Abstract
Background
People with haematological disorders are frequently at risk of severe or life‐threatening bleeding as a result of thrombocytopenia (reduced platelet count). This is despite the routine use of prophylactic platelet transfusions to prevent bleeding once the platelet count falls below a certain threshold. Platelet transfusions are not without risk and adverse events may be life‐threatening. A possible adjunct to prophylactic platelet transfusions is the use of antifibrinolytics, specifically the lysine analogues tranexamic acid (TXA) and epsilon aminocaproic acid (EACA). This is an update of a Cochrane review first published in 2013.
Objectives
To determine the efficacy and safety of antifibrinolytics (lysine analogues) in preventing bleeding in people with haematological disorders.
Search methods
We searched for randomised controlled trials (RCTs) in the Cochrane Central Register of Controlled Trials (The Cochrane Library 2016, Issue 3), MEDLINE (from 1946), Embase (from 1974), CINAHL (from 1937), the Transfusion Evidence Library (from 1950) and ongoing trial databases to 07 March 2016.
Selection criteria
We included RCTs involving participants with haematological disorders, who would routinely require prophylactic platelet transfusions to prevent bleeding. We only included trials involving the use of the lysine analogues TXA and EACA.
Data collection and analysis
Two review authors independently screened all electronically‐derived citations and abstracts of papers, identified by the review search strategy, for relevancy. Two review authors independently assessed the full text of all potentially relevant trials for eligibility, completed the data extraction and assessed the studies for risk of bias using The Cochrane Collaboration's 'Risk of bias' tool. We requested missing data from one author but the data were no longer available. The outcomes are reported narratively: we performed no meta‐analyses because of the heterogeneity of the available data.
Main results
We identified three new studies in this update of the review. In total seven studies were eligible for inclusion, three were ongoing RCTs and four were completed studies. The four completed studies were included in the original review and the three ongoing studies were included in this update. We did not identify any RCTs that compared TXA with EACA.
Of the four completed studies, one cross‐over TXA study (eight participants) was excluded from the outcome analysis because it had very flawed study methodology. Data from the other three studies were all at unclear risk of bias due to lack of reporting of study methodology.
Three studies (two TXA (12 to 56 participants), one EACA (18 participants) reported in four articles (published 1983 to 1995) were included in the narrative review. All three studies compared the drug with placebo. All three studies included adults with acute leukaemia receiving chemotherapy. One study (12 participants) only included participants with acute promyelocytic leukaemia. None of the studies included children. One of the three studies reported funding sources and this study was funded by a charity.
We are uncertain whether antifibrinolytics reduce the risk of bleeding (three studies; 86 participants; very low‐quality evidence). Only one study reported the number of bleeding events per participant and there was no difference in the number of bleeding events seen during induction or consolidation chemotherapy between TXA and placebo (induction; 38 participants; mean difference (MD) 1.70 bleeding events, 95% confidence interval (CI) ‐0.37 to 3.77: consolidation; 18 participants; MD ‐1.50 bleeding events, 95% CI ‐3.25 to 0.25; very low‐quality evidence). The two other studies suggested bleeding was reduced in the antifibrinolytic study arm, but this was statistically significant in only one of these two studies.
Two studies reported thromboembolism and no events occurred (68 participants, very low‐quality evidence).
All three studies reported a reduction in platelet transfusion usage (three studies, 86 participants; very low‐quality evidence), but this was reported in different ways and no meta‐analysis could be performed. No trials reported the number of platelet transfusions per participant. Only one study reported the number of platelet components per participant and there was a reduction in the number of platelet components per participant during consolidation chemotherapy but not during induction chemotherapy (consolidation; 18 participants; MD ‐5.60 platelet units, 95% CI ‐9.02 to ‐2.18: induction; 38 participants, MD ‐1.00 platelet units, 95% CI ‐9.11 to 7.11; very low‐quality evidence).
Only one study reported adverse events of TXA as an outcome measure and none occurred. One study stated side effects of EACA were minimal but no further information was provided (two studies, 74 participants, very low‐quality evidence).
None of the studies reported on the following pre‐specified outcomes: overall mortality, adverse events of transfusion, disseminated intravascular coagulation (DIC) or quality of life (QoL).
Authors' conclusions
Our results indicate that the evidence available for the use of antifibrinolytics in haematology patients is very limited. The trials were too small to assess whether or not antifibrinolytics decrease bleeding. No trials reported the number of platelet transfusions per participant. The trials were too small to assess whether or not antifibrinolytics increased the risk of thromboembolic events or other adverse events. There are three ongoing RCTs (1276 participants) due to be completed in 2017 and 2020.
Keywords: Humans, Aminocaproic Acid, Aminocaproic Acid/therapeutic use, Antifibrinolytic Agents, Antifibrinolytic Agents/therapeutic use, Erythrocyte Transfusion, Erythrocyte Transfusion/statistics & numerical data, Hematologic Diseases, Hematologic Diseases/complications, Hematologic Diseases/drug therapy, Hemorrhage, Hemorrhage/etiology, Hemorrhage/prevention & control, Lysine, Lysine/analogs & derivatives, Platelet Transfusion, Platelet Transfusion/adverse effects, Thrombocytopenia, Thrombocytopenia/etiology, Thrombocytopenia/therapy, Thromboembolism, Tranexamic Acid, Tranexamic Acid/therapeutic use
Plain language summary
Antifibrinolytics (tranexamic acid and epsilon‐aminocaproic acid) to prevent bleeding in people with low platelets due to bone marrow failure
Review question
We evaluated the evidence about whether giving antifibrinolytics (tranexamic acid or epsilon‐aminocaproic acid) to people with a low platelet count prevents bleeding and whether these antifibrinolytics are associated with side effects. Our target population was people with haematological disorders who have a low platelet count and would usually be treated with platelet transfusions. We did not include people with immune thrombocytopenia because they are not usually treated with platelet transfusions.
Background
People with haematological (blood) cancers and other blood disorders (for example, aplastic anaemia) are frequently at risk of severe or life‐threatening bleeding from having low platelet counts (thrombocytopenia). This may be from bone marrow failure due to an underlying blood disorder but also from the toxic effect of treatment (chemotherapy) on the bone marrow. These people can be given prophylactic platelet transfusions (from donations) to prevent bleeding if their own platelet counts are too low. These transfusions are not without risks, ranging from mild reactions like fevers to more serious, or even life‐threatening, consequences such as infections transmitted to the patient from the transfused platelets, despite stringent attempts to prevent this.
Clearly, ways to safely prevent bleeding in people with thrombocytopenia whilst also minimising exposure to transfused platelets would be welcome. One possible way of achieving these goals is the use of antifibrinolytics, known as lysine analogues: tranexamic acid (TXA) and epsilon aminocaproic acid (EACA). These medications help to stabilise the clots that form after bleeding, therefore reducing the chances of further bleeding as well as the need for transfusing platelets. There may be risks associated with the use of TXA and EACA; the most important being an increased risk of forming unwanted blood clots (such as deep vein thrombosis (DVT), which could be potentially life‐threatening.
Study characteristics
The evidence is current to March 2016. In this update, seven randomised controlled trials were identified. Three trials are either not yet recruiting or still recruiting participants and and have not been completed. Four randomised controlled trials with a total of 95 participants were reviewed. These trials were conducted between 1983 and 1995. Data from one of the trials (eight participants) was excluded from the outcome analysis because the conduct of the study was so flawed.
All three trials (86 participants) included in the outcome analysis were of adults with acute leukaemia receiving chemotherapy. None of the studies included children.
One of these three studies reported funding sources and this study was funded by a charity.
Key results
In people with haematological disorders who have a low platelet count and would usually be treated with platelet transfusions, we are uncertain whether antifibrinolytics decrease the risk of bleeding and the use of platelet transfusions. We are uncertain whether antifibrinolytics increase the risk of developing a clot. We are uncertain whether antifibrinolytics increases the risk of adverse events.
None of the studies reported several of this review's outcomes including overall mortality, adverse events of transfusion, and quality of life.
Quality of the evidence
The quality of the evidence was very low, making it difficult to draw conclusions or make recommendations regarding the usefulness and safety of antifibrinolytics. The only evidence available is for adults with acute leukaemia receiving chemotherapy. We await the results of the three ongoing trials that are expected to recruit 1276 participants in total by 2020.
Summary of findings
Summary of findings for the main comparison. Antifibrinolytics (lysine analogues) compared to placebo to prevent bleeding in people with haematological disorders.
| Antifibrinolytics (lysine analogues) compared to placebo to prevent bleeding in people with haematological disorders | ||||||
| Patient or population: people with haematological disorders Settings: hospital Intervention: antifibrinolytics (lysine analogues) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risksa (95% CI) | Relative effect (95% CI) | No of participants (studies)b | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Antifibrinolytics (lysine analogues) | |||||
| Number of participants with any bleeding | See comment | See comment | Not estimable | 56 (1 study) | ⊕⊝⊝⊝ very low1,2 | We are uncertain whether antifibrinolytics reduce bleeding. Only Shpilberg 1995 reported the number of bleeding episodes per participant, results were reported separately for induction and consolidation chemotherapy and no difference was seen. |
| Number of participants with thromboembolism | See comment | See comment | Not estimable | 68 (2 studies) | ⊕⊝⊝⊝ very low1,2 | We are uncertain whether antifibrinolytics increase the risk of thromboembolism. No patients within the Avvisati 1989 or Shpilberg 1995 studies had an episode of thromboembolism. Gallardo 1983 only reported no deaths due to thrombosis. |
| Mortality (all‐cause) ‐ not reported | Not estimable | Not estimable | 0 (0) | See comment | None of the studies reported all‐cause mortality. Shpilberg 1995 reported no deaths due to bleeding and Gallardo 1983 reported no deaths due to thrombosis. |
|
| Number of platelet transfusions per participant ‐ not reported | Not estimable | Not estimable | 0 (0) | See comment | None of the studies reported the number of platelet transfusions per participant | |
| Adverse events of transfusions ‐ not reported | Not estimable | Not estimable | 0 (0) | See comment | None of the studies reported adverse events due to blood transfusions | |
| Adverse events of antifibrinolytic agents | See comment | See comment | Not estimable | 74 (2 studies) | ⊕⊝⊝⊝ very low1,2 | We are uncertain whether antifibrinolytics increase the risk of adverse events. Shpilberg 1995 reported no side effects were observed and Gallardo 1983 stated side effects were minimal but no further information was provided |
| Quality of life ‐ not reported | Not estimable | Not estimable | 0 (0) | See comment | None of the studies reported quality of life. | |
|
aNo meta‐analyses were performed within this review and therefore no comparative risks could be calculated. bOne study (Fricke 1991) was included within the review but no data were extracted from this study. This was because Fricke 1991 had significant flaws within the study design (see Risk of bias in included studies and Characteristics of included studies) and no viable data could be extracted from the study report. CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1We downgraded the quality of the evidence by 1 for risk of bias. A full assessment of the quality of the evidence was limited by a lack of reporting. However, selective outcome reporting was present in Gallardo 1983. 2We downgraded the quality of evidence by 2 for imprecision. Within all three studies there were only 86 participants. This is significantly below the optimal information size (OIS) (Pogue 1997).
Background
Description of the condition
People with haematological disorders are frequently at risk of severe or life‐threatening bleeding as a result of thrombocytopenia (reduced platelet count). This is commonly a result of the underlying pathology, a side effect of treatment with chemotherapeutic agents, or both. People are administered therapeutic platelet transfusions to treat bleeding and prophylactic platelet transfusions to prevent bleeding once the platelet count falls below a certain threshold (10 x 109/L, or higher if the risk of haemorrhage is raised, e.g. sepsis or the presence of another bleeding diathesis) (BCSH 2003). A large, recent randomised controlled trial (RCT) of platelet transfusions involving 1272 participants with haematological or solid tumours showed that the baseline level of clinically significant bleeding does not appear to depend on the platelet count once it is above 5 x 109/L. The risk of bleeding was 17% per day at platelet counts above 5 x 109/L, compared with 25% per day at counts below this level (Slichter 2010). This confirms earlier work from a large, retrospective observational study of 2942 adults with thrombocytopenia that showed that the platelet count did not affect the risk of severe or life‐threatening bleeding (World Health Organization (WHO) scale 3 or 4) (Friedmann 2002).
Platelet transfusions are not without risks. Adverse events may range from mild reactions, such as fever (one in five transfusions) (Heddle 2009), to more serious and even life‐threatening events such as bacterial sepsis from transfusion‐transmitted infection (one in 10,000 transfusions) (Heddle 2009), or transfusion‐related acute lung injury (TRALI) (Popovsky 1985). Patients may also become refractory to platelet transfusions; the incidence increases with the number of platelet transfusions administered (Slichter 2005). Once refractory, the ability to treat bleeding with platelet transfusions becomes more difficult, requiring expensive, specially matched platelets that can be difficult to source. Furthermore, the financial cost of platelet transfusions is considerable. Around 302,000 adult doses of platelets are issued in the UK each year (Bolton‐Maggs 2012) at an annual cost of approximately GBP 68.5 million (Llewelyn 2009) and up to two‐thirds (67%) of these are given to people with haematological malignancies (Cameron 2007; Greeno 2007; Pendry 2011).
Clearly interventions that can safely prevent bleeding in people with thrombocytopenia, whilst minimising exposure to allogeneic platelets and reducing financial costs, would be welcomed. One possible adjunct, or even an alternative, to prophylactic platelet transfusions is the use of antifibrinolytics, specifically lysine analogues, such as tranexamic acid (TXA) and epsilon aminocaproic acid (EACA).
This systematic review has been designed to establish the safety and efficacy of these agents specifically in people with haematological disorders who are at risk of thrombocytopenia and bleeding, either due to the disorder itself, its treatment, or both.
Description of the intervention
There have been several Cochrane reviews examining the efficacy of antifibrinolytics in preventing bleeding in other patient groups (Gurusamy 2011; Henry 2011; Martin‐Hirsch 2010; Novikova 2011; Roos 2008; Tzortzopoulou 2008). The largest of these involved over 25,000 participants, and assessed the use of TXA, EACA, and another type of antifibrinolytic, aprotinin, with respect to the minimisation of perioperative allogeneic blood transfusions (Henry 2011). However, no systematic review has addressed the use of antifibrinolytics to prevent bleeding in people with haematological disorders.
TXA and EACA are effective in surgical patients (Henry 2011; Ker 2012). They have been used widely in both elective and emergency surgery and have been shown to reduce both blood loss and the need for blood transfusions. In the largest Cochrane review, 65 trials compared TXA with control and comprised a total of 4842 participants of whom 2528 were randomised to TXA. TXA versus control showed a relative reduction in the need for allogeneic blood transfusion of 39% (Henry 2011). A significant effect was also observed for EACA; in 16 trials comparing EACA with control (with a total of 1035 participants, of whom 530 were randomised to EACA), there was a relative reduction in the need for allogeneic blood transfusion of 19% (Henry 2011). In the literature there appears to be a paucity of direct comparisons between TXA and EACA but they appear comparable in terms of safety and efficacy. A recent study comparing TXA and EACA in 234 children undergoing cardiac surgery found no significant differences between the two in terms of transfusion requirement, rates of revision for re‐bleeding, postoperative complications (such as seizures, renal failure and thrombosis) and in‐house mortality (Martin 2011).
TXA is effective in trauma patients. In a recent large RCT (CRASH‐2), TXA has been shown to significantly reduce the risk of death due to bleeding in trauma patients with significant haemorrhage (Shakur 2010). TXA has also been found to be highly cost‐effective: it is relatively inexpensive and its use in preventing bleeding may obviate the need for additional transfusion of blood products and longer stays in hospital. In a recent cost‐effectiveness analysis of the CRASH‐2 trial, Guerriero 2011 reported that the administration of TXA within three hours of injury to bleeding trauma patients has been estimated to save 755 life‐years (LYs) per 1000 trauma patients in the UK, and the incremental cost of giving TXA versus not giving TXA was estimated at USD 48,002 in the UK, equivalent of a cost of around USD 64 per life‐year saved.
TXA and EACA are commonly used to treat bleeding in patients with haematological disorders (Lozano 2013). They are also used to prevent bleeding in patients who are refractory to platelet transfusions (Lozano 2013). It therefore seems possible that lysine analogues may also be cost‐effective in preventing bleeding in patients with haematological disorders with severe thrombocytopenia who are not refractory to platelet transfusions.
TXA and EACA are the only antifibrinolytics in common use. Aprotinin, a naturally occurring serine protease inhibitor, was once commonly used as a blood‐sparing agent, particularly in cardiac surgery. However, it is now used rarely due to concerns of an increased risk of cardiovascular complications and death (Henry 2011). This was because the BART (Blood Conservation Using Antifibrinolytics in a Randomized Trial) multi‐centre blinded RCT was terminated early when a higher rate of death was seen in patients receiving aprotinin (Fergusson 2008). This study was designed to determine whether aprotinin was superior to either TXA or EACA in decreasing massive postoperative bleeding in patients undergoing high‐risk cardiac surgery. A modest and non‐significant reduction in the risk of massive bleeding was observed in the aprotinin arm compared to TXA or EACA, but the rate of death from any cause at 30 days was 6.0% in the aprotinin group, compared with 3.9% (relative risk 1.55; 95% CI 0.99 to 2.42) and 4.0% (relative risk 1.52; 95% CI 0.98 to 2.36) in the TXA and EACA groups, respectively. The authors concluded the negative mortality trend associated with aprotinin, as compared with the lysine analogues, precluded its use in high‐risk cardiac surgery (Fergusson 2008).
Although TXA and EACA have been shown to be effective in other patient groups, there is a concern that these drugs may increase the rate of thromboembolism (Henry 2011). This is particularly important in haematology patients, as patients with an underlying malignancy already have a higher rate of thromboembolic disease than the general population. In a retrospective cohort study of thromboembolism in hospitalised neutropenic cancer patients, 4% (593/14,600) of acute leukaemia patients developed venous thromboembolism and 1.9% (279/14,600) of acute leukaemia patients developed arterial thromboembolism (Khorana 2006).
Furthermore, TXA and EACA may increase the risk of disseminated intravascular coagulation (DIC). In a subsequent exploratory analysis of the CRASH‐2 trial, late treatment with TXA (> three hours) seemed to increase the risk of death in trauma patients due to bleeding (Roberts 2011). The mechanism underlying this could not be readily explained, but the authors noted that one possibility related to the evolution of DIC, a condition in which lysine analogues could be contraindicated (Prentice 1980; Roberts 2011; Sawamura 2009). This highlights a serious need for caution in the use of these agents in patients with haematological malignancies as they are at increased risk of DIC (Franchini 2010). Overt cases of DIC are diagnosed in approximately 15% of people with acute leukaemia and bleeding manifestations tend to prevail over thrombosis (Franchini 2010).
