Abstract
The present study identified differentially-expressed genes (DEGs) between pancreatic cancer (PC) tissues and normal tissues, and assessed genetic factors associated with the pathogenesis of PC. The mRNA expression microarray dataset, GSE16515, containing 52 samples, including 16 paired tumor and normal tissue samples, and 20 tumor samples, was downloaded from Gene Expression Omnibus. Raw data were normalized and DEGs were identified. Subsequently, clustering was performed, protein-protein interaction networks were drawn, and functional and pathway enrichment analyses of the DEGs were performed. Copy number variations of DEGs were also identified. A total of 1,765 DEGs between PC and normal tissues were identified, including 1,312 upregulated and 453 downregulated DEGs. Upregulated DEGs were associated with the regulation of nucleocytoplasmic and intracellular transport, whereas downregulated DEGs were associated with the response to organic substances and hormone stimulus. The pancreatic cancer pathway was connected to three DEGs, namely transforming growth factor β1 (TGFB1), TGFβ receptor 1 (TGFBR1) and epidermal growth factor (EGF), which had 2, 3 and 5 CNVs, respectively. These results indicated the important roles of TGFB1, TGFBR1 and EGF in the pathogenesis of PC. These genes may be potential therapeutic targets for the treatment of PC.
Keywords: pancreatic cancer, differentially-expressed genes, TGFB1, TGFBR1, EGF
Introduction
Pancreatic cancer (PC; OMIM 260350) is a highly lethal disease, with an incidence rate that is constantly increasing (1). The 5-year survival rate of PC is <5% (2), and almost all patients with primary PC develop metastases.
Previous studies have indicated that PC has a complex genomic landscape with frequent copy number variations (CNVs) or copy number polymorphisms (CNPs) (3). Biankin et al (4) defined 16 significantly mutated genes [e.g., Kirsten rat sarcoma viral oncogene homolog (KRAS), tumor protein p53 (TP53), SMAD family member 4 (SMAD4) and transforming growth factor β receptor 2 (TGFBR2)] that were reaffirmed known mutations associated with PC. The commonly mutated genes, such as KRAS (74–100%), cyclin-dependent kinase inhibitor 2A (up to 98%), TP53 (43–76%), erb-b2 receptor tyrosine kinase 2 (ERBB2; ~65%) and fragile histidine triad (~70%) have been found in PC (5–9). Among these genes, KRAS and ERBB2 are proto-oncogenes, whereas the other genes are tumor suppressors (3). The progression of PC is correlated with the activation of oncogenes and the inactivation of tumor suppressor genes, as well as the deregulation of a number of signaling pathways, among which the epidermal growth factor receptor (EGFR), v-akt murine thymoma viral oncogene homolog 1 (v-AKT1) and nuclear factor of κ light polypeptide gene enhancer in B-cells 1 (NFκB1) pathways appear to be most relevant (10).
AKT1 is a central regulator of cell growth. AKT1 has been shown to inhibit apoptosis and promote cell survival, thus contributing to the pathogenesis of cancer (11,12). Pei et al (13) showed that FK506-binding protein 51 (FKBP51) acted as a scaffolding protein for Akt and that it promoted the activation of Akt. The expression of FKBP51 was downregulated in PC tissues. Decreased FKBP51 expression resulted in the hyper-phosphorylation of Akt, and then decreased the level of cell death in the PC tissues. Thus, Pei et al demonstrated FKBP51 to be a negative regulator of the Akt pathway (13). Pei et al also released the mRNA expression microarray dataset, GSE16515, consisting of 36 pancreatic tumor and 16 normal tissue samples. Numerous studies have since been performed using this dataset (14–16). For example, using the GSE16515 dataset, Yang et al (14) screened the differentially-expressed genes (DEGs), such as TGF α (TGFA) and EGF, between PC tumor tissues and normal tissues, and selected the important single nucleotide polymorphisms (SNPs) of A/G and C/T in the DEGs. However, none of the studies based on the GSE16515 dataset performed an analysis of CNVs in the DEGs.
