Abstract
Background
The Model for End-Stage Liver Disease (MELD) score, which estimates mortality within 90 days, determines priority for liver transplantation (LT). However, longer-term outcomes on the waitlist for patients who are initially listed with low MELD scores are not well-characterized.
Methods
All adults listed for primary liver transplantation at a single, high-volume center from 2005-12 with an initial laboratory MELD 22 or lower were evaluated. Excluded were those listed with MELD exception points, who underwent living donor LT (LDLT) or transplant at another center, or who were removed from the waitlist for non-medical reasons. Outcomes and causes of death were identified by UNOS, the National Death Index, and an electronic medical record review. Multivariable competing risk analysis evaluated predictors of death compared to deceased donor LT (DDLT).
Results
893 patients were listed from 2005-12. By the end of follow-up, 27% had undergone DDLT, and 31% were removed from the waitlist for death or clinical deterioration. In competing risks assessment, only MELD 6-9, older age, lower serum albumin, lower BMI, and diabetes conferred increased risk of waitlist dropout compared to DDLT. Listing for SLK was protective against waitlist dropout. 275 patients died or were delisted for being too sick; 87% of the identifiable causes of death were directly related to end-stage liver disease or hepatocellular carcinoma.
Conclusion
Patients with low listing MELD scores remain at significant risk for death due to liver-related causes and may benefit from early access to transplantation, such as LDLT or acceptance of high-risk donor livers. Predictors of death compared to transplantation may allow for early identification of patients who are at risk for waitlist mortality.
Keywords: liver transplantation, waitlist mortality, MELD, clinical outcomes, cirrhosis
INTRODUCTION
The Model for End-Stage Liver Disease (MELD) score estimates short-term mortality and has been used to determine priority for liver transplantation in the United States since 2002 (1). Though this model has overall created a more equitable allocation process, there remains significant mortality on the waitlist while awaiting liver transplantation (2).
Of the 15,308 patients awaiting transplant at the end of 2012, 64% had MELD ≤ 19 (2). In theory, these liver transplant candidates have a relatively low predicted risk of 90-day mortality related to liver disease. The observed three-month waitlist dropout for either death or clinical deterioration is 7.7% for patients with MELD 10-19, and 2.9% for those with MELD <9 (1). Nonetheless, even at low MELD scores, patients listed for liver transplantation experience complications of liver disease and are thus at risk of death from their liver disease. Long-term outcomes for this substantial cohort of patients are not yet well-characterized.
For certain populations, the MELD score may not accurately predict liver-related mortality (3). Candidates with clinically significant complications of liver disease, but in whom MELD parameters may not be very abnormal, may be disadvantaged by the current allocation system and experience disproportionate rates of waitlist dropout and mortality (4). Low serum sodium, hypoalbuminemia, and the presence of ascites have been found to be predictors of waitlist mortality independent of the MELD score. Revised models incorporating these variables, such as the MELD-Na score and the five-variable MELD, have been validated to better predict short-term mortality, particularly among those with low native MELD scores (5-10).
Our aim is to characterize outcomes and causes of death for liver transplant candidates listed with a low initial MELD on the waitlist, as well as to identify and investigate predictive factors for various outcomes in liver transplant candidates, including transplantation, removal from the waitlist, and death.
METHODS
All adults (≥18 years) listed for primary liver transplant with a laboratory MELD score ≤22 at the University of California, San Francisco between January 1, 2005 and December 31, 2012 were included. Excluded were patients listed with MELD exception points. Patients were followed through December 31, 2014. The study was approved by the Institutional Review Board at the University of California, San Francisco.
Demographic information, relevant dates (e.g., listing, death), and reasons for removal from the waitlist were obtained from the United Network for Organ Sharing (UNOS)/Organ Procurement and Transplantation Network (OPTN) (11). Also provided were laboratory data, including serum bilirubin, INR, creatinine, albumin, and sodium at the time of registration, as well as grades of ascites and encephalopathy. In the UNOS database, ascites is reported as “Absent,” “Slight,” or “Moderate,” and encephalopathy is reported as “None,” “1-2,” or “3-4.”
