Abstract
Background:
While there is increasing evidence that Advance Care Planning has the potential to strengthen patient autonomy and improve quality of care near the end of life, it remains unclear whether it could also reduce net costs of care.
Aim:
This study aims to describe the cost implications of Advance Care Planning programmes and discusses ethical conflicts arising in this context.
Design:
We conducted a systematic review based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.
Data sources:
We systematically searched the databases PubMed, NHS EED, EURONHEED, Cochrane Library and EconLit. We included empirical studies (no limitation to study type) that investigated the cost implications of Advance Care Planning programmes involving professionally facilitated end-of-life discussions.
Results and discussion:
Seven studies met our inclusion criteria. Four of them used a randomised controlled design, one used a before-after design and two were observational studies. Six studies found reductions in costs of care ranging from USD1041 to USD64,827 per patient, depending on the study period and the cost measurement. One study detected no differences in costs. Studies varied considerably regarding the Advance Care Planning intervention, patient selection and costs measured which may explain some of the variations in findings.
Normative appraisal:
Looking at the impact of Advance Care Planning on costs raises delicate ethical issues. Given the increasing pressure to reduce expenditures, there may be concerns that cost considerations could unduly influence the sensitive communication process, thus jeopardising patient autonomy. Safeguards are proposed to reduce these risks.
Conclusion:
The limited data indicate net cost savings may be realised with Advance Care Planning. Methodologically robust trials with clearly defined Advance Care Planning interventions are needed to make the costs and returns of Advance Care Planning transparent.
Keywords: Advance care planning, advance directives, healthcare costs, economics, ethics, review
What is already known about the topic?
Advance Care Planning (ACP), here defined as an advance decision-making process involving a professionally facilitated conversation, has been proposed as a strategy to overcome the shortcomings of traditional advance directives.
Evidence is accumulating that facilitated ACP can strengthen patient autonomy and improve quality of care near the end of life.
It is yet unclear whether ACP may also reduce healthcare expenditures.
What this paper adds?
Findings indicate that facilitated ACP has the potential to reduce net costs of care.
The impact on costs of care may depend on the details of the ACP programme.
To protect end-of-life discussions from undue influences of cost considerations, adequate training of facilitators, clearly defined ACP standards and transparency concerning programme objectives and conflicts of interest should be ensured.
Implications for practice, theory or policy
Policy makers might consider investment in professionally facilitated ACP a good use of scarce healthcare resources if safeguards guarantee the openness of the planning process.
Methodologically robust trials are needed to determine which ACP elements are decisive for improving clinical outcomes and potentially reducing healthcare expenditures in accordance with patients’ preferences.
Introduction
Most healthcare systems in high-income countries face rising expenditures due to medical innovations and an increasing number of multimorbid, chronically ill patients in ageing societies. Empirical studies show that healthcare costs rise exponentially in the last year of life,1–5 while high-cost treatment near the end of life (EOL) may not improve quality of care.6,7 In addition, aggressive and expensive medical care is often inconsistent with the treatment preferences of seriously ill patients.8
Decisions about life-sustaining treatment near the EOL often have to be made when patients lack decision-making capacity.9,10 Advance directives (ADs) have been proposed as a means to respect patients’ treatment preferences in these situations. As many people do not request all available life-sustaining interventions,10,11 ADs have also been discussed – controversially – as a means to achieve cost savings at the EOL.12–15 ADs as legal documents, however, have fallen short of their high expectations.16–19 They are not widely used, not available when needed, often not relevant, of dubious validity and frequently not honoured by medical staff.20 It is therefore not surprising that ADs also largely failed to reduce resource use and costs of care at the EOL.11,12,21–23
Over the last decades, however, the new concept of Advance Care Planning (ACP) has emerged.24,25 Instead of just completing and signing a legal form, ACP is understood as a life-long communication process based on two fundamental components:25,26 (1) Specifically qualified healthcare professionals (‘facilitators’) assist individuals and their families/friends to develop, articulate and document preferences for future medical care. (2) A systematic regional implementation ensures that the resulting plans are available and honoured reliably across all healthcare institutions in the community. A growing body of literature shows that these comprehensive ACP programmes are effective in increasing the number of meaningful and valid ADs,26 respecting patients’ treatment preferences near the EOL27 and improving quality of care for patients and their families.28 A recent systematic review found that complex ACP interventions (20 studies out of 113) may be more effective than ADs alone in improving compliance with patient’s EOL wishes and satisfaction with care.29
If such ACP programmes improve consistency between patients’ preferences and the delivered care, they could also have a larger impact on costs of care; at the same time, they require a considerable investment for implementation and maintenance. Systematic reviews have assessed the effects of ACP on other outcome parameters (e.g. reduction of hospital days),29,30 and a recent review explored the economic evidence for ACP in a broad sense of the term that counts any completion of ADs as ACP.31 However, the available evidence on the specific cost effects of comprehensive ACP programmes based on a professionally facilitated communication process has never been evaluated systematically. We therefore conducted a systematic review on the cost implications of such comprehensive ACP programmes.