Using the GSE16515 dataset downloaded from Gene Expression Omnibus (GEO), the DEGs between PC tumor tissues and normal tissues were screened in the present study. Next, clustering analysis and construction of a protein-protein interaction (PPI) network of DEGs was performed. The underlying functions of these DEGs were investigated by functional and pathway enrichment analyses. Finally, the CNVs of these DEGs were also analyzed. This will be beneficial for developing therapeutic strategies for patients with PC.
Materials and methods
mRNA microarray data
The mRNA expression microarray data from the GSE16515 dataset (6) was downloaded from GEO (http://www.ncbi.nlm.nih.gov/geo/), based on the platform of GPL570 [HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array (Affymetrix, Santa Clara, CA, USA). GSE16515 was composed of 52 samples (from 34 males and 18 females). In total, 32 samples consisted of tumor and normal expression data, whereas 20 samples consisted of only tumor data. These samples were obtained during clinically indicated surgical procedures and consent was obtained for experimental purposes. The raw data and the probe annotation files were downloaded for further analysis. The microarray data of the GSE16515 dataset was analyzed following the procedures presented in Fig. 1.
Figure 1.
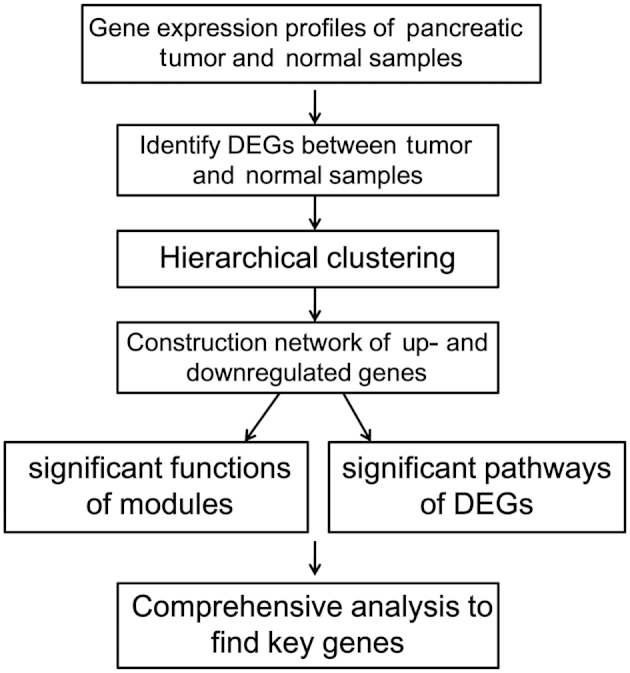
Flowchart of the analysis of the microarray data from the GSE16515 dataset. DEG, differentially-expressed gene.
Data preprocessing and DEG identification
The Robust Multiarray Average in Affy package of R (http://www.biocon-ductor.org/packages/release/bioc/html/affy.html), provided by Bioconductor project (17), was applied to process the raw micro-array data. The processing included background correction, quantile normalization and probe summarization of expression values. The gene expression matrices were obtained for further analysis. Afterwards, the Linear Models for Microarray Data package was used to identify the gene signatures between the tumor and normal tissues, with significant differences indicated using a P-value of <0.05. Next, the Bonferroni correction (18) was applied to adjust the raw P-value for the false discovery rate (FDR) and to calculate the fold change (FC). In the present study, the cut-off criteria for the statistically significant DEGs were |log2FC| >1 and FDR <0.05.
Clustering analysis of DEGs
Based on the Euclidean distance between the expression profile of each DEG filtered from the samples, hierarchical clustering can be used to build a hierarchy of clusters of DEGs (19). The heatmap figure of the DEGs was drawn with the R package pheatmap (http://cran.r-project.org/web/packages/pheatmap/index.html) function. DEGs with the same signatures were clustered together, indicating the specificity of the DEGs.
Identification of PPIs of DEGs
Identification of protein complexes and functional modules from PPI networks is crucial to predict protein functions and to understand the principles of cellular organization (20). The Search Tool for the Retrieval of Interacting Genes (STRING; http://string-db.org/) database provides uniquely comprehensive coverage and ease of access for the prediction of interaction information (21). To better understand the interactions of the DEGs, the PPI network of their encoding products was predicted using the STRING database, with the reliability threshold of >0.9. Cytoscape software (http://cytoscape.org/), a standard tool for the integrated analysis and visualization of biological networks, was used to visualize the PPI network (22).
