Abstract
Ultrasound Doppler and near-infrared spectroscopy (NIRS) are routinely used for noninvasive monitoring of peripheral hemodynamics in both clinical and experimental settings. However, the comparative ability of these methodologies to detect changes in microvascular and whole limb hemodynamics during pharmacological manipulation of vascular smooth muscle receptors located at varied locations within the arterial tree is unknown. Thus, in 10 healthy subjects (25 ± 2 yr), changes in resting leg blood flow (ultrasound Doppler; femoral artery) and muscle oxygenation (oxyhemoglobin + oxymyoglobin; vastus lateralis) were simultaneously evaluated in response to intra-arterial infusions of phenylephrine (PE, 0.025–0.8 μg·kg−1·min−1), BHT-933 (2.5–40 μg·kg−1·min−1), and angiotensin II (ANG II, 0.5–8 ng·kg−1·min−1). All drugs elicited significant dose-dependent reductions in leg blood flow and oxyhemoglobin + oxymyoglobin. Significant relationships were found between ultrasound Doppler and NIRS changes across doses of PE (r2 = 0.37 ± 0.08), BHT-933 (r2 = 0.74 ± 0.06), and ANG II (r2 = 0.68 ± 0.13), with the strongest relationships evident with agonists for receptors located preferentially “downstream” in the leg microcirculation (BHT-933 and ANG II). Analyses of drug potency revealed similar EC50 between ultrasound Doppler and NIRS measurements for PE (0.06 ± 0.02 vs. 0.10 ± 0.01), BHT-933 (5.0 ± 0.9 vs. 4.5 ± 1.3), and ANG II (1.4 ± 0.8 vs. 1.3 ± 0.3). These data provide evidence that both ultrasound Doppler and NIRS track pharmacologically induced changes in peripheral hemodynamics and are equally capable of determining drug potency. However, considerable disparity was observed between agonist infusions targeting different levels of the arterial tree, suggesting that receptor landscape is an important consideration for proper interpretation of hemodynamic monitoring with these methodologies.
Keywords: vascular imaging, α-adrenergic, angiotensin II, vasoconstriction, microcirculation
a variety of noninvasive methods have been devised to assess peripheral hemodynamics in humans. Ultrasound Doppler has grown in popularity because of its noninvasive nature and relative ease of use. However, the spatial resolution of this methodology limits measurements to larger caliber vessels and, in the case of the arm or leg, provides determination of bulk limb blood flow that includes perfusion of skin, bone, and skeletal muscle. In contrast, near-infrared (NIR) spectroscopy (NIRS) has been developed as a viable method of assessing tissue oxygenation and, under steady-state conditions, microcirculatory blood flow (16). While our group (26) and others (4, 7, 18) have concomitantly used these noninvasive methodologies in an effort to comprehensively evaluate peripheral hemodynamics, little work has been done to determine to what degree these methods are related or to establish whether one method is preferable to another under certain experimental or clinical conditions.
Knowledge of the interchangeability between these methodologies may be particularly informative when determining changes in vascular tone elicited by pharmacological agents that target specific receptor subtypes located at distinct levels of the arterial tree. One of the best-described examples of this heterogeneous distribution of vascular smooth muscle receptors in the peripheral vasculature is the α-adrenergic pathway. Using only ultrasound Doppler, we have identified a unique spatial distribution for α-adrenergic receptor subtypes in humans, with α1-adrenergic receptors preferentially localized proximally and α2-adrenergic receptors located more distally in the leg vasculature (27). However, a clear indication of how bulk limb blood flow relates to microcirculatory blood flow is needed to fully understand the functional consequence and potential therapeutic implications for this heterogeneity in the vascular smooth muscle receptor landscape.
Therefore, the purpose of the current study was to determine the relationship between ultrasound Doppler and NIRS assessments of skeletal muscle hemodynamics and to evaluate potential differences in this relationship using pharmacological agents acting on different portions of the arterial tree. We hypothesized that these methods would be significantly related in terms of drug-induced changes in peripheral hemodynamics in response to local drug delivery and that both methodologies would detect similar levels of drug potency (EC50). However, we expected a difference in the nature of the relationship between methods depending on the drug used; specifically, we anticipated the best relationship between ultrasound Doppler and NIRS in response to BHT-933 and angiotensin II (ANG II), drugs that primarily target distal portions of the leg microcirculation as opposed to phenylephrine (PE), which preferentially targets the more proximal vasculature of the leg (2, 27).
