Abstract
Background
Use of robotic systems for minimally invasive surgery has rapidly increased during the last decade. Understanding the causes of adverse events and their impact on patients in robot-assisted surgery will help improve systems and operational practices to avoid incidents in the future.
Methods
By developing an automated natural language processing tool, we performed a comprehensive analysis of the adverse events reported to the publicly available MAUDE database (maintained by the U.S. Food and Drug Administration) from 2000 to 2013. We determined the number of events reported per procedure and per surgical specialty, the most common types of device malfunctions and their impact on patients, and the potential causes for catastrophic events such as patient injuries and deaths.
Results
During the study period, 144 deaths (1.4% of the 10,624 reports), 1,391 patient injuries (13.1%), and 8,061 device malfunctions (75.9%) were reported. The numbers of injury and death events per procedure have stayed relatively constant (mean = 83.4, 95% confidence interval (CI), 74.2–92.7 per 100,000 procedures) over the years. Surgical specialties for which robots are extensively used, such as gynecology and urology, had lower numbers of injuries, deaths, and conversions per procedure than more complex surgeries, such as cardiothoracic and head and neck (106.3 vs. 232.9 per 100,000 procedures, Risk Ratio = 2.2, 95% CI, 1.9–2.6). Device and instrument malfunctions, such as falling of burnt/broken pieces of instruments into the patient (14.7%), electrical arcing of instruments (10.5%), unintended operation of instruments (8.6%), system errors (5%), and video/imaging problems (2.6%), constituted a major part of the reports. Device malfunctions impacted patients in terms of injuries or procedure interruptions. In 1,104 (10.4%) of all the events, the procedure was interrupted to restart the system (3.1%), to convert the procedure to non-robotic techniques (7.3%), or to reschedule it (2.5%).
Conclusions
Despite widespread adoption of robotic systems for minimally invasive surgery in the U.S., a non-negligible number of technical difficulties and complications are still being experienced during procedures. Adoption of advanced techniques in design and operation of robotic surgical systems and enhanced mechanisms for adverse event reporting may reduce these preventable incidents in the future.
Introduction
During the last 14 years, over 1.75 million robotic procedures were performed in the United States across various surgical specialties [1]. Surgical robots enable conducting complex minimally invasive procedures with better visualization, increased precision, and enhanced dexterity compared to laparoscopy. Robotic devices provide 3-dimensional magnified views of the surgical field and translate the surgeon’s hand, wrist, and finger movements into precisely engineered movements of miniaturized surgical instruments inside patient’s body. The Intuitive Surgical’s da Vinci robot [2] is currently the only surgical robot approved by the U.S. Food and Drug Administration (FDA), for performing various types of procedures in urologic, gynecologic, general, cardiothoracic, and head and neck surgery. There are also other robotic systems designed for minimally invasive surgery in areas such as neurosurgery and orthopedic surgery (e.g. MAKO Surgical’s RIO Robotic Arm Interactive System for orthopedic surgery [3]) or for research in tele-operated robotic surgery (e.g. the da Vinci research kit [4] and the RAVEN II surgical robot [5][6]).
This study focuses on assessing the safety and effectiveness of robotic surgical systems used in minimally invasive surgery, by analyzing safety incidents experienced during robotic procedures. We retrieved all the nation-wide adverse event reports collected by the publicly available FDA MAUDE database [7] over the 14-year period of 2000–2013. We estimated the prevalence of incidents, including deaths, injuries, and device malfunctions over the years and across six major surgical specialties of gynecology, urology, general, colorectal, cardiothoracic, and head and neck surgery. We further characterized the potential causes for incidents and measured their impact on patients and on the progress of surgery.
There have been previous studies on safety and effectiveness of robotic surgery based on the experience of different surgical institutions as well as analyses of the FDA MAUDE data. However, an important question left unanswered is whether the evolution of the robotic systems with new technologies and safety features over the years has improved the safety of robotic systems and their effectiveness across different surgical specialties.
Our goal is to use the knowledge gained from the analysis of past incidents to provide insights on design of future robotic surgical systems that by taking advantage of advanced safety mechanisms, improved human machine interfaces, and enhanced safety training and operational practices can minimize the adverse impact on both the patients and surgical teams.
Background and Related Work
Previous studies on the safety and effectiveness of minimally invasive robotic surgery focused on its comparison to non-robotic minimally invasive surgical methods (laparoscopy) and analysis of system failures experienced during procedures or the adverse events reported to the FDA MAUDE database. The FDA MAUDE data collected and analyzed in this manuscript cannot be used to determine the relative complication rates between robotic and non-robotic surgery. A summary of related work on such comparison is provided in S1 Table. This section presents an overview on the FDA MAUDE database and previous analyses of adverse events in robotic surgery.
Failures of Robotic Surgical Systems
There have been several reports by individual surgical institutions on the various software-related, mechanical, and electrical failures experienced before or during robotic procedures [8]-[21]. S2 Table summarizes these reports by providing the number and types of procedures performed at each center as well as the failure rates and number of cases in which failures led to conversion or rescheduling of procedures. The rates of device malfunctions and failure-related conversions reported by these studies varied between 0.4–8.0% and 0.1–2.7%, with an average of 3% (95% confidence interval (CI), 1.9–4.2) and 0.9% (95% confidence interval (CI), 0.4–1.4), respectively.
Adverse Events Reports from the FDA MAUDE Database
The Manufacturer and User Facility Device Experience (“MAUDE”) database is a publicly available collection of suspected medical device-related adverse event reports, submitted by mandatory (user facilities, manufacturers, and distributors) and voluntary (health care professionals, patients, and customers) reporters to the FDA [7]. Manufacturers and the FDA regularly monitor these reports to detect and correct device-related safety issues in a timely manner. Each adverse event report contains information such as Device Name; Manufacturer Name; Event Type (“Malfunction,” “Injury,” “Death,” or “Other”); Event Date; Report Date; and human-written Event Description and Manufacturer Narrative fields, which provide a short description of the incident, as well as any comments made or follow-up actions taken by the manufacturer to detect and address device problems [7]. Fig 1 shows an example MAUDE report on an adverse event occurred during a robotic cardiothoracic procedure.
Fig 1. An example adverse event report from the publicly available FDA MAUDE database.
This report is accessible through the online FDA MAUDE database at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/Detail.cfm?MDRFOI__ID=2240665. Other MAUDE reports can also be accessed through searching the online database: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/TextSearch.cfm.
The FDA MAUDE database, as a spontaneous reporting system, suffers from underreporting and inconsistencies [22][23][24]. However, it provides valuable insights on real incidents that occurred during the robotic procedures and impacted patient safety. The reported data on deaths, injuries, and device malfunctions provided by the MAUDE can be treated as a sample set to estimate the lower bounds on the prevalence of adverse events and identify examples of their major causes and patient impacts (see S1 File for more details on the problem of under-reporting).
Table 1 shows summary of related work on analysis of the FDA adverse event reports on robotic surgical systems. All these studies were performed by manual extraction and review of subsets of the MAUDE data. Almost half of the studies only focused on specific types of device failures (e.g., electro-cautery failures [25], electrosurgical injuries [26], and instrument failures [27]) and only considered the gynecology and urology specialties in their analysis. To the best of our knowledge, none of the previous studies considered the total volume of procedures performed in their analysis period and none of them assessed the risk of adverse events across different surgical specialties and their impact on the progress of surgery. This is in part due to the fact that adverse event descriptions in the FDA MAUDE database [7] are mainly composed of free-form natural language text, written by the manufacturers and healthcare professionals, and are often difficult to analyze without understanding semantics and the contextual factors involved in the events. Therefore, (i) manual analysis of incident reports requires significant amount of human effort, and (ii) often not all the contributing causes for incidents can be identified from the reports.