Functional enrichment analysis of DEGs
Gene Ontology (GO; http://www.geneontology.org/) analysis is an functional study method for large-scale transcriptomic or genomic data (23). In order to investigate the biofunctions of DEGs in tumor progression, the Database for Annotation, Visualization and Integrated Discovery (http://david.abcc.Ncifcrf.gov/), a high-throughput and integrated data-mining environment (24), was used to identify the enriched GO biological processes that the DEGs were associated with (FDR<0.05).
Pathway analysis of DEGs
The Kyoto Encyclopedia of Genes and Genomes (KEGG; http://www.genome.jp/kegg/pathway.html) pathway database provides information on how molecules or genes function (25). Pathway analysis of all the DEGs was performed using the KEGG database. The KEGG maps of biological functions associated with DEGs were obtained (P<0.05).
CNV analysis of DEGs
The Database of Genomic Variants (DGV; http://dgv.tcag.ca/) (26) was used to identify the CNVs in the DEGs, including deletions, insertions, duplications, complex multi-site variants and SNPs.
Results
Data processing and identification of DEGs
After the normalization, the DEGs between the tumor and normal tissues of the 52 samples were identified, with the cut-off criteria of |log2FC| >1 and FDR <0.05. A total of 1,765 DEGs were identified between the PC and normal tissues, of which 1,312 were upregulated and 453 were downregulated.
Hierarchical clustering of DEGs
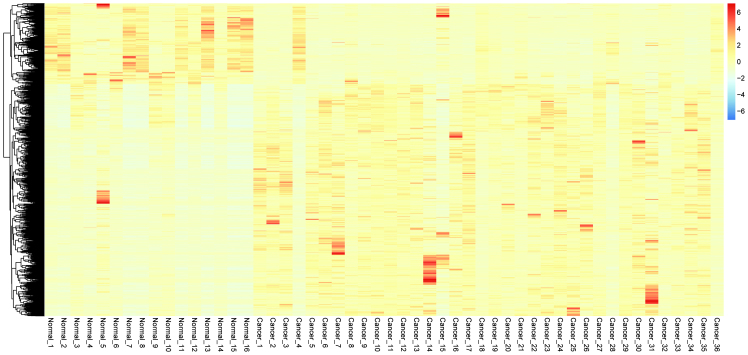
Hierarchical clustering of the 1,765 DEGs is presented in Fig. 2. The LogFC values of the DEGs ranged from 6-fold downregulated and 6-fold upregulated. The majority of the DEGs were upregulated in the PC tumors compared with the normal tissues. The tumor samples and the normal control samples could easily be distinguished from the characteristics of the DEGs.
Figure 2.
Heat map clustering of the differentially-expressed genes between two samples. The x-axis represents normal and tumor samples, and the y-axis represents genes. Blue (<0) indicates downregulation and orange (>0) indicates upregulation of gene expression in the pancreatic and normal tissues.
PPIs analysis of DEGs
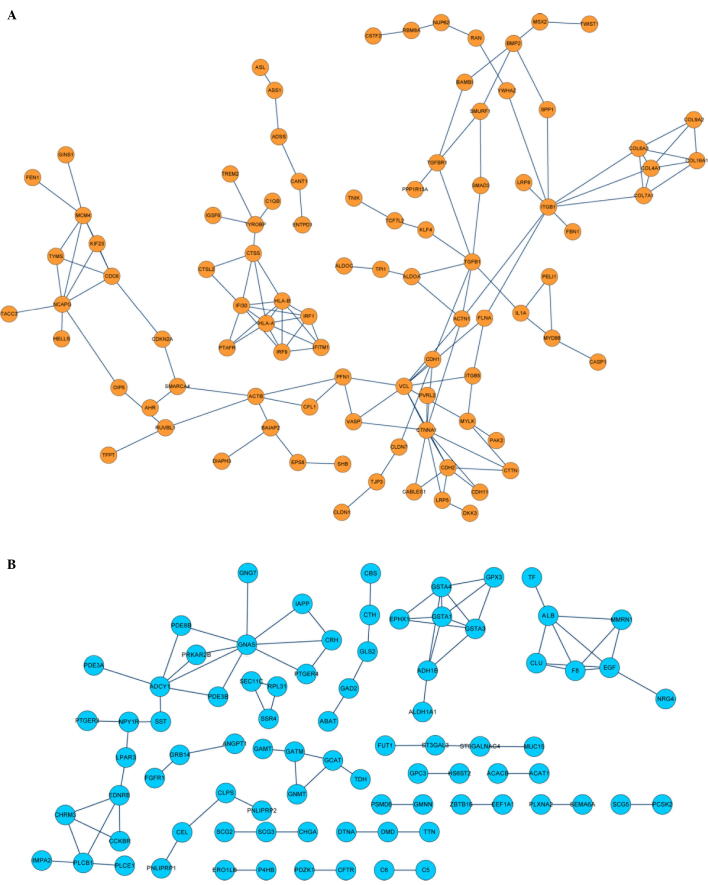
To identify the PPIs and predict protein functions, PPI network analysis was performed using the STRING database (threshold >0.9). The resulting PPI network of upregulated DEGs connected to 92 nodes (proteins) through 171 PPIs, whereas the PPI network of the downregulated DEGs connected to 82 nodes through 83 PPIs (Fig 3).
