenal gland (16). The hypothesis that one neuron can control more than one organ was also supported by observations from Buijs et al. (6) demonstrating that many presympathetic neurons project to the liver and adrenal gland; however, the above-discussed issue regarding vasomotor and nonvasomotor sympathetic innervation of the liver has to be kept in mind. On the contrary, complete segregation of preautonomic neurons projecting to the intra-abdominal fat and subcutaneous fat tissue was shown by Kreier et al. (25), whereas command neurons were projecting to intra-abdominal organs, suggesting an organization based on body compartments. Interaction between neurons of the sympathetic and parasympathetic pathways also has to be considered, and connections have been proposed at the level of presympathetic PVN neurons, which can have collaterals projecting to parasympathetic-related neurons (6, 59, 66).
There is much less information available about the cellular properties of preautonomic liver-related PVN neurons. PVN consists of magnocellular neurons, parvocellular neuroendocrine cells, and parvocellular preautonomic neurons (31, 32, 60). These neurons can be distinguished based on their electrophysiological (e.g., pre- and postsynaptic) properties (63). Preautonomic PVN neurons express low-threshold spikes (LTS) and strong inward rectification, which are common features (32, 58). However, besides the common characteristics like LTS, the characterization of the electrophysiological and morphological properties of the preautonomic PVN neurons revealed a heterogeneous neuronal population (58). This heterogeneity could be associated with their target of innervation and/or differential modulation of sympathetic and parasympathetic outflow or related to the neurochemical phenotype of the preautonomic neurons, as suggested by Stern (58). The segregation of the presympathetic and preparasympathetic PVN neurons innervating organs related to glucose metabolism is also demonstrated by retrograde tracing studies that revealed no overlap between spinally projecting neurons and neurons projecting to the dorsal motor nucleus of the vagus (42, 43).
Our laboratory used retrograde viral labeling to identify liver-related PVN neurons (10, 74). The excitatory neurotransmission of preautonomic liver-related PVN neurons was investigated in a control and a type 1 diabetic mouse model (10). In this study, we did not find a significant difference between the frequencies of spontaneous or miniature excitatory postsynaptic currents among the groups. On the other hand, the transient receptor potential vanilloid type 1 (TRPV1)-dependent regulation of liver-related PVN neurons was diminished in type 1 diabetic mice (10). We revealed that in vitro and in vivo insulin application restored TRPV1 activity in a phosphatidylinositol 3-kinase/PKC-dependent manner and also stimulated TRPV1 trafficking to the plasma membrane (10). These data suggested that TRPV1 plays an important role in the regulation of liver-related PVN neurons and that the diabetic condition alters the TRPV1-driven excitatory neurotransmitter release. We have to note that in these experiments liver-related PVN neurons were identified and recordings were conducted from these neurons, but our study does not differentiate the presympathetic or preparasympathetic nature of the preautonomic neurons. Despite these data, there is still limited information on the cellular characteristics of liver-related PVN neurons.
Future Perspectives
During the past few decades, significant effort has been made to further delineate brain-dependent regulation of glucose metabolism; however, despite this effort, more detailed studies are necessary to further understand the underlying mechanisms. In general, the information available about the tissue-specific subset of presympathetic and preparasympathetic neurons in the hypothalamus is limited. The retrograde viral labeling technique, used in combination with sympathectomy and/or parasympathectomy, has identified specific brain areas, but due to the above-mentioned technical limitations, many questions are still not answered. The phenotype of the majority of liver-related preautonomic neurons is not known. It would also be intriguing to know the neuromodulators, neurotransmitters, nutrients, hormones, and receptors that are able to regulate the activity of the liver-related neurons (Fig. 1). Determining these regulators in a control condition would be important to identify their potential involvement in the development of pathophysiological conditions or ways of restoring normal neuronal functions. Since it does appear that the diabetic state alters autonomic function, both sympathetic and parasympathetic, once the fundamental regulatory players involved in the control of liver-related neurons and outflow are identified, it will be possible to further investigate how those transmitters/regulators are altered in a diabetic state or other disease conditions affecting the ANS. Then, with a greater understanding of how the ANS may go awry in a diabetic state, it may be possible to intervene on some of those processes. Furthermore, despite the importance of organ-related circuits, including the brain-liver pathway, our understanding of the mechanisms controlling these circuits, the origin of inputs to liver-related PVN neurons, and factors modulating these inputs is limited. The recent thriving of optogenetic and pharmacogenetic approaches in combination with transgenic animals has been useful and successful in revealing brain circuits for homeostatic control such as feeding (2, 23, 24) and opens new avenues to discover the elements of brain circuits regulating glucose homeostasis.
