Abstract
Background
Comorbidities have been shown to play an important role in prognostic assessment of several hematologic conditions; however, the role of comorbidities in primary myelofibrosis has not been studied. The aim of our study was to evaluate the prevalence and impact of comorbidities in patients with primary myelofibrosis (PMF) using the Adult Comorbidity Evaluation-27 (ACE-27).
Methods
In this retrospective observational cohort study, we evaluated 349 consecutive patients with a confirmed diagnosis of PMF who presented to our institution from 2000 to 2008. We evaluated the frequency and severity of comorbidities in these patients and assessed their impact on survival in a bivariable model that included the ACE-27 and Dynamic International Prognostic Scoring System (DIPSS) scores as covariates.
Results
Sixty-four percent of patients had at least one comorbid condition, and diseases of the cardiovascular system (63%) were most common. Comorbidities had a significant negative impact on survival (P < 0.001). Patients with severe comorbidities had twice the risk of death as those with no comorbidities. When stratified by demographic and clinical characteristics, comorbidities were significantly associated with worse survival in patients younger than 65 years (P < 0.001) and those with performance status < 1 (P < 0.001). In a multivariable model that included the ACE-27 and Dynamic-International Prognostic Scoring System scores, comorbidities retained a significant association with shorter survival (P ≤ 0.001).
Conclusions
Assessment of comorbid conditions in patients with PMF, particularly those who are younger and with good performance status, has important implications for overall prognosis and treatment planning.
Introduction
Primary myelofibrosis (PMF), the most aggressive of the BCR-ABL1-negative myeloproliferative neoplasms is characterized by extramedullary hematopoiesis, progressive cytopenias, and bone marrow fibrosis.1 Typical clinical manifestations include progressive anemia and splenomegaly, leading to several debilitating symptoms, including fatigue, night sweats, itching, loss of appetite, and bone pain.2 The International Prognostic Scoring System (IPSS),3 developed by the International Working Group for MPN Research and Treatment, and the Dynamic-IPSS (D-IPSS),4 which allows assessment of patients at any time during their clinical course, are the main tools used for prognostic and treatment planning in patients with PMF. Both scores stratify patients into four risk categories based on the presence of five prognostic factors: age > 65 years, presence of constitutional symptoms, hemoglobin < 10 g/dL, white blood cell count >25 × 109/L, and ≥ 1% peripheral blood blasts. Average overall survival (OS) for patients classified as low-risk is approximately 11 years, while those classified as high-risk disease have much worse prognosis (median survival < 2 years).3 Importantly, most patients with PMF present in the fifth and sixth decade of life (median age at presentation is 67 years)5 and often have existing comorbid conditions, which is not surprising given that the incidence of comorbidities increases with age.6
Comorbidity is a well-known independent prognostic factor for patients with cancer that negatively affects overall survival.7 Furthermore, the presence of comorbid diseases can also have an impact on detection and diagnosis of cancer, treatment decisions, and assessment of outcomes in studies of novel therapies.8, 9 Several studies have evaluated the impact of comorbidities on patients with solid tumors, including prostate,10 colon,11 head and neck,12 and breast cancer.13 More recently, evaluation of comorbidities as part of prognostic risk assessment has been shown to improve risk stratification in patients with myelodysplastic syndromes.14–16 Given that patients with PMF are generally older and often have several comorbidities, including comorbidities as part of the prognostic assessment may be useful. However, neither the IPSS nor the D-IPSS includes comorbidities as part of the assessment. Furthermore, as treatment options for patients with PMF have expanded in recent years with the development of JAK2 inhibitors and other targeted therapies, including comorbidities as part of the prognostic assessment may allow better treatment planning and ultimately improve outcomes.8, 9
Thus, the aim of our study was to evaluate the prevalence and impact of comorbidities in patients with PMF using the Adult Comorbidity Evaluation-27 (ACE-27) and to determine whether combining information on comorbidities with standard D-IPSS risk score can improve predictions about prognosis.