If ACP programmes turn out to reduce overall expenditures and cost containment becomes an explicit goal of ACP, this raises delicate ethical issues.32,33 Given the increasing pressure to reduce healthcare expenditures, cost considerations could unduly influence the sensitive communication process, thus jeopardising informed consent and patient autonomy.14 We have therefore added a section on the ethical implications because we consider it essential to openly address these ethical tensions – for the sake of patient autonomy.
Methods
We first developed an informal review protocol that was based on the Preferred Reporting Items For Systematic Reviews And Meta-Analyses (PRISMA) statement recommendations.34
Inclusion criteria
In accordance with the patients, interventions, comparators, outcomes, study design (PICOS) approach, we used the following inclusion criteria. Patients: We included all patient groups. Interventions: A preliminary literature search revealed that hardly any studies had assessed the impact on costs of comprehensive ACP programmes with both a facilitated communication process and a systematic community implementation as described above. We therefore decided to broaden the inclusion criterion to any intervention containing the first element of comprehensive ACP interventions: a communication process facilitated by a professional caregiver involving the patient and/or legal proxy about the patient’s preferences for future medical care. Comparators: We accepted any intervention as comparator. Outcomes: We included all studies that assessed healthcare costs or cost-effectiveness as primary or secondary outcome measures. We excluded studies investigating other endpoints like hospitalisation rates or days spent in the intensive care unit (ICU) as mere indicators for cost reductions because such studies do not provide an account of the net resource use resulting from ACP interventions. Study design: We included all original empirical studies on cost effects of ACP. We did not restrict our search to prospective controlled intervention studies to give a full overview of all studies conducted so far.
Search strategy and data sources
Our search strategy comprised terms describing the intervention (ACP and its synonyms) and the outcome (costs and synonyms) linked by the Boolean operator ‘and’. The search strategy for PubMed is described in Table 1 and was adapted to the specificities of each database. We included studies published in English or German. Our search was conducted in April 2013. In addition to PubMed, we searched NHS EED, EURONHEED, the Cochrane Library and EconLit, as recommended.35 We also screened the references of relevant articles we identified prior to and during the literature search.
Table 1.
Search strategy in PubMed.
| Search strategy in PubMed | |
|---|---|
| Intervention | 1. (MeSH terms): Advance Care Planning (due to automatic explosion includes advance directives and living will) OR resuscitation order |
| 2. (Title/abstract): resuscitation order* OR advance directive* OR advanced directive* OR advance care plan* OR advanced care plan* OR living will* OR end-of-life decision* OR end-of-life conversation* OR end-of-life discussion* | |
| 3. (1 OR 2) | |
| Outcome | 4. (MeSH terms): costs and cost analysis OR economics, hospital OR economics, medical |
| 5. (MeSH subheading): economics | |
| 6. (Title/abstract): cost* OR price* OR economic* OR resource* OR efficien* | |
| 7. (4 OR 5 OR 6) | |
| 8. (3 AND 7) | |
Study selection
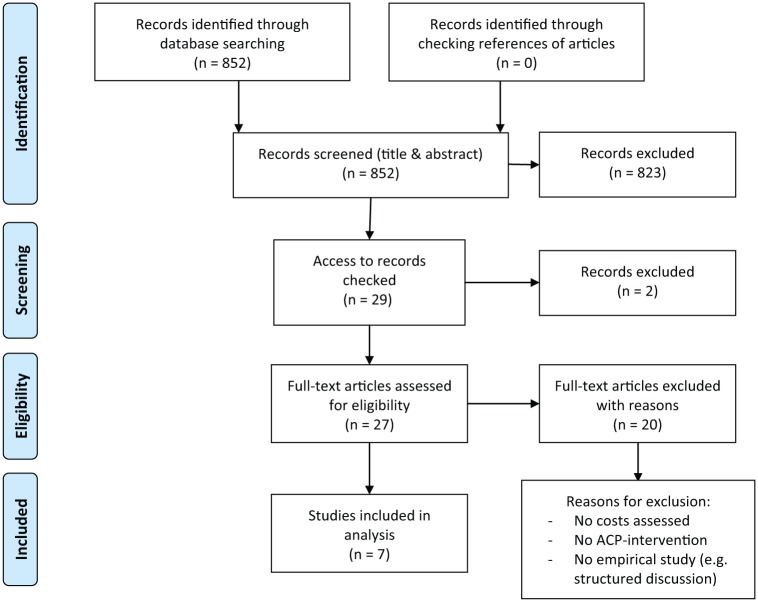
Based on the inclusion criteria, two reviewers screened titles and abstracts of all articles independently. In case of disagreement, the inclusion was decided consensually within the research team. We then sought access via the local university and the Bavarian State Library for the articles that were considered potentially relevant after the first screening. The Bavarian State Library provides access to one of the most comprehensive online international journal collections as well as print media available in Germany. Authors were also contacted when necessary. The full texts were screened by G.M. and J.i.d.S. independently, and disagreement was resolved consensually. The screening and inclusion process is depicted in a flowchart as recommended by the PRISMA statement (see Figure 1).
Figure 1.
PRISMA 2009 flow diagram depicting the screening and inclusion process.
