Abstract
The liver is unique in that it is able to regenerate. This regeneration occurs without formation of a scar in the case of non-iterative hepatic injury. However, when the liver is exposed to chronic liver injury, the purely regenerative process fails and excessive extracellular matrix proteins are deposited in place of normal liver parenchyma. While much has been discovered in the past three decades, insights into fibrotic mechanisms have not yet lead to effective therapies; liver transplant remains the only cure for advanced liver disease. In an effort to broaden the collection of possible therapeutic targets, this review will compare and contrast the liver wound healing response to that found in two types of wound healing: scarless wound healing of fetal skin and oral mucosa and scar-forming wound healing found in adult skin. This review will examine wound healing in the liver and the skin in relation to the role of humoral and cellular factors, as well as the extracellular matrix, in this process. While several therapeutic targets are similar between fibrotic liver and adult skin wound healing, others are unique and represent novel areas for hepatic anti-fibrotic research. In particular, investigations into the role of hyaluronan in liver fibrosis and fibrosis resolution are warranted.
Keywords: Extracellular matrix, fibrosis, hepatic stellate cell, inflammation, hyaluronan, macrophage
1. INTRODUCTION
Liver disease consists of a spectrum of hepatic pathologies beginning with simple steatosis. While exacerbations of liver disease can manifest as acute hepatitis superimposed on any stage of liver disease, typically, a progression from steatosis to fibrosis, cirrhosis and even hepatocellular carcinoma over several decades is recognized [1].
Liver fibrosis is the end result of chronic hepatic injury and inflammation coupled to incomplete tissue repair. The net effect of incomplete repair over several cycles of tissue injury is accumulation of extracellular matrix (ECM) proteins. When prolonged, excessive hepatic ECM accumulation impacts hepatic architecture and function. Several agents cause liver fibrosis including chronic alcohol consumption, viruses, congenital disorders, cholestasis, parasites, drugs and toxins [2, 3]. Liver fibrosis is also a common feature of obesity and associated metabolic derangements [2]; it is this patient population which is expected to increase with the burgeoning number of overweight, obese and morbidly obese people found in our over-nourished, sedentary Western societies [4]. Regardless of etiologic agent, the progression to liver fibrosis occurs in only a subset of patients and is clearly affected by a host of additional factors including genetics, environment, behavior and various comorbidities [1]. While removal of the etiologic agent can attenuate disease progression and even lead to fibrosis reversal in some patients, no pharmacologic strategy yet exists to ‘cure’ liver disease [5, 6]. Indeed, liver transplantation is the only recuperative strategy for advanced liver disease not responsive to etiologic agent removal.
There are two therapeutic areas which could have a profound impact on how liver fibrosis is managed in patients: preventing fibrosis progression and hastening fibrosis reversal. This review will focus on the biology of fibrosis progression and fibrosis resolution with respect to the specific involvement that inflammation, hepatic macrophages and hepatic stellate cells (HSC) have in this process. This review will also compare the hepatic response to injury with scarless wound healing found in fetal skin and the oral mucosa with wound healing in adult skin. Through this discussion, the reader will gain an appreciation of how mechanisms of scarless wound healing may help us develop novel therapeutic strategies to treat liver fibrosis. Steatosis predisposes the liver to fibrosis; some mechanisms contribute to both stages of liver disease. Therefore, we will also include a review of those shared mechanisms. This review will not cover, in any significant detail, the contributions of reactive oxygen species, portal fibroblasts, additional innate or adaptive immunity or various cell death pathways to liver fibrosis, in an effort to not recapitulate recent excellent reviews on this subject [7–12] and to keep the review relatively concise and focused.
1.1. Wound Healing Overview
Wound healing refers to a generalized response of a tissue, whether it be the skin or a solid organ such as the liver, to injury. A cascade of sequential steps are involved in this process whose outcome is to reinstate tissue integrity and function. In brief, the steps include: hemostasis to prevent prolonged blood loss, inflammation to debride the wound of pathogens or dead cellular material, granulation tissue formation (including the activation and proliferation of fibroblasts and synthesis of a provisional ECM on which the new tissue organizes), angiogenesis, wound contraction and finally, ECM remodeling during which the injured tissue is reorganized closely approximating its original architecture and strength [13]. This is a very well-described process in the skin, but broadly applicable to other sites of tissue injury. Perturbations at any point of this process can lead to abnormally healing wounds. For example, in people with diabetes dermal wound healing is impaired due to persistent inflammation, reduced growth factor production, fibroblast activation, cell migration, angiogenesis and increased matrix degradation leading to wounds which do not heal [14]. In contrast, overly robust wound healing is characterized by excessive ECM deposition without adequate remodeling the end result of which is a robust fibrotic response (e.g. keloid scar formation or solid organ fibrosis) [15]. In the next few sections, we will focus on the role of the macrophage and hepatic stellate cell and the ECM in the maladaptive wound healing found in liver fibrosis.
1.2. Hepatic Macrophages in Health and Disease
1.2.1. Kupffer Cell Function in Healthy Liver
The liver consists of many different immune cell populations involved in both innate and adaptive immunity. In particular, the innate immune system predominates as macrophages, natural killer, natural killer T and γδ T cells are enriched in this organ and critical for normal hepatic function, protection from pathogens and tumor development, and in liver injury and repair [16]. Most organs contain a resident population of macrophages [17]. The Kupffer cell, the liver resident macrophage, constitutes 20% of the liver non-parenchymal population [18]. Kupffer cells are found in greatest numbers in the periportal area of the liver [19]. This is an anatomically critical location: portal blood from the intestine enters the liver at this location. Portal blood is rich in antigens from dietary sources, but also contains a small amount of antigens derived from components of the gut microbiome, including gram negative (lipopolysaccharide, LPS) and gram positive (lipoteichoic acid) bacterial cell wall molecules. Broadly speaking, these and other molecules derived from bacteria, and other gut residents, are called pathogen-associated molecular pattern (PAMP) molecules [20, 21]. This perhaps striking inclusion of bacterial products in portal blood is due to the fact that the intestine is normally somewhat permeable [22–24]. Kupffer cells screen this large antigenic load, in part, via their pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) and under normal circumstances, do not initiate an inflammatory response [25]. Macrophage hyporesponsiveness of TLR signaling likely evolved due to continuous exposure to components produced by normal gut microbiome. Instead of producing proinflammatory molecules in response to various antigens from the gut, Kupffer cells respond by producing IL10, a cytokine which reduces inflammation [26]. Indeed, Kupffer cells are largely responsible for the seemingly immunologically tolerant nature of the healthy liver [27].
1.2.2. Role of Kupffer Cells and Alterations in the Gut Microbiome in Liver Disease
In response to chronic ethanol exposure, or other toxic or metabolic perturbations, HCV infection, autoimmune hepatitis or hereditary diseases, the liver will exhibit an inflammatory response; this inflammatory response, is generated, at least in part, by innate immune effector cells and activation of the complement system, and contributes to liver injury and later dysfunction [28, 29]. While many immune cell types are clearly involved in chronic hepatic inflammation, one might argue that the Kupffer cell plays a predominant role in the initiation and progression of liver disease [30–32]. For example, published reports demonstrate a reduction in ethanol-induced steatosis, liver injury and inflammation in rats after depleting macrophages using gadolinium chloride [33, 34]. There are several mechanisms by which the normally immunologically subdued Kupffer cell shifts from a state tolerant of gut-derived PAMPs to exquisitely sensitive to those same, and other, PAMPs. In animal models of liver disease, accumulating evidence suggests that the gut microbiome changes, favoring ‘pathogenic’ strains and limiting beneficial strains; this is referred to as dysbiosis. For example, Fouts et al. have shown a relative increase of Firmicutes and Actinobacteria in mice after chronic CCl4 exposure [35]. Similarly, obese mice (ob/ob mice) exhibit increased Firmicutes and reduced Bacteroidetes after high fat diet feeding when compared to lean littermate controls [36]. Finally, in obese humans, Bacteroidetes is also reduced in comparison to lean people [37–39] providing relevance of animal models to human disease.
In addition to bacterial population shifts, bacterial overgrowth is also purported to contribute to liver disease; this is observed in mouse models of alcoholic liver disease [40] and non-alcoholic fatty liver disease [41]. Slower transition time of intestine luminal contents may contribute to this observation [42]. The overall impact of these changes is increased PAMP delivery to the liver; this is observed in animal models of liver disease as well as in liver disease patients [40, 41, 43–45].
The gut microbiome consists of a plethora of commensal organisms which contribute to immunity, nutrition, metabolism and behavior [46]. The vast majority of published literature focuses on the role of LPS in liver disease. Significant evidence linking LPS and LPS signaling to development of steatosis after chronic ethanol exposure has accumulated over several years. Specifically, deficiency of TLR4 [47, 48] or CD14 [49], two necessary components of the LPS receptor complex, or animals whose gut microbiome is depleted using antibiotics, exhibit reduced steatosis after chronic ethanol exposure [50]. Similarly, TLR4 contributes to development of non-alcoholic steatohepatitis: liver injury, inflammation and steatosis are reduced in TLR4-deficient (C3H/HeJ) mice fed a methionine/choline deficient diet [51]. Further evidence suggests that therapeutic modulation of the gut microbiome via supplementation with probiotics (live microorganisms that have beneficial effects on health), prebiotics (indigestible food ingredients which alter the gut microbiome), synbiotics (nutritional supplements which are combinations of pre and probiotics), or even fecal transplants may improve liver disease and are therefore attractive therapeutic approaches [40, 52–54].
1.2.3. Increased Gut Permeability in Liver Disease
In addition to the increases in bacterial load and shifts in the ratio of beneficial to pathogenic bacterial species, additional changes in the gut promote liver disease. For example, gut permeability is compromised, allowing bacteria or bacterial products increased access to the portal circulation [22, 46, 55]. Several proposed mechanisms exist by which the gut becomes leaky. First, in alcoholic and non-alcoholic liver disease, presence or synthesis [56] of ethanol in the gut as well as ethanol metabolism to acetaldehyde, disrupts tight junctions [57]. In addition, recent evidence also suggests that gut permeability induced by chronic ethanol exposure can be attenuated by treatment with a probiotic strain of bacteria Lactobacillus rhamnosus GG [58]. Regardless of etiology, changes in gut permeability are appreciated to influence alcoholic and non-alcoholic fatty liver disease initiation and progression in mouse and man.
Gut permeability is also implicated in other liver diseases. For example, a strong association exists between primary sclerosing cholangitis (PSC) and inflammatory bowel disease (IBD), with nearly 75% of PSC patients also affected with IBD [59] and are associated with poorer outcomes than when either disease occurs in isolation [60]; changes in the gut microbiome, and gut permeability are often associated with IBD [61]. Likewise, considerable evidence in clinical and preclinical models suggest that increases in gut permeability, intestinal dysbiosis and increased delivery of PAMPs to the liver as found in IBD contribute to PSC, although increases in gut permeability are not always found in PSC patients [59]. Additional support for a role for the gut in PSC is found in published clinical studies in which patients were treated with antibiotics. In many, but not all of these studies, improvements in disease parameters were observed [59, 62]. Further optimization with respect to which antibiotic is used, dosage and dosing regimen are required to improve response of PSC to antibiotic-mediated disease therapy.
1.2.4. Changes in Kupffer Cell LPS Sensitivity Contribute to Fatty Liver Disease
Inherent changes in the Kupffer cell are also responsible for increased inflammation found in liver disease. For example, Kupffer cells from ethanol-fed rats accumulate increased TNFα mRNA and secrete more TNFα protein in response to LPS relative to Kupffer cells isolated from control rats [63]. Similarly, after chronic ethanol feeding to mice, hepatic TNFα mRNA and plasma TNFα protein are both increased relative to LPS-stimulated TNFα in controls [64]. Additional studies revealed that the increased sensitization of Kupffer cells to LPS is due enhanced ERK1/2 signaling and Egr-1 binding to the TNFα promoter [65] and p38-dependent TNFα mRNA stability [66, 67]. Consistently, mice deficient in Egr-1 are protected from ethanol-induced steatosis suggesting the relevance of in vitro studies to animal models of fatty liver disease [64].
While the studies discussed above as well as others, implicate TLR4 in development of fatty liver disease, additional studies demonstrated that the MyD88-independent, also known as the TRIF-dependent, pathway downstream of TLR4 is critical for increased TNFα synthesis and secretion after chronic ethanol exposure in mice [68, 69]. Consistently, LPS-stimulated TNFα protein and steatosis after chronic ethanol exposure are attenuated in Trif−/− mice [69, 70]. Finally, although SNPs in the TLR4 gene predispose patients to septic shock and gram negative infections [71, 72], those same SNPs confer protection from fibrosis progression in people with HCV [73]. Specific anti-inflammatory and anti-fibrotic effects of these SNPs were identified in HSC in a subsequent study [74]. Collectively, these data suggest a critical contribution of Kupffer cell sensitivity to LPS and TLR signaling to inflammation and steatosis, changes which predispose the liver to disease progression.