Methods
Patients
We performed a retrospective chart review of 349 consecutive patients with a confirmed diagnosis of PMF who presented to The University of Texas MD Anderson Cancer Center from 2000 to 2008. Diagnosis of PMF was confirmed using the 2008 World Health Organization criteria.1 We restricted our cohort to patients who presented before the general availability of ruxolitinib, which was approved by the US Food and Drug Administration for MF in 2011, to minimize the confounding effects of treatment on overall survival. With the exception of stem-cell transplantation, which is used in fewer than 10% of patients, standard treatment for MF before the advent of ruxolitinib has had no effect on survival and thus, treatment was not considered in our analysis. The ACE-27 form, a validated 27-item comorbidity index for cancer patients,7 was used to assess the presence and severity of comorbid conditions in these patients at the time of presentation to MD Anderson. A comorbid condition was defined as one that had been diagnosed before the patient presented to MD Anderson. The ACE-27, which is derived from the Kaplan-Feinstein comorbidity index,17 grades each comorbid disease and condition into 1 of 3 levels according to the severity of individual organ decompensation and prognostic impact: grade 1 (mild), grade 2 (moderate), or grade 3 (severe). For each patient, an overall comorbidity score (none=0, mild=1, moderate=2, or severe=3) is then assigned based on the highest-ranked single comorbidity. For patients with 2 or more moderate comorbid conditions occurring in different organ systems or disease groupings, the overall score is designated as severe. Patients were also assigned a D-IPSS score,4 based on the presence of risk factors including age > 65 years, presence of constitutional symptoms, hemoglobin < 10 g/dL, white blood cell count >25 × 109/L, and ≥ 1% peripheral blood blasts. Clinical parameters (blood cell counts, laboratory values, and disease-related symptoms) and demographic information (age, gender, and race) were collected from the patients’ charts. This study was based on a chart review protocol approved by the Institutional Review Board at MD Anderson Cancer Center.
Statistical Analyses
The primary endpoint of the study was OS, which was calculated from the date of presentation until death from any cause. Patients were censored on the date of last follow-up. Kaplan-Meier analysis was used to determine median survival, and association of clinical and demographic variables with survival was performed using univariate and multivariate Cox proportional hazard regression. Prior to the development of the multivariable prognostic model, we attempted to separate the data into a training and validation set. However, we were unable to develop the prognostic model using the training set because of the small number of events observed. Therefore, data from all patients were used to determine the relative weights for the ACE-27 and D-IPSS scores in the final prognostic model. Weighted scores derived from the coefficients in the Cox multivariable regression model were used to predict survival. Categorical variables were compared using the χ2 test and continuous variables using the Mann-Whitney test. A p-value of <0.05 (two-tailed) was considered statistically significant. Statistical analyses were carried out using IBM SPSS Statistics 21 for Windows (SPSS Inc., Chicago, Illinois).
Results
Patient Characteristics
Of 349 patients analyzed, the median age at presentation was 64 years (range, 20–83 years) and 64% of patients were men (Table 1). The median time from diagnosis to presentation at MD Anderson was 5 months (range, 0 – 352 months), and median follow-up time was 72 months (95% CI, 63–81 months). At presentation, 45% of patients had constitutional symptoms (significant as per D-IPSS: weight loss > 10% of the baseline value in the year preceding PMF diagnosis and/or unexplained fever or excessive sweats persisting for more than 1 month), and 60% had splenomegaly by palpation as recorded in the medical records (5% had undergone splenectomy prior to presentation). Thirteen percent of patients were classified as low-risk by D-IPSS, 44% as intermediate-1, 36% as intermediate-2, and 7% as high-risk.
Table 1.