Despite these important concerns, it should be noted that in the recent large Cochrane review of over 25,000 participants (Henry 2011), the use of TXA or EACA was not associated with an increased risk of mortality, myocardial infarction, deep vein thrombosis, stroke, incidence of renal dysfunction or length of hospital stay, although the data were sparse. In addition, there have been small RCTs assessing the efficacy of TXA versus placebo in haematology patients that did not report an increase in thromboembolic complications, although sample sizes were small (Avvisati 1989; Shpilberg 1995).
How the intervention might work
TXA and EACA are synthetic analogues of the amino acid lysine that act by blocking the lysine binding sites on plasminogen. This inhibits the formation of plasmin and therefore prevents fibrinolysis, leading to improved haemostasis (Okamoto 1997). In vitro TXA is approximately 10 times more potent than aminocaproic acid and binds much more strongly to the sites on the plasminogen molecule (Faught 1998). It is plausible that if these lysine analogues are effective and safe, the bleeding risk in people with haematological disorders could be reduced, and the requirement for prophylactic platelet transfusions could be minimised.
Why it is important to do this review
Clearly, it is essential to reduce the risk of bleeding in patients with haematological disorders and thrombocytopenia as effectively and as safely as possible. Since the CRASH‐2 trial (Roberts 2011) and two large systematic reviews (Henry 2011; Ker 2012) have shown antifibrinolytics to be effective in other patient groups there has been renewed interest in using this drug to prevent bleeding in patients with haematological disorders.
The following key questions need to be addressed.
What is the efficacy of lysine analogues in preventing bleeding people with haematological disorders who are thrombocytopenic?
Can the number of prophylactic platelet transfusions be minimised?
Does the use of lysine analogues lead to a significant increase in the incidence of thromboembolism?
If lysine analogues are shown to be effective whilst demonstrating an acceptable safety profile, there would be a strong case for their routine use in patients with haematological disorders at significant risk of severe thrombocytopenia. A systematic review is therefore required before any proposed introduction of these agents in patients with haematological disorders.
Objectives
To determine the efficacy and safety of antifibrinolytics (lysine analogues) in preventing bleeding in people with haematological disorders.
Methods
Criteria for considering studies for this review
Types of studies
We only included RCTs in this review, irrespective of language or publication status.
Types of participants
People of any age, with a haematological disorder (malignant or non‐malignant) who were severely thrombocytopenic due to bone marrow failure (secondary to the disease or to its treatment) and required platelet transfusions. We excluded people with immune thrombocytopenic purpura (ITP) because they are not usually treated with platelet transfusions.
Types of interventions
We only reviewed antifibrinolytic agents that act by competitively inhibiting the conversion of plasminogen to plasmin (lysine analogues), i.e. tranexamic acid (TXA) and epsilon aminocaproic acid (EACA). Aprotinin is a serine protease and has a different mechanism of action. We included the following comparisons:
TXA versus placebo;
EACA versus placebo;
TXA versus EACA.
We included any dose of the medication, administered either orally or intravenously.
Types of outcome measures
Primary outcomes
Number, site and severity of bleeding (i.e. any bleeding, clinically significant bleeding, life‐threatening bleeding)
Thromboembolism (venous and arterial)
Secondary outcomes
Mortality (all causes)
Mortality (secondary to bleeding)
Mortality (secondary to thromboembolism)
Laboratory assessment of fibrinolysis
Number of platelet transfusions or platelet components
Number of red cell transfusions or red cell components
Adverse events of antifibrinolytic agents
Adverse events of transfusions (e.g. transfusion reactions, antibody development)
Disseminated intravascular coagulation (DIC)
Quality of life (QoL)
We listed both primary outcomes in the 'Summary of findings' table, as well as the number of red cell and platelet transfusions.
Search methods for identification of studies
We formulated search strategies in collaboration with the Cochrane Haematological Malignancies Group.
Electronic searches
The Systematic Review Initiative Information Specialist (CD) updated search strategies in collaboration with the Cochrane Haematological Malignancies Review Group based on those used in the previous version of this review (Wardrop 2013). We searched for relevant RCTs in the following electronic databases:
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2016, Issue 3 to 07 March 2016) (Appendix 1);
MEDLINE (from 1948 to 07 March 2016) (Appendix 2);
Embase (from 1974 to 07 March 2016) (Appendix 3);
CINAHL (from 1937 to 07 March 2016) (Appendix 4);
PubMed (e‐publications as of 07 March 2016 only) (Appendix 5);
LILACS (from 1982 to 07 March 2016) (Appendix 6);
KoreaMed (from 1982 to 07 March 2016) (Appendix 6);
PakMediNet (from 2001 to 07 March 2016) (Appendix 6);
IndMed (from 1985 to 07 March 2016) (Appendix 6);
Transfusion Evidence Library (www.transfusionevidencelibrary.com) (from 1950 to 07 March 2016) (Appendix 7);
Web of Science, Conference Proceedings Citation Index (from 1990 to 07 March 2016) (Appendix 8).
We updated searches from the original search in January 2013 (Wardrop 2013). We did not re‐screen the original search strategies and instead placed date restrictions from the date of the final search in the preceding review (10 January 2013) to 07 March 2016 for four databases (CENTRAL, MEDLINE, Embase and CINAHL). The other databases had no date restrictions.
We combined searches in MEDLINE, Embase, and CINAHL with adaptations of the Cochrane RCT search filters, as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011).
Databases of ongoing trials
We also searched ongoing trial databases (all years) on 07 March 2016:
ClinicalTrials.gov (Appendix 9);
WHO International Clinical Trials Registry Platform (ICTRP) (Appendix 9);
ISRCTN Register (Appendix 10);
EU Clinical Trials Register (EUDRACT) (Appendix 10);
UMIN‐CTR Japanese Clinical Trials Registry and the Hong Kong Clinical Trials Registry (Appendix 10).
Searching other resources
We augmented database searching with the following.
-
Handsearching of reference lists
We checked references of all identified trials, relevant review articles and current treatment guidelines for further literature.
We limited these searches to the 'first generation' reference lists.
-
Personal contacts
We contacted authors of relevant studies, study groups and experts worldwide who are known to be active in the field for unpublished material or further information on ongoing studies.
Data collection and analysis
Selection of studies
The selection of studies was updated from the selection of studies performed for the original version of this review (Wardrop 2013).
Two review authors (MD, LE) screened independently all electronically‐derived citations and abstracts of papers identified by the review search strategy for relevance. Studies clearly irrelevant were excluded at this stage. The full texts of all potentially‐relevant trials were formally assessed independently for eligibility by two review authors (MD, LE) against the criteria outlined above. All disagreements were resolved by discussion without the need for a third review author (SS). Further information was sought from study authors if the article contained insufficient data to make a decision about eligibility. A study eligibility form was designed for this review to help in the assessment of relevance. The reasons why potentially‐relevant studies failed to meet the eligibility criteria were recorded.
Data extraction and management
We updated the data extraction from the data extraction performed for the previous version of this review (Wardrop 2013). This included data extraction for the new ongoing studies included since the previous review.
Two review authors (LE, MD) conducted the data extraction according to the guidelines proposed by The Cochrane Collaboration (Higgins 2011a). Potential disagreements between the review authors were resolved by consensus. The review authors were not blinded to names of authors, institutions, journals, or the outcomes of the trials. The data extraction forms were piloted in the previous version of this review (Wardrop 2013). We were not blinded to the names of authors, institutions, journals or the outcomes of the trials. We used a standardised data extraction form to record the following items.
General information: review author's name, date of data extraction, study ID, first author of study, author's contact address (if available), citation of paper, objectives of the trial.
Trial details: trial design, location, setting, sample size, power calculation, inclusion and exclusion criteria, reasons for exclusion, comparability of groups, length of follow‐up, stratification, stopping rules described, results, conclusion and funding.
'Risk of bias' assessment: sequence generation, allocation concealment, blinding (participants, personnel, outcome assessors), incomplete outcome data, selective outcome reporting and other sources of bias.
Characteristics of participants: age, gender, ethnicity, total number recruited, total number randomised, total number analysed, types of haematological disease, lost to follow‐up numbers, drop outs (percentage in each arm) with reasons, protocol violations, current treatment, previous treatments.
Interventions: experimental and control interventions, type of antifibrinolytic given, timing of intervention, compliance to interventions, additional interventions given especially in relation to platelet and red cell transfusions, any differences between interventions.
Outcomes measured: number, site and severity of bleeding episodes; thromboembolism (venous and arterial); mortality (all causes); mortality due to haemorrhage; mortality due to thromboembolism; laboratory assessment of fibrinolysis; number of platelet transfusions; number of red cell transfusions; adverse effects of antifibrinolytic agents; adverse effects of transfusions (e.g. transfusion reactions, development of platelet antibodies); DIC.
We retrieved the data from both full‐text and abstract reports of studies. Where these sources did not provide sufficient information, we contacted authors and study groups for additional details.
Assessment of risk of bias in included studies
The risk of bias assessment was not updated from the previous version of this review because no new completed studies were identified (Wardrop 2013). We assessed all completed studies for possible risk of bias as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). The assessment included information about the design, conduct and analysis of the trial. We evaluated whether the studies are at a low risk, high risk or unclear risk of bias. To assess risk of bias, the following questions were included in the 'Risk of bias' table for each included study:
Was the allocation sequence adequately generated?
Was allocation adequately concealed?
Was the knowledge of the allocated intervention adequately prevented during the study?
Were incomplete outcome data adequately addressed?
Are reports of the study free of selective outcome reporting?
Was the study apparently free of other problems that could put it at risk of bias?
Measures of treatment effect
We performed this according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). For dichotomous outcomes we recorded the numbers of outcomes in treatment and control groups.
For continuous outcomes, we recorded the mean and standard deviations.
Unit of analysis issues
We did not pre‐specify in the protocol how we would deal with any unit of analysis issues. No unit of analysis issues arose in this review.
Dealing with missing data
We performed this according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We contacted one author by email in order to obtain information that was missing or unclear in the published report. This author responded to our email request but was unable to provide any further information (Gallardo 1983). We were unable to contact the other authors. We recorded the number of patients lost to follow‐up for each trial.
Assessment of heterogeneity
We did not perform a formal assessment of heterogeneity because it was not possible to perform meta‐analyses due to the nature of the data reported by the included studies. (Deeks 2011).
Assessment of reporting biases
We did not perform a formal assessment of reporting biases because there were not enough data to support such an assessment and no meta‐analyses of outcome data were performed (Sterne 2011).
Data synthesis
We performed a narrative synthesis of the findings from the included studies, structured around the type of antifibrinolytic. No statistical analyses were performed because the studies reported outcomes in different ways and these results could not be integrated.
We used the GRADE profiler to create a 'Summary of findings' table as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011). We did not pre‐specify in the protocol the outcomes to be reported in the 'Summary of findings' table (Wardrop 2012).
Subgroup analysis and investigation of heterogeneity
We pre‐specified three subgroup analyses.
Age (children/adults).
Underlying haematological diagnoses.
Type of treatment (e.g. chemotherapy, autologous and allogeneic transplantation, immunosuppression).
However, we did not perform any subgroup analyses due to a lack of outcome data. The three studies included in the outcome analysis only included adults who had acute leukaemia who were receiving chemotherapy.
Sensitivity analysis
We did not perform a formal sensitivity analysis because we performed no meta‐analyses.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies and Table 2.
1. Study characteristics.
| Study | Type of study | No. of participants | No. of participants receiving antifibrinolytic | No. of participants platelet refractory/ allo‐immunisation | Diagnosis of patients | Treatment of underlying disease | Antifibrinolytic dose | Antifibrinolytic frequency | Antifibrinolytic route | Treatment started | Treatment stopped | Platelets given |
| Tranexamic acid studies | ||||||||||||
| Avvisati 1989 | RCT | 12 | 6 | NR |
APML | Chemo | 2 g | 8‐hourly | IV in 500 mL 5% glucose | 1st day of antileukaemic Rx | After 6 days | Prophylactic |
| Fricke 1991 | RCT Cross‐over | 8 Only 3 completed study |
8 | At least 3 | 7 AA 1 MDS |
NR | 20 mg/kg | 8‐hourly | Oral | After 4‐day trial period to assess drug tolerance | Successive 4/52 courses or until WHO grade 2 bleeding | Therapeutic |
| Shpilberg 1995 | RCT | 56 | 26 | NR | AML 38 induction 18 consolidation |
Chemo | 1 g | 6‐hourly | Oral | Platelets < 20 or rapidly falling and < 50 | Platelet count > 20 for 2 consecutive counts | Therapeutic |
| EACA studies | ||||||||||||
| Gallardo 1983 | RCT | 19 | 9 | NR | 15 AML 4 ALL (1 patient not evaluable) |
Chemo | Loading dose 100 mg/kg 12 to 24 g/day thereafter |
NR | NR | Platelet count < 20 x 109/L | Platelet count ≥ 20 x 109/L | Platelet count < 20 x 109/L |
AA: aplastic anaemia ALL: acute lymphoblastic leukaemia AML: acute myeloid leukaemia APML: acute promyelocytic leukaemia EACA: epsilon aminocaproic acid IV: intravenous MDS: myelodysplastic syndrome NR: not reported RCT: randomised controlled trial TXA: tranexamic acid WHO: World Health Organization
Results of the search
See PRISMA diagram Figure 1. The original search (conducted January 2013) identified 953 records through database searching with an additional 12 records identified through other sources (principally the handsearching of reference lists of included studies). After duplicates were removed, we screened 470 records in abstract form for eligibility and excluded 436 records. Of the remaining records, we retrieved and assessed 34 full‐text articles for eligibility and excluded 29 due to either: not being an RCT (N = 12), wrong participant group (N = 8), because the article was a review (N = 6) or ineligible intervention (N = 3). The original search identified four included studies (Avvisati 1989; Fricke 1991; Gallardo 1983; Shpilberg 1995).
1.
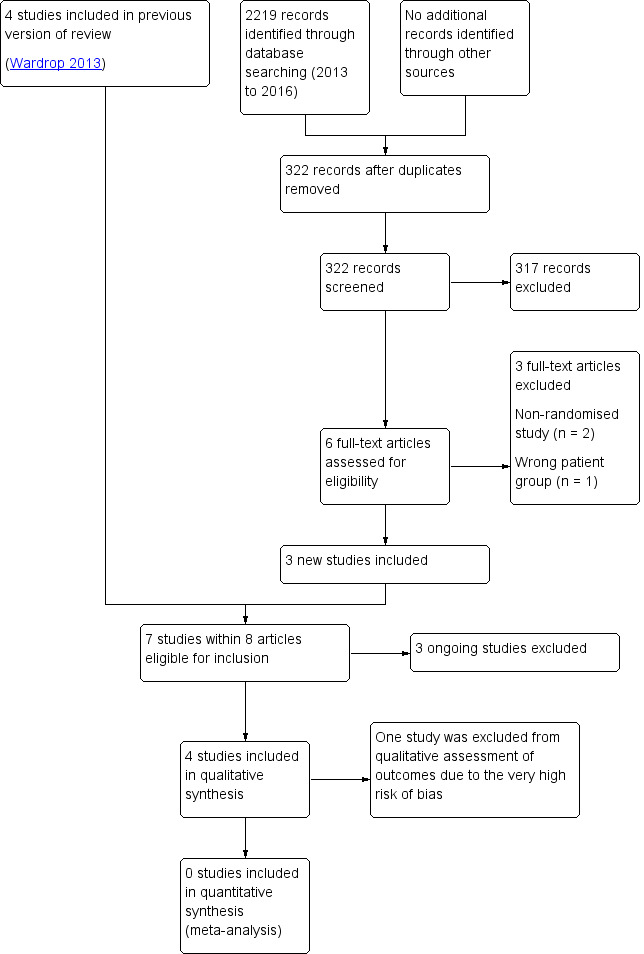
Study flow diagram.
This updated search (conducted 07 March 2016) identified an additional 2219 potentially relevant records. There were 322 records after duplicates were removed and 317 records were able to be excluded on the basis of the abstract by two review authors (LE, MD). Six full‐text articles were retrieved for relevance and assessed by two review authors (LE, MD). This updated search identified three studies (A‐TREAT 2015; PROBLEMA 2014; TREATT 2015) that are either recruiting participants or are not yet recruiting participants and are due to be completed in 2017 and 2020. We excluded three studies.
No studies were identified that compared tranexamic acid (TXA) with epsilon aminocaproic acid (EACA).
In total, six studies were assessed and deemed eligible for inclusion (Avvisati 1989; Fricke 1991; Gallardo 1983; Shpilberg 1995; PROBLEMA 2014; TREATT 2015).
Included studies
There were seven trials (within eight articles) eligible for inclusion (A‐TREAT 2015; Avvisati 1989; Fricke 1991; Gallardo 1983; PROBLEMA 2014; Shpilberg 1995; TREATT 2015). Two of these trials are still recruiting participants (PROBLEMA 2014; TREATT 2015), and one trial has not yet started recruiting participants (A‐TREAT 2015).
Completed studies
The four completed trials were published between 1983 and 1995. Two were conducted in the USA, one in Israel, and a further one in Italy and The Netherlands. The trials randomised a total of 95 participants (8 to 56). See Characteristics of included studies for full details of each study and Table 2 for a comparison between studies.
Three studies evaluated the effect of TXA therapy in the reduction of bleeding during treatment of acute myeloid leukaemia (AML) (Avvisati 1989; Fricke 1991; Shpilberg 1995). One cross‐over TXA study (eight patients) was excluded from the outcome analysis because the data were uninterpretable due to major methodological flaws in the study design (see Table 2 for details of the study design) (Fricke 1991). We included data from this study in the 'Risk of bias' assessment.
One study evaluated the effect of EACA for bleeding control during induction chemotherapy for acute leukaemia (Gallardo 1983).
In the remainder of this review, these sub‐categories will be reported in separate sections.
Tranexamic acid (TXA) versus placebo
Three trials evaluated this comparison (Avvisati 1989; Fricke 1991; Shpilberg 1995) (Table 2).
Participants
In total, 76 participants were randomised to receive TXA or placebo (Table 2). The population characteristics varied between the studies. In Avvisati 1989, the 12 participants randomised were all diagnosed with acute promyelocytic leukaemia (APML) and were all undergoing induction chemotherapy. In Fricke 1991, seven participants had aplastic anaemia (AA) and one participant had myelodysplastic syndrome (MDS); all were out‐patients but no other treatments were reported. In Shpilberg 1995, all 56 participants had acute myeloid leukaemia (AML), however, only one of the participants randomised was diagnosed with APML (consolidation group). Thirty‐eight of the participants randomised were undergoing induction chemotherapy and 18 were undergoing consolidation chemotherapy. None of the participants were children.
Intervention
All three trials compared TXA versus placebo (Table 2). In Avvisati 1989, TXA or placebo began at the same time as the antileukaemic therapy (day 1) and lasted for 14 days. In Fricke 1991, all patients served as their own control and, after a four‐day trial period to test drug tolerance (followed by a one‐week interval without the drug), each patient began a course of either TXA or placebo that lasted for four weeks or until a platelet transfusion was required to control bleeding. In Shpilberg 1995, TXA or placebo was given when the platelet count was less than 20 x 109/L or in a falling trend and less than 50 x 109/L.