Table 1. Related work on analysis of the FDA adverse event reports on robotic surgical systems.
| Study | No. Reports (Years) | System Under Study | Surgical Specialties | Major Results |
|---|---|---|---|---|
| Murphy et al. [25] | 38 system failures, 78 adverse events (2006–2007) | da Vinci system | N/A | Most of these events were related to broken instrument tips or failures of electrocautery elements. |
| Andonian et al. [28] | 189 (2000–2007) | ZEUS and da Vinci systems | N/A | Estimated failure rate of 0.38% for robotic-assisted laparoscopic surgeries. |
| Lucas et al. [29] | 1,914 (2003 − 2009) | da Vinci system models dV and dVs | N/A | Both device malfunctions and open conversions were reduced by increased robotic experience and newer surgical systems. The number of patient injuries did not change and the number of deaths increased. |
| Fuller et al. [26] | 605 (2001–2011) | da Vinci system | N/A | 24 (3.9%) of reports were related to electrosurgical injuries (ESI), of which 37.5% resulted in surgical intervention. |
| Friedman et al. [27] | 565 (2009–2010) | da Vinci Instruments | N/A | The majority of events were related to the instrument wrist or tip (285), 174 were related to cautery problems, 76 were shaft failures, and the rest were cable and control housing failures (36). |
| Gupta et al. [30] | 741 (2009–2010) | da Vinci system | Urology, Gynecology | The events were related to the use of energy instruments (43.5%), surgical systems (19.3%), and the instruments (11.7%). The severity of events was correlated with the type of surgery and the type of device. |
| Manoucheri et al. [31] | 50 injuries/deaths (2006–2012) | da Vinci system | Gynecology | The majority of injuries (65%) were not directly related to use of robot; 21% were related to operator error; and 14% were due to technical system failures. |
Methods
We developed a natural language processing tool that automatically retrieves all the reported events on robotic surgical systems from the FDA MAUDE database and extracts important safety information from the reports, including types of patient complications, surgical specialties and types of robotic procedures, most common types of system malfunctions, and the actions taken by the surgical teams to recover from failures. Our natural language parsing tool enables large-scale analysis of nation-wide incidents reported to the FDA over any timing period and, thus, facilitates more accurate estimation of the prevalence of incidents over the years and more effective evaluation of robotic surgical systems across different surgical specialties.
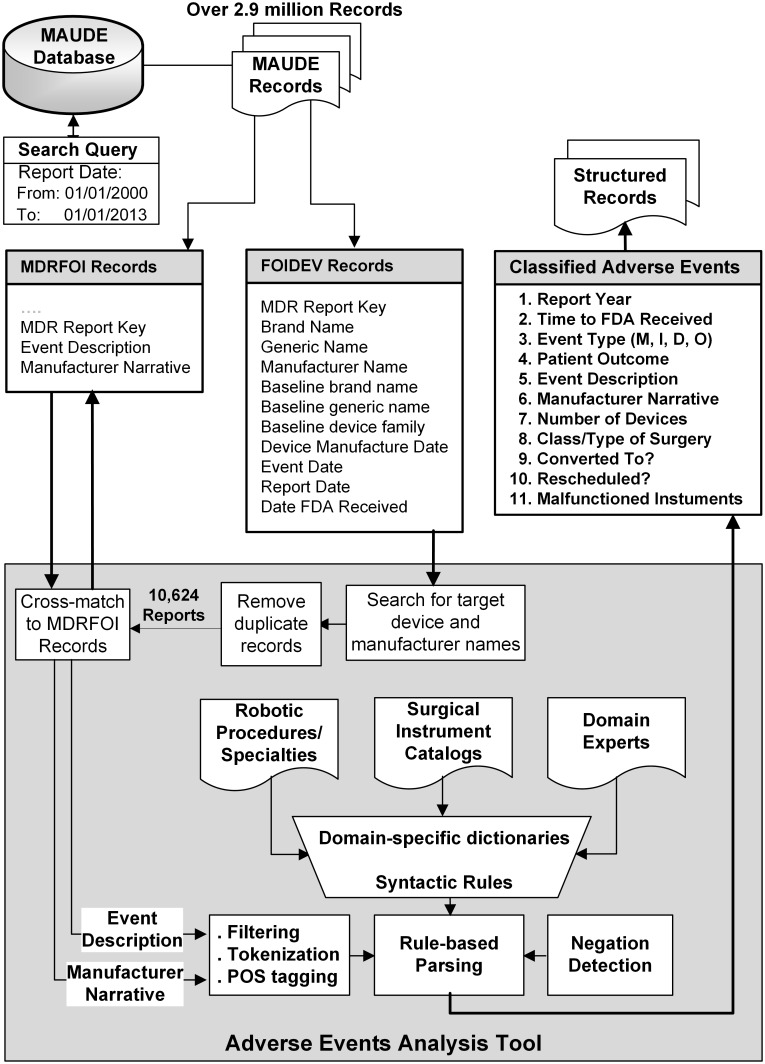
We extracted the reports related to the systems and instruments used in minimally invasive robotic surgery by searching for related keywords (e.g. device and manufacturer names) in the Device Name and Manufacturer Name fields of over 2.9 million MAUDE records posted between January 2000 and December 2013. That led us to an initial list of adverse event reports, from which we filtered out those with duplicate database keys (reporting the same adverse event for multiple devices). In addition to the structured information that was directly available from the reports, we extracted further information from the unstructured human-written descriptions of events by natural language parsing of the Event Description and Manufacturer Narrative fields (see Fig 1). As shown in Fig 2, our natural language processing tool combines domain knowledge with linguistic rules to interpret the semantics of the event descriptions. This was done by creating several domain-specific dictionaries (e.g., for patient complications, surgery types [32], surgical instruments [33], and malfunction types) as well as syntactic rules, parts-of-speech (POS) taggers, and negation detectors [34]. The results generated by each step of our automated analysis tool were manually reviewed for accuracy and validity.
Fig 2. Data extraction and analysis flow from the FDA MAUDE database.
The dictionaries of keywords for surgery types/specialties and surgical instruments were constructed based on the online information available on the da Vinci surgeries [32] and instruments catalog [33] from the manufacturer. The analysis tool was developed using open-source Python libraries for natural language processing, data analysis, and machine learning.
More specifically, we extracted the following information from the reports:
Patient injury (such as burns, cuts, or damage to organs) and death events that were reported under another Event Type, such as “Malfunction” or “Other”.
Surgical specialty and type of robotic procedure during which the adverse events occurred.
Major types of device or instrument malfunctions (e.g., falling of burnt/broken pieces of instruments into patients’ bodies or electrical arcing of instruments)
Adverse events that caused an interruption in the progress of surgery, by leading the surgical team to troubleshoot technical problems (e.g., restarting the system), convert the procedure to non-robotic surgical approaches (e.g., laparoscopy or open surgery), or abort the procedure and reschedule it to a later time.
We compared the number of adverse events (in general) and injury/death events and procedure conversions (in particular) per 100,000 procedures across different surgical specialties. The rate of events was estimated by dividing the number of adverse events that occurred in each year (based on the Event Date) by the annual number of robotic procedures performed in the U.S. The total number of procedures per year was extracted from the device manufacturer’s reports [1][35] for 2004–2013 (see S1 Fig). The annual number of procedures per surgical specialty was available only for gynecology, urology, and general surgery after 2007. So we estimated a combined annual number of procedures for cardiothoracic and head and neck surgery by assuming that the majority of the remaining procedures (other than genecology, urology, and general) were related to these specialties, as, according to the manufacturer reports, they are the only other specialties for which the robot has been used [1]. Due to the possible underreporting of the adverse events in the FDA MAUDE database (the numerator) and the over-reporting of the annual number of robotic procedures in the manufacturing company investor presentations (the denominator), the estimated rates of adverse events per procedures are conservative and represent the lower-bounds on the prevalence of events.