Outcomes and reasons for removal were confirmed through an electronic medical record review. Data including MELD component values, the etiology of liver disease, the development of hepatocellular carcinoma (HCC), human immunodeficiency virus (HIV) status, placement of a transjugular intrahepatic portosystemic shunt (TIPS), and listing for simultaneous-liver kidney transplantation (SLK), were collected from review of our local UCSF databases. Patients listed with HCV in addition to other diagnoses were considered to have HCV as the primary diagnosis.
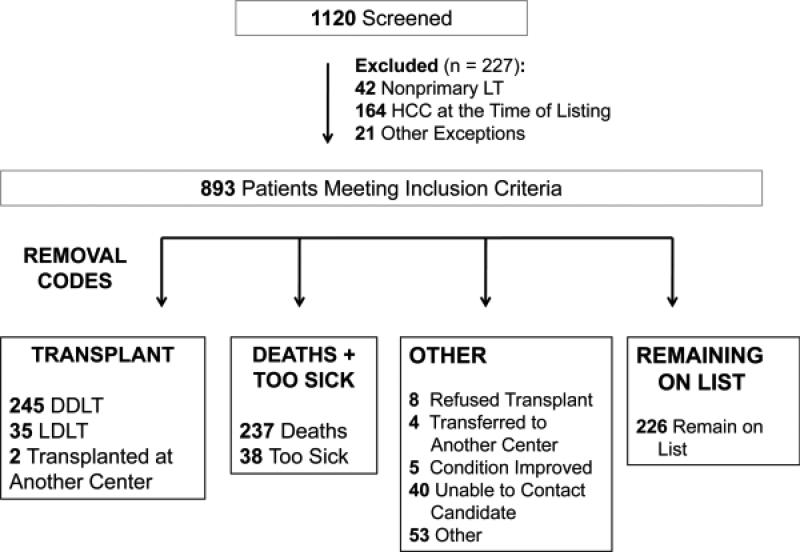
Excluded from the analysis were 37 patients who underwent living donor LT (LDLT) or transplant at another center, as they do not participate in the usual priority and allocation process at our center. Also excluded from the analysis were 110 patients removed from the waitlist for non-medical reasons, including “Condition Improved,” “Other,” “Refused transplant,” “Transferred to another center,” and “Unable to contact candidate.”
Identification of patient outcomes and cause of death was conducted by individual record review, supplemented by data from UNOS and the National Death Index. Our transplant program records patient data and outcomes through an electronic custom-designed database, with regular updates and input, when available, from outside hospital records, telephone calls to patients, and audits through the Social Security Master Death File. Delta-MELD was calculated as the difference in MELD scores at the time of death and 30 days prior.
Statistical analysis
Clinical characteristics and laboratory data were summarized by median and interquartile range for continuous variables. Comparisons between groups were performed using Chi-square and Wilcoxon tests, as appropriate.
The primary outcome was death defined as death while on the waiting list or delisting for deteriorating condition resulting in the patient being too sick for transplant. Patients with UNOS removal code “too sick” were combined with patients dying on the waitlist. Of patients too sick for transplant, 79% was confirmed to have died by NDI query and review of local databases. Therefore, we classified these patient outcomes as deaths. 55% had a known death date, within a median of 29 days (IQR 4-182) after waitlist removal.
Patients were followed from the date of listing to the date of removal from the waitlist. Date of removal corresponded with the date of death while on the waitlist (event), date of delisting for too sick (event), or the date of DDLT (competing risk). Patients remaining on the waitlist after December 31, 2014, were censored at this date.
Cumulative incidence and 95% confidence intervals (CI) for waitlist death or delisting for too sick and DDLT were calculated while accounting for competing risks. Risk of waitlist death or delisting for too sick was evaluated using competing risks regression with the Fine Gray model (12). Single predictor subhazard ratios (HRs) and 95% CIs were estimated by modeling the cumulative incidence function. Predictors evaluated included patient demographics, clinical characteristics, and laboratory data at the time of listing. All co-variables with a p-value <0.1 in univariable analysis were evaluated for inclusion in the final multivariable model. The final model was selected using backward elimination removing factors with p-values > 0.05.
Two-sided p-values < 0.05 were considered statistically significant. Analyses were performed using Stata 13.1 statistical software (College Station, Texas).
RESULTS
Included were 893 patients listed for liver transplantation without MELD exception points and with an initial laboratory MELD ≤22 (Figure 1). The median age was 55.3 years (IQR 50-60), 64% were male, and the median MELD score at listing was 15 (IQR 12-17). By the end of follow-up, 27% had undergone DDLT, and 31% had died or were delisted for being too sick for liver transplantation. Of the 275 patients in the death/too sick group, 38 (14%) patients were removed for “too sick.”