Co‐interventions
In Avvisati 1989, platelet transfusions (6 to 8 units/m2) were given routinely during the first seven days and additionally for overt haemorrhage, and packed red cells were administered to maintain the haemoglobin concentration above 90 g/L. In Fricke 1991, each patient's personal physician was permitted to determine the need for platelet transfusion based on "some form of bleeding, such as severe petechiae, blood blisters, and gum or nose bleeding". No red cell transfusion policy was stated. In Shpilberg 1995, platelet transfusions (4 units/m2) were given irrespective of the count, but only when clinically significant bleeding occurred and packed red cells were given to maintain the haemoglobin concentration above 90 g/L.
Outcomes
Efficacy endpoints in Avvisati 1989 were severity of bleeding, thromboembolism, laboratory assessment of fibrinolysis, packed red cell and platelet concentrate transfusion requirement. In Fricke 1991, the endpoints were number of bleeding episodes, severity of bleeding episodes, site of bleeding episodes, red cell and platelet transfusion requirements, and drug side effects. Shpilberg 1995 reported the number of bleeding events and severity of bleeding (using a scoring system), red cell and platelet concentrate transfusion requirement, thromboembolism and adverse events of drug, duration of hospitalisation, duration of significant thrombocytopenia (< 20 x 109/L) and days with fever.
Epsilon aminocaproic acid (EACA) versus placebo
There was only one trial evaluating this comparison (Gallardo 1983). It was an abstract published in 1983 detailing a randomised two‐arm study with adults undergoing remission induction for acute leukaemia.
Participants
In total 19 adults undergoing remission induction for acute leukaemia were randomised to receive EACA or not; 15 with AML and four with acute lymphoblastic leukaemia (ALL). One patient was not evaluable for unstated reasons, leaving nine patients in each study arm.
Intervention
All patients received platelet transfusions (multiple, single donor or human leukocyte antigen (HLA)‐matched) in the event of thrombocytopenia (< 20 x 109/L); this count defined the "days at risk of bleeding". One arm received EACA (100 mg/kg loading dose and 12 to 24 g/day thereafter in divided doses) alongside platelet transfusions whilst the other arm did not.
Co‐interventions
All patients received platelet transfusions (multiple, single donor or HLA‐matched) in the event of thrombocytopenia (< 20 x 109/L); this count defined the "days at risk of bleeding".
This study reported no other co‐interventions.
Outcomes
Outcomes reported included bleeding; either as capillary bleeding (CB; skin, mucous membranes, conjunctivae, nose, occult blood in gastrointestinal (GI) or genitourinary (GU) tract), or major bleeding (MB; nose bleeding requiring posterior packing, gross GI or GU bleeding, and central nervous system (CNS) bleeding), monitoring of antifibrinolytic therapy using I125 fibrinogen plasma clot lysis, platelet transfusion requirement, adverse events of drug and thromboembolism.
Tranexamic acid versus epsilon aminocaproic acid
No RCTs that evaluated this comparison were identified.
Ongoing Studies
There are three ongoing clinical trials (A‐TREAT 2015; PROBLEMA 2014; TREATT 2015). Please see Characteristics of ongoing studies for further details.
Excluded studies
In this update, we excluded a further three trials.(Antun 2013; NCT01980355; Rathi 2015) See Characteristics of excluded studies for further details.
Fourteen studies were not randomised controlled trials.
(Antun 2013; Bartholomew 1989; Ben‐Bassat 1990; Cattan 1963; Chakrabarti 1998; Dean 1997; Fossa 1978; Gardner 1980; Garewal 1985; Kalmadi 2006; Rathi 2015; Sanz 2010; Schwartz 1986; Wassenaar 2008)
Nine studies examined different patient groups.
(Amar 2003; Byams 2007; Celebi 2006; McConnell 2011; McConnell 2012; Mevio 1983; Movafegh 2011; NCT01980355; Yang 2001)
Six studies were reviews.
(Bates 2011; Breen 2012; Brown 2002; Levy 2005; Marti‐Carvajal 2011; Rickles 2007)
Three studies examined a different intervention.
Risk of bias in included studies
See Figure 2 and Characteristics of included studies for further details.
2.
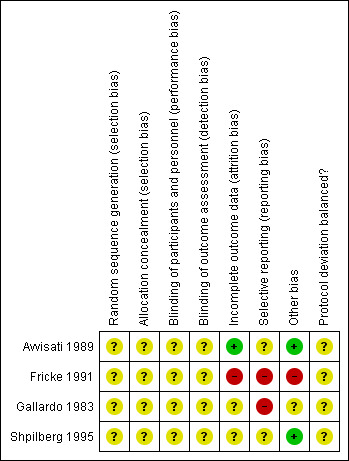
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
All completed studies (Avvisati 1989; Fricke 1991; Gallardo 1983; Shpilberg 1995) had some threats to validity. The majority of these threats were due to a lack of detail provided on the specific criteria and we therefore judged them as 'unclear' using the Cochrane grading system. However Fricke 1991 had significant flaws in study design which we considered 'high risk', including attrition bias, reporting bias and other sources of bias (see Figure 2 and Characteristics of included studies).
Allocation
None of the studies reported the method of sequence generation or allocation concealment and we deemed all studies reported in this review as having an unclear risk of bias.
Blinding
We deemed all studies to have an unclear risk of bias (Avvisati 1989; Fricke 1991; Gallardo 1983; Shpilberg 1995).
In Avvisati 1989, the risk of performance bias and detection bias was unclear as the article states that attending physicians were blinded to the treatment groups and that bleeding assessments were examined by the same investigator, but it is not stated whether the investigator was one of the attending physicians. In Gallardo 1983, the risk of performance and detection bias was unclear as the abstract did not state whether the investigators and or patients were blinded and does not state who carried out the assessments of bleeding. In Shpilberg 1995, the threat of performance and detection bias was unclear as although the study states that it was double‐blinded, no further details were given as to who was blinded. In Fricke 1991, although it was stated that study was double‐blinded, the article states that drug levels were obtained during 38 of the 49 courses. It does not state that the investigators or patients were blinded to this information. It also states that the study defined overall success of TXA in a patient as either five failures of placebo and none of drug or seven failures of placebo and one of drug and defined overall failure of TXA as two failed courses of drug. Sequential courses continued until overall success or failure of TXA could be determined. However, it did not state how this assessment of success or failure was performed without un‐blinding study personnel.
Incomplete outcome data
There were no missing outcome data in Avvisati 1989, so we deemed the article to have a low risk of attrition bias. We deemed Fricke 1991 to have a high risk of bias ‐ the article states that "three [of eight] patients completed the randomised portion of the study ... five of the eight patients did not complete enough courses to determine the efficacy of the drug". The two remaining studies (Gallardo 1983; Shpilberg 1995) were deemed to have an unclear risk of bias as there were insufficient data to assess incomplete outcome data.
Selective reporting
We deemed two studies to have an unclear risk of bias (Avvisati 1989; Shpilberg 1995) and two studies to have a high risk of bias (Fricke 1991; Gallardo 1983).
There were insufficient data to assess the risk of selective reporting (reporting bias) in Avvisati 1989 and in Shpilberg 1995 and we deemed these to have an unclear risk of bias. There was a high risk of reporting bias in two studies (Fricke 1991; Gallardo 1983).
In Fricke 1991, one patient died of intracranial haemorrhage four days after starting the first randomised course. Data from this course were not reported. There were two courses of TXA or placebo interrupted in two separate patients; one due to an upper respiratory tract infection and the other in which the patient developed an oesophageal haematoma after starting antibiotic treatment for an infection. Data from these courses were not included in the analysis as the investigators felt that the infection/antibiotic treatment may have compromised haemostasis. Furthermore, in Fricke 1991, the article states that severity of bleeding (as well as number and site) were recorded by the assessor (the patient), but this outcome is not reported in the article. Finally, five of the eight patients were reported as not completing enough sessions to determine the effectiveness of TXA.
In Gallardo 1983, there are data for thromboembolism and death ("no patient died of thrombosis"), but no data given on the number episodes of thromboembolism or number of deaths. There were also no data reported on the monitoring of antifibrinolytic therapy using I125 fibrinogen plasma clot lysis. This may have been because the article was an abstract and there was limited space available.
Other potential sources of bias
We deemed two studies to have a low risk of bias (Avvisati 1989; Shpilberg 1995), one an unclear risk of bias (Gallardo 1983) and one a high risk of bias (Fricke 1991).
Avvisati 1989 and Shpilberg 1995 seemed to be free of other sources of bias and we deemed them to be at low risk of bias. In Gallardo 1983, the “at risk of bleeding days” were much higher in the EACA group – 158 versus 80 due to more severe thrombocytopenia and more cycles of chemotherapy for refractory disease. There may be bias in the randomisation procedure but the method of randomisation is not stated and we deemed the study overall to have an unclear risk of bias.
There were other sources of potential bias in Fricke 1991. The overall success of TXA was defined as either five failures of placebo and none of the drug or seven failures of placebo and one of the drug and overall failure of TXA was defined as two failed courses of drug. Failure of a course was defined as a patient receiving a platelet transfusion for bleeding during a four‐week study period. Patients received a variable number of courses of drug/placebo. The three patients who completed the study received between three (two TXA, one placebo) and nine courses (five TXA, four placebo) of treatment. The five patients who did not complete the study received between zero and 20 courses (10 TXA, 10 placebo) of treatment. Of the three patients who completed the study, two did not have any successful courses of treatment. The third patient had three of five successful courses with TXA and one of four successful courses with placebo, however this was classified by the study as a failure of TXA (two failed courses with TXA). Interim analysis of the data was therefore performed after each course of treatment for each patient, with completion of the study being biased against TXA (only two failures of TXA are required, whereas five failures of placebo and none of TXA for study to be classified as completed).
In Fricke 1991, failure of a course of treatment would be classified in the same way whether the patient was on study drug for one day before bleeding or 27 days before bleeding that required treatment with a platelet transfusion. More bleeding episodes seen in the TXA arm may have been due to more days on study drug before bleeding requiring a platelet transfusion. Number of days on study drug before bleeding was not reported for individual courses.
No protocol deviations were commented upon in any of the studies. However, in Fricke 1991, one patient began receiving HLA‐matched platelet transfusions two months after enrolment and was kept in the study because these platelet transfusions failed to control bleeding. Definition of failure of a course of treatment for this patient was the need for additional platelet transfusions. It is unclear whether this represented a protocol violation but two other patients were withdrawn from the study after they started to receive HLA‐matched platelet transfusions.
Effects of interventions
See: Table 1
2. Results of studies (primary outcomes of review).
| Study | Type of study | Number of participants | Type of participants | Number, site and severity of bleeding | Thromboembolism (venous and arterial) | |
| Tranexamic acid studies | ||||||
| Avvisati 1989 | RCT | 12 | APML | Cumulative haemorrhagic scores TXA 3 C 42 (P = 0.0045) |
No thromboembolic events observed | |
| Shpilberg 1995 | RCT | 56 | AML 38 induction 18 consolidation |
Mean number of bleeding events per participant: Induction TXA 6.2 SD 2.9 C 4.5 SD 3.6 Consolidation TXA 1.1 SD 1.4 C 2.6 SD 2.2 (P < 0.05) |
Cumulative haemorrhagic scores: Induction TXA 8.3 SD 4.8 C 5.6 SD 4.8 Consolidation TXA 1.3 SD 1.8 C 5.1 SD 3.6 (P < 0.05) |
No thromboembolic events observed |
| EACA Studies | ||||||
| Gallardo 1983 | RCT | 19 | 15 AML 4 ALL |
Capillary bleedinga EACA 31% of days at riskb Placebo 50% of days at riskb |
Major bleedingc EACA 15% Placebo 19% |
No deaths due to thromboembolic disease |
aCapillary bleeding defined as bleeding in skin, mucous membranes, conjunctivae, nose and guaiac in gastrointestinal or genitourinary tract.
bDays at risk defined as days when platelet count fewer than 20 x 109/ L. cMajor bleeding defined as nose bleeding requiring posterior packing, gross gastrointestinal or genitourinary tract bleeding and central nervous system bleeding.
ALL: acute lymphoblastic leukaemia AML: acute myeloid leukaemia APML: acute promyelocytic leukaemia C: control EACA: epsilon aminocaproic acid RCT: randomised controlled trial SD: standard deviation TXA: tranexamic acid
3. Results of studies (secondary outcomes of review).
| Study | Type of study | Number of participants | Type of participants | Mortality (all causes) | Mortality (secondary to bleeding) | Mortality (secondary to thromboembolism) | Laboratory assessment of fibrinolysis | Number of platelet transfusions | Number of red cell components | Adverse events of antifibrinolytic agents |
Adverse events of transfusions (e.g. transfusion reactions, antibody development) |
DIC | QoL |
| Tranexamic acid studies | |||||||||||||
| Avvisati 1989 | RCT | 12 | APML | NR | NR | NR | No difference in the coagulation and fibrinolysis indices between the 2 groups apart from FDPsa. FDPs decreased in TXA arm but increased in the placebo arm (P < 0.01) |
Platelet Tx TXA = 45 Tx C = 246 Tx (P = 0.045) |
Reduction in overall RBC components required in TXA group: (units) TXA = 28 C = 56 (P = 0.016) |
NR | NR | NR | NR |
| Shpilberg 1995 | RCT | 56 | AML 38 induction 18 consolidation |
NR | No fatal bleeding in either group | NR | NR | Induction (units) TXA 22.1 SD 13.2 C 23.1 SD 11.7 Consolidation (units) TXA 3.7 SD 4.1 C 9.3 SD 3.3 (P < 0.05) |
No reduction RBC transfusion requirements Induction (units) TXA 7.5 SD 4.7 C 7.3 SD 3.3 Consolidation (units) TXA 4.1 SD 2.8 C 4.1 SD 3.4 |
No side effects were observed | NR | NR | NR |
| EACA studies | |||||||||||||
| Gallardo 1983 | RCT | 19 | 15 AML 4 ALL |
NR | NR | No participant died of thrombosis | Monitored with the I125 fibrinogen plasma clot lysis assay but no further data described. | EACA 1 every 13.3 days at riskb Placebo 1 every 10.5 days at riskb |
NR | Side effects were stated as minimal | NR | NR | NR |
aBlood coagulation tests were prothrombin time, activated partial thromboplastin time, fibrinogen, FDP, antithrombin III activity, thrombin‐antithrombin III complexes, protein C activity and α2‐antiplasmin. These were carried out daily for the first 10 days. bDays at risk defined as days when platelet count fewer than 20 x 109/L.
ALL: acute lymphoblastic leukaemia AML: acute myeloid leukaemia APML: acute promyelocytic leukaemia C: control DIC: intravascular coagulation EACA: epsilon aminocaproic acid FDPs: fibrin degradation products GI: gastrointestinal GU: genitourinary QoL: quality of life NR: not reported RBC: red blood cell RCT: randomised controlled trial SD: standard deviation TXA: tranexamic acid Tx: transfusion
Tranexamic acid (TXA) versus placebo
Three studies evaluated this comparison (Avvisati 1989; Fricke 1991; Shpilberg 1995).
We did not extract any data from the Fricke 1991 study due to major methodological problems in the study design. In addition to the high risk of bias in terms of attrition bias, reporting bias and other bias (see text section above, Figure 2 and the Risk of bias in included studies table), there was a variable number of study cycles depending on the results of previous cycles of treatment. All these factors meant that it was impossible to fully understand the data in this trial and we took the decision to not include this trial in the assessment of 'effects of interventions'.
Number, site and severity of bleeding (i.e. any bleeding, clinically significant bleeding, life‐threatening bleeding)
The two remaining studies (68 participants) both reported bleeding (Table 3), but bleeding was reported in different ways.
Shpilberg 1995 reported the mean number of bleeding events per participant. During induction chemotherapy, there was no difference in the number of bleeding events per participant between TXA and placebo (38 participants, mean difference (MD) 1.70 bleeding events, 95% confidence interval (CI) ‐0.37 to 3.77) (Analysis 1.1). During consolidation chemotherapy, there was no difference in the number of bleeding events per participant between TXA and placebo (18 participants; MD ‐1.50 bleeding events (95% CI ‐3.25 to 0.25) (Analysis 1.1). This differs from the study authors who reported a statistically significant difference in the number of bleeding events per participant (P < 0.05). We are unable to explain the reason for this difference because we do not know what statistical analyses were performed by the study authors.
1.1. Analysis.
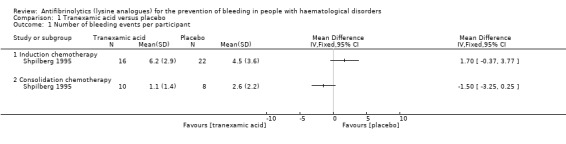
Comparison 1 Tranexamic acid versus placebo, Outcome 1 Number of bleeding events per participant.
Avvisati 1989 (12 participants) did not report bleeding per participant and instead reported bleeding as a cumulative score in the first observation period (days two to seven), second observation period (days eight to 14) and as an overall cumulative score (Table 3). The study stated that there was a reduction in the overall cumulative haemorrhagic scores in the TXA study arm (P = 0.0045).
Thromboembolism (venous and arterial)
Both studies (68 participants) reported thromboembolism but did not distinguish between arterial or venous events (Table 3). Shpilberg 1995 (56 participants) reported that no thromboembolic events occurred in either group throughout the study. Avvisati 1989 (12 participants) reported that there were no thromboembolic complications and there was no evidence of enhanced thrombin generation (as assessed by thrombin‐antithrombin‐III complexes). TXA was only given for the first six days out of 14 days of observation because of what the authors described as the "known increase of cerebral thromboembolic disease with prolonged therapy".
Mortality (all causes)
Neither study reported all‐cause mortality.
Mortality (secondary to bleeding)
Only Shpilberg 1995 (56 participants) reported mortality (secondary to bleeding) and stated that there was no fatal bleeding in either group (Table 4).
Mortality (secondary to thromboembolism)
Neither study reported mortality secondary to thromboembolism.
Laboratory assessment of fibrinolysis
Only Avvisati 1989 (12 participants) reported laboratory assessment of fibrinolysis. There were no statistically significant differences in the coagulation and fibrinolysis indices between the two groups apart from the results for fibrin degradation products. The study stated that median fibrin degradation products decreased in the TXA arm but increased in the placebo arm during the first week of observation (P < 0.01) (Table 5).
