Abstract
D-Serine is an endogenous NMDA receptor co-agonist that activates synaptic NMDA receptors modulating neuronal networks in the cerebral cortex and plays a key role in long-term potentiation of synaptic transmission. D-serine is associated with NMDA receptor neurotoxicity and neurodegeneration and elevated D-serine concentrations have been associated with Alzheimer’s and Parkinsons’ diseases and amyotrophic lateral sclerosis. Previous studies have demonstrated that the ketamine metabolites (rac)-dehydronorketamine and (2S,6S)-hydroxynorketamine decrease intracellular D-serine concentrations in a concentration dependent manner in PC-12 cells. In the current study, PC-12 cells were incubated with a series of ketamine metabolites and the IC50 values associated with attenuated intracellular D-serine concentrations were determined. The results demonstrate that structural and stereochemical features of the studied compounds contribute to the magnitude of the inhibitory effect with (2S,6S)-hydroxynorketamine and (2R,6R)-hydroxynorketamine displaying the most potent inhibition with IC50 values of 0.18 ± 0.04 nM and 0.68 ± 0.09 nM. The data was utilized to construct a preliminary 3D-QSAR/pharmacophore model for use in the design of new and more efficient modulators of D-serine.
Introduction
(rac)-Ketamine (Ket) is a chiral phencyclidine derivative that produces rapid and short-lived anesthesia via inhibition of the N-methyl-D-aspartate (NMDA) receptor [1,2]. (rac)-Ket is rapidly, extensively and stereoselectively transformed into an array of N-demethylated and hydroxylated metabolites including norketamine (norKet), dehydronorketamine (DHNK) and diastereomeric hydroxynorketamines (HNK) [3,4]. Initial studies of (rac)-Ket induced anesthesia demonstrated that the effect was produced by the parent compound and (R,S)-norKet and that the (2S,6S;2R,6R)-HNK metabolite was inactive in this test [5]. However, recent studies have demonstrated that while the HNK and DHNK metabolites may not significantly contribute to the anesthetic effects of Ket, they are associated with the antidepressant effects in patients suffering from treatment resistant major depressive disorder (MDD) and bipolar depression produced by subanesthetic dosing of (R,S)-Ket [6]. In addition, administration of (2S,6S)-HNK stimulated phosphorylation of the mammalian target of rapamycin (mTOR) and its downstream targets in Wistar rat pre-frontal cortex tissue [7]. This effect was also observed after the administration of (R,S)-Ket and was associated with (R,S)-Ket’s antidepressant activity in the Wistar rat [8,9].
We have demonstrated that racemic (2S,6S;2R,6R)-HNK, (2S,6R;2R,6S)-HNK and (rac)-DHNK have a low affinity for the NMDA receptor [10], and, as a result, it is unlikely that the direct inhibition of the NMDA receptor is the source of the observed activation of the mTOR pathway. A potential pharmacological mechanism explaining mTOR activation is an “indirect” inhibition of NMDA receptor produced by a reduction in the concentration of D-serine. D-Serine, an endogenous NMDA receptor co-agonist, plays a critical role in long-term potentiation and NMDA-induced neurotoxicity and a decrease in D-serine concentration has been associated with reduced NMDA receptor activity [11–14]. We have previously demonstrated that incubation of PC-12 pheochromocytoma and 1321N1 astrocytoma cells with (rac)-DHNK and (2S,6S)-HNK reduces intracellular D-serine concentrations [7,15]. Paradoxically, these compounds also stimulate mTOR signaling resulting in increased expression of serine racemase (SR), the enzyme that mediates racemization of L-serine to D-serine. The effect of (2S,6S)-HNK and (R,S)-Ket on mTOR signaling in PC-12 and 1321N1 cells was similar to the effect observed was in rat pre-frontal cortex tissues [7].
The in vitro effects of the HNK and DHNK metabolites are consistent with results obtained in MDD patients. In these patients, antidepressant response to treatment with (R,S)-Ket was associated with pre-dose plasma D-serine concentrations, as basal D-serine levels are significantly lower in MDD patients that respond to treatment relative to non-responders [16]. The administration of (R,S)-Ket resulted in a ~20–25% decrease in D-serine plasma levels immediately following its 40-min infusion followed by a recovery to pre-dose levels at 120 min and then a slow decreased over the next 24h [16]. The rapid fall in plasma D-serine levels is clinically relevant as it is associated with increased dissociative side effects reflected as increased scores on the Clinician-Administered Dissociative States Scale (CADDS) which peak at 40 min after the initiation of the (R,S)-Ket infusion and return to baseline at 80 min [16,17]. We have recently demonstrated that the rapid decrease in plasma D-serine concentrations was produced by (S)-Ket inhibition of the alanine-serine-cysteine transporter (ASCT2), which mediates D-serine cellular export, while the latter, slower decease in D-serine plasma concentrations has been attributed to the activities of (R,S)-Ket metabolites [17].