Demographic and clinical characteristics of patients with PMF at presentation
| N=349 n (%) |
|
|---|---|
| Age at presentation | |
| ≤ 65 years | 191 (55) |
| > 65 years | 158 (45) |
| Median age, y (range) | 64 (20–83) |
| Median time from diagnosis to presentation, months (range) | 5 (0–352) |
| Sex | |
| Women | 127 (36) |
| Men | 122 (64) |
| Race | |
| White | 311 (89) |
| Non-White | 38 (11) |
| Constitutional symptoms | 156 (45) |
| Splenomegaly | 209 (60) |
| Transfusion dependence, n (percent) | 104 (30) |
| Cytogenetic type | |
| Diploid | 193 (61) |
| Complex | 125 (39) |
| Median HGB, g/dL (range) | 10.3 (5.9–18.7) |
| Median WBC, 10−9/L (range) | 9.5 (1–200) |
| Median PLT, 10−9/L (range) | 194 (1–1283) |
| Median PB blast, percent (range) | 1 (0–17) |
| Median LDH, IU/L (range) | 1255 (251–7593) |
| Median creatinine, mg/dL (range) | 1 (0.5–7.4) |
| Median PS (range) | 1 (0–3) |
| ACE-27 score | |
| No comorbidities | 125 (36) |
| Grade 1 = Mild | 128 (37) |
| Grade 2 = Moderate | 63 (18) |
| Grade 3 = Severe | 33 (9) |
| D-IPSS | |
| Low | 44 (13) |
| Intermediate -1 | 153 (44) |
| Intermediate -2 | 127 (36) |
| High | 25 (7) |
| Comorbidities by organ system | |
| Cardiovascular | 221 (63) |
| Endocrine | 49 (14) |
| Malignancy | 41 (12) |
| Alcohol or substance abuse | 25 (7) |
| Psychiatric | 19 (5) |
| Neurological | 18 (5) |
| Respiratory | 16 (5) |
| Gastrointestinal | 14 (4) |
| Obesity | 10 (3) |
| Rheumatologic | 9 (3) |
| End stage renal disease | 5 (1) |
Prevalence of comorbidities and association with demographic and clinical characteristics