We assumed that the rate of underreporting for injury and death events are low and are independent from the type of surgery, because the device manufacturers are required and monitored by the FDA to report serious injury and death events to the MAUDE database. However, due to possible changes in the reporting rates during the years, the total number of events per procedure in the whole study period (rather than per year) was compared across different surgical specialties. The 2-sided P values (< 0.05) and 95% confidence intervals were used to determine the statistical significance of the results. The cumulative number of malfunctions per procedure was used to evaluate the trends in malfunction rates over 2004–2013.
To characterize the major causes to which injury and death events were attributed, we performed a manual review of event descriptions for all the reports made before 2013. The cumulative number of malfunctions per procedure was used to evaluate the trends in malfunction rates over 2004–2013.
Results
We identified a total of 10,624 events related to the robotic systems and instruments, reported over 2000–2013. About 98% of the events were reported by device manufacturers and distributors, and the rest (2%) were voluntary reports. In the same period, over 1,745,000 robotic procedures were performed in the U.S., so the estimated number of adverse events per procedure was less than 0.6% (95% confidence interval (CI), 0.6–0.62).
Data included 1,535 (14.4%) adverse events with significant negative patient impacts, including injuries (1,391 cases) and deaths (144 cases), and over 8,061 (75.9%) device malfunctions. For the rest of the events (1,028 cases), the Event Type information either was not available or was indicated as “Other.” We identified 160 adverse events (1.5%) that included some kind of patient injuries but were reported as a “Malfunction” or “Other.”
Trends in Adverse Event Reports
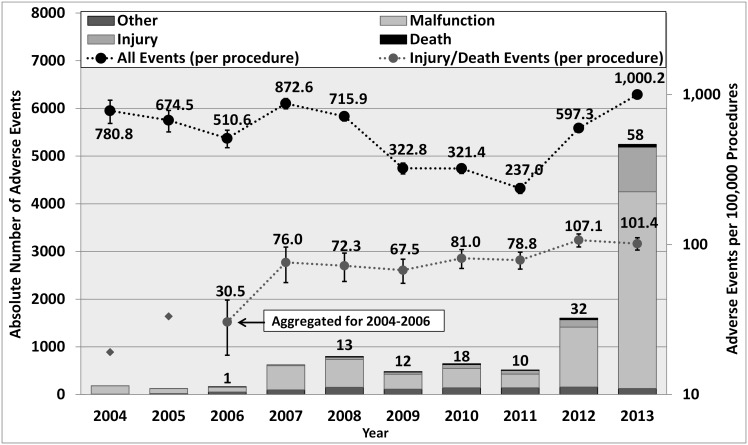
Fig 3 shows the overall trends in the annual numbers of reports and the estimated rates of events per 100,000 procedures over 2004–2013:
Fig 3. Annual Numbers of Adverse Event Reports and Rates of Events per Procedure.
The left Y-axis corresponds to the bars showing the absolute numbers of adverse events (based on the years that reports were received by the FDA). The right Y-axis corresponds to the trend lines showing (in logarithmic scale) the annual number of adverse events per 100,000 procedures (based on the year the events occurred). Numbers on the bars indicate number of deaths reported per year. Error bars represent 95% confidence intervals for the proportion estimates. Because of the small number of injury and death events reported for 2004 and 2005, a combined rate was calculated for 2004–2006. Note that of all the events, 40 were reported as part of the articles or the legal disputes received by the manufacturer.
The absolute number of reports made per year has significantly increased (about 32 times) since 2006, reaching 58 deaths, 938 patient injuries, and 4,124 malfunctions in 2013. The number of robotic procedures performed per year has increased 10-fold in the same period [1][35].
While the annual average number of adverse events was about 550 per 100,000 procedures (95% confidence interval (CI), 410–700) between 2004 and 2011, in 2013 it peaked at about 1,000 events per 100,000 procedures (1 event reported in every 100 procedures).
The numbers of injury and death events per procedure have stayed relatively constant since 2007 (mean = 83.4 per 100,000 procedures, 95% CI, 74.2–92.7).
Adverse Events across Different Surgical Specialties
Table 2 shows the numbers of adverse events reported in different surgical specialties and their impact on patients (injuries or deaths) and progress of surgery (procedure conversion or rescheduling). The last row shows examples of the most common types of procedures reported in each specialty.
Table 2. Adverse events in different surgical specialties: Deaths, injuries, malfunctions, procedure conversion or rescheduling, common types of surgery.
| No. (%) [95% Confidence Interval] | |||||||
|---|---|---|---|---|---|---|---|
| Gynecology | Urology | Cardiothoracic | Head & Neck | Colorectal | General | N/A | |
| 3,194 | 1,565 | 393 | 71 | 301 | 197 | 4,903 | |
| Overall a | (30.1) | (14.7) | (3.7) | (0.7) | (2.8) | (1.9) | (46.2) |
| [29.2–31.0] | [14.0–15.4] | [3.3–4.1] | [0.5–0.9] | [2.5–3.1] | [1.6–2.2] | [45.3–47.1] | |
| Event Type b | |||||||
| Death | 46 | 30 | 25 | 14 | 11 | 11 | 7 |
| (1.4) | (1.9) | (6.4) | (19.7) | (3.7) | (5.6) | (0.1) | |
| [1.0–1.8] | [1.2–2.6] | [4.0–8.8] | [10.4–29.0] | [1.6–5.8] | [2.4–8.8] | [0.0–0.2] | |
| Injury | 818 | 272 | 64 | 14 | 58 | 56 | 109 |
| (25.6) | (17.4) | (16.3) | (19.7) | (19.3) | (28.4) | (2.2) | |
| [24.1–27.1] | [15.5–19.3] | [12.6–20.0] | [10.4–29.0] | [14.8–23.8] | [22.1–34.7] | [1.8–2.6] | |
| Malfunction | 2,103 | 902 | 226 | 35 | 209 | 110 | 4,476 |
| (65.8) | (57.6) | (57.5) | (49.3) | (69.4) | (57.8) | (91.3) | |
| [64.2–67.4] | [55.2–60.0] | [52.6–62.4] | [37.7–60.9] | [64.2–74.6] | [48.9–62.7] | [90.5–92.1] | |
| Other | 227 | 361 | 78 | 8 | 23 | 20 | 311 |
| (7.1) | (23.1) | (19.8) | (11.3) | (7.6) | (10.2) | (6.3) | |
| [6.2–8.0] | [21.0–25.2] | [15.9–23.8] | [3.9–18.7] | [4.6–10.6] | [6.0–14.4] | [5.6–7.0] | |
| 236 | 212 | 66 | 6 | 29 | 14 | 217 | |
| Conversion | (7.4) | (13.5) | (16.8) | (8.5) | (9.6) | (7.1) | (4.4) |
| [6.5–8.3] | [11.8–15.2] | [13.1–20.5] | [2.0–15.0] | [6.3–12.9] | [3.5–10.7] | [3.8–5.0] | |
| 26 | 148 | 11 | 1 | 1 | 6 | 77 | |
| Rescheduling | (0.8) | (9.5) | (2.8) | (1.4) | (0.3) | (3.0) | (1.6) |
| [0.5–1.1] | [8.1–10.9] | [1.2–4.4] | [0–4.1] | [0–1.0] | [0.6–5.4] | [1.3–1.9] | |
| Common Surgery Types | Hysterectomy (2,331) | Prostatectomy (1,291) | Thoracic (110) | Thyroidectomy (19) | Cholecyst-ectomy (118) | Hernia repair (37) | |
| Myomectomy (328) | Nephrectomy (138) | Lobectomy (67) | Tongue base resection (19) | Colectomy (61) | Nissen fundoplication (34) | ||
| Sacrocolpopexy (170) | Pyeloplasty (31) | Mitral valve repair (54) | Transoral robotic (18) | Low anterior resection (44) | Gastric bypass (28) | ||
| Oophorectomy (120) | Cystectomy (48) | Coronary artery bypass (23) | Colon resection (25) | Gastrectomy (15) | |||
a Percentages are over all the adverse event reports (n = 10,624).
b Percentages are over the total adverse events reported for a surgical specialty.