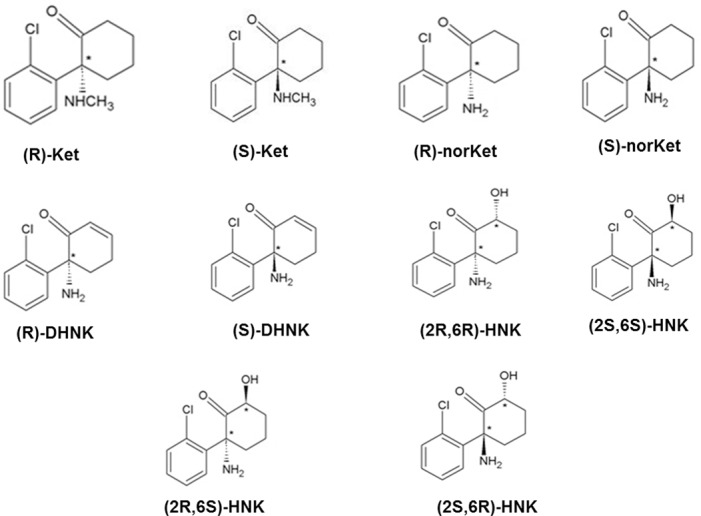
Since D-Serine concentrations have been correlated with a number of CNS diseases such as amyotrophic lateral sclerosis (ALS), Alzheimer’s and schizophrenia, the development of drugs that can modulate D-serine expression and distribution is an area of pharmacological and clinical interest [18,19]. The data from our previous studies of the effect of (R,S)-DHNK and (2S,6S)-HNK on intracellular D-serine concentrations suggest that these compounds might be a starting point for a D-serine-targeted drug discovery program. We now report the expansion of our initial observations through the determination of the effect of the individual enantiomers of (R)- and (S)-norKet, (R)- and (S)-DHNK, (2S,6S)- and (2R,6R)-HNK and (2S,6R)- and (2R,6S)-HNK, Fig 1, on intracellular D-serine concentration and SR expression in PC-12 cells. The limited data set demonstrated that structural, steric and stereochemical factors contributed to the modulation of intracellular D-serine concentration and SR expression. The data was utilized to construct a preliminary 3D-QSAR/pharmacophore model for use in the design of new and more efficient modulators of D-serine.
Fig 1. The structures of the compounds used in this study.
Materials and Methods
Ketamine (Ket) Metabolites
The Ket metabolites used in this study were (R)- and (S)-norKet, (R)- and (S)-DHNK, (2R,6R)- and (2S,6S)-HNK, and (2R,6S)- and (2S,6R)-HNK. These metabolites were prepared as previously described [20].
Maintenance and Treatment of Cell Lines
The PC-12 cell line, which is derived from rat adrenal medulla, was obtained from American Type Culture Collection (Manassas, VA, USA). The PC-12 cells were maintained in RPMI-1640 (Quality Biological, Gaithersburg, MD, USA) supplemented with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer [1 mM, pH 7.4] (Mediatech, Inc., Manassas, VA, USA), 10% heat-inactivated horse serum (Biosource, Rockville, MD, USA), 5% fetal bovine serum (FBS), 1% sodium pyruvate, 5% L-glutamine and 1% penicillin/streptomycin all purchased from Quality Biological.
Incubation of PC-12 Cells with Ket Metabolites
Cells were seeded on 100 x 20 mm tissue culture plates and maintained at 37°C under humidified 5% CO2 in air until they reached >70% confluence. Serial dilutions of the test compounds were prepared in the media using stock solutions of the compounds (10 mM in ethanol). The original media was replaced with media containing the test compounds and the plates were incubated for an additional 36 h, unless otherwise indicated. The medium was removed, and the cells collected for analysis. In the first series of experiments, (R)-norKet and (S)-norKet (0–1μM); (R)-DHNK and (S)-DHNK (0–0.45μM); (2R,6R)-HNK, (2S,6S)-HNK, (2R,6S)-HNK, and (2S,6R)-HNK (0–0.1μM) were tested. The cells were assessed for intracellular and extracellular D-serine levels, and expression of monomeric and dimeric forms of serine racemase (SR). The intracellular D-serine levels were determined in triplicate dishes while the determination of SR protein expression was carried out on one set of dishes. Additionally, extracellular D-serine levels were determined in cells treated with (2S,6S)-HNK. All analyses were repeated in three independent cell cultures (n = 3).
Determination of Intracellular and Extracellular D-Ser Concentrations
Intracellular D-serine concentrations were measured using a previously described and validated capillary electrophoresis-laser induced fluorescence (CE-LIF) method using a P/ACE MDQ system equipped with a laser-induced fluorescence detector (Beckman Instruments, Fullerton, CA, USA) [21]. The extracellular D-serine levels were determined using a previously reported assay employing liquid chromatography with mass spectrometric detection [17].
Measurement of Monomeric-SR (m-SR) and Dimeric-SR (d-SR) Expression by Western Blotting
The expression of m-SR and d-SR in PC-12 cells was determined using a previously described procedure [15]. The primary antibody for d-SR was obtained from Santa Cruz Biotechnology (Dallas, TX, USA), and the antibody that recognizes both m-SR and d-SR was purchased from Abcam, Inc. (Cambridge, MA). The primary antibody for β-actin was from Abcam. The antibodies were used at a dilution recommended by the manufacturer. Immunoreactive bands were detected using the ECL Plus Western Blotting Detection System (GE Healthcare, Piscataway, NJ, USA) and quantification was accomplished by volume densitometry using ImageJ software (National Institutes of Health, Bethesda, MD) and normalization to β-actin.