1.2.5. Role of Kupffer Cells and DAMPs in Liver Disease
In addition to LPS and other PAMPs from the gut, an additional class of molecules released during tissue injury generates inflammation and alerts the innate immune system to tissue damage or danger. This class of molecules is called danger or damage-associated molecular pattern molecules (DAMPs) and DAMP release is likely the earliest event leading to a fibrotic response [75]. DAMPs come from injured cells (e.g. HMGB1, mitochondrial and nuclear DNA, ATP, heat shock proteins) or the ECM (e.g. low molecular weight (LMW) hyaluronan (HA), laminin and elastin) and induce inflammation via binding to PRRs on macrophages and other cells. In the liver, considerable evidence links damage or death of hepatocytes and subsequent DAMP release to liver inflammation and liver disease progression [10]. In light of Polly Matzinger’s Danger Model [76], it is very appealing to implicate these and other molecules signaling ‘danger’ to initiation of inflammation and promotion of fibrogenic programs in the liver. For a good review on the role of DAMPs and sterile inflammation in the liver see [75].
1.3. Macrophages and Fibrosis
1.3.1. Kupffer Cells and Initiation of Fibrosis: Lessons from an Animal Model of Hepatotoxin-Induced Injury and Fibrosis
Macrophage dysregulation and chronic inflammation is a hallmark of liver fibrosis. While Kupffer cells likely initiate hepatic response to injury as described above, infiltrating cells clearly contribute to disease progression. Chronic exposure to carbon tetrachloride (CCl4), a well-studied hepatotoxicant, is used to model liver injury and fibrosis in rodents [77]. Single (acute) exposure to CCl4 provides a model for hepatotoxicity and complete regenerative response, while multiple (chronic) exposures to CCl4 induces hepatic fibrosis [77]. This animal model is also useful to explore mechanisms of fibrosis resolution [77]. The pathologic mechanism behind CCl4-induced hepatotoxicity and fibrosis rely, in large part, on the bioactivation of CCl4 by CYP2E1 in the liver [78–81]. Indeed, in the absence of CYP2E1, CCl4-mediated hepatotoxicity is significantly attenuated [79]. The reactive trichloromethyl and trichloromethylperoxy radicals generated by CCl4 metabolism in vivo cause injury and cell death through lipid and protein modification [80, 82]. In particular, lipid peroxidation is associated with hepatocyte cell death by necrosis after CCl4 exposure [83] resulting in DAMP release. Despite the role for CYP2E1 in CCl4-induced liver injury and fibrosis, considerable additional evidence exists that Kupffer cells also contribute. Indeed, after Kupffer cell depletion using gadolinium chloride (GdCl3), CCl4-induced liver fibrosis is attenuated [84]. These findings were associated with reductions in TGFβ, a Kupffer cell-synthesized pro-fibrotic molecule. Finally, Nolan and Leibowitz clearly demonstrated that concurrent administration of CCl4 with polymyxin B, an antibiotic, could attenuate hepatic injury after acute CCl4 exposure [85]. Consistently, antibiotic treatment of mice attenuated fibrosis in mice on a choline-deficient diet [86]. Collectively these data strongly implicate the Kupffer cell, and response to bacterial components, in the initiation of fibrosis.
1.3.2. Infiltrating Macrophage and Fibrosis Progression
Hepatic inflammation and fibrosis are not regulated solely by Kupffer cells. Indeed, macrophage subset analysis during progression of liver disease in mice revealed that macrophage populations are dynamic, exhibit incredible plasticity and rely on the recruitment of bone marrow-derived monocytes to the liver for disease progression. Kupffer cells contribute to this early phase by recruiting additional monocytic cells to the liver. The recruited macrophages are characterized phenotypically as Gr1hi/Ly-6chi, CD11b+ F4/80+ in mice (CD14hi, CD16lo, in humans [87]) and produce TGFβ and TNFα, a cytokine which plays dual roles in inflammation and fibrosis [88]; these cells originate from the bone marrow and localize to the injured liver in a CCR2-dependent manner and contribute to fibrosis [88]. Therefore, the infiltrating macrophages, and the activated Kupffer cells which attract them, exhibit phenotypic and functional characteristics of M1 (classically activated) macrophages [89]. In addition to TGFβ and TNFα, these M1-like macrophages produce PDGF and IL1β and recruit HSC to the area of hepatic injury [90]. Additional mechanisms which contribute to HSC activation are discussed in Section 1.4.2 – 1.4.5 below.
When hepatic recruitment of Gr1hi/Ly-6C+ mononuclear cells is inhibited, CCl4-induced hepatic stellate cell activation and fibrosis is attenuated [88]. Intriguingly, the recruited cells are not pro-fibrotic directly harvested from bone marrow, but require specific cues from the injured and inflamed hepatic microenvironment to fully develop this phenotype [88]. Additional evidence exists for a pro-fibrotic role of recruited macrophages. Specifically, macrophage depletion using diphtheria toxin in a novel CD11b-human diphtheria toxin receptor (DTR) transgenic mouse during progression of liver disease, attenuates fibrosis [91]. Collectively, these data support the hypothesis that macrophage phenotype is not static and resident and recruited macrophages contribute to progression of fibrotic disease.
1.3.3. Infiltrating Macrophages and Fibrosis Resolution
Macrophages also play an integral role in the resolution of fibrosis. In some instances, after chronic liver injury ceases, the fibrotic liver can complete the wound healing process [5, 77]. Degradation of fibrotic scar is one important aspect of fibrosis resolution and depends upon the activity of matrix metalloproteinases (MMPs), Zn-dependent enzymes able to degrade collagen and other ECM components [92, 93]. There is convincing evidence linking macrophages to the production of MMPs during both the inflammatory and resolution portions of the wound healing response. During fibrosis resolution in mice, MMP13, an interstitial collagenase (collagenase 3, MMP1 is likely the human functional equivalent of mouse MMP13 [94]), appears critical in this regard [95–97]. MMP13 degrades fibrillar collagen and is responsible for facilitating gelatinase (MMP2, MMP9) activity necessary for continued collagen polymer degradation. Evidence supporting a role of MMP13 in resolution of fibrosis includes the observation that MMP13 is localized to fibrotic septae during the recovery from CCl4-induced fibrosis. Dual labeling approaches reveal that MMP13 in the scar is localized to macrophages [95]. This particular wound-healing macrophage population has different names: scar-, or resolution-associated and restorative macrophages (we will use restorative macrophages to refer to this population the remainder of this review); the M2 (alternatively activated) macrophage nomenclature most closely describes this restorative macrophage subset [89].
Restorative macrophages are identified phenotypically as Gr-1−/Ly-6Clo, CD11bhi, F4/80int (CD14lo, CD16hi in humans, [87]) and acquire their matrix resolving function after phagocytosing cellular debris in the injured liver [98]. Temporal depletion of macrophages during fibrosis resolution using CD11b–DTR transgenic mice results in reduced MMP13 transcript levels and attenuated ECM remodeling [91]. Interestingly, using a model of pig serum-induced liver fibrosis which lacks a hepatocyte death and inflammation, Kupffer cells express MMP13 and contribute to the degradation of type I collagen in fibrotic liver [99], suggesting matrix degradation is not restricted to recruited macrophages. Regardless or origin, if restorative macrophages are lost, further resolution ceases and fibrotic scar is maintained [95].
As mentioned above, macrophages gain fibrosis resolution activity after phagocytosing cellular debris and upregulating MMP13 [100]. Removal of debris, such as apoptotic bodies, limits HSC activation and fibrosis [90], providing a second way by which macrophage-mediated phagocytosis is anti-fibrotic. Macrophages also limit the number of activated HSC by inducing their apoptosis [101]; this apoptosis requires cell contact, caspase 9 and perhaps tumor necrosis factor-related apoptosis-inducing ligand receptor 2 after LPS stimulation [102]. Interestingly, macrophage-synthesized MMP9 also promotes myofibroblast apoptosis [90]. Once the HSC are removed, overproduction of ECM synthesis and TIMPs is reduced, facilitating fibrosis resolution.
1.3.4. Macrophage Recruitment to the Liver
As discussed above, recruitment of monocytes to the liver contributes to liver fibrosis and fibrosis resolution. Signals facilitating monocyte recruitment emanate from the liver; in part, these signals are mediated by chemokines. Several chemokines are synthesized in the injured liver and actively participate in cell recruitment; monocyte chemoattractant protein (MCP1), also known as CCL2, is recognized as a major player. MCP1 expression is increased after toxin-induced fibrosis or fibrosis induced by nutritional deficiencies [29, 100] while interventions which reduce fibrosis, such as activation of PPARβ/δ, also reduce MCP1 [103]. Conversely, in studies which block MCP1 activity via over expression of a dominant-negative MCP1 [104] or use of MCP1 receptor (CCR2)-deficient mice [105], liver fibrosis is attenuated. Modulation of MCP1 levels is also important for fibrosis resolution. Indeed, when MCP1 is blocked using a Spiegelmer-based inhibitor (mNOX-E36) during the resolution phase after toxic or metabolic fibrosis, further infiltration of Ly-6Chi cells is reduced favoring a shift toward the restorative Ly-6Clo subset [106]; this shift facilitates accelerated fibrosis resolution. The relevance of MCP1 in human inflammation and fibrosis is also under investigation. The available literature suggest that MCP1 levels are positively correlated with disease severity [107, 108].
In addition to MCP1, recent evidence suggests that another chemokine, macrophage migration inhibitory factor (MIF) is critically important in liver fibrosis. Despite its seemingly contradictory name, MIF is required for restorative macrophage recruitment to liver and fibrosis resolution [109]. Barnes, et al. have recently demonstrated that female MIF-deficient mice exhibit reduced CCl4-induced type I collagen gene expression but not frank fibrosis when compared to wild-type mice. This apparent discrepancy is associated with a reduced capacity for restorative macrophage recruitment. Consistently, matrix metabolism mediated by MMP13 is also reduced in female Mif−/− mice. These data contrast with those of Heinrichs, et al. who demonstrated enhanced fibrosis in Mif−/− mice after CCl4 and thioacetamine-induced liver fibrosis. Reduced fibrosis in this study was associated with an increase in HSC activation, inhibitable by MIF supplementation, in vitro [110]. Differences between the two studies may involve the genders of the mice employed, or the nature of MIF deletion (whole gene deletion vs deletion in exon 3 alone).
MIF also participates in high-fat diet (HFD) induced insulin resistance associated with fatty liver disease. Indeed, in the absence of MIF, recruitment of inflammatory macrophages to adipose tissue is reduced after HFD feeding [111]. These data parallel MIF’s role in macrophage recruitment after CCl4 shown by Barnes, et al. [109]. Strikingly, the changes in adipose inflammation are associated with improved insulin sensitivity in obese Mif−/− mice assessed by insulin-stimulated 3H-glucose uptake into adipose tissue, ex vivo. Consistently, obese Mif−/− mice exhibit reduced hepatic steatosis, increased insulin sensitivity and reduced inflammation. These data are consistent with other reports linking liver pathobiology to infiltration of macrophages into adipose tissue [112]. While fibrosis was not examined in these animal models of obesity and metabolic syndrome, an additional study in humans found an association between MIF expression in mononuclear cells and fibrosis stage [113]. Finally, MIF promoter polymorphisms associated with increased MIF production in inflammatory diseases of the liver and other organs may have critical impact on disease pathogenesis and progression [114, 115]. Additional studies evaluating MIF in liver disease in humans are underway. Taken together, these data strongly suggest that macrophages are integral not only to the establishment of fibrosis, but also the resolution of fibrosis.
1.4. Contribution of Hepatic Stellate Cells to Liver Fibrosis
1.4.1. Hepatic Stellate Cell Biology
Signals emanating from activated macrophages and other innate immune effector cells and dead or dying hepatocytes are clearly required for the inflammatory stages of the wound-healing response after tissue injury. Another subset of cells is required for the later, wound-healing stages. Regardless of organ identity, tissue-resident fibroblast populations contribute to ECM remodeling after tissue injury and parenchymal cell loss [9]. Hepatic stellate cells (HSC) and portal fibroblasts (PF) are the main fibroblast populations which synthesize extracellular matrix in response to liver injury [9]. In general, experimental evidence suggests a predominant role for HSC in hepatic fibrosis, but important contributions by PFs cannot be ignored, particularly in hepatic injury involving the periportal area of the liver [116]. Excellent reviews on this important cell population were recently published [116–118].