We assessed the number and severity of comorbidities at presentation using the ACE-27 comorbidity index (Table 1 and Supplementary Table 1). Thirty-six percent (n=125) of patients had no comorbidities, 37% (n=128) mild, 18% (n=63) moderate, and 9% (n=33) severe. Hypertension (n=109; 31%) was the most common comorbid condition followed by diabetes mellitus (n=49; 14%), previous solid tumors (n=37; 11%), and angina/coronary artery disease (n=37; 11%). When we compared the distribution of patients in each ACE-27 category by clinical characteristics, we found that comorbidity burden was not significantly associated with constitutional symptoms (P=0.43), splenomegaly (P=0.14), JAK2 mutation status (P=0.34), or disease duration (i.e., time from diagnosis to presentation; P=0.10).
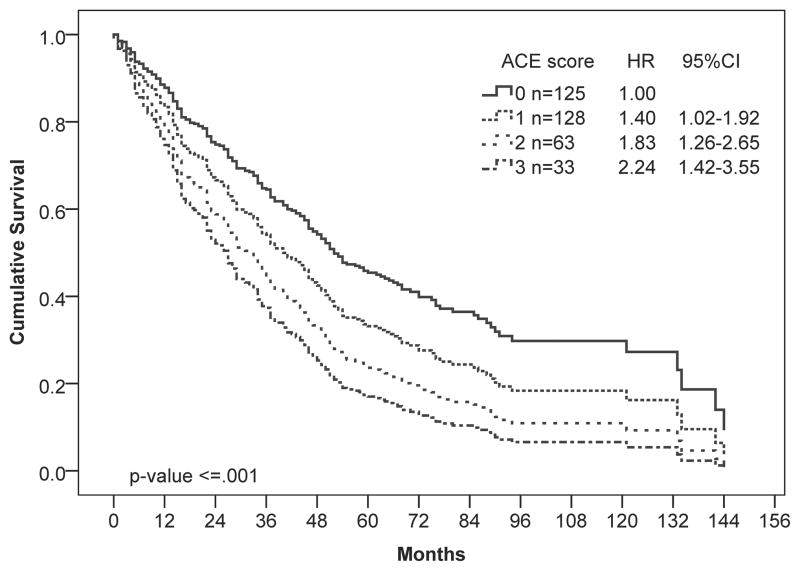
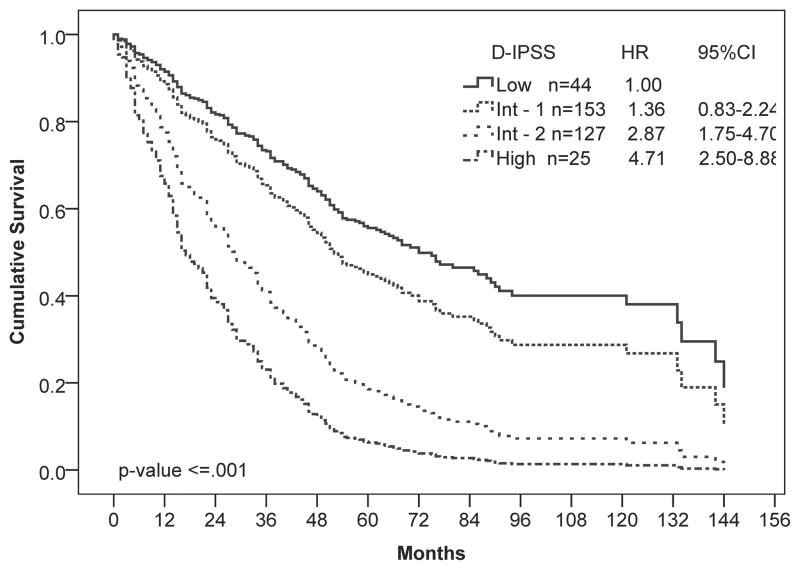
At the time of analysis, the median OS for the total population was 41 months (95% CI, 35 – 47 months), and 230 (66%) patients had died. To determine whether the ACE-27 index was prognostic in our patient population, we performed univariate Cox regression analysis of survival by ACE score. The ACE score was significantly associated with worse OS (P ≤ 0.001; Figure 1A). Patients with severe comorbidities had twice the hazard of death as those with no comorbidities. As expected, the D-IPSS score was also associated with survival in our patient population (P ≤ 0.001; Figure 1B).
Figure 1.
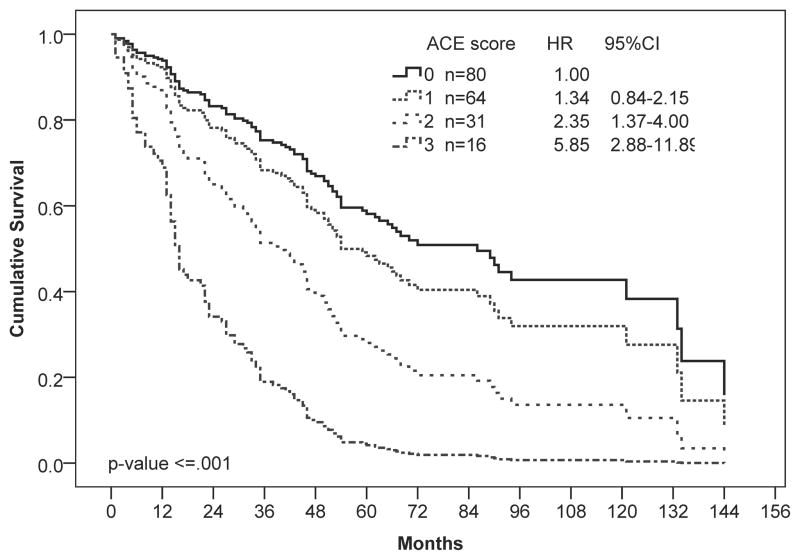
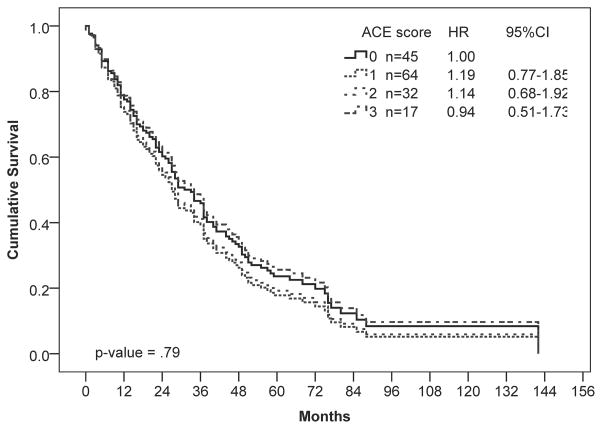
Univariate Cox regression analysis of overall survival by (A) ACE-27, (B) D-IPSS, and (C, D) ACE-27 stratified by age: (C) 65 years and younger and (D) older than 65 years. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated using Cox-proportional hazards regression. Number of patients in each risk category and Hazard ratios (HR) with 95% confidence intervals (CI) are shown.