MATERIALS AND METHODS
Subjects and General Procedures
Ten young, healthy males participated in the present study (Table 1). All subjects were nonsmokers, normotensive, and free from overt cardiovascular disease, as determined by health history questionnaire and physical examination. Protocols were approved by the local ethics committee of Copenhagen and Frederiksberg, in accordance with the Declaration of Helsinki, and written informed consent from subjects was obtained according to the local ethics committee's guidelines. All studies were performed in a thermoneutral environment with subjects in a semirecumbent position. Subjects reported to the laboratory in a fasted state and without caffeine or alcohol use for 12 and 24 h, respectively. They also had not performed any exercise within the past 24 h. Arterial and venous catheters were placed under local anesthesia (lidocaine, 5 ml, 20 mg/ml) in a retrograde fashion in the right common femoral artery and vein using sterile technique. After catheter placement, subjects recovered for 30 min before any drug infusions. A portion of the ultrasound Doppler data were generated from previous published studies by our group (2, 27); additional analyses were applied to address the novel hypothesis of this study, making direct comparisons to NIRS-derived measurements.
Table 1.
Subject Characteristics
| Variable | |
|---|---|
| Age, yr | 26 ± 2 |
| Height, cm | 187 ± 2 |
| Weight, kg | 81 ± 4 |
| Body mass index, kg/m2 | 23 ± 1 |
| Heart rate, beats/min | 60 ± 3 |
| Mean arterial blood pressure, mmHg | 88 ± 2 |
| Leg blood flow, ml/min | 403 ± 60 |
| Leg vascular conductance, ml·min−1·mmHg−1 | 4.6 ± 0.7 |
Values are means ± SE.
Drugs
PE (Danish County Pharmaceutical Corporation, SAD) was used as a specific α1-adrenergic agonist. BHT-933 (Sigma-Aldrich, Denmark) was used as a specific α2-adrenergic agonist. Angiotensin-II (ANG II, Clinalfa, Switzerland) was used as an AT receptor agonist. A range of drug doses were administered (PE, 0.025, 0.05, 0.1, 0.2, 0.4, 0.8 μg·kg−1·min−1; BHT-933, 2.5, 5, 10, 20, 40 μg·kg−1·min−1; and ANG II, 0.5, 1, 2, 4, 8 ng·kg−1·min−1). Each dose was infused for 2 min to achieve a steady-state hemodynamic response. Ultrasound Doppler and NIRS measurements were performed concurrently and continuously during each drug infusion.
Measurements
Femoral blood flow.
The ultrasound machine (model CFM 800, GE Medical) was equipped with a mechanical sector transducer operating at an imaging frequency of 7.5 MHz. Vessel diameter was determined at a perpendicular angle along the central axis of the scanned area, where the best spatial resolution was achieved. The femoral artery was insonated distal to the inguinal ligament for dynamic recordings of diameter throughout a cardiac cycle. The maximum diameter (systole) was used for calculation of blood flow. The blood velocity profile was obtained using the same transducer with a Doppler frequency of 4.0–6.0 MHz, operated in the high-pulsed repetition frequency mode (4–36 kHz) with a sample volume of 5 mm. All blood velocity measurements were obtained with a 46–50 insonation angle. At all sample points, we obtained both diameter of the femoral artery and, ∼20–30 s later, an angle-corrected, time- and space-averaged, and intensity-weighted mean blood velocity (Vmean) (Echopac Software, GE Medical and PowerLab, ADInstruments). With the use of arterial diameter and Vmean, femoral blood flow was calculated as: FBF = Vmean·π (vessel diameter/2)2·60, where blood flow is in milliliters per minute.
Near-infrared spectroscopy.
NIRS (NIRO300, Hamamatsu, Japan) was used to determine muscle oxygenation of the vastus lateralis muscle. Muscle oxygenation was determined by the oxyhemoglobin signal (6), which cannot differentiate between oxyhemoglobin (HbO2) and oxymyoglobin (MbO2); thus we express the data as a conglomerate signal (HbO2 + MbO2). The site over the vastus lateralis was cleaned, and double-sided adhesive tape was used to secure the optodes in place. Optodes were positioned inside a rubber holder with a fixed distance of 4 cm between emitting and receiving optodes for an effective penetrating depth of 2 cm. The optodes were then covered and further secured with an opaque wrap. The data were acquired at 0.5 Hz, and 30-s averages were created at baseline and during the last minute of drug infusion for each dose. To normalize the data to individual maximal physiological changes, the total labile signal was determined by placing a cuff proximal to the NIRS probes inflated to suprasystolic levels (250 mmHg) for 10 min to elicit complete deoxygenation. The pharmacologically induced changes in the NIRS signal were then expressed as a percentage of this maximal change (%total labile signal).