Comparative Molecular Field Analysis (CoMFA)
The CoMFA model was generated using methodology implemented in Sybyl-X 2.1.1 (Certara, L.P., Princeton, NJ, USA). The molecular models of structures were prepared in HyperChem v. 6.03 (HyperCube Inc., Gainesville, FL) using Model Build procedure to ensure the same conformation of the common scaffold. The models were extracted to Sybyl and the Gasteiger-Huckel atomic charges were calculated. The models were aligned using 2-chlorobenzyl moiety as a common substructure. Two types of molecular fields (steric and electrostatic) were sampled on the grid lattice surrounding each structure. In the procedure default settings were used. The pIC50 values presenting effects on the intracellular D-serine levels in PC-12 cells of ketamine metabolites were subjected to 3D-QSAR modeling
Statistical Analysis
Prism 4 (GraphPad Software, Inc., La Jolla, CA, USA) running on a personal computer was used to perform all statistical data analysis, including IC50 value calculations. The effect of test compounds on intracellular D-serine concentration is reported as ‘average percent change ± standard deviation’ compared to control values. Differences between two groups were analyzed using Student’s t-test (unpaired, two-tailed). A P value ≤ 0.05 was considered significant.
Results
Ket Metabolites Reduce Intracellular D-Serine Concentrations
The molecular structures of the compounds used in this study are presented in Fig 1. All of the tested compounds, (R)-norKet, (S)-norKet, (R)-DHNK, (S)-DHNK, (2S,6S)-HNK, (2R,6R)-HNK, (2S,6R)-HNK and (2R,6S)-HNK significantly reduced intracellular D-serine concentration. The maximum effects, determined as percent decrease from control, were produced by (2S,6R)-HNK and (2R,6S)-HNK with reductions of 51% and 57%, respectively with the remaining decreases in the range of 29% ((S)-norKet) to 39% ((2R,6R)-HNK) (Table 1). The reductions in intracellular D-serine levels were concentration-dependent and the potency of the compounds, expressed as IC50 value, ranged from ~100nM to 0.18nM (Table 1).
Table 1. The concentration-dependent decrease of intracellular D-Ser concentrations produced by incubation of PC-12 cells with the enantiomers of ketamine (Ket) and its major metabolites expressed as IC50 values and percent (%) maximum decrease from vehicle-treated PC-12 cells.
The effect of the configuration at the chiral centers on the magnitude of the IC50 value represented as the enantioselectivity factor α is derived by IC50(2R isomer)/IC50(2S isomer). The values for (R)-Ket were obtained from [17] and ‘NA’ indicates that (S)-Ket increases intracellular D-Ser concentrations [17]. Results are expressed as means ± SD, n = 3 independent experiments.
| Compound | IC50 (nM) | Decrease | Compound | IC50 (nM) | Decrease | (α) |
|---|---|---|---|---|---|---|
| (R)-Ket | 940 ± 160 | -33 ± 8% | (S)-Ket | NA | NA | NA |
| (R)-norKet | 91.0 ± 5.9 | -39 ± 2% | (S)-norKet | 62.4 ± 1.0 | -29 ± 1% | 1.5 |
| (R)-DHNK | 102 ± 11 | -32 ± 9% | (S)-DHNK | 50.9 ± 6.2 | -30 ± 2% | 2.0 |
| (2R,6R)-HNK | 0.68±0.09 | -35 ± 3% | (2S,6S)-HNK | 0.18 ± 0.04 | -39 ± 5% | 3.8 |
| (2R,6S)-HNK | 2.34±0.32 | -57 ± 5% | (2S,6R)-HNK | 1.11 ± 0.24 | -51 ± 4% | 2.1 |
The Attenuation of Intracellular D-Serine Concentration is Stereospecific
The stereochemical configuration at the C2 carbon on the cyclohexanone ring affected the relative IC50 values associated with the decrease in intracellular D-serine concentration (Table 1). The metabolites with an S- configuration at the C2 carbon were more potent than the corresponding R-isomers, and the enantioselectivity determined as the ratio of the IC50 of the R-isomer to that of the S-isomer ranged from 1.5 ((R/S)-norKet) to 3.8 ((2R,6R/2S,6S)-HNK) (Table 1).
The addition of the hydroxyl moiety at the C6 position in the cyclohexanone ring of norKet produces a second chiral center and a significant increase in the inhibitory activity as measured by decreased IC50 values (Table 1). This effect was produced regardless of the configuration at the C2 or C6 position, but there was an important relationship between activity and the stereochemistry at two chiral centers. In the HNK molecules, a cis stereochemical relationship between the C2 and C6 chiral centers on the cyclohexanone ring, i.e. (2S,6S)-HNK and (2R,6R)-HNK, produced a more potent inhibition when compared to the corresponding HNK metabolites with a trans relationship between the two chiral centers. The diastereoselective effect on intracellular D-serine concentration (αD) ranged from 1.6 to 13, calculated as IC50(trans-isomer)/IC50(cis-isomer) (Table 2).