HSC account for approximately 5–8% of the total cells, and 30% of non-parenchymal cells in healthy liver [119, 120]. HSC localize to the space of Dissé, an anatomical location between hepatocytes and liver sinusoidal endothelial cells. HSC are analogous to the pericytes of normal blood vessels in that they wrap around the hepatic sinusoids and participate in normal LSEC function [121]. However, HSC are unique from pericytes in many ways including their ability to store vitamin A. Indeed, 80% of our vitamin A is stored as retinyl esters in HSC [122]. In addition to vitamin A storage, HSC also synthesize ECM in normal liver. Specifically, HSC synthesize type III and IV collagen, laminin and low amounts of type I collagen [121]. These and other ECM proteins are found in several areas of the liver including the space of Dissé, portal triads and Glisson’s capsule. Even under normal circumstances, ECM proteins are remodeled. HSCs, and other cell types, are involved in normal hepatic EMC turnover [121]. Finally, and similar to vascular pericytes, anatomical and experimental evidence exists that HSC regulate sinusoidal blood flow [121].
1.4.2. Hepatic Stellate Cell Activation and Hepatic Extracellular Matrix Accumulation during Fibrosis
When the liver is injured, quiescent HSC are ‘activated’. Activation results in an HSC phenotypic change from a vitamin A storage cell to a contractile, highly proliferative and migratory myofibroblast capable of increasing matrix remodeling enzyme (MMPs and TIMPs) and matrix protein (collagen, elastin) synthesis required for reorganization of damaged hepatic tissue [123]. At first glance, the induction and activity of MMPs and TIMPs by HSC during fibrosis may appear like a contradiction, but even in the face of frank fibrosis, matrix remodeling occurs, but favors ECM accumulation over degradation [90].
In addition to macrophages (see Section 1.3.2), several molecules participate in HSC activation, including, but not limited to, reactive oxygen species, LPS and hepatocyte apoptotic bodies [120, 123]. These early mediators prepare the HSC to better respond to later signals required for fibro-genesis; this early stage of HSC activation is called initiation [120]. Initiation, is associated with loss of vitamin A and increased expression platelet-derived growth factor receptor β chain (PDGFRβ), a component of the PDGF receptor [124–126]. Activated HSC, also called myofibroblasts (MFB), proliferate in response to PDGF, the primary HSC mitogen, and produce additional molecules which promote full engagement of the fibrotic response; this stage of HSC activation is called perpetuation [120]. Molecules produced by activated HSC include chemokines which recruit additional immune cells and HSC to site of hepatic injury (e.g. MCP1 and RANTES) and profibrotic molecules (e.g. TGFβ, PDGF, CTGF) which facilitate HSC proliferation and ability to synthesize matrix proteins [127–129]. Critically, if signals perpetuating HSC activation are maintained, development of fibrosis is favored. When stimuli promoting perpetuation of HSC activation subside, HSC undergo NK cell-mediated apoptosis [130], senescence [131] or reversion [132], limiting further ECM production. Reduced expression and activity of HSC-derived TIMPs subsequently allows increased macrophage-mediated MMP activity, facilitating matrix degradation [90].
1.4.3. Role of PAMPs and DAMPs in Hepatic Stellate Cell Activation
In addition to a role in macrophage activation, LPS contributes to HSC activation. A relationship between LPS and HSC activation was revealed in 2007 when Seki et al. demonstrated that HSC express high levels of TLR4, and when exposed to LPS, expression of BAMBI, a pseudoreceptor for TGFβ, is reduced, facilitating better TGFβ signaling in HSC [133]. Mechanistically, LPS-mediated sensitization of HSC by LPS requires TLR4, MyD88, ERK phosphorylation and NFκB activation. In addition, LPS-stimulated HSC leads to the expression and secretion of MCP1, TGFβ1 and IL6 [134]. HSC activation is not limited to TLR4 or TLR4 ligands. For example, HSC stimulation with lipoteichoic acid or peptidoglycan fragment (N-acetyl muramyl peptide) results in the production of the same effector molecules downstream of TLR2 [134], suggesting profibrotic roles for other gut-derived PAMPs.
Despite the clear links between LPS, and other bacterial products, and HSC activation and fibrosis, it is intriguing to note that some gut-derived molecules may provide protection from liver disease. Indeed, a very recent study demonstrated that germ-free mice had worse thioacetamide and CCl4-induced fibrosis than conventional mice [135]. In parallel, mice deficient in MyD88 and Trif, two important adaptor proteins downstream of TLR4, also exhibit worse fibrosis when compared to wild-type mice [135]. These data suggest that the commensal microbiota, or other gut-derived molecules dependent on those microbiota, help protect the liver against heptotoxin-induced liver fibrosis.
Finally, direct contribution of DAMPs to HSC activation is currently unknown [136], but implicated. However, the indirect activation of HSC by DAMP-stimulated macrophages is clearly relevant to fibrosis. Future work should focus on DAMP-mediated HSC activation and fibrosis.
1.4.4. Role of the Extracellular Matrix in Facilitating HSC Activation and Fibrosis
The ECM is a critical component of normal organ architecture. It provides structural support to cells and is necessary for establishing and maintaining cell polarity in epithelial cell sheets. In the liver, the ECM is composed of proteins such as fibrillar and non-fibrillar collagens, elastin, fibronectin, laminin, tenascin and thrombospondin as well as glycosaminoglycans with (heparan sulfate, chondroitin sulfate) or without (HA) core proteins [137, 138]. Each of these molecules is altered in liver fibrosis, the most striking changes of which occur in increased synthesis of type I and type III, fibrillar collagens; the hepatic stellate cell is a major producer of theses ECM components. Overall, it is estimated that up to a 10-fold increase in ECM components occurs in fibrotic liver [137] and this can ultimately lead to hepatic dysfunction and disease progression.
The fibrotic ECM is not static and is constantly remodeled. Indeed, production of ECM fragments exhibit different physiological or pathophysiological properties when compared to native ECM proteins. These bioactive fragments are able to affect fibroblast and macrophage function and can promote inflammation and fibrosis. For example, LMW-HA fragments generated from high molecular weight (HMW)-HA when tissues are injured promote inflammation after binding to TLRs on the surface of macrophages [139–141]. Likewise, laminin [138, 142] and elastin [143, 144] protolytic fragments bind to EGFR and facilitate cell migration and proliferation. While no studies to date have explored the effect of ECM degradation products on HSC function, sufficient evidence from other in vivo and in vitro systems which explore how these fragments affect fibroblasts isolated from other tissues would suggest similar changes occur in the HSC.
Changes in structural ECM components are paralleled by changes in the synthesis of growth factors and other molecules. Many of these molecules are intimately associated with the ECM and are sequestered and protected by the ECM or whose activity is enhanced by ECM interactions. For example, fibroblast growth factor (FGF) 2, a molecule involved in HSC activation, binds to heparan sulfate and is required for FGF’s activity [138]. Likewise, certain isoforms of vascular endothelial cell growth factor (VEGF), a growth factor associated with pathogenic angiogenesis in fibrosis, are also sequestered by heparan sulfate in the ECM. ECM-sequestered VEGF is released by plasmin found in wounded tissue thereby facilitating angiogenesis [138, 145]. ECM-growth factor interactions are not limited to FGF2 and VEGF. Indeed, TGFβ, found in a large latent complex with latency-associated peptide and latent-TGFβ–binding protein, is anchored to the ECM and is required for proper TGFβ activation. Release of active TGFβ can be mediated by thrombospondin 1, αvβ6 integrin, MMP2 and MMP9, and plasmin each of which contribute to activation of resident fibroblast populations and synthesis of ECM [146]. Taken together, the dynamic nature of ECM remodeling during fibrosis has considerable capacity to affect HSC function and should be explored in future studies.
1.4.5. Matrix Stiffness and Hepatic Stellate Cell Activation
In addition to biochemical signals, mechanical signals contribute to initiation and perpetuation of HSC activation. Specifically, matrix rigidity or stiffness is an important factor. In vitro, quiescent primary HSC spontaneously activate over time in culture (7–10d for full activation) on tissue culture plastic, a stiff environment compared to a healthy liver. Culturing quiescent HSC on a basement membrane-like substrate (Matrigel), maintains quiescence, while culturing myofibroblasts on a similar Matrigel substrate attenuates their activated phenotype [147–149]. Following these observations, Olsen et al. carefully dissected the effect of defined polyacrylamide stiffness on HSC activation and found that soft substrates (0.4–1.0 kPa) maintained quiescence while stiff substrates (8–12 kPa) promoted activation. These effects where independent of the matrix protein coating the different polyacrylamide supports. Substrate stiffness did not just slow down the activation/differentiation process, for even cells cultured on substrates with intermediate stiffness (1.75–2.5 kPa) exhibited an intermediate activation phenotype even after 2 weeks of culture [150].
A relationship between matrix stiffness and activation of HSC, in vivo, is also appreciated. For example, Georges et al. convincingly demonstrated that increased liver stiffness occurred before indications of myofibroblast differentiation and ECM synthesis [151] and that this early increase in mechanical stiffness is absolutely required for HSC and PF activation [150, 152]. Subsequent work by the same group showed using a mouse model that the early increase in hepatic stiffness was due to induction of lysyl oxidase (LOX) activity and not simply edema after CCl4 exposure. These data are supported by the observation that treatment of mice with β-aminoproprionitrile (BAPN), an inhibitor of LOX family cross-linking enzymes, reduces early increases in liver stiffness, fibrosis and numbers of myofibroblasts [151]. Finally, HSC and PF are the major sources of LOX isoforms prior to their activation and differentiation into myofibroblasts [153] suggesting that they are major catalysts to their own activation upon liver injury. Taken together, these data clearly demonstrate that HSC are able to sense the mechanical properties of the microenvirontment and respond by engaging either an activation or quiescence program based on substrate stiffness.
1.4.6. Role of Liver Sinusoidal Endothelial Cells (LSECs) in Hepatic Stellate Cell Activation
Changes in LSECs are also critical determinants of HSC activation. Indeed, LSECs lose their fenestrae and develop a basement membrane akin to that found in blood capillaries in other places of the body [154]. This phenotypic change is called capillarization. Changes in LSEC morphology are associated with increased expression of CD31, a marker for vascular endothelial cells, and laminin, a protein found in vascular endothelium [155–157]; these changes contribute to fibrosis. Critically, LSECs regulate HSC activation independent of capillarization status. For example, when quiescent HSC are co-cultured with differentiated (noncapillarized) LSECs, with or without cell contact, HSC quiescence is maintained [158]; this effect is mediated, at least in part, by VEGF-induced nitric oxide production. Likewise, when differentiated LSEC are co-cultured with activated HSC, HSC revert to a quiescent phenotype. Taken together, these data suggest that cross-talk between LSEC and HSC is a critical determinant of liver fibrogenesis.
1.5. What Can We Learn About Liver Fibrosis from Fetal/Oral vs Dermal Wound Healing?
The wound-healing response, as described earlier in this review, is very well characterized in the adult skin, the end result of which is imperfect repair characterized by scar formation and reduced tissue strength. In that earlier discussion, three outcomes of tissue injury were discussed: normal repair in which tissue equilibrium is reestablished, deficient healing as found in chronic wounds from diabetic patients and excessive healing associated with fibrosis [15]. However, there is a fourth outcome in which complete replacement of injured tissue occurs in the absence of scar formation. This describes “ideal tissue repair” [159] or regeneration and occurs without scar formation. While this is most clearly demonstrated in limb regrowth in lower vertebrates such as salamanders [160], scarless wound healing is maintained under some circumstances in more recently evolved vertebrates including mouse and man [161]. Specifically, cutaneous wounds in fetal skin, especially in the first 24 weeks of gestation in humans or first 18 days in mice, and in oral mucosa, heal without scar formation [162–164]. In the dermal wound healing literature, considerable attention is given to this special response to tissue injury. The expectation is that understanding how wounds heal without a scar should provide insights on how to limit dermatologic scarring and its associated functional, structural and aesthetic deficits. It is important to point out that liver repair should also be classified in this special category of scarless wound healing in the context of non-iterative tissue injury. Therefore, the insights revealed from examination of fetal/oral wound healing could also apply to non-iterative wound healing in the liver. By contrast, adult skin wound healing more closely reflects liver fibrosis after chronic liver injury. Interestingly, this association has not yet been explored.
In the discussion which follows, a comparison between fetal/oral wound healing and dermal wound healing will be presented. Several differences between the two processes are illustrated which shed light on why scars are established in adult skin, but not in fetal skin or oral mucosa. In the liver, injury is repaired completely as long as liver injury does not persist paralleling fetal skin and oral wound healing. Conversely, unrelenting cycles of injury and coupled to incomplete repair favor fibrotic wound healing and morphologically reflect wound repair in adult skin. It is possible that novel insights into why and how the liver shifts from a regenerative process after acute liver injury to an unrelenting fibrotic response in response to chronic liver injury could be achieved through a careful examination of the skin wound healing literature.