Figure 3.
Protein-protein interaction networks of (A) upregulated DEGs (orange) and (B) downregulated DEGs (blue). The nodes represent proteins and the lines between nodes represent interactions. DEG, differentially-expressed gene.
Functional enrichment analysis of DEGs
To obtain the enriched GO biological processes of the DEGs in the PPI networks, GO functional enrichment analysis was performed for the up- and downregulated DEGs, respectively (FDR <0.05). The upregulated DEGs (including TGFB1 and TGFBR1) were associated with significant biological processes, such as the regulation of nucleocytoplasmic transport, protein localization and intracellular transport (Table I), whereas the downregulated DEGs (e.g., EGF) were correlated with the biological processes of the positive regulation of catalytic activity, the response to organic substances and the response to hormone stimuli (Table I).
Table I.
Enriched GO biological processes of the DEGs in the protein-protein interaction networks.
| Term and function | Count | Genes | P-value | FDR |
|---|---|---|---|---|
| Upregulated DEGs | ||||
| GO:0046822 - Regulation of nucleocytoplasmic transport | 7 | CDKN2A, TGFBR1, SMAD3, CDH1, TACC3, FLNA, TGFB1 | 1.72×10−6 | 0.002848 |
| GO:0032880 - Regulation of protein localization | 9 | TGFBR1, SMAD3, CDH1, CDH2, CASP1, TACC3, FLNA, TGFB1, IL1A | 2.60×10−6 | 0.004308 |
| GO:0051222 - Positive regulation of protein transport | 7 | TGFBR1, SMAD3, CDH1, CASP1, FLNA, TGFB1, IL1A | 4.05×10−6 | 0.006710 |
| GO:0032386 - Regulation of intracellular transport | 7 | CDKN2A, TGFBR1, SMAD3, CDH1, TACC3, FLNA, TGFB1 | 5.69×10−6 | 0.009436 |
| Downregulated DEGs | ||||
| GO:0043085 - Positive regulation of catalytic activity | 13 | ADCY1, PTGER3, CCKBR, C6, C5, LPAR3, EDNRB, PRKAR2B, PLCE1, CLPS, GNAS, EGF, PSMD6 | 1.72×10−5 | 0.027131 |
| GO:0010033 - Response to organic substances | 15 | TF, ADCY1, PNLIPRP1, GATM, PDE3B, EPHX1, CFTR, PDE3A, NPY1R, PRKAR2B, ABAT, ANGPT1, GNAS, SST, GNG7 | 2.20×10−5 | 0.034801 |
| GO:0009725 - Response to hormone stimuli | 11 | PRKAR2B, PNLIPRP1, ADCY1, GATM, PDE3B, GNAS, ANGPT1, CFTR, NPY1R, SST, GNG7 | 2.29×10−5 | 0.036206 |
| GO:0006575 - Cellular amino acid derivative metabolic processes | 8 | GSTA1, P4HB, CTH, GATM, GPX3, ABAT, GAMT, GNMT | 2.78×10−5 | 0.043955 |
FDR, false discovery rate; DEG, differentially-expressed genes; GO, gene ontology.