Together, delineating the brain-visceral organ circuits, including the brain-liver circuitry, may have potential therapeutic value via opening new strategies for better control of glucose homeostasis via the ANS.
GRANTS
We acknowledge funding support from the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-099598) to A. Zsombok.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.D.O. and A.Z. prepared figures; J.D.O. and A.Z. drafted manuscript; J.D.O. and A.Z. edited and revised manuscript; J.D.O. and A.Z. approved final version of manuscript.
REFERENCES
- 1.Bernard C. Lecons de Physiologie Experimentale Appliques a la Medicine. Paris: Eds Baillere et Fils, 1854. [Google Scholar]
- 2.Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell 155: 1337–1350, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bobbioni E, Marre M, Helman A, Assan R. The nervous control of rat glucagon secretion in vivo. Horm Metab Res 15: 133–138, 1983. [DOI] [PubMed] [Google Scholar]
- 4.Brodows RG, Pi S, Campbell RG. Sympathetic control of hepatic glycogenolysis during glucopenia in man. Metabolism 24: 617–624, 1975. [DOI] [PubMed] [Google Scholar]
- 5.Bruinstroop E, Fliers E, Kalsbeek A. Hypothalamic control of hepatic lipid metabolism via the autonomic nervous system. Best Pract Res Clin Endocrinol Metab 28: 673–684, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Buijs RM, la Fleur SE, Wortel J, Van Heyningen C, Zuiddam L, Mettenleiter TC, Kalsbeek A, Nagai K, Niijima A. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J Comp Neurol 464: 36–48, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Carlsson S, Skarphedinsson JO, Delle M, Hoffman P, Thoren P. Differential responses in post- and pre-ganglionic adrenal sympathetic nerve activity and renal sympathetic nerve activity after injection of 2-deoxy-d-glucose and insulin in rats. Acta Physiol Scand 145: 169–175, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Carnethon MR, Golden SH, Folsom AR, Haskell W, Liao D. Prospective investigation of autonomic nervous system function and the development of type 2 diabetes: the Atherosclerosis Risk In Communities study, 1987–1998. Circulation 107: 2190–2195, 2003. [DOI] [PubMed] [Google Scholar]
- 8a.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: US Department of Health and Human Services, 2014. [Google Scholar]
- 9.Dimitriadis GD, Raptis SA. Thyroid hormone excess and glucose intolerance. Exp Clin Endocrinol Diabetes 109, Suppl 2: S225–S239, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Gao H, Miyata K, Bhaskaran MD, Derbenev AV, Zsombok A. Transient receptor potential vanilloid type 1-dependent regulation of liver-related neurons in the paraventricular nucleus of the hypothalamus diminished in the type 1 diabetic mouse. Diabetes 61: 1381–1390, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.German J, Kim F, Schwartz GJ, Havel PJ, Rhodes CJ, Schwartz MW, Morton GJ. Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology 150: 4502–4511, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grassi G, Quarti-Trevano F, Seravalle G, Dell'Oro R, Dubini A, Mancia G. Differential sympathetic activation in muscle and skin neural districts in the metabolic syndrome. Metabolism 58: 1446–1451, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Hartmann H, Beckh K, Jungermann K. Direct control of glycogen metabolism in the perfused rat liver by the sympathetic innervation. Eur J Biochem 123: 521–526, 1982. [DOI] [PubMed] [Google Scholar]
- 14.Huggett RJ, Burns J, Mackintosh AF, Mary DA. Sympathetic neural activation in nondiabetic metabolic syndrome and its further augmentation by hypertension. Hypertension 44: 847–852, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Ionescu E, Coimbra CC, Walker CD, Jeanrenaud B. Paraventricular nucleus modulation of glycemia and insulinemia in freely moving lean rats. Am J Physiol Regul Integr Comp Physiol 257: R1370–R1376, 1989. [DOI] [PubMed] [Google Scholar]