4. Laboratory assessment of fibrinolysis ‐ Avvisati 1989.
| Coagulation factors | Timing |
Treatment groups Median (range) |
|
| Tranexamic acid | Placebo | ||
| Fibrinogen (mg/dL) | Baseline | 80 (55 to 395) | 70 (50 to 190) |
| Day 3 | 55 (20 to 125) | 62 (45 to 150) | |
| Day 5 | 53 (35 to 80) | 78 (36 to 150) | |
| Day 7 | 60 (50 to 65) | 85 (17 to 160) | |
| Thrombin‐antithrombin complex (ng/mL) | Baseline | 32 (4 to 58) | 22 (15 to 26) |
| Day 3 | 50 (20 to 100) | 22 (11 to 52) | |
| Day 5 | 41 (5 to 51) | 17 (5 to 34) | |
| Day 7 | 10 (5 to 31) | 21 (3 to 37) | |
| α2‐antiplasmin (%) | Baseline | 39 (29 to 80) | 50 (29 to 58) |
| Day 3 | 27 (17 to 40) | 35 (27 to 47) | |
| Day 5 | 32 (27 to 34) | 36 (29 to 51) | |
| Day 7 | 33 (19 to 53) | 42 (32 to 59) | |
| Fibrin/fibrinogen degradation products (μg/dL) | Baseline | 40 (10 to 80) | 80 (40 to 160) |
| Day 3 | NR | NR | |
| Day 5 | NR | NR | |
| Day 7 | NR | NR | |
NR: not reported
Number of platelet transfusions or number of platelet components
Neither study reported the number of platelet transfusions per participant.
Both studies reported the number of platelet components (Table 4) but only Shpilberg 1995 (56 participants) reported the number of platelet components per participant. During induction chemotherapy, there was no difference in the number of platelet components per participant in the TXA arm versus the placebo arm (38 participants, MD ‐1.00 platelet units, 95% CI ‐9.11 to 7.11) (Analysis 1.2). During consolidation chemotherapy there was a decrease in the number of platelet components per participant (18 participants; MD ‐5.60 platelet units, 95% CI ‐9.02 to ‐2.18) (Analysis 1.2).
1.2. Analysis.
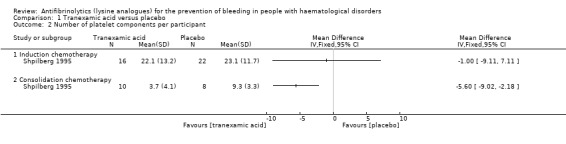
Comparison 1 Tranexamic acid versus placebo, Outcome 2 Number of platelet components per participant.
Avvisati 1989 (12 participants) did not report the number of platelet transfusions per participant and instead reported the total number of platelet transfusions used in each study arm (Table 4). The study stated that there was a reduction in the number of platelet transfusions in the TXA study arm (P = 0.045).
Number of red cell transfusions or number of red cell components
Neither study reported the number of red cell transfusions per participant.
Both studies reported the number of red cell components (Table 4), but only Shpilberg 1995 (56 participants) reported the number of red cell components per participant. During induction and consolidation chemotherapy, there was no difference in the number of red cell components per participant in the TXA arm versus the placebo arm (induction chemotherapy; 38 participants, MD 0.20 red cell components, 95% CI ‐2.48 to 2.88: consolidation chemotherapy; 18 participants, MD 0.00 red cell components, 95% CI ‐2.93 to 2.93) (Analysis 1.3).
1.3. Analysis.
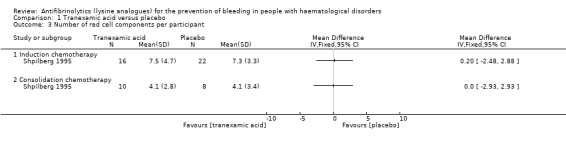
Comparison 1 Tranexamic acid versus placebo, Outcome 3 Number of red cell components per participant.
Avvisati 1989 (12 participants) did not report the number of red cell components per participant and instead reported the total number of red cell components used in each study arm (Table 4). The study stated that there was a reduction in the number of red cell components used in the TXA study arm (P = 0.016).
Adverse events of antifibrinolytic agents
Avvisati 1989 did not report adverse events of antifibrinolytic agents. Shpilberg 1995 (56 participants) reported that no side effects were observed (Table 4).
Adverse events of transfusions (e.g. transfusion reactions, antibody development)
Neither study reported the adverse events of transfusions.
Disseminated intravascular coagulation (DIC)
Neither study reported DIC.
Quality of life
Neither study reported quality of life.
Epsilon aminocaproic acid (EACA) versus placebo
There was only one study evaluating this comparison (Gallardo 1983). It was a randomised two‐arm study published in 1983 involving participants undergoing remission induction for acute leukaemia.
Number, site and severity of bleeding (i.e. any bleeding, clinically significant bleeding, life‐threatening bleeding)
Gallardo 1983 did not report bleeding per participant instead it reported bleeding as the proportion of days at risk of bleeding (defined as where the platelet count was < 20 x 109/L) (Table 3). This was 158 days for the group on EACA compared to only 80 for the group on no EACA, but the abstract noted that the patients on EACA had more severe thrombocytopenia and more cycles of chemotherapy for refractory disease. Capillary bleeding (i.e. bleeding in skin, mucous membranes, conjunctivae, nose and occult blood in gastrointestinal (GI) or genitourinary (GU) tract) was present in 31% of days at risk with patients on EACA compared to 50% of patients not receiving EACA (P value not reported). There was no difference in major bleeding (defined as nose bleeding requiring posterior packing, gross GI or GU bleeding and bleeds within the central nervous system (CNS)) between the two groups (15% versus 19%) (P value not reported).
Thromboembolism (venous and arterial)
There were no reports of thromboembolism although the study stated that no patient died of thrombosis (18 participants).
Mortality (all causes)
The study did not report all‐cause mortality.
Mortality (secondary to bleeding)
The study did not report mortality secondary to bleeding.
Mortality (secondary to thromboembolism)
The study stated that no patient died of thrombosis (18 participants).
Laboratory assessment of fibrinolysis
The study reported that antifibrinolytic therapy was monitored with the I125fibrinogen plasma clot lysis assay although no further data were described regarding this outcome.
Number of platelet transfusions or platelet components
The study did not report the number of platelet transfusions or platelet components per participant.
The study reported that platelet transfusions per days at risk were decreased in the patients on EACA, one every 13.3 days at risk versus one every 10.5 days at risk. However, the authors noted that these were not statistically significant (P value not reported). The abstract detailed a projection that the results would achieve statistical significance at a P value of < 0.05 with 25 patients in each group. However, no subsequent study has since been published. It is therefore important to note that there were insufficient patients within this study to show statistical significance for any clinically meaningful true difference (Table 4).
Number of red cell transfusions or platelet components
The study did not report the number of red cell transfusions per participant.
Adverse events of antifibrinolytic agents
No specific adverse events were described, although the study stated that side effects were minimal (18 participants).
Adverse events of transfusions (e.g. transfusion reactions, antibody development)
The study did not report adverse events of transfusions.
Disseminated intravascular coagulation (DIC)
The study did not report DIC.
Quality of life
The study did not report quality of life.
Tranexamic acid versus epsilon aminocaproic acid
We did not identify any RCTs that evaluated this comparison.
Discussion
The overall aim of this review was to determine the efficacy and safety of antifibrinolytics (lysine analogues) in the prevention of bleeding in people with haematological disorders.
Specifically, we aimed to address the following questions:
i) Do lysine analogues help to prevent bleeding in people with haematological disorders who are thrombocytopenic?
ii) Can the number of prophylactic platelet transfusions be minimised?
iii) Do lysine analogues increase the incidence of thromboembolism?
Our primary outcomes were bleeding and the occurrence of thromboembolism. Our secondary outcomes were mortality, laboratory assessment of fibrinolysis, number of platelet transfusions, number of red cell transfusions, adverse events of antifibrinolytic agents, adverse events of transfusions (e.g. transfusion reactions, antibody development), disseminated intravascular coagulation (DIC) and quality of life.
Summary of main results
Seven trials met our inclusion criteria and three of these trials are on‐going. Of the four completed trials, one had to be excluded from the assessment of Effects of interventions due to major methodological flaws in its design and a high risk of bias across several criteria. Of the remaining three randomised controlled trials (RCTs), a total of 86 patients were investigated. There were two studies comparing tranexamic acid (TXA) and placebo (Avvisati 1989; Shpilberg 1995) and one trial comparing epsilon aminocaproic acid (EACA) with placebo (Gallardo 1983). There were no studies comparing TXA with EACA.
i) Do lysine analogues help to prevent bleeding in people with haematological disorders who are thrombocytopenic?
We are uncertain whether antifibrinolytics reduce the risk of bleeding. Only one trial reported the number of bleeding episodes per participant and no difference was seen (Shpilberg 1995). The two other trials suggested bleeding was reduced in the antifibrinolytic study arm (Avvisati 1989; Gallardo 1983), but this was statistically significant in only one of these studies.
ii) Can the number of prophylactic platelet transfusions be minimised?
No trial reported the number of prophylactic platelet transfusions per participant. Only one trial reported the number of platelet components per participant and there was a reduction in platelet component usage during consolidation chemotherapy but not during induction chemotherapy (Shpilberg 1995). One of the other two trials also found that the total number of platelet components administered to participants in the antifibrinolytic arm was lower than in the placebo arm (Avvisati 1989).
iii) Do lysine analogues increase the incidence of thromboembolism?
We are uncertain whether antifibrinolytics increase the risk of developing a thromboembolism as the quality of the evidence has been assessed as very low. Two trials reported the presence or absence of thromboembolism and no events occurred in either of these trials (Avvisati 1989; Shpilberg 1995). It was reported in Gallardo 1983 that no patient died of thrombosis.
Other results
Two of the trials (Avvisati 1989; Shpilberg 1995) reported red blood cell transfusion requirements. Only one trial reported the number of red cell components per participant and there was no difference in red cell component usage between the TXA and placebo arms (Shpilberg 1995).
None of the trials reported on overall (all‐cause) mortality. However, one of the trials reported on mortality due to bleeding (Shpilberg 1995) and only one reported on mortality due to thromboembolism (Gallardo 1983); none occurred in either category.
Two trials (Gallardo 1983; Shpilberg 1995) reported on the side effects of antifibrinolytics. Gallardo 1983 stated that the side effects of EACA "were minimal" but did not provide any further detail on this within the abstract. Shpilberg 1995 also reported on side effects of TXA and stated that none were observed.
None of the trials reported on our other outcomes of interest: adverse events of transfusion, presence or development of DIC, or quality of life.
Overall completeness and applicability of evidence
This review provides the most up‐to‐date assessment of the effectiveness and safety of antifibrinolytics in people with haematological disorders who are thrombocytopenic and would usually be treated with platelet transfusions. This review identified three ongoing RCTs that are due to be completed by 2020.
There is very low quality evidence that adults with acute leukaemia who are receiving chemotherapy may have a decreased risk of bleeding with antifibrinolytics and may decrease their use of platelet components.
The results of this review should take into consideration the impact of the following factors.
The completed studies included in this review were all conducted at least twenty years ago (1983 to 1995), during which time chemotherapy protocols, predicted overall survival rates, and supportive care, including transfusion, have changed substantially.
Only 86 participants were included in the three studies that provided data for this review's outcomes. Two studies (68 participants) compared TXA with placebo and one study (18 participants) compared EACA with placebo.
The recording of bleeding is subjective, and between centres there is variability in the assessment, grading, investigation and recording of bleeding. The same bleeding scale may even be interpreted and applied differently, particularly with respect to red cell transfusion.
We could not perform any meta‐analyses because of the various ways in which the outcomes had been reported.
We were unable to obtain any additional data from study authors to be used quantitatively in a meta‐analysis. Due to the age of all three studies only one author could be located. This author no longer had data available because the trial was conducted over 30 years ago (Gallardo 1983).
There were several review outcomes that were not reported by any of the studies. These included: adverse events of transfusion, the presence or development of DIC and quality of life. This highlights the paucity of data in this area.
All of the evidence in this review was in adults with acute leukaemia receiving chemotherapy, there is currently no evidence to support the use of antifibrinolytics in adults with other haematological disorders, people requiring haematopoietic stem cell transplants, or children.
All studies were suggestive of a role of antifibrinolytics in reducing platelet usage, but it is important to note that in Shpilberg 1995 the effect was only seen in the consolidation group and not in the induction group, and in Gallardo 1983, although the number of platelet transfusions required per days at risk was less for the EACA arm, the results were not statistically significant. The study authors projected that their results would "achieve statistical significance at a P value of < 0.05 with 25 patient in each group". However, despite an extensive literature search, and direct contact with the study authors no further data for Gallardo 1983 were available.
Two studies (68 patients) reported the presence or absence of thromboembolic events and no events occurred in either study (Avvisati 1989; Shpilberg 1995). It was reported in Gallardo 1983 that no patient died of thrombosis, but it is unclear whether any non‐fatal thromboembolic events occurred at all. Although there is no evidence within these three studies to suggest that there is an increased risk of thromboembolism with antifibrinolytics there are insufficient data to conclude that this risk does not exist
Quality of the evidence
We assessed the quality of the evidence using the GRADE approach and this was either very low or the outcome was not reported by any of the studies (Table 1). We were unable to gain additional information via direct author contact and therefore could not improve the quality of the data.
Only three outcomes could be assessed using the GRADE approach.
Number of participants with any bleeding
Number of participants with thromboembolism
Adverse events of antifibrinolytic agents
All were assessed as very low grade quality evidence due to serious risk of bias and very serious imprecision.
One study (Fricke 1991) had significant methodological problems with its design (seeRisk of bias in included studies and Figure 2). The overall success of TXA was defined as either five failures of placebo and none of the drug, or seven failures of placebo and one of the drug. Overall failure of TXA was defined as two failed courses of the drug. Failure of a course was defined as a patient receiving a platelet transfusion for bleeding during a four‐week study period. Patients received a variable number of courses of drug/placebo. The three patients who completed the study received between three (two TXA, one placebo) and nine courses (five TXA, four placebo) of treatment. The five patients who did not complete the study received between zero and 20 courses (10 TXA, 10 placebo) of treatment. Of the three patients who completed the study, two did not have any successful courses of treatment. The third patient had three of five successful courses with TXA and one of four successful courses with placebo, however this was classified by the study as a failure of TXA (two failed courses with TXA). Interim analysis of the data was therefore performed after each course of treatment for each patient, with completion of the study being biased against TXA (only two failures of TXA are required, whereas five failures of placebo and none of TXA for study to be classified as completed).
All of the other three included studies (Avvisati 1989; Gallardo 1983; Shpilberg 1995) had some threats to validity and in most cases this was graded as 'unclear' due to lack of detail in the study to determine the level of risk. One of these studies (Gallardo 1983) was at high risk of selective reporting. The data were presented in an abstract form and the problem was likely to have been due to limited space. However, important data were omitted, most notably number of episodes of thrombosis since the comment "no patient died of thrombosis" is suggestive of the presence of thrombosis in the study. This is clearly of particular importance when considering the safety of antifibrinolytics when thrombosis is a noted side effect of these agents.
Avvisati 1989 appeared to be the study most free of bias with low risk when considering attrition bias and other bias. Other than those mentioned, the other parameters for bias were listed as unclear due to a lack of data to determine risk as high or low.
One negative aspect that all the studies had in common was small sample sizes which reduced their statistical power. This means that even if a clinically meaningful true difference was present it may not be detected due to the small number of patients within each study. This could not be overcome via the use of meta‐analysis because the data had been reported in different ways. In one study in particular (Gallardo 1983), the small sample size was insufficient to permit statistical significance for at least one outcome (number of platelet transfusions in each arm)
Potential biases in the review process
There were no clear biases identified in the review process. We conducted a comprehensive search of data sources (including multiple databases and clinical trial registries) to ensure that we would capture all relevant trials. We made no restrictions for the language in which the paper was originally published. We carefully assessed the relevance of each paper identified and performed all screening and data extractions in duplicate.
The systematic methods of searching, data extraction and result analysis were followed with reference to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
One important consideration was whether our decision not to include Fricke 1991 in the narrative review of the included studies may represent some risk of publication bias. Fricke 1991 was the only study that showed a lack efficacy of the antifibrinolytic (TXA). However, given the methodological flaws and high levels of risk across several criteria as mentioned in Assessment of risk of bias in included studies, it was felt that it should be excluded from the narrative review.
Two of the authors of this review are primary investigators of an ongoing study included in the systematic review (TREATT 2015). This study has not been completed yet and 'Risk of bias' assessment and analysis of the completed studies was performed prior to the initiation of TREATT 2015 in the previous version of this review (Wardrop 2013).
Agreements and disagreements with other studies or reviews
The fact that there were so few RCTs that were suitable for inclusion in our narrative review demonstrates the lack of efficacy and safety data for the use of antifibrinolytics in haematology patients who are thrombocytopenic.
Comparison to other systematic reviews
To our knowledge, there are no other systematic reviews examining this topic.
Comparison to non‐randomised trials
Several small non‐randomised studies have used TXA or EACA in haematology patients (Bartholomew 1989; Ben‐Bassat 1990; Chakrabarti 1998; Dean 1997; Gardner 1980; Garewal 1985; Kalmadi 2006; Schwartz 1986; Wassenaar 2008). However, virtually all of these studies did not have a comparator arm, making it difficult to draw any valid conclusions on the effectiveness and safety of antifibrinolytics. One larger study of TXA in patients with acute promyelocytic leukaemia (APML) used an historical control (Sanz 2010); there was no difference in deaths due to bleeding between those patients who received TXA and those who did not. Death due to bleeding is a rare event and this study may not have had sufficient power to detect a difference. This study (Sanz 2010) did show a statistically significant increase in the number of patients who developed thromboembolic complications. However, because Sanz 2010 used an historical control there may have been other confounding factors including changes to the chemotherapy regimen used that could have also affected the number of thromboembolic events.
Authors' conclusions
Implications for practice.
Our results indicate that the evidence available for the use of antifibrinolytics in haematology patients is very limited. The only data available suggest that tranexamic acid (TXA) and epsilon aminocaproic acid may (EACA) help to reduce bleeding and might therefore be useful adjuncts to platelet transfusions but it was not possible to perform a meta‐analysis. Two of the three studies suggested a reduction in bleeding and two of the three studies suggested a reduction in platelet usage. The trials were too small to assess whether antifibrinolytics increased the risk of thromboembolic events. Although the available evidence from the included studies appears consistent, the quality of the evidence was very low, making it difficult to draw conclusions or make recommendations regarding the usefulness and safety of antifibrinolytics.
Implications for research.
The only evidence available is for adults with acute leukaemia receiving chemotherapy. We await the results of the two ongoing trials that are expected to recruit 916 participants in total by 2020. These studies are recruiting adults with a mixture of haematological malignancies.
There is currently no evidence for the use of antifibrinolytics in children with haematological disorders who are thrombocytopenic and usually require treatment with platelet transfusions and there are no ongoing studies that include children.
What's new
| Date | Event | Description |
|---|---|---|
| 7 March 2016 | New citation required but conclusions have not changed | Three new studies were identified and added to Ongoing studies (A‐TREAT 2015; PROBLEMA 2014; TREATT 2015); two are recruiting participants and are due to be completed in 2017 and 2020 (PROBLEMA 2014; TREATT 2015), and one is not yet recruiting participants (A‐TREAT 2015). Three additional studies were excluded (Antun 2013; NCT01980355; Rathi 2015). Two new review authors joined the review team. |
| 7 March 2016 | New search has been performed | Searches re‐run. Review updated. |
Acknowledgements
We thank the editorial base of the Cochrane Haematological Malignancies Review Group.