The majority of reports were related to gynecology (30.1%), urology (14.7%), and cardiothoracic (3.7%) surgeries, such as hysterectomy (2,331), prostatectomy (1,291), and thoracic (110) procedures, respectively. The higher percentage of adverse events in gynecologic and urologic surgeries could be explained by the higher number of these procedures performed (86% of all the robotic procedures performed in the U.S.) compared to other surgical specialties (less than 14.2% of all procedures) [1].
Cardiothoracic and head and neck surgeries involved a higher number of deaths per adverse event report (6.4% and 19.7%) than gynecology and urology (1.4 and 1.9%).
The highest number of procedure conversions per adverse event was for cardiothoracic (16.8%) and urology (13.5%), and the highest rates of procedure rescheduling were for urology (9.5%), general (3.0%), and cardiothoracic (2.8%) surgeries.
Of all the reports, only 5,721 (53.8%) indicated the class and type of surgery involved. However, the majority of reports with missing information on the type of surgery were related to device malfunctions and “Other” events (97.6%). In order to compare the rate of adverse events across different specialties, we focused only on reports related to injuries, deaths, and procedure conversions. For the majority of these events (92.2% of injury reports, 95.1% of deaths, and 72.2% of procedure conversions), the surgery type information was available and the rest (with ‘N/A’ surgical specialty) were removed from our analysis. In order to estimate the rate of events per procedure, we regrouped the events into four major categories of “Gynecology,” “Urology,” “General,” and “Cardiothoracic and Head and Neck,” according to the manufacturer’s reports [1][35]. The “General” category includes both colorectal and general specialties.
As shown in Table 3, for cardiothoracic and head and neck surgery, the rates of injuries, deaths, and procedure conversions have been significantly higher than other specialties. During 2007–2013, the estimated rate of deaths have been 52.2 per 100,000 procedures for cardiothoracic and head and neck specialties vs. 5.7 in gynecology, urology, and general surgeries (RR = 9.23, 95% CI, 6.35–13.40, P < 0.0001). Also, the rate of injuries and procedure conversions in these specialties have been 91.0 and 89.7 per 100,000 procedures vs. 71.5 (RR = 1.27, 95% CI, 0.99–1.63, P < 0.052) and 29.2 (RR = 3.07, 95% CI, 2.38–3.97, P < 0.0001) in the other surgical categories.
Table 3. Comparsion of adverse events rates in different surgical specialities (2007–2013).
| No. (rate per 100,000 procedures) a [95% CI] | ||||||
|---|---|---|---|---|---|---|
| Gynecology, Urology, General | Cardiothoracic, Head and Neck, Other | Cardiothoracic and Head and Neck vs. Gynecology, Urology, and General | ||||
| Total Procedures | 1,661,891 | 74,709 | Relative Risk (95% Cl)b | |||
| Total Adverse Events | 5,209 | 447 | P Value | |||
| Event Type | ||||||
| Death | 94 | (5.7) | 39 | (52.2) | 9.23 (6.35–13.40) | < 0.0001 |
| Injury | 1188 | (71.5) | 68 | (91.0) | 1.27 (0.99–1.63) | < 0.052 |
| Conversion | 485 | (29.2) | 67 | (89.7) | 3.07 (2.38–3.97) | < 0.0001 |
| Rescheduling | 180 | (10.8) | 12 | (16.1) | 1.48 (0.83–2.66) | < 0.19 c |
a Percentages are over total number of procedures in each column.
b Assuming that the level of underreporting across different surgical specialties is similar.
c Not statistically significant because of the small number of samples (12) in the cardiothoracic and head and neck surgery.
Device and Instrument Malfunctions
We identified five major categories of device and instrument malfunctions that impacted the patients, either by causing injuries and complications or by interrupting the progress of surgery and/or prolonging procedure times. Table 4 shows the numbers of events in each category, the event types as indicated by reporters (including “Malfunction” (M), “Injury” (IN), “Death” (D), and “Other” (O)), and the actions taken by the surgical team to resolve the problems. The malfunction categories and actions taken by the surgical teams are not mutually exclusive, and in many cases two or three different malfunctions or two actions were reported in a single event. S2 and S3 Figs. use Venn diagrams to depict the intersections among different malfunction categories and actions taken by the surgical team.
Table 4. Major categories of malfunctions.
(Note that the malfunction and surgical team action categories are not mutually exclusive, i.e., in many cases more than one malfunction or action were reported in a single event.)
| Malfunction Category | No. of Reports | Surgical Team Actions (% of malfunction category) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Description | Total | Event Type | System Reset | ProcedureConverted | Procedure Rescheduled | ||||
| (% of all) | M | IN | D | O | |||||
| System | - System error codes and faults | 536 | 231 | 330 | 133 | ||||
| Errors | - System transferred into a recoverable or non-recoverable safety state | (5.0%) | 44 | 23 | 1 | 468 | (43.1%) | (61.6%) | (24.8%) |
| Video/ | - Loss of video | 275 | 21 | 18 | 0 | 236 | 53 | 145 | 94 |
| Imaging Problems | - Display of blurry images at surgeon’s console or assistant’s touchscreen | (2.6%) | (19.3%) | (52.7%) | (34.2%) | ||||
| Broken | - Burnt/broken parts and components | ||||||||
| Pieces | - Fell into surgical field or body cavity | 1,557 | 1,396 | 119 | 1 | 41 | 3 | 38 | 5 |
| Falling Into Patients | - Required additional procedure time to be found/removed from the patient | (14.7%) | (0.2%) | (2.4%) | (0.3%) | ||||
| Broken Tip | - Tears, burns, splits, holes on tip cover | 1,111 | 900 | 193 | 0 | 18 | 2 | 18 | 0 |
| Covers or Elec. Arcing | - Electrical arcing, sparking, charring | (10.5%) | (0.2%) | (1.6%) | (0.0%) | ||||
| Unintended Instrument | - Unintended or unstoppable movements started without the surgeon’s command | 1,078 | 919 | 52 | 2 | 105 | 31 | 93 | 21 |
| Operation | - Instruments not recognized by system | (10.1%) | (2.9%) | (8.6%) | (1.9%) | ||||
| - Instruments not working, open/closed | |||||||||
| Other | - Cable, wire, tube, or instrument damages and breakages | 5,092 | 4,962 | 55 | 1 | 74 | 20 | 62 | 13 |
| - Issues with electrosurgical units, power supplies/cords, patient-side manipulators, etc. | (47.9%) | (0.4%) | (1.2%) | (0.3%) | |||||
| - Other events reported as “Malfunction | |||||||||
| Total | - All malfunctions | 9,377 | 8,061 | 443 | 5 | 868 | 305 | 630 | 246 |
| (% of all) | (88.3%) | (3.3%) | (6.7%) | (2.6%) | |||||
| All Adverse Events | 10,624 | 8,061 | 1,391 | 144 | 1,028 | 334 | 780 | 270 | |
| (100%) | (3.1%) | (7.3%) | (2.5%) | ||||||
System errors and video/imaging problems contributed to 787 (7.4%) of the adverse events and were the major contributors to procedure interruptions, including system resets (274 cases, 82% of all system resets), conversion of the procedures to a non-robotic approach (462 cases, 59.2% of all conversions), and aborting/rescheduling of the procedures (221 cases, 81.8% of all cases). System errors are raised by the existing safety mechanisms of the robot upon detection of device problems that cannot be autonomously recovered from and either require manual system reset (recoverable system errors) or stopping the robotic procedure (non-recoverable system errors). S3 Table lists the descriptions and frequencies of the most common system error codes that we extracted by natural language parsing of the adverse event descriptions in the reports.