Figure 1.
Chart showing patient selection.
Baseline characteristics of the 746 patients eligible for the analysis by outcome are shown in Table 1. Compared to those who underwent DDLT, patients who died or were delisted were older (56.4 v. 55.6 years), had lower BMI (27.3 v. 29.2), and were more likely to have diabetes, but were otherwise similar in terms of sex, race, and etiology of liver disease. At a BMI cutoff of less than 30, patients were more likely to be removed for death or being “too sick” (p=0.003).
Table 1.
Baseline demographics for patients listed at UCSF between 2005-2012 with MELD 22 or less.
| Death + too sick (n=275) | DDLT (n=245) | p-value | Still waiting (n=226) | |
|---|---|---|---|---|
| Age, median (IQR), years | 56.3 (51.3-61.9) | 55.5 (50.1-59.6) | p=0.03 | 55.1 (50.2-60.4) |
| Sex | p=0.85 | |||
| Male, No. (%) | 183 (67) | 165 (67) | 138 (61) | |
| Female, No. (%) | 92 (33) | 80 (33) | 88 (39) | |
| Race | ||||
| White, No. (%) | 145 (53) | 132 (54) | p=0.41 | 122 (54) |
| Hispanic/Latino, No. (%) | 78 (28) | 75 (31) | p=0.37 | 62 (27) |
| Asian, No. (%) | 31 (11) | 18 (7) | p=0.85 | 28 (12) |
| Black, No. (%) | 11 (4) | 13 (5) | p=0.29 | 8 (4) |
| MELD at listing, median (IQR) | 15 (12-18) | 15 (13-18) | p=0.02 | 14 (12-16) |
| MELD at listing, No. (%) | ||||
| 6-9 | 25 (9) | 6 (2) | p=0.002 | 18 (8) |
| 10-13 | 73 (27) | 63 (26) | p=0.39 | 86 (38) |
| 14-17 | 107 (39) | 103 (42) | p=0.71 | 87 (38) |
| 18-22 | 70 (25) | 73 (30) | p=0.61 | 35 (15) |
| Etiology | ||||
| Hepatitis C, No. (%) | 137 (50) | 129 (53) | p=0.85 | 98 (43) |
| Alcohol, No. (%) | 55 (20) | 24 (10) | p=0.11 | 44 (19) |
| Hepatitis B, No. (%) | 12 (4) | 14 (6) | p=0.59 | 11 (5) |
| NASH, No. (%) | 21 (8) | 26 (11) | p=0.40 | 21 (9) |
| PSC, No. (%) | 7 (3) | 17 (7) | p=0.08 | 7 (3) |
| AIH/PBC, No. (%) | 20 (7) | 14 (6) | p=0.76 | 13 (6) |
| Diabetes, No. (%) | 83 (30) | 53 (22) | p=0.03 | 51 (23) |
| BMI, median (IQR) | 27.3 (23.7-31.2) | 29.2 (25.8-32.8) | p<0.001 | 28.5 (24.6-32.3) |
| BMI < 30, No. (%) | 196 (71) | 141 (58) | p=0.003 | 141 (62) |
| BMI > 40, No. (%) | 5 (2) | 13 (5) | p=0.07 | 9 (4) |
| Development of HCC, No. (%) | 38 (14) | 63 (26) | p<0.001 | 17 (8) |
| TIPS, No. (%) | 38 (14) | 26 (11) | p=0.34 | 43 (19) |
| Listed for SLK, No. (%) | 4 (1) | 17 (7) | p=0.004 | (4) |
| HIV, No. (%) | 12 (4) | 9 (4) | p=0.69 | 8 (4) |
| Initial albumin (g/dL), median (IQR) | 2.8 (2.4-3.2) | 2.8 (2.4-3.1) | p=0.68 | 3.1 (2.8-3.5) |
| Initial bilirubin (mg/dL), median (IQR) | 2.4 (1.7-3.8) | 2.7 (1.9-4.3) | p=0.008 | 2.1 (1.3-3.0) |
| Initial creatinine (mg/dL), median (IQR) | 0.9 (0.7-1.2) | 0.9 (0.8-1.2) | p=0.53 | 0.9 (0.7-1.1) |
| Initial INR, median (IQR) | 1.4 (1.2-1.6) | 1.4 (1.3-1.6) | p=0.28 | 1.3 (1.2-1.5) |
| Initial sodium (mmol/L), median (IQR) | 137 (134-139) | 137 (134-138) | p=0.59 | 138 (135-139) |
| Encephalopathy at listing | p=0.39 | |||
| None, No. (%) | 113 (41) | 91 (37) | 120 (53) | |
| 1-2, No. (%) | 160 (58) | 151 (62) | 104 (46) | |
| 3-4, No. (%) | 2 (1) | 3 (1) | 2 (1) | |
| Ascites at listing | p=0.62 | |||
| Absent, No. (%) | 59 (21) | 56 (23) | 74 (33) | |
| Slight, No. (%) | 188 (68) | 162 (66) | 19 (8) | |
| Moderate, No. (%) | 28 (10) | 27 (11) | 133 (59) | |
P-value compares deceased-donor liver transplantation to delisting for death or clinical deterioration.