Source: Adapted from Moher et al.34
Data extraction and analysis
A data extraction tabloid was developed and tested. We extracted information on the following characteristics: study design, participants, intervention and comparator, outcome measure used, further effects measured, results and conclusion, and further information like funding source. C.K. extracted the data from studies meeting inclusion criteria and assessed study quality, while G.M. reviewed her decisions. We considered data pooling in a meta-analysis inappropriate due to study heterogeneity in design, intervention, setting and outcome measures. We therefore preferred a narrative synthesis to describe the study results.
Results
The electronic database search yielded 852 potentially relevant articles. Based on the inclusion criteria, we identified seven empirical studies assessing the costs of care with ACP in comparison with standard care, the first published in 1994, the last in 2010 (see Table 2 for an overview of all studies). So far, no cost-effectiveness study on ACP has been published.
Table 2.
Overview of included studies.
| Study | Study design | Participants/setting | Further patient characteristicsa | Intervention/comparator | Cost components measured | Results: costs over observation period (per patient) | Results: other effects | Study qualityb |
|---|---|---|---|---|---|---|---|---|
| Chambers et al.36 | Retrospective cohort study | 474 Medicare decedents in a large US hospital | Average age: 73 yearsGender: 53% male and 47% femaleEthnicity: 77% White, 19% Black and 4% otherReligion: 38% Catholic, 39% Protestant, 16% Jewish, 5% other and 2% none | Advance planning was assumed when discussion with patient about AD was documented in medical record | Total inpatient healthcare charges during last hospitalisation | Mean costs with AD discussion: USD30,478Mean cost without AD discussion: USD95,305Reduction: USD64,827 (68%) (p not given) | No further effects measured | III |
| Edes et al.37 | Before-after study | 43 US veterans suffering from end-stage heart and/or lung disease and being cared for at home | Average age: 75 yearsGender: 100% maleEthnicity: 97% White and 3% other | Intervention: advance directive discussion conducted by nurse/social worker as part of a home-based primary care programmeComparator: standard care received before | Inpatient and outpatient care costs (including ACP intervention costs) incurred 6 months before and 6 months after intervention start | Median costs before enrolment: USD16,750Median costs after enrolment: USD5511Reduction: USD11,239 (67%) (significance and p not reported) | Patient satisfaction improved; reduction of hospital days (p = 0.0019) | II |
| Engelhardt et al.38 | Randomised controlled trial | US Veterans Affairs medical centres; 275 patients suffering from COPD, CHF, cancer | Average age: 71 yearsGender: 79% male and 21% femaleEthnicity: 87% White, 11% Black and 2% otherReligion: 54% Catholic, 39% Protestant, 2% Jewish and 5% other | Intervention: care coordinators assisted in formulating and documenting ADs as part of intervention to improve care coordinationComparator: usual care | Inpatient, outpatient, nursing home, inpatient hospice and ‘other’ care cost (time frame for measurement unclear) | Mean costs intervention group: USD12,123Mean costs in control group: USD16,295Reduction: USD4172 (25.6%) (not significant, p not reported) | Patients and surrogates more satisfied with care, more ADs completed (overall and per patient), less days until completion of AD, no difference in survival rates | I |
| Hamlet et al.39 | Secondary analysis of data from randomised controlled study | 4742 US Medicare decedents that suffered from diabetes and/or heart failure with high risk of death | Average age: 77 yearsGender: 52% male and 48% femaleEthnicity: 79% White, 20% Black and 1% other | Intervention: telephonic EOL counselling with trained nurses given alongside a chronic care management interventionComparator: usual care | All Medicare claims incurred during the 6 months prior to death except those incurred after hospice enrolment | Mean adjusted costs intervention group: USD40,363Mean adjusted costs control group: USD42,276Reduction: USD1913 (4.5%) (p = 0.05) | No effect on hospice admission or length of stay in hospice | I |
| Molloy et al.40 | Randomised controlled trial | Six nursing homes with 1292 residents (1133 agreed to participate) in Canada | Average age: 83 yearsGender: 26% male and 74% femaleEthnicity: 97% White and 3% other | Intervention: education about ADs facilitated by specifically trained nurses, offer of AD and system interventionComparator: standard care | Hospitalisation, nursing home drug and programme implementation costs over 18 months | Mean costs intervention homes: CAD3490Mean costs control homes: CAD5239Reduction: CAD1748 (33.4%) (p = 0.013) | No effect on resident and family satisfaction, less hospitalisations/days spent in hospital in intervention group, similar death rates | I |
| SUPPORT41 | Cluster-randomised controlled trial | 4804 US teaching hospital patients with serious illnesses | Average age: 65 yearsGender: 56% male and 44% femaleEthnicity: 79% White, 16% Black and 5% other | Intervention: trained nurses elicited and documented patient and family preferences/ADs as part of an intervention to improve communication and decision-makingComparator: usual care | Patients’ hospital charges during hospital stays | Median cost estimates given only for major disease categories (e.g. advanced cancer → Intervention: USD6100; Control: USD5100)Overall no impact on costs (adjusted ratio: 1.05) | No effect on incidence and timing of written DNR orders, physicians’ awareness of patients’ preferences, level of pain, days spent in ICU, coma, or receiving mechanical ventilation | I |
| Zhang et al.42 | Prospective cohort study | 627 US hospital patients with advanced cancer | Average age: 59 yearsGender: 51% male and 49% femaleEthnicity: 71% White, 15% Black, 12% Hispanic and 2% otherReligion: 43% Catholic, 19% Protestant, 3% Jewish, 11% Baptist, 17% other and 5% none | Advance planning was assumed when patients reported EOL discussion with physician. Controls reported no EOL discussion. | Costs for hospital stays and hospice use in the last week of life | Mean costs with EOL discussion: USD1876Mean cost without EOL discussion: USD2917Reduction: USD1041 (37.5%) (p = 0.002) | Intervention group experienced less physical distress, less ventilations, resuscitations or ICU admissions, more outpatient hospice care and longer stays in outpatient hospice; no difference in survival rates, psychological distress, quality of death, chemotherapy, and inpatient hospice services utilised | III |
AD: advance directive; ACP: Advance Care Planning; COPD: chronic obstructive pulmonary disease; CHF: congestive heart failure; SUPPORT: Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments; DNR: do-not-resuscitate; ICU: intensive care unit; EOL: end of life.