Falling of the broken/burnt pieces into the patient’s body constituted about 1,557 (14.7%) of the adverse events. In almost all these cases, the procedure was interrupted, and the surgical team spent some time searching for the missing pieces and retrieving them from the patient (in 119 cases, a patient injury, and in one case a death, was reported).
Electrical arcing, sparking, or charring of instruments and burns or holes developed in the tip cover accessories constituted 1,111 reports (10.5% of the events), leading to nearly 193 injuries, such as burning of tissues.
Unintended operation of instruments, such as uncontrolled movements and spontaneous powering on/off, happened in 1,078 of the adverse events (10.1%), including 52 injuries and 2 deaths.
The Other category in Table 4 represents the malfunctions that could not be classified in any of the classes, including cable and instrument breakages that did not lead to other types of malfunctions, electrosurgical unit and power supply problems, and patient-side manipulator issues.
In total, 9,377 reports were about technical problems, including 1,104 cases (10.4% of all the adverse events) in which the procedure was interrupted and additional time was spent on troubleshooting the errors, resetting the system, and/or converting the procedure to a traditional technique, or rescheduling the procedure to a later time. In 1,019 of cases (10.9% of all the malfunctions), the device or instrument malfunction was detected prior to start of the procedure, of which in 20 cases the procedure was rescheduled to a later time and in 2 cases it was converted to a non-robotic approach.
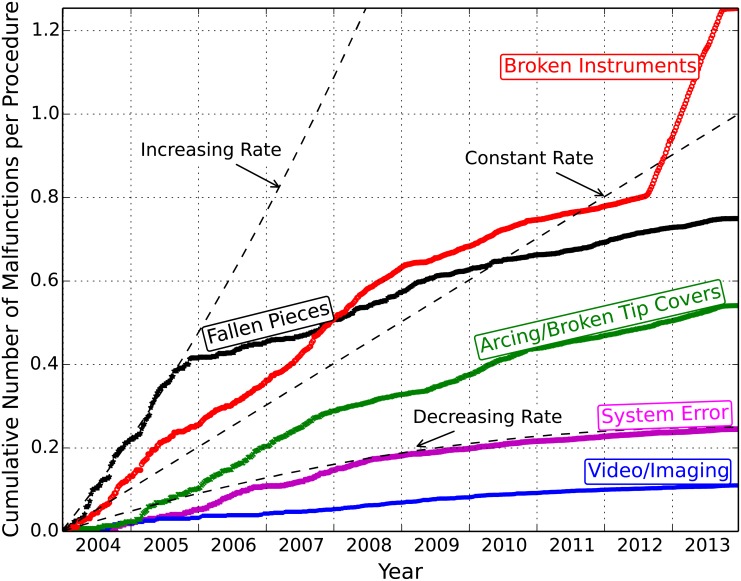
Fig 4 shows the cumulative rates of malfunctions per procedure over 2004–2013. Overall, the malfunction rates decreased after 2006, but the rate of cases with arcing instruments and broken instruments followed a relatively constant trend. The sudden increase in the rate of broken instruments after the middle of 2012 could be related to changes made to the adverse event reporting practices by the manufacturer in 2012 (mostly related to instrument cable breaks) [36], as well as increased reporting of adverse events after concerns about the safety of robotic surgery were raised by the FDA [37][38] and public media in early 2013 [39][40][41].
Fig 4. Cumulative rates of malfunctions per procedure.
The rates of malfunctions per procedure were obtained for each week (see S1 Fig for more details on the estimation of the number of procedures).
Injury and Death Causes
A manual review of a sample set of injury and death reports (from 2000–2012) allowed us to classify the causes indicated by reporters into three main categories: inherent risks associated with surgery, technical issues with the robot, and mistakes made by the surgical team. For the majority of death events, little or no information was provided in the reports. About 50% of the death events were indicated by the reporters to be related to inherent risks or complications of surgery, 11.6% due to patient’s history or health state, and 7% were attributed to surgeon/staff mistakes (e.g., incorrect instrument change or accidental cuts of artery). Of all the reported deaths in different classes of surgery, at least 75.3% (64 of 86) happened after the procedure (mainly due to patient history, infection/sepsis, or uncontrollable and heavy bleeding) and 17.4% (15 of 86) happened during the procedure. Of the deaths that occurred during the procedures, five were due to inadvertent cuts or punctures of organs and the others were related to complications such as uncontrolled bleeding, pulmonary embolism, and cardiac arrest.
As Table 5 shows, about 62% of the injury events involved device malfunctions and the rest were related to operator errors (7.1%), improper positioning of patient or port incisions (6.3%), inherent risks of surgery (3.9%), or problems with grounding the equipment (1.5%). S4 Table lists example reports on device malfunctions that impacted patients during cardiothoracic procedures.
Table 5. Summary of death and injury reports (2000–2012).
| Death Reports (Total = 86) | ||
| Example Causes | Number of Reports (%) | |
| Surgeon/staff mistake | 6 (7.0%) | |
| Patient’s history | 10 (11.6%) | |
| Inherent risks and complications | 43 (50.0%) | |
| N/A | 27 (31.4%) | |
| During Procedure | Punctures, bleeding, pulmonary embolism, cardiac arrest | 15 (17.4%) |
| After Procedure | Infection/sepsis, heavy bleeding | 64 (75.3%) |
| Injury Reports (Total = 410) | ||
| Example Causes | Number of Reports (%) | |
| Device malfunctions | 254 (62.0%) | |
| Surgeon/staff mistake | 29 (7.1%) | |
| Improper positioning of the patient led to post-operation complications such as nerve damage | 17 (4.1%) | |
| Inherent risks of surgery and patient history | 16 (3.9%) | |
| Burning of tissues near port incisions | 9 (2.2%) | |
| Possible passing of the electrosurgical unit currents through instruments to the patient body | 6 (1.5%) | |
| Surgeon felt shocking at the surgeon-side console | 2 (0.5%) | |
| N/A | 77 (18.8%) | |
A more in-depth review of several injury and death reports showed that adverse events are often not due to a single root cause (e.g., a given component failure or a human error), but a combination of multiple causal factors and underlying conditions that lead to safety hazards and incidents. For example, the report shown in Fig 1 (MAUDE report no. 2240665), describes an event where an electronic component failure, a non-recoverable system error, inadequate training and troubleshooting procedures for dealing with technical problems (non-recoverable system errors), and possibly ineffective decisions made by human operators (surgeon deciding to manually operate the endoscopic camera instead of converting the procedure) each played a role in a very long procedure time, loss of carbon dioxide insufflation, and consequently, a patient injury. The following are the common flawed operational practices used by the surgical team that contributed to catastrophic events during surgery:
Inadequate experience with handling emergency situations
Lack of training with specific system features
Inadequate troubleshooting of technical problems
Inadequate system/instrument checks before procedure
Incorrect port placements
Incorrect electro-cautery settings
Incorrect cable connections
Inadequate manipulation of robot master controls
Inadequate coordination between hand & foot movements
Incorrect manipulation or exchange of instruments
A more comprehensive analysis of multi-dimensional causes of incidents is the topic of the future research.