Abbreviations: AIH, autoimmune hepatitis; BMI, body-mass index; HIV, human immunodeficiency virus; DDLT, deceased donor liver transplantation; INR, international normalized ratio; NASH, non-alcoholic steatohepatitis; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis; TIPS, transhepatic intrahepatic portosystemic shunt
Candidates who died or were delisted had a lower MELD score at the time of listing and lower initial bilirubin (2.4 v. 2.7 mg/dL), compared to those who were ultimately transplanted. Initial creatinine, INR, sodium, and albumin were comparable between the two groups, as were the degrees of ascites or encephalopathy at the time of listing, as reported to UNOS. A greater proportion of patients who underwent DDLT compared to death or delisting developed HCC while on the waitlist, as well as listing for simultaneous liver-kidney transplant.
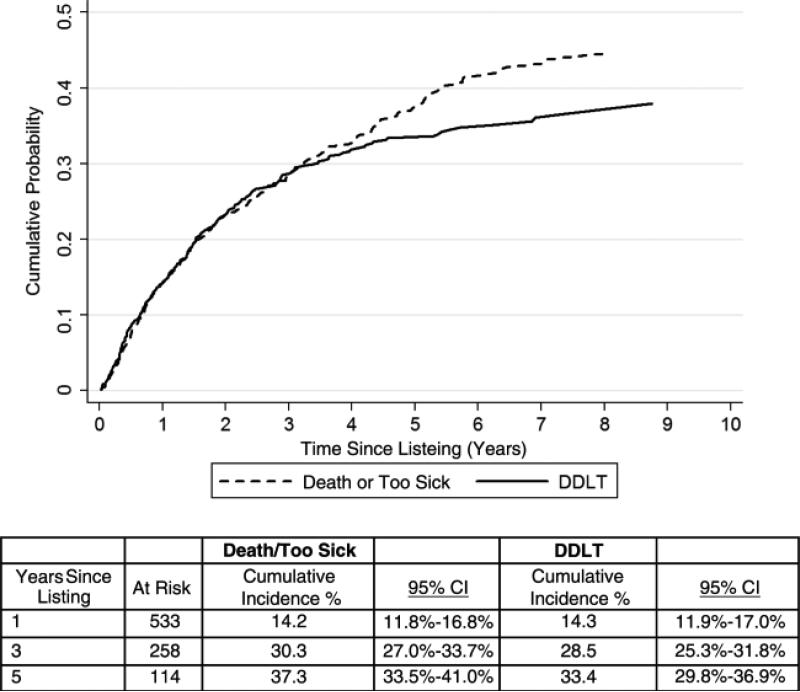
Cumulative probabilities of death or delisting and DDLT after listing for LT are shown in Figure 2. The cumulative probability of death or delisting within 1 and 5 years was 14% (95% CI 12-17) and 37% (95% CI 34-41), respectively. Similarly, the cumulative probability of DDLT within 1 and 5 years was 14% (95% CI 12-17) and 33% (95% CI 30-37), respectively.
Figure 2.
Cumulative probabilities of death or delisting and DDLT after listing for LT.
