Abstract
Galangin, a flavonoid extracted from the root of the Alpinia officinarum Hence, has been shown to have anticancer properties against several types of cancer cells. However, the influence of galangin on human renal cancer cells remains to be elucidated. In the present study, proliferation of 786-0 and Caki-1 cells was suppressed following exposure to various doses of galangin. Cell invasion and wound healing assays were used to observe the effect of galangin on invasion and migration. The results demonstrated that Galangin inhibited cell invasion by suppressing the epithelial mesenchymal transition (EMT), with an increase in the expression of E-cadherin and decreased expression levels of N-cadherin and vimentin. The apoptosis induced by galangin was analyzed by flow cytometry. The results revealed that galangin induced apoptosis in a dose-dependent manner. The accumulation of reactive oxygen species (ROS) is an important contributing factor for the apoptosis of various types of cancer cell. The dichlorofluorescein-diacetate method was used to determine the level of ROS. Galangin induced the accumulation of intracellular ROS and malondialdehyde, and decreased the activities of total antioxidant and superoxide dismutase in renal cell carcinoma cells. Galangin exerted an antiproliferative effect and inhibited renal cell carcinoma invasion by suppressing the EMT. This treatment also induced apoptosis, accompanied by the production of ROS. Therefore, the present data suggested that galangin may have beneficial effects by preventing renal cell carcinoma growth, inhibiting cell invasion via the EMT and inducing cell apoptosis.
Keywords: galangin, renal cell carcinoma, epithelial-mesenchymal transition, invasion, apoptosis
Introduction
Renal cell carcinoma (RCC) accounts for 2–3% of all malignant diseases in adults, and is the most common type of kidney cancer. The incidence of this cancer has increased over several years, contributing to a steady increase in mortality rate in developing and developed countries (1–3). Cigarette smoking (4,5), obesity (6,7), hypertension (8) and certain environmental factors (9) are well-known risk factors for RCC. However, the majority of patients exhibit no identifiable risk factor, and the underlying pathogenic mechanisms of risk factors remain obscure. Roughly 1/3 patients with RCC are diagnosed at the late phase of the disease, missing the opportunity of surgical management. Almost all RCC pathological types are resistent to chemotherapeutics and radiation therapy. At present, immunotherapy is the major treatment for the late phase disease, however, the response rate is <20% (10). Although sorafenib and sunitinib have shown better response in metastatic RCC, the overall survival remains markedly poor (11,12). Consequently, novel antitumor agents and methods with high efficiency are urgently required.
Galangin (3,5,7-trihydroxyflavone; Fig. 1A) is a naturally active flavonoid from the root of Alpinia officinarum Hance, which has been used as a herbal medicine in Asian cultures for a variety of symptoms for centuries (13,14). Several previous studies have demonstrated that galangin has anticancer effects against several cancer types. Galangin induces apoptosis in gastric cancer cells via the regulation of ubiquitin carboxy-terminal hydrolase isozyme L1 and glutathione S-transferase (15). Galangin also inhibits the growth and metastasis of B16F10 melanoma cells (16). However, little is understood about its influence on RCC.
Figure 1.
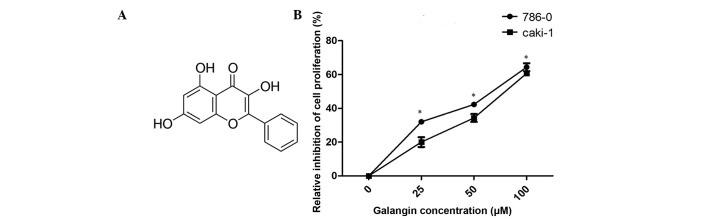
Effect of galangin on 786-0 and Caki-1 cell proliferation. (A) The chemical structure of galangin. (B) A CCK-8 assay revealed that cell growth was suppressed in the 786-0 and Caki-1 cells following treatment with Galangin (*P<0.05, vs. control group). Data are presented as mean ± standard deviation.
The present study investigated the effect of galangin on RCC and demonstrated that galangin inhibited RCC cell proliferation, cell invasion and induced apoptosis in vitro. It was also revealed that galangin inhibited RCC cell invasion by suppressing the epithelial-mesenchymal transition (EMT) and induced apoptosis, accompanied by the production of reactive oxygen species (ROS).
