Abstract
Recent resurgence of the bedbug Cimex lectularius is a global problem on the public health. On account of the worldwide rise of insecticide-resistant bedbug populations, exploration of new approaches to the bedbug control and management is anticipated. In this context, gene silencing by RNA interference (RNAi) has been considered for its potential application to pest control and management, because RNAi enables specific suppression of target genes and thus flexible selection of target traits to be disrupted. In this study, in an attempt to develop a control strategy targeting reproduction of the bedbug, we investigated RNAi-mediated gene silencing of vitellogenin (Vg), a major yolk protein precursor essential for oogenesis. From the bedbug transcriptomes, we identified a typical Vg gene and a truncated Vg gene, which were designated as ClVg and ClVg-like, respectively. ClVg gene was highly expressed mainly in the fat body of adult females, which was more than 100 times higher than the expression level of ClVg-like gene, indicating that ClVg gene is the primary functional Vg gene in the bedbug. RNAi-mediated suppression of ClVg gene expression in adult females resulted in drastically reduced egg production, atrophied ovaries, and inflated abdomen due to hypertrophied fat bodies. These phenotypic consequences are expected not only to suppress the bedbug reproduction directly but also to deteriorate its feeding and survival indirectly via behavioral modifications. These results suggest the potential of ClVg gene as a promising target for RNAi-based population management of the bedbug.
Introduction
An important factor that makes insects serious pests is their high reproductive ability. Hence, understanding how insects produce plentiful offspring and how the reproductive capability can be suppressed have been among major subjects in researches on pest control and management [1–3]. In most insects, as in oviparous animals in general, females deliver a considerable amount of nutritious resources to their eggs, which is mainly mediated by a major yolk precursor protein called vitellogenin (Vg) [4–7]. Vg protein, a member of the large lipid transfer protein superfamily [8], is mainly synthesized in the fat body and subjected to a variety of post-translational processing. Typically, Vg protein is proteolytically cleaved at particular sites, and the subunits are assembled together with lipids, carbohydrates and other nutrients, thereby forming a large oligomeric phosphoglycolipoprotein [6,7]. The complex is subsequently secreted into hemolymph and taken up by growing oocytes through endocytosis mediated by Vg receptors (VgR) [5].
The common bedbug Cimex lectularius (Hemiptera: Cimicidae) is a nuisance pest that feeds on blood of humans and other warm-blooded animals [9,10]. Although the bedbugs do not transmit fatal disease agents of humans, their bites annoy people by causing cutaneous manifestations, urticarial reactions and, occasionally, anaphylaxis [11]. The domestic infestation of this nuisance pest may result in adverse effects on mental health of residents, such as emotional distress, anxiety, insomnia and paranoia [12]. A single female bedbug can produce 200–500 eggs during its lifetime, which underlies its rapid population growth once infestation occurs [13]. The recent resurgence of the bedbug across the world, which might have been facilitated by increasing international travel and trade, is regarded as a global problem on public health [10,11]. Conventional eradication means are nowadays not effective, mainly because of the rise of insecticide-resistant populations of the bedbug [12]. Therefore, exploration of new approaches to the bedbug control and management is of urgent need.
In this context, gene silencing by RNA interference (RNAi) has recently attracted much attention not only in characterizing gene functions but also in controlling insect pests [14–17]. The high specificity of RNAi machinery against target nucleotide sequences conceptually enables eradication programs with minimal ecological side effects. It can also provide an alternative strategy for pest management without direct lethal actions, in which key genes responsible for pest status including fecundity, ability to utilize peculiar plant/animal hosts, pathogen transmitting capacity, resistance to chemical pesticides, etc., are targeted [14,18,19].
In diverse pest arthropods including cockroaches, fire ants and ticks, previous studies reported successful instances of RNAi targeting Vg gene [20,21] or VgR gene [22–24], which generally caused negative consequences in the ovarial development. Several studies reported that RNAi works in the bedbug, although those studies are not intended to population control of the nuisance pest [25,26]. In this study, in an attempt to develop a novel approach to reproduction control of the bedbug, we investigated Vg genes as a target of RNAi-mediated gene silencing.
Materials and Methods
Insect
A laboratory strain of the bedbug JESC, which had been maintained at the Japan Environmental Sanitation Center for decades [27], was used in this study. Adults and nymphs were kept in plastic Petri dishes (9 cm in diameter and 2 cm in depth) with several pieces of pleated filter paper (2 cm x 3 cm) at 25°C under constant darkness. The insects were fed once a week on commercially-purchased rabbit blood (Kohjin Bio, Japan) warmed at 35–36°C using a membrane feeding system as described previously [27].
Identification of Bedbug Vg Genes
Vg genes of the bedbug were surveyed in the expression sequence tag (EST) database [28] and the RNA sequencing (RNAseq) database [29]. The short RNAseq reads (SRA accession number, SRP008480) were assembled using Trinity v2.0.2 [30]. Using BLASTX similarity searches against the Uniprot protein database (www.uniprot.org), we obtained partial fragments of the Vg genes. Then, full-length transcript sequences were obtained by PCR amplification and DNA sequencing using the primers listed in Table 1 and the bedbug cDNA libraries constructed previously [28]. The Vg gene sequences determined in this study were deposited in the DNA Data Bank of Japan with the accession numbers LC115022 and LC115023.
Table 1. Primers used in this study.