1.5.1. Role of Inflammation in Scarless vs. Scarring Wound Healing
Relative to adult wound repair, the hallmark of fetal repair is reduced inflammation [165, 166]. For example, proinflammatory cytokines such as interleukin (IL)1, IL6 and IL8 are reduced in fetal wound repair [167]. Consistently, if inflammation is increased in fetal wounds, those wounds recruit macrophages, induce fibroblast proliferation and collagen deposition similar to adult wounds [168]. In part, increased production of IL10, a cytokine with potent anti-inflammatory activity, may be responsible for reduced inflammatory cytokine production in fetal wounds [169]. Indeed, fetal wounds in IL10 knockout mice heal with scars [170], supporting an important role of IL10 in scarless wound healing. Conversely, in adult tissues, over-expression of IL10 attenuates inflammation and scar tissue formation [171]. In addition to differential cytokine production, fundamental differences in wound cellular infiltrate exist between fetal and adult wounds. Specifically, wounds which heal without a scar exhibit reduced or absent neutrophil and macrophage infiltration [165]. Interestingly, although wounds in the aged heal more slowly, they do so with less inflammation and reduced scar formation [163]. In the liver, persistent inflammation after chronic injury parallels adult skin wound healing, while a temporally-regulated inflammatory response after acute injury parallels fetal/oral wound healing. Collectively, these data suggest that reduced humoral and cellular inflammation is critical for scarless wound healing.
Recent data suggest that another mechanism by which fetal wounds heal scarlessly involves the absence of microbial colonization. Evidence for this hypothesis is provided in a study which used germ-free (GF) mice. Indeed, germ-free mice exhibit scarless dermal wound healing which can be reversed by bacterial colonization [172]. Even though the parallel between skin bacterial colonization and gut-derived PAMP delivery to the liver is not perfect, it is tempting to speculate that changes in the gut microbiome and increased bacterial product delivery to the liver in liver disease may somewhat mimic the role the dermal microbiome has in skin wound healing. While intrahepatic sources of inflammation also exist (e.g. cell death/DAMP-mediated sterile inflammation), limiting the contribution of extrahepatic sources of inflammation-inducing molecules (i.e. gut-derived PAMPs) may still limit hepatic inflammation and fibrogenesis. Many additional features are associated with scarless wound healing in GF mice including high expression of IL10 and TNFα, sustained wound-healing macrophage infiltration and reduced TGFβ suggesting additional mechanisms are critically important in scarless wound healing [172].
It is not just the accumulation of proinflammatory or profibrotic mediators which dictates whether or not the wound heals with or without a scar, but, perhaps more importantly, the temporal expression pattern of those molecules. For example, a low-level TGFβ1 is induced in fetal wounds. However, its expression is rapid and transient, in contrast to adult wounds where expression of TGFβ1 is delayed and sustained [173]. The importance of sustained TGFβ1 and TGFβ2 to scar formation is revealed when both isoforms are blocked using neutralizing antibodies; in dermal wounds receiving this treatment, scar formation is reduced [174]. Another TGFβ isoform, TGFβ3, appears to have anti-fibrotic roles. For example, addition of TGFβ3 can reduce scarring in animals and humans [174]. Also, while TGFβ3 induction is delayed in adult wounds, its levels are increased in fetal wounds and its expression is prolonged [175, 176]. Indeed, fetal wounds exhibit an increased ratio of TGFβ3 to TGFβ2 or TGFβ1, presumably favoring reduced scar formation after wounding [177, 178].
Regulation of profibrotic growth factor expression is not limited to TGFβ. For example, PDGF levels are reduced in fetal wounds relative to adult wounds [179] and exposure of fetal wounds to PDGF not only increases inflammation but also recruitment of fibroblasts and results in a fibrotic response [180]. Taken together, this evidence suggests that wound healing in the absence of scar formation (e.g. in fetal/oral tissues or after acute liver injury) is associated with precisely regulated inflammation and pro-fibrotic growth factor production. By contrast, in adult wounds which heal with a scar or in liver in the context of chronic liver injury, persistent inflammation and profibrotic growth factor production predominate.
1.5.2. ECM Composition and Remodeling in Scarless Wound Healing
In addition to altered inflammatory and fibrotic processes described above, differences in the wound ECM composition dictate whether or not wounds heal with a scar. In particular, increased content of type III collagen relative to type I collagen is characteristic of scarless wound healing [181]. In fetal wounds, type III collagen is rapidly deposited in a reticular network similar to that found in unwounded tissue. By contrast, collagen synthesis is delayed in adult wounds and consists of more type I collagen than type III collagen [164, 182]. Generally speaking, a type I collagen matrix provides more strength but impedes cell migration and regeneration relative to a matrix consisting predominantly of type III collagen [164]. Collectively, these data are consistent with differential roles for type I and type III collagen in the outcome of the wound healing response.
Other ECM components differentially participate in scarring vs scarless wound healing. For example, fibronectin (Fn), an ECM protein expressed early after wounding, provides a provisional matrix on which the wounded tissue can regenerate and also participates in crosstalk between the ECM and cells [183, 184]. Fn has splice variants with distinct roles in wound healing and fibrosis; up to 20 different variants are observed in humans [184]. In particular, prolonged Fn extra domain A (Fn ED-A) tissue accumulation is associated with tissue fibrosis. Fn ED-A can activate ECM-bound latent TGFβ [183], which is crucial for myofibroblast differentiation [185]. In this way, Fn ED-A directly supports fibrogenesis. For example, Fn ED-A is increased in scarring dermal and non-scarring oral wounds in pigs and humans, but its levels are reduced more rapidly in oral wounds of both species [186]. Likewise, the accumulation and maintenance of Tenacsin C, another ECM component, in wounded tissues is also associated with scarring in human and pig wounds [186].
How wounded tissue remodels its ECM dictates the outcome of wound healing. Fetal wounds are characterized by an increase in MMPs compared to TIMPs, favoring complete tissue regeneration instead of ECM accumulation [187, 188]. By contrast, fibroblasts found in keloid scars and keratinocytes found in hypertrophic scars exhibit increased production of TIMP1 favoring scar accumulation [189, 190]. This is also found for urokinase-type plasminogen activator and its inhibitor plasminogen activator inhibitor 1 [191]. Finally, fetal scarless wounds have reduced expression of LOX, a matrix stabilizing molecule which is also a current target of liver fibrosis therapies. Consistently, in transition from scarless fetal wound healing to adult wound healing, type I collagen crosslinking increases [192].
The proteases involved in ECM remodeling also play important roles in the activation of latent growth factors [138, 162, 169]; activation of these growth factors could therefore be differentially regulated in wounds which heal with or without a scar. However, aside from data linking activation of TGFβ by MMPs and other enzymes during wound healing [146], direct studies which compare matrix-associated enzymes with growth factor sequestration, activation or deactivation in wounds which heal with or without a scar have not yet been conducted. However, based on differences in temporal accumulation of various growth factors including platelet derived growth factor and FGF which are intricately associated with the ECM, this is an important area for investigation.
These and other studies suggest that fundamental differences in ECM components, content, crosslinking and remodeling determine whether or not wounds heal with a scar. HA is another ECM component with differential roles in fetal vs adult wound healing. The remainder of this review article will concentrate on HA, and its roles in wound healing, and conclude with how this information may be leveraged to explore how HA content and molecular mass could be manipulated to attenuate liver fibrosis.
1.6. Hyaluronan: A Novel Target for Therapeutic Manipulation in Liver Fibrosis?
1.6.1. Basic Biology of HA
HA is an anionic glycosaminoglycan consisting of repeating N-acetylglucosamine and glucuronic acid disaccharide units [193]. It is a ubiquitous molecule found predominantly in the ECM and is a major component of articular joints, vitreous humor of the eye and skin. Three mammalian HA synthases exist: (HAS)1, 2 and 3 [194]. They differ based on biosynthetic capacity, induction profiles and also based on tissue expression levels [194–196]. In healthy tissue, HA is a large molecule, upwards of 1 MDa and is purported to exhibit homeostatic and anti-inflammatory activity [139]. However, HA can be degraded by hyaluronidases or reactive oxygen species into LMW fragments with considerable polydispersity [140]; this occurs when tissues are injured and contributes to inflammation through HA’s DAMP function [140]. LMW-HA fragments signal through TLR2, TLR4 and CD44 to promote inflammation and fibrosis [141]. Indeed, in an animal model of idiopathic pulmonary fibrosis, mice deficient in CD44, and therefore unable to clear LMW-HA fragments, exhibit increased inflammation and fibrosis when compared to wild-type mice.
1.6.2. Differential Roles for Hyaluronan in Scaring vs. Non-Scaring Wounds
HA’s synthesis and function differs between scarless and scar-forming wounds [169]. For example, Has enzyme expression differs between oral mucosa and adult skin: oral mucosal fibroblasts do not express Has1 mRNA, while Has3 mRNA is abundant [197]. Conversely, Has1 is, while Has3 is not, expressed by dermal fibroblasts [197]. Accumulation of Has2 mRNA is not different between these two fibroblast populations. Has enzyme expression, therefore, is associated with scarless (Has3) vs scar-forming (Has1) wound healing responses. Our unpublished data support these observations. Specifically, HSC activation, measured by hepatic type I collagen and αSMA mRNA accumulation, is increased in livers from mice deficient in HAS3 after acute CCl4 exposure [198].
Differences in HA polymer content in wounded tissue also help differentiate between wounds that heal with or without a scar. For example, HA is persistently enriched in fetal wounds in comparison to adult wounds [199, 200]. This is, in part, regulated by IL10, and requires STAT3 [201, 202]. Interestingly, a specific HA synthesis-stimulating activity is found in fetal wounds, but not adult wounds [203, 204] consistent with increased HA accumulation in fetal tissue. Oral wounds also exhibit increased HA [186], supporting the idea that similarities in HA content exist between fetal and oral scarless wound healing. Another mechanism which supports HA persistence in fetal wounds is reduced hyaluronidase activity. Reduced hyaluronidase activity limits HA degradation and maintains high levels of HMW-HA [159]. These changes foster an extracellular environment which is permissive of cell motility and cell proliferation facilitating tissue regeneration [203, 205].
On its own, HMW-HA exhibits anti-inflammatory and homeostatic properties [139]. Given reduced inflammation and reduced presence of HA-degrading enzymes, it is likely that the HA found in fetal wounds is predominantly HMW while adult wounds likely contain proportionally more LMW-HA [206]. Consistently, fibrotic healing of adult and late gestational fetal wounds is associated with increased hyaluronidase activity relative to fetal wounds. Notably, LMW-HA has potent proinflammatory and angiogenic activity [207] as found in adult dermal wound healing. In further support of a beneficial role for HMW-HA in wound healing, it is possible to attenuate scar formation in adults through application of HA to dermal wounds [169]. Beyond these rolls for HA in scarless wound healing, one might argue that increasing a wound’s content of HMW-HA creates a mechanically soft environment limiting fibroblast activation. Collectively, these data suggest that increasing the content of HA in a wound promotes scarless wound healing and phenocopies complete tissue regeneration [208].
1.6.3. Hyaluronan in Liver Disease
Despite a well-appreciated role for plasma HA as a biomarker for liver disease [209], few studies have explored the role of HA in the pathogenesis or resolution of liver disease. Importantly, no studies determined HA polydispersity in plasma or liver tissue from healthy people or patients with liver disease. What is known includes the observation that HSC can synthesize HA and do so in response to partial hepatectomy, a surgical model of liver regeneration [210]. In addition, treating mice with HMW-HA, but not LMW-HA, can prevent apoptotic liver injury (concanavalin A and galactosamine + LPS models) and is associated with reduced proinflammatory cytokines, including TNFα, interferon gamma, macrophage inflammatory protein 2 and IL4, in mice [211]. HA also exerts some protection against CCl4-induced liver injury, lipid peroxidation and fibrosis in rats [212], but this study is not clear on the molecular mass of HA employed, making clear interpretation of the results difficult. Additional evidence suggests that LMW-HA promotes the expression and function of MMP13 [213, 214] as well as MMP9 [215], suggesting a possible target for HA-mediated therapeutic intervention in liver fibrosis. Our own work supports this hypothesis. Has3−/− mice who exhibit an increase in LMW-HA relative to HMW-HA after CCl4 exposure exhibit increased MMP13 mRNA, active form of the protein and MMP13-mediated matrix metabolizing activity [198]. Collectively, these data provide strong rationale for continued explorations into the role HA may play in liver fibrosis, the modulation of which may provide a novel therapeutic avenue to explore in the future.