Materials and methods
Cell culture
The human RCC cell lines, Caki-1 and 786-0, were obtained from the Chinese Academy of Sciences Cell Bank (Shanghai, China). The cells were cultured in McCoy's 5A medium and RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), respectively, containing 100 mg/ml penicillin and 100 mg/ml streptomycin (Beyotime Institute of Biotechnology, Shanghai, China), and supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a humidified atmosphere with 5% CO2.
Agents and chemicals
All chemicals and reagents used in the present study were molecular biology grade. Galangin was purchased from Sigma-Aldrich (St. Louis, MO, USA), at a purity of 98%, and was dissolved in dimethyl sulfoxide (DMSO). Malondialdehyde (MDA), total antioxidant capacity (T-AOC) and superoxide dismutase (SOD) assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The production of ROS was measured using dichlorofluorescein-diacetate (DCFH-DA; Molecular Probes; Sigma-Aldrich). The primary antibodies for monoclonal rabbit anti-human SOD (1:1,000; cat. no. 2770), monoclonal rabbit anti-human catalase (1:1,000; cat. no. 12980), monoclonal rabbit anti-human E-cadherin (1:1,000; cat. no. 3195), monoclonal rabbit anti-human N-cadherin (1:1,000; cat. no. 13116), monoclonal rabbit anti-human vimentin (1:1,000; cat. no. 5741) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Cell proliferation assay
The Caki-1 and 786-0 cell lines were incubated with different concentrations of galangin (0 µM, 25 µM, 50 µM, 100 µM). Following a 24-h incubation the cells were seeded into 96-well plates at a density of 2×103 cells/well. Cell proliferation was determined using a Cell Counting Kit-8 (CCK-8; Beyotime Institute of Biotechnology) following the manufacturer's protocol. Absorbance was detected at the wavelength of 450 nm using a spectophotometer (Multiskan FC; Thermo Fisher Scientific, Inc.) Three wells were measured for cell viability per group.
Cell invasion assays
For the invasion assays, 5×104 cells in 200 ml serum-free medium were placed in the upper chamber of the Transwell (pore size, 8 mm; BD Biosciences, Franklin Lakes, NJ, USA) coated with Matrigel (BD Biosciences), according to the manufacturer's protocol. Medium containing 20% FBS was added to the lower chamber. Following incubation for 24 h at 37°C, the cells remaining on the upper membrane were removed and those on the lower surface of the membrane were fixed in 95% ethanol and stained with crystal violet (Beyotime Institute of Biotechnology). A total of 10 random fields were counted. All of the experiments were performed in triplicate.
Wound healing assay
The 786-0 and Caki-1 cells were grown to confluent monolayers, which were serum starved for 12 h. A 1 ml pipette tip was drawn across the center of the well to produce a clean wound area and the wounded cell layer was washed with fresh medium to remove loose cells. Immediately following wounding and an incubation for 24 h at 37°C in the presence or absence of 100 µM galangin, images of the wound healing process were captured digitally (magnification, ×200). The gap distance was normalized against the control level and was compared between 0 and 24 h. The mean values were obtained from at least three separate experiments.
Analysis of apoptosis
The RCC cells were seeded into 6-well plates overnight and were subsequently treated with different concentrations of galangin for 48 h. The cells were collected by trypsinization (Gibco; Thermo Fisher Scientific, Inc.) and were washed at least twice with cold phosphate-buffered saline (PBS). The cells were resuspended in 1X binding buffer (Beyotime Institute of Biotechnology) at a concentration of 1×106 cells/ml. A total of 5 µl annexin V-fluorescein isothiocyanate reagent and 10 µl propidium iodide were added to the cell suspension, and were incubated for 15 min at room temperature in the dark. The stained cells were analyzed by flow cytometry (Becton-Dickinson, San Jose, CA, USA). The data was analysed using FlowJo software (version 7.6.1; FlowJo LLC, OR, USA)
Detection of ROS
For measurement of intracellular ROS levels, the DCFH-DA method was used. The cells were harvested following treatment with Galangin and were subsequently washed once with ice-cold PBS. The cells were treated with DCFH-DA (at a final concentration of 10 mol/l in serum-free medium). Following incubation for 20 min at 37°C in the dark, the cells were washed twice with PBS. Intracellular ROS accumulation was measured by flow cytometry. The median fluorescence intensity values were calculated. All experiments were performed in triplicate.