| Name | Gene | Sequence (5’ to 3’) | Usagea |
|---|---|---|---|
| ClVg_235_F | ClVg | GGA AAA CTC ACC GTC CAA CC | cloning |
| ClVg_370_R | ClVg | TTG TTG GAA AAG GGG AGT TG | cloning |
| ClVg_1660_F | ClVg | CCA TAC TCC AAA GCA TCA CC | cloning |
| ClVg_1686_R | ClVg | CGT TCG GCA TCA GAA GTG | cloning |
| ClVg_3341_R | ClVg | TTC GAC ATC AAC ATC GAC AC | cloning |
| ClVg_4021_F | ClVg | CGA TTC CTC TTC TTC GTG AG | cloning |
| ClVg_4106_R | ClVg | AAG TGG GAC ATT GGG TGA AG | cloning |
| ClVg_4501_R | ClVg | TCA AAC CTG ACA ACG ACA TCT AC | cloning |
| ClVg_4634_F | ClVg | TCC ACC CAC ATT ACA ACA CC | cloning/dsRNA |
| ClVg_4942_R | ClVg | GCT GTC TGT CCG TTG ACC TT | cloning/dsRNA |
| ClVg_5342_F | ClVg | ACA GCC GCA AAT CAC AAC AC | qPCR |
| ClVg_5474_R | ClVg | GGC ACT CGG GCA TCT TTC | qPCR |
| ClVgL_13_F | ClVg-like | ATT TTT AAT CGT CCC GCC GC | cloning |
| ClVgL_83_R | ClVg-like | AGG GAC GAA TGA TCC ATC GC | cloning |
| ClVgL_1380_F | ClVg-like | ACA ACG AGA CAG TGT CAT CCC | cloning |
| ClVgL_1431_R | ClVg-like | TGG TTT TCA AGC CTC CCA TG | cloning |
| ClVgL_2691_F | ClVg-like | TGC ATA CTC AAG CAG TCC GG | cloning |
| ClVgL_2778_R | ClVg-like | GCA ACG AAA CCT GGA AAG GC | cloning |
| ClVgL_4033_F | ClVg-like | ACC GAC AAG ATG AGC AGA GC | cloning/pPCR |
| ClVgL_4119_R | ClVg-like | TGC AAG AGC GAG TTT GAT CG | cloning/pPCR |
| ClEF1alpha_Fb | Ef1α | TGG TAT CGA CAA ACG TAC CAT C | qPCR |
| ClEF1alpha_Rb | Ef1α | GCT CGG CCT TGA GCT TGT C | qPCR |
a Primers were designed for the following purposes: cloning, Vg gene sequence identification; dsRNA, gene fragment amplification for dsRNA synthesis; qPCR, gene expression quantification by RT-qPCR.
b These primers were originated from Moriyama et al. [28].
Molecular Phylogenetic and Evolutionary Analyses
Multiple alignment of Vg protein sequences was conducted using MUSCLE program [31]. Phylogenetic trees were constructed by maximum-likelihood and neighbor-joining methods using MEGA ver. 6.0 with 1,000 bootstrap replications [32]. Relative rate tests were performed using RRTree [33].
Quantification of Vg Gene Expression
Reverse-transcription quantitative PCR (RT-qPCR) was conducted for evaluating expression levels of the Vg genes in different tissues of the bedbug essentially as described previously [28]. Adult females of 1–2 months after emergence were dissected in a phosphate buffered saline. Total RNA samples of the isolated tissues were purified and reverse-transcribed using RNAiso plus (TaKaRa), RNeasy columns (QIAGEN) and Improm II Reverse Transcription System (Promega), and subjected to RT-qPCR using the primers listed in Table 1. A standard curve was drawn using the PCR fragment cloned into the pT7 blue plasmid (Novagene). Expression levels of the Vg genes were normalized by quantifying expression levels of elongation factor 1α (EF1α) gene using the primers listed in Table 1. Each biologically independent sample was measured twice and averaged.
RNAi Treatment and Phenotype Inspection
Double-stranded RNA (dsRNA) was synthesized from the PCR product of the Vg gene using MEGAscript RNAi kit (Ambion), in which fragment amplification was conducted using the gene specific primers (see Table 1) attached to T7 promoter sequence. For control experiments, dsRNA of β-lactamase gene fragment from pT7 blue plasmid was synthesized. Adult insects within two weeks after emergence were collected and assigned to each treatment group. Three females and three males were placed in each plastic petri dish (6 cm in diameter and 1.5 cm in depth) with two pieces of pleated filter paper, where they were allowed to mate freely. After two days from the first blood meal, the females were injected with 20 ng or 200 ng of dsRNA dissolved in 0.5 μl of distilled water from the basement membrane of a hind leg using a fine glass capillary needle. These bedbugs were fed once a week, when the number of eggs and hatched nymphs were recorded. Four weeks after the injection, these females were subjected to histological analysis or RNA extraction. The ovaries of some females were dissected out, photographed, and measured under a stereoscopic microscope and digital camera system (S8Apo and EC3, Leica Microsystems). Total RNA was extracted from the whole body, and expression levels of the Vg gene were quantified as described above.
Statistics
Gene expression levels were compared by fitting to a generalized linear model (GLM) [34], where Gamma error structure with log link function was assumed. The following models were selected for each GLM analysis: binomial error structure with logit link function for survival and hatching rates; Gaussian error structure for oocyte size; and Poisson error structure with log link function for number of eggs. When sample overdispersion was observed, we adopted a generalized linear mixed model (GLMM) that considers individual variation as a random effect. If deviance reduction due to the treatment term was significant in chi-square test, we compared its effect between each treatment group by Tukey-type multiple comparisons. All statistical analyses were performed using R ver. 3.2 [35].
Results
Bedbug Vg and Vg-like Genes
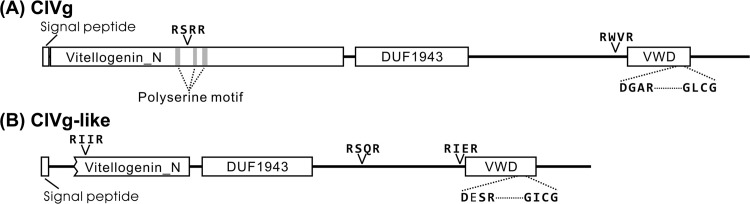
From transcriptome databases of the bedbug [28,29], we obtained partial gene sequences exhibiting significant similarities to known Vg genes. By making use of the sequences, we cloned and identified full-length cDNA sequences of two putative Vg genes. Fig 1A shows the amino acid sequence (1,863 residues) deduced from the first Vg gene, which encoded all conserved structures typical of insect Vg proteins [6]. In addition to the well-conserved RXXR cleavage site flanked by polyserine motifs at the N-terminal region, this protein contained another RXXR cleavage site at the C-terminal region (Fig 1A), indicating possible multiple cleavages as known for Vg protein of the bean bug Riptortus pedestris [36]. The amino acid sequence inferred from the other Vg gene (1,449 residues) showed 36% sequence identity to the sequence of the former Vg protein. This sequence was somewhat diverged from those of conventional Vg proteins known from diverse insects, with a truncated lipid binding domain, so-called Vitellogenin_N, and without the polyserine motif (Fig 1B). In addition, there was an amino acid substitution in the well-conserved DGXR-GL/ICG motif at the C-terminal region [6]. Hereafter we refer to the former gene as ClVg, and the latter gene as ClVg-like.
Fig 1. Schematic representation of protein structures encoded by vitellogenin genes, ClVg and ClVg-like, of the bedbug.
(A) ClVg protein. (B) ClVg-like protein. The following domains typical of vitellogenin proteins are illustrated: Vitellogenin_N, a lipoprotein amino terminal region; DUF1943, a domain of unknown function consisting of several large open beta-sheets; VWD, a von Willebrand factor type D domain. The consensus RXXR cleavage sites and DGXR-GL/ICG motifs are also indicated.