Data Analysis
The EC50 (half-maximal effective concentration) was calculated on an individual basis using a sigmoidal parameter to estimate the vascular sensitivity to the pharmacological agonists (Biodatafit, v.1.02, Castro, CA). To determine the relationship between methods, the slopes and coefficient of determinations were calculated on an individual basis and compared between drug trials. Repeated-measures ANOVA and paired t-tests were used where appropriate. The level of significance was established at P ≤ 0.05. Data are presented as means ± SE.
RESULTS
Subject characteristics are presented in Table 1. The dose-response curves and drug potency (EC50) for PE, BHT-933, and ANG II are presented in Fig. 1. There was a significant relationship between ultrasound Doppler and NIRS changes for all drugs (Fig. 2), although the nature of this relationship was significantly different between drugs, with PE having the lowest coefficient of determination. A significant drug-related difference in the femoral arterial diameter response to each pharmacological agonist was also observed, with the highest doses of PE inducing a much greater change in diameter (8.66 ± 0.27 to 5.79 ± 0.51 mm) compared with ANG II (8.75 ± 0.28 to 8.36 ± 0.53 mm) and BHT-933 (8.63 ± 0.22 to 7.79 ± 0.22 mm). Ultrasound Doppler responses presented in Fig. 1, D–F, and Fig. 2 have been previously reported (2, 27) and are presented here for the purposes of comparison with NIRS assessment.
Fig. 1.
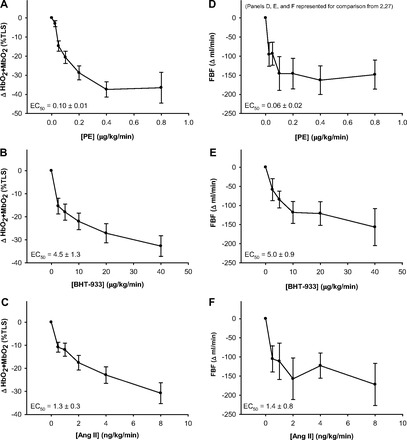
Dose response curves for the α1-agonist phenylephrine (PE), the α2-agonist BHT-933, and the AT receptor agonist angiotensin II (ANG II) assessed via near-infrared spectroscopy (NIRS; A–C) and femoral blood flow (FBF, ultrasound Doppler; D–F). Ultrasound Doppler responses illustrated in D–F have been previously reported (2, 27) and are presented here for the purposes of comparison with NIRS assessment.
Fig. 2.
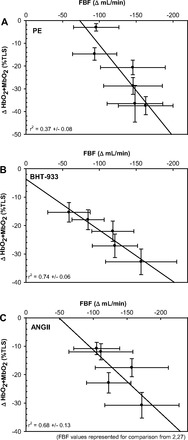
Relationship between femoral blood flow (FBF, ultrasound Doppler) and muscle oxygenation (NIRS) during pharmacological vasoconstriction induced by the α1-agonist phenylephrine (PE; A), the α2-agonist BHT-933 (B), and the AT receptor agonist angiotensin II (ANG II; C). Ultrasound Doppler responses have been previously reported (2, 27) and are presented here for the purposes of comparison with NIRS assessment.
DISCUSSION
The main finding of the current study was that ultrasound Doppler and NIRS methodologies detect similar changes in limb hemodynamics in response to intra-arterial infusion of three distinct vasoconstrictor drugs (PE, BHT-933, and ANG II), both in terms of drug potency (EC50) and efficacy (dose-dependent changes). Significant relationships were found between the two methodologies for all drugs. However, considerable disparity in correlative analysis was observed between agonist infusions targeting different levels of the arterial tree. The best relationships were evident with agonists preferentially targeting receptor groups located more distal in the leg microcirculation (BHT-933 and ANG II) (2, 27), while a more modest correlation was observed for PE, which likely reflects the greater distribution of α1-receptors in the proximal compared with distal portions of the arterial tree. Together, these data provide evidence that both ultrasound Doppler and NIRS are equally sensitive to detecting pharmacologically induced changes in peripheral hemodynamics and drug potency and also emphasize that receptor landscape is an important consideration for proper interpretation of hemodynamic monitoring with these methodologies.