Table 2. The stereochemical configurations at the C2 and C6 carbons of hydroxynorketamine (HNK) affect the concentration-dependent decrease in intracellular D-Ser concentration in PC-12 cells.
The relative pharmacological activity (αD) is derived by IC50(trans-isomer)/IC50(cis-isomer). The IC50 values are expressed as means ± SD, n = 3 independent experiments.
| Compound | IC50 (nM) | Compound | IC50 (nM) | Stereoselectivity (αΔ) |
|---|---|---|---|---|
| (2R,6R)-HNK | 0.68 ± 0.09 | (2R,6S)-HNK | 2.34 ± 0.32 | 3.4 |
| (2R,6R)-HNK | 0.68 ± 0.09 | (2S,6R)-HNK | 1.11 ± 0.24 | 1.6 |
| (2S,6S)-HNK | 0.18 ± 0.04 | (2S,6R)-HNK | 1.11 ± 0.24 | 6.2 |
| (2S,6S)-HNK | 0.18 ± 0.04 | (2R,6S)-HNK | 2.34 ± 0.32 | 13 |
Ket Metabolites Increase SR Expression Is a Stereospecific Manner
We recently demonstrated that incubation of PC-12 cells with (R,S)-Ket, (R)-Ket, (S)-Ket, (R,S)-norKet, (R,S)-DHNK or (2S,6S)-HNK produced an ∼2-fold increase in the expression of the monomeric form of SR (m-SR) in a concentration-dependent inverted-U-shaped manner [7,15,17]. The effect was enantioselective with respect to (S)-Ket and (R)-Ket, as the minimum concentration required to elicit the maximum response with (S)-Ket was 200 nM while (R)-Ket required a concentration of 4,000 nM [17]. Here, similar inverted-U-shaped response curves were observed for all of the compounds examined in this study (Fig 2). The concentrations required to elicit maximal expression of m-SR ranged from 100 nM ((R)-norKet) to 0.25 nM ((2S,6S)-HNK) and were enantioselective as the compounds with an S- configuration at C2 were more potent than the corresponding enantiomer (Table 3).
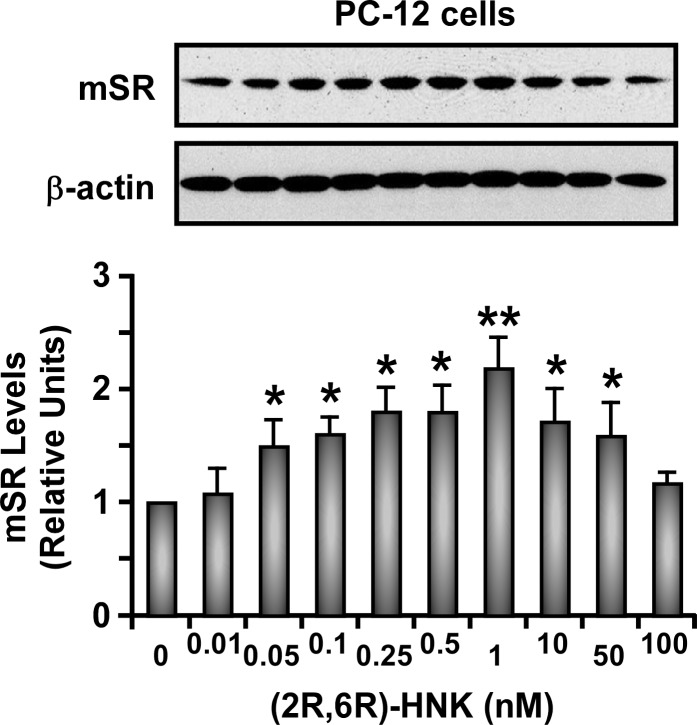
Fig 2. Effect of (2R,6R)-hydroxynorketamine (HNK) on the levels of serine racemase protein in PC-12 cells.
Cells were treated with various concentrations of HNK for 36 h and then total cell lysates were prepared for Western blot analysis. Top panel: representative immunoblot of monomeric serine racemase (m-SR; 36-kilodalton band). The blot was reprobed for β-actin, which was used as loading control. Bottom panel: relative levels of m-SR after quantification and normalization with β-actin. Bars represent the means ± SD of three independent experiments. * P< 0.05 and ** P< 0.01 as compared with control cells.
Table 3. The minimum concentration (nM) of ketamine (Ket) and Ket metabolites required to elicit maximum increase in the expression of the monomeric form of SR (m-SR) in PC-12 cells.