Only information that was presented in all studies is given here with the exception of religion as a potentially influential factor for EOL decision-making.
The study quality was assessed in levels of evidence ranging from I (randomised controlled trials) over II (other interventional studies) to III (observational studies).
Study quality
The seven studies varied considerably regarding study design, setting and participants, details of the intervention and cost components included in the assessment. Four studies used a randomised controlled trial design.38–41 The other three studies were more prone to risk of bias: One study used a before-after design37 and two used a cohort design (one prospective42 and one retrospective36).
Study setting and participants
The studies were conducted in quite different settings: six in the United States and one in Ontario, Canada.40 Three studies were performed in hospitals,36,41,42 one in nursing homes40 and three in home care settings.37–39 The studies were performed with different patient populations. Zhang et al. included patients suffering from advanced cancer (mostly lung, colorectal, breast and different gastro-intestinal cancers), Edes et al. included patients with chronic obstructive pulmonary disease (COPD) and congestive heart failure (CHF) and Engelhardt et al. included COPD, CHF and cancer patients. The Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT) study included patients suffering from one of nine life-threatening diseases, while Hamlet et al. included patients with heart failure and/or severe diabetes at high risk of death as identified by an EOL predictive model. The other two studies did not limit their analysis to certain diagnostic groups. However, the largest classes of admitting diagnoses during last hospitalisation found by Chambers et al. were malignant neoplasms, respiratory diseases and cardiopulmonary diseases. See Table 2 for further information on patient characteristics.
ACP intervention administered
The ACP interventions varied considerably among the seven studies and were often not described in sufficient detail. Some studies did not use the term ACP but rather talked about EOL discussion,42 discussion of ADs36,37 or EOL counselling.39 Only Molloy et al. implemented a comprehensive ACP programme (while calling it an ‘advance directive programme’): In addition to facilitating EOL conversations, the project team educated the staff in the local hospitals and nursing homes about ADs and implemented routines for archiving and transferring the ADs across care settings. In all other studies, facilitated discussions of EOL issues were a core element of the ACP intervention without a systematic regional implementation. Nurses, nurse practitioners or social workers supported the discussions about EOL care and ADs. Most of them were specifically trained for this task;38–41 however, the content of the training sessions was not specified. In one study, the nurses conducted the EOL counselling via telephone,39 while in all other studies the advance planning conversations were led face to face. None of the papers clearly specified content, length and style of the conversations; often it was deliberately left open to the facilitators how to conduct the conversations.
In four studies, ACP was part of a more comprehensive intervention to improve EOL care.37–39,41 The two observational studies used indicators for the communication process as they did not implement the intervention themselves. Chambers et al. assumed the presence of EOL conversations if a discussion of the patient’s AD within the first 48 h after admission had been documented. Zhang et al. asked the patients whether they had discussions with their physicians about EOL care.
Cost data analysed
The cost components included in the calculations also varied considerably between the seven studies. None of the studies assessed overall costs for the healthcare system. Costs accruing outside the healthcare system to family and friends from providing care themselves were also never included in the calculations. The most comprehensive cost assessment was performed by Engelhardt et al. who gathered cost data on inpatient, outpatient, nursing home and inpatient hospice care as well as other cost components (e.g. diagnostic services, prosthetics and administrative overhead). Zhang et al. only included costs for hospitalisation, hospice care and life-sustaining procedures thereby excluding costs, for example, for outpatient or home care. Edes et al. incorporated costs of inpatient and outpatient care including allied health and ancillary services, but excluded costs for medication. Hamlet et al. included all Medicare costs, but did not collect cost data incurred after hospice enrolment. The SUPPORT study as well as Chambers et al. focused on inpatient costs ignoring outpatient and other care costs. Molloy et al. collected data on hospitalisation and nursing home drug costs and included the programme costs, but did not consider costs of nursing home care and outpatient physician services.