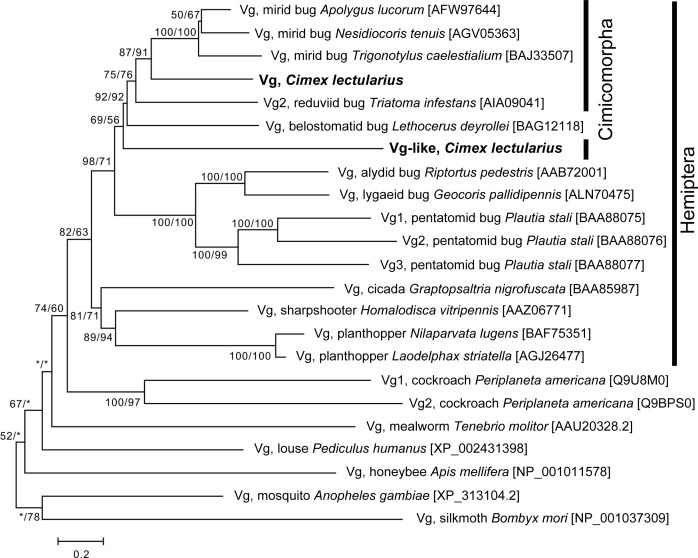
In the NCBI non-redundant protein database, the ClVg protein was the most similar to Vg2 of Triatoma infestans (Hemiptera: Reduviidae) with 52% sequence identity, while the ClVg-like protein was the most similar to Vg of Apolygus lucorum (Hemiptera: Miridae) with 36% sequence identity. Molecular phylogenetic analyses were performed by including Vg protein sequences of hemipterans and other insects after removing the N-terminal region that was lacking in the ClVg-like protein (Fig 2). While both the ClVg protein and the ClVg-like protein were placed within the clade of the order Hemiptera, the ClVg-like protein was located outside the infraorder Cimicomorpha to which bedbugs, reduviid bugs, and mirid bugs belong. On the phylogeny, the ClVg-like branch was elongated (Fig 2), and relative rate tests showed accelerated molecular evolution in the lineage of ClVg-like gene (Table 2). Hence, although the ClVg-like protein was placed even outside of the belostomatid water bug Lethocerus deyrollei of the infraorder Nepomorpha, it seems doubtful that the ClVg-like gene diverged from the common ancestor of the Cimicomorpha and the Nepomorpha, considering the low resolution of the phylogeny and the accelerated molecular evolution in the lineage of the ClVg-like gene.
Fig 2. Phylogenetic placement of vitellogenin proteins, ClVg and ClVg-like, of the bedbug.
The two Vg protein sequences of the bedbug (bold face) were analyzed with fourteen Vg protein sequences of hemipteran insects and seven Vg protein sequences of other insects, from which the N-terminal region lacking in ClVg-like was removed. Pairwise alignments of 1,387–1,562 amino acid sites were subjected to the analysis. A maximum-likelihood phylogeny is shown with bootstrap probabilities in the order of neighbor-joining/maximum likelihood. Asterisks indicate statistical values lower than 50%. The ranges of the infraorder Cimicomorpha and the order Hemiptera are shown on the right side.
Table 2. Relative rate tests for comparing the molecular evolutionary rate of ClVg protein sequence of the bedbug with those of ClVg-like protein sequence and other Vg protein sequences of hemipteran insects based on 1,235 unambiguously aligned amino acid sites.
| Lineage 1a | Lineage 2b | Outgroup | K1 | K2 | K1-K2 | K1/K2 | P-valuec |
|---|---|---|---|---|---|---|---|
| Vg-like, Cimex lectularius | Vg, Cimex lectularius | Vg, Pediculus humanus; Vg, Nilaparvata lugens | 0.643 | 0.360 | 0.283 | 1.79 | 1.0 x 10−7 |
| Vg-like, Cimex lectularius | Vg, Apolygus lucorum | Vg, Pediculus humanus; Vg, Nilaparvata lugens | 0.648 | 0.330 | 0.318 | 1.96 | 1.0 x 10−7 |
| Vg-like, Cimex lectularius | Vg, Nesidiocoris tenuis | Vg, Pediculus humanus; Vg, Nilaparvata lugens | 0.639 | 0.336 | 0.303 | 1.90 | 1.0 x 10−7 |
| Vg-like, Cimex lectularius | Vg, Trigonotylus caelestialium | Vg, Pediculus humanus; Vg, Nilaparvata lugens | 0.656 | 0.407 | 0.249 | 1.61 | 2.1 x 10−7 |
| Vg-like, Cimex lectularius | Vg2, Triatoma infestans | Vg, Pediculus humanus; Vg, Nilaparvata lugens | 0.647 | 0.354 | 0.293 | 1.83 | 1.0 x 10−7 |
| Vg-like, Cimex lectularius | Vg, Lethocerus deyrollei | Vg, Pediculus humanus; Vg, Nilaparvata lugens | 0.613 | 0.360 | 0.253 | 1.70 | 1.5 x 10−7 |
| Vg-like, Cimex lectularius | Vg, Riptortus pedestris | Vg, Pediculus humanus; Vg, Nilaparvata lugens | 0.666 | 0.499 | 0.167 | 1.33 | 2.8 x 10−4 |
| Vg-like, Cimex lectularius | Vg, Geocoris pallidipennis | Vg, Pediculus humanus; Vg, Nilaparvata lugens | 0.659 | 0.509 | 0.150 | 1.29 | 1.2 x 10−3 |
| Vg-like, Cimex lectularius | Vg1, Plautia stali | Vg, Pediculus humanus; Vg, Nilaparvata lugens | 0.637 | 0.522 | 0.115 | 1.22 | 0.014 |
a Estimated mean distance between lineage 1 and the last common ancestor of lineages 1 and 2.
b Estimated mean distance between lineage 2 and the last common ancestor of lineages 1 and 2.
c P-value was generated using the program package RRTree [33].