Discussion
Our analysis shows an increasing number of adverse events related to the robotic surgical systems being reported. As cautioned by the FDA [7][37], the number of MAUDE reports may not be used to evaluate the changes in rates of events over time, because the increased reporting of events may be due to different factors, e.g., the increasing use of surgical systems [1], changes in the manufacturers’ reporting practices [36], and/or better awareness and increased publicity resulting from product recalls, media coverage, and litigation [37]. Therefore, we measured the prevalence of adverse events in each year by estimating the number of events reported per procedure. We found that despite a relatively high number of reports, the vast majority of procedures were successful and did not involve any problems and the number of injuries/death events per procedure has stayed relatively constant since 2007. However, total number of malfunctions reported per procedure (0.46%, 95% confidence interval (CI), 0.45–0.47%) was about 6 times lower than the average number of malfunctions per procedure (3%, 95% confidence interval (CI), 1.9–4.2) published by different surgical institutions (see S2 Table). Also the total number of injuries and deaths reported per procedure (0.08%, 95% confidence interval (CI), 0.08–0.09%) was about the same as the predicted complication rates for robotic surgery [42] but an order of magnitude less than the lowest rate of complications reported for robotic surgery in previous studies (2% [43]) (see S1 Table). This further confirms the uncertainty in the rates of events due to possible under-reporting in MAUDE data and possible changes in reporting practices.
Our analysis shows that estimated number of events per procedure in complex surgical areas, such as cardiothoracic and head and neck surgery were significantly higher than gynecology, urology, and general surgeries. Although not all the reported injuries and deaths were due to device problems, and the procedure conversions, of themselves, cannot be considered adverse events [44][45], the estimated numbers of injury/death events and conversions per procedure can be used as a metric to measure the difficulty experienced in different surgical specialties. The best that we can tell from the available data is that the higher number of injury, death, and conversion per adverse event, in cardiothoracic and head and neck surgeries, could be indirectly explained by the higher complexity of the procedures, less frequent use of robotic devices, and less robotic expertise in these fields. Although the use of robotic technology has rapidly grown in urology and gynecology for prostatectomy and hysterectomy, it has been slow to percolate into more complex areas of cardiothoracic and head and neck surgery. Between 2007 and 2013, over 1.4 million (86%) robotic procedures in gynecology and urology were performed in the U.S., while the number of procedures in other surgical specialties altogether was less than 250,000 (14.2%) [1]. The limitations of the robotic user interface [46], long procedure times [47], steep learning curve [48][49], and higher costs for purchase and maintenance of robotic systems and instruments [50] are some factors that may have contributed to the lower utilization of the robotic approach in more complex surgical procedures. For example, only a select type of robotic cardiac procedures are reported to have been successfully performed using the robots, such as mitral valve repair and internal mammary artery harvest [51][52][53]. The experiences of highly competent robotic teams that performed multi-vessel coronary artery bypass grafting (CABG) showed that the robotic approach may be associated with higher mortality and morbidity rates compared to open surgery [54]. In head and surgery, there may be the problem of close anatomical proximity to many vascular and neurological structures that may increase procedural complexity. Also, bleeding when it occurs may be difficult to control because of increased vascularity.
In practice, the use of the robotic platform involves the interface of a sophisticated machine with surgical teams, in an area of patient care that is safety-critical. From a technology perspective, employing substantially improved safety practices and controls in the design, operation, and validation of robotic surgical systems could prevent some of the reported events. Some examples include:
Improved human-machine interfaces and surgical simulators that train surgical teams for handling technical problems [55][56] and assess their actions in real-time during the surgery.
Providing real-time feedback to the surgeon on the safe surgical paths that can be taken or safety barriers that prevent the robotic tools to enter to certain portions in the surgical workspace [57], based on the patient-specific anatomical models, as well as surgeon-specific modeling and monitoring of robotic surgical motions [58], to minimize the risk of approaching dangerous limits and inadvertent patient injuries.
New safety engines for monitoring of procedures (including surgeon, patient, and device status) and providing comprehensive feedback to surgical team on upcoming events and troubleshooting procedures to prevent long procedure interruptions.
Improved mechanisms for logging and reporting of incidents experienced during procedures to enable more accurate validation of safety and effectiveness of surgical systems.
Limitations
The results of our study come with the caveats that: (i) inherent risks exist in all surgical procedures (more so in complex procedures), (ii) the non-device-related adverse events (e.g., caused by human errors) are less likely to be reported to the MAUDE database, and (iii) the MAUDE database suffers from underreporting and inconsistencies. Thus, the estimated number of adverse events per procedure are likely to be lower than the actual numbers in robotic surgery. Further, the lack of detailed information in the reports makes it difficult to determine the exact causes and circumstances underlying the events. Therefore, the sensitivity of adverse event trends to changes in reporting mechanisms, surgical team expertise, and inherent risks of surgery could not be assessed based on this data.
Conclusions
While the robotic surgical systems have been successfully adopted in many different specialties, this study demonstrates several important findings: (i) the overall numbers of injury and death events per procedure have stayed relatively constant over the years, (ii) the probability of events in complex surgical specialties of cardiothoracic and head and neck surgery has been higher than other specialties, (iii) device and instrument malfunctions have affected thousands of patients and surgical teams by causing complications and prolonged procedure times.
As the surgical systems continue to evolve with new technologies, uniform standards for surgical team training, advanced human machine interfaces, improved accident investigation and reporting mechanisms, and safety-based design techniques should be developed to reduce incident rates in the future.
Supporting Information
The annual numbers of procedures performed in the U.S. for 2010–2013 were extracted from the annual reports of the manufacturer. For 2004–2009, we estimated the numbers of procedures by measuring the graphs in the robot manufacturer’s investor presentations. Whenever the estimated numbers from two different sources did not match or the data were available only for the total worldwide procedures, we chose the maximum number of procedures for that year in order to achieve a lower bound on the likelihood of events.
We estimated the number of procedures per week from annual number of procedures by fitting a 4-degree polynomial curve (R2 = 0.999) to the bar graph of annual procedures and calculating the area under the fitted curve for every week.
(EPS)
A total of 3,067 adverse event reports were not classified by MedSafe in any of the malfunction categories.
(TIFF)
For 9,520 of adverse events, no system reset, conversion, or rescheduling were reported.
(TIFF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
This paper was presented as the J. Maxwell Chamberlain Memorial Paper for adult cardiac surgery at the 50th annual meeting of The Society of Thoracic Surgeons (STS) in 2014. Earlier drafts of the manuscript have been deposited in arXiv at http://arxiv.org/abs/1507.03518. We would like to express our special thanks to anonymous reviewers for their constructive comments and to Prof. Ditlev Monrad from the Department of Statistics at the University of Illinois at Urbana-Champaign for reviewing the manuscript and many helpful suggestions. We also thank Carol Bosley and Jenny Applequist for their editing of the manuscript.
Data Availability
Data are available from: https://figshare.com/articles/FDA_MAUDE_Robotic_Surgery_Raw_Data_2000_2013_csv/3084655. The source code for the software tool used for deriving the statistics in the manuscript is available from: https://github.com/homa-alem/MedSafe_MAUDE.