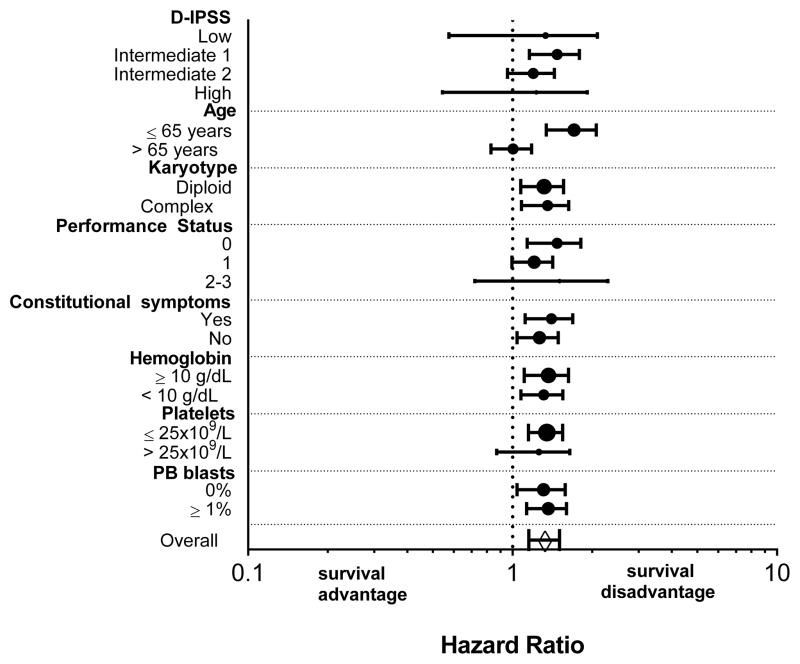
We next performed a subgroup analysis examining the effect of comorbidities on survival of patients stratified by demographic and clinical characteristics (Figure 2). When stratified by age, comorbidities significantly reduced survival in patients 65 and younger (P ≤ 0.001; Figure 1C) but not those older than 65 (P = 0.97; Figure 1D). In younger patients, moderate and severe comorbidities were associated with 2-fold and 6-fold higher hazards for death, respectively. When stratified by D-IPSS score, comorbidities were only significantly associated with shorter survival for patients in the intermediate-1 risk category (P ≤ 0.001), with a trend towards shorter survival for patients in the intermediate-2 risk category (P = 0.06). Similarly, comorbidities were only associated with a survival disadvantage in patients with good performance status (PS < 1; P = 0.002).
Figure 2.
Univariate analysis of association of ACE-27 score with survival by demographic and clinical characteristics. Circles represent the Hazard ratio and 95% confidence intervals are shown. The size of the circles are proportional to the number of patients in each subgroup. The diamond shows the Hazard ratio for the whole patient population.
Combining the ACE-27 and D-IPSS scores
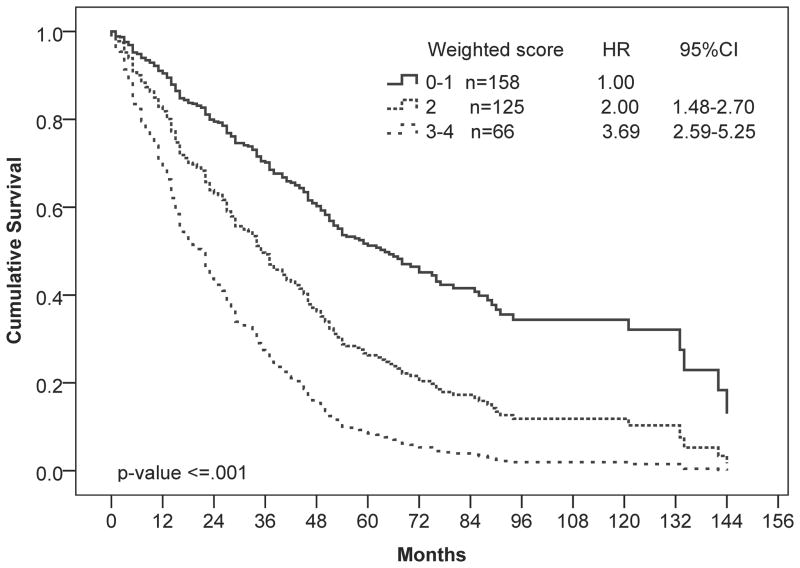
Because comorbidities were shown to have a significant impact on survival of patients in the univariate analysis, we sought to determine whether information about comorbidities could be used to improve prognostic risk stratification. We constructed a multivariable Cox regression model that included the ACE-27 and D-IPSS scores, which were assigned weights that were proportional to the regression coefficient of the multivariable Cox model (Table 2). The final weighted combined score (CS), allowed the stratification of patients into 3 risk categories with median OS ranging from 17 to 65 months (P ≤ 0.001; Figure 3 and Table 3). Using this CS, 45% of patients were categorized as low risk, 36% as intermediate risk, and 19% as high risk. Of the 66 patients with the poorest survival (CS = 3–4), all were defined as either intermediate-2 or high-risk by D-IPSS.