SOD, T-AOC and MDA determination
The cells were seeded at 70% confluence into 6-well plates. After 24 h incubation, the cells were treated with different concentrations of galangin for 48 h. Following treatment, the cells were detached by trypsinization, collected by centrifugation (878 × g, for 10 min at 4°C) and resuspended in PBS. The suspensions were used immediately for SOD, T-AOC and MDA assays, according to the manufacturer's protocol.
Western blot analysis
The cells were lysed in radioimmunoprecipitation buffer (Nanjing KeyGen BioTech Co., Ltd., Nanjing, China), supplemented with protease inhibitors at 4°C for 1 h. The protein samples were collected from cell lysates. The protein content of the supernatants was determined with a BCA Protein Assay kit (Beyotime Institute of Biotechnology). The protein samples (40 µg per lane) were electrophoresed in 10% SDS-PAGE gels (Beyotime Institute of Biotechnology) and transferred onto polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). Following transfer, the membranes were blocked for 2 h at room temperature with 5% non-fat milk. The membranes were subsequently incubated with primary antibodies at 4°C overnight. The membranes were washed three times with Tris-buffered saline [20 mM Tris-HCl (pH 7.6), 137 mM NaCl], containing 0.01% Tween-20, and were subsequently incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:1,000; cat. no. A0208; Beyotime Institute of Biotechnology) at room temperature for 2 h. Following three washes with TBST the blots were detected using chemiluminescence using a microplate photometer (Multiskan FC; Thermo Fisher Scientific, Inc.), they were visualized Gel Doc XR+ system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The protein levels were determined by normalizing against the levels of GAPDH using a monoclonal rabbit anti-human antibody (1:1,000; cat. no. 5174; Cell Signaling Technology, Inc.).
Statistical analysis
The data are presented as the mean ± standard deviation from at least three independent experiments. Statistical calculations were performed using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). Statistical analysis was performed by Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Galangin inhibits the proliferation of RCC cells
The antiproliferative effects of galangin on RCC cells were measured using a CCK-8 assay (Fig. 1B). Galangin had a significant inhibitory effect on 786-0 and Caki-1 cell growth in a dose-dependent manner. The cell viabilities of the two cell lines, 786-0 and Caki-1, at 100 µM concentrations were 64.1 and 59.2%, respectively (Fig. 1B).
Galangin suppresses cell migration and invasion in vitro by inhibiting the EMT
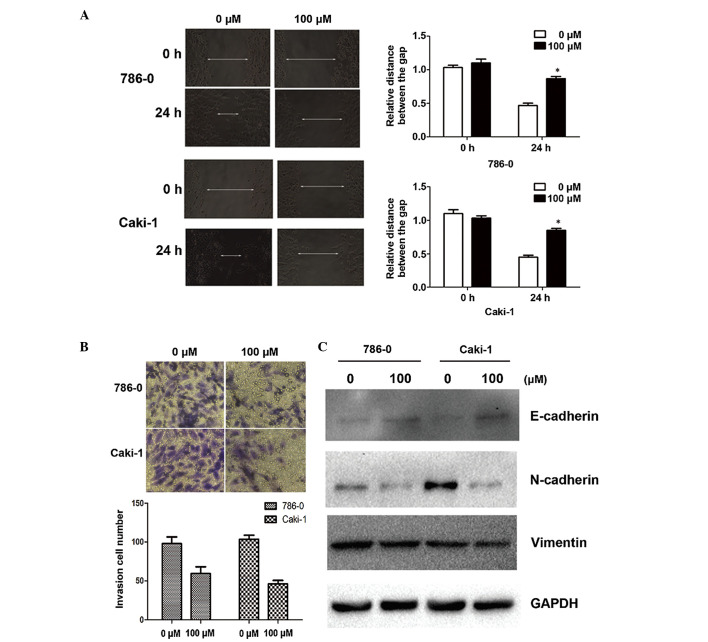
Since cell adhesion is one of the essential steps involved in cancer metastasis, the present study investigated the effect of galangin on cancer cell migration and invasion. As shown by the wound healing assay, 100 µM galangin significantly slowed the rate by which the cells migrated to the wounded area compared with the control group at 24 h (Fig. 2A). Furthermore, using an invasion assay, a marked reduction in the number of invasive cells was observed when the cells were treated with galangin at a concentration of 100µM for 24 h (Fig. 2B). Alterations in epithelial and mesenchymal markers, including N-cadherin, E-cadherin and vimentin, were determined by immunoblotting. The results demonstrated a reduction in the expression levels of N-cadherin and vimentin, and an increase in the expression of E-cadherin (Fig. 2C).