Funding Statement
This research was supported, in part, by grants no. NSF CNS 15-45069 and NSF CNS 13-14891 from the National Science Foundation, Jump ARCHES Endowment for Engineering and Health Care Innovation from the OSF Foundation (Peoria, IL), an unrestricted grant from Infosys, and a faculty award from IBM (HA, ZK, and RKI received the funding). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1."Annual Report 2013," Intuitive Surgical; http://phx.corporate-ir.net/External.File?item=UGFyZW50SUQ9MjIzOTk3fENoaWxkSUQ9LTF8VHlwZT0z&t=1.
- 2."The da Vinci Surgical System," Intuitive Surgical Inc.; http://www.intuitivesurgical.com/products/davinci_surgical_system/.
- 3."RIO® Robotic Arm Interactive System," MAKO Surgical Corp., http://www.makosurgical.com/physicians/products/rio.
- 4.Kazanzidesf, Peter, et al. "An open-source research kit for the da Vinci® Surgical System." Robotics and Automation (ICRA), 2014 IEEE International Conference on. IEEE, 2014.
- 5.Lum Mitchell JH, et al. "The RAVEN: Design and validation of a telesurgery system." The International Journal of Robotics Research 289 (2009): 1183–1197. [Google Scholar]
- 6.Hannaford Blake, et al. "Raven-II: an open platform for surgical robotics research." IEEE Transactions on Biomedical Engineering, 604 (2013): 954–959. 10.1109/TBME.2012.2228858 [DOI] [PubMed] [Google Scholar]
- 7."MAUDE: Manufacturer and User Facility Device Experience," U.S. Food and Drug Administration, http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/search.CFM. [Google Scholar]
- 8.Eichel L, Ahlering TE, Clayman RV. "Robotics in Urologic Surgery: Risks and Benefits." AUA Update Series 2005; 24(lesson 13): 106–111. [Google Scholar]
- 9.Kozlowski PM, Porter CR, Corman JM. "Mechanical failure rate of DaVinci robotic system: Implications for pre-op patient counseling." Journal of Urology 1754 (2006):372–373. [Google Scholar]
- 10.Borden L. S. Jr, Kozlowski P. M., Porter C. R., and Corman J. M.. "Mechanical failure rate of da Vinci robotic system." The Canadian Journal of Urology 142 (2007): 3499–3501. [PubMed] [Google Scholar]
- 11.Zorn Kevin C., et al. "Da Vinci robot error and failure rates: single institution experience on a single three-arm robot unit of more than 700 consecutive robot-assisted laparoscopic radical prostatectomies." Journal of Endourology 2111 (2007): 1341–1344. [DOI] [PubMed] [Google Scholar]
- 12.Fischer Boris, Engel Nadja, Fehr Jean-Luc, and John Hubert. "Complications of robotic assisted radical prostatectomy." World journal of urology 266 (2008): 595–602. 10.1007/s00345-008-0287-7 [DOI] [PubMed] [Google Scholar]
- 13.Lavery Hugh J., et al. "Robotic equipment malfunction during robotic prostatectomy: a multi-institutional study." Journal of Endourology 229 (2008): 2165–2168. 10.1089/end.2007.0407 [DOI] [PubMed] [Google Scholar]
- 14.Ham Won Sik, et al. "Malfunction of da Vinci robotic system-disassembled surgeon's console hand piece: Case report and review of the literature." Urology 731 (2009): 209–e7. [DOI] [PubMed] [Google Scholar]
- 15.Kim Won Tae, et al. "Failure and malfunction of da Vinci Surgical systems during various robotic surgeries: experience from six departments at a single institute." Urology 746 (2009): 1234–1237. 10.1016/j.urology.2009.05.071 [DOI] [PubMed] [Google Scholar]
- 16.Kaushik Dharam, et al. "Malfunction of the Da Vinci robotic system during robot-assisted laparoscopic prostatectomy: an international survey." Journal of Endourology 244 (2010): 571–575. 10.1089/end.2009.0489 [DOI] [PubMed] [Google Scholar]
- 17.Finan Michael A., and Rocconi Rodney P.. "Overcoming technical challenges with robotic surgery in gynecologic oncology." Surgical Endoscopy 246 (2010): 1256–1260. 10.1007/s00464-009-0756-0 [DOI] [PubMed] [Google Scholar]
- 18.Mues Adam C., Box Geoffrey N., and Abaza Ronney. "Robotic instrument insulation failure: initial report of a potential source of patient injury." Urology 771 (2011): 104–107. 10.1016/j.urology.2010.01.093 [DOI] [PubMed] [Google Scholar]
- 19.Agcaoglu Orhan, et al. "Malfunction and failure of robotic systems during general surgical procedures." Surgical Endoscopy 2612 (2012): 3580–3583. 10.1007/s00464-012-2370-9 [DOI] [PubMed] [Google Scholar]
- 20.Chen Cheng-Che, et al. "Malfunction of the da Vinci robotic system in urology." International Journal of Urology 198 (2012): 736–740. 10.1111/j.1442-2042.2012.03010.x [DOI] [PubMed] [Google Scholar]
- 21.Buchs Nicolas C., et al. "Reliability of robotic system during general surgical procedures in a university hospital." The American Journal of Surgery 2071 (2014): 84–88. 10.1016/j.amjsurg.2013.06.007 [DOI] [PubMed] [Google Scholar]
- 22."Adverse Event Reporting of Medical Devices," U.S. Department of Health and Human Services, Office of Inspector General (OEI-01-08-00110), October 2009; https://oig.hhs.gov/oei/reports/oei-01-08-00110.pdf.
- 23.Hauser Robert G., et al. "Deaths and cardiovascular injuries due to device-assisted implantable cardioverter-defibrillator and pacemaker lead extraction." Europace 123 (2010):395–401. 10.1093/europace/eup375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper Michol A., et al. "Underreporting of Robotic Surgery Complications." Journal of Healthcare Quality (2013). [DOI] [PubMed] [Google Scholar]
- 25.Murphy D., et al. "Complications in robotic urological surgery." Minerva urologica e nefrologica = The Italian journal of urology and nephrology 592 (2007): 191–198. [PubMed] [Google Scholar]
- 26.Fuller Andrew, Vilos George A., and Pautler Stephen E.. "Electrosurgical Injuries during Robot Assisted Surgery: Insights from the FDA MAUDE Database." in SPIE BiOS 8207 (2012): 820714. [Google Scholar]
- 27.Friedman Diana CW, Lendvay Thomas S., and Hannaford Blake. "Instrument Failures for the da Vinci Surgical System: a Food and Drug Administration MAUDE Database Study." Surgical endoscopy 275 (2013): 1503–1508. 10.1007/s00464-012-2659-8 [DOI] [PubMed] [Google Scholar]
- 28.Andonian Sero, et al. "Device Failures Associated with Patient Injuries during Robot-assisted Laparoscopic Surgeries: A Comprehensive Review of FDA MAUDE Database." Canadian Journal of Urology 15, no. 1 (2008): 3912. [PubMed] [Google Scholar]
- 29.Lucas Steven M., Pattison Erik A., and Sundaram Chandru P.. "Global Robotic Experience and the Type of Surgical System Impact the Types of Robotic Malfunctions and their Clinical Consequences: An FDA MAUDE Review." BJU International 1098 (2012): 1222–1227. 10.1111/j.1464-410X.2011.10692.x [DOI] [PubMed] [Google Scholar]
- 30.Gupta Priyanka, et al. "855 adverse events associated with the davinci surgical system as reported in the fda maude database." The Journal of Urology 1894 (2013): e351. [Google Scholar]
- 31.Manoucheri Elmira, et al. "MAUDE-Analysis of Robotic-Assisted Gynecologic Surgery." Journal of Minimally Invasive Gynecology 214 (2014): 592–595. 10.1016/j.jmig.2013.12.122 [DOI] [PubMed] [Google Scholar]
- 32."da Vinci® Procedures," da Vinci Surgery, http://www.davincisurgery.com/da-vinci-surgery/da-vinci-procedures/.