We thank the National Institute of Health Research (NIHR). This review is part of a series of reviews that have been funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components. This research was also supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre Programme. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Douglas Wardrop and Marialena Trivella were authors on the previous version of this review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Antifibrinolytic Agents, this term only #2 MeSH descriptor Tranexamic Acid, this term only #3 MeSH descriptor Aminocaproic Acids explode all trees #4 (antifibrinolytic* or anti fibrinolytic* or antiplasmin* or plasmin inhibitor* or tranexamic or cyclohexanecarboxylic acid* or trans‐4‐aminomethyl‐cyclohexanecarboxylic acid* or t‐amcha or kabi 2161 or transamin or exacyl or anvitoff or spotof or cyklokapron or ugurol or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or AMCHA or amchafibrin or amikapron or aminomethyl cyclohexane carboxylic acid or aminomethyl cyclohexanecarboxylic acid or aminomethylcyclohexane carbonic acid or aminomethylcyclohexane carboxylic acid or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or aminomethylcyclohexanocarboxylic acid or aminomethylcyclohexanoic acid or amstat or anvitoff or cl?65336 or cl65336 or cyclocapron or cyclokapron or cyklocapron or exacyl or frenolyse or hexacapron or hexakapron or TXA) #5 (Agretax or Bio‐Stat or Capiloc or Capitrax or Clip Inj or Clot‐XL or Clotawin‐T or Cymin or Dubatran or Examic or Existat or Extam or Fibran or Hemstate or Menogia or Monitex or Nestran or Nexamic or Nexi‐500 or Nexmeff or Nixa‐500 or Rheonex or Sylstep TX or Synostat or T‐nex or T Stat or T Stat or Tanmic or Temsyl‐T or Texakind or Texanis or Texapar or Texid or Thams or Tonopan or Traklot or Tramic or Tramix or Tranarest or Trance Inj or Tranecid or Tranee or Tranemic or Tranex or Tranexa or Tranfib or Tranlok or Transtat or Transys or Tranxi or Trapic or Traxage or Traxamic or Trenaxa or Trexamic or Trim Inj or Tx‐1000 or Tx 500 or Wistran or X‐Tran or Xamic) #6 ((aminocaproic or aminacaproic or aminohexanoic or aminohexanoic or epsilon‐aminocaproic or E‐aminocaproic or amino caproic or amino‐n‐hexanoic) NEAR/2 acid*) #7 (epsikapron or cy‐116 or cy116 or epsamon or amicar or caprocid or acikaprin or afibrin or capracid or capramol or caprogel or caprolest or caprolisine or caprolysin or capromol or hemocaprol or caproamin or EACA or caprolest or capralense or hexalense or hamostat or hemocid) #8 (cl 10304 or ecapron or ekaprol or epsamon or epsicapron or epsilcapramin or epsilon amino caproate or epsilon aminocaproate or epsilonaminocaproic or etha?aminocaproic or ethaaminocaproich or emocaprol or hepin or ipsilon or jd?177or neocaprol or nsc?26154 or tachostyptan) #9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8) #10 MeSH descriptor Hematologic Neoplasms explode all trees #11 MeSH descriptor Leukemia explode all trees #12 MeSH descriptor Lymphoma explode all trees #13 MeSH descriptor Multiple Myeloma explode all trees #14 MeSH descriptor Anemia, Aplastic explode all trees #15 MeSH descriptor Bone Marrow Diseases explode all trees #16 MeSH descriptor Thrombocytopenia explode all trees #17 (thrombocytopeni* or thrombocytopaeni* or leukemia or leukaemia or lymphoma* or aplastic anemia or aplastic anaemia or myelodysplas* or myeloproliferat* or multiple myeloma or plasma cell myeloma or thrombocythemi* or thrombocythaemi* or polycythemi* or polycythaemi* or myelofibros* or AML or CLL or CML or Hodgkin*) #18 ((haematolog* or hematolog* or blood or red cell* or white cell* or lymph* or marrow or platelet*) NEAR/3 (malignan* or oncolog* or cancer* or neoplasm*)) #19 MeSH descriptor Antineoplastic Agents explode all trees #20 MeSH descriptor Stem Cell Transplantation explode all trees #21 MeSH descriptor Bone Marrow Transplantation, this term only #22 MeSH descriptor Radiotherapy explode all trees #23 (chemotherap* or radiotherap* or chemoradiotherap* or stem cell* or bone marrow transplant* or rituximab) #24 ((haematolog* or hematolog*) NEAR/2 patients) #25 (malignan* or oncolog* or cancer*):ti #26 (#10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25) #27 (#9 AND #26)
Appendix 2. MEDLINE (Ovid) search strategy
1. Antifibrinolytic Agents/ 2. Tranexamic Acid/ 3. exp Aminocaproic Acids/ 4. (antifibrinolytic* or anti fibrinolytic* or antiplasmin* or plasmin inhibitor*).tw. 5. (tranexamic or cyclohexanecarboxylic acid* or trans‐4‐aminomethyl‐cyclohexanecarboxylic acid* or t‐amcha or kabi 2161 or transamin or exacyl or anvitoff or spotof or cyklokapron or ugurol or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or AMCHA or amchafibrin or amikapron or aminomethyl cyclohexane carboxylic acid or aminomethyl cyclohexanecarboxylic acid or aminomethylcyclohexane carbonic acid or aminomethylcyclohexane carboxylic acid or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or aminomethylcyclohexanocarboxylic acid or aminomethylcyclohexanoic acid or amstat or anvitoff or cl?65336 or cl65336 or cyclocapron or cyclokapron or cyklocapron or exacyl or frenolyse or hexacapron or hexakapron or TXA).tw. 6. (Agretax or Bio‐Stat or Capiloc or Capitrax or Clip Inj or Clot‐XL or Clotawin‐T or Cymin or Dubatran or Examic or Existat or Extam or Fibran or Hemstate or Menogia or Monitex or Nestran or Nexamic or Nexi‐500 or Nexmeff or Nixa‐500 or Rheonex or Sylstep TX or Synostat or T‐nex or T Stat or T Stat or Tanmic or Temsyl‐T or Texakind or Texanis or Texapar or Texid or Thams or Tonopan or Traklot or Tramic or Tramix or Tranarest or Trance Inj or Tranecid or Tranee or Tranemic or Tranex or Tranexa or Tranfib or Tranlok or Transtat or Transys or Tranxi or Trapic or Traxage or Traxamic or Trenaxa or Trexamic or Trim Inj or Tx‐1000 or Tx 500 or Wistran or X‐Tran or Xamic).tw. 7. ((aminocaproic or amino?caproic or aminohexanoic or amino?hexanoic or epsilon‐aminocaproic or E‐aminocaproic or amino caproic or amino‐n‐hexanoic) adj2 acid*).tw. 8. (epsikapron or cy‐116 or cy116 or epsamon or amicar or caprocid or acikaprin or afibrin or capracid or capramol or caprogel or caprolest or caprolisine or caprolysin or capromol or hemocaprol or caproamin or EACA or caprolest or capralense or hexalense or hamostat or hemocid).tw. 9. (cl 10304 or ecapron or ekaprol or epsamon or epsicapron or epsilcapramin or epsilon amino caproate or epsilon aminocaproate or epsilonaminocaproic or etha?aminocaproic or ethaaminocaproich or emocaprol or hepin or ipsilon or jd?177or neocaprol or nsc?26154 or tachostyptan).tw. 10. or/1‐9 11. exp Hematologic Neoplasms/ 12. exp leukemia/ or exp lymphoma/ 13. exp Multiple Myeloma/ 14. exp Anemia, Aplastic/ 15. exp Bone Marrow Diseases/ 16. exp Thrombocytopenia/ 17. (thrombocytopeni* or thrombocytopaeni* or leukemia or leukaemia or lymphoma* or aplastic anemia or aplastic anaemia or myelodysplas* or myeloproliferat* or multiple myeloma or plasma cell myeloma or thrombocythemi* or thrombocythaemi* or polycythemi* or polycythaemi* or myelofibros* or AML or CLL or CML or Hodgkin*).tw. 18. ((haematolog* or hematolog* or blood or red cell* or white cell* or lymph* or marrow or platelet*) adj3 (malignan* or oncolog* or cancer* or neoplasm*)).tw. 19. exp Antineoplastic Agents/ 20. exp Stem Cell Transplantation/ or Bone Marrow Transplantation/ or exp Radiotherapy/ 21. (chemotherap* or radiotherap* or chemoradiotherap* or stem cell* or bone marrow transplant* or rituximab).tw. 22. ((haematolog* or hematolog*) adj2 patients).tw. 23. (malignan* or oncolog* or cancer*).ti. 24. or/11‐23 25. 10 and 24
Appendix 3. EMBASE (Ovid) search strategy
1. Antifibrinolytic Agent/ 2. Tranexamic Acid/ 3. Aminocaproic Acid/ 4. (antifibrinolytic* or anti fibrinolytic* or antiplasmin* or plasmin inhibitor*).tw. 5. (tranexamic or cyclohexanecarboxylic acid* or trans‐4‐aminomethyl‐cyclohexanecarboxylic acid* or t‐amcha or kabi 2161 or transamin or exacyl or anvitoff or spotof or cyklokapron or ugurol or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or AMCHA or amchafibrin or amikapron or aminomethyl cyclohexane carboxylic acid or aminomethyl cyclohexanecarboxylic acid or aminomethylcyclohexane carbonic acid or aminomethylcyclohexane carboxylic acid or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or aminomethylcyclohexanocarboxylic acid or aminomethylcyclohexanoic acid or amstat or anvitoff or cl?65336 or cl65336 or cyclocapron or cyclokapron or cyklocapron or exacyl or frenolyse or hexacapron or hexakapron or TXA).tw. 6. (Agretax or Bio‐Stat or Capiloc or Capitrax or Clip Inj or Clot‐XL or Clotawin‐T or Cymin or Dubatran or Examic or Existat or Extam or Fibran or Hemstate or Menogia or Monitex or Nestran or Nexamic or Nexi‐500 or Nexmeff or Nixa‐500 or Rheonex or Sylstep TX or Synostat or T‐nex or T Stat or T Stat or Tanmic or Temsyl‐T or Texakind or Texanis or Texapar or Texid or Thams or Tonopan or Traklot or Tramic or Tramix or Tranarest or Trance Inj or Tranecid or Tranee or Tranemic or Tranex or Tranexa or Tranfib or Tranlok or Transtat or Transys or Tranxi or Trapic or Traxage or Traxamic or Trenaxa or Trexamic or Trim Inj or Tx‐1000 or Tx 500 or Wistran or X‐Tran or Xamic).tw. 7. ((aminocaproic or amino?caproic or aminohexanoic or amino?hexanoic or epsilon‐aminocaproic or E‐aminocaproic or amino caproic or amino‐n‐hexanoic) adj2 acid*).tw. 8. (epsikapron or cy‐116 or cy116 or epsamon or amicar or caprocid or acikaprin or afibrin or capracid or capramol or caprogel or caprolest or caprolisine or caprolysin or capromol or hemocaprol or caproamin or EACA or caprolest or capralense or hexalense or hamostat or hemocid).tw. 9. (cl 10304 or ecapron or ekaprol or epsamon or epsicapron or epsilcapramin or epsilon amino caproate or epsilon aminocaproate or epsilonaminocaproic or etha?aminocaproic or ethaaminocaproich or emocaprol or hepin or ipsilon or jd?177or neocaprol or nsc?26154 or tachostyptan).tw. 10. or/1‐9 11. Hematologic Malignancy/ 12. Lymphoma/ 13. NonHodgkin Lymphoma/ 14. Hodgkin Disease/ 15. exp Myeloproliferative Disorder/ 16. exp Aplastic Anemia/ 17. exp Thrombocytopenia/ 18. (thrombocytopeni* or thrombocytopaeni* or leukemia or leukaemia or lymphoma* or aplastic anemia or aplastic anaemia or myelodysplas* or myeloproliferat* or multiple myeloma or plasma cell myeloma or thrombocythemi* or thrombocythaemi* or polycythemi* or polycythaemi* or myelofibros* or AML or CLL or CML or Hodgkin*).tw. 19. ((haematolog* or hematolog* or blood or red cell* or white cell* or lymph* or marrow or platelet*) adj3 (malignan* or oncolog* or cancer* or neoplasm*)).tw. 20. exp Chemotherapy/ 21. exp Stem Cell Transplantation/ 22. exp Bone Marrow Transplantation/ 23. exp Radiotherapy/ 24. (chemotherap* or radiotherap* or chemoradiotherap* or stem cell* or bone marrow transplant* or rituximab).tw. 25. ((haematolog* or hematolog*) adj2 patients).tw. 26. (malignan* or oncolog* or cancer*).ti. 27. or/11‐26 28. 10 and 27
Appendix 4. CINAHL (EBSCOhost) search strategy
1. (MH Antifibrinolytic Agent) 2. (MH Aminocaproic Acids+) 3. (antifibrinolytic* or “anti fibrinolytic*” or antiplasmin* or “plasmin inhibitor*”) 4. TI (tranexamic or "cyclohexanecarboxylic acid*" or "trans‐4‐aminomethyl‐cyclohexanecarboxylic acid*" or "t‐amcha" or "kabi 2161" or transamin or exacyl or anvitoff or spotof or cyklokapron or ugurol or aminomethylcyclohexanecarbonic acid or "aminomethylcyclohexanecarboxylic acid" or AMCHA or amchafibrin or amikapron or "aminomethyl cyclohexane carboxylic acid" or "aminomethyl cyclohexanecarboxylic acid" or "aminomethylcyclohexane carbonic acid" or "aminomethylcyclohexane carboxylic acid" or "aminomethylcyclohexanecarbonic acid" or "aminomethylcyclohexanecarboxylic acid" or "aminomethylcyclohexanocarboxylic acid" or "aminomethylcyclohexanoic acid" or amstat or anvitoff or cl65336 or cyclocapron or cyclokapron or cyklocapron or exacyl or frenolyse or hexacapron or hexakapron or TXA) OR AB (tranexamic or "cyclohexanecarboxylic acid*" or "trans‐4‐aminomethyl‐cyclohexanecarboxylic acid*" or "t‐amcha" or "kabi 2161" or transamin or exacyl or anvitoff or spotof or cyklokapron or ugurol or aminomethylcyclohexanecarbonic acid or "aminomethylcyclohexanecarboxylic acid" or AMCHA or amchafibrin or amikapron or "aminomethyl cyclohexane carboxylic acid" or "aminomethyl cyclohexanecarboxylic acid" or "aminomethylcyclohexane carbonic acid" or "aminomethylcyclohexane carboxylic acid" or "aminomethylcyclohexanecarbonic acid" or "aminomethylcyclohexanecarboxylic acid" or "aminomethylcyclohexanocarboxylic acid" or "aminomethylcyclohexanoic acid" or amstat or anvitoff or cl65336 or cyclocapron or cyclokapron or cyklocapron or exacyl or frenolyse or hexacapron or hexakapron or TXA) 5. TI (Agretax or "Bio‐Stat" or Capiloc or Capitrax or "Clip Inj" or "Clot‐XL" or "Clotawin‐T" or Cymin or Dubatran or Examic or Existat or Extam or Fibran or Hemstate or Menogia or Monitex or Nestran or Nexamic or "Nexi‐500" or Nexmeff or Nixa‐500 or Rheonex or "Sylstep TX" or Synostat or "T‐nex" or "T Stat" or "T Stat" or Tanmic or "Temsyl‐T" or Texakind or Texanis or Texapar or Texid or Thams or Tonopan or Traklot or Tramic or Tramix or Tranarest or "Trance Inj" or Tranecid or Tranee or Tranemic or Tranex or Tranexa or Tranfib or Tranlok or Transtat or Transys or Tranxi or Trapic or Traxage or Traxamic or Trenaxa or Trexamic or "Trim Inj" or "Tx‐1000" or "Tx 500" or Wistran or "X‐Tran" or Xamic) OR AB (Agretax or "Bio‐Stat" or Capiloc or Capitrax or "Clip Inj" or "Clot‐XL" or "Clotawin‐T" or Cymin or Dubatran or Examic or Existat or Extam or Fibran or Hemstate or Menogia or Monitex or Nestran or Nexamic or "Nexi‐500" or Nexmeff or Nixa‐500 or Rheonex or "Sylstep TX" or Synostat or "T‐nex" or "T Stat" or "T Stat" or Tanmic or "Temsyl‐T" or Texakind or Texanis or Texapar or Texid or Thams or Tonopan or Traklot or Tramic or Tramix or Tranarest or "Trance Inj" or Tranecid or Tranee or Tranemic or Tranex or Tranexa or Tranfib or Tranlok or Transtat or Transys or Tranxi or Trapic or Traxage or Traxamic or Trenaxa or Trexamic or "Trim Inj" or "Tx‐1000" or "Tx 500" or Wistran or "X‐Tran" or Xamic) 6. TI ((aminocaproic or amino‐caproic or aminohexanoic or amino‐hexanoic or epsilon‐aminocaproic or E‐aminocaproic or amino caproic or amino‐n‐hexanoic) and acid*) OR AB ((aminocaproic or amino‐caproic or aminohexanoic or amino‐hexanoic or epsilon‐aminocaproic or E‐aminocaproic or amino caproic or amino‐n‐hexanoic) and acid*) 7. TI (epsikapron or cy‐116 or cy116 or epsamon or amicar or caprocid or acikaprin or afibrin or capracid or capramol or caprogel or caprolest or caprolisine or caprolysin or capromol or hemocaprol or caproamin or EACA or caprolest or capralense or hexalense or hamostat or hemocid) OR AB (epsikapron or cy‐116 or cy116 or epsamon or amicar or caprocid or acikaprin or afibrin or capracid or capramol or caprogel or caprolest or caprolisine or caprolysin or capromol or hemocaprol or caproamin or EACA or caprolest or capralense or hexalense or hamostat or hemocid) 8. TI (cl 10304 or ecapron or ekaprol or epsamon or epsicapron or epsilcapramin or epsilon amino caproate or epsilon aminocaproate or epsilonaminocaproic or etha‐aminocaproic or ethaaminocaproich or emocaprol or hepin or ipsilon or jd‐177or neocaprol or nsc?26154 or tachostyptan) OR AB (cl 10304 or ecapron or ekaprol or epsamon or epsicapron or epsilcapramin or epsilon amino caproate or epsilon aminocaproate or epsilonaminocaproic or etha‐aminocaproic or ethaaminocaproich or emocaprol or hepin or ipsilon or jd‐177or neocaprol or nsc‐26154 or tachostyptan) 9. S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 10. (MH Hematologic Neoplasms+) 11. (MH Leukemia+) 12. (MH Lymphoma+) 13. (MH Bone Marrow Diseases+) 14. (MH Thrombocytopenia+) 15. TI (thrombocytopeni* or thrombocytopaeni* or leukemia or leukaemia or lymphoma* or aplastic anemia or aplastic anaemia or myelodysplas* or myeloproliferat* or multiple myeloma or plasma cell myeloma or thrombocythemi* or thrombocythaemi* or polycythemi* or polycythaemi* or myelofibros* or AML or CLL or CML or Hodgkin*) 16. TI ((haematolog* or hematolog* or blood or red cell* or white cell* or lymph* or marrow or platelet*) and (malignan* or oncolog* or cancer* or neoplasm*)) 17. (MH Antineoplastic Agents) 18. (MH Chemotherapy, Cancer+) 19. (MH Hematopoietic Stem Cell Transplantation) 20. (MH Bone Marrow Transplantation+) 21. (MH Radiotherapy+) 22. TI (chemotherap* or radiotherap* or chemoradiotherap* or stem cell* or bone marrow transplant* or rituximab) 23. TI ((haematolog* N2 patients) or (hematolog* N2 patients)) 24. TI (malignan* or oncolog* or cancer*) 25. S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 26. S9 AND S25
Appendix 5. PubMed (epublications only)