Figure 2.
Galangin suppresses invasion and migration in renal cell carcinoma cells. (A) Effects of galangin on cell invasion in RCC cell lines (magnification, ×200). (B) Expression of genes involved in the epithelial-mesenchymal transition were assessed following 48 h exposure to galangin (magnification, ×200). GAPDH was used as a loading control. (C) The effects of galangin on cell migration were assessed using a cell migration assay 48 h after treatment with galagin. GAPDH was used as a loading control (magnification ×200). The data are expressed as the mean ± standard deviation of the mean of at least three independent experiments (*P<0.05). RCC, renal cell carcinoma.
Effect of galangin on RCC cell apoptosis
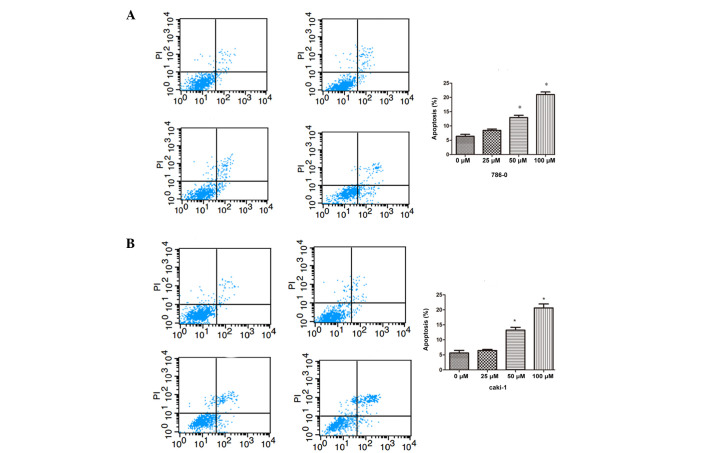
To assess the antitumor effect of different concentrations of galangin in 786-0 and Caki-1 cells, apoptosis was assessed by flow cytometry. As shown in Fig. 3, the number of apoptotic cells were significantly increased compared with the control group in a dose-dependent manner, in both the 786-0 and Caki-1 cells.
Figure 3.
Galangin induces cell apoptosis in 786-0 and Caki-1 cells. Apoptosis was measured by flow cytometric analysis using annexin V-fluorescein isothiocyanate and PI staining following treatment with different concentrations of Galangin for 48 h in (A) 786-0 and (B) Caki-1 cells (*P<0.05, compared with the control group). The data are expressed as the mean ± standard deviation of the mean of at least three independent experiments. PI, propidium iodide; AV, annexin V.
Increase of intracellular ROS following galangin treatment
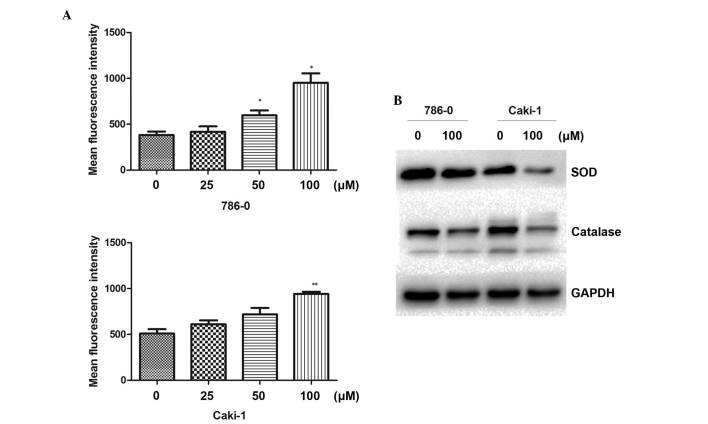
Following treatment of the cells with different concentrations of galangin for 48 h, the DCFH-DA mean fluorescence intensity was markedly increased and positively correlated with galangin concentration (Fig. 4A). The result revealed that galangin enhanced intracellular ROS levels in the RCC cells. To understand the molecular mechanisms involved in the ROS induced by galangin, the effect of galangin on the expression of ROS-associated proteins was next investigated. As shown in Fig. 4B, the galangin treatment group significantly downregulated the levels of SOD and catalase. These data demonstrated that the antitumor apoptotic effect of galangin was associated with intracellular ROS in RCC cells.