In univariable competing risks analysis, age (HR 1.02 per year; p=0.02), MELD 6-9 (HR 1.54, p=0.03) or MELD 18-22 (HR 1.40; p=0.046), and slight ascites documented at listing (HR 1.30; p=0.07) were significantly associated with death or delisting, while higher BMI (HR 0.96 per unit increase; p<0.001), higher initial serum albumin (HR 0.66 per g/dL; p<0.001), higher initial serum sodium (HR 0.96 per mmol/L; p=0.02), and listing for SLK (HR 0.30, p=0.01) were associated with a reduced risk of waitlist removal for death or clinical deterioration. In multivariable analysis, older age (HR 1.02 per year; p=0.009), MELD 6-9 (HR 1.68; p=0.008) and diabetes (HR 1.40; p=0.01) remained significant predictors of death or delisting. Higher BMI (HR 0.96 per unit increase; p<0.001), higher initial serum albumin (HR 0.63 per g/dL; p<0.001), and listing for SLK (HR 0.30; p=0.02 remained protective against death or delisting (Table 2).
Table 2.
Competing risks assessment of risk of delisting for death or being “too sick” among patients with MELD 22 or lower at listing.
| Univariable analysis | Multivariable analysis (backward elimination) | |||||
|---|---|---|---|---|---|---|
| Characteristics at listing | HR | 95% CI | p | HR | 95% CI | p |
| Age (per year) | 1.02 | 1.00-1.03 | 0.02 | 1.02 | 1.00-1.03 | 0.01 |
| Female (versus male) | 0.90 | 0.70-1.15 | 0.40 | |||
| Race | ||||||
| White | 1.00 | |||||
| Hispanic/Latino | 0.97 | 0.74-1.27 | 0.83 | |||
| Asian | 1.08 | 0.73-1.59 | 0.69 | |||
| Black | 0.94 | 0.51-1.74 | 0.85 | |||
| Other | 1.27 | 0.67-2.42 | 0.46 | |||
| MELD (per 1 unit increase) | 1.02 | 0.98-1.05 | 0.34 | |||
| MELD | ||||||
| 6-9 | 1.54 | 1.04-2.30 | 0.03 | 1.61 | 1.10-2.34 | 0.01 |
| 10-13 | 1.00 | 1.00 | ||||
| 14-17 | 1.21 | 0.90-1.62 | 0.20 | |||
| 18-22 | 1.40 | 1.01-1.95 | 0.046 | |||
| Etiology | ||||||
| Hepatitis C | 1.00 | |||||
| Alcohol | 1.28 | 0.94-1.74 | 0.12 | |||
| Hepatitis B | 0.77 | 0.43-1.40 | 0.39 | |||
| NASH | 0.71 | 0.45-1.12 | 0.14 | |||
| PSC | 0.56 | 0.25-1.23 | 0.15 | |||
| AIH/PBC | 1.18 | 0.73-1.92 | 0.49 | |||
| Other | 0.80 | 0.52-1.22 | 0.30 | |||
| Diabetes | 1.38 | 1.07-1.78 | 0.01 | 1.42 | 1.10-1.84 | 0.01 |
| BMI (per 1 unit increase) | 0.96 | 0.94-0.98 | <0.001 | 0.96 | 0.94-0.98 | <0.001 |
| Development of HCC | 0.76 | 0.54-1.07 | 0.11 | |||
| TIPS | 0.89 | 0.64-1.25 | 0.51 | |||
| SLK | 0.30 | 0.12-0.78 | 0.01 | 0.31 | 0.11-0.82 | 0.02 |
| HIV | 1.40 | 0.72-2.69 | 0.32 | |||
| Initial albumin (per g/dL) | 0.66 | 0.54-0.82 | <0.001 | 0.63 | 0.51-0.79 | <0.001 |
| Initial bilirubin (per mg/dL) | 1.01 | 0.96-1.06 | 0.74 | |||
| Initial creatinine (per mg/dL) | 0.96 | 0.86-1.08 | 0.50 | |||
| Initial INR (per unit) | 1.38 | 0.86-2.20 | 0.18 | |||
| Initial sodium (per mmol/L) | 0.96 | 0.93-0.99 | 0.02 | |||
| Encephalopathy at listing | ||||||
| None | 1.00 | |||||
| 1-2 | 1.12 | 0.89-1.43 | 0.33 | |||
| 3-4 | 0.80 | 0.18-3.52 | 0.77 | |||
| Ascites at listing | ||||||
| Absent | 1.00 | |||||
| Slight | 1.30 | 0.98-1.73 | 0.07 | |||
| Moderate | 1.40 | 0.87-2.24 | 0.16 | |||
BMI, body-mass index; INR, international normalized ratio; NASH, non-alcoholic steatohepatitis; PSC, primary sclerosing cholangitis
Patients who develop HCC while awaiting transplantation are provided an alternate pathway to transplantation in the current allocation model. In a sensitivity analysis excluding 118 patients who developed HCC, older age, lower BMI, diabetes, hypoalbuminemia, and listing for SLK remain significant, and subhazard ratios are not meaningfully changed. MELD 6-9 is no longer significant in this model.