Tissue Specific Expression of Vg and Vg-like Genes
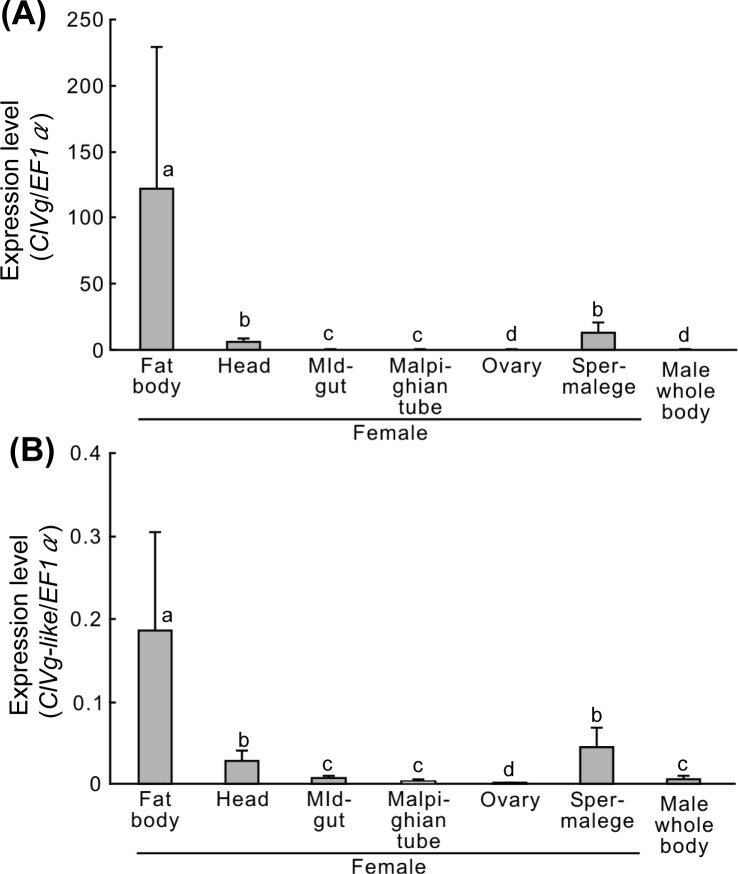
We investigated expression levels of the ClVg gene and the ClVg-like gene in dissected tissues of adult bedbugs. In adult females, the ClVg gene was highly expressed in the fat body, whereas it exhibited a low level of expression in the spermalege, a female-specific abdominal organ involved in traumatic insemination [37] (Fig 3A). Little expression of the ClVg gene was detected in adult males (Fig 3A). Expression patterns of the ClVg-like gene were similar to those of the ClVg gene, but the expression levels were 102−103 times lower (Fig 3B). These results strongly suggest that the ClVg gene represents the primary functional Vg gene in the bedbug.
Fig 3. Tissue-specific expression patterns of vitellogenin genes, ClVg and ClVg-like, in the bedbug.
Expression levels of the ClVg gene (A) and the ClVg-like gene (B) in dissected tissues were quantified by RT-qPCR, where expression levels of EF1α gene were used as an internal standard. Means and standard deviations of six biological replicates are shown. Different alphabetical letters (a-d) indicate statistically significant differences (a likelihood-ratio test of GLM and post-hoc multiple comparisons, P < 0.05).
RNAi-Mediated Silencing of Vg Gene Expression
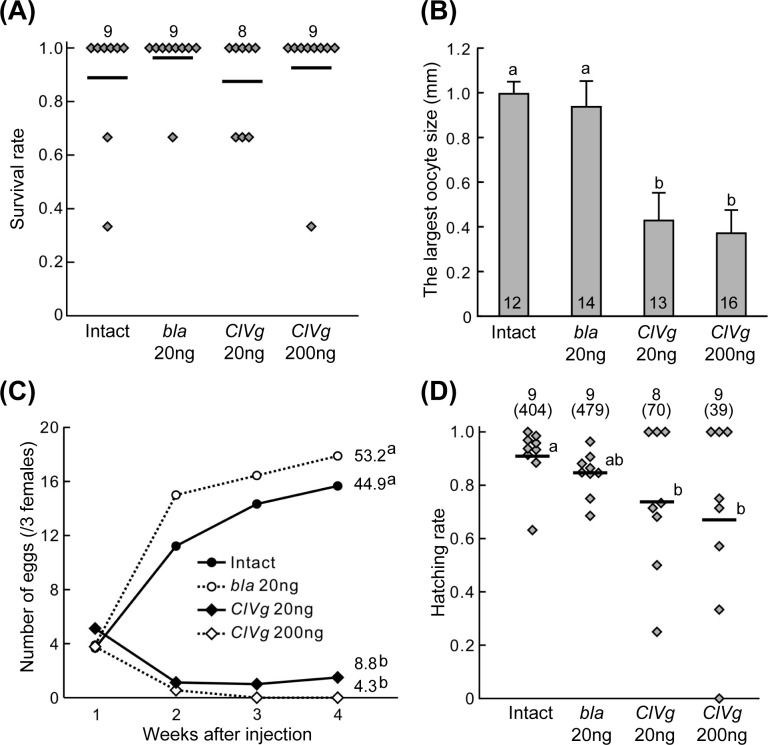
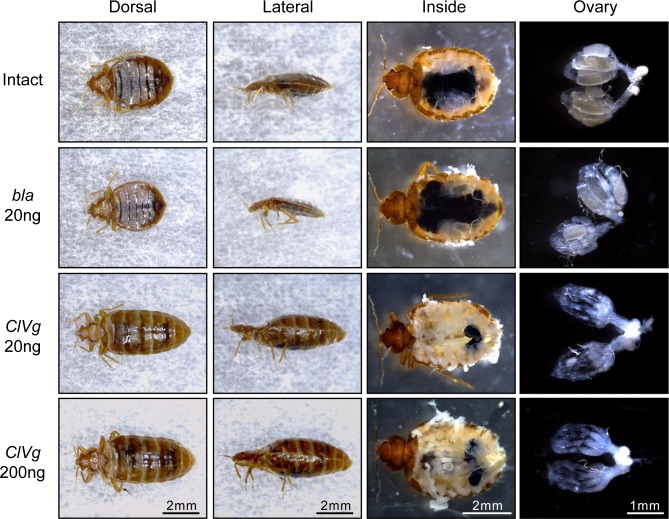
For RNAi-mediated silencing of the ClVg gene expression, dsRNA targeting the ClVg gene was injected into the hemocoel of adult females at the dose of 20 ng or 200 ng per insect. Expression levels of the ClVg gene were drastically suppressed even four weeks after the dsRNA injection (Fig 4). During the experimental period of four weeks, mortality rates were generally low (4–12%), and survival rates were statistically not different among the treatment groups (Fig 5A). On the other hand, the ClVg dsRNA injection resulted in remarkable phenotypic consequences. The abdomen of adult females subjected to the ClVg RNAi was conspicuously swollen like fully-engorged bedbugs (Fig 6, “Dorsal” and “Lateral” columns). The swollen abdomen of the ClVg RNAi females was full of hypertrophied fat bodies, which was in contrast to much less developed fat bodies adhering to the inner wall of the thinner abdomen of the control females (Fig 6, “Inside” column). In the ClVg RNAi females, ovaries were atrophied with no mature oocytes, which were in contrast to well-developed ovaries containing mature oocytes in the control females (Figs 5B and 6, “Ovary” column). In the ClVg RNAi females, egg production was drastically and significantly suppressed in comparison with the control females (Fig 5C). Notably, in all eight replicate groups (three females each) injected with 200 ng dsRNA, and six of eight replicate groups injected with 20 ng dsRNA, females completely ceased egg production two weeks after the dsRNA injection. It is also notable that eggs produced by the ClVg RNAi females tended to suffer low hatching success (Fig 5D). Taken together, it was concluded that RNAi targeting the ClVg gene effectively inhibited the bedbug reproduction.