Figure 4.
Effect of Galangin on intracellular ROS. (A) Flow cytometry revealed that Galangin induced intracellular ROS at a large dose (*P<0.05; **P<0.01, compared with the control group). (B) 786-0 and Caki-1 cells were treated with 100 µM Galangin for 48 h and were subsequently lysed for Western blot analysis for SOD and catalase. The data are expressed as the mean ± standard deviation of the mean of at least three independent experiments. ROS, reactive oxygen species; SOD, superoxide dismutase.
SOD, T-AOC and MDA activity
The activity of the antioxidant enzyme, SOD, was markedly decreased in the galangin treatment group in both 786-0 and Caki-1 cells. The activity of T-AOC was significantly reduced compared with the control group in the RCC cell lines. MDA, the stable metabolite of lipid peroxidation products, was markedly increased following exposure to 100 µM galangin (Table I).
Table I.
Activity of SOD, T-AOC and MDA at different concentrations of galangin in 786-0 and Caki-1 cells.
| Galangin (µM) | SOD (U/mg) | T-AOC (nmol/mg) | MDA (nmol/mg) |
|---|---|---|---|
| 786-0 | |||
| 0 | 35.85±2.10 | 3.20±0.21 | 7.31±0.18 |
| 25 | 35.22±1.79 | 2.81±0.17 | 8.45±0.49 |
| 50 | 29.12±1.99a | 2.30±0.25a | 8.20±1.04 |
| 100 | 25.70±2.07b | 1.65±0.38b | 13.68±0.50c |
| Caki-1 | |||
| 0 | 31.2±1.04 | 1.88±0.19 | 3.76±0.43 |
| 25 | 32.1±1.05 | 1.65±0.16 | 4.08±.28 |
| 50 | 28.11±1.80 | 1.46±0.26 | 5.87±0.58b |
| 100 | 25.18±1.05b | 0.97±0.21b | 7.01±0.41c |
The data are expressed as the mean ± standard deviation.
P<0.05;
P<0.01;
P<0.001, compared with the untreated cells. SOD, superoxide dismutase; T-AOC, total antioxidant capacity; MDA, malondialdehyde.
Discussion
Numerous previous studies have concentrated on the effects of flavonoids in cancer treatment. Flavonoids are regarded as possible chemopreventive agents against various cancer types, which are generally non-toxic and reveal a diverse range of beneficial biological activities (17). It has been recognized as a promising cancer chemopreventive agent (17). However, no study has investigated the influence of galangin on RCC. The aim of the present study was to assess the effect of galangin on 786-0 and Caki-1 cells, and to gain preliminary insight into the underlying molecular mechanism.
In the present study, the anticancer activities of galangin against human 786-0 and Caki-1 RCC cells were assessed. Firstly, it was determined that galangin inhibited RCC cell proliferation in a dose-dependent manner. It was also revealed that galangin inhibited RCC invasion by suppressing the EMT and induced apoptosis, accompanied by the production of ROS.
The EMT is the differentiation switch to change epithelial polarized cells into motile mesenchymal cells, which is vital in embryonic development, fibrotic diseases, and invasion and metastasis of human cancer. The EMT allows cells to obtain fibroblast-like properties and reduces intercellular adhesion and increases motility (18–20). In addition, the EMT is dysregulated in cancer cells and is characterized by the acquisition of a mesenchymal phenotype, leading to increased motility, allowing the tumor cells to metastasize (21). The EMT is regulated by a variety of signaling pathways, including tumor growth factor-β, epidermal growth factor and hepatocyte growth factor (22). Decreased expression of E-cadherin is considered an important step in the progression of tumor metastasis and is a fundamental event in the EMT (23). In the present study, exposure of 786-0 and Caki-1 cells to galangin resulted in increased expression of E-cadherin and decreased expression levels of N-cadherin and vimentin. These results suggested that galangin suppressed the EMT in 786-0 and Caki-1 cells. The present study used wound healing and invasion assays to evaluate the migration and invasion of the cells. Treatment with100 µM significantly decreased the migratory and invasive capabilities of the RCC cells in vitro. Taken together, this data revealed that galangin inhibited tumor cell invasion and migration, which may modulate the EMT process.