The effect of the configuration at the chiral centers at saturating concentration of each compound is represented as the enantioselectivity factor α derived from Concentration(2R isomer)/Concentration(2S isomer). The values for (R)-Ket and (S)-Ket were obtained from [17].
| Compound | Concentration (nM) | Compound | Concentration (nM) | Enantioselectivity (α) |
|---|---|---|---|---|
| (R)-Ket | 4,000 | (S)-Ket | 200 | 20 |
| (R)-norKet | 100 | (S)-norKet | 25 | 4 |
| (R)-DHNK | 75 | (S)-DHNK | 25 | 3 |
| (2R,6R)-HNK | 1 | (2S,6S)-HNK | 0.25 | 4 |
| (2R,6S)-HNK | 1 | (2S,6R)-HNK | 0.50 | 2 |
(2S,6S)-HNK Does Not Inhibit ASCT2 Transport of D-Serine
We have previously shown that incubation of PC-12 cells with (S)-Ket increased the amount of intracellular D-serine by ∼59% while decreasing extracellular D-serine levels by ∼41% [17]. This effect was due to the enantioselective inhibition of ASCT2-mediated cellular export of D-serine. (R)-Ket had no effect on ASCT2 export of D-serine and incubation of PC-12 cells with (R)-Ket resulted in equivalent decreases in intracellular and extracellular D-serine concentrations [17]. In order to determine if inhibition of ASCT2 activity contributed to the observed results, PC-12 cells were incubated with varying concentrations of (2S,6S)-HNK, the most potent of the tested metabolites. A concentration-dependent reduction in extracellular D-serine levels was observed with a maximum decrease of 33.5 ± 2.5% and an IC50 value of 0.21 ± 0.08 nM (Table 4) as compared to the effect on intracellular D-serine concentration where the maximum decrease was 39 ± 5% and an IC50 value of 0.18 ± 0.04 nM (Table 1). The results indicate that (2S,6S)-HNK had no significant effect on ASCT2-mediated cellular export of D-serine. Since this stereoisomer is the most potent metabolite of (S)-Ket it is reasonable to assume that the other compounds examined in this study also do not inhibit ASCT2-mediated transport of D-serine.
Table 4. The concentration-dependent decrease of extracellular D-serine concentrations produced by incubation of PC-12 cells with the (2S,6S)-HNK and the associated IC50 value.
The results are expressed as percent (%) maximum decrease from vehicle-treated PC-12 cells, means ± SD, n = 3 independent experiments.
| % Change in Extracellular D-Serine levels in PC-12 cells | |
|---|---|
| Conc. (nM) | (2S,6S)-HNK |
| 0 | 100.00 |
| 0.01 | 98.23 ± 0.30 |
| 0.05 | 91.40 ± 2.52 |
| 0.1 | 87.77 ± 1.99 |
| 0.25 | 81.86 ± 3.00 |
| 0.5 | 76.36 ± 3.25 |
| 1 | 73.40 ± 1.18 |
| 10 | 71.47 ± 0.79 |
| 50 | 67.89 ± 0.91 |
| 100 | 66.47 ± 1.52 |
| IC50 (nM) | 0.21 ± 0.08 |
Relationship between Structure and Pharmacological Effect
Although there were a limited number of compounds, a comparative molecular field analysis (CoMFA) was performed using the IC50 values and corresponding molecular structures. The resulting model identified several steric fields around the studied molecules that reached statistical significance in the analysis (Fig 3). The placement of (R)-norKet on the CoMFA model (Fig 3A) shows two fields, one sterically favorable (depicted in green) located on the left and another sterically unfavorable (yellow) on the right of the molecule. The model suggests that the N-methyl substituent on (R)-Ket would occupy a sterically unfavorable space, depicted as the top yellow region, resulting in a weak inhibition of D-serine production and that the removal of the N-methyl moiety relieves the steric interaction producing the stronger inhibitory effects observed with (R)-norKet and (R)-DHNK. The lack of a significant difference between the IC50 values of (R)-norKet and (R)-DHNK (Table 1) indicates that the effect on the conformation of cyclohexanone ring and electronic distribution at the carbonyl moiety produced by the introduction of the C5-C6 double bond had no impact on pharmacological activity. The inhibitory potencies of the norKet and DHNK molecules were enhanced by inversion of the stereochemistry at C2 as the IC50 values of the respective S-enantiomers, which strongly suggest that the cyclohexanone ring is in a favorable steric environment when the C2 carbon is in an S configuration.
Fig 3. CoMFA models derived from the potency (determined as IC50 value) of ketamine metabolites on intracellular D-Ser concentrations.
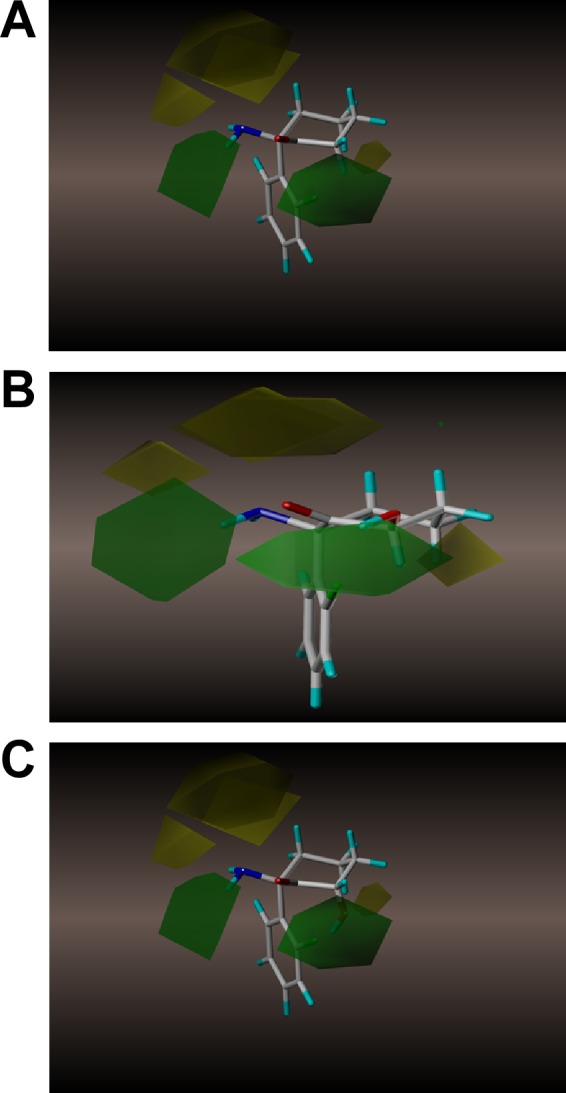
Nine compounds were considered in this study. The inclusion of (R)-norKet (A); (2R,6R)-HNK (B) and (2R,6S)-HNK (C) are depicted.