Table 2.
Multivariate Cox regression model and weighted scores
| Prognostic factor | Coefficient | P-value | HR | 95% CI | Weighted Score* |
|---|---|---|---|---|---|
| ACE score | |||||
| 0–1 | Reference | 0 | |||
| 2–3 | .48 | 0.001 | 1.62 | 1.22–2.14 | 1 |
| D-IPSS score | |||||
| Low (0) | Reference | 0 | |||
| Intermediate-1 (1–2) | .25 | 0.277 | 1.32 | 0.80–2.17 | 1 |
| Intermediate-2 (3–4) | 1.018 | <0.001 | 2.77 | 1.69–4.54 | 2 |
| High (5–6) | 1.538 | <0.001 | 4.66 | 2.47–8.79 | 3 |
Weighted score was obtained by dividing the estimated coefficients by 0.5 and rounding to the nearest integer.
Figure 3.
Multivariate Cox regression analysis of overall survival by weighted combined ACE-27 and D-IPSS scores. The model stratified patients into 3 risk categories: low (combined score [CS]=0–1), intermediate (CS=2), and high (CS=3–4) risk. Number of patients in each risk category and Hazard ratios (HR) with 95% confidence intervals (CI) are shown.
Table 3.
Cox regression analysis of survival by weighted combined score
| Combined score values | N=349 (%) | Events N (%) | Median OS (months) | Coefficient | Hazard Ratio (95% CI) | P value |
|---|---|---|---|---|---|---|
| Low (0–1) | 158 (45) | 80 (51%) | 65 | - | Reference | - |
| Intermediate (2) | 125 (36) | 95 (76%) | 35 | 0.693 | 2.00 (1.48–2.70) | ≤ 0.001 |
| High (3–4) | 66 (19) | 55 (83%) | 17 | 1.306 | 3.69 (2.59–5.25) | ≤ 0.001 |
Discussion
In this retrospective study of a cohort of patients with PMF, we found that comorbidities had a significant, independent negative impact on OS. Patients with severe comorbidities (ACE-27 score = 3) had twice the risk of death as those with no comorbidities. In addition, we developed a weighted score that could stratify patients into three risk categories on the basis of their D-IPSS and ACE-27 comorbidity scores.
In the univariate subgroup analysis comorbidities had no significant effect on survival in patients older than 65. This observation is likely driven by the short survival time observed in this group of patients (median, 29 months), suggesting that the aggressiveness of the disease in these patients may minimize or nullify the effect of comorbidities. In agreement with our findings, comorbidities have been shown to have more prognostic importance among patients with more indolent cancers (e.g., prostate and breast cancers) who have longer mean survival than in those with more aggressive cancers, such as lung cancer.18 In addition, age > 65 years is an independent negative prognostic factor for PMF, suggesting that comorbidities may be acting as an effect modifier of the association with survival in older patients.3 However, a larger study ideally with patients from multiple sites would be necessary to delineate the relative contributions of age and comorbidity to survival. Regardless, our finding suggests that for patients with PMF, optimal management of comorbid conditions would have the highest impact in patients younger than 65, though the benefits could be extended to those older than 65 years.