CONCLUSION
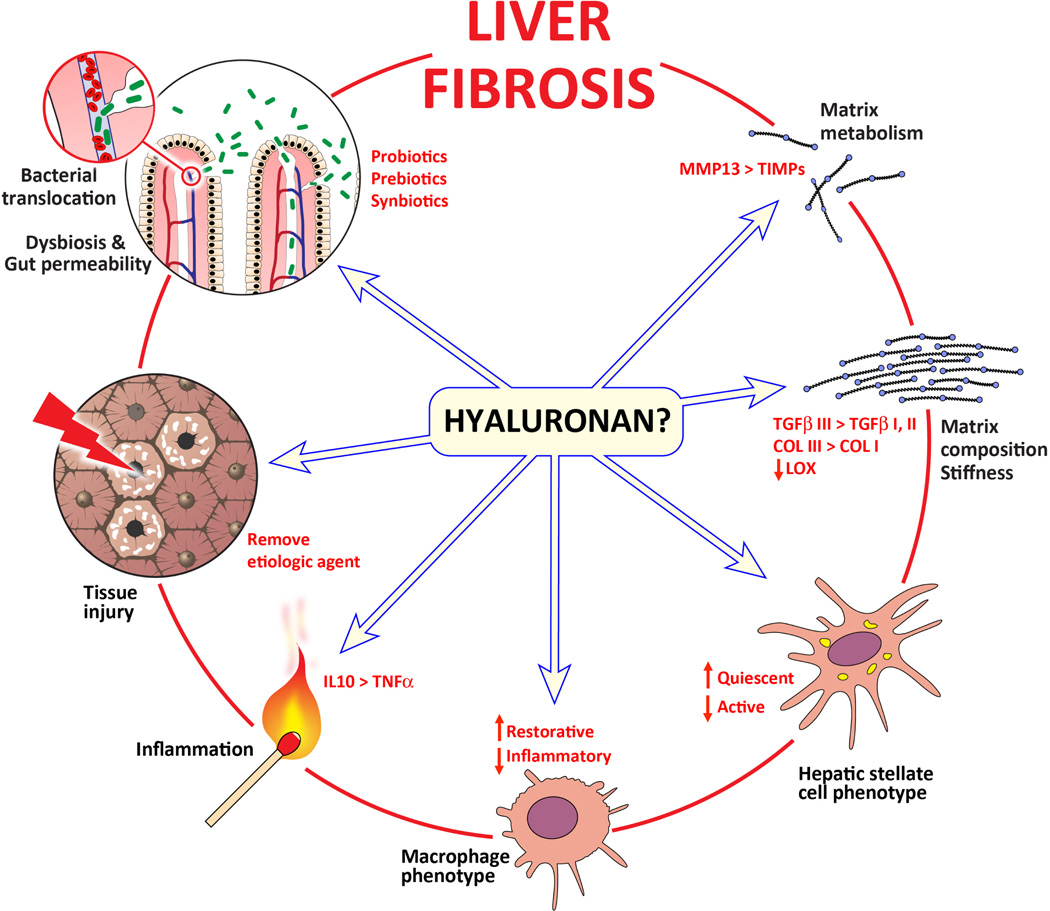
The liver is unique in that it can fully regenerate after non-iterative injury. This is most clearly demonstrated in liver regeneration after acute hepatotoxicant exposure or after partial hepatectomy [216, 217]. It is only after chronic injury that the liver loses this ability, becomes fibrotic and loses its functional capacity. Macrophages, both resident and infiltrating, as well as HSC, play critical roles in fibrogenesis, fibrosis progression and fibrosis resolution; the temporal regulation of macrophage and HSC function dictates the outcome of hepatic wound healing response. In this review, several parallels between wounds which heal with or without a scar and the regenerative vs fibrotic wound healing of the liver were presented. Evaluation of the mechanisms by which scarless wound healing occurs in fetal and oral wounds provides additional support for the development of therapeutic approaches targeting the microbiome, inflammation, macrophage recruitment and phenotype, HSC activation and the ECM composition and remodeling to halt fibrosis progression and accelerate fibrosis resolution. While additional therapeutic approaches which target each of these distinct aspects of scarless wound healing, could be (or are being) developed, focusing attention on HA which may unify several of those profibrotic features makes it an attractive therapeutic target. Specifically, evidence from the scarless wound healing literature suggests that HA could dampen inflammation (both cellular and humoral) by shifting the balance away from sustained tissue injury and towards tissue homeostasis as well as through altering matrix composition and remodeling. Each of these could be therapeutically manipulated to attenuate fibrogenesis, halt fibrosis progression, or accelerate fibrosis resolution (Fig. 1). Therefore, additional studies which interrogate HA’s protective and pathogenic roles in the context of liver injury, inflammation, fibrosis and fibrosis resolution are warranted and may support further development of HA-based therapeutic strategies which target liver disease.
Fig. (1).
Therapeutic targets for liver fibrosis. Several intervention points exist for which therapeutic strategies for liver fibrosis can be or are being developed. Some of the factors which contribute to liver fibrosis are written in black and found outside the circle. Some therapeutic strategies/targets are written in red and found inside the circle. From the discussion presented in this review, published evidence suggests that the extracellular matrix glycosaminoglycan, hyaluronan (HA), in its native, high molecular weight form, has the potential to reduce chronic inflammation, facilitate tissue repair and improve the extracellular matrix compliance. Leveraging HA’s anti-inflammatory and pro-homeostatic functions may ‘fetalize’ the liver response to chronic injury and therefore improve hepatic wound healing and attenuate fibrosis or facilitate fibrosis resolution.
Acknowledgments
Special thanks to Stanton Fernald, BFA, MA of the Kansas Intellectual and Developmental Disabilities Research Center’s Integrative Imaging Core at the University of Kansas Medical Center who created the model diagram in Fig. (1). This work was supported by grants to M.T.P. (P20 GM103549, P20 GM103549, R00 AA017918) and J.M.M. (T32 ES007079).
Biography

Michele T. Pritchard
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141(5):1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bataller R, Rombouts K, Altamirano J, Marra F. Fibrosis in alcoholic and nonalcoholic steatohepatitis. Best Pract Res Clin Gastroenterol. 2011;25(2):231–244. doi: 10.1016/j.bpg.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Gunay-Aygun M. Liver and kidney disease in ciliopathies. Am J Med Genet C Semin Med Genet. 2009;151C(4):296–306. doi: 10.1002/ajmg.c.30225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masarone M, Federico A, Abenavoli L, Loguercio C, Persico M. Non alcoholic Fatty liver: epidemiology and natural history. Rev Recent Clin Trials. 2014;9(3):126–133. doi: 10.2174/1574887109666141216111143. [DOI] [PubMed] [Google Scholar]
- 5.Ismail MH, Pinzani M. Reversal of hepatic fibrosis: pathophysiological basis of antifibrotic therapies. Hepat Med. 2011;3:69–80. doi: 10.2147/HMER.S9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Invest. 2013;123(5):1887–1901. doi: 10.1172/JCI66028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceni E, Mello T, Galli A. Pathogenesis of alcoholic liver disease: Role of oxidative metabolism. World J Gastroenterol. 2014;20(47):17756–17772. doi: 10.3748/wjg.v20.i47.17756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paik YH, Kim J, Aoyama T, De Minicis S, Bataller R, Brenner DA. Role of NADPH oxidases in liver fibrosis. Antioxid Redox Signal. 2014;20(17):2854–2872. doi: 10.1089/ars.2013.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells RG. The portal fibroblast: not just a poor man’s stellate cell. Gastroenterology. 2014;147(1):41–47. doi: 10.1053/j.gastro.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. 2014;147(4):765–83. doi: 10.1053/j.gastro.2014.07.018. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wick G, Grundtman C, Mayerl C, et al. The immunology of fibrosis. Annu Rev Immunol. 2013;31:107–135. doi: 10.1146/annurev-immunol-032712-095937. [DOI] [PubMed] [Google Scholar]
- 12.Ni HM, Williams JA, Yang H, Shi YH, Fan J, Ding WX. Targeting autophagy for the treatment of liver diseases. Pharmacol Res. 2012;66(6):463–474. doi: 10.1016/j.phrs.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127(3):514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 14.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117(5):1219–1222. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 16.Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008;47(2):729–736. doi: 10.1002/hep.22034. [DOI] [PubMed] [Google Scholar]
- 17.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41(1):21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freudenberg N, Schalk J, Galanos C, et al. Identification and percentage frequency of isolated non-parenchymal liver cells (NPLC) in the mouse. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;57(2):109–115. doi: 10.1007/BF02899071. [DOI] [PubMed] [Google Scholar]
- 19.Sleyster EC, Knook DL. Relation between localization and function of rat liver Kupffer cells. Lab Invest. 1982;47(5):484–490. [PubMed] [Google Scholar]
- 20.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41(3):422–433. doi: 10.1002/hep.20632. [DOI] [PubMed] [Google Scholar]
- 23.Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol. 1987;4(1):8–14. doi: 10.1016/s0168-8278(87)80003-x. [DOI] [PubMed] [Google Scholar]
- 24.Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol. 1991;12(2):162–169. doi: 10.1016/0168-8278(91)90933-3. [DOI] [PubMed] [Google Scholar]
- 25.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43(2 Suppl 1):S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 26.Knolle P, Schlaak J, Uhrig A, Kempf P, Meyer zum Buschenfelde KH, Gerken G. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J Hepatol. 1995;22(2):226–229. doi: 10.1016/0168-8278(95)80433-1. [DOI] [PubMed] [Google Scholar]
- 27.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 28.Gao B, Seki E, Brenner DA, et al. Innate immunity in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2011;300(4):G516–G525. doi: 10.1152/ajpgi.00537.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014;147(3):577–94. doi: 10.1053/j.gastro.2014.06.043. e1. [DOI] [PubMed] [Google Scholar]
- 30.Thurman RG., II Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275(4 Pt 1):G605–G611. doi: 10.1152/ajpgi.1998.275.4.G605. [DOI] [PubMed] [Google Scholar]
- 31.Nagy LE. Recent insights into the role of the innate immune system in the development of alcoholic liver disease. Exp Biol Med (Maywood) 2003;228(8):882–890. doi: 10.1177/153537020322800803. [DOI] [PubMed] [Google Scholar]
- 32.Dixon LJ, Barnes M, Tang H, Pritchard MT, Nagy LE. Kupffer cells in the liver. Compr Physiol. 2013;3(2):785–797. doi: 10.1002/cphy.c120026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20(2):453–460. [PubMed] [Google Scholar]
- 34.Koop DR, Klopfenstein B, Iimuro Y, Thurman RG. Gadolinium chloride blocks alcohol-dependent liver toxicity in rats treated chronically with intragastric alcohol despite the induction of CYP2E1. Mol Pharmacol. 1997;51(6):944–950. doi: 10.1124/mol.51.6.944. [DOI] [PubMed] [Google Scholar]
- 35.Fouts DE, Torralba M, Nelson KE, Brenner DA, Schnabl B. Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. J Hepatol. 2012;56(6):1283–1292. doi: 10.1016/j.jhep.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 38.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut micro-biome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol. 2009;587(Pt 17):4153–4158. doi: 10.1113/jphysiol.2009.174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan AW, Fouts DE, Brandl J, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53(1):96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miele L, Valenza V, La Torre G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49(6):1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 42.Chang CS, Chen GH, Lien HC, Yeh HZ. Small intestine dysmotility and bacterial overgrowth in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 1998;28(5):1187–1190. doi: 10.1002/hep.510280504. [DOI] [PubMed] [Google Scholar]
- 43.Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48(2):206–211. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaeta GB, Perna P, Adinolfi LE, Utili R, Ruggiero G. Endotoxemia in a series of 104 patients with chronic liver diseases: prevalence and significance. Digestion. 1982;23(4):239–244. doi: 10.1159/000198756. [DOI] [PubMed] [Google Scholar]
- 45.Lin RS, Lee FY, Lee SD, et al. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;22(2):165–172. doi: 10.1016/0168-8278(95)80424-2. [DOI] [PubMed] [Google Scholar]
- 46.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 47.Inokuchi S, Tsukamoto H, Park E, Liu ZX, Brenner DA, Seki E. Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol Clin Exp Res. 2011;35(8):1509–1518. doi: 10.1111/j.1530-0277.2011.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34(1):101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 49.Yin M, Bradford BU, Wheeler MD, et al. Reduced early alcohol-induced liver injury in CD14-deficient mice. J Immunol. 2001;166(7):4737–4742. doi: 10.4049/jimmunol.166.7.4737. [DOI] [PubMed] [Google Scholar]
- 50.Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108(1):218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 51.Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47(4):571–579. doi: 10.1016/j.jhep.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miura K, Ohnishi H. Role of gut microbiota and Toll-like receptors in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20(23):7381–7391. doi: 10.3748/wjg.v20.i23.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chassaing B, Etienne-Mesmin L, Gewirtz AT. Microbiota-liver axis in hepatic disease. Hepatology. 2014;59(1):328–339. doi: 10.1002/hep.26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cani PD, Delzenne NM. The gut microbiome as therapeutic target. Pharmacol Ther. 2011;130(2):202–212. doi: 10.1016/j.pharmthera.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 55.Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148(1):30–36. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu L, Baker SS, Gill C, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57(2):601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 57.Rao RK, Seth A, Sheth P. Recent advances in alcoholic liver disease I. role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286(6):G881–G884. doi: 10.1152/ajpgi.00006.2004. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, Kirpich I, Liu Y, et al. Lactobacillus rhamnosus GG treatment potentiates intestinal hypoxia-inducible factor, promotes intestinal integrity and ameliorates alcohol-induced liver injury. Am J Pathol. 2011;179(6):2866–2875. doi: 10.1016/j.ajpath.2011.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tabibian JH, O’Hara SP, Lindor KD. Primary sclerosing cholangitis and the microbiota: current knowledge and perspectives on etiopathogenesis and emerging therapies. Scand J Gastroenterol. 2014;49(8):901–908. doi: 10.3109/00365521.2014.913189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ngu JH, Gearry RB, Wright AJ, Stedman CA. Inflammatory bowel disease is associated with poor outcomes of patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2011;9(12):1092–1097. doi: 10.1016/j.cgh.2011.08.027. quiz e135. [DOI] [PubMed] [Google Scholar]