Assessment of Macro vs. Microcirculatory Hemodynamics
While various methods have been devised for assessment of peripheral hemodynamics in humans, ultrasound Doppler and NIRS have emerged as gold standards of noninvasive testing. When equipped with a duplex linear array probe, ultrasound Doppler is capable of simultaneous, high-resolution measurements of both vessel diameter (12 MHz) and blood velocity (5 MHz), enabling beat-to-beat determination of limb blood flow. However, ultrasound Doppler measurements are limited to large conduit vessels and thus are most often used to determine bulk limb blood flow. In contrast, NIRS exploits the principle that NIR light easily penetrates tissues and is maximally absorbed by large vessels to provide measurements of oxygenated and deoxygenated hemoglobin and myoglobin in the microcirculation (20). Since changes in the absorption of NIR light are proportional to changes in the relative concentrations of these molecules under steady-state conditions when oxygen demand is constant, NIR absorption is thought to reflect changes in oxygen supply and thus provide an index of microcirculatory blood flow under resting conditions (9).
Though these noninvasive methodologies are often used in an effort to comprehensively evaluate peripheral hemodynamics, the degree to which the two methods are able to track hemodynamic changes, and in particular the sensitivity to detect pharmacologically induced vasoconstriction, is not well understood. In one of the only studies directly comparing these methodologies, Fadel et al. (10) investigated the potential link between ultrasound Doppler and NIRS in both humans and anesthetized rats. In this study, reflex sympathetic vasoconstriction measured in the forearm with ultrasound Doppler and NIRS were significantly related. These results were confirmed in a rodent model, revealing a significant relationship between the methodologies elicited by direct sympathetic nerve stimulation (10).
Findings from the present study extend these earlier findings in several important ways. First, we have identified that ultrasound Doppler and NIRS are equally efficacious in detecting changes in blood flow in the leg (Fig. 1), an ambulatory limb with distinct differences in both vascular function (21) and vascular smooth muscle receptor sensitivity (19) compared with the arm. We have also used an array of discrete pharmacological agents to elicit robust vasoconstriction via various vascular smooth muscle receptor types with differing distribution across the arterial tree. This pharmacological approach also afforded the opportunity to examine the ability of these two methodologies to determine drug potency, as quantified by half-maximal effective concentration (EC50). To our knowledge, this is the first study using both ultrasound Doppler and NIRS to assess EC50 and to report comparable values between the two methods (Fig. 1).
Receptor-Specific Hemodynamic Responses
Though a clear relationship was present between ultrasound Doppler and NIRS for all drugs, a clear disparity in the strength of the relationship between the two methods was identified. The best relationships were seen with BHT-933 (an α2-adrenergic receptor agonist) and ANG II (an AT receptor agonist), where coefficients of determination exceeded 0.7 for all subjects (Fig. 2). In contrast, the relationship between methodologies was substantially lower for PE (an α1-adrenergic receptor agonist). This difference is, at least in part, mediated by differential receptor landscapes across the leg arterial tree. Indeed, we have previously identified functional α1-adrenergic receptors in the upstream portions of the femoral artery capable of reducing arterial diameter by nearly 50% in response to PE, whereas postjunctional α2-adrenergic receptors are preferentially expressed in the more distal portions of the femoral artery and produce minimal changes in conduit artery diameter (27). These previous findings in humans are supported by earlier work in animals, indicating a similar pattern of α-adrenergic receptor distribution (1), and together indicate a hierarchy of receptor subtypes that may be relevant to the regulation of blood flow and arterial blood pressure.
In the present study, we observed a 30–40% reduction in femoral artery diameter during the highest doses of PE, whereas no significant reductions in femoral artery diameter were observed during any dose of ANG II or BHT-933, as reported previously (2, 27). These findings providing evidence for a paucity of α2-adrenergic and ANG II receptors at the level of the conduit vessel, providing evidence for a differential “receptor landscape” may partially explain why the relationship between ultrasound Doppler and NIRS is lowest during PE infusion (Fig. 2). Indeed, an assessment of microvascular hemodynamics (i.e., NIRS) may not track perfectly with conduit artery limb blood flow measurements because of the ability of PE to bind at multiple sites along the arterial tree, whereas better agreement would be expected when infusing drugs acting predominantly in the skeletal muscle microcirculation (i.e., ANG II and BHT-933).