Interestingly, our findings also show that patients classified as intermediate risk by D-IPSS with comorbidities have a worse prognosis. However, for patients in the low- and high-risk D-IPSS categories, the association with comorbidities was not seen, possibly due to the small number of patients in these risk categories (n=44, low risk; n=25, high risk). An alternative explanation may be that the relative impact of comorbidities and other risk factors are affected by the complex interaction of the various clinical parameters: the natural disease course, treatments, performance status, age, comorbidities, and other unknown risk factors. For example, age over 65 years or high-risk disease by DIPSS may eclipse the effect of comorbidity on OS. A future study conducted in a larger population is necessary to better delineate the relative contributions of these risk factors to OS.
Cardiovascular comorbidities, including hypertension (31%), angina/coronary artery disease (11%), and arrhythmia (6%), myocardial infarction (4%), venous disease (3%) and peripheral arterial disease (3%), were observed in 63% of patients in this study. Given the known increased risk of cardiovascular events in patients with PMF,19 it is unclear whether these represent true comorbidities (i.e, a medical condition unassociated with the index disease) or whether they may be better defined as “complications” of MF (i.e., an additional disorder/disease co-occurring with the primary disease). This distinction may be especially important, given that myelofibrosis is sometimes described as an “inflammatory disorder,” 20 which suggests an increased risk of cardiovascular complications. However, a population-based prospective cohort study would be necessary to determine whether cardiovascular comorbidities/complications are indeed associated with an increased relative risk of death in patients with MF. Nevertheless, future studies that measure comorbidities before and during treatment with JAK inhibitors, which are known to reduce levels of inflammatory cytokines, are particularly important for understanding whether JAK inhibitor therapy can improve inflammation-mediated comorbidities.
Comorbidities alone are clearly associated with survival in patients with PMF, especially in those younger than 65 years and those with good PS, a subgroup that might benefit not only from optimal management of the comorbid conditions but also from earlier treatment interventions. The impact of comorbidities in younger patients and those with good PS may be especially important for those who might otherwise be eligible for allogeneic stem cell transplantation, which is currently the only curative treatment for PMF.21 Importantly, 9% of the patients in this sample had severe organ decompensation and were scored as grade 3 according to the ACE-27 index. These patients are at a significant survival disadvantage even when their MF is classified as low-risk by D-IPSS. Therefore, close collaboration between the oncologist and other medical specialists to monitor and control the comorbid conditions is important for optimal outcomes.
Despite the relatively large sample size, our study has several limitations. Retrospective reviews of medical charts are limited to the information collected: details of the comorbid disorders, patients’ medical history and prior treatments may not always available, especially given that patients were referred to our institution for PMF and not for the comorbid illnesses. In addition, because we are at a tertiary care center, we often see patients many months or years after their initial MF diagnosis. Therefore, estimated survival times are likely shorter than those expected had we followed patients from initial diagnosis. Finally, because we only analyzed patients from a single institution, generalization of our results is limited to groups of patients with PMF who share similar characteristics to our sample. Future studies in a larger, independent cohort need to be performed to validate our findings.
In conclusion, our study in a large cohort of PMF patients highlights the importance of comorbidities in OS; comorbidities were associated with a 2- to nearly 6-fold (for patients ≤ 65) increased risk of death for patients in this cohort. Therefore, including comorbidities is critical for individualizing assessment and optimizing treatment and outcomes for patients with PMF.
Supplementary Material
Acknowledgments
Funding sources: This work was funded in part by philanthropic funding to support the Clinical Research Center for Myeloproliferative Neoplasia at MD Anderson Cancer Center.
P30 CA016672, Ronald DePinho
Footnotes
Author contributions
K. J. Newberry analyzed the data and wrote the paper. K. Naqvi designed the study, analyzed the data and provided critical review of the manuscript. M. Cardenas-Turanzas analyzed the data and provided critical review of the manuscript. K. T-T. Nguyen critically reviewed the data and the manuscript. F. Tanaka and S. Pierce participated in the data collection and annotation. S. Verstovsek designed the study, provided oversight for the study, and wrote the manuscript.
Conflict of interest disclosure: The authors declare no conflicts of interest.