Pathway analysis of DEGs
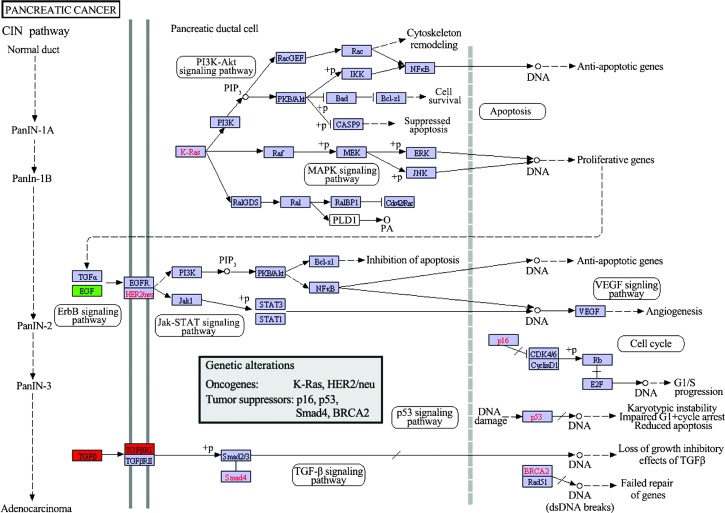
The KEGG maps of biological functions associated with DEGs in the PPI networks were obtained (P<0.05). The results showed that only the pancreatic cancer pathway was associated with DEGs in the PPI networks, including upregulated TGFB1 and TGFBR1, and downregulated EGF (Fig. 4).
Figure 4.
Molecular pathways in pancreatic cancer involving the DEGs in the protein-protein interaction networks. Red boxes represent upregulated DEGs and the green box represents a downregulated DEG. Red letters represent tumor suppressors or oncogenes that have been validated in previous studies. CIN, chromosomal instability; DEG, differentially-expressed gene; ds, double strand; PanIN, pancreatic intraepithelial neoplasia.
CNV analysis of DEGs
The CNVs of the TGFB1, TGFBR1 and EGF genes were further identified using the DGV. The identification of CNVs of DEGs included deletions, insertions, duplications and complex multi-site variants. Finally, 2, 3 and 5 CNVs, were identified in the TGFB1, TGFBR1 and EGF genes, respectively (Table II) (27–34). In total, 1 of the CNVs of TGFB1 was insertin; all 3 of the CNVs of TGFBR1 were insertins; and 1 of the 5 CNVs of EGF was insertin.
Table II.
Copy number variations in TGFB1, TGFBR1 and EGF genes.
| First author, year | Gene | Variant ID | Subtype | (Ref.) |
|---|---|---|---|---|
| Xu et al, 2011 | TGFB1 | nsv911769 | Loss | (27) |
| Shaikh et al, 2009 | nsv521311 | Insertion | (28) | |
| Xu et al, 2011 | TGFBR1 | nsv893619 | Insertion | (27) |
| nsv893618 | Insertion | |||
| Wong et al, 2007 | nsv831666 | Insertion | (29) | |
| Abecasis et al, 2012 | EGF | esv2672203 | Deletion | (30) |
| McKernan et al, 2009 | esv2618042 | Insertion | (31) | |
| Conrad et al, 2010 | esv22936 | Loss | (32) | |
| Mills et al, 2006 | nsv290769 | Loss | (33) | |
| Kim et al, 2009 | nsv820232 | Loss | (34) |
Discussion
The early stages of PC are usually asymptomatic, and the majority of patients with PC are diagnosed at an advanced stage. The pathogenesis of PC is involved in a number of biological processes. Advanced studies of genetic factors have greatly improved our understanding of the pathogenesis of PC, which is associated with gene mutations, continuous changes to nuclei, loss of polarity and changes in cellular architecture (35).
In the present study, the DEGs between PC tumor tissues and normal tissues were systematically investigated. A total of 1,765 DEGs, including 1,312 upregulated and 453 down-regulated DEGs, were identified. The majority of DEGs were upregulated in the tumor tissues. The upregulated DEGs (including SMAD3, TGFB1 and TGFBR1) were associated with the regulation of nucleocytoplasmic and intracellular transport, and protein localization, whereas the downregulated DEGs (e.g., EGF) were associated with regulation of catalytic activity, and the responses to organic substances and hormone stimuli. All 4 of these DEGs were connected to the pancreatic cancer pathway. In addition, TGFB1, TGFBR1 and EGF exhibited 2, 3 and 5 CNVs, respectively. These results proposed an important role for these DEGs in PC development.