- 61.Welcker K, Martin A, Kolle P, Siebeck M, Gross M. Increased intestinal permeability in patients with inflammatory bowel disease. Eur J Med Res. 2004;9(10):456–460. [PubMed] [Google Scholar]
- 62.Tabibian JH, Talwalkar JA, Lindor KD. Role of the microbiota and antibiotics in primary sclerosing cholangitis. Biomed Res Int. 2013;2013:389537. doi: 10.1155/2013/389537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kishore R, McMullen MR, Cocuzzi E, Nagy LE. Lipopolysaccharide-mediated signal transduction: Stabilization of TNF-alpha mRNA contributes to increased lipopolysaccharide-stimulated TNF-alpha production by Kupffer cells after chronic ethanol feeding. Comp Hepatol. 2004;(3 Suppl 1):S31. doi: 10.1186/1476-5926-2-S1-S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McMullen MR, Pritchard MT, Wang Q, Millward CA, Croniger CM, Nagy LE. Early growth response-1 transcription factor is essential for ethanol-induced fatty liver injury in mice. Gastroenterology. 2005;128(7):2066–2076. doi: 10.1053/j.gastro.2005.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kishore R, Hill JR, McMullen MR, Frenkel J, Nagy LE. ERK1/2 and Egr-1 contribute to increased TNF-alpha production in rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastroin-test Liver Physiol. 2002;282(1):G6–G15. doi: 10.1152/ajpgi.00328.2001. [DOI] [PubMed] [Google Scholar]
- 66.McMullen MR, Cocuzzi E, Hatzoglou M, Nagy LE. Chronic ethanol exposure increases the binding of HuR to the TNFalpha 3’-untranslated region in macrophages. J Biol Chem. 2003;278(40):38333–38341. doi: 10.1074/jbc.M304566200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kishore R, McMullen MR, Nagy LE. Stabilization of tumor necrosis factor alpha mRNA by chronic ethanol: role of A + U-rich elements and p38 mitogen-activated protein kinase signaling pathway. J Biol Chem. 2001;276(45):41930–41937. doi: 10.1074/jbc.M107181200. [DOI] [PubMed] [Google Scholar]
- 68.Hritz I, Mandrekar P, Velayudham A, et al. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48(4):1224–1231. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao XJ, Dong Q, Bindas J, et al. TRIF and IRF-3 binding to the TNF promoter results in macrophage TNF dysregulation and steatosis induced by chronic ethanol. J Immunol. 2008;181(5):3049–3056. doi: 10.4049/jimmunol.181.5.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Petrasek J, Dolganiuc A, Csak T, et al. Interferon regulatory factor 3 and type I interferons are protective in alcoholic liver injury in mice by way of crosstalk of parenchymal and myeloid cells. Hepatology. 2011;53(2):649–660. doi: 10.1002/hep.24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lorenz E, Mira JP, Frees KL, Schwartz DA. Relevance of mutations in the TLR4 receptor in patients with gram-negative septic shock. Arch Intern Med. 2002;162(9):1028–1032. doi: 10.1001/archinte.162.9.1028. [DOI] [PubMed] [Google Scholar]
- 72.Agnese DM, Calvano JE, Hahm SJ, et al. Human toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of gram-negative infections. J Infect Dis. 2002;186(10):1522–1525. doi: 10.1086/344893. [DOI] [PubMed] [Google Scholar]
- 73.Huang H, Shiffman ML, Friedman S, et al. A 7 gene signature identifies the risk of developing cirrhosis in patients with chronic hepatitis C. Hepatology. 2007;46(2):297–306. doi: 10.1002/hep.21695. [DOI] [PubMed] [Google Scholar]
- 74.Guo J, Loke J, Zheng F, et al. Functional linkage of cirrhosis-predictive single nucleotide polymorphisms of Toll-like receptor 4 to hepatic stellate cell responses. Hepatology. 2009;49(3):960–968. doi: 10.1002/hep.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143(5):1158–1172. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 76.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 77.Constandinou C, Henderson N, Iredale JP. Modeling liver fibrosis in rodents. Methods Mol Med. 2005;117:237–250. doi: 10.1385/1-59259-940-0:237. [DOI] [PubMed] [Google Scholar]
- 78.Avasarala S, Yang L, Sun Y, et al. A temporal study on the histopathological, biochemical and molecular responses of CCl(4)-induced hepatotoxicity in Cyp2e1-null mice. Toxicology. 2006;228(2–3):310–322. doi: 10.1016/j.tox.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 79.Wong FW, Chan WY, Lee SS. Resistance to carbon tetrachloride-induced hepatotoxicity in mice which lack CYP2E1 expression. Toxicol Appl Pharmacol. 1998;153(1):109–118. doi: 10.1006/taap.1998.8547. [DOI] [PubMed] [Google Scholar]
- 80.Gruebele A, Zawaski K, Kaplan D, Novak RF. Cytochrome P4502E1- and cytochrome P4502B1/2B2-catalyzed carbon tetrachloride metabolism: effects on signal transduction as demonstrated by altered immediate-early (c-Fos and c-Jun) gene expression and nuclear AP-1 and NF-kappa B transcription factor levels. Drug Metab Dispos. 1996;24(1):15–22. [PubMed] [Google Scholar]
- 81.Johansson I, Ingelman-Sundberg M. Carbon tetrachloride-induced lipid peroxidation dependent on an ethanol-inducible form of rabbit liver microsomal cytochrome P-450. FEBS Lett. 1985;183(2):265–269. doi: 10.1016/0014-5793(85)80790-0. [DOI] [PubMed] [Google Scholar]
- 82.Luckey SW, Petersen DR. Activation of Kupffer cells during the course of carbon tetrachloride-induced liver injury and fibrosis in rats. Exp Mol Pathol. 2001;71(3):226–240. doi: 10.1006/exmp.2001.2399. [DOI] [PubMed] [Google Scholar]
- 83.Recknagel RO, Glende EA, Jr, Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther. 1989;43(1):139–154. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 84.Rivera CA, Bradford BU, Hunt KJ, et al. Attenuation of CCl(4)-induced hepatic fibrosis by GdCl(3) treatment or dietary glycine. Am J Physiol Gastrointest Liver Physiol. 2001;281(1):G200–G207. doi: 10.1152/ajpgi.2001.281.1.G200. [DOI] [PubMed] [Google Scholar]
- 85.Nolan JP, Leibowitz AI. Endotoxin and the liver. III. Modification of acute carbon tetrachloride injury by polymyxin b--an antiendotoxin. Gastroenterology. 1978;75(3):445–449. [PubMed] [Google Scholar]
- 86.Rutenburg AM, Sonnenblick E, Koven I, Aprahamian HA, Reiner L, Fine J. The role of intestinal bacteria in the development of dietary cirrhosis in rats. J Exp Med. 1957;106(1):1–14. doi: 10.1084/jem.106.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ingersoll MA, Spanbroek R, Lottaz C, et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115(3):e10–e19. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Karlmark KR, Weiskirchen R, Zimmermann HW, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50(1):261–274. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- 89.Sica A, Invernizzi P, Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology. 2014;59(5):2034–2042. doi: 10.1002/hep.26754. [DOI] [PubMed] [Google Scholar]
- 90.Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14(3):181–194. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- 91.Duffield JS, Forbes SJ, Constandinou CM, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115(1):56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274(31):21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 93.Duarte S, Baber J, Fujii T, Coito AJ. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. 2015 doi: 10.1016/j.matbio.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Knauper V, Lopez-Otin C, Smith B, Knight G, Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996;271(3):1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- 95.Fallowfield JA, Mizuno M, Kendall TJ, et al. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J Immunol. 2007;178(8):5288–5295. doi: 10.4049/jimmunol.178.8.5288. [DOI] [PubMed] [Google Scholar]
- 96.Uchinami H, Seki E, Brenner DA, D’Armiento J. Loss of MMP 13 attenuates murine hepatic injury and fibrosis during cholestasis. Hepatology. 2006;44(2):420–429. doi: 10.1002/hep.21268. [DOI] [PubMed] [Google Scholar]
- 97.Endo H, Niioka M, Sugioka Y, et al. Matrix metalloproteinase-13 promotes recovery from experimental liver cirrhosis in rats. Pathobiology. 2011;78(5):239–252. doi: 10.1159/000328841. [DOI] [PubMed] [Google Scholar]
- 98.Ramachandran P, Pellicoro A, Vernon MA, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci USA. 2012;109(46):E3186–E3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hironaka K, Sakaida I, Matsumura Y, Kaino S, Miyamoto K, Okita K. Enhanced interstitial collagenase (matrix metalloproteinase-13) production of Kupffer cell by gadolinium chloride prevents pig serum-induced rat liver fibrosis. Biochem Biophys Res Commun. 2000;267(1):290–295. doi: 10.1006/bbrc.1999.1910. [DOI] [PubMed] [Google Scholar]
- 100.Ramachandran P, Iredale JP. Macrophages: central regulators of hepatic fibrogenesis and fibrosis resolution. J Hepatol. 2012;56(6):1417–1419. doi: 10.1016/j.jhep.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 101.Elsharkawy AM, Oakley F, Mann DA. The role and regulation of hepatic stellate cell apoptosis in reversal of liver fibrosis. Apoptosis. 2005;10(5):927–939. doi: 10.1007/s10495-005-1055-4. [DOI] [PubMed] [Google Scholar]
- 102.Fischer R, Cariers A, Reinehr R, Haussinger D. Caspase 9-dependent killing of hepatic stellate cells by activated Kupffer cells. Gastroenterology. 2002;123(3):845–861. doi: 10.1053/gast.2002.35384. [DOI] [PubMed] [Google Scholar]
- 103.Shan W, Palkar PS, Murray IA, et al. Ligand activation of peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) attenuates carbon tetrachloride hepatotoxicity by downregulating proinflammatory gene expression. Toxicol Sci. 2008;105(2):418–428. doi: 10.1093/toxsci/kfn142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Imamura M, Ogawa T, Sasaguri Y, Chayama K, Ueno H. Suppression of macrophage infiltration inhibits activation of hepatic stellate cells and liver fibrogenesis in rats. Gastroenterology. 2005;128(1):138–146. doi: 10.1053/j.gastro.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 105.Mitchell C, Couton D, Couty JP, et al. Dual role of CCR2 in the constitution and the resolution of liver fibrosis in mice. Am J Pathol. 2009;174(5):1766–1775. doi: 10.2353/ajpath.2009.080632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baeck C, Wei X, Bartneck M, et al. Pharmacological inhibition of the chemokine C-C motif chemokine ligand 2 (monocyte chemoattractant protein 1) accelerates liver fibrosis regression by suppressing Ly-6C(+) macrophage infiltration in mice. Hepatology. 2014;59(3):1060–1072. doi: 10.1002/hep.26783. [DOI] [PubMed] [Google Scholar]
- 107.Rovin BH, Lu L, Saxena R. A novel polymorphism in the MCP-1 gene regulatory region that influences MCP-1 expression. Biochem Biophys Res Commun. 1999;259(2):344–348. doi: 10.1006/bbrc.1999.0796. [DOI] [PubMed] [Google Scholar]
- 108.Muhlbauer M, Bosserhoff AK, Hartmann A, et al. A novel MCP-1 gene polymorphism is associated with hepatic MCP-1 expression and severity of HCV-related liver disease. Gastroenterology. 2003;125(4):1085–1093. doi: 10.1016/s0016-5085(03)01213-7. [DOI] [PubMed] [Google Scholar]