- 16.Jansen AS, Nguyen XV, Karpitskiy V, Mettenleiter TC, Loewy AD. Central command neurons of the sympathetic nervous system: basis of the fight-or-flight response. Science 270: 644–646, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Kalsbeek A, Bruinstroop E, Yi CX, Klieverik LP, La Fleur SE, Fliers E. Hypothalamic control of energy metabolism via the autonomic nervous system. Ann NY Acad Sci 1212: 114–129, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Kalsbeek A, La Fleur S, Van Heijningen C, Buijs RM. Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J Neurosci 24: 7604–7613, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaneto A, Kajinuma H, Kosaka K. Effect of splanchnic nerve stimulation on glucagon and insulin output in the dog. Endocrinology 96: 143–150, 1975. [DOI] [PubMed] [Google Scholar]
- 20.Kaneto A, Miki E, Kosaka K. Effects of vagal stimulation on glucagon and insulin secretion. Endocrinology 95: 1005–1010, 1974. [DOI] [PubMed] [Google Scholar]
- 21.Klieverik LP, Janssen SF, van Riel A, Foppen E, Bisschop PH, Serlie MJ, Boelen A, Ackermans MT, Sauerwein HP, Fliers E, Kalsbeek A. Thyroid hormone modulates glucose production via a sympathetic pathway from the hypothalamic paraventricular nucleus to the liver. Proc Natl Acad Sci USA 106: 5966–5971, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klieverik LP, Sauerwein HP, Ackermans MT, Boelen A, Kalsbeek A, Fliers E. Effects of thyrotoxicosis and selective hepatic autonomic denervation on hepatic glucose metabolism in rats. Am J Physiol Endocrinol Metab 294: E513–E520, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Kong D, Tong Q, Ye C, Koda S, Fuller PM, Krashes MJ, Vong L, Ray RS, Olson DP, Lowell BB. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell 151: 645–657, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest 121: 1424–1428, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kreier F, Kap YS, Mettenleiter TC, van Heijningen C, van der Vliet J, Kalsbeek A, Sauerwein HP, Fliers E, Romijn JA, Buijs RM. Tracing from fat tissue, liver, and pancreas: a neuroanatomical framework for the role of the brain in type 2 diabetes. Endocrinology 147: 1140–1147, 2006. [DOI] [PubMed] [Google Scholar]
- 26.la Fleur SE, Kalsbeek A, Wortel J, Buijs RM. Polysynaptic neural pathways between the hypothalamus, including the suprachiasmatic nucleus, and the liver. Brain Res 871: 50–56, 2000. [DOI] [PubMed] [Google Scholar]
- 27.La Fleur SE, Kalsbeek A, Wortel J, Buijs RM. A suprachiasmatic nucleus generated rhythm in basal glucose concentrations. J Neuroendocrinol 11: 643–652, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L, Schwartz GJ, Rossetti L. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med 11: 320–327, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence D, Pittman QJ. Interaction between descending paraventricular neurons and vagal motor neurons. Brain Res 332: 158–160, 1985. [DOI] [PubMed] [Google Scholar]
- 30.Licht CM, Vreeburg SA, van Reedt Dortland AK, Giltay EJ, Hoogendijk WJ, DeRijk RH, Vogelzangs N, Zitman FG, de Geus EJ, Penninx BW. Increased sympathetic and decreased parasympathetic activity rather than changes in hypothalamic-pituitary-adrenal axis activity is associated with metabolic abnormalities. J Clin Endocrinol Metab 95: 2458–2466, 2010. [DOI] [PubMed] [Google Scholar]
- 31.Liposits Z. Ultrastructure of hypothalamic paraventricular neurons. Crit Rev Neurobiol 7: 89–162, 1993. [PubMed] [Google Scholar]
- 32.Luther JA, Tasker JG. Voltage-gated currents distinguish parvocellular from magnocellular neurones in the rat hypothalamic paraventricular nucleus. J Physiol 523: 193–209, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medvedev OS, Delle M, Thoren P. 2-Deoxy-d-glucose-induced central glycopenia differentially influences renal and adrenal nerve activity in awake SHR rats. Clin Exp Hypertens A 10, Suppl 1: 375–381, 1988. [DOI] [PubMed] [Google Scholar]