Clinical Implications
There is now considerable evidence supporting the concept that peripheral artery disease is characterized by formation of atherosclerotic lesions at the level of the conduit vessels in the lower limbs (15, 22, 24). However, these medium and large caliber vessels are inexorably linked to the downstream skeletal muscle microcirculation, where the large majority of vasomotor regulation occurs. This obvious but often overlooked association between different levels of the arterial tree is increasingly recognized as an important consideration in the etiology and progression of cardiovascular disease, particularly with respect to therapies targeting the vascular endothelium and the sympathetic nervous system (17). In this context, the present data concerning the simultaneous determination of macro- and microcirculatory hemodynamics in the peripheral circulation may be particularly important as we seek to better define the integrative relationship between conduit and resistance vessel beds. Findings from the present study also support the utility of concurrent ultrasound Doppler and NIRS measurements for exploring regional patterns of adaptation in patients with peripheral artery disease.
Experimental Considerations
We recognize the known effect of adipose tissue thickness on NIR light absorption and scatter (13, 25) and therefore cannot exclude the possibility that differences in adipose thickness may have affected our NIRS measurements. This concern is somewhat mitigated by use of a large separation between source and detector optodes in the present study, which provides a maximum measurement depth of ∼2 cm. This depth is more than sufficient to reach skeletal muscle tissue in young healthy adults, where normal adipose tissue thickness of the vastus lateralis is <10 mm (3, 23). We also recognize the potential of high-irradiance NIR light to provoke nitric oxide (NO) release (5, 12). While a very lower power (<2 mW/m2) NIR device was employed in the present study, there are currently no data addressing the potential impact of this device on NO bioavailability. Further work is necessary to elucidate the interaction between low-power NIR and NO release in humans. By design, the present study compared the capabilities of ultrasound Doppler and NIRS-derived measures to detect hemodynamics changes at varying portions of the arterial tree. Although these are the two most commonly used methods for hemodynamic monitoring, we acknowledge that the addition of magnetic resonance imaging (MRI) or contrast perfusion ultrasound measures could provide a more comprehensive examination of skeletal muscle microvascular function. Finally, it is noteworthy that the technology of both ultrasound Doppler (8) and NIRS devices (11) is continually advancing, including the development of multichannel, spatially resolved NIR devices (14). Thus caution is warranted when extrapolating results from the present study to other measurement devices that may differ in spatial or temporal resolution.
Conclusions
These data provide evidence that both ultrasound Doppler and NIRS track pharmacologically induced changes in peripheral hemodynamics to a similar degree and are equally capable of determining drug potency. However, disparity in responses with different drugs suggests that receptor location in the arterial tree is an important consideration for proper interpretation of hemodynamic monitoring with these methodologies.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.J.I. and D.W.W. analyzed data; S.J.I., P.J.F., M.S., and D.W.W. interpreted results of experiments; S.J.I. and D.W.W. prepared figures; S.J.I. and D.W.W. drafted manuscript; S.J.I., P.J.F., M.B., and D.W.W. edited and revised manuscript; S.J.I., P.J.F., M.B., and D.W.W. approved final version of manuscript; P.J.F., M.B., M.S., and D.W.W. conception and design of research; P.J.F., M.B., M.S., and D.W.W. performed experiments.
ACKNOWLEDGMENTS
This work was funded in part by American Heart Association Grant 0835209N (to D. W. Wray).