CNVs or CNPs, such as deletions, insertions, duplications and complex multi-site variants, have been found in all humans and in other mammals (36). CNVs can be simple in structures like tandem duplication, or may be complex in the genome, such as in gains or losses of homologous sequences at multiple sites (37). Yang et al (14) screened the DEGs between PC tissues and normal tissues using the GSE16515 dataset, and selected the important SNPs of A/G and C/T in DEGs such as TGFA and EGF. In the present study, 5 CNVs of EGF were identified, including 3 losses, 1 deletion and 1 insertion. These results demonstrate that the CNVs of EGF may be significant in the pathogenesis of PC.
EGFR is required for KRAS-induced PC (38). Accordingly, the overexpression of EGFR and TGFA has been reported as an important molecular abnormality in human PCs (39). EGF and TGFA are essential molecules for the VEGF signaling pathway (Fig. 4). The inhibition of the vascular endothelial growth factor (VEGF) signaling pathway is beneficial for the suppression of tumor metastasis and invasion, such as required in PC (40). In the present study, it was found that the expression of EGF was downregulated in the PC tissues. Taken together, these results suggest the significant role of EGF in PC and indicate that EGF may be a novel target for the therapy of PC.
TGFB1 and TGFBR1 were overexpressed in the PC tumors in the present study. The two genes are elements of the TGFB signaling pathway, which is a potent inhibitor of cell growth (41). There is growing evidence that members of the TGFB family are frequently mutated in cancer. CNVs, DNA segments that are ≥1 kb and present at variable copy number in comparison with a reference genome, affect the expression of genes, the variation and adaptation of phenotypes, and the pathogenesis of diseases, such as human immunodeficiency virus-1 infection, by disrupting genes and altering gene dosage in microdeletion or microduplication disorders (42,43). The present study identified 3 insertion CNVs in TGFBR1, and 1 insertion CNV and 1 loss CNV in TGFB1. Moreover, 3 of the CNVs in TGFBR1 and TGFB1 were located at the same segment as in a previous study (27). These indicate the vital roles of TGFBR1 and TGFB1 in PC development. The genes may be potential therapeutic targets for the treatment of PC.
In summary, 1,765 DEGs, including 1,312 upregulated (e.g., TGFB1 and TGFBR1) and 453 downregulated (e.g., EGF) DEGS, were identified in the PC tissues compared with the normal tissues in the present study. The upregulated DEGs were associated with the regulation of nucleocytoplasmic and intracellular transport, whereas the downregulated DEGs were associated with the regulation of catalytic activity, and the response to organic substances and hormone stimuli. A pancreatic cancer pathway was connected to the DEGs of TGFB1, TGFBR1 and EGF. In addition, TGFB1, TGFBR1 and EGF exhibited 2, 3 and 5 CNVs, respectively. These results suggested the significance of the DEGs in PC. TGFB1, TGFBR1 and EGF may be potential therapeutic targets for the treatment of PC. However, further clinical trials are required to validate these conclusions and hypotheses.