- 109.Barnes MA, McMullen MR, Roychowdhury S, et al. Macrophage migration inhibitory factor is required for recruitment of scar-associated macrophages during liver fibrosis. J Leukoc Biol. 2015;97(1):161–169. doi: 10.1189/jlb.3A0614-280R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Heinrichs D, Knauel M, Offermanns C, et al. Macrophage migration inhibitory factor (MIF) exerts antifibrotic effects in experimental liver fibrosis via CD74. Proc Natl Acad Sci USA. 2011;108(42):17444–17449. doi: 10.1073/pnas.1107023108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Finucane OM, Reynolds CM, McGillicuddy FC, et al. Macrophage migration inhibitory factor deficiency ameliorates high-fat diet induced insulin resistance in mice with reduced adipose inflammation and hepatic steatosis. PLoS One. 2014;9(11):e113369. doi: 10.1371/journal.pone.0113369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macro-phage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Akyildiz M, Gunsar F, Nart D, et al. Macrophage migration inhibitory factor expression and MIF gene −173 G/C polymorphism in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2010;22(2):192–198. doi: 10.1097/MEG.0b013e328331a596. [DOI] [PubMed] [Google Scholar]
- 114.Assis DN, Leng L, Du X, et al. The role of macrophage migration inhibitory factor in autoimmune liver disease. Hepatology. 2014;59(2):580–591. doi: 10.1002/hep.26664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang FF, Huang XF, Shen N, et al. A genetic role for macrophage migration inhibitory factor (MIF) in adult-onset Still’s disease. Arthritis Res Ther. 2013;15(3):R65. doi: 10.1186/ar4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dranoff JA, Wells RG. Portal fibroblasts: Underappreciated mediators of biliary fibrosis. Hepatology. 2010;51(4):1438–1444. doi: 10.1002/hep.23405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Forbes SJ, Parola M. Liver fibrogenic cells. Best Pract Res Clin Gastroenterol. 2011;25(2):207–217. doi: 10.1016/j.bpg.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 118.Iwaisako K, Brenner DA, Kisseleva T. What’s new in liver fibro-sis? The origin of myofibroblasts in liver fibrosis. J Gastroenterol Hepatol. 2012;27(Suppl 2):65–68. doi: 10.1111/j.1440-1746.2011.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Blouin A, Bolender RP, Weibel ER. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. J Cell Biol. 1977;72(2):441–455. doi: 10.1083/jcb.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88(1):125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis. 2001;21(3):311–335. doi: 10.1055/s-2001-17550. [DOI] [PubMed] [Google Scholar]
- 122.Blomhoff R, Green MH, Berg T, Norum KR. Transport and storage of vitamin A. Science. 1990;250(4979):399–404. doi: 10.1126/science.2218545. [DOI] [PubMed] [Google Scholar]
- 123.Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25(2):195–206. doi: 10.1016/j.bpg.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wong L, Yamasaki G, Johnson RJ, Friedman SL. Induction of beta-platelet-derived growth factor receptor in rat hepatic lipocytes during cellular activation in vivo and in culture. J Clin Invest. 1994;94(4):1563–1569. doi: 10.1172/JCI117497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pinzani M, Gesualdo L, Sabbah GM, Abboud HE. Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. J Clin Invest. 1989;84(6):1786–1793. doi: 10.1172/JCI114363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Friedman SL, Arthur MJ. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J Clin Invest. 1989;84(6):1780–1785. doi: 10.1172/JCI114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Inagaki Y, Okazaki I. Emerging insights into transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut. 2007;56(2):284–292. doi: 10.1136/gut.2005.088690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Paradis V, Dargere D, Bonvoust F, Vidaud M, Segarini P, Bedossa P. Effects and regulation of connective tissue growth factor on hepatic stellate cells. Lab Invest. 2002;82(6):767–774. doi: 10.1097/01.lab.0000017365.18894.d3. [DOI] [PubMed] [Google Scholar]
- 129.Paradis V, Dargere D, Vidaud M, et al. Expression of connective tissue growth factor in experimental rat and human liver fibrosis. Hepatology. 1999;30(4):968–976. doi: 10.1002/hep.510300425. [DOI] [PubMed] [Google Scholar]
- 130.Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130(2):435–452. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 131.Krizhanovsky V, Yon M, Dickins RA, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134(4):657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Troeger JS, Mederacke I, Gwak GY, et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology. 2012;143(4):1073–83. doi: 10.1053/j.gastro.2012.06.036. e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13(11):1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 134.Brun P, Castagliuolo I, Pinzani M, Palu G, Martines D. Exposure to bacterial cell wall products triggers an inflammatory phenotype in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2005;289(3):G571–G578. doi: 10.1152/ajpgi.00537.2004. [DOI] [PubMed] [Google Scholar]
- 135.Mazagova M, Wang L, Anfora AT, et al. Commensal microbiota is hepatoprotective and prevents liver fibrosis in mice. FASEB J. 2015;29(3):1043–1053. doi: 10.1096/fj.14-259515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Seki E, Schwabe RF. Hepatic inflammation and fibrosis: Functional links and key pathways. Hepatology. 2015;61(3):1066–1079. doi: 10.1002/hep.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gressner OA, Rizk MS, Kovalenko E, Weiskirchen R, Gressner AM. Changing the pathogenetic roadmap of liver fibrosis? Where did it start; where will it go? J Gastroenterol Hepatol. 2008;23(7 Pt 1):1024–1035. doi: 10.1111/j.1440-1746.2008.05345.x. [DOI] [PubMed] [Google Scholar]
- 138.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17(2):153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 139.Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur J Cell Biol. 2006;85(8):699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 140.Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91(1):221–264. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Taylor KR, Yamasaki K, Radek KA, et al. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem. 2007;282(25):18265–18275. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- 142.Tran KT, Griffith L, Wells A. Extracellular matrix signaling through growth factor receptors during wound healing. Wound Repair Regen. 2004;12(3):262–268. doi: 10.1111/j.1067-1927.2004.012302.x. [DOI] [PubMed] [Google Scholar]
- 143.Duca L, Floquet N, Alix AJ, Haye B, Debelle L. Elastin as a matrikine. Crit Rev Oncol Hematol. 2004;49(3):235–244. doi: 10.1016/j.critrevonc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 144.Maquart FX, Pasco S, Ramont L, Hornebeck W, Monboisse JC. An introduction to matrikines: extracellular matrix-derived peptides which regulate cell activity. Implication in tumor invasion. Crit Rev Oncol Hematol. 2004;49(3):199–202. doi: 10.1016/j.critrevonc.2003.06.007. [DOI] [PubMed] [Google Scholar]
- 145.Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992;267(36):26031–26037. [PubMed] [Google Scholar]
- 146.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGF-beta activation. J Cell Sci. 2003;116(Pt 2):217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 147.Gaca MD, Zhou X, Issa R, Kiriella K, Iredale JP, Benyon RC. Basement membrane-like matrix inhibits proliferation and collagen synthesis by activated rat hepatic stellate cells: evidence for matrix-dependent deactivation of stellate cells. Matrix Biol. 2003;22(3):229–239. doi: 10.1016/s0945-053x(03)00017-9. [DOI] [PubMed] [Google Scholar]
- 148.Sohara N, Znoyko I, Levy MT, Trojanowska M, Reuben A. Reversal of activation of human myofibroblast-like cells by culture on a basement membrane-like substrate. J Hepatol. 2002;37(2):214–221. doi: 10.1016/s0168-8278(02)00103-4. [DOI] [PubMed] [Google Scholar]
- 149.Friedman SL, Roll FJ, Boyles J, Arenson DM, Bissell DM. Maintenance of differentiated phenotype of cultured rat hepatic lipocytes by basement membrane matrix. J Biol Chem. 1989;264(18):10756–10762. [PubMed] [Google Scholar]
- 150.Olsen AL, Bloomer SA, Chan EP, et al. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol. 2011;301(1):G110–G118. doi: 10.1152/ajpgi.00412.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Georges PC, Hui JJ, Gombos Z, et al. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;293(6):G1147–G1154. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- 152.Li Z, Dranoff JA, Chan EP, Uemura M, Sevigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. 2007;46(4):1246–1256. doi: 10.1002/hep.21792. [DOI] [PubMed] [Google Scholar]
- 153.Perepelyuk M, Terajima M, Wang AY, et al. Hepatic stellate cells and portal fibroblasts are the major cellular sources of collagens and lysyl oxidases in normal liver and early after injury. Am J Physiol Gastrointest Liver Physiol. 2013;304(6):G605–G614. doi: 10.1152/ajpgi.00222.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schaffner F, Poper H. Capillarization of hepatic sinusoids in man. Gastroenterology. 1963;44:239–242. [PubMed] [Google Scholar]
- 155.Braet F, Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp Hepatol. 2002;1(1):1. doi: 10.1186/1476-5926-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.DeLeve LD, Wang X, Hu L, McCuskey MK, McCuskey RS. Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation. Am J Physiol Gastrointest Liver Physiol. 2004;287(4):G757–G763. doi: 10.1152/ajpgi.00017.2004. [DOI] [PubMed] [Google Scholar]
- 157.Couvelard A, Scoazec JY, Feldmann G. Expression of cell-cell and cell-matrix adhesion proteins by sinusoidal endothelial cells in the normal and cirrhotic human liver. Am J Pathol. 1993;143(3):738–752. [PMC free article] [PubMed] [Google Scholar]
- 158.Deleve LD, Wang X, Guo Y. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology. 2008;48(3):920–930. doi: 10.1002/hep.22351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Adzick NS, Longaker MT. Scarless fetal healing. Therapeutic implications. Ann Surg. 1992;215(1):3–7. doi: 10.1097/00000658-199201000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brockes JP. Amphibian limb regeneration: rebuilding a complex structure. Science. 1997;276(5309):81–87. doi: 10.1126/science.276.5309.81. [DOI] [PubMed] [Google Scholar]
- 161.Colwell AS, Longaker MT, Lorenz HP. Mammalian fetal organ regeneration. Adv Biochem Eng Biotechnol. 2005;93:83–100. doi: 10.1007/b99972. [DOI] [PubMed] [Google Scholar]
- 162.Glim JE, van Egmond M, Niessen FB, Everts V, Beelen RH. Detrimental dermal wound healing: what can we learn from the oral mucosa? Wound Repair Regen. 2013;21(5):648–660. doi: 10.1111/wrr.12072. [DOI] [PubMed] [Google Scholar]
- 163.Namazi MR, Fallahzadeh MK, Schwartz RA. Strategies for prevention of scars: what can we learn from fetal skin? Int J Dermatol. 2011;50(1):85–93. doi: 10.1111/j.1365-4632.2010.04678.x. [DOI] [PubMed] [Google Scholar]
- 164.Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Plast Reconstr Surg. 2010;126(4):1172–1180. doi: 10.1097/PRS.0b013e3181eae781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cowin AJ, Brosnan MP, Holmes TM, Ferguson MW. Endogenous inflammatory response to dermal wound healing in the fetal and adult mouse. Dev Dyn. 1998;212(3):385–393. doi: 10.1002/(SICI)1097-0177(199807)212:3<385::AID-AJA6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 166.Redd MJ, Cooper L, Wood W, Stramer B, Martin P. Wound healing and inflammation: embryos reveal the way to perfect repair. Philos Trans R Soc Lond B Biol Sci. 2004;359(1445):777–784. doi: 10.1098/rstb.2004.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu W, Cao Y, Longaker MT. Gene therapy of scarring: a lesson learned from fetal scarless wound healing. Yonsei Med J. 2001;42(6):634–645. doi: 10.3349/ymj.2001.42.6.634. [DOI] [PubMed] [Google Scholar]
- 168.Frantz FW, Bettinger DA, Haynes JH, et al. Biology of fetal repair: the presence of bacteria in fetal wounds induces an adult-like healing response. J Pediatr Surg. 1993;28(3):428–433. doi: 10.1016/0022-3468(93)90243-e. [DOI] [PubMed] [Google Scholar]