Only three studies included the costs of the intervention in their calculation,37,38,40 thus allowing an assessment of the net cost savings. It is often not clearly stated whether all costs or only costs arising for the payer/insurer were incorporated in the calculation with the exception of Zhang et al. and Hamlet et al. who chose the payer’s perspective. The time frames for the cost measurement also differed considerably ranging from 1 week before death42 over 6 months37,39 to 18 months40 after implementing the intervention. Engelhardt et al. do not explicitly report the time frame of their cost calculation, while Chambers et al. and the SUPPORT study calculated the costs during one hospital stay. Furthermore, the studies used different approaches for calculating the included cost components.
Effects of ACP on costs
Except for the SUPPORT trial, all studies found reduced costs through the professionally facilitated discussions about future medical care, three of them statistically significant (p-value ⩽ 0.05).39,40,42 One study showed a non-significant gross cost reduction,38 while the remaining two studies did not report whether the cost reductions reached statistical significance. Chambers et al. found the relationship between costs and AD discussions to be statistically significant in the logistic regression.
The reported cost reductions range from USD1041 to USD64,827 per patient. These figures cannot be compared (let alone pooled in a meta-analysis), however, because the studies differ substantially with respect to the costs included in the calculation, the time frame of analysis and the patient population, setting and intervention chosen for the respective study. Yet, an overall appraisal of these figures and their respective frames of reference indicates that cost savings of ACP programmes may be substantial: The relative cost reduction ranged from 68%36 to 5%.39 The two highest cost reductions, both absolute and relative, were achieved in rather sick patient populations who used a considerable amount of hospital care.36,37 Only three studies reported the costs of implementing facilitated ACP conversations. In the study of Edes et al., programme costs amounted to USD1968 per patient with a net saving of USD11,239 per patient over 6 months. Molloy et al. found a net cost reduction of CAD1748 per resident with programme implementation costs of CAD113 per resident. In the study of Engelhardt et al., the programme costs were USD452 per case compared to gross savings of USD4172 per patient.
Discussion
Key findings
To our knowledge, this is the first systematic review evaluating the cost implications of ACP, operationalised as at least comprising one professionally facilitated conversation about individuals’ preferences for future medical care. While traditional ADs, merely understood as the presence of a signed legal document, have not been found to reduce costs consistently,12,21,23,31 six of the seven studies included in this review demonstrated cost savings through ACP ranging from USD1041 to USD64,830 per patient, depending on the study period and the cost measurement. Programme implementation costs were small compared to savings realised, amounting to 6%, 11% and 15% of gross savings. However, the results of this review have to be interpreted with caution as the studies varied considerably with respect to study design, ACP intervention, setting, patient selection and cost components included. None of the studies provided a comprehensive cost-effectiveness analysis of ACP.
Relation between effectiveness and cost impact of ACP
Can we conclude that ACP programmes with qualified facilitation reduce costs? Not from the SUPPORT study. However, leaving aside that SUPPORT estimated costs using an algorithm based on prior research which might be less reliable, another observation warrants some thoughts: The SUPPORT study was disappointingly ineffective in reaching any of its goals (like increasing the completion of ADs or improving physicians’ understanding of patient preferences). The other six studies were more successful on all outcome parameters, not just costs. It seems evident that an intervention which is ineffective in reaching its primary goals cannot lead to any cost reductions. But why did the SUPPORT study fail? A potential explanation could be the lack of standards for the ACP intervention: content and style of communication, training of facilitators and essential components of a regional implementation all remained vague. Apparently, the details of the ACP programme’s structure and realisation matter: We need to understand better, therefore, which specific elements make ACP programmes effective in eliciting and respecting patients’ treatment preferences and consequently might lead to cost reductions.
In most studies, however, ACP was part of a more comprehensive intervention to improve communication and EOL care. It therefore remains unclear which part of the intervention was responsible for the observed cost reductions. Only one intervention study40 and the two observational studies36,42 assessed the specific cost effects of ACP in isolation and are therefore able to attribute the observed cost reductions to the ACP intervention. The evidence, however, is limited by a small sample size40 or by the observational study design and the inability to control the intervention.36,42 In addition, the two observational studies did not include the costs for ACP, and it is therefore unclear whether the savings at the EOL can offset costs for EOL conversations, although the cost savings in Chambers et al.’s study are big enough to assume that they would exceed any costs for communicative interventions. The strengths of Molloy et al.’s study are the randomised controlled design and the comprehensive cost assessment, although they apparently excluded outpatient physician costs. Overall, the evidence base remains limited and no final conclusion can be drawn regarding the cost implications of ACP; especially Molloy et al.’s study, however, seems to justify the hypothesis that ACP may be able to reduce costs of care at the EOL.
Limitations of the review and generalisability of results
Our review has several limitations. First, it was difficult to identify ACP programmes because there is no established common understanding of ‘ACP’. On the one hand, the term ‘ACP’ referred to a wide spectrum of phenomena ranging from merely signing AD documents to fully fletched ACP programmes with a regionally implemented, professionally facilitated communication process. On the other hand, the ACP elements were often integrated into a wide variety of communicative interventions with different overriding goals. We tried to be rather broad in our search strategy, but still may not have captured all relevant articles. Furthermore, we may have missed potentially interesting studies due to our limitation to articles published in English or German. Finally, we were not able to access two potentially relevant articles43,44 for full-text screening.