REFERENCES
- 1.Anderson KM, Faber JE. Differential sensitivity of arteriolar alpha 1- and alpha 2-adrenoceptor constriction to metabolic inhibition during rat skeletal muscle contraction. Circ Res 69: 174–184, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Brothers RM, Haslund ML, Wray DW, Raven PB, Sander M. Exercise-induced inhibition of angiotensin II vasoconstriction in human thigh muscle. J Physiol 577: 727–737, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardinale M, Ferrari M, Quaresima V. Gastrocnemius medialis and vastus lateralis oxygenation during whole-body vibration exercise. Med Sci Sports Exerc 39: 694–700, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Chin LM, Heigenhauser GJ, Paterson DH, Kowalchuk JM. Pulmonary O2 uptake and leg blood flow kinetics during moderate exercise are slowed by hyperventilation-induced hypocapnic alkalosis. J Appl Physiol 108: 1641–1650, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng 40: 516–533, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeLorey DS, Kowalchuk JM, Paterson DH. Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate-intensity exercise. J Appl Physiol 95: 113–120, 2003. [DOI] [PubMed] [Google Scholar]
- 7.DeLorey DS, Shaw CN, Shoemaker JK, Kowalchuk JM, Paterson DH. The effect of hypoxia on pulmonary O2 uptake, leg blood flow and muscle deoxygenation during single-leg knee-extension exercise. Exp Physiol 89: 293–302, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Ducas R, Tsang W, Chong AA, Jassal DS, Lang RM, Leong-Poi H, Chan KL. Echocardiography and vascular ultrasound: new developments and future directions. Can J Cardiol 29: 304–316, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Edwards AD, Richardson C, van der Zee P, Elwell C, Wyatt JS, Cope M, Delpy DT, Reynolds EO. Measurement of hemoglobin flow and blood flow by near-infrared spectroscopy. J Appl Physiol 75: 1884–1889, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Fadel PJ, Keller DM, Watanabe H, Raven PB, Thomas GD. Noninvasive assessment of sympathetic vasoconstriction in human and rodent skeletal muscle using near-infrared spectroscopy and Doppler ultrasound. J Appl Physiol 96: 1323–1330, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari M, Muthalib M, Quaresima V. The use of near-infrared spectroscopy in understanding skeletal muscle physiology: recent developments. Philos Trans A Math Phys Eng Sci 369: 4577–4590, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Hashmi JT, Huang YY, Osmani BZ, Sharma SK, Naeser MA, Hamblin MR. Role of low-level laser therapy in neurorehabilitation. Pm R 2: S292–S305, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Homma S, Fukunaga T, Kagaya A. Influence of adipose tissue thickness on near infrared spectroscopic signal in the measurement of human muscle. J Biomed Opt 1: 418–424, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Kek K, Samizo M, Miyakawa T, Kudo N, Yamamoto K. Imaging of regional differences of muscle oxygenation during exercise using spatially resolved NIRS. Conf Proc IEEE Eng Med Biol Soc 3: 2622–2625, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Kroger K, Kucharczik A, Hirche H, Rudofsky G. Atherosclerotic lesions are more frequent in femoral arteries than in carotid arteries independent of increasing number of risk factors. Angiology 50: 649–654, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Mancini DM, Bolinger L, Li H, Kendrick K, Chance B, Wilson JR. Validation of near-infrared spectroscopy in humans. J Appl Physiol 77: 2740–2747, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Padilla J, Jenkins NT, Laughlin MH, Fadel PJ. Blood pressure regulation. VIII: resistance vessel tone and implications for a pro-atherogenic conduit artery endothelial cell phenotype. Eur J Appl Physiol 2013. July 17 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker BA, Smithmyer SL, Ridout SJ, Ray CA, Proctor DN. Age and microvascular responses to knee extensor exercise in women. Eur J Appl Physiol 103: 343–351, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Pawelczyk JA, Levine BD. Heterogeneous responses of human limbs to infused adrenergic agonists: a gravitational effect? J Appl Physiol 92: 2105–2113, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Piantadosi CA, Duhaylongsod FG. Near infrared spectroscopy: in situ studies of skeletal and cardiac muscle. Adv Exp Med Biol 361: 157–161, 1994. [DOI] [PubMed] [Google Scholar]
- 21.Proctor DN, Newcomer SC. Is there a difference in vascular reactivity of the arms and legs? Med Sci Sports Exerc 38: 1819–1828, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Ross R, Wight TN, Strandness E, Thiele B. Human atherosclerosis. I. Cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am J Pathol 114: 79–93, 1984. [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan TE, Erickson ML, Brizendine JT, Young HJ, McCully KK. Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: correcting for blood volume changes. J Appl Physiol 113: 175–183, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol 15: 1512–1531, 1995. [DOI] [PubMed] [Google Scholar]
- 25.van Beekvelt MC, Borghuis MS, van Engelen BG, Wevers RA, Colier WN. Adipose tissue thickness affects in vivo quantitative near-IR spectroscopy in human skeletal muscle. Clin Sci (Lond) 101: 21–28, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Wray DW, Fadel PJ, Keller DM, Ogoh S, Sander M, Raven PB, Smith ML. Dynamic carotid baroreflex control of the peripheral circulation during exercise in humans. J Physiol 559: 675–684, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wray DW, Fadel PJ, Smith ML, Raven P, Sander M. Inhibition of alpha-adrenergic vasoconstriction in exercising human thigh muscles. J Physiol 555: 545–563, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