Causes of death
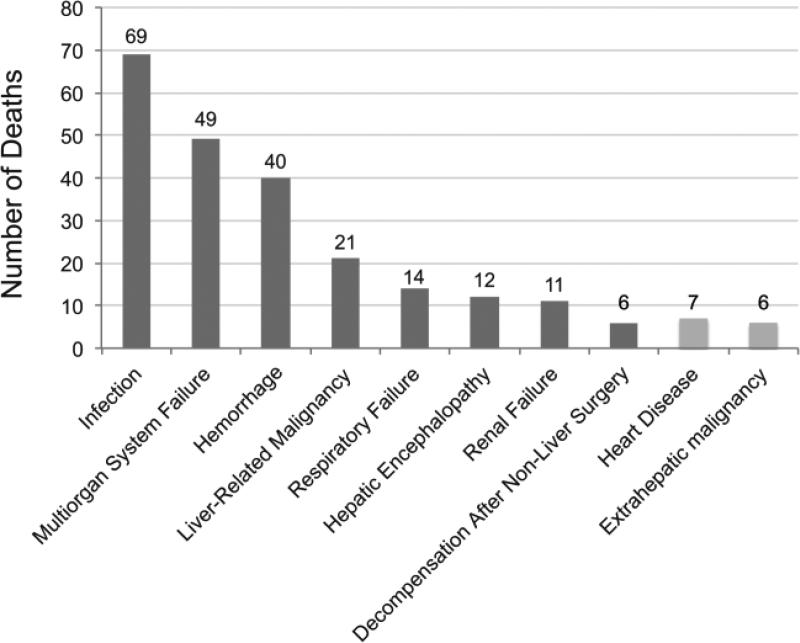
Of the 275 patients removed from the list for death or for clinical deterioration, the ultimate cause of death was identified in 255 (93%) candidates using data requested from local databases, UNOS, and the National Death Index. Two-hundred and twenty-two (87%) patients died of a cause that could be directly related to liver disease. The leading cause of death was infection, followed by multi-organ system failure, bleeding, and liver-related malignancy (Figure 3). Causes of delisting for the 33 patients removed for non-hepatic reasons were varied, but were most commonly due to cardiac disease or extrahepatic malignancy.
Figure 3.
Identified causes of death in patients removed from the waitlist for death or being “too sick.”
Of the 95 deceased patients for whom MELD data were available in the months leading up to death, 71 (75%) patients experienced an increase in MELD of 5 or greater in the 30 days prior to their date of death.
Patients with very low initial laboratory MELD, i.e. 6-9, nonetheless experienced a substantial risk of late waitlist dropout compared to those with MELD 10-22. In our cohort, candidates with initial MELD 6-9 remained on the waitlist for significantly longer prior to death (median 40.0 months; IQR 18.5-53.8, p<0.001) and had lower grades of ascites and encephalopathy as reported to UNOS at listing. Patients with MELD 6-9 were less likely to die of causes related to liver disease compared to patients with MELD 10-22 (72% v. 89%, p=0.02).
Ninety-three liver transplant candidates were removed from the waitlist for reasons including “Unable to contact candidate” or “Other.” Thirty-four (36%) of these patients were later confirmed by the National Death Index to be deceased.
DISCUSSION
Patients with low laboratory MELD make up a significant proportion of patients on the transplant waitlist. Based on OPTN data as of April 10, 2015, 8,972 (71.9%) of 12,487 active liver transplant candidates had a MELD 18 or lower. By definition, the current allocation model grants these patients lowest priority for liver transplantation based on a low predicted 90-day mortality. Nevertheless, they remain vulnerable to death – not only from complications of liver disease but potentially also from non-liver related causes.