The present study has also revealed that different concentrations of galangin may induce apoptosis of RCC cells. The effect of galangin on increasing the intracellular ROS level was noted. Accumulation of ROS is an important contributing factor for the apoptosis of various types of cancer cell (24,25). ROS, including O2−, H2O2 and hydroxyl radical, which are the side products of normal metabolism or environmental stress, can cause mitogenic to proliferative effects at low concentrations, and induce cell damage and cell death when ROS generation exceeds the cellular antioxidant defenses (26). In the present study, the intracellular ROS levels in 786-0 and Caki-1 cells treated with galangin were revealed to be increased at a high concentrations. However, previous reports demonstrate that flavonoids have certain antioxidant properties (27,28). To further confirm the present result, the expression levels of T-AOC, SOD and MDA were determined. MDA, a biomarker of ROS damage, was significantly increased following treatment. By contrast, the production of the antioxidant enzymes, T-AOC and SOD, were significantly decreased in the 786-0 and Caki-1 cells. Western blotting also demonstrated that treatment with 100 µM galangin suppressed the activity of two antioxidant enzymes, SOD and catalase. These results suggested that the proapoptotic effects of galangin may be mediated by the production of intracellular ROS at a large concentration.
Surgery has been the mainstay treatment for early stage RCC tumors. For advanced RCC, traditional chemotherapeutic agents are generally considered to be inefficient. Therefore, novel therapeutic approaches against RCC are necessary. The present research demonstrated that galangin exerted an antiproliferative property, inhibited cell invasion and induced apoptosis in RCC, which suggested that galangin may be a novel approach for the treatment of RCC.
In conclusion, galangin exerted an antiproliferative property in a dose-dependent manner. In addition, galangin induced cell apoptosis by increasing the intracellular concentration of ROS at a large dose and inhibited cell invasion by suppressing the EMT. Therefore, combining galangin with other drugs may increase the therapeutic potential. Further studies are required to determine the influence of galangin in the progression of RCC.
Acknowledgments
The present study was supported by the National Natural Science Foundation of China (no. 81270685).
References
- 1.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628–1631. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 2.Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): A literature review. Cancer Treat Rev. 2008;34:193–205. doi: 10.1016/j.ctrv.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Five-year survival after surgical treatment for kidney cancer: A population-based competing risk analysis. Cancer. 2007;109:1763–1768. doi: 10.1002/cncr.22600. [DOI] [PubMed] [Google Scholar]
- 4.Hunt JD, van der Hel OL, McMillan GP, Boffetta P, Brennan P. Renal cell carcinoma in relation to cigarette smoking: Meta-analysis of 24 studies. Int J Cancer. 2005;114:101–108. doi: 10.1002/ijc.20618. [DOI] [PubMed] [Google Scholar]
- 5.Yuan JM, Castelao JE, Gago-Dominguez M, Yu MC, Ross RK. Tobacco use in relation to renal cell carcinoma. Cancer Epidemiol Biomarkers Prev. 1998;7:429–433. [PubMed] [Google Scholar]
- 6.Bjørge Tand Tretli S, Engeland A. Relation of height and body mass index to renal cell carcinoma in two million Norwegian men and women. Am J Epidemiol. 2004;160:1168–1176. doi: 10.1093/aje/kwh345. [DOI] [PubMed] [Google Scholar]
- 7.van Dijk BA, Schouten LJ, Kiemeney LA, Goldbohm RA, van den Brandt PA. Relation of height, body mass, energy intake and physical activity to risk of renal cell carcinoma: Results from the Netherlands cohort study. Am J Epidemiol. 2004;160:1159–1167. doi: 10.1093/aje/kwh344. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin JK, Chow WH, Mandel JS, Mellemgaard A, McCredie M, Lindblad P, Schlehofer B, Pommer W, Niwa S, Adami HO. International renal-cell cancer study. VIII. Role of diuretics, other anti-hypertensive medications and hypertension. Int J Cancer. 1995;63:216–221. doi: 10.1002/ijc.2910630212. [DOI] [PubMed] [Google Scholar]