References
- 1.Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:197–209. doi: 10.1016/j.bpg.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Thomas JK, Kim MS, Balakrishnan L, Nanjappa V, Raju R, Marimuthu A, Radhakrishnan A, Muthusamy B, Khan AA, Sakamuri S, et al. Pancreatic Cancer Database: An integrative resource for pancreatic cancer. Cancer Biol Ther. 2014;15:963–967. doi: 10.4161/cbt.29188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strimpakos A, Saif MW, Syrigos KN. Pancreatic cancer: From molecular pathogenesis to targeted therapy. Cancer Metastasis Rev. 2008;27:495–522. doi: 10.1007/s10555-008-9134-y. [DOI] [PubMed] [Google Scholar]
- 4.Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/S1535-6108(03)00309-X. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein AM, Fraser MC, Struewing JP, Hussussian CJ, Ranade K, Zametkin DP, Fontaine LS, Organic SM, Dracopoli NC, Clark WH., Jr Increased risk of pancreatic cancer in melanoma-prone kindreds with p16INK4 mutations. N Engl J Med. 1995;333:970–974. doi: 10.1056/NEJM199510123331504. [DOI] [PubMed] [Google Scholar]
- 7.Pellegata NS, Sessa F, Renault B, Bonato M, Leone BE, Solcia E, Ranzani GN. K-ras and p53 gene mutations in pancreatic cancer: Ductal and nonductal tumors progress through different genetic lesions. Cancer Res. 1994;54:1556–1560. [PubMed] [Google Scholar]
- 8.Grau AM, Zhang L, Wang W, Ruan S, Evans DB, Abbruzzese JL, Zhang W, Chiao PJ. Induction of p21waf1 expression and growth inhibition by transforming growth factor beta involve the tumor suppressor gene DPC4 in human pancreatic adenocarcinoma cells. Cancer Res. 1997;57:3929–3934. [PubMed] [Google Scholar]
- 9.Sorio C, Baron A, Orlandini S, Zamboni G, Pederzoli P, Huebner K, Scarpa A. The FHIT gene is expressed in pancreatic ductular cells and is altered in pancreatic cancers. Cancer Res. 1999;59:1308–1314. [PubMed] [Google Scholar]
- 10.Sarkar FH, Banerjee S, Li Y. Pancreatic cancer: Pathogenesis, prevention and treatment. Toxicol Appl Pharmacol. 2007;224:326–336. doi: 10.1016/j.taap.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: Rationale and promise. Cancer Cell. 2003;4:257–262. doi: 10.1016/S1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 12.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pei H, Li L, Fridley BL, Jenkins GD, Kalari KR, Lingle W, Petersen G, Lou Z, Wang L. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. 2009;16:259–266. doi: 10.1016/j.ccr.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang D, Zhu Z, Wang W, Shen P, Wei Z, Wang C, Cai Q. Expression profiles analysis of pancreatic cancer. Eur Rev Med Pharmacol Sci. 2013;17:311–317. [PubMed] [Google Scholar]
- 15.Zhao LL, Zhang T, Zhuang LW, Yan BZ, Wang RF, Liu BR. Uncovering the pathogenesis and identifying novel targets of pancreatic cancer using bioinformatics approach. Mol Biol Rep. 2014;41:4697–4704. doi: 10.1007/s11033-014-3340-1. [DOI] [PubMed] [Google Scholar]
- 16.Söderquist F, Hellström PM, Cunningham JL. Human gastroenteropancreatic expression of melatonin and its receptors MT1 and MT2. PLoS One. 2015;10:e0120195. doi: 10.1371/journal.pone.0120195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 18.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 19.Deza MM, Deza E. Encyclopedia of distances. Springer; Heidelberg: 2009. [DOI] [Google Scholar]
- 20.Li M, Wu X, Wang J, Pan Y. Towards the identification of protein complexes and functional modules by integrating PPI network and gene expression data. BMC Bioinformatics. 2012;13:109. doi: 10.1186/1471-2105-13-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szklarczyk D, Franceschini A, Kuhn M, Simonovic M, Roth A, Minguez P, Doerks T, Stark M, Muller J, Bork P, et al. The STRING database in 2011: Functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 2011;39(Database issue):D561–D568. doi: 10.1093/nar/gkq973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hulsegge I, Kommadath A, Smits MA. Globaltest and GOEAST: Two different approaches for Gene Ontology analysis. BMC Proc. 2009;3(Suppl 4):S10. doi: 10.1186/1753-6561-3-s4-s10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 25.Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacDonald JR, Ziman R, Yuen RK, Feuk L, Scherer SW. The Database of Genomic Variants: A curated collection of structural variation in the human genome. Nucleic Acids Res. 2014;42(Database issue):D986–D992. doi: 10.1093/nar/gkt958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu H, Poh WT, Sim X, Ong RT, Suo C, Tay WT, Khor CC, Seielstad M, Liu J, Aung T, et al. SgD-CNV, a database for common and rare copy number variants in three Asian populations. Hum Mutat. 2011;32:1341–1349. doi: 10.1002/humu.21601. [DOI] [PubMed] [Google Scholar]