Our study, evaluating outcomes in only low MELD patients at our center, found that patient factors such as older age, lower BMI, diabetes, and lower initial serum albumin, appear to contribute to an increased probability of waitlist dropout for death or clinical deterioration. Awareness of such risk factors may justify closer monitoring and vigilance for higher risk patients awaiting transplantation.
Patients with very low initial laboratory MELD, i.e. 6-9, nonetheless experienced a substantial risk of late waitlist dropout compared to those with MELD 10-22. This effect may represent a population who were listed with serious complications of liver disease but without evidence of significantly abnormal MELD component values.
Listing for SLK was protective against death or delisting, which may reflect MELD scores driven primarily by creatinine and renal dysfunction more than hepatic failure, and thus a lower risk of liver-related waitlist mortality.
The causes of death in our cohort were primarily related to complications of liver disease, including infection, bleeding, malignancy, and multi-organ failure. Patients with cirrhosis are at increased risk for bacterial infections, which can precipitate multi-organ failure and death. Once infected, patients have a significantly higher risk of waitlist dropout and death compared to liver transplantation (13, 14).
Three-quarters of our cohort experienced an increase in MELD of 5 or greater in the 30 days prior to their date of death or removal from the waitlist. Increase in MELD score by greater than 5 points over 30 days has been shown to confer a threefold greater risk of waitlist mortality (15). A rapid increase in MELD score should, in theory, prioritize patients in greater need for transplant; unfortunately for these patients, it could represent a precipitous decompensating event that precluded transplant. This statistic can be difficult to interpret, however, since most causes of death, both hepatic and non-hepatic, are associated with circulatory failure and a rising creatinine.
Under-estimation of waitlist mortality in our cohort is a potential limitation of our study. Nationally, removals from the waitlist are often misclassified, and thus removals for death or clinical deterioration are under-reported (16). In addition, region 5, to which our center belongs, has one of the highest median MELD scores at the time of transplant (2).
In our cohort, the causes of death among liver transplant candidates remain primarily related to liver disease, despite a low predicted 90-day mortality based on their initial MELD score. These patients may benefit from closer pre-transplant monitoring and more timely access to transplantation, such as LDLT or acceptance of high-risk donor livers.
For low MELD patients, expanded models using certain non-MELD variables may be able to more accurately predict mortality on the waitlist and risk-stratify patients awaiting liver transplantation. Various scoring symptoms for patients with low MELD have been proposed and validated in order to predict the longer-term risk of waitlist dropout or liver-related death, incorporating variables such as sodium, albumin, bilirubin, renal function, hepatic encephalopathy, ascites, or varices with or without bleeding (17, 18).
The current organ allocation model is designed to capture the severity of liver disease, but it is increasingly recognized that factors beyond the MELD also influence waitlist outcomes, mortality, and peri-operative risk. Identification and recognition of predictive factors for these outcomes may allow for earlier transplantation or revised allocation policies.
Acknowledgements
This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C and the Biostatistics Core of the UCSF Liver Center (P30 DK026743). The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Abbreviations
- AIH
autoimmune hepatitis
- BMI
body-mass index
- DDLT
deceased donor liver transplantation
- HCC
hepatocellular carcinoma
- HIV
human immunodeficiency virus
- INR
international normalized ratio
- LT
liver transplantation
- MELD
Model for End-Stage Liver Disease
- NASH
non-alcoholic steatohepatitis
- PBC
primary biliary cirrhosis
- PSC
primary sclerosing cholangitis
- SLK
simultaneous liver-kidney transplantation
- TIPS
transhepatic intrahepatic portosystemic shunt
- UNOS
United Network for Organ Sharing
Footnotes
None of the authors have any specific grants or financial interests relevant to the subject of this manuscript.
None of the authors have conflicts of interest relevant to the subject of this manuscript.