- 9.McCredie M, Pommer W, McLaughlin JK, Stewart JH, Lindblad P, Mandel JS, Mellemgaard A, Schlehofer B, Niwa S. International renal-cell cancer study. II. Analgesics. Int J Cancer. 1995;60:345–349. doi: 10.1002/ijc.2910600312. [DOI] [PubMed] [Google Scholar]
- 10.McDermott DF. Immunotherapy of metastatic renal cell carcinoma. Cancer. 2009;115(Suppl 10):2298–2305. doi: 10.1002/cncr.24236. [DOI] [PubMed] [Google Scholar]
- 11.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 13.Zhang HT, Luo H, Wu J, Lan LB, Fan DH, Zhu KD, Chen XY, Wen M, Liu HM. Galangin induces apoptosis of hepatocellular carcinoma cells via the mitochondrial pathway. World J Gastroenterol. 2010;16:3377–3384. doi: 10.3748/wjg.v16.i27.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capasso R, Mascolo N. Inhibitory effect of the plant flavonoid galangin on rat vas deferens in vitro. Life Sci. 2003;72:2993–3001. doi: 10.1016/S0024-3205(03)00232-7. [DOI] [PubMed] [Google Scholar]
- 15.Kim DA, Jeon YK, Nam MJ. Galangin induces apoptosis in gastric cancer cells via regulation of ubiquitin carboxy-terminal hydrolase isozyme L1 and glutathione S-transferase P. Food Chem Toxicol. 2012;50:684–688. doi: 10.1016/j.fct.2011.11.039. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Tang B, Huang Q, Hua Z. Galangin inhibits tumor growth and metastasis of B16F10 melanoma. J Cell Biochem. 2013;114:152–161. doi: 10.1002/jcb.24312. [DOI] [PubMed] [Google Scholar]
- 17.Heo MY, Sohn SJ, Au WW. Anti-genotoxicity of galangin as a cancer chemopreventive agent candidate. Mutat Res. 2001;488:135–150. doi: 10.1016/S1383-5742(01)00054-0. [DOI] [PubMed] [Google Scholar]
- 18.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 19.Shi J, Wang DM, Wang CM, Hu Y, Liu AH, Zhang YL, Sun B, Song JG. Insulin receptor substrate-1 suppresses transforming growth factor-beta1-mediated epithelial-mesenchymal transition. Cancer Res. 2009;69:7180–7187. doi: 10.1158/0008-5472.CAN-08-4470. [DOI] [PubMed] [Google Scholar]
- 20.Fan F, Samuel S, Evans KW, Lu J, Xia L, Zhou Y, Sceusi E, Tozzi F, Ye XC, Mani SA, Ellis LM. Overexpression of snail induces epithelial-mesenchymal transition and a cancer stem cell-like phenotype in human colorectal cancer cells. Cancer Med. 2012;1:5–16. doi: 10.1002/cam4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito RA, Watabe T, Horiguchi K, Kohyama T, Saitoh M, Nagase T, Miyazono K. Thyroid transcription factor-1 inhibits transforming growth factor-beta-mediated epithelial-to-mesenchymal transition in lung adenocarcinoma cells. Cancer Res. 2009;69:2783–2791. doi: 10.1158/0008-5472.CAN-08-3490. [DOI] [PubMed] [Google Scholar]
- 22.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 24.El-Najjar N, Chatila M, Moukadem H, Vuorela H, Ocker M, Gandesiri M, Schneider-Stock R, Gali-Muhtasib H. Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis. 2010;15:183–195. doi: 10.1007/s10495-009-0421-z. [DOI] [PubMed] [Google Scholar]
- 25.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems and apoptosis. Free Radic Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gamaley IA, Klyubin IV. Roles of reactive oxygen species: Signaling and regulation of cellular functions. Int Rev Cytol. 1999;188:203–255. doi: 10.1016/S0074-7696(08)61568-5. [DOI] [PubMed] [Google Scholar]
- 27.Parhiz H, Roohbakhsh A, Soltani F, Rezaee R, Iranshahi M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother Res. 2015;29:323–31. doi: 10.1002/ptr.5256. [DOI] [PubMed] [Google Scholar]
- 28.Agati G, Azzarello E, Pollastri S, Tattini M. Flavonoids as antioxidants in plants: location and functional significance. Plant Sci. 2012 Nov;196:67–76. doi: 10.1016/j.plantsci.2012.07.014. [DOI] [PubMed] [Google Scholar]