- 34.Morrison SF. Differential control of sympathetic outflow. Am J Physiol Regul Integr Comp Physiol 281: R683–R698, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Morrison SF. Differential regulation of brown adipose and splanchnic sympathetic outflows in rat: roles of raphe and rostral ventrolateral medulla neurons. Clin Exp Pharmacol Physiol 28: 138–143, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia 43: 533–549, 2000. [DOI] [PubMed] [Google Scholar]
- 37.O'Hare JD, Zielinski E, Cheng B, Scherer T, Buettner C. Central endocannabinoid signaling regulates hepatic glucose production and systemic lipolysis. Diabetes 60: 1055–1062, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 8: 1376–1382, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic K(ATP) channels control hepatic glucose production. Nature 434: 1026–1031, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Pocai A, Morgan K, Buettner C, Gutierrez-Juarez R, Obici S, Rossetti L. Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes 54: 3182–3189, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab 1: 53–61, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Portillo F, Carrasco M, Vallo JJ. Hypothalamic neuron projection to autonomic preganglionic levels related with glucose metabolism: a fluorescent labelling study in the rat. Neurosci Lett 210: 197–200, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Portillo F, Carrasco M, Vallo JJ. Separate populations of neurons within the paraventricular hypothalamic nucleus of the rat project to vagal and thoracic autonomic preganglionic levels and express c-Fos protein induced by lithium chloride. J Chem Neuroanat 14: 95–102, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Püschel GP. Control of hepatocyte metabolism by sympathetic and parasympathetic hepatic nerves. Anat Rec A Discov Mol Cell Evol Biol 280: 854–867, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Rogers RC, Nelson DO. Neurons of the vagal division of the solitary nucleus activated by the paraventricular nucleus of the hypothalamus. J Auton Nerv Syst 10: 193–197, 1984. [DOI] [PubMed] [Google Scholar]
- 46.Sandoval DA, Obici S, Seeley RJ. Targeting the CNS to treat type 2 diabetes. Nat Rev Drug Discov 8: 386–398, 2009. [DOI] [PubMed] [Google Scholar]
- 47.Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res 117: 305–312, 1976. [DOI] [PubMed] [Google Scholar]
- 48.Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol 205: 260–272, 1982. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz MW, Marks JL, Sipols AJ, Baskin DG, Woods SC, Kahn SE, Porte D Jr. Central insulin administration reduces neuropeptide Y mRNA expression in the arcuate nucleus of food-deprived lean (Fa/Fa) but not obese (fa/fa) Zucker rats. Endocrinology 128: 2645–2647, 1991. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz MW, Seeley RJ, Tschöp MH, Woods SC, Morton GJ, Myers MG, D'Alessio D. Cooperation between brain and islet in glucose homeostasis and diabetes. Nature 503: 59–66, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seoane-Collazo P, Fernø J, Gonzalez F, Diéguez C, Leis R, Nogueiras R, López M. Hypothalamic-autonomic control of energy homeostasis. Endocrine 50: 276–291, 2015. [DOI] [PubMed] [Google Scholar]
- 52.Shimazu T. Glycogen synthetase activity in liver: regulation by the autonomic nerves. Science 156: 1256–1257, 1967. [DOI] [PubMed] [Google Scholar]
- 53.Shimazu T. Innervation of the liver and glucoregulation: roles of the hypothalamus and autonomic nerves. Nutrition 12: 65–66, 1996. [DOI] [PubMed] [Google Scholar]
- 54.Shimazu T. Neuronal regulation of hepatic glucose metabolism in mammals. Diabetes Metab Rev 3: 185–206, 1987. [DOI] [PubMed] [Google Scholar]
- 55.Shimazu T, Fukuda A. Increased activities of glycogenolytic enzymes in liver after splanchnic-nerve stimulation. Science 150: 1607–1608, 1965. [DOI] [PubMed] [Google Scholar]