- 33."EndoWrist® Instrument & Accessory Catalog," Intuitive Surgical, Inc., January 2013; http://www.intuitivesurgical.com/products/871145_Instrument_Accessory_%20Catalog.pdf.
- 34.Manning Christopher D. Foundations of statistical natural language processing. Edited by Hinrich Schütze. Cambridge: MIT press, 1999. [Google Scholar]
- 35.Investor Presentation, Intuitive Surgical, Inc., Q1 2013, http://phx.corporate-ir.net/External.File?item=UGFyZW50SUQ9MTcwOTQ5fENoaWxkSUQ9LTF8VHlwZT0z&t=1.
- 36."Intuitive Surgical Comments on Medical Device Reporting Practices," Intuitive Surgical, Inc., March 13, 2013; http://investor.intuitivesurgical.com/phoenix.zhtml?c=122359&p=irol-newsArticle&id=1796011. [Google Scholar]
- 37."Computer-Assisted (Robotic) Surgical Systems: What are Computer-Assisted (Robotic) Surgical Systems?" U.S. Food and Drug Administration, November 2013; http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/SurgeryandLifeSupport/ComputerAssistedRoboticSurgicalSystems/default.htm. [Google Scholar]
- 38.U.S. Food and Drug Administration, Warning Letters, July 16, 2013; http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2013/ucm363260.htm.
- 39."Has the Halo Been Broken on Intuitive Surgical?" Citron Research, December 19, 2012; http://www.citronresearch.com/wp-content/uploads/2012/12/isrg-final1.pdf.
- 40."Intuitive Surgical: Angel with Broken Wings, or the Devil in Disguise?" Citron Research, January 17, 2013; http://www.citronresearch.com/wp-content/uploads/2013/01/Intuitive-Surgical-part-two-final.pdf.
- 41."FDA Investigates Robotic Surgery System after Adverse Event Spike," Medscape Today; http://www.medscape.com/viewarticle/803339.
- 42.Robertson Clare, et al. "Relative effectiveness of robot?assisted and standard laparoscopic prostatectomy as alternatives to open radical prostatectomy for treatment of localized prostate cancer: a systematic review and mixed treatment comparison meta?analysis." BJU International 1126 (2013): 798–812. 10.1111/bju.12247 [DOI] [PubMed] [Google Scholar]
- 43.Breitenstein Stefan, et al. "Robotic-assisted versus laparoscopic cholecystectomy: outcome and cost analyses of a case-matched control study." Annals of Surgery 2476 (2008): 987–93. 10.1097/SLA.0b013e318172501f [DOI] [PubMed] [Google Scholar]
- 44.Marusch Frank, et al. "Importance of conversion for results obtained with laparoscopic colorectal surgery." Diseases of the colon & rectum 442 (2001): 207–214. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez Rodrigo, et al. "Consequences of conversion in laparoscopic colorectal surgery." Diseases of the colon & rectum 492 (2006): 197–204. [DOI] [PubMed] [Google Scholar]
- 46.Simorov Anton, et al. "Review of surgical robotics user interface: what is the best way to control robotic surgery?." Surgical Endosopy 268 (2012): 2117–2125. [DOI] [PubMed] [Google Scholar]
- 47.Seco Michael, et al. "Systematic review of robotic minimally invasive mitral valve surgery". Annals of Cardiothoracic Surgery 26 (2013): 704–716. 10.3978/j.issn.2225-319X.2013.10.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holzhey David M., et al. "Learning minimally invasive mitral valve surgery: a cumulative sum sequential probability analysis of 3895 operations from a single high-volume center." Circulation 1285 (2013):483–491. 10.1161/CIRCULATIONAHA.112.001402 [DOI] [PubMed] [Google Scholar]
- 49.Lee Jandee, et al. "The learning curve for robotic thyroidectomy: a multicenter study." Annals of Surgical Oncology 181 (2011): 226–232. 10.1245/s10434-010-1220-z [DOI] [PubMed] [Google Scholar]
- 50.Barbash Gabriel I., and Glied Sherry A.. "New technology and health care costs-the case of robot-assisted surgery." New England Journal of Medicine 3638 (2010): 701–704. 10.1056/NEJMp1006602 [DOI] [PubMed] [Google Scholar]
- 51.Damiano Ralph J. "Robotics in cardiac surgery: the emperor's new clothes." The Journal of thoracic and cardiovascular surgery 1343 (2007): 559–561. [DOI] [PubMed] [Google Scholar]
- 52.Robicsek Francis. "Robotic cardiac surgery: Time told!." The Journal of thoracic and cardiovascular surgery 1352 (2008): 243–246. 10.1016/j.jtcvs.2007.05.072 [DOI] [PubMed] [Google Scholar]
- 53.Modi Paul, Hassan Ansar, and Chitwood Walter Randolph. "Minimally invasive mitral valve surgery: a systematic review and meta-analysis." European Journal of Cardio-Thoracic Surgery 345 (2008): 943–952. 10.1016/j.ejcts.2008.07.057 [DOI] [PubMed] [Google Scholar]
- 54.Dhawan Richa, et al. "Multivessel beating heart robotic myocardial revascularization increases morbidity and mortality." The Journal of thoracic and cardiovascular surgery 1435 (2012): 1056–1061. 10.1016/j.jtcvs.2011.06.023 [DOI] [PubMed] [Google Scholar]
- 55.Alemzadeh Homa, et al. "A Software Framework for Simulation of Safety Hazards in Robotic Surgical Systems," SIGBED Review (Special Issue on Medical Cyber Physical Systems Workshop) 124 (2015). [Google Scholar]
- 56.Alemzadeh, Homa, et al. "Simulation-based Training for Safety Incidents: Lessons from Analysis of Adverse Events in Robotic Surgical Systems," American College of Surgeons' 8th Annual Meeting of the Consortium of ACS-accredited Education Institutes, March 2015.
- 57.Taylor Russell H., et al. "Medical robotics and computer-integrated surgery" Springer handbook of robotics. Springer Berlin; Heidelberg, 2008. 1199–1222. [Google Scholar]
- 58.Lin Henry C., Shafran Izhak, Yuh David, and Hager Gregory D.. "Towards automatic skill evaluation: Detection and segmentation of robot-assisted surgical motions." Computer Aided Surgery 115 (2006): 220–230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The annual numbers of procedures performed in the U.S. for 2010–2013 were extracted from the annual reports of the manufacturer. For 2004–2009, we estimated the numbers of procedures by measuring the graphs in the robot manufacturer’s investor presentations. Whenever the estimated numbers from two different sources did not match or the data were available only for the total worldwide procedures, we chose the maximum number of procedures for that year in order to achieve a lower bound on the likelihood of events.
We estimated the number of procedures per week from annual number of procedures by fitting a 4-degree polynomial curve (R2 = 0.999) to the bar graph of annual procedures and calculating the area under the fitted curve for every week.
(EPS)
A total of 3,067 adverse event reports were not classified by MedSafe in any of the malfunction categories.
(TIFF)
For 9,520 of adverse events, no system reset, conversion, or rescheduling were reported.
(TIFF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
Data are available from: https://figshare.com/articles/FDA_MAUDE_Robotic_Surgery_Raw_Data_2000_2013_csv/3084655. The source code for the software tool used for deriving the statistics in the manuscript is available from: https://github.com/homa-alem/MedSafe_MAUDE.