#1 antifibrinolytic*[tiab] OR "anti fibrinolytic*"[tiab] OR antiplasmin*[tiab] OR “plasmin inhibitor*”[tiab] OR tranexamic[tiab] OR amcha[tiab] OR transamin[tiab] OR exacyl[tiab] OR amchafibrin[tiab] OR anvitoff[tiab] OR spotof[tiab] OR cyklokapron[tiab] OR ugurol[tiab] OR amikapron[tiab] OR amstat[tiab] OR anvitoff[tiab] OR OR cyclocapron[tiab] OR cyclokapron[tiab] OR cyklocapron[tiab] OR exacyl[tiab] OR frenolyse OR hexacapron[tiab] OR hexakapron[tiab] OR TXA[tiab] OR agretax[tiab] OR "Bio‐Stat"[tiab] OR Capiloc[tiab] OR Capitrax[tiab] OR "Clip Inj"[tiab] OR “Clot‐XL”[tiab] OR "Clotawin‐T"[tiab] OR Cymin[tiab] OR Dubatran[tiab] OR Examic[tiab] OR Existat[tiab] OR Extam[tiab] OR Fibran[tiab] OR Hemstate[tiab] OR Menogia[tiab] OR Monitex[tiab] OR Nestran[tiab] OR Nexamic[tiab] OR “Nexi‐500”[tiab] OR Nexmeff[tiab] OR Nixa‐500[tiab] OR Rheonex[tiab] OR “Sylstep TX”[tiab] OR Synostat[tiab] OR "T‐nex"[tiab] OR "T Stat"[tiab] OR Tanmic OR “Temsyl‐T”[tiab] OR Texakind[tiab] OR Texanis[tiab] OR Texapar[tiab] OR Texid[tiab] OR Thams[tiab] OR Tonopan[tiab] OR Traklot[tiab] OR Tramic[tiab] OR Tramix[tiab] OR Tranarest[tiab] OR “Trance Inj”[tiab] OR Tranecid[tiab] OR Tranee[tiab] OR Tranemic[tiab] OR Tranex[tiab] OR Tranexa[tiab] OR Tranfib[tiab] OR Tranlok[tiab] OR Transtat[tiab] OR Transys[tiab] OR Tranxi[tiab] OR Trapic[tiab] OR Traxage[tiab] OR Traxamic[tiab] OR Trenaxa[tiab] OR Trexamic[tiab] OR "Trim Inj"[tiab] OR "Tx‐1000"[tiab] OR "Tx 500"[tiab] OR Wistran[tiab] OR "X‐Tran"[tiab] OR Xamic[tiab] OR aminocaproic[tiab] OR aminohexanoic[tiab] OR amino caproic[tiab] OR amino‐n‐hexanoic[tiab] OR epsikapron[tiab] OR cy‐116[tiab] OR cy116[tiab] OR epsamon[tiab] OR amicar[tiab] OR caprocid[tiab] OR acikaprin[tiab] OR afibrin[tiab] OR capracid[tiab] OR capramol[tiab] OR caprogel[tiab] OR caprolest[tiab] OR caprolisine[tiab] OR caprolysin[tiab] OR capromol[tiab] OR hemocaprol[tiab] OR caproamin[tiab] OR EACA[tiab] OR caprolest[tiab] OR capralense[tiab] OR hexalense[tiab] OR hamostat[tiab] OR hemocid[tiab] OR ecapron[tiab] OR ekaprol[tiab] OR epsamon[tiab] OR epsicapron[tiab] OR epsilcapramin[tiab] OR “epsilon amino caproate”[tiab] OR “epsilon aminocaproate”[tiab] OR epsilonaminocaproic[tiab] OR ethaaminocaproich[tiab] OR emocaprol[tiab] OR hepin[tiab] OR ipsilon[tiab] OR neocaprol[tiab] OR nsc26154[tiab] OR tachostyptan[tiab] #2 thrombocytopeni*[tiab] OR thrombocytopaeni*[tiab] OR leukemia[tiab] OR leukaemia[tiab] OR lymphoma*[tiab] OR aplastic anemia[tiab] OR aplastic anaemia[tiab] OR myelodysplas*[tiab] OR myeloproliferat*[tiab] OR multiple myeloma[tiab] OR plasma cell myeloma[tiab] OR thrombocythemi*[tiab] OR thrombocythaemi*[tiab] OR polycythemi*[tiab] OR polycythaemi*[tiab] OR myelofibros*[tiab] OR AML[tiab] OR CLL[tiab] OR CML[tiab] OR Hodgkin*[tiab] #3 (haematolog*[tiab] OR hematolog*[tiab] OR blood[tiab] OR red cell*[tiab] OR white cell*[tiab] OR lymphom*[tiab] OR marrow[tiab] OR platelet*[tiab]) and (malignan*[tiab] OR oncolog*[tiab] OR cancer*[tiab] OR neoplasm*[tiab]) #4 chemotherap*[tiab] OR radiotherap*[tiab] OR chemoradiotherap*[tiab] OR stem cell*[tiab] OR bone marrow transplant*[tiab] OR rituximab[tiab] #5 "haematology patients"[tiab] OR "hematology patients"[tiab] OR "haematological patients"[tiab] OR "hematological patients"[tiab] #6 malignan*[ti] OR oncolog*[ti] OR cancer*[ti] #7 #2 OR #3 OR #4 OR #5 OR #6 #8 #1 AND #7 #9 publisher[sb] NOT pubstatusnihms #10 #8 AND #9
Appendix 6. LILACS, KoreaMed, IndMed, PakMediNet search strategy
antifibrinolytic OR antifibrinolytics OR "anti fibrinolytic" OR "anti fibrinolytics" OR antiplasmin OR "plasmin inhibitor" OR tranexamic OR cyklokapron OR aminocaproic OR EACA OR amcha
Appendix 7. UKBTS SRI Transfusion Evidence Library search strategy
#1 (antifibrinolytic OR antifibrinolytics OR anti‐fibrinolytic OR anti fibrinolytics OR antiplasmin OR plasmin inhibitor OR tranexamic OR amcha OR transamin OR exacyl OR amchafibrin OR anvitoff OR spotof OR cyklokapron OR ugurol OR amikapron OR amstat OR anvitoff OR cyclocapron OR cyclokapron OR cyklocapron OR exacyl OR frenolyse OR hexacapron OR hexakapron OR agretax OR Capiloc OR Capitrax OR Cymin OR Dubatran OR Examic OR Existat OR Extam OR Fibran OR Hemstate OR Monitex OR Nestran OR Nexamic OR Nexmeff OR Nixa‐500 OR Rheonex OR Synostat OR aminocaproic OR aminohexanoic OR amino caproic OR EACA OR amino‐n‐hexanoic OR epsikapron OR epsamon OR amicar OR caprocid OR acikaprin OR afibrin OR capracid OR capramol OR caprogel OR caprolest OR caprolisine OR caprolysin OR capromol OR hemocaprol OR caproamin OR caprolest OR capralense OR hexalense OR hamostat OR hemocid OR ecapron OR ekaprol OR epsamon OR epsicapron OR epsilcapramin OR epsilon amino caproate OR epsilon aminocaproate OR epsilonaminocaproic OR ethaaminocaproich OR emocaprol OR hepin OR ipsilon OR neocaprol OR tachostyptan) #2 ((haematolog* OR hematolog* OR marrow OR platelet*) AND (malignan* OR oncolog* OR cancer* OR neoplasm*)) #3 (leukemi* or leukaemi* or lymphoma* or chemotherap* OR radiotherap* OR chemoradiotherap* OR stem cell* OR bone marrow transplant* OR rituximab OR "haematology patients" OR "hematology patients" OR "haematological patients" OR "hematological patients") #4 (malignan* OR oncolog* OR cancer*)[In Title] #5 #2 OR #3 OR #4 #6 #1 AND #5
Appendix 8. Web of Science, Conference Proceedings Citation Index search strategy
(malignan* OR oncolog* OR cancer* OR neoplasm* OR chemotherap* OR radiotherap* OR chemoradiotherap* OR stem cell* OR bone marrow transplant* OR rituximab OR "haematology patients" OR "hematology patients" OR "haematological patients" OR "hematological patients") AND (antifibrinolytic OR antifibrinolytics OR anti‐fibrinolytic OR anti fibrinolytics OR tranexamic OR aminocaproic OR EACA)
Appendix 9. ClinicalTrials.gov and ICTRP search strategy
Condition: malignan* OR oncolog* OR cancer* OR neoplasm* OR chemotherap* OR radiotherap* OR chemoradiotherap* OR stem cell* OR bone marrow transplant* OR rituximab OR "haematology patients" OR "hematology patients" OR "haematological patients" OR "hematological patients" AND Intervention: antifibrinolytic OR antifibrinolytics OR anti‐fibrinolytic OR anti fibrinolytics OR tranexamic OR aminocaproic OR EACA
Appendix 10. ISRCTN, EUDRACT, UMIN and Hong Kong Registry search strategy
antifibrinolytic OR antifibrinolytics OR anti‐fibrinolytic OR anti fibrinolytics OR tranexamic OR aminocaproic OR EACA
Data and analyses
Comparison 1. Tranexamic acid versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of bleeding events per participant | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Induction chemotherapy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Consolidation chemotherapy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of platelet components per participant | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Induction chemotherapy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Consolidation chemotherapy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of red cell components per participant | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Induction chemotherapy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Consolidation chemotherapy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Avvisati 1989.
| Methods | Parallel RCT. 2 centres (Italy and The Netherlands). Enrolment period not stated. | |
| Participants |
Inclusion criteria: people with newly diagnosed APML who met the following criteria: age 15 to 60 yrs; ventricular ejection fraction > 50%; serum alanine aminotransferase (ALT) < 4 x upper limit of normal; creatinine < 177 μmol/L. Exclusion criteria: not stated Arm 1 N = 6 (APML N = 6) Arm 2 N = 6 (APML N = 6) Age range 17 to 59 years |
|
| Interventions | Comparison between TXA and placebo Arm 1 TXA (2 g given as a continuous infusion every 8 hours for the first 6 days of antileukaemic treatment) Arm 2 Placebo (equal volume of 5% glucose) RBC transfusion thresholds: packed red cells given to maintain Hb > 90 g/L Platelet transfusion threshold: 6 to 8 U/m2 (source not stated) routinely given during first 7 days and additionally for overt haemorrhage Packed red cells and additional platelet concentrates given at the discretion of the attending physician |
|
| Outcomes | Main or primary outcome not stated Outcomes reported:
Number of days participants from both arms on study: 14 |
|
| Notes |
Patients randomised at: not reported Follow‐up of participants: for 14 days from start of antileukaemic treatment Stopping guidelines: not reported Funding: "This work was supported by grants from the Amstol Foundation (A 584), Amsterdam, the Netherlands and the Italian National Research Council (86.00466.44); and by a Royal Dutch Academy of Sciences Fellowship (H. R. B.)." Declarations of interest: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised to either TXA or placebo. The article does not state how participants were randomised. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The article states that "Patients and attending physicians were blinded to the treatment groups" and that bleeding assessments were examined by the same investigator but it is not clear whether the investigator was one of the attending physicians. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Each patient was examined daily by the same investigator (G.A.) for clinically manifest haemorrhage during the entire study of 14 days". Unclear whether G.A. was blinded to the treatment groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | The article states that their efficacy endpoints were "severity of bleeding" and the packed red cell and platelet concentrate transfusion requirement but also reports outcome data for laboratory assessment of coagulation and fibrinolysis. It also states that there were no episodes of thromboembolism. We would be concerned as to what other outcomes were measured and not reported. |
| Other bias | Low risk | The study seemed to be free of other sources of bias |
| Protocol deviation balanced? | Unclear risk | Protocol deviations or violations were not commented on |
Fricke 1991.
| Methods | Cross‐over RCT. USA. Enrolment period and centres not stated. | |
| Participants |
Inclusion criteria: people with "amegakaryocytic thrombocytopenia" who met the following criteria: platelet count < 20 x 109/L with no immediate prospect of recovery and absent/rare megakaryocytes in the bone marrow aspirate/biopsy; at least 1 bleeding episode per month (excluding skin bleeding); a history of platelet transfusions for such bleeding episodes Exclusion criteria: people who had any of the following: active bleeding from an anatomical lesion (e.g. peptic ulcer); personal/family history of hypercoagulopathy; pregnancy; DIC; liver failure; personal history of a congenital bleeding disorder Arms (cross‐over RCT): N = 8 (aplastic anaemia N = 7; myelodysplastic syndrome N = 1) |
|
| Interventions | Comparison between TXA and placebo Arm 1 TXA (20 mg/kg) 3 x daily for 4 weeks or until a platelet transfusion was required to control bleeding. Followed by a 1‐week rest period. Placebo (equivalent number of identical placebo tablets) for 4 weeks or until a platelet transfusion was required to control bleeding. Followed by a 1‐week rest period. The method of allocating the randomised patients to further courses of TXA or placebo was not stated. Arm 2 Placebo (equivalent number of identical placebo tablets) for 4 weeks or until a platelet transfusion was required to control bleeding. Followed by a 1‐week rest period. TXA (20 mg/kg) 3 x daily for 4 weeks or until a platelet transfusion was required to control bleeding. Followed by a 1‐week rest period. The method of allocating the randomised patients to further courses of TXA or placebo was not stated. Further cycles of TXA and placebo were repeated until TXA was deemed a success or a failure. RBC transfusion thresholds: not stated Platelet transfusion thresholds: platelets (dose and source not stated) given in the event of bleeding as each participant's personal physician deemed necessary |
|
| Outcomes | Main or primary outcome not stated Outcomes reported
Number of days patients on study: not stated Defined overall success of TXA in a participant as either 5 failures of placebo and none of drug or 7 failures of placebo and 1 of drug. Defined overall failure of TXA as 2 failed courses of drug. Sequential courses continued until overall success or failure of TXA could be determined. |
|
| Notes |
Participants randomised at: not reported Follow‐up of participants: not reported Stopping guidelines: not reported Funding: "Supported in part by KabiVitrum, Inc" Declarations of interest: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States that participants were randomised but does not state the mechanism of randomisation. Nor does it state whether they were re‐randomised after the initial 2 courses of TXA and placebo or what other method was used to allocate them to successive courses of TXA or placebo. |
| Allocation concealment (selection bias) | Unclear risk | States that participants were randomised but does not state the mechanism of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States that study was double‐blinded (and the interventions were identical) However, the article states that drug levels were obtained during 38 of the 49 courses. It does not state that the investigators or participants were blinded to this information during the study. "Plasma tranexamic acid levels were taken weekly and before each platelet transfusion, if possible". It also states that the study defined overall success of TXA in a participant as either 5 failures of placebo and none of drug or 7 failures of placebo and 1 of drug. Defined overall failure of TXA as 2 failed courses of drug. Sequential courses continued until overall success or failure of TXA could be determined. However, it did not state how this assessment of success or failure was performed without unblinding study personnel. It is unlikely that participants were informed of these results and therefore blinding of participants is assumed to be at low risk of bias. However, it is not clear that study personnel were not informed of the results and therefore overall risk of bias was classified as unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | States that study was double‐blinded (and the interventions were identical) However, the article states that drug levels were obtained during 38 of the 49 courses. It does not state that the outcome assessors were blinded to this information during the study. "Plasma tranexamic acid levels were taken weekly and before each platelet transfusion, if possible". It also states that the study defined overall success of TXA in a patient as either 5 failures of placebo and none of drug or 7 failures of placebo and 1 of drug. Defined overall failure of TXA as 2 failed courses of drug. Sequential courses continued until overall success or failure of TXA could be determined. However, it did not state how this assessment of success or failure was performed without unblinding study personnel (including outcome assessors). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | States that "Three patients completed the randomised portion of the study". "Five of the eight patients did not complete enough courses to determine the efficacy of the drug". |
| Selective reporting (reporting bias) | High risk | The article states that severity of bleeding (as well as number and site) were recorded by the assessor (the patient) but this outcome was not reported in the article. 1 patient died of intracranial haemorrhage 4 days after starting the first randomised course. Data from this course were not included in the analysis. There were 2 courses of TXA or placebo interrupted in 2 participants. One due to an upper respiratory tract infection and the other in which the participant developed an oesophageal haematoma after starting antibiotic treatment for an infection. Data from these courses were not included in the analysis as the investigators felt that the infection/antibiotic treatment may have compromised haemostasis. |
| Other bias | High risk | One participant began receiving HLA‐matched platelet transfusions 2 months after enrolment and was kept in the study as these transfusions did not completely control the bleeding. This creates bias as the other participants were deemed to have "failed" the course if bleeding necessitating platelet transfusions occurred. 2 other participants were withdrawn after they started to receive HLA‐matched platelet transfusions. The overall success of TXA was defined as either 5 failures of placebo and none of the drug or 7 failures of placebo and 1 of the drug. Overall failure of TXA was defined as 2 failed courses of the drug. Failure of a course was defined as a participant receiving a platelet transfusion for bleeding during a 4‐week study period. Participants received a variable number of courses of drug/placebo. The 3 patients who completed the study received between 3 (2 TXA, 1 placebo) and 9 courses (5 TXA, 4 placebo) of treatment. The 5 patients who did not complete the study received between 0 and 20 courses (10 TXA, 10 placebo) of treatment. Of the 3 participants who completed the study, 2 did not have any successful courses of treatment. The third participants had 3/5 successful courses with TXA and 1/4 successful courses with placebo, however this was classified by the study as a failure of TXA (2 failed courses with TXA). Failure of a course of treatment would be classified in the same way whether patient was on study drug for 1 day before bleeding or 27 days before bleeding that required treatment with a platelet transfusion. More bleeding episodes seen in TXA arm may have been due to more days on study before bleeding requiring a platelet transfusion. Number of days on study drug before bleeding was not reported for individual courses. |
| Protocol deviation balanced? | Unclear risk | Insufficient information to determine |
Gallardo 1983.