- 56.Shimazu T, Fukuda A, Ban T. Reciprocal influences of the ventromedial and lateral hypothalamic nuclei on blood glucose level and liver glycogen content. Nature 210: 1178–1179, 1966. [DOI] [PubMed] [Google Scholar]
- 57.Stanley S, Pinto S, Segal J, Perez CA, Viale A, DeFalco J, Cai X, Heisler LK, Friedman JM. Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically. Proc Natl Acad Sci USA 107: 7024–7029, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stern JE. Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus. J Physiol 537: 161–177, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol 194: 555–570, 1980. [DOI] [PubMed] [Google Scholar]
- 60.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci 6: 269–324, 1983. [DOI] [PubMed] [Google Scholar]
- 61.Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology 31: 410–417, 1980. [DOI] [PubMed] [Google Scholar]
- 62.Swanson LW, Sawchenko PE, Wiegand SJ, Price JL. Separate neurons in the paraventricular nucleus project to the median eminence and to the medulla or spinal cord. Brain Res 198: 190–195, 1980. [DOI] [PubMed] [Google Scholar]
- 63.Tasker JG, Dudek FE. Electrophysiological properties of neurones in the region of the paraventricular nucleus in slices of rat hypothalamus. J Physiol 434: 271–293, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uyama N, Geerts A, Reynaert H. Neural connections between the hypothalamus and the liver. Anat Rec A Discov Mol Cell Evol Biol 280: 808–820, 2004. [DOI] [PubMed] [Google Scholar]
- 65.van den Hoek AM, van Heijningen C, Schroder-van der Elst JP, Ouwens DM, Havekes LM, Romijn JA, Kalsbeek A, Pijl H. Intracerebroventricular administration of neuropeptide Y induces hepatic insulin resistance via sympathetic innervation. Diabetes 57: 2304–2310, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van den Pol AN. The magnocellular and parvocellular paraventricular nucleus of rat: intrinsic organization. J Comp Neurol 206: 317–345, 1982. [DOI] [PubMed] [Google Scholar]
- 67.Wulsin LR, Horn PS, Perry JL, Massaro J, D'Agostino R. Autonomic Imbalance as a Predictor of Metabolic Risks, Cardiovascular Disease, Diabetes, and Mortality Autonomic Imbalance Predicts CVD, DM, Mortality. J Clin Endocrinol Metab 100: 2443–2448, 2015. [DOI] [PubMed] [Google Scholar]
- 68.Yamashita H, Inenaga K, Koizumi K. Possible projections from regions of paraventricular and supraoptic nuclei to the spinal cord: electrophysiological studies. Brain Res 296: 373–378, 1984. [DOI] [PubMed] [Google Scholar]
- 69.Yi CX, la Fleur SE, Fliers E, Kalsbeek A. The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochim Biophys Acta 1802: 416–431, 2010. [DOI] [PubMed] [Google Scholar]
- 70.Yi CX, Serlie MJ, Ackermans MT, Foppen E, Buijs RM, Sauerwein HP, Fliers E, Kalsbeek A. A major role for perifornical orexin neurons in the control of glucose metabolism in rats. Diabetes 58: 1998–2005, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yi CX, Sun N, Ackermans MT, Alkemade A, Foppen E, Shi J, Serlie MJ, Buijs RM, Fliers E, Kalsbeek A. Pituitary adenylate cyclase-activating polypeptide stimulates glucose production via the hepatic sympathetic innervation in rats. Diabetes 59: 1591–1600, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zsombok A. Autonomic control and bariatric procedures. Auton Neurosci 177: 81–86, 2013. [DOI] [PubMed] [Google Scholar]
- 73.Zsombok A. Vanilloid receptors—do they have a role in whole body metabolism? Evidence from TRPV1. J Diabetes Complications 27: 287–292, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zsombok A, Gao H, Miyata K, Issa A, Derbenev AV. Immunohistochemical localization of transient receptor potential vanilloid type 1 and insulin receptor substrate 2 and their co-localization with liver-related neurons in the hypothalamus and brainstem. Brain Res 1398: 30–39, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zsombok A, Smith BN. Plasticity of central autonomic neural circuits in diabetes. Biochim Biophys Acta 1792: 423–431, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