With respect to the generalisability of the results, it can be questioned whether the observed effects can be replicated in other settings. The observed cost savings of ACP may depend on the structure of the healthcare systems and the cultural background which determines personal preferences and values: If individuals, for example, request all possible life-sustaining treatments for religious reasons, ACP will not reduce costs of care. Most studies were conducted in the United States with an overall high level of healthcare spending and an individualistic culture. It therefore remains unclear whether the same effects could be observed in national healthcare systems with lower overall healthcare expenditures or other cultural preferences. This is supported by another study,45 which found that ADs are associated with lower levels of Medicare spending only in regions with comparatively high levels of EOL expenditures. In regions where expenditures were already low, ADs seemed to have no effect on healthcare costs.
Implications for future research
First, the cost effects of ACP should be studied in other settings outside the United States or Canada, especially in healthcare systems with lower levels of spending and stricter cost-control policies. In addition, methodologically robust randomised controlled intervention trials of ACP are necessary that employ a comprehensive, validated ACP approach (including facilitated EOL conversations and a regional implementation) to accurately assess programme costs, cost savings and clinical outcomes (e.g. efficacy). It might be difficult to estimate the full impact of ACP on costs because cost reductions may be realised in one healthcare sector (e.g. intensive care) while additional costs arise in another sector (e.g. hospice care and nursing homes), but efforts should be made to make those effects visible in future studies evaluating costs and returns of ACP.
Furthermore, the reporting of such studies has to be improved: Questions that need to be addressed are:46,47 Which perspective is chosen (societal, healthcare system, payer, etc.)? What cost components are included in the analysis? How are costs calculated (measurement of resource consumption, estimation of prices)? Were adjustments made (e.g. for market imperfections)? Consolidated reporting standards for health economic evaluations have been published in 2013 and give helpful guidance to future authors.48 Likewise, the ACP interventions should be explicitly defined and described in sufficient detail to allow an assessment of which elements determine ACP effectiveness.
Ethical considerations
This review provides preliminary empirical evidence that ACP programmes with facilitated EOL discussions may offset their costs or even produce net cost savings near the EOL. It therefore might appear an attractive tool for payers and policy makers at different levels of the healthcare system. From an ethical standpoint, however, it is questionable whether reducing costs of care should be a reason for implementing ACP interventions. At the surface, this does not appear to be a problem, on the contrary: Where else in highly developed healthcare systems can we strengthen patient autonomy, improve quality of care and save a considerable amount of resources at the same time? It seems that the cost argument just strengthens the case for implementing ACP programmes.
Risks for patient autonomy and patient-centred care
At a closer look, however, the relationship between ACP and costs of care is much more delicate involving a considerable potential for ethical conflict. ACP programmes will only reduce healthcare expenditures if patients opt for limiting life-sustaining treatment. Consequently, there is no firm and systematic link between ACP and net cost savings. Under economic pressure, the primary goal of ACP – promoting patient-centred care near the EOL – could therefore compete with the goal of reducing healthcare expenditures. Facilitators might be incentivised to advise individuals to choose less invasive and therefore less costly treatment in their ADs. At the same time, facilitators employed by a certain institution (e.g. a nursing home or a hospital) could be tempted to push individuals towards advance treatment decisions that are likely to increase the revenues of their respective institution. These conflicts of interest jeopardise the openness of the communication process as individuals may be unduly influenced to choose more limited or more extensive care than truly desired.14 A proliferating suspicion of biased ACP conversations will erode patient trust in the process and prompt fears of undertreatment which could eventually undermine the efforts to introduce ACP into regular health care.32,49,50
Managing the risks
In light of these concerns, ACP must remain an instrument to ensure that patients’ wishes are honoured reliably when they have lost decision-making capacity, irrespective of the effects on the costs of care. However, costs of care are an important driver for payers and policy makers, and it would therefore be naïve not to take into account that cost containment may also be a reason for implementing ACP. In Australia, for example, ACP in residential homes for the elderly was funded in order to reduce hospital days for acute care.33 Policy makers should therefore openly acknowledge the potential interference with patient autonomy and introduce safeguards to ensure the openness of the ACP process:32,49,50 (1) make programme objectives and potential conflicts of interest transparent, (2) ensure adequate training and supervision of facilitators, (3) establish clearly defined standards for the facilitation process to guarantee its high quality and (4) educate facilitators to identify and manage conflicts of interest.
Conclusion
The studies included in this review allow only preliminary conclusions regarding the cost implications of ACP because of poorly defined and heterogeneous interventions and incomplete cost assessments. The available evidence, however, indicates that ACP may reduce net health expenditures – despite the costs of implementation and maintenance. Methodologically rigorous prospective intervention trials are necessary to produce more reliable evidence about the cost implications of ACP and to identify those ACP elements that are essential for improving clinical outcomes and possibly reducing net healthcare expenditures. The ethical tension between the primary goal of ACP, promoting patient autonomy and patient-centred care, and the goal of reducing costs of care requires certain safeguards – above all ensuring the quality of the facilitation process. It therefore should be a priority to develop evidence-based standards for comprehensive ACP programmes that effectively align the care delivered near the EOL with patients’ preferences.