Fig 4. Inhibition of ClVg gene expression by RNAi in adult females of the bedbug.
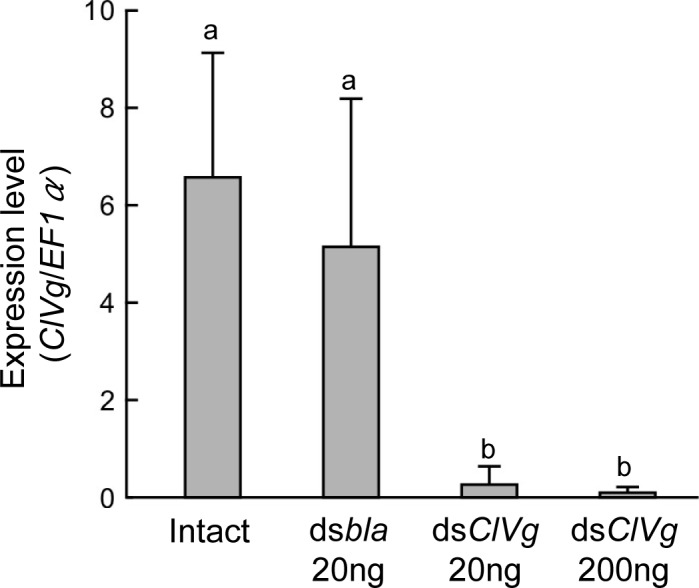
Expression levels of the ClVg gene were quantified by RT-qPCR four weeks after the following experimental treatments: Intact, no injection control; dsbla 20 ng, injection with 20 ng dsRNA of β-lactamase gene; dsClVg 20 ng, injection with 20 ng dsRNA of ClVg gene; dsClVg 200 ng, injection with 200 ng dsRNA of ClVg gene. Means and standard deviations of nine biological replicates are shown. Different alphabetical letters (a, b) indicate statistically significant differences (a likelihood-ratio test of GLM and post-hoc multiple comparisons, P < 0.05).
Fig 5. Effects of ClVg RNAi on reproductive performance of adult females of the bedbug.
(A) Survival rates after dsRNA injection. Numbers of replicates each containing three adult females are shown at top. Rhombic points and bars indicate survival rates of individual replicates and their means, respectively. (B) Effects on oocyte size. The longitudinal length of the largest oocyte in the ovary was compared among the treatment groups. Means and standard deviations are shown. Numbers of adult females inspected are shown at the bottom of the columns. (C) Effects on oviposition. Mean egg numbers of 8–9 replicates each containing three females are shown. Mean accumulative egg numbers for the treatment groups are indicated on the right side. (D) Effects on egg hatching rate. Rhombic points and bars indicate replicates and their means, respectively. Numbers of replicates (and total egg numbers inspected for each treatment) are shown at top. In (A), there are no statistical differences among the treatment groups (a likelihood-ratio test of GLM, P > 0.05). In (B)-(D), different alphabetical letters (a, b) indicate statistically significant differences (a likelihood-ratio test of GLM followed by multiple comparisons, P < 0.05).
Fig 6. Phenotypic effects of ClVg RNAi on adult females of the bedbug.
The insects were photographed and dissected four weeks after the following experimental treatments. From top to bottom: Intact, no injection control; dsbla 20 ng, injection with 20 ng dsRNA of β-lactamase gene; dsClVg 20 ng, injection with 20 ng dsRNA of ClVg gene; dsClVg 200 ng, injection with 200 ng dsRNA of ClVg gene. From left to right: Dorsal, dorsal view of whole insect; Lateral, lateral view of whole insect; Inside, dorsal view of insect whose dorsal plates were removed; Ovary, image of dissected ovaries.
Discussion
Vg proteins are the major yolk precursor proteins whose structure and function are conserved among diverse insect species, although there are some diversity in number of cleavage sites, number of duplicated genes, and tissue-specific expression patterns [6,7]. In this study, we identified two Vg genes, ClVg and ClVg-like, in the bedbug (Fig 1). Some insects possess multiple Vg genes, whose sequences are usually highly similar to each other with retaining conserved functional domains and participating in vitellogenesis [38–41]. However, the two Vg genes of the bedbug are considerably different in their sequences. The ClVg gene exhibited canonical insect Vg protein structures including RXXR cleavage sites and conserved binding sites for a variety of nutrients (Fig 1A), and its transcription was highly up-regulated in the fat body of adult females (Fig 3A). RNAi-mediated silencing of the ClVg gene expression confirmed its primary contribution to oocyte maturation (Figs 5 and 6). These results indicate that the ClVg gene encodes the principal Vg protein involved in the conventional vitellogenic function in the bedbug. On the other hand, the ClVg-like gene lacked the N-terminal lipid-binding region (Fig 1B), although the ClVg gene and the ClVg-like gene are presumably derived from the common ancestral Vg gene (Fig 2). Since the ClVg-like gene still retains putative cleavage sites and some domains typical of insect Vg genes (Fig 1B), the possibility cannot be ruled out that the ClVg-like protein may be subjected to posttranslational processing and also incorporated into the oocytes as typical Vg proteins. However, expression levels of the ClVg-like gene in the fat body were incomparably lower than those of the ClVg gene (Fig 3), refuting the possibility of substantial contribution of ClVg-like protein to vitellogenesis. Considering its structural divergence (Fig 1) and accelerated molecular evolution (Table 2), it is conceivable, although speculative, that the ClVg-like gene might have acquired a novel biological function distinct from the vitellogenic role of the ClVg gene in the evolutionary course of the bedbug. In this context, previous studies suggested that Vg proteins may also be involved in hemolymph clotting [8,42], hormonal regulation [43], innate immune responses [44], and other biological roles. Further studies are needed to address what roles the ClVg-like gene plays in the bedbug.