The addition of a hydroxyl moiety at the C6 position of norKet dramatically increased the potency of the resulting HNK compounds with IC50 values that were from 40-fold to 300-fold lower than norKet (Table 1). This suggests that an additional stabilizing interaction, most probably hydrogen bonding, occurs between the HNK molecule and the target protein as depicted in the placement of (2R,6R)-HNK on the CoMFA model (Fig 3B). The observed difference in the IC50 values between the cis and trans isomers are consistent with the closer proximity to the sterically favorable spaces produced by the cis orientation as illustrated by a comparison of (2R,6R)-HNK and (2R,6S)-HNK in the CoMFA model (Fig 2B and 2C, respectively).
Discussion
D-Serine and glycine are endogenous NMDA receptor co-agonists that act on different NMDA receptor populations [13,22]. Glycine has a demonstrated preference for extrasynaptic NMDA receptors associated with long term depression of synaptic signaling [13], while D-serine activates synaptic NMDA receptors modulating neuronal networks in the cerebral cortex [23] and plays a key role in long-term potentiation of synaptic transmission [12,13]. Recent studies have associated D-serine with NMDA receptor neurotoxicity and neurodegeneration [11,24] and elevated D-serine concentrations have been associated with Alzheimer’s and Parkinsons’ diseases and ALS [18,19]. In ALS, it has been suggested that increased levels of D-serine in the CNS is the primary cause of neuronal death associated with the disease [25]. In Alzheimer’s disease, elevated D-serine concentrations have been associated with increased expression of SR induced by amyloid β-peptide (Aβ1–42) [26] and secreted amyloid precursor protein [27].
The association of elevated D-serine with a number of CNS diseases and pathological states has resulted in drug development programs aimed at modulating the endogenous concentrations of this compound [18,19]. Since, the primary source of endogenous D-serine is SR-mediated racemization of L-serine these programs have targeted this enzyme using competitive and suicidal inhibitors [18,19]. SR is a pyridoxal-5’-phosphate-dependent enzyme whose activation is dependent upon the binding of divalent cations such as Mg2+ and Ca2+ to a metal binding site on the molecule [19] and intracellular Ca2+ concentrations affect the production of D-Ser [19,28,29]. For example, incubation of rat cortical astrocytes with the calcium ionophore A23187 increases D-Ser secretion [28] while the addition of a calcium chelator to the incubation media decreases D-Ser release from rat neuronal cultures [29]. The sensitivity of SR to changes in intracellular Ca2+ concentration suggests that the development of small molecule drugs designed attenuate intracellular Ca2+ concentration is a viable approach to the treatment of D-serine-related diseases. This approach is supported by our recent observation that the treatment of PC-12 cells with gabapentin and (S)-pregabalin produced significant decreases in intracellular D-Ser concentrations [30]. This effect was attributed to decreased intracellular Ca2+ flux resulting from the interaction of gabapentin and (S)-pregabalin with the α2-δ subunit of the voltage-gated Cavα2-δ calcium channel [31].
We have recently demonstrated that incubation of PC-12 cells with (rac)-DHNK and (2S,6S)-HNK decreases the intracellular concentration of D-serine [7,15]. This effect was associated with the negative allosteric modulation of α7-nAChR activity, which results in lower intracellular Ca2+, which, in turn, reduces the magnitude of Ca2+–activated SR and consequently the intracellular D-Ser concentrations [7,10,15]. In the current study, we investigated the effect of the structure and stereochemistry of a series of Ket metabolites on the IC50 values associated with the decrease in intracellular D-serine in PC-12 cells. When compared to the IC50 value previously determined for (R)-Ket [17], the IC50 values of the N-demethylated metabolites, (R)-norKet and (R)-DHNK, were reduced by ~10-fold (Table 1). Hydroxylation of (R)-norKet at the C6 position on the cyclohexanone ring further reduced the IC50 values relative to (R)-Ket by more than 1400-fold for (2R,6R)-HNK and ~400-fold for (2R,6S)-HNK. A similar comparison for (S)-Ket was not possible as the incubation of PC-12 cells with (S)-Ket increased the intracellular pool of D-serine through enantioselective inhibition of ASCT2-mediated cellular export of D-serine [17]. However, the IC50 values of (2S,6S)-HNK and (2S,6R)-HNK were reduced by 344% and 56%, respectively, relative to (S)-norKet (Table 1).