| Methods | Parallel RCT. Abstract. Single centre: USA. Enrolment period not stated | |
| Participants |
Inclusion criteria: adults undergoing remission induction for acute leukaemia. No other inclusion criteria stated. Exclusion criteria: not stated N = 19 were eligible. N = 9 in each arm (AML N = 15; ALL N = 4). N = 1 was not evaluable (reason not stated). Distribution of subtypes in to each arm not stated. Arm 1 N = 9 Arm 2 N = 9 |
|
| Interventions | Comparison between EACA therapy and no EACA therapy. Arm 1 N = 9 (to receive EACA 100 mg/kg loading dose and 12 to 24 g/day in divided doses) Arm 2 N = 9 (did not receive EACA) RBC transfusion threshold: not stated Platelet transfusion threshold: participants in both arms were administered platelet transfusion (dose and source not stated) when platelet count < 20 x 109/L. This threshold defined the "days at risk of bleeding". |
|
| Outcomes | Main or primary outcomes not stated. Outcomes reported:
Number of days participants on study: not reported |
|
| Notes |
Participants randomised at: not reported Follow‐up of participants: not reported Stopping guidelines: not reported Funding: not reported Declarations of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit a judgement of 'high' or 'low' risk. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit a judgement of 'high' or 'low' risk |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit a judgement of 'high' or 'low' risk. The abstract does not state whether investigators and participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit a judgement of 'high' or 'low' risk. The abstract does not state who carried out the bleeding assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit a judgement of 'high' or 'low' risk |
| Selective reporting (reporting bias) | High risk | There are data for thromboembolism and death ("no patient died of thrombosis"), but no data given on number episodes of thromboembolism or number of deaths. No data reported monitoring of antifibrinolytic therapy using I125 fibrinogen plasma clot lysis |
| Other bias | Unclear risk | The “at risk of bleeding days” were much higher in the EACA group – 158 vs. 80 due to more severe thrombocytopenia and more cycles of chemotherapy for refractory disease. There may be bias in the randomisation procedure but method of randomisation is not stated. |
| Protocol deviation balanced? | Unclear risk | Protocol deviations or violations were not commented on |
Shpilberg 1995.
| Methods | Parallel RCT. Enrolment period 1990 to 1992. 2 centres (Israel) | |
| Participants |
Inclusion criteria: de novo AML. All ages. Exclusion criteria: laboratory signs of DIC; recent history of a thromboembolic event; clinical evidence or suspicion of thromboembolism. There were 2 parts to the study. The first part investigated patients undergoing induction chemotherapy. The second part investigated people undergoing consolidation chemotherapy Induction chemotherapy: N = 38 (FAB M1 N = 5; FAB M2 N = 8; FAB M4 N = 25) Arm 1 N = 16 (FAB M1 N = 2; FAB M2 N =2; FAB M4 N = 12) Arm 2 N = 22 (FAB M1 N = 3; FAB M2 N = 6; FAB M4 N = 13) Consolidation chemotherapy: N = 18 (FAB M2 = 8; FAB M3 = 1; FAB M4 = 9) Arm 1 N = 10 (FAB M2 N = 5; FAB M3 N =1; FAB M4 N = 4) Arm 2 N = 8 (FAB M2 N = 3; FAB M4 N = 5) Age range of participants 18 to 71 years |
|
| Interventions | Comparison between TXA and placebo in participants receiving (i) induction chemotherapy and (ii) consolidation chemotherapy. Both parts of the investigation had the same intervention. Arm 1 TXA 1 g every 6 hours Arm 2 Identically appearing placebo RBC transfusion threshold: administered to maintain > 90 g/L Platelet transfusion threshold: participants in both arms were administered platelet transfusions. (Random donor pooled; 4 units/m2) in the event of clinically significant bleeding, irrespective of platelet count. |
|
| Outcomes | Main or primary outcomes not stated Outcomes reported:
Number of days participants on study: not stated |
|
| Notes |
Participants randomised at: not reported Follow‐up of participants: not reported Stopping guidelines: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study states that the trial was randomised but no further details are given as to how this was done |
| Allocation concealment (selection bias) | Unclear risk | Study states that the trial was randomised but no further details are given as to how this was done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study states that the trial was double‐blind but no further details are given as to who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study states that the participants were carefully examined daily by one of the investigators. The trial was double‐blind but no further details are given as to who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient data to assess |
| Selective reporting (reporting bias) | Unclear risk | Insufficient data to assess |
| Other bias | Low risk | The study seemed to be free of other forms of bias |
| Protocol deviation balanced? | Unclear risk | Insufficient information to determine |
ALL: acute lymphoblastic leukaemia AML: acute myeloid leukaemia APML: acute promyelocytic leukaemia CNS: central nervous system DIC: disseminated intravascular coagulation EACA: epsilon aminocaproic acid FAB: French American British Classification GI: gastrointestinal GU: genitourinary Hb: haemoglobin HLA: human leukocyte antigen RBC: red blood cells RCT: randomised controlled trial TXA: tranexamic acid
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amar 2003 | Wrong participant group ‐ a randomised controlled trial examining the usefulness of antifibrinolytic therapy in patients with non‐haematological malignancy undergoing major orthopaedic surgery |
| Antun 2013 | Not an RCT ‐ an observational study of thrombocytopenic patients who received EACA for the prevention of bleeding |
| Bartholomew 1989 | Not an RCT ‐ a non‐randomised controlled trial on the control of bleeding in people with immune and non‐immune thrombocytopenia with aminocaproic acid |
| Bates 2011 | Review |
| Bedirhan 2001 | Wrong intervention ‐ a randomised controlled trial investigating the use of aprotinin in postoperative bleeding and the need for blood products in thoracic surgery |
| Ben‐Bassat 1990 | Not an RCT ‐ non‐randomised and non‐controlled study of TXA therapy in AML |
| Breen 2012 | Review |
| Brown 2002 | Review |
| Byams 2007 | Wrong participant group ‐ a cross‐over study evaluating the use of desmopressin and TXA in women with menorrhagia |
| Cattan 1963 | Not an RCT ‐ non‐randomised study of EACA in people with thrombocytopenia |
| Celebi 2006 | Wrong participant group ‐ a randomised, double‐blind prospective study that examined the role of antifibrinolytic agents in gynaecologic cancer surgery |
| Chakrabarti 1998 | Not an RCT ‐ non‐randomised and non‐controlled trial of EACA in people with acute leukaemia |
| Dean 1997 | Not an RCT ‐ non‐randomised and non‐controlled trial of EACA and TXA for cancer‐associated bleeding problems |
| Fossa 1978 | Not an RCT ‐ non‐randomised, controlled pilot study on the effect of TXA in patients being treated for various advanced malignancies |
| Gardner 1980 | Not an RCT ‐ a series of cases of patients with amegakaryocytic thrombocytopenia treated with EACA to control bleeding |
| Garewal 1985 | Not an RCT ‐ non‐randomised and non‐controlled trial of EACA for the control of bleeding in thrombocytopenic people |
| Jeserschek 2003 | Wrong intervention ‐ a randomised controlled trial examining the role of high‐dose aprotinin in the reduction of bleeding in major orthopaedic surgery |
| Kalmadi 2006 | Not an RCT ‐ retrospective study of the effect of EACA on transfusion requirements in patients with thrombocytopenic haemorrhage |
| Katzel 1998 | Wrong intervention ‐ a prospective, controlled, double‐blind pilot study examining the role of aprotinin during thoracic surgery for malignant lung disease |
| Levy 2005 | Review |
| Marti‐Carvajal 2011 | Review |
| McConnell 2011 | Wrong participant group ‐ a randomised controlled trial examining the reduction of blood loss in primary hip arthoplasty with TXA fibrin spray |
| McConnell 2012 | Wrong participant group ‐ a randomised controlled trial examining the reduction of blood loss in primary knee arthoplasty with TXA fibrin spray |
| Mevio 1983 | Wrong participant group ‐ a double‐blind study examining the role of tranexamic acid in the prevention of radiomucositis in head and neck cancer patients submitted for radiotherapy |
| Movafegh 2011 | Wrong participant group ‐ a randomised, double‐blind study examining the effect of intravenous tranexamic acid on blood loss during and after caesarian delivery |
| NCT01980355 | Wrong participant group ‐ patients undergoing major oncological surgery for standard of care purposes (to include: liver resections, radical cholecystectomy, pancreaticoduodenectomy [Whipple procedure], oesophagectomy, gastrectomy, colectomy, and debulking with hyperthermic intraperitoneal chemotherapy, prostatectomies, nephrectomies, and partial nephrectomies) |
| Rathi 2015 | Not an RCT ‐ non‐randomised study of use of EACA in people with diffuse alveolar haemorrhage |
| Rickles 2007 | Review |
| Sanz 2010 | Not an RCT ‐ non‐randomised with historical control evaluating the use of TXA in people with promyelocytic leukaemia |
| Schwartz 1986 | Not an RCT ‐ a non‐randomised, non‐controlled trial of the effect of EACA in people with APML and acquired alpha‐2‐plasmin inhibitor deficiency |
| Wassenaar 2008 | Not an RCT ‐ a retrospective study of the use of EACA in people with APML and acquired alpha‐2‐plasmin inhibitor deficiency |
| Yang 2001 | Wrong participant group ‐ a randomised, comparative trial examining the efficacy of TXA in reducing post‐partum blood loss in primigravid women |
AML: acute myeloid leukaemia APML: acute promyelocytic leukaemia EACA: epsilon aminocaproic acid RCT: randomised controlled trial TXA: tranexamic acid
Characteristics of ongoing studies [ordered by study ID]
A‐TREAT 2015.
| Trial name or title | American Trial Using Tranexamic Acid in Thrombocytopenia (ATREAT) (NCT02578901) |
| Methods | Multi‐centre (USA), randomised parallel, double‐blind placebo‐controlled study |
| Participants |
Inclusion Criteria:
Exclusion Criteria:
|
| Interventions |
Intervention: Tranexamic acid. Doses will be given IV or PO per the discretion of the treating investigator. Doses are administered every 8 hours. When given IV, TXA 1.0 gram will be administered. Comparator: Placebo. Doses will be given IV or PO per the discretion of the treating investigator. Doses are administered every 8 hours. When given IV, Normal Saline will be administered. When given PO, placebo pills will be administered |
| Outcomes |
Primary outcome:
Proportion of patients with bleeding of WHO grade 2 or above, over the study period of 30 days after activation of study drug Secondary outcomes:
|
| Starting date | March 2016 |
| Contact information | Heather Herren 8003320586 hherren@uw.edu University of North Carolina Chapel Hill, North Carolina, United States, 21599 Contact: Nigel Key, MD nigel_key@med.unc.edu University of Pittsburgh Pittsburgh, Pennsylvania, United States, 15213 Contact: Darrell Triulzi, MD Dtriulzi@itxm.org University of Washington Seattle, Washington, United States, 98195 Contact: Terry Gernsheimer, MD bldbuddy@u.washington.edu |
| Notes | University of North Carolina |
PROBLEMA 2014.
| Trial name or title | Randomized Trial of Epsilon Aminocaproic Acid Versus Platelet Transfusions for the Prevention of Bleeding in Thrombocytopenic Patients With Hematological Malignancies (PROBLEMA) (NCT02074436) |
| Methods | Single‐centre (USA), randomised parallel, open‐label study Duration of patient participation: 6 months |
| Participants |
Inclusion Criteria:
Exclusion Criteria: APML Person receiving anticoagulation Person receiving antiplatelet or antifibrinolytic agents Person receiving procoagulant agent including 1‐deamino‐8‐D‐arginine vasopressin (DDAVP), recombinant factor VIIa or prothrombin complex concentrate within 24 hours of enrolment Person with known congenital bleeding disorders or platelet dysfunction DIC Fibrinogen level < 150 mg/dL Person with known lupus anticoagulant or positive antiphospholipid antibody History of arterial or venous thromboembolic disease 6 months prior to screening Person requiring platelet transfusion threshold of > 20 x 10⁹/L Active WHO grade ≥ 2 bleeding at the time of randomisation, including haematuria History of WHO grade 4 bleeding Haematopoietic stem cell transplant recipient within 100 days posttransplant Pregnancy Known allergy to EACA History of veno‐occlusive disease of the liver Myocardial infarction 6 months prior to screening |
| Interventions |
Intervention: Prophylactic EACA 1000 mg PO twice daily if platelet count < 20 x 10⁹/L. Additional platelet transfusion will be administered in case of WHO grade 3 or 4 bleeding. Comparator: Platelet transfusion if platelet count is < 20 x 10⁹/L. Additional platelet transfusion will be administered in case of grade 3 or 4 bleeding. |
| Outcomes |
Primary outcome:
Proportion of patients who develop major bleeding episodes (WHO grades 3 or 4) [ Time Frame: 6 months ] Secondary outcomes:
|
| Starting date | May 2014 |
| Contact information | Ana G. Antun, MD 4045936732
ana.antun@emoryhealthcare.org Ann Shen, CRN 4047785982 ann.shen@emory.edu Emory University Hospital Atlanta, Georgia, United States, 30322 |
| Notes |
Planned enrolment: 100 Planned study completion: May 2017 |
TREATT 2015.
| Trial name or title | A double blind, randomised controlled TRial EvaluAting the safety and efficacy of Tranexamic acid in patients with haematological malignancies with severe Thrombocytopenia (TREATT) (ISRCTN73545489) |
| Methods | Multinational (Australia and United Kingdom), randomised, parallel double‐blind placebo‐controlled trial |
| Participants |
Inclusion criteria:
Exclusion criteria
Concentrates (PCC) within 48 hours of enrolment, or with known hypercoagulable state
|
| Interventions |
Intervention: TXA 1 g every eight hours IV or 1.5 g every eight hours PO once platelet count drops to ≤ 30 x 109/L Comparator: Placebo to match once platelet count drops to ≤ 30 x 109/L. Study treatment will be stopped if platelet count rises spontaneously above 50 x 109/L, the platelet count is ≥ 30 x 109/L for at least 3 days or participant has received 30 days of study treatment. |
| Outcomes |
Primary outcome: Proportion of patients who died or had bleeding of WHO grade 2 or above during the first 30 days of the trial. Secondary outcomes: Secondary efficacy outcomes all measured during first 30 days of the trial.
Secondary Safety Outcomes
Other outcomes all measured during first 30 days of the trial.
|
| Starting date | June 2015 |
| Contact information | Dr Simon Stanworth simon.stanworth@nhsbt.nhs.uk Dr Lise Estcourt lise.estcourt@nhsbt.nhs.uk Dr Erica Wood erica.wood@monash.edu Gillian Powter gillian.powter@nhsbt.nhs.uk |
| Notes |
Planned enrolment: 816 Planned study completion: December 2020 |
APML: acute promyelocytic leukaemia DIC: disseminated intravascular coagulation EACA: epsilon aminocaproic acid IV: intravenous PO: by mouth QoL: quality of life TXA: tranexamic acid WHO: World Health Organization
Differences between protocol and review
Postprotocol changes to the review
We have changed the outcome "number of platelet transfusions" to the "number of platelet transfusions or platelet components". The number of platelet components had been reported in the previous version of the review (Wardrop 2013) under the original heading.
We have changed the outcome "number of red cell transfusions" to the "number of red cell transfusions or red cell components". The number of red cell components had been reported in the previous version of the review (Wardrop 2013) under the original heading.
We did not pre‐specify in the protocol (Wardrop 2012) how we would deal with multi‐arm studies. None of the studies were multi‐arm studies
We did not pre‐specify in the protocol (Wardrop 2012) how we would deal with any unit of analysis issues. No unit of analysis issues arose.
We did not pre‐specify in the protocol which outcomes we would report in the 'Summary of findings' table. The outcomes we reported were: number of participants with any bleeding, number of participants with thromboembolism, mortality (all causes), number of platelet transfusions per participant, adverse events of transfusions, adverse events of antifibrinolytic agents, and quality of life.
Aspects of the protocol that were not implemented due to lack of data
We performed a narrative synthesis of the findings from the included studies, structured around the type of antifibrinolytic. No overall statistical analyses were performed because the studies reported outcomes in different ways and these results could not be integrated.
We did not perform a formal assessment of heterogeneity because it was not possible to perform meta‐analyses due to the nature of the data reported by the included studies. (Deeks 2011).
We did not perform a formal assessment of potential publication bias (small‐trial bias) because no meta‐analysis was performed (Sterne 2011).
Secondary outcomes: No study reported:
mortality (all causes);
adverse events of transfusions (e.g. transfusion reactions, antibody development);
disseminated intravascular coagulation (DIC);
quality of life (QoL).
Subgroup analyses: We pre‐specified three subgroup analyses:
age (children/adults);
underlying haematological diagnoses;
type of treatment (e.g. chemotherapy, autologous and allogeneic transplantation, immunosuppression).
However, we did not perform any subgroup analyses due to a lack of outcome data. The included studies only included adults who had acute myeloid leukaemia and were receiving chemotherapy.
Sensitivity analyses: We did not perform a formal sensitivity analysis because we performed no meta‐analyses.
Contributions of authors
Lise Estcourt: protocol development and content expert.
Michael Desborough: content expert.
Susan Brunskill: protocol development and methodological expert.
Sally Hopewell: methodological expert.
Carolyn Doree: protocol development and search strategy expert.
Simon Stanworth: protocol development and content expert.
Mike Murphy: protocol development and content expert.
Sources of support
Internal sources
NHS Blood and Transplant, UK.
External sources
-
Cochrane Haematological Malignancies Group, Germany.
Editorial support
-
National Institute of Health Research (NIHR) Cochrane Programme Grant, UK.
To provide funding for systematic reviewers and methodological support from the Centre for Statistics in Medicine, Oxford
Declarations of interest
Lise Estcourt: chief investigator on one of the ongoing studies, partly funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
Michael Desborough: none declared.
Susan Brunskill: none declared.
Sally Hopewell: partly funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
Carolyn Doree: none declared.
Simon Stanworth: chief investigator on one of the ongoing studies.