References
- 1.Swerdlow SH International Agency for Research on Cancer., World Health Organization. WHO classification of tumours of haematopoietic and lymphoid tissues. 4. Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 2.Tefferi A. Primary myelofibrosis: 2013 update on diagnosis, risk-stratification, and management. Am J Hematol. 2013;88:141–150. doi: 10.1002/ajh.23384. [DOI] [PubMed] [Google Scholar]
- 3.Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113:2895–2901. doi: 10.1182/blood-2008-07-170449. [DOI] [PubMed] [Google Scholar]
- 4.Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment) Blood. 2010;115:1703–1708. doi: 10.1182/blood-2009-09-245837. [DOI] [PubMed] [Google Scholar]
- 5.Mesa RA, Silverstein MN, Jacobsen SJ, Wollan PC, Tefferi A. Population-based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: an Olmsted County Study, 1976–1995. Am J Hematol. 1999;61:10–15. doi: 10.1002/(sici)1096-8652(199905)61:1<10::aid-ajh3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 6.Extermann M. Measurement and impact of comorbidity in older cancer patients. Crit Rev Oncol Hematol. 2000;35:181–200. doi: 10.1016/s1040-8428(00)00090-1. [DOI] [PubMed] [Google Scholar]
- 7.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 8.Feinstein AR. Pre-therapeutic classification of co-morbidity in chronic disease. J Chron Dis. 1970;23:455–468. doi: 10.1016/0021-9681(70)90054-8. [DOI] [PubMed] [Google Scholar]
- 9.Degroot V, Beckerman H, Lankhorst G, Bouter L. How to measure comorbidity a critical review of available methods. J Clin Epidemiol. 2003;56:221–229. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- 10.Albertsen PC, Fryback DG, Storer BE, Kolon TF, Fine J. The impact of co-morbidity on life expectancy among men with localized prostate cancer. J Urol. 1996;156:127–132. [PubMed] [Google Scholar]
- 11.Yancik R, Wesley MN, Ries LA, et al. Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients: a population-based study. Cancer. 1998;82:2123–2134. [PubMed] [Google Scholar]
- 12.Piccirillo JF. Importance of comorbidity in head and neck cancer. Laryngoscope. 2000;110:593–602. doi: 10.1097/00005537-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Yancik R, Wesley MN, Ries LG, Havlik RJ, Edwards BK, Yates JW. EFfect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 14.Della Porta MG, Malcovati L, Strupp C, et al. Risk stratification based on both disease status and extra-hematologic comorbidities in patients with myelodysplastic syndrome. Haematologica. 2011;96:441–449. doi: 10.3324/haematol.2010.033506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naqvi K, Garcia-Manero G, Sardesai S, et al. Association of comorbidities with overall survival in myelodysplastic syndrome: development of a prognostic model. J Clin Oncol. 2011;29:2240–2246. doi: 10.1200/JCO.2010.31.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daver N, Naqvi K, Jabbour E, et al. Impact of comorbidities by ACE-27 in the Revised-IPSS for patients with myelodysplastic syndromes. Am J Hematol. 2014 doi: 10.1002/ajh.23675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan MH, Feinstein AR. The importance of classifying initial co-morbidity in evaluating the outcome of diabetes mellitus. J Chronic Dis. 1974;27:387–404. doi: 10.1016/0021-9681(74)90017-4. [DOI] [PubMed] [Google Scholar]
- 18.Read WL, Tierney RM, Page NC, et al. Differential prognostic impact of comorbidity. J Clin Oncol. 2004;22:3099–3103. doi: 10.1200/JCO.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 19.Barbui T, Carobbio A, Cervantes F, et al. Thrombosis in primary myelofibrosis: incidence and risk factors. Blood. 2010;115:778–782. doi: 10.1182/blood-2009-08-238956. [DOI] [PubMed] [Google Scholar]
- 20.Hasselbalch HC. Perspectives on chronic inflammation in essential thrombocythemia, polycythemia vera, and myelofibrosis: is chronic inflammation a trigger and driver of clonal evolution and development of accelerated atherosclerosis and second cancer? Blood. 2012;119:3219–3225. doi: 10.1182/blood-2011-11-394775. [DOI] [PubMed] [Google Scholar]
- 21.Sorror ML. Comorbidities and hematopoietic cell transplantation outcomes. Hematology Am Soc Hematol Educ Program. 2010;2010:237–247. doi: 10.1182/asheducation-2010.1.237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.