The results of this study also indicate that the molecular structure of the HNK metabolites presents a template for the development of new and potent modifiers of endogenous concentrations of D-serine for use in the treatment of depression, Alzheimer’s disease, ALS and Parkinson’s disease. The initial CoMFA model derived from this data is a positive step in this direction. The potential clinical use of these compounds is supported by recent studies of the metabolism and disposition of (2S,6S)-HNK in the Wistar rat [32]. The results demonstrate that (2S,6S)-HNK is rapidly distributed with a volume of distribution (Vd) of 7352 ±736 ml.kg-1 and a half-life of drug elimination during the terminal phase (t1/2) of 8.0 ± 4.0 h. Significant concentrations of (2S,6S)-HNK are present in brain tissue samples 10 min after an intravenous administration and the compound has an oral bioavailability of 46.3%. The antidepressant activities of (2S,6S)-HNK and other Ket metabolites are under investigation in a number of mouse models and the data will be reported elsewhere. The data from the functional studies and the initial CoMFA model will be used to direct the synthesis of compounds in the next iteration in our program to develop highly effective and selective therapies.
Data Availability
All relevant data are within the paper.
Funding Statement
The work was funded by the Intramural Research Programs of the National Institute on Aging and by The Foundation for Polish Science (TEAM Programme 2009 – 4/5).
References
- 1.Domino EF. Taming the ketamine tiger. Anesthesiology 2010; 113: 678–84. 10.1097/ALN.0b013e3181ed09a2 . [DOI] [PubMed] [Google Scholar]
- 2.Hirota K, Lambert DG. Ketamine: new uses for an old drug. Br J Anaesth 2011; 107:123–6. 10.1093/bja/aer221 . [DOI] [PubMed] [Google Scholar]
- 3.Adams JD, Baille TA, Trevor AJ, Castagnoli N Jr. Studies on the biotransformation of ketamine: Identification of metabolites produced in vitro from rat liver microsomal preparations. Biomed Mass Spec 1981; 8:527–38. . [DOI] [PubMed] [Google Scholar]
- 4.Desta Z, Moaddel R, Ogburn ET, Xu C, Ramamoorthy A, Venkata SLV, et al. Stereoselective and regiospecific hydroxylation of ketamine and norketamine. Xenobiotica 2012; 42:1076–87. 10.3109/00498254.2012.685777 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leung LY, Baillie TA. Comparative pharmacology in the rat of ketamine and its two principal metabolites, norketamine and (Z)-6-hydroxynorketamine. J Med Chem 1986; 29:2396–9. . [DOI] [PubMed] [Google Scholar]
- 6.Zarate CA Jr, Brutsche N, Laje G, Luckenbaugh DA, Ramamoorthy A, Moaddel R, et al. Relationship of Ketamine’s Plasma Metabolites with Response and Diagnosis, and Side Effects in Major Depression. Biol Psychiatry 2012; 72:331–8. 10.1016/j.biopsych.2012.03.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paul RK, Singh NS, Khadeer M, Moaddel R, Sanghvi M, Green CE, et al. (R,S)-Ketamine metabolites (R,S)-norketamine and (2S,6S)-hydroxynorketamine increase the mammalian target of rapamycin (mTOR) function. Anesthesiology 2014; 121:149–59. 10.1097/ALN.0000000000000285 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li N, Lee B, Lui R-J, Banasr M, Dwyer JM, Iwata X-Y, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010; 329:959–64. 10.1126/science.1190287 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dwyer JM, Duman RS. Activation of mammalian target of rapamycin and synaptogenesis: role in the actions of rapid-acting antidepressants. Biol Psychiatry 2013; 73:1189–98. 10.1016/j.biopsych.2012.11.011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moaddel R, Abdrakhmanova G, Kozak J, Jozwiak K, Toll L, Jimenez L, et al. Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in a7 nicotinic acetylcholine receptors. Eur J Pharmacol 2013; 698:228–34. 10.1016/j.ejphar.2012.11.023 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolosker H, Dumin E, Balan L, Foltyn V. D-Amino acids in the brain: D-Serine in neurotransmission and neurodegeneration. FEBS J 2008; 275:3514–26. 10.1111/j.1742-4658.2008.06515.x . [DOI] [PubMed] [Google Scholar]
- 12.Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010; 463: 232–6. 10.1038/nature08673 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henneberger C, Bard L, King C, Jennings A, Rusahov DA. NMDA receptor activation: Two targets for two co-agonists. Neurochem Res 2013; 38: 1156–62. 10.1007/s11064-013-0987-2 . [DOI] [PubMed] [Google Scholar]
- 14.Papouin T, Ladépêche L, Ruel J, Sacchi S, Labasque M, Hanini M, et al. Synaptic and Extrasynaptic NMDA Receptors Are Gated by Different Endogenous Coagonists. Cell. 2012; 150: 633–46. 10.1016/j.cell.2012.06.029 . [DOI] [PubMed] [Google Scholar]