Acknowledgments
The authors wish to thank Katja Kühlmeyer, Galia Assadi, Sabine Petri, Marion Frobenius and Christine Marhold for their help in screening the literature. They also sincerely thank Julia Lotz for her help in building the search strategy and Bernd Richter for valuable feedback on a draft of the manuscript.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
References
- 1. Polder JJ, Barendregt JJ, van Oers H. Health care costs in the last year of life–the Dutch experience. Soc Sci Med 2006; 63: 1720–1731. [DOI] [PubMed] [Google Scholar]
- 2. Hoover DR, Crystal S, Kumar R, et al. Medical expenditures during the last year of life: findings from the 1992–1996 Medicare current beneficiary survey. Health Serv Res 2002; 37: 1625–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zweifel P, Felder S, Meiers M. Ageing of population and health care expenditure: a red herring? Health Econ 1999; 8: 485–496. [DOI] [PubMed] [Google Scholar]
- 4. Seshamani M, Gray AM. A longitudinal study of the effects of age and time to death on hospital costs. J Health Econ 2004; 23: 217–235. [DOI] [PubMed] [Google Scholar]
- 5. Riley GF, Lubitz JD. Long-term trends in Medicare payments in the last year of life. Health Serv Res 2010; 45: 565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Skinner J, Chandra A, Goodman D, et al. The elusive connection between health care spending and quality. Health Aff 2009; 28: w119–w123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yasaitis L, Fisher ES, Skinner JS, et al. Hospital quality and intensity of spending: is there an association? Health Aff 2009; 28: w566–w572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Teno JM, Fisher ES, Hamel MB, et al. Medical care inconsistent with patients’ treatment goals: association with 1-year Medicare resource use and survival. J Am Geriatr Soc 2002; 50: 496–500. [DOI] [PubMed] [Google Scholar]
- 9. van der Heide A, Deliens L, Faisst K, et al. End-of-life decision-making in six European countries: descriptive study. Lancet 2003; 362: 345–350. [DOI] [PubMed] [Google Scholar]
- 10. Silveira MJ, Kim SY, Langa KM. Advance directives and outcomes of surrogate decision making before death. N Engl J Med 2010; 362: 1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kelley AS, Ettner SL, Morrison RS, et al. Determinants of medical expenditures in the last 6 months of life. Ann Intern Med 2011; 154: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Emanuel EJ, Emanuel LL. The economics of dying. The illusion of cost savings at the end of life. N Engl J Med 1994; 330: 540–544. [DOI] [PubMed] [Google Scholar]
- 13. Emanuel EJ. Cost savings at the end of life. What do the data show? JAMA 1996; 275: 1907–1914. [PubMed] [Google Scholar]
- 14. Levinsky NG. The purpose of advance medical planning–autonomy for patients or limitation of care? N Engl J Med 1996; 335: 741–743. [DOI] [PubMed] [Google Scholar]
- 15. Halpern SD, Emanuel EJ. Advance directives and cost savings: greater clarity and perpetual confusion. Arch Intern Med 2012; 172: 266–268. [DOI] [PubMed] [Google Scholar]
- 16. Fagerlin A, Schneider CE. Enough. The failure of the living will. Hastings Cent Rep 2004; 34: 30–42. [PubMed] [Google Scholar]
- 17. Perkins HS. Controlling death: the false promise of advance directives. Ann Intern Med 2007; 147: 51–57. [DOI] [PubMed] [Google Scholar]
- 18. Castillo LS, Williams BA, Hooper SM, et al. Lost in translation: the unintended consequences of advance directive law on clinical care. Ann Intern Med 2011; 154: 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sommer S, Marckmann G, Pentzek M, et al. Advance directives in nursing homes: prevalence, validity, significance, and nursing staff adherence. Dtsch Arztebl Int 2012; 109: 577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Teno JM, Gruneir A, Schwartz Z, et al. Association between advance directives and quality of end-of-life care: a national study. J Am Geriatr Soc 2007; 55: 189–194. [DOI] [PubMed] [Google Scholar]
- 21. Schneiderman LJ, Kronick R, Kaplan RM, et al. Effects of offering advance directives on medical treatments and costs. Ann Intern Med 1992; 117: 599–606. [DOI] [PubMed] [Google Scholar]
- 22. Teno J, Lynn J, Connors AF, Jr, et al. The illusion of end-of-life resource savings with advance directives. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment. J Am Geriatr Soc 1997; 45: 513–518. [DOI] [PubMed] [Google Scholar]
- 23. Taylor JS, Heyland DK, Taylor SJ. How advance directives affect hospital resource use. Systematic review of the literature. Can Fam Physician 1999; 45: 2408–2413. [PMC free article] [PubMed] [Google Scholar]
- 24. Singer PA, Robertson G, Roy DJ. Bioethics for clinicians: 6. Advance care planning. CMAJ 1996; 155: 1689–1692. [PMC free article] [PubMed] [Google Scholar]