References
- 1.Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003 Jan;124(1):91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 2.Kim WR, Smith JM, Skeans MA, Schladt DP, Schnitzler MA, Edwards EB, et al. OPTN/SRTR 2012 Annual Data Report: liver. Am J Transplant. 2014 Jan;14(Suppl 1):69–96. doi: 10.1111/ajt.12581. [DOI] [PubMed] [Google Scholar]
- 3.Huo TI, Lin HC, Wu JC, Hou MC, Lee FY, Lee PC, et al. Limitation of the model for end-stage liver disease for outcome prediction in patients with cirrhosis-related complications. Clin Transplant. 2006 Mar-Apr;20(2):188–194. doi: 10.1111/j.1399-0012.2005.00463.x. [DOI] [PubMed] [Google Scholar]
- 4.Biggins SW, Bambha K. MELD-based liver allocation: who is underserved? Semin Liver Dis. 2006 Aug;26(3):211–220. doi: 10.1055/s-2006-947291. [DOI] [PubMed] [Google Scholar]
- 5.Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008 Sep 4;359(10):1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somsouk M, Kornfield R, Vittinghoff E, Inadomi JM, Biggins SW. Moderate ascites identifies patients with low model for end-stage liver disease scores awaiting liver transplantation who have a high mortality risk. Liver Transpl. 2011 Feb;17(2):129–136. doi: 10.1002/lt.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heuman DM, Abou-Assi SG, Habib A, Williams LM, Stravitz RT, Sanyal AJ, et al. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology. 2004 Oct;40(4):802–810. doi: 10.1002/hep.20405. [DOI] [PubMed] [Google Scholar]
- 8.Porrett PM, Baranov E, Ter Horst M. Serum Hypoalbuminemia Predicts Late Mortality on the Liver Transplant Waiting List. Transplantation. 2014 Jul 21; doi: 10.1097/TP.0000000000000299. [DOI] [PubMed] [Google Scholar]
- 9.Myers RP, Shaheen AA, Faris P, Aspinall AI, Burak KW. Revision of MELD to include serum albumin improves prediction of mortality on the liver transplant waiting list. PLoS One. 2013;8(1):e51926. doi: 10.1371/journal.pone.0051926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myers RP, Tandon P, Ney M, Meeberg G, Faris P, Shaheen AA, et al. Validation of the five-variable Model for End-stage Liver Disease (5vMELD) for prediction of mortality on the liver transplant waiting list. Liver Int. 2014 Sep;34(8):1176–1183. doi: 10.1111/liv.12373. [DOI] [PubMed] [Google Scholar]
- 11.Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation . Transplant Data. United Network for Organ Sharing; University Renal Research and Education Association; U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients; Rockville, MD: Richmond, VA: Ann Arbor, MI: 2005-2013. [Google Scholar]
- 12.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 13.Cardenas A, Gustot T. Delisting of liver transplant candidates because of bacterial sepsis. Liver Transpl. 2015;21:866–867. doi: 10.1002/lt.24174. [DOI] [PubMed] [Google Scholar]
- 14.Reddy KR, O'Leary JG, Kamath PS, Fallon MB, Biggins SW, Wong F, et al. for NACSELD High risk of delisting or death in liver transplant candidates following infections: Results from NACSELD. Liver Transpl. 2015;21(7):881–8. doi: 10.1002/lt.24139. [DOI] [PubMed] [Google Scholar]
- 15.Merion RM, Wolfe RA, Dykstra DM, Leichtman AB, Gillespie B, Held PJ. Longitudinal assessment of mortality risk among candidates for liver transplantation. Liver Transpl. 2003 Jan;9(1):12–18. doi: 10.1053/jlts.2003.50009. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg D, French B, Trotter J, Shetty K, Schiano T, Reddy KR, et al. Underreporting of liver transplant waitlist removals due to death or clinical deterioration: results at four major centers. Transplantation. 2013 Jul 27;96(2):211–216. doi: 10.1097/TP.0b013e3182970619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biselli M, Dall'agata M, Gramenzi A, Gitto S, Liberati C, Brodosi L, et al. A new prognostic model to predict dropout from the waiting list in cirrhotic candidates for liver transplantation with MELD score <18. Liver Int. 2014 Mar 20; doi: 10.1111/liv.12538. [DOI] [PubMed] [Google Scholar]
- 18.Wedd J, Bambha KM, Stotts M, Laskey H, Colmenero J, Gralla J, et al. Stage of cirrhosis predicts the risk of liver-related death in patients with low Model for End-Stage Liver Disease scores and cirrhosis awaiting liver transplantation. Liver Transpl. 2014 Oct;20(10):1193–1201. doi: 10.1002/lt.23929. [DOI] [PMC free article] [PubMed] [Google Scholar]