- 15.Singh NS, Paul RK, Ramamoorthy A, Torjman MC, Moaddel R, Bernier M, et al. Nicotinic acetylcholine receptor antagonists alter the function and expression of serine racemase in PC-12 and 1321N1 cells. Cell Signal 2013; 25:2634–45. 10.1016/j.cellsig.2013.08.025 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moaddel R, Luckenbaugh DA, Xie Y, Villaseñor A, Brutsche NE, Machado-Vieira R et al. D-serine plasma concentration is a potential biomarker of (R,S)-ketamine antidepressant response in subjects with major depressive disorder. Psychopharmacology. 2015; 232: 399–409. 10.1007/s00213-014-3669-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh NS, Bernier M, Camandola S, Khadeer MA, Moaddel R, Mattson MP, et al. Enantioselective inhibition of D-serine transport by (S)-ketamine. Br J Pharmacol 2015; 172: 4546–59. 10.1111/bph.13239 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sethuraman R, Lee T, Tachibana S. D-Serine regulation: A possible therapeutic approach for central nervous diseases and chronic pain. Mini Rev Med Chem 2009; 9:813–9. . [DOI] [PubMed] [Google Scholar]
- 19.Jirásková-Vanícková J, Ettrich R, Vorlová B, Hoffman H, Lepšík M, Jansa P, et al. Inhibition of human serine racemase, an emerging target for medicinal chemistry, Curr Drug Targets 2011; 12:1037–55. . [DOI] [PubMed] [Google Scholar]
- 20.Moaddel R, Venkata SLV, Tanga MJ, Bupp JE, Green CE, LaIyer L, et al. A parallel chiral-achiral liquid chromatographic method for the determination of the stereoisomers of ketamine and ketamine metabolites in the plasma and urine of patients with complex regional pain syndrome. Talanta 2010; 82: 1892–904. 10.1016/j.talanta.2010.08.005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh NS, Paul RK, Sichler M, Moaddel R, Bernier M, Wainer IW. Capillary electrophoresis-laser-induced fluorescence (CE-LIF) assay for measurement of intracellular D-serine and serine racemase activity. Anal Biochem 2012; 421: 460–466. 10.1016/j.ab.2011.10.003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balu DT, Basu AC, Corradi JP, Cacace AM, Coyle JT. The NMDA receptor co-agonists, D-serine and glycine, regulate neuronal dendritic architecture in the somatosensory cortex. Neurobiol Disease 2012; 45: 671–82. 10.1016/j.nbd.2011.10.006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fossat P, Turpin FR, Sacchi S, Dulong J, Shi T, Rivet JM, et al. Glial D-serine gates NMDA receptors at excitatory synapses in prefrontal cortex. Cereb Cortex 2012; 22: 595–606. 10.1093/cercor/bhr130 . [DOI] [PubMed] [Google Scholar]
- 24.Shelper M, Kartvelishvily E, Wolosker H. D-Serine is the dominant endogenous coagonist for NMDA receptor neurotoxicity in organotypic hippocampal slices. J Neurosci. 2005; 25: 9413–17. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crow JP, Marecki JC, Thompson M. D-Serine production, degradation and transport in ALS: critical role of methodology. Neuro Res Inter 2012; 2012: 625245 10.1155/2012/625245 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu S, Barger SW. Induction of serine racemase by inflammatory stimuli is dependent on AP-1. Ann NY Acad Sci 2004; 1035: 133–46. . [DOI] [PubMed] [Google Scholar]
- 27.Wu S, Basile AS, Barger SW. Induction of serine racemase expression and D-serine release from microglia by secreted amyloid precursor protein (sAPP). Curr Alzheimer Res 2007; 4: 243–51. . [DOI] [PubMed] [Google Scholar]
- 28.Cook S, Galve-Roperh I, Martinez del Pozo A, Rodriguez-Crespo I. Direct calcium binding results in activation of brain serine racemase, J Biol Chem 2002; 27782–92. . [DOI] [PubMed] [Google Scholar]
- 29.Kartvelishvily E, Shleper M, Balan L, Dumin E, Wolosker H. Neuron-derived D-serine release provides a novel means to activate N-methyl-D-aspartate receptors, J Biol Chem. 2006; 281: 14151–62. . [DOI] [PubMed] [Google Scholar]
- 30.Singh NS, Paul RK, Torjman MC, Wainer IW. Gabapentin and (S)-pregabalin decrease intracellular D-serine concentrations in PC-12 cells. Neurosci Lett 2013; 535:90–4. 10.1016/j.neulet.2012.12.024 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor C.P., Mechanisms of analgesia by gabapentin and pregabalin–Calcium channel α2-δ [Cavα2-δ] ligands, Pain 2009; 142: 13–6. 10.1016/j.pain.2008.11.019 . [DOI] [PubMed] [Google Scholar]
- 32.Moaddel R, Sanghvi S, Dossou KS, Ramamoorthy A, Green C, Bupp J, et al. The distribution and clearance of (2S,6S)-hydroxynorketamine, an active ketamine metabolite, in Wistar rats. Pharmacol Res Perspect 2015; 3: e00157 10.1002/prp2.157 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.