- 25. Hammes BJ, Rooney BL. Death and end-of-life planning in one midwestern community. Arch Intern Med 1998; 158: 383–390. [DOI] [PubMed] [Google Scholar]
- 26. in der Schmitten J, Lex K, Mellert C, et al. Implementing an advance care planning program in German nursing homes: results of an inter-regionally controlled intervention trial. Dtsch Arztebl Int 2014; 111: 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hammes BJ, Rooney BL, Gundrum JD. A comparative, retrospective, observational study of the prevalence, availability, and specificity of advance care plans in a county that implemented an advance care planning microsystem. J Am Geriatr Soc 2010; 58: 1249–1255. [DOI] [PubMed] [Google Scholar]
- 28. Detering KM, Hancock AD, Reade MC, et al. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ 2010; 340: c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brinkman-Stoppelenburg A, Rietjens JA, van der Heide A. The effects of advance care planning on end-of-life care: a systematic review. Palliat Med 2014; 28: 1000–1025. [DOI] [PubMed] [Google Scholar]
- 30. Houben CH, Spruit MA, Groenen MT, et al. Efficacy of advance care planning: a systematic review and meta-analysis. J Am Med Dir Assoc 2014; 15: 477–489. [DOI] [PubMed] [Google Scholar]
- 31. Dixon J, Matosevic T, Knapp M. The economic evidence for advance care planning: systematic review of evidence. Palliat Med. Epub ahead of print 9 June 2015. DOI: 10.1177/0269216315586659. [DOI] [PubMed] [Google Scholar]
- 32. Billings JA. The need for safeguards in advance care planning. J Gen Intern Med 2012; 27: 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Robins-Browne K. Why we need to acknowledge the multiple aims of advance care planning. Hastings Cent Rep 2014; 44: 3. [DOI] [PubMed] [Google Scholar]
- 34. Moher D, Liberati A, Tetzlaff J, et al. ; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–269, w64. [DOI] [PubMed] [Google Scholar]
- 35. Shemilt I, Mugford M, Byford S, et al. Chapter 15. Incorporating economics evidence. In: Higgins J, Green S. (eds) Cochrane handbook for systematic reviews of interventions. Chichester: John Wiley & Sons, 2008, p. 449–479. [Google Scholar]
- 36. Chambers CV, Diamond JJ, Perkel RL, et al. Relationship of advance directives to hospital charges in a Medicare population. Arch Intern Med 1994; 154: 541–547. [PubMed] [Google Scholar]
- 37. Edes TE, Lindbloom EJ, Deal JL, et al. Improving care at lower cost for end-stage heart and lung disease: integrating end of life planning with home care. Mo Med 2006; 103: 146–151. [PubMed] [Google Scholar]
- 38. Engelhardt JB, McClive-Reed KP, Toseland RW, et al. Effects of a program for coordinated care of advanced illness on patients, surrogates, and healthcare costs: a randomized trial. Am J Manag Care 2006; 12: 93–100. [PubMed] [Google Scholar]
- 39. Hamlet KS, Hobgood A, Hamar GB, et al. Impact of predictive model-directed end-of-life counseling for Medicare beneficiaries. Am J Manag Care 2010; 16: 379–384. [PubMed] [Google Scholar]
- 40. Molloy DW, Guyatt GH, Russo R, et al. Systematic implementation of an advance directive program in nursing homes: a randomized controlled trial. JAMA 2000; 283: 1437–1444. [DOI] [PubMed] [Google Scholar]
- 41. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). The SUPPORT Principal Investigators. JAMA 1995; 274: 1591–1598. [PubMed] [Google Scholar]
- 42. Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med 2009; 169: 480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Renick O, Hanson S, Nelson M. Advance patient directives: are they worth it? Med Interface 1994; 7: 149–152. [PubMed] [Google Scholar]
- 44. Improved end-of-life care proves worthwhile, even at higher cost. Exec Solut Healthc Manag 1998; 1: 17–19. [PubMed] [Google Scholar]
- 45. Nicholas LH, Langa KM, Iwashyna TJ, et al. Regional variation in the association between advance directives and end-of-life Medicare expenditures. JAMA 2011; 306: 1447–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brouwer W, Rutten F, Koopmanschap M. Costing in economic evaluations. In: Drummond M, McGuire A. (eds) Economic evaluation in health care: merging theory with practice. Oxford; New York: Oxford University Press, 2001, pp. 68–93. [Google Scholar]
- 47. Drummond M, Sculpher MJ, Torrance GW, et al. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford; New York: Oxford University Press, 2005. [Google Scholar]
- 48. Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Int J Technol Assess Health Care 2013; 29: 117–122. [DOI] [PubMed] [Google Scholar]
- 49. Stark M, Fins JJ. What’s not being shared in shared decision-making? Hastings Cent Rep 2013; 43: 13–16. [DOI] [PubMed] [Google Scholar]
- 50. Piemonte NM, Hermer L. Avoiding a ‘death panel’ redux. Hastings Cent Rep 2013; 43: 20–28. [DOI] [PubMed] [Google Scholar]