Although the main location of Vg protein synthesis is the fat body in diverse insects [6,7], there are several reports on Vg protein production outside the fat body. In a blood-sucking reduviid bug Rhodnius prolixus, Vg protein is partially synthesized in the follicle cells, which is also incorporated into the oocytes together with the major Vg protein derived from the fat body [45]. In a blood-sucking tick Haemaphysalis longicornis, one Vg gene is exclusively expressed in the midgut, the other two Vg genes are mainly expressed in the fat body, and all the three genes are involved in oocyte maturation [21]. In the bedbug, we found that the ClVg gene is also expressed in a female-specific paragenital organ called the spermalege (Fig 3). The spermalege is a pouched mesodermal tissue attached to the abdominal inner wall of adult females of cimicid bugs [37], whose function is postulated as a counter adaptation against the traumatic insemination, a peculiar reproductive habit typical of cimicids [46,47]. Although the possibility that the ClVg protein synthesis in the spermalege may somehow contribute to vitellogenesis cannot be ruled out, a more likely possibility is that the ClVg protein may be recruited as a clotting element in this organ [8,42], considering that the spermalege is the site of frequent wounding by traumatic insemination.
We found that RNAi-mediated silencing of the ClVg gene expression effectively inhibits egg production in the bedbug (Figs 5 and 6). The significant inhibitory effects on egg laying became evident two weeks after the dsRNA injection (Fig 5C). The initial egg production is likely attributable to the Vg protein accumulated before the dsRNA injection. Note that we performed the dsRNA injection two days after blood meal, and the bedbugs normally start laying eggs three days after blood feeding [48]. It is also notable that the eggs produced by the ClVg RNAi females tended to suffer low hatching success (Fig 5D), which is also likely attributable to insufficient accumulation of the Vg protein during oogenesis. Furthermore, all females injected with 200 ng dsRNA and most females injected with 20 ng dsRNA completely stopped laying eggs two weeks after the dsRNA injection, in which no mature oocytes were found in the ovaries (Figs 5 and 6). On the basis of these results, we propose that the ClVg gene can be a promising target for RNAi-based control by inducing reproductive arrest of the bedbug.
We also found that abdominal inflation is another remarkable symptom of the ClVg RNAi in the bedbug. The external appearance of the insects looked like that just after full engorgement, but their abdomen was actually full of hypertrophied fat bodies instead of a blood-filled stomach (Fig 6). Such a phenotypic syndrome associated with Vg-RNAi was not observed in the cockroach Blattella germanica [20] and the tick Haemaphysalis longicornis [21], although in the latter species the Malpighian tubules and the rectal sac were abnormally filled with white liquid [21]. The peculiar abdominal inflation may be relevant to feeding habit of the bedbug. Adult bedbugs ingest blood about two times of their own weight in a single meal by expanding intersegmental membranes of the abdomen, and the acquired nutritional resource is equivalent to production of 15–19 eggs [48,49]. The ClVg RNAi may inhibit not only the synthesis of Vg protein in the fat body but also the transportation of associated nutrients from the fat body to the oocytes. Conceivably, nutritional resources derived from blood meals are stagnated in the fat body, thereby resulting in its hypertrophy and consequent abdominal inflation.
In addition to the nutritional stagnation, the abdominal inflation due to the ClVg RNAi may influence the pest status of the bedbug by way of its behavioral modifications. First, because of the stuffed abdomen, the insects may have little room for further blood ingestion (see Fig 6), thereby presumably suppressing their blood-sucking activity. Second, considering the fact that fully-engorged female bedbugs frequently receive traumatic insemination because their swollen abdomen makes it difficult to take a guard posture against sexually aggressive males [37], it seems likely that the ClVg RNAi females with the inflated abdomen may suffer continuous and repetitive harassment by conspecific males. It was reported that accumulated traumas reduce female longevity not only by damage of integument piercing but also by increasing risks of microbial infections [46,47,50,51]. Experimental verification of these behavior-mediated effects of the ClVg RNAi deserves future studies.
The recent global resurgence of bedbug populations with resistance to broad-spectrum chemical insecticides has prompted exploration of new pest management strategies [12]. Our study demonstrated that an RNAi approach targeting the ClVg gene causes drastically suppressed egg production and remarkable abdominal inflation in adult females of the bedbug, which possibly reduces reproduction, feeding frequency and longevity of this notorious pest. These findings suggest the possibility that the ClVg gene is a promising candidate for RNAi-based population management of the bedbug, especially in an early phase of population growth on account of its non-acute effects on the insect reproduction. What needs to be solved for enabling practical applications is the development of technologies for efficient delivery of dsRNA into the bedbugs. Microinjection into individual insects is practically not feasible. The effectiveness of oral ingestion of dsRNA has been reported in several hemipteran insects including the blood-sucking reduviid bug Rhodnius prolixus [52–54]. For agricultural insect pests, application of transgenic plants that produce dsRNA of target insect genes has been attempted [55,56]. For blood-sucking insect pests, however, using transgenic animals for supplying dsRNA-containing blood meal is unrealistic both practically and ethically. Therefore, future studies should be directed to the development of alternative dsRNA delivery methods like chemo-attractive feeding traps [57] and microbial vectors [58,59], in parallel with survey of other target genes for more efficient control of bedbug populations.
Data Availability
The Vg gene sequences determined in this study were deposited in the DNA Data Bank of Japan with the accession numbers LC115022 and LC115023.
Funding Statement
This study was financially supported by the Program for Promotion of Basic and Applied Research for Innovations in Bio-oriented Industry (BRAIN) to TF and NN, and by the Japan Society for the Promotion of Science KAKENHI grant no. 25221107 to TF and NN. MM and MT were supported by the Japan Society for the Promotion of Science Fellowship for Young Scientists.