- 28.Shaikh TH, Gai X, Perin JC, Glessner JT, Xie H, Murphy K, O'Hara R, Casalunovo T, Conlin LK, D'Arcy M, et al. High-resolution mapping and analysis of copy number variations in the human genome: a data resource for clinical and research applications. Genome Res. 2009;19:1682–1690. doi: 10.1101/gr.083501.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong KK, deLeeuw RJ, Dosanjh NS, Kimm LR, Cheng Z, Horsman DE, MacAulay C, Ng RT, Brown CJ, Eichler EE, Lam WL. A comprehensive analysis of common copy-number variations in the human genome. Am J Hum Genet. 2007;80:91–104. doi: 10.1086/510560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abecasis GR, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR. 1000 Genomes Project Consortium: An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKernan KJ, Peckham HE, Costa GL, McLaughlin SF, Fu Y, Tsung EF, Clouser CR, Duncan C, Ichikawa JK, Lee CC, et al. Sequence and structural variation in a human genome uncovered by short-read, massively parallel ligation sequencing using two-base encoding. Genome Res. 2009;19:1527–1541. doi: 10.1101/gr.091868.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conrad DF, Pinto D, Redon R, Feuk L, Gokcumen O, Zhang Y, Aerts J, Andrews TD, Barnes C, Campbell P, et al. Origins and functional impact of copy number variation in the human genome. Nature. 2010;464:704–712. doi: 10.1038/nature08516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mills RE, Luttig CT, Larkins CE, Beauchamp A, Tsui C, Pittard WS, Devine SE. An initial map of insertion and deletion (INDEL) variation in the human genome. Genome Res. 2006;16:1182–1190. doi: 10.1101/gr.4565806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JI, Ju YS, Park H, Kim S, Lee S, Yi JH, Mudge J, Miller NA, Hong D, Bell CJ, et al. A highly annotated whole-genome sequence of a Korean individual. Nature. 2009;460:1011–1015. doi: 10.1038/nature08211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Klöppel G, Longnecker DS, et al. v Am J Surg Pathol. 2001;25:579–586. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Feuk L, Carson AR, Scherer SW. Structural variation in the human genome. Nat Rev Genet. 2006;7:85–97. doi: 10.1038/nrg1767. [DOI] [PubMed] [Google Scholar]
- 37.Redon R, Ishikawa S, Fitch KR, Feuk L, Perry GH, Andrews TD, Fiegler H, Shapero MH, Carson AR, Chen W, et al. Global variation in copy number in the human genome. Nature. 2006;444:444–454. doi: 10.1038/nature05329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ardito CM, Grüner BM, Takeuchi KK, Lubeseder-Martellato C, Teichmann N, Mazur PK, DelGiorno KE, Carpenter ES, Halbrook CJ, Hall JC, et al. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell. 2012;22:304–317. doi: 10.1016/j.ccr.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barton CM, Hall PA, Hughes CM, Gullick WJ, Lemoine NR. Transforming growth factor alpha and epidermal growth factor in human pancreatic cancer. J Pathol. 1991;163:111–116. doi: 10.1002/path.1711630206. [DOI] [PubMed] [Google Scholar]
- 40.Sennino B, Ishiguro-Oonuma T, Wei Y, Naylor RM, Williamson CW, Bhagwandin V, Tabruyn SP, You WK, Chapman HA, Christensen JG, et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov. 2012;2:270–287. doi: 10.1158/2159-8290.CD-11-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pasche B, Pennison MJ, Jimenez H, Wang M. TGFBR1 and cancer susceptibility. Trans Am Clin Climatol Assoc. 2014;125:300–312. [PMC free article] [PubMed] [Google Scholar]
- 42.McCarroll SA, Hadnott TN, Perry GH, Sabeti PC, Zody MC, Barrett JC, Dallaire S, Gabriel SB, Lee C, Daly MJ, et al. Common deletion polymorphisms in the human genome. Nat Genet. 2006;38:86–92. doi: 10.1038/ng1696. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen DQ, Webber C, Ponting CP. Bias of selection on human copy-number variants. PLoS Genet. 2006;2:e20. doi: 10.1371/journal.pgen.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]