- 169.Rolfe KJ, Grobbelaar AO. A review of fetal scarless healing. ISRN Dermatol. 2012;2012:698034. doi: 10.5402/2012/698034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liechty KW, Kim HB, Adzick NS, Crombleholme TM. Fetal wound repair results in scar formation in interleukin-10-deficient mice in a syngeneic murine model of scarless fetal wound repair. J Pediatr Surg. 2000;35(6):866–872. doi: 10.1053/jpsu.2000.6868. discussion 72–3. [DOI] [PubMed] [Google Scholar]
- 171.Peranteau WH, Zhang L, Muvarak N, et al. IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J Invest Dermatol. 2008;128(7):1852–1860. doi: 10.1038/sj.jid.5701232. [DOI] [PubMed] [Google Scholar]
- 172.Canesso MC, Vieira AT, Castro TB, et al. Skin wound healing is accelerated and scarless in the absence of commensal microbiota. J Immunol. 2014;193(10):5171–5180. doi: 10.4049/jimmunol.1400625. [DOI] [PubMed] [Google Scholar]
- 173.Cowin AJ, Holmes TM, Brosnan P, Ferguson MW. Expression of TGF-beta and its receptors in murine fetal and adult dermal wounds. Eur J Dermatol. 2001;11(5):424–431. [PubMed] [Google Scholar]
- 174.Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108(Pt 3):985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- 175.Levine JH, Moses HL, Gold LI, Nanney LB. Spatial and temporal patterns of immunoreactive transforming growth factor beta 1, beta 2, and beta 3 during excisional wound repair. Am J Pathol. 1993;143(2):368–380. [PMC free article] [PubMed] [Google Scholar]
- 176.Soo C, Beanes SR, Hu FY, et al. Ontogenetic transition in fetal wound transforming growth factor-beta regulation correlates with collagen organization. Am J Pathol. 2003;163(6):2459–2476. doi: 10.1016/s0002-9440(10)63601-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hsu M, Peled ZM, Chin GS, Liu W, Longaker MT. Ontogeny of expression of transforming growth factor-beta 1 (TGF-beta 1), TGF-beta 3, and TGF-beta receptors I and II in fetal rat fibroblasts and skin. Plast Reconstr Surg. 2001;107(7):1787–1794. doi: 10.1097/00006534-200106000-00023. discussion 95–6. [DOI] [PubMed] [Google Scholar]
- 178.Eslami A, Gallant-Behm CL, Hart DA, et al. Expression of integrin alphavbeta6 and TGF-beta in scarless vs scar-forming wound healing. J Histochem Cytochem. 2009;57(6):543–557. doi: 10.1369/jhc.2009.952572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Olutoye OO, Yager DR, Cohen IK, Diegelmann RF. Lower cytokine release by fetal porcine platelets: a possible explanation for reduced inflammation after fetal wounding. J Pediatr Surg. 1996;31(1):91–95. doi: 10.1016/s0022-3468(96)90326-7. [DOI] [PubMed] [Google Scholar]
- 180.Haynes JH, Johnson DE, Mast BA, et al. Platelet-derived growth factor induces fetal wound fibrosis. J Pediatr Surg. 1994;29(11):1405–1408. doi: 10.1016/0022-3468(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 181.Merkel JR, DiPaolo BR, Hallock GG, Rice DC. Type I and type III collagen content of healing wounds in fetal and adult rats. Proc Soc Exp Biol Med. 1988;187(4):493–497. doi: 10.3181/00379727-187-42694. [DOI] [PubMed] [Google Scholar]
- 182.Cuttle L, Nataatmadja M, Fraser JF, Kempf M, Kimble RM, Hayes MT. Collagen in the scarless fetal skin wound: detection with picrosirius-polarization. Wound Repair Regen. 2005;13(2):198–204. doi: 10.1111/j.1067-1927.2005.130211.x. [DOI] [PubMed] [Google Scholar]
- 183.White ES, Baralle FE, Muro AF. New insights into form and function of fibronectin splice variants. J Pathol. 2008;216(1):1–14. doi: 10.1002/path.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.White ES, Muro AF. Fibronectin splice variants: understanding their multiple roles in health and disease using engineered mouse models. IUBMB Life. 2011;63(7):538–546. doi: 10.1002/iub.493. [DOI] [PubMed] [Google Scholar]
- 185.Serini G, Bochaton-Piallat ML, Ropraz P, et al. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142(3):873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wong JW, Gallant-Behm C, Wiebe C, et al. Wound healing in oral mucosa results in reduced scar formation as compared with skin: evidence from the red Duroc pig model and humans. Wound Repair Regen. 2009;17(5):717–729. doi: 10.1111/j.1524-475X.2009.00531.x. [DOI] [PubMed] [Google Scholar]
- 187.Peled ZM, Phelps ED, Updike DL, et al. Matrix metalloproteinases and the ontogeny of scarless repair: the other side of the wound healing balance. Plast Reconstr Surg. 2002;110(3):801–811. doi: 10.1097/00006534-200209010-00013. [DOI] [PubMed] [Google Scholar]
- 188.Dang CM, Beanes SR, Lee H, Zhang X, Soo C, Ting K. Scarless fetal wounds are associated with an increased matrix metalloproteinase-to-tissue-derived inhibitor of metalloproteinase ratio. Plast Reconstr Surg. 2003;111(7):2273–2285. doi: 10.1097/01.PRS.0000060102.57809.DA. [DOI] [PubMed] [Google Scholar]
- 189.Ulrich D, Ulrich F, Unglaub F, Piatkowski A, Pallua N. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with different types of scars and keloids. J Plast Reconstr Aesthet Surg. 2010;63(6):1015–1021. doi: 10.1016/j.bjps.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 190.Simon F, Bergeron D, Larochelle S, et al. Enhanced secretion of TIMP-1 by human hypertrophic scar keratinocytes could contribute to fibrosis. Burns. 2012;38(3):421–427. doi: 10.1016/j.burns.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 191.Huang EY, Wu H, Island ER, et al. Differential expression of urokinase-type plasminogen activator and plasminogen activator inhibitor-1 in early and late gestational mouse skin and skin wounds. Wound Repair Regen. 2002;10(6):387–396. doi: 10.1046/j.1524-475x.2002.t01-1-10608.x. [DOI] [PubMed] [Google Scholar]
- 192.Lovvorn HN, 3rd, Cheung DT, Nimni ME, Perelman N, Estes JM, Adzick NS. Relative distribution and crosslinking of collagen distinguish fetal from adult sheep wound repair. J Pediatr Surg. 1999;34(1):218–223. doi: 10.1016/s0022-3468(99)90261-0. [DOI] [PubMed] [Google Scholar]
- 193.Weissmann B, Meyer K, Sampson P, Linker A. Isolation of oligosaccharides enzymatically produced from hyaluronic acid. J Biol Chem. 1954;208(1):417–429. [PubMed] [Google Scholar]
- 194.Spicer AP, McDonald JA. Characterization and molecular evolution of a vertebrate hyaluronan synthase gene family. J Biol Chem. 1998;273(4):1923–1932. doi: 10.1074/jbc.273.4.1923. [DOI] [PubMed] [Google Scholar]
- 195.Itano N, Sawai T, Yoshida M, et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem. 1999;274(35):25085–25092. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- 196.Jacobson A, Brinck J, Briskin MJ, Spicer AP, Heldin P. Expression of human hyaluronan synthases in response to external stimuli. Biochem J. 2000;348(Pt 1):29–35. [PMC free article] [PubMed] [Google Scholar]
- 197.Yamada Y, Itano N, Hata K, Ueda M, Kimata K. Differential regulation by IL-1beta and EGF of expression of three different hyaluronan synthases in oral mucosal epithelial cells and fibro-blasts and dermal fibroblasts: quantitative analysis using real-time RT-PCR. J Invest Dermatol. 2004;122(3):631–639. doi: 10.1111/j.0022-202X.2004.22332.x. [DOI] [PubMed] [Google Scholar]
- 198.McCracken JM, Deshpande KT, Pritchard MT. Liver injury and pro-fibrotic markers, but not frank fibrosis, are enhanced in Has3−/− mice after carbon tetrachloride exposure: potential role for enhanced matrix metabolism. Hepatol. 2014;60(1S):578A. [Google Scholar]
- 199.Longaker MT, Chiu ES, Adzick NS, Stern M, Harrison MR, Stern R. Studies in fetal wound healing. V. A prolonged presence of hyaluronic acid characterizes fetal wound fluid. Ann Surg. 1991;213(4):292–296. doi: 10.1097/00000658-199104000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sawai T, Usui N, Sando K, et al. Hyaluronic acid of wound fluid in adult and fetal rabbits. J Pediatr Surg. 1997;32(1):41–43. doi: 10.1016/s0022-3468(97)90089-0. [DOI] [PubMed] [Google Scholar]
- 201.King A, Balaji S, Le LD, Marsh E, Crombleholme TM, Keswani SG. Interleukin-10 regulates fetal extracellular matrix hyaluronan production. J Pediatr Surg. 2013;48(6):1211–1217. doi: 10.1016/j.jpedsurg.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.King A, Balaji S, Marsh E, et al. Interleukin-10 regulates the fetal hyaluronan-rich extracellular matrix via a STAT3-dependent mechanism. J Surg Res. 2013;184(1):671–677. doi: 10.1016/j.jss.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Longaker MT, Chiu ES, Harrison MR, et al. Studies in fetal wound healing. IV. Hyaluronic acid-stimulating activity distinguishes fetal wound fluid from adult wound fluid. Ann Surg. 1989;210(5):667–672. doi: 10.1097/00000658-198911000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Longaker MT, Harrison MR, Crombleholme TM, et al. Studies in fetal wound healing: I. A factor in fetal serum that stimulates deposition of hyaluronic acid. J Pediatr Surg. 1989;24(8):789–792. doi: 10.1016/s0022-3468(89)80538-x. [DOI] [PubMed] [Google Scholar]
- 205.Tolg C, McCarthy JB, Yazdani A, Turley EA. Hyaluronan and RHAMM in wound repair and the “cancerization” of stromal tissues. Biomed Res Int. 2014;2014:103923. doi: 10.1155/2014/103923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Leung A, Crombleholme TM, Keswani SG. Fetal wound healing: implications for minimal scar formation. Curr Opin Pediatr. 2012;24(3):371–378. doi: 10.1097/MOP.0b013e3283535790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.West DC, Shaw DM, Lorenz P, Adzick NS, Longaker MT. Fibrotic healing of adult and late gestation fetal wounds correlates with increased hyaluronidase activity and removal of hyaluronan. Int J Biochem Cell Biol. 1997;29(1):201–210. doi: 10.1016/s1357-2725(96)00133-1. [DOI] [PubMed] [Google Scholar]
- 208.Mast BA, Flood LC, Haynes JH, et al. Hyaluronic acid is a major component of the matrix of fetal rabbit skin and wounds: implications for healing by regeneration. Matrix. 1991;11(1):63–68. doi: 10.1016/s0934-8832(11)80228-3. [DOI] [PubMed] [Google Scholar]
- 209.Adams LA. Biomarkers of liver fibrosis. J Gastroenterol Hepatol. 2011;26(5):802–809. doi: 10.1111/j.1440-1746.2010.06612.x. [DOI] [PubMed] [Google Scholar]
- 210.Vrochides D, Papanikolaou V, Pertoft H, Antoniades AA, Heldin P. Biosynthesis and degradation of hyaluronan by nonparenchymal liver cells during liver regeneration. Hepatology. 1996;23(6):1650–1655. doi: 10.1002/hep.510230648. [DOI] [PubMed] [Google Scholar]
- 211.Nakamura K, Yokohama S, Yoneda M, et al. High, but not low, molecular weight hyaluronan prevents T-cell-mediated liver injury by reducing proinflammatory cytokines in mice. J Gastroenterol. 2004;39(4):346–354. doi: 10.1007/s00535-003-1301-x. [DOI] [PubMed] [Google Scholar]
- 212.Campo GM, Avenoso A, Campo S, D’Ascola A, Ferlazzo AM, Calatroni A. The antioxidant and antifibrogenic effects of the glycosaminoglycans hyaluronic acid and chondroitin-4-sulphate in a subchronic rat model of carbon tetrachloride-induced liver fibro-genesis. Chem Biol Interact. 2004;148(3):125–138. doi: 10.1016/j.cbi.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 213.Ohno S, Im HJ, Knudson CB, Knudson W. Hyaluronan oligosaccharides induce matrix metalloproteinase 13 via transcriptional activation of NFkappaB and p38 MAP kinase in articular chondrocytes. J Biol Chem. 2006;281(26):17952–17960. doi: 10.1074/jbc.M602750200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Fieber C, Baumann P, Vallon R, et al. Hyaluronan-oligosaccharide-induced transcription of metalloproteases. J Cell Sci. 2004;117(Pt 2):359–367. doi: 10.1242/jcs.00831. [DOI] [PubMed] [Google Scholar]
- 215.Horton MR, Shapiro S, Bao C, Lowenstein CJ, Noble PW. Induction and regulation of macrophage metalloelastase by hyaluronan fragments in mouse macrophages. J Immunol. 1999;162(7):4171–4176. [PubMed] [Google Scholar]
- 216.Higgins GM, Anderson RM. Experimental pathology of liver: Restoration of liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 217.Mehendale HM. Tissue repair: an important determinant of final outcome of toxicant-induced injury. Toxicol Pathol. 2005;33(1):41–51. doi: 10.1080/01926230590881808. [DOI] [PubMed] [Google Scholar]