References
- 1.Hoffmann KH, Lorenz MW. Recent advances in hormones in insect pest control. Phytoparasitica. 1998;26: 323–330. [Google Scholar]
- 2.Gäde G, Goldsworthy GJ. Insect peptide hormones: a selective review of their physiology and potential application for pest control. Pest Manag Sci. 2003;59: 1063–1075. [DOI] [PubMed] [Google Scholar]
- 3.Santos D, Broeck J Vanden, Wynant N. Systemic RNA interference in locusts: reverse genetics and possibilities for locust pest control. Curr Opin Insect Sci. 2014;6: 9–14. [DOI] [PubMed] [Google Scholar]
- 4.Hagedorn HH, Kunkel JG. Vitellogenin and Vitellin in Insects. Annu Rev Entomol. 1979;24: 475–505. [Google Scholar]
- 5.Sappington TW, Raikhel AS. Molecular characteristics of insect vitellogenins and vitellogenin receptors. Insect Biochem Mol Biol. 1998;28: 277–300. [DOI] [PubMed] [Google Scholar]
- 6.Tufail M, Takeda M. Molecular characteristics of insect vitellogenins. J Insect Physiol. 2008;54: 1447–1458. 10.1016/j.jinsphys.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 7.Tufail M, Nagaba Y, Elgendy AM, Takeda M. Regulation of vitellogenin genes in insects. Entomol Sci. 2014;17: 269–282. [Google Scholar]
- 8.Avarre JC, Lubzens E, Babin PJ. Apolipocrustacein, formerly vitellogenin, is the major egg yolk precursor protein in decapod crustaceans and is homologous to insect apolipophorin II/I and vertebrate apolipoprotein B. BMC Evol Biol. 2007;7: 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Usinger RL. Monograph of Cimicidae (Hemiptera, Heteroptera). Entomological Society of America; 1966. [Google Scholar]
- 10.Reinhardt K, Siva-Jothy MT. Lanham MD: Biology of the bed bugs (Cimicidae). Annu Rev Entomol. 2007;52: 351–374. [DOI] [PubMed] [Google Scholar]
- 11.Delaunay P, Blanc V, Giudice PD, Levy-Bencheton A, Chosidow O, Marty P, et al. Bedbugs and infectious diseases. Clin Infect Dis. 2011;52: 200–210. 10.1093/cid/ciq102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies TGE, Field LM, Williamson MS. The re-emergence of the bed bug as a nuisance pest: implications of resistance to the pyrethroid insecticides. Med Vet Entomol. 2012;26: 241–254. 10.1111/j.1365-2915.2011.01006.x [DOI] [PubMed] [Google Scholar]
- 13.Harlan HJ. Bed Bugs 101: the Basics of Cimex lectularius. Am Entomol. 2006;52: 99–101. [Google Scholar]
- 14.Burand JP, Hunter WB. RNAi: future in insect management. J Invertebr Pathol. 2013;112 Suppl: S68–S74. 10.1016/j.jip.2012.07.012 [DOI] [PubMed] [Google Scholar]
- 15.Yu N, Christiaens O, Liu J, Niu J, Cappelle K, Caccia S, et al. Delivery of dsRNA for RNAi in insects: an overview and future directions. Insect Sci. 2013;20: 4–14. 10.1111/j.1744-7917.2012.01534.x [DOI] [PubMed] [Google Scholar]
- 16.Gu L, Knipple DC. Recent advances in RNA interference research in insects: Implications for future insect pest management strategies. Crop Prot. 2013;45: 36–40. [Google Scholar]
- 17.Smagghe G, Swevers L. Editorial overview: Pests and resistance—RNAi research in insects. Curr Opin Insect Sci. 2014;6: iv–v. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Li HC, Miao XX. Feasibility, limitation and possible solutions of RNAi-based technology for insect pest control. Insect Sci. 2013;20: 15–30. 10.1111/j.1744-7917.2012.01513.x [DOI] [PubMed] [Google Scholar]
- 19.Kim YH, Issa MS, Cooper AMW, Zhu KY. RNA interference: Applications and advances in insect toxicology and insect pest management. Pestic Biochem Physiol. 2015;120: 109–117. 10.1016/j.pestbp.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 20.Martín D, Maestro O, Cruz J, Mané-Padrós D, Bellés X. RNAi studies reveal a conserved role for RXR in molting in the cockroach Blattella germanica. J Insect Physiol. 2006;52: 410–416. [DOI] [PubMed] [Google Scholar]
- 21.Boldbaatar D, Umemiya-Shirafuji R, Liao M, Tanaka T, Xuan X, Fujisaki K. Multiple vitellogenins from the Haemaphysalis longicornis tick are crucial for ovarian development. J Insect Physiol. 2010;56: 1587–1598. 10.1016/j.jinsphys.2010.05.019 [DOI] [PubMed] [Google Scholar]
- 22.Ciudad L, Piulachs M, Bellés X. Systemic RNAi of the cockroach vitellogenin receptor results in a phenotype similar to that of the Drosophila yolkless mutant. FEBS J. 2006;273: 325–335. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell RD, Ross E, Osgood C, Sonenshine DE, Donohue K V, Khalil SM, et al. Molecular characterization, tissue-specific expression and RNAi knockdown of the first vitellogenin receptor from a tick. Insect Biochem Mol Biol. 2007;37: 375–388. [DOI] [PubMed] [Google Scholar]
- 24.Lu HL, Vinson SB, Pietrantonio P V. Oocyte membrane localization of vitellogenin receptor coincides with queen flying age, and receptor silencing by RNAi disrupts egg formation in fire ant virgin queens. FEBS J. 2009;276: 3110–3123. 10.1111/j.1742-4658.2009.07029.x [DOI] [PubMed] [Google Scholar]
- 25.Zhu F, Sams S, Moural T, Haynes KF, Potter MF, Palli SR. RNA interference of NADPH-cytochrome P450 reductase results in reduced insecticide resistance in the bed bug, Cimex lectularius. PLoS One. 2012;7: e31037 10.1371/journal.pone.0031037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mamidala P, Mittapelly P, Jones SC, Piermarini PM, Mittapalli O. Molecular characterization of genes encoding inward rectifier potassium (Kir) channels in the bed bug (Cimex lectularius). Comp Biochem Physiol B Biochem Mol Biol. 2013;164: 275–279. 10.1016/j.cbpb.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 27.Hosokawa T, Koga R, Kikuchi Y, Meng XY, Fukatsu T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci USA. 2010;107: 769–774. 10.1073/pnas.0911476107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moriyama M, Koga R, Hosokawa T, Nikoh N, Futahashi R, Fukatsu T. Comparative transcriptomics of the bacteriome and the spermalege of the bedbug Cimex lectularius (Hemiptera: Cimicidae). Appl Entomol Zool. 2012:47:233–243. [Google Scholar]