Mike Murphy: investigator on one of the ongoing studies.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Avvisati 1989 {published data only}
- Avvisati G, Cate JW, Buller HR, Mandelli F. Tranexamic acid for control of haemorrhage in acute promyelocytic leukaemia. Lancet 1989;334(8655):122‐4. [DOI] [PubMed] [Google Scholar]
Fricke 1991 {published data only}
- Fricke W, Alling D, Kimble J, Griffith P, Klein H. Lack of efficacy of tranexamic acid in thrombocytopenic bleeding. Transfusion 1991;31(4):345‐8. [DOI] [PubMed] [Google Scholar]
Gallardo 1983 {published data only}
- Gallardo RL, Gardner FH. Antifibrinolytic therapy for bleeding control during remission induction for acute leukemia. Blood 1983;62:202a. [Google Scholar]
Shpilberg 1995 {published data only}
- Shpilberg O, Blumenthal R, Sofer O, Eldor A, Ben‐Bassat I. A controlled trial of tranexamic acid (TA) treatment for reduction of bleeding during acute myeloid leukemia (AML) induction and consolidation. Blood 1993;82:547a. [DOI] [PubMed] [Google Scholar]
- Shpilberg O, Blumenthal R, Sofer O, Katz Y, Chetrit A, Ramot B, et al. A controlled trial of tranexamic acid therapy for the reduction of bleeding during treatment of acute myeloid leukemia. Leukemia and Lymphoma 1995;19:141‐4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Amar 2003 {published data only}
- Amar D, Grant FM, Zhang H, Boland PJ, Leung DH, Healey JA. Antifibrinolytic therapy and perioperative blood loss in cancer patients undergoing major orthopedic surgery. Anesthesiology 2003;98:337‐42. [DOI] [PubMed] [Google Scholar]
Antun 2013 {published data only}
- Antun AG, Gleason S, Arellano M, Langston AA, McLemore ML, Gaddh M, et al. Epsilon aminocaproic acid prevents bleeding in severely thrombocytopenic patients with hematological malignancies. Cancer 2013;119(21):3784‐7. [DOI] [PubMed] [Google Scholar]
Bartholomew 1989 {published data only}
- Bartholomew J, Salgia R, Bell W. Control of bleeding in patients with immune and non‐immune thrombocytopenia with aminocaproic acid. Archives of Internal Medicine 1989;149:1959‐61. [PubMed] [Google Scholar]
Bates 2011 {published data only}
- Bates JS, Buie LW, Woodis CB. Management of menorrhagia associated with chemotherapy‐induced thrombocytopenia in women with hematologic malignancy. Pharmacotherapy 2011;11:1092‐110. [DOI] [PubMed] [Google Scholar]
Bedirhan 2001 {published data only}
- Bedirhan MA, Turna A, Yagan N, Tasci O. Aprotinin reduces postoperative bleeding and the need for blood products in thoracic surgery: results of a randomized double‐blind study. European Journal of Cardio‐thoracic Surgery 2001;20:1122‐7. [DOI] [PubMed] [Google Scholar]
Ben‐Bassat 1990 {published data only}
- Ben‐Bassat I, Douer D, Ramot B. Tranexamic acid therapy in acute myeloid leukemia: possible reduction of platelet transfusions. European Journal of Haematology 1990;45:86‐9. [DOI] [PubMed] [Google Scholar]
Breen 2012 {published data only}
- Breen KA, Grimwade D, Hunt BJ. The pathogenesis and management of the coagulopathy of acute promyelocytic leukaemia. British Journal of Haematology 2011;156:24‐36. [DOI] [PubMed] [Google Scholar]
Brown 2002 {published data only}
- Brown SA, Aledort LM, Lee CA. Haemostasis: from bench to bedside. Haemophilia 2002;8:685‐93. [DOI] [PubMed] [Google Scholar]
Byams 2007 {published data only}
- Byams VR, Dowling NF, Heit JA, Kouides PA, Philipp CS, Stein SF, et al. Quality of life (QOL) evaluation in women with menorrhagia and abnormal hemostasis testing participating in a cross‐over study of intranasal desmopressin and oral tranexamic acid. Journal of Thrombosis and Hemostasis 2007;5(2):P‐S‐189. [Google Scholar]
Cattan 1963 {published data only}
- Cattan A, Schwarzenberg L, Schneider M, Amiel J‐L, Mathé G. Trial of epsilon‐amino‐caproic acid in patients with a bleeding disorder, in particular thrombocytopenic disorders [Essai de traitement par l'acide epsilon‐amino‐caproique de patients atteints de syndrome hémorragiques, en particulier de syndromes liés a une thrombocytopénie]. La Presse Medicale 1963;71(43):2037‐8. [PubMed] [Google Scholar]
Celebi 2006 {published data only}
- Celebi N, Celebioglu B, Selcuk M, Canbay O, Karagoz AH, Aypar U. The role of antifibrinolytic agents in gynecologic cancer surgery. Saudi Medical Journal 2006;27(5):637‐41. [PubMed] [Google Scholar]
Chakrabarti 1998 {published data only}
- Chakrabarti S. Low dose bolus aminocaproic acid: an alternative to platelet transfusion in thrombocytopenia. European Journal of Haematology 1998;60:313‐4. [DOI] [PubMed] [Google Scholar]
Dean 1997 {published data only}
- Dean A, Tuffin P. Fibrinolytic inhibitors for cancer‐associated bleeding problems. Journal of Pain and Symptom Management 1997;3(1):20‐4. [DOI] [PubMed] [Google Scholar]
Fossa 1978 {published data only}
- Fossa SD, Solheim OO, Fossa JJ. Cyklokapron (tranexamic acid) in the treatment of patients with advanced neoplasms. Tidsskr Nor Laegeforen 1978;98(28):1368‐70. [PubMed] [Google Scholar]
Gardner 1980 {published data only}
- Gardner FH, Helmer RE. Aminocaproic acid: use in control of hemorrhage in patients with amegakaryocytic thrombocytopenia. JAMA 1980;243(1):35‐7. [DOI] [PubMed] [Google Scholar]
Garewal 1985 {published data only}
- Garewal HS, Durie BGM. Anti‐fibrinolytic therapy with aminocaproic acid for the control of bleeding in thrombocytopenic patients. Scandinavian Journal of Haematology 1985;35:497‐500. [DOI] [PubMed] [Google Scholar]
Jeserschek 2003 {published data only}
- Jeserschek R, Clar H, Aigner C, Rehak P, Primus B, Windhager R. Reduction of blood loss using high‐dose aprotinin in major orthopaedic surgery. Journal of Bone and Joint Surgery 2003;85(2):174‐7. [DOI] [PubMed] [Google Scholar]
Kalmadi 2006 {published data only}
- Kalmadi S, Tiu R, Lowe C, Jin T, Kalaycio M. Epsilon aminocaproic acid reduces transfusion requirements in patients with thrombocytopenic hemorrhage. Cancer 2006;107(1):136‐40. [DOI] [PubMed] [Google Scholar]
Katzel 1998 {published data only}
- Katzel R, Keuper H, Wiedemann B, Brethner L, Viogt B. The effects of intraoperative aprotinin on perioperative haemostasis and fibrinolysis and their effects on blood loss and transfusion requirements in lung surgery [Effekte der intraoperativen Aprotininapplikation auf perioperative Haemostase und Fibrinolyseveraenderungen und ihre Auswirkungen auf Blutverlust und Transfusionsbedarf bei lungenchirurgischen Eingriffen]. Infusionstherapie und Transfusionsmedizin 1998;25:236‐45. [Google Scholar]
Levy 2005 {published data only}
- Levy JH. Novel concepts in treatment and prevention of bleeding. IARS Review Course Lectures. International Anesthesia Research Society, 2005:43‐7. [Google Scholar]
Marti‐Carvajal 2011 {published data only}
- Marti‐Carvajal AJ, Simancas D, Cardona AF. Treatment for disseminated intravascular coagulation in patients with acute and chronic leukemia. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD008562.pub2] [DOI] [PubMed] [Google Scholar]
McConnell 2011 {published data only}
- McConnell JS, Shewale S, Munro NA, Shah K, Deakin AH, Kinninmonth AWG. Reduction of blood loss in primary hip arthroplasty with tranexamic acid or fibrin spray. Acta Orthopaedica 2011;82(6):660‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
McConnell 2012 {published data only}
- McConnell JS, Shewale S, Munro NA, Shah K, Deakin AH, Kinninmonth AWG. Reducing blood loss in primary knee arthroplasty: a prospective randomised controlled trial of tranexamic acid and fibrin spray. Knee 2012;19(4):295‐8. [DOI] [PubMed] [Google Scholar]
Mevio 1983 {published data only}
- Mevio E, Santarelli P. Tranexamic acid in prevention of oropharyngeal radiomucositis [L'acido tranexamico nella prevenzione delle radiomucositi a livello oro‐faringeo]. La Radiologia Medica 1983;69:325‐8. [PubMed] [Google Scholar]
Movafegh 2011 {published data only}
- Movafegh A, Eslamian L, Dorabadi A. Effects of intravenous tranexamic acid administration on blood loss during and after cesarian delivery. International Journal of Gynecology and Obstetrics 2011;115:224‐6. [DOI] [PubMed] [Google Scholar]
NCT01980355 {published data only}
- Chung M. TXA study in major oncologic surgery. ClinicalTrials.gov November 4 2013, update January 21 2015; Vol. [Accessed August 12th 2015].
Rathi 2015 {published data only}
- Rathi NK, Tanner AR, Dinh A, Dong W, Feng L, Ensor J, et al. Low‐, medium‐ and high‐dose steroids with or without aminocaproic acid in adult hematopoietic SCT patients with diffuse alveolar hemorrhage. Bone Marrow Transplantation 2015;50:420‐6. [DOI] [PubMed] [Google Scholar]
Rickles 2007 {published data only}
- Rickles FR, Falanga A, Montesinos P, Sanz MA, Brenner B, Barbui T. Bleeding and thrombosis in acute leukemia: what does the future of therapy look like?. Thrombosis Research 2007;120(2):99‐106. [DOI] [PubMed] [Google Scholar]
Sanz 2010 {published data only}
- Sanz MA, Montesinos P. Open issues on bleeding and thrombosis in acute promyelocytic leukemia. Thrombosis Research 2010;125(2):51‐4. [DOI] [PubMed] [Google Scholar]
Schwartz 1986 {published data only}
- Schwartz BS, Williams EC, Conlan MG, Mosher DF. Epsilon‐aminocaproic acid in the treatment of patients with acute promyelocytic leukaemia and acquired alpha‐2‐plasmin inhibitor deficiency. Annals of Internal Medicine 1986;105:873‐7. [DOI] [PubMed] [Google Scholar]
Wassenaar 2008 {published data only}
- Wassenaar T, Black J, Kahl B, Schwartz B, Longo W, Mosher D, et al. Acute promyelocytic leukemia and acquired alpha‐2‐plasmin inhibitor deficiency: a retrospective look at the use of epsilon‐aminocaproic acid (Amicar) in 30 patients. Hematological Oncology 2008;26:241‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2001 {published data only}
- Yang H, Zheng S, Shi C. Clinical study on the efficacy of tranexamic acid in reducing postpartum blood loss: a randomized, comparative, multicenter trial. Chinese Journal of Obstetrics and Gynecology 2001;36(10):590‐2. [PubMed] [Google Scholar]
References to ongoing studies
A‐TREAT 2015 {published data only}
- NCT02578901. American Trial Using Tranexamic Acid in Thrombocytopenia (A‐TREAT). ClinicalTrials.gov 15 October 2015 [Accessed 09 March 2016].
PROBLEMA 2014 {published data only}
- NCT02074436. PRevention Of BLeeding in hEmatological Malignancies With Antifibrinolytic (Epsilon Aminocaproic Acid) (PROBLEMA). ClinicalTrials.gov 26 February 2014, last updated 31 May 31 2015; Vol. [Accessed 12/08/2015].
TREATT 2015 {published data only}
- ISRCTN 73545489. A double blind, randomised controlled TRial EvaluAting the safety and efficacy of Tranexamic acid in patients with haematological malignancies with severe Thrombocytopenia (TREATT). ISRCTN registry 25th March 2015 [Accessed 12 August 2015].
Additional references
BCSH 2003
- British Committee for Standards in Haematology. Guidelines for the use of platelet transfusions. British Journal of Haematology 2003;122(1):10‐23. [DOI] [PubMed] [Google Scholar]
Bolton‐Maggs 2012
- Bolton‐Maggs PHB (editor) and Cohen H on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group. The 2011 Annual SHOT Report. Serious Hazards of Transfusion (SHOT), 2012. [Google Scholar]
Cameron 2007
- Cameron B, Rock G, Olberg B, Neurath D. Evaluation of platelet transfusion triggers in a tertiary‐care hospital. Transfusion 2007;47(2):206‐11. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Faught 1998
- Faught C, Wells P, Fergusson D, Laupacis A. Adverse effects of methods for minimizing perioperative allogeneic transfusion: a critical review of the literature. Transfusion Medicine Reviews 1998;12(3):206‐25. [DOI] [PubMed] [Google Scholar]
Fergusson 2008
- Fergusson DA, Hebert PC, Mazer CD, Fremes S, MacAdams C, Murkin JM, et al. A comparison of aprotinin and lysine analogues in high‐risk cardiac surgery. New England Journal of Medicine 2008;358(22):2319‐31. [DOI] [PubMed] [Google Scholar]
Franchini 2010
- Franchini M, Dario Di Minno MN, Coppola A. Disseminated intravascular coagulation in hematologic malignancies. Seminars in Thrombosis and Haemostasis 2010;36(4):388‐403. [DOI] [PubMed] [Google Scholar]
Friedmann 2002
- Friedmann AM, Sengul H, Lehmann H, Schwartz C, Goodman S. Do basic laboratory tests or clinical observations predict bleeding in thrombocytopenic oncology patients? A reevaluation of prophylactic platelet transfusions. Transfusion Medicine Reviews 2002;16(1):34‐45. [DOI] [PubMed] [Google Scholar]
Greeno 2007
- Greeno E, McCullough J, Weisdorf D. Platelet utilisation and the transfusion trigger: a prospective analysis. Transfusion 2007;72(2):201‐5. [DOI] [PubMed] [Google Scholar]
Guerriero 2011
- Guerriero C, Cairns J, Perel P, Shakur H, Roberts I. Cost‐effectiveness analysis of administering tranexamic acid to bleeding trauma patients using evidence from the CRASH‐2 trial. PLoS One 2011;6(5):e18987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurusamy 2011
- Gurusamy KS, Pissanou T, Pikhart H, Vaughan J, Burroughs AK, Davidson BR. Methods to decrease blood loss and transfusion requirements for liver transplantation. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD009052.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heddle 2009
- Heddle NM, Webbert K. Chapter 6: Investigation of acute transfusion reactions. In: Murphy MF, Pamphilion DH editor(s). Practical Transfusion Medicine. 3rd Edition. Blackwell, 2009:63‐89. [Google Scholar]
Henry 2011
- Henry DA, Carless PA, Moxey AJ, O'Connell D, Stokes BJ, Fergusson DA, et al. Anti‐fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD001886.pub4] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in Statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ker 2012
- Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta‐analysis. BMJ 2012;344:e3054. [PUBMED: 22611164] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khorana 2006
- Khorana AA, Francis CW, Culakova E, Fisher RI, Kuderer NM, Lyman GH. Thromboembolism in hospitalized neutropenic cancer patients. Journal of Clinical Oncology 2006;24(3):484‐90. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Llewelyn 2009
- Llewelyn CA, Wells AW, Amin M, Casbard A, Johnson A J, Ballard S, et al. The EASTR study: a new approach to determine the reasons for transfusion in epidemiological studies. Transfusion Medicine 2009;19(2):89‐98. [DOI] [PubMed] [Google Scholar]
Lozano 2013
- Lozano M, Sweeney JD. Avoiding platelet transfusions. In: Sweeney J, Lozano M editor(s). Platelet Transfusion Therapy. Bethesda, Maryland: AABB Press, 2013:567‐89. [Google Scholar]
Martin 2011
- Martin K, Gertler R, Sterner A, MacGuill M, Schreiber C, Horer J, et al. Comparison of blood‐sparing efficacy of epsilon‐aminocaproic acid and tranexamic acid in newborns undergoing cardiac surgery. The Thoracic and Cardiovascular Surgeon 2011;59(5):276‐80. [DOI] [PubMed] [Google Scholar]
Martin‐Hirsch 2010
- Martin‐Hirsch PPL, Keep SL, Bryant A. Interventions for preventing blood loss during the treatment of cervical intraepithelial neoplasia. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD001421.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Novikova 2011
- Novikova N, Hofmeyr GJ. Tranexamic acid for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD007872.pub2] [DOI] [PubMed] [Google Scholar]
Okamoto 1997
- Okamoto S, Hijikata‐Okunomiya A, Wanaka K, Okada Y, Okamoto U. Enzyme‐controlling medicines: introduction. Seminars in Thrombosis and Hemostasis 1997;23(6):493‐501. [DOI] [PubMed] [Google Scholar]
Pendry 2011
- Pendry K, Davies T. An audit of the use and wastage in the North West of England and North Wales ‐ where have all the platelets gone?. Blood and Transfusion Matters 2011;34:17‐9. [Google Scholar]
Pogue 1997
- Pogue JM, Yusuf S. Cumulating evidence from randomized trials: utilizing sequential monitoring boundaries for cumulative meta‐analysis. Controlled Clinical Trials 1997;18(6):580‐93. [DOI] [PubMed] [Google Scholar]
Popovsky 1985
- Popovsky MA, Moore SB. Diagnostic and pathogenetic considerations in transfusion‐related acute lung injury. Transfusion 1985;25(6):573‐7. [DOI] [PubMed] [Google Scholar]
Prentice 1980
- Prentice CR. Basis of antifibrinolytic therapy. Journal of Clinical Pathology Supplement 1980;14:35‐40. [PMC free article] [PubMed] [Google Scholar]
Roberts 2011
- Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, Dewan Y, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH‐2 randomised controlled trial. Lancet 2011;377(9771):1096‐101, 1101.e1‐2. [DOI] [PubMed] [Google Scholar]
Roos 2008
- Roos YB, Rinkel GJE, Vermeulen M, Algra A, Gijn J. Antifibrinolytic therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD001245] [DOI] [PubMed] [Google Scholar]
Sawamura 2009
- Sawamura A, Hayakawa M, Gando S, Kubota N, Sugano M, Wada T, et al. Disseminated intravascular coagulation with a fibrinolytic phenotype at an early phase of trauma predicts mortality. Thrombosis Research 2009;124(5):608‐13. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Shakur 2010
- Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH‐2): a randomised, placebo‐controlled trial. Lancet 2010;376(9734):23‐32. [DOI] [PubMed] [Google Scholar]
Slichter 2005
- Slichter SJ, Davis K, Enright H, Braine H, Gernsheimer T, Kao KJ, et al. Factors affecting posttransfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood 2005;105(10):4106‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Slichter 2010
- Slichter SJ, Kaufman RM, Assmann SF, McCullough J, Triulzi DJ, Strauss RG, et al. Dose of prophylactic platelet transfusions and prevention of hemorrhage. New England Journal of Medicine 2010;362(7):600‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Tzortzopoulou 2008
- Tzortzopoulou A, Cepeda MS, Schumann R, Carr DB. Antifibrinolytic agents for reducing blood loss in scoliosis surgery in children. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD006883.pub2] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Wardrop 2012
- Wardrop D, Estcourt L, Doree C, Hopewell S, Stanworth S, Murphy MF. Anti‐fibrinolytics (lysine analogues) for the prevention of bleeding in patients with haematological disorders. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD009733] [DOI] [PubMed] [Google Scholar]
Wardrop 2013
- Wardrop D, Estcourt LJ, Doree C, Hopewell S, Stanworth S, Murphy MF. Antifibrinolytics (lysine analogues) for the prevention of bleeding in patients with haematological disorders. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD009733.pub2] [DOI] [PubMed] [Google Scholar]