- 29.Mamidala P, Wijeratne AJ, Wijeratne S, Kornacker K, Sudhamalla B, Rivera-Vega LJ, et al. RNA-Seq and molecular docking reveal multi-level pesticide resistance in the bed bug. BMC Genomics. 2012;13: 6 10.1186/1471-2164-13-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29: 644–652. 10.1038/nbt.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32: 1792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30: 2725–2529. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson-Rechavi M, Huchon D. RRTree: relative-rate tests between groups of sequences on a phylogenetic tree. Bioinformatics. 2000;19: 296–297. [DOI] [PubMed] [Google Scholar]
- 34.Crawley MJ. Statistics: an Introduction Using R. West Sussex: John Wiley & Sons; 2005. [Google Scholar]
- 35.R Core Team. R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 36.Hirai M, Watanabe D, Kiyota A, Chinzei Y. Nucleotide sequence of vitellogenin mRNA in the bean bug, Riptortus clavatus: analysis of processing in the fat body and ovary. Insect Biochem Mol Biol. 1998;28: 537–547. [DOI] [PubMed] [Google Scholar]
- 37.Siva-Jothy MT. Trauma, disease and collateral damage: conflict in cimicids. Philos Trans R Soc Lond B Biol Sci. 2006;361: 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JM, Hatakeyama M, Oishi K. A simple and rapid method for cloning insect vitellogenin cDNAs. Insect Biochem Mol Biol. 2000;30: 189–194. [DOI] [PubMed] [Google Scholar]
- 39.Tufail M, Hatakeyama M, Takeda M. Molecular evidence for two vitellogenin genes and processing of vitellogenins in the American cockroach, Periplaneta americana. Arch Insect Biochem Physiol. 2001;48: 72–80. [DOI] [PubMed] [Google Scholar]
- 40.Tufail M, Bembenek J, Elgendy AM, Takeda M. Evidence for two vitellogenin-related genes in Leucophaea maderae: the protein primary structure and its processing. Arch Insect Biochem Physiol. 2007;66: 190–203. [DOI] [PubMed] [Google Scholar]
- 41.Provost-Javier KN, Chen S, Rasgon JL. Vitellogenin gene expression in autogenous Culex tarsalis. Insect Mol Biol. 2010;19: 423–429. 10.1111/j.1365-2583.2010.00999.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall M, Wang R, van Antwerpen R, Sottrup-Jensen L, Söderhäll K. The crayfish plasma clotting protein: a vitellogenin-related protein responsible for clot formation in crustacean blood. Proc Natl Acad Sci USA. 1999;96: 1965–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guidugli KR, Nascimento AM, Amdam G V, Barchuk AR, Omholt S, Simões ZLP, et al. Vitellogenin regulates hormonal dynamics in the worker caste of a eusocial insect. FEBS Lett. 2005;579: 4961–4965. [DOI] [PubMed] [Google Scholar]
- 44.Zhang S, Wang S, Li H, Li L. Vitellogenin, a multivalent sensor and an antimicrobial effector. Int J Biochem Cell Biol. 2011;43:303–305. 10.1016/j.biocel.2010.11.003 [DOI] [PubMed] [Google Scholar]
- 45.Melo AC, Valle D, Machado EA, Salerno AP, Paiva-Silva GO, Silva NLCE, et al. Synthesis of vitellogenin by the follicle cells of Rhodnius prolixus. Insect Biochem Mol Biol. 2000;30: 549–557. [DOI] [PubMed] [Google Scholar]
- 46.Reinhardt K, Naylor R, Siva-Jothy MT. Reducing a cost of traumatic insemination: female bedbugs evolve a unique organ. Proc Biol Sci. 2003;270: 2371–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrow EH, Arnqvist G. Costly traumatic insemination and a female counter-adaptation in bed bugs. Proc Biol Sci. 2003;270: 2377–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davis N. Studies of the reproductive physiology of Cimicidae (Hemiptera)—I. Fecundation and egg maturation. J Insect Physiol. 1964;10: 947–963. [DOI] [PubMed] [Google Scholar]
- 49.Johnson CG. The ecology of the bed-bug, Cimex lectularius L., in Britain: Report on Research, 1935–40. J Hyg (Lond). 1941;41: 345–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stutt a D, Siva-Jothy MT. Traumatic insemination and sexual conflict in the bed bug Cimex lectularius. Proc Natl Acad Sci USA. 2001;98: 5683–5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reinhardt K, Naylor RA, Siva-Jothy MT. Potential sexual transmission of environmental microbes in a traumatically inseminating insect. Ecol Entomol. 2005;30: 607–611. [Google Scholar]
- 52.Araujo RN, Santos A, Pinto FS, Gontijo NF, Lehane MJ, Pereira MH. RNA interference of the salivary gland nitrophorin 2 in the triatomine bug Rhodnius prolixus (Hemiptera: Reduviidae) by dsRNA ingestion or injection. Insect Biochem Mol Biol. 2006;36: 683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J, Wang XP, Wang MQ, Ma WH, Hua HX. Advances in the use of the RNA interference technique in Hemiptera. Insect Sci. 2013;20: 31–39. 10.1111/j.1744-7917.2012.01550.x [DOI] [PubMed] [Google Scholar]
- 54.Christiaens O, Smagghe G. The challenge of RNAi-mediated control of hemipterans. Curr Opin Insect Sci. 2014;6: 15–21. [DOI] [PubMed] [Google Scholar]
- 55.Baum J, Bogaert T, Clinton W. Control of coleopteran insect pests through RNA interference. Nat Biotechnol. 2007;25: 1322–1326. [DOI] [PubMed] [Google Scholar]
- 56.Mao YB, Cai WJ, Wang JW, Hong GJ, Tao XY, Wang LJ, et al. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat Biotechnol. 2007;25: 1307–1313. [DOI] [PubMed] [Google Scholar]
- 57.Weeks ENI, Birkett MA, Cameron MM, Pickett JA, Logan JG. Semiochemicals of the common bed bug, Cimex lectularius L. (Hemiptera: Cimicidae), and their potential for use in monitoring and control. Pest Manag Sci. 2011;67: 10–20. 10.1002/ps.2024 [DOI] [PubMed] [Google Scholar]
- 58.Tian H, Peng H, Yao Q, Chen H, Xie Q, Tang B, et al. Developmental control of a lepidopteran pest Spodoptera exigua by ingestion of bacteria expressing dsRNA of a non-midgut gene. PLoS One. 2009;4: e6225 10.1371/journal.pone.0006225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gu J, Liu M, Deng Y, Peng H, Chen X. Development of an efficient recombinant mosquito densovirus-mediated RNA interference system and its preliminary application in mosquito control. PLoS One. 2011;6: e21329 10.1371/journal.pone.0021329 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Vg gene sequences determined in this study were deposited in the DNA Data Bank of Japan with the accession numbers LC115